Abstract
Salmonella enterica serovar Typhimurium melibiose permease (MelBSt) is a prototype of the Na+-coupled major facilitator superfamily transporters, which are important for the cellular uptake of molecules including sugars and small drugs. Although the symport mechanisms have been well-studied, mechanisms of substrate binding and translocation remain enigmatic. We have previously determined the sugar-binding site of outward-facing MelBSt by crystallography. To obtain other key kinetic states, here we raised camelid single-domain nanobodies (Nbs) and carried out a screening against the WT MelBSt under 4 ligand conditions. We applied an in vivo cAMP-dependent two-hybrid assay to detect interactions of Nbs with MelBSt and melibiose transport assays to determine the effects on MelBSt functions. We found that all selected Nbs showed partial to complete inhibitions of MelBSt transport activities, confirming their intracellular interactions. A group of Nbs (714, 725, and 733) was purified, and isothermal titration calorimetry measurements showed that their binding affinities were significantly inhibited by the substrate melibiose. When titrating melibiose to the MelBSt/Nb complexes, Nb also inhibited the sugar-binding. However, the Nb733/MelBSt complex retained binding to the coupling cation Na+ and also to the regulatory enzyme EIIAGlc of the glucose-specific phosphoenolpyruvate/sugar phosphotransferase system. Further, EIIAGlc/MelBSt complex also retained binding to Nb733 and formed a stable supercomplex. All data indicated that MelBSt trapped by Nbs retained its physiological functions and the trapped conformation is similar to that bound by the physiological regulator EIIAGlc. Therefore, these conformational Nbs can be useful tools for further structural, functional, and conformational analyses.
Keywords: Nanobody, conformational binder, protein-protein interactions, ITC, binding, sugar fermentations, two-hybrid assay, EIIAGlc
Nanobodies (Nbs) are the variable domains of single polypeptide heavy-chain antibodies from camelid mammals such as camels and llamas (1). Nbs are as small as one-tenth of common antibodies and are characteristic of longer complementarity-determining region 3 (CDR-3) in length (2). Their finger-like motifs can recognize cleft or hidden epitopes unavailable to monoclonal antibodies. They are known to exhibit high specificity and affinity. Conformation-specific Nbs can trap the membrane transport proteins of interest at high-energy states by binding to epitopes that might be transiently available. Nbs have been successfully applied for structural and functional studies of many membrane proteins (3, 4, 5, 6, 7).
Major facilitator superfamily (MFS) transporters are widely expressed from bacteria to mammalian cells and play important roles in health and disease (8). The MFS transporters contain a large group of cation-coupled symporters or antiporters. The melibiose permease of Salmonella enterica serovar Typhimurium (MelBSt) catalyzes the symport of a galactopyranoside with a cation (H+, Li+, or Na+). It is a prototype for Na+-coupled MFS transporters and serves as a model system for studying cation-coupled transport mechanisms (9, 10, 11, 12, 13, 14). The computational predictions (15, 16) and high-resolution 3D X-ray crystal structure determination (10, 14) are consistent with the canonical MFS fold. The crystal structures with a bound sugar analog are also determined, which reveals the sugar specificity determinant pocket and the sugar recognition mechanism (14). MelBSt in all solved X-ray crystal structures is at an outward-facing conformation; the substrate-binding site is only accessible from the periplasmic side (Fig. 1). The presence of a physical barrier called inner barrier at this conformation prevents the substrate from moving into the cytoplasm (Fig. 1A).
Figure 1.
Crystal structure of the D59C uniporter MelBStmutant with a sugar bound [PDB ID, 7L17]. MelBSt exhibits a canonical MFS fold. A, the sugar-bound outward-facing MelBSt as shown by a cross-section of the surface representation. The sugar substrate α-nitrophenyl galactoside (α-NPG) highlighted in dark gray is bound at the apex of the outward-open cavity, and its access to the cytoplasm is blocked by the thick inner barrier. B, overall fold, the transmembrane helices I-VI and VII-XII form two helix bundles and the cytoplasmic ends are closed that is stabilized by the C-terminal tail helix. Molecular α-NPG and the residues involved in its binding are highlighted in the sticks. C, viewed from the periplasmic side. The transmembrane helices were labeled in Roman numerals. The cation-binding pocket is indicated by a pink triangle. The D59C uniporter mutant does not bind a cation. MelBSt,Salmonella enterica serovar Typhimurium melibiose permease; MFS, major facilitator superfamily; Nbs, nanobodies; PDB, Protein Data Bank.
It is well-known that the MFS transporters should change their conformation to allow the substrate translocation from one side of the membrane to the other. To analyze these structurally unresolved states of melibiose permease (MelB), 10 Nbs were raised and selected from the WT MelBSt. To determine the binding sidedness of all Nbs with the membrane protein MelBSt, we adapted a bacterial adenylate cyclase (CyaA)-based two-hybrid system, which has been widely used for soluble protein-protein interaction studies (17, 18, 19, 20, 21); however, this method has not been well explored for soluble-membrane protein interaction studies yet. This assay relies on two hybrid proteins containing the T18 and T25 fragments of the adenylate domain of a Bordetella pertussis toxin (Fig. 2A). Co-expression of both fragments in Escherichia coli cells does not generate CyaA activity unless both are fused with a pair of interacting proteins (17, 22). Nb and MelBSt interactions were examined by this in vivo assay.
Figure 2.
Two-hybrid assay with Escherichia coli DH5α ΔcyaA strain.A, a scheme for adenylate cyclase-based two-hybrid system. When the T18 and T25 fragments are expressed separately, no cAMP is produced. The interaction of two hybrid proteins with X and Y brings out the T18 and T25 fragments together and restores adenylate cyclase activities and cAMP production. The framed images in blue color for the two hybrid results containing the Nb732:T18 fusion and Nb738:T18 fusion were obtained separately due to the slow development of the red color. B, DNA-binding leucine zipper (ZIP) and a transcription factor MelR were fused to T18 and T25 vectors, respectively. Co-expression encoded by two compatible vectors was carried out in a created DH5α ΔcyaA strain (Table S1) and incubated on MacConkey agar plates containing 30 mM maltose as the sole carbohydrate source. The pictures were taken after 4 days or 7 days of incubation at 30 °C. C, interaction of MelR and ANK-N5C-281. Two polarities of fusions were tested. The plasmids encoding T25:ANK-N5C fusion (ANK-N5C was fused to the C terminus of T25 fragment) and ANK-N5C:T18 fusion (ANK-N5C was fused to the N terminus of T18 fragment) were individually co-transformed with a compatible vector pCS19/MelR-T18 or pACYC/T25:MelR into the cyaA- DH5α cells. The results are shown in patching from a single colony on the MacConkey agar plates containing 30 mM maltose. Only those cells containing a pair of fusions with ANK-N5C-281 and MelR restored the maltose fermentation in the ΔcyaA strain. CyaA, adenylate cyclase.
We also performed extensive in vivo and in vitro characterizations of Nbs binding and effects on MelBSt functions, including transport activities, binding affinities for substates melibiose, Na+, and the regulatory enzyme EIIAGlc in the glucose-specific phosphoenolpyruvate/sugar phosphotransferase system (23, 24, 25). Few Nbs have been identified to trap MelB at a possible Na+-bound and sugar low-affinity conformation. Those Nbs could serve as a useful tool for structural analysis.
Results
Generation of MelBSt Nbs
The WT MelBSt proteins were purified and functionally reconstituted into proteoliposomes as described (13), and llama immunization and library construction were also described (1). The selections by phage display using WT MelBSt proteoliposomes were performed under 4 conditions (Na+ or Li+ in the absence or presence of melibiose). A total of 11 representative Nbs (ca. 126–132 amino acid residues) were obtained (Table 1), which can be grouped into 6 groups based on the amino acid sequence similarity in the CDR-3. It is likely that Nb from the same group might bind to the same target epitope. Two Nbs CA10718 and CA10833 (the short ID Nb718 and Nb733), which were obtained from 2 different selection conditions, are silent variants with identical amino acid sequences. Thus, 10 Nbs with unique amino acid sequences were collected for further studies. The group 1 were found from all 4 selection conditions with a low occurrence (5 times), and group 4 members were obtained from both melibiose-containing conditions or Li+ alone, with a high occurrence (99 times from each condition). The selection results indicate that MelBSt bound with either cation Na+ or Li+ alone or together with melibiose could generate a similar epitope to be recognized by the group 1 Nbs even with a low occurrence.
Table 1.
Nb selection
| Reference No. | Nb ID No. | Group | Occurrencea | Selection condition | Inhibition on melibiose active transport | Binding site |
|---|---|---|---|---|---|---|
| CA10714 | Nb714 | 1 | 5 | Li+ | Full inhibition | Cytoplasmic side |
| CA10718 | Nb718b | 5 | Li+ and melibiose | Full inhibition | Cytoplasmic side | |
| CA10725 | Nb725 | 5 | Na+ | Full inhibition | Cytoplasmic side | |
| CA10733 | Nb733b | 5 | Na+ and melibiose | Full inhibition | Cytoplasmic side | |
| CA10732 | Nb732 | 2 | 2 | Na+ and melibiose | Partial inhibition | Cytoplasmic side |
| CA10721 | Nb721 | 3 | 17 | Na+ | Full inhibition | Cytoplasmic side |
| CA10712 | Nb712 | 4 | 99 | Li+ | Partial inhibition | Cytoplasmic side |
| CA10715 | Nb715 | 99 | Li+ and melibiose | Partial inhibition | Cytoplasmic side | |
| CA10728 | Nb728 | 99 | Na+ and melibiose | Partial inhibition | Cytoplasmic side | |
| CA10738 | Nb738 | 5 | 6 | Na+ and melibiose | Partial inhibition | Cytoplasmic side |
| CA10723 | Nb723 | 6 | 2 | Na+ | Full inhibition | Cytoplasmic side |
The number of group members under each selection condition was obtained.
Identical amino acid sequence but different at one nucleobase position.
In vivo protein-protein interactions analyzed by a bacterial two-hybrid assay
To analyze intracellular protein-protein interactions, a bacterial two-hybrid assay based on CyaA toxin T18/T25 fragments of B. pertussis was established. Three cyaA gene-deleted E. coli strains including DH5α, DW2, and T7 Express were created (Table S1; Fig. 2A). In E. coli, cAMP was required for these cAMP-dependent sugar utilizations, such as maltose, melibiose, lactose, and so on. To assess the cyaA- phenotype, the cAMP-dependent sugar fermentations were carried out on MacConkey agar plates containing maltose or melibiose as a sole carbohydrate source. Two of the parent strains DH5α and T7 Express fermented both sugars, as shown by the red-forming colonies after incubation overnight (Fig. S1A). The DW2 strain (melA+, melB-, and lacZ-Y-) only fermented maltose, not melibiose, since lacking melibiose transport, so grew as yellow colonies (26).
The created DH5α cyaA- strain grew slowly and formed yellow colonies on the melibiose plates after 18-h incubation at 30 °C (Fig. S1A); interestingly, the colonies grown on the maltose plates showed transient red for 3 to 4 days after the transformation, and the color eventually changed to yellow with further incubation. The results indicated no further maltose utilization, which is consistent with the lack of cAMP production due to the cyaA gene deletion. With the supplement of 0.5 mM cAMP into the media, the cyaA- strain formed red colonies on both sugar plates after 2 to 3 days and maintained the red color for at least 10 days. The T7 Express cyaA- strain showed similar phenotypes with more robust fermentation of both sugars. The DW2 cyaA- strain also exhibited cAMP-dependent maltose fermentation but had no melibiose fermentation in the absence or presence of supplement cAMP as expected.
The disaccharide utilization by the DH5α cyaA strain was further tested by monitoring the cell-growth curves in M9 minimal media containing melibiose or maltose as the sole carbon source. The cyaA- cells did not grow on either sugar (Fig. S1B) unless 0.5 mM cAMP was added into the media; even then, the growth rate was much slower than that of the WT in the absence or presence of cAMP. All data support the conclusion that the cyaA gene-deleted mutants lost the capability to utilize either melibiose or maltose due to the lack of cAMP production, which agrees with the previous reports (17, 18, 22).
To verify the two-hybrid interaction assay, 2 DNA-binding dimeric proteins leucine zipper (ZIP) (22) and the transcription activator of melibiose operon melAB in E. coli (MelR) (27) were used as the positive control. Two pairs of compatible fusion plasmids encoding 2 ZIP (or 2 MelR) fused with T18 and T25, respectively, were co-transformed into the DH5α cyaA strain (Fig. 2B). As described for another indicator strain (17, 18), the cells formed small colonies after overnight incubation on the maltose MacConkey agar plates at 30 °C. For the ZIP:T18 and T25:ZIP hybrids, or MelR:T18 and T25:MelR hybrids, few red cells showed up after a 4 to 5 incubation. These red colonies became larger with irregular shapes and maintained the color for at least 10 days, which indicated the homodimeric formation of ZIP or MelR proteins, respectively. The protein-protein interactions of either monomeric ZIP or MelR facilitated the interactions of T18 with T25 fragments, reconstituting the CyaA activities. As the negative control, the cells expressing T18 and T25 fragments without a fusion, or fused with only the ZIP or MelR, appeared yellow. The colony’s color remained longer even after incubation for more than 10 days. The final results, which were represented in patches including varying negative controls (Fig. S1C), validated this cAMP-dependent two-hybrid assay.
A previously created transcription inhibitor ankyrin ANK-N5C-281 by a combinatorial approach has been proposed to interact with MelR and inhibit its function, but direct evidence of their interaction was missing (27). The two-hybrid assay supported the interactions of the ANK-N5C-281 with MelR regardless of the polarity of fusion (Fig. 2C). Five other ankyrin N5C proteins (62, 64, 88, 317, or 429), which are not the mel operon inhibitor, showed no interaction with MelR (27). This study provided evidence for the direct interaction of N5C-281 and MelR.
Intracellular interactions of Nbs with MelBSt
The 10 Nbs were individually fused to the N-terminus of T18 and then transformed into the DH5α cyaA- cells pre-transformed with a compatible plasmid carrying T25:MelBSt (Table S1). All, except the Nb723:T18 fusion, showed positive maltose fermentation as indicated by the appearance of red colonies (Fig. 3A). There was no colony growth on the plates containing the Nb723:T18 fusion. The results indicated that at least 9 Nbs bind to the cytoplasmic side of MelBSt, which validated this method for soluble and membrane protein interactions.
Figure 3.
In vivo Nbs binding and effect on MelB transport activities.A, in vivo cAMP-based two-hybrid assay. Each Nb was fused to the N-terminus of the T18 fragment on the plasmid pCS19/X:T18/FX vector to generate the Nbs:T18 fusions, and MelBSt was fused to the C terminus of the T25 on the plasmid pACYC/T25:X/FX to generate the T25:MelBSt fusion. The 2 types of compatible plasmids containing the T18 (labeled on the top) and T25 (labeled at the left side) without or with a fusion were co-transformed at all possible combinations into the indicator strain Escherichia coli DH5α ΔcyaA strain. The cells were grown on maltose-containing MacConkey agar plates as described in the Experimental procedures. The picture was taken after a 7-day incubation at 30 °C. Red colonies indicate a positive result. B, inhibition of [3H]]melibiose active transport. E. coli DW2 (melB-lacY-Z-) was transformed with 2 compatible expression vectors pACYC/MelBSt and pCS19/Nbs, encoding WT MelBSt and Nbs (without any fusion), respectively. With intact cells, the Na+-coupled melibiose uptake was stopped at the indicated time points. Error bar, SEM; the number of tests = 2 to 3. The DW2 cells containing the pACYC/MelBSt and pCS19 without Nb were used as the positive control, and pACYC and pCS19 with no MelB nor Nb were used as the negative control. C, Western blot, membranes expressing MelBSt alone or co-expressed with Nbs in a buffer containing 50 mM NaPi, pH 7.5, 100 mM NaCl, and 10% glycerol were prepared as described in Experimental procedures. An aliquot of 50 μg membrane proteins of each sample was analyzed by SDS-15%PAGE, and MelBSt proteins were detected by Western blotting using anti-His tag antibody as described in Experimental procedures. Control, membranes isolated from the DW2 cells carrying the 2 empty vectors pCS19 and pACYC with no protein. D, inhibition of melibiose fermentation. E. coli DW2 was transformed with pACYC/MelBSt and pCS19/Nbs encoding the WT MelBSt and an Nb without any fusion, respectively. The cells with pACYC/MelBSt and pCS19 were used as the positive control, and empty vectors, pACYC and pCS1, were used as the negative control. MacConkey agar plates containing 30 mM melibiose as the sole carbohydrate source were incubated at 30 °C, and photos were taken after 18 h incubation. This conventional melibiose fermentation assay detects the melibiose transport rate that limits the fermentation rate and the degree of acidification as indicated by the color shades. Red colonies, normal melibiose fermentation; yellow colonies, no fermentation; and pink colonies, reduced fermentation rates. CyaA, adenylate cyclase; MelBSt,Salmonella enterica serovar Typhimurium melibiose permease; Nbs, nanobodies.
Effects of Nbs on MelBSt transport activity
E. coli DW2 cells (melA+, melB-, and lacZ-Y-), which were used for the expression and functional characterization of MelB, were transformed with 2 compatible expression plasmids encoding for MelBSt and the Nbs, respectively, and subjected to the Na+-coupled [3H]melibiose active transport assay against melibiose concentration gradient as described in Methods (26). Compared with the WT MelBSt, all Nbs inhibited the melibiose active transport activities to varying extents (Fig. 3B). Five Nbs (Nbs714, 725, 733, 721, and 723) nearly inactivated MelBSt, and the other 5 Nbs (Nb712, 715, 728, 732, and 738) showed 60 to 85% inhibitions. The Western blot of membranes prepared from the cells co-expressing both MelBSt alone or with Nbs showed that MelBSt protein expression when co-expressed with each individual Nb is comparable to the WT MelBSt proteins. However, the expression levels were very low (Fig. 3C).
The melibiose fermentation assay with DW2 (lacY-melB-), which was routinely used to report the melibiose down-concentration transport activities, was also applied to test the effects of intracellular expressed Nbs on the co-expressed MelBSt (Fig. 3D). Out of the 5 Nbs (Nbs714, 725, 733, 721, 723) that completely inhibited the MelBSt active transport, 4 also completely inhibited melibiose fermentation and one (Nb723) showed partial inhibition since the colonies were pink. The other 5 Nbs with partial inhibitions on active transport showed little or no inhibition of melibiose fermentation. While each of the Nbs binds to the cytoplasmic side of MelBSt, the inhibition of the MelBSt function varied.
Nb binding and substrate effects determined by ITC
All Nbs were subjected to cytoplasmic expression and metal affinity purification. Only 3 Nbs from group 1 (Nbs714, 725, and 733), which inhibited both active transport and melibiose fermentation, can be concentrated for isothermal titration calorimetry (ITC) measurements to determine the dissociation constants (Kd) (Fig. 4; Table 2).
Figure 4.
Measurements of Nbs binding to MelBStand melibiose effect by ITC. Nbs binding to the Na+-bound WT MelBSt in the absence or presence of 50 mM melibiose was collected with ITC calorimeters (TA Instruments) at 25 °C. For each measurement, an aliquot of 30 μM of MelBSt in the buffer of 20 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.01% DDM, and 10% glycerol without or with the addition of 50 mM melibiose was placed in the sample cell. Solutions containing Nbs of 180 to 200 μM were prepared in a matching buffer, and placed in the syringe, and incrementally injected with 2 μl aliquots into the sample cell at 300-s intervals as described in the Experimental procedures. A, Nb714 binding to WT MelBSt. B, Nb725 binding to WT MelBSt. C, Nb733 binding to WT MelBSt. The thermogram was plotted as the baseline-corrected heat rate (μJ/s; left axis) versus time (bottom axis) for the titrant to MelBSt (black) or to buffer (red) under an identical scale. The enthalpy change (ΔH) (kJ/mol; filled green symbol) was plotted against the Nb/MelBSt molar ratio (top/right axes). DDM, n-dodecyl-β-D-maltopyranoside; ITC, isothermal titration calorimetry; MelBSt,Salmonella enterica serovar Typhimurium melibiose permease; Nbs, nanobodies.
Table 2.
Nb binding thermodynamics
| Syringe | Sample cell | Kd (μM) | ΔG (kJ/mol) | ΔH (kJ/mol) | -TΔS (kJ/mol) | N | Increase in Kd | Decrease in ΔH (kJ/mol) |
|---|---|---|---|---|---|---|---|---|
| Nb714 | MelBSt/Na+ | 0.69 ± 0.11a | −35.21 ± 0.40 | −78.83 ± 4.52 | 43.63 ± 4.13 | 0.92 ± 0.09 | 9.2-fold | 6.11 |
| MelBSt/Na+/Mel | 6.35 ± 0.42 | −29.69 ± 0.42 | −72.72 ± 6.46 | 43.03 ± 6.28 | 0.96 ± 0.03 | |||
| Nb725 | MelBSt/Na+ | 1.61 ± 0.08 | −33.08 ± 0.13 | −88.24 ± 0.84 | 55.16 ± 20.72 | 1.01 ± 0.09 | 4.08-fold | 4.27 |
| MelBSt/Na+/Mel | 6.56 ± 0.83 | −29.61 ± 0.31 | −83.97 ± 1.42 | 54.35 ± 0.06 | 0.85 ± 0.06 | |||
| Nb733 | MelBSt/Na+ | 0.52 ± 0.07 | −35.90 ± 0.32 | −89.92 ± 2.64 | 54.01 ± 2.97 | 0.98 ± 0.03 | 11.73-fold | 4.78 |
| MelBSt/Na+/Mel | 6.10 ± 0.03 | −29.77 ± 0.01 | −85.14 ± 3.67 | 55.38 ± 3.68 | 0.98 ± 0.03 |
Error bar, SEM; the number of tests = 2 for all.
The binding of the Nbs to MelBSt in the presence of Na+, or Na+ and melibiose, released heat, showing exothermic thermographs. In the absence of the melibiose, the curve shape for the binding exhibited a near-ideal sigmoidal feature and can be fitted to stoichiometric numbers close to 1. The Kd values for Nb714, Nb725, and Nb733 in the presence of Na+ were 0.69 ± 0.11, 1.61 ± 0.08, and 0.52 ± 0.07 μM, respectively. In either case, the higher binding enthalpy (ΔH) made the sole favorable contribution to the binding free energy (ΔG) after compensating for the unfavorable large entropic changes. The Nbs/MelBSt protein interactions were also supported by gel-filtration chromatography (Fig. S2). The Nb733 and MelBSt migrate differently at 13.02 ml and 16.66 ml, respectively (Fig. S2A upper panel). When mixed with MelBSt, Nb733 results in a slightly left shift by 0.11 ml of the MelBSt peak (Fig. S2A upper panel).
The effects of MelB substrates (melibiose and Na+) on the Nb binding were also carried out (Fig. 4; Table 2). In the presence of both melibiose and Na+, all of the 3 binding affinities were significantly reduced by approximately 10-fold for Nbs714 and 733 and by 4-fold for Nb725. The decrease in the binding enthalpy contributed to the major affinity decrease. These results indicated that the sugar substrate inhibits the Nbs binding.
Differential effects of Nbs on MelBSt affinities to melibiose and Na+
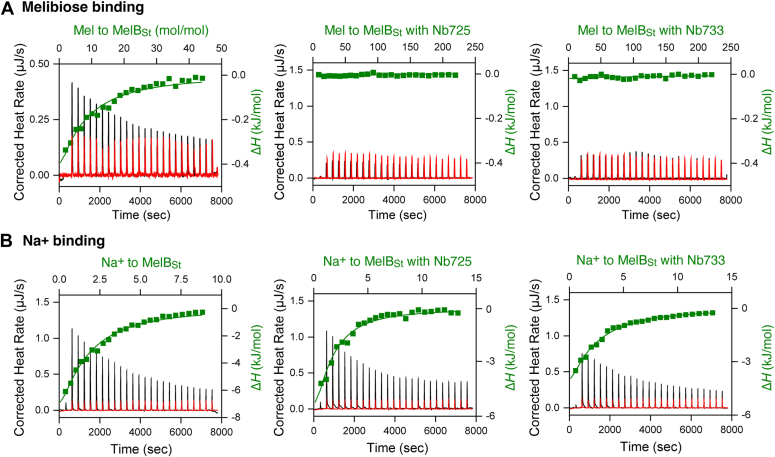
Nb714, 725, and 733 completely inhibited melibiose transport activity when co-expressed with MelBSt (Fig. 3, A and C). The Nb733 and Nb714 only differ in one side chain, and Nb733 and Nb725 have further been analyzed for their effects on MelB binding affinities to melibiose and Na+ (Fig. 5; Table 3). In the presence of Na+, melibiose binding to MelBSt in n-dodecyl-β-D-maltopyranoside (DDM) buffer exhibits Kd of 1.43 ± 0.03 mM, which is slightly higher than that with MelBSt in undecyl-β-D-maltopyranoside (UDM) buffer (12, 24, 28). Surprisingly, when performing melibiose binding to the MelBSt complexed with Nb725 or Nb733 in the presence of Na+, even with an increased sugar concentration, both the peak height and change pattern are indistinguishable from that derived from the injection of melibiose at the same concentration into the buffer without a protein (Fig. 5).
Figure 5.
Effect of Nbs on melibiose and Na+binding to MelBStby ITC. Melibiose or Na+ binding to Nb-bound or free WT MelBSt was collected with ITC calorimeters (TA Instruments) at 25 °C. For each measurement, an aliquot of 80 μM of MelBSt without or with an Nb (725 or 733) at a concentration of 120 μM (molar ratio of 1:1.5) in the buffer of 20 mM Tris–HCl, pH 7.5, 50 mM choline chloride, 0.01% DDM, and 10% glycerol was placed in the sample cell. Solutions containing melibiose (10 mM or 50 mM free MelBSt or MelBSt/Nb733 complex, respectively), or Na+ (5 or 2 mM for free MelBSt or MelBSt/Nb733 complex, respectively) were prepared in a matching buffer and placed in the syringe and incrementally injected in 2 μl aliquots into the sample cell. A, melibiose binding to free WT MelBSt or complexed with Nb725 or Nb733. B, Na+ binding to free WT MelBSt or complexed with Nb725 or Nb733. The thermogram was plotted as the baseline-corrected heat rate (μJ/s; left axis) versus time (bottom axis) for the titrant to MelBSt or MelB/Nb complex (black) or to buffer (red) under an identical scale. ΔH (kJ/mol; filled green symbol) was plotted against the Nb/MelBSt molar ratio based on the top/right axes. DDM, n-dodecyl-β-D-maltopyranoside; ITC, isothermal titration calorimetry; Mel, melibiose; MelBSt,Salmonella enterica serovar Typhimurium melibiose permease; Nbs, nanobodies.
Table 3.
Nb effects on MelBSt binding for melibiose or Na+
| Syringe | Sample cell | Kd (mM) | ΔG (kJ/mol) | ΔH (kJ/mol) | -TΔS (kJ/mol) | N |
|---|---|---|---|---|---|---|
| Melibiose | MelBSt/Na+ | 1.43 ± 0.03a | −16.23 ± 0.04 | −6.95 ± 0.64 | −9.28 ± 0.69 | 1 |
| MelBSt/Na+/Nb725 | /b | / | / | / | / | |
| MelBSt/Na+/Nb733 | / | / | / | / | / | |
| Na+ | MelBSt | 0.17 ± 0.02 | −21.15 ± 0.36 | −22.12 ± 2.06 | 0.61 ± 2.42 | 1 |
| MelBSt/Nb725 | 0.18 ± 0.01 | −21.44 ± 0.14 | −16.14 ± 0.28 | −5.3 ± 0.13 | 1 | |
| MelBSt/Nb733 | 0.13 ± 0.01 | −22.22 ± 0.36 | −17.00 ± 3.52 | −5.21 ± 3.87 | 1 | |
| MelBSt/EIIAGlcc | 0.25 ± 0.01 | −20.53 ± 0.11 | −31.54 ± 2.96 | 11.01 ± 2.85 | 1 | |
| EIIAGlc | MelBSt | 3.33 ± 0.49 | −31.31 ± 0.45 | −39.93 ± 2.97 | 8.62 ± 2.51 | 0.81 ± 0.01 |
| MelBSt/Nb733 | 8.12 ± 0.14 | −29.06 ± 0.04 | −28.23 ± 0.34 | −0.83 ± 0.30 | 0.76 ± 0.04 | |
| Nb733 | MelBSt | 0.52 ± 0.07 | −35.90 ± 0.32 | −89.92 ± 2.64 | 54.01 ± 2.97 | 0.98 ± 0.03 |
| MelBSt/EIIAGlc | 0.75 ± 0.06 | −34.98 ± 0.02 | −40.22 ± 3.04 | 5.25 ± 3.05 | 0.98 ± 0.03 |
Error bar, SEM; the number of tests = 2 for all.
Not detectable.
Detergent in the buffer in this assay is UDM.
Interestingly, Na+ binding to MelBSt in DDM buffer exhibits Kd of 1.17 ± 0.02 mM, slightly lower than that with MelBSt in UDM buffer (12, 24, 28). When titrating Na+ to MelBSt/Nb725 or Nb733 complexes, the Kd values are indistinguishable from the binding with the free MelBSt, 0.18 ± 0.02 or 0.13 ± 0.01 μM, respectively (Table 3). The entropic components contributed favorably to the binding-free energy and compensated for the reduced enthalpic change.
Effects of Nb733 on MelBSt binding to its physiological binder EIIAGlc
EIIAGlc, which is the central regulator in the glucose-specific phosphoenolpyruvate/sugar phosphotransferase system in certain bacteria (23). Previous studies showed that EIIAGlc binds readily to the apo (28) and Na+-bound MelBSt at a Kd of ∼3 μM (24). Surprisingly, EIIAGlc also binds to the MelBSt/Nb733 complex with a 2-fold reduced affinity (Fig. 6A; Table 3); similarly, the Nb733 binding affinity to MelBSt was also not affected significantly by the prebinding with EIIAGlc (Fig. 6B). The supercomplex MelBSt/Nb733/EIIAGlc can be isolated from gel-filtration chromatography. EIIAGlc protein does not absorb at 280 nm due to a lack of Trp and Tyr residues; however, EIIAGlc shifted the MelBSt peak from 13.02 ml to 12.91 ml and further shifted the MelBSt/Nb733 complex peak to 12.73 ml. The peak fractions analyzed by SDS-15%PAGE showed that MelBSt, Nb733, and EIIAGlc are present in all fraction of the supercomplex peak at 12.73 ml, providing strong evidence that both Nb733 and EIIAGlc can cocurrently interact with MelBSt (Fig. S2, A and B).
Figure 6.
Regulatory protein EIIAGlcand Nb form a super-complex with MelBSt.A, the Nb effect of Nb733 on the binding of the native binding protein EIIAGlc to MelBSt was determined by ITC measurement at 25 °C as described in Experimental procedures. An aliquot of 30 μM of free MelBSt or complexed with EIIAGlc at a concentration of 60 μM (molar ratio of 1:2) in the buffer of 20 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.01% DDM, and 10% glycerol was placed in the sample cell. Solutions containing 180 μM Nb were prepared in a matching buffer and placed in the syringe. B, the EIIAGlc effect on the Nb733 binding to MelBSt. An aliquot of 50 μM of free MelBSt or complexed with Nb733 at a concentration of 75 μM (molar ratio of 1:1.5) in the buffer 20 mM Tris–HCl, pH 7.5, 100 mM NaCl, 0.01% DDM, and 10% glycerol was placed in the sample cell. Solutions containing EIIAGlc (450 μM) were prepared in a matching buffer and placed in the syringe. C, Na+ binding to free WT MelBSt or complexed with EIIAGlc. An aliquot of 80 μM of MelBSt without or with EIIAGlc at a concentration of 120 μM (molar ratio of 1:1.5) in the buffer of 20 mM Tris–HCl, pH 7.5, 50 mM choline chloride, 0.01% DDM, and 10% glycerol was placed in the sample cell. Solutions containing Na+ (5 mM) were prepared in a matching buffer and placed in the syringe. The thermogram was plotted as the baseline-corrected heat rate (μJ/s; left axis) versus time (bottom axis) for the titrant to free MelBSt, MelBSt/Nb733, or MelBSt/EIIAGlc complex (black) or to buffer (red) under an identical scale. ΔH (kJ/mol; filled green symbol) was plotted against the Nb/MelBSt molar ratio based on the top/right axes. DDM, n-dodecyl-β-D-maltopyranoside; ITC, isothermal titration calorimetry; MelBSt,Salmonella enterica serovar Typhimurium melibiose permease.
Previously published results also showed that EIIAGlc largely inhibits the sugar binding in MelB (24) and another sugar transport lactose permease LacY (25). To test if the EIIAGlc binding behaves similarly to the Nb733, Na+ binding to MelBSt/EIIAGlc complex was also analyzed (Fig. 6C). Similarly, the MelBSt/EIIAGlc complex also retains the Na+ binding affinity at a Kd value of just 2-fold higher than that of the free MelBSt (Table 3).
Discussion
Nbs have been shown to be useful for membrane protein structure determination by X-ray crystallography or cryo-EM single-particle analysis (3, 4, 5, 29, 30). Membrane transporters have intrinsically conformational flexibility necessary for their functions. Current advances in structural biology have confirmed that a solvent-access path on both sides of the membranes is alternatively formed and closed for substrate binding and translocation across the membranes (31, 32, 33, 34). The available knowledge also suggests that most membrane transporters have their favored conformations. As such, it is challenging to determine high-resolution structures in their transient, less populated states. In addition, the MFS transporters are a group of so-called hydrophobic membrane proteins with the majority mass embedded within the membranes. All those structural features increase the difficulties in high-resolution structure determination for the mechanistic studies of transport processes. Nbs usually bind to a tertiary epitope with high affinities and can select/stabilize conformations and reduce intrinsic conformation dynamics/motions (1, 3). Nbs have attracted increasing attention in protein research.
It is challenging to obtain Nbs to target a specific conformation. In this study, we tested if a specific selection condition can provide conformation-targeted Nbs. We applied 4 selection conditions and analyzed the data based on the Nb group, occurrence under each selection condition, and extensive characterizations of interactions with the targeted protein, both in vivo and in vitro. Interestingly, the group 1 members were isolated from all 4 conditions at only 5 occurrences (Table 1), and Nbs with identical amino acid sequences were obtained from different selection conditions, such as the Nb718 and Nb733. In contrast, the group 4 members with higher 99 occurrences were from 3 conditions including melibiose-containing and Li+ alone, but not Na+ alone conditions. This high occurrence implies that the group 4 members might bind to an epitope that is available at all or most conformational states of the transporter. This is also supported by their partial inhibition of the melibiose transport (Table 1; Fig. 3). The low occurrences from group 1 might suggest that this group member might bind MelBSt at certain transient high-energy states. Notably, the group 1 Nbs can be obtained from either condition, Na+ or Li+, alone or together with melibiose. The same group Nbs might bind to a similar epitope, which did not exclude the possibility that a similar epitope can be recognized by Nbs from more than one group. To establish a general method to analyze soluble protein and membrane protein interactions in vivo, a widely used soluble protein-protein interaction assay based on reconstituted CyaA activities by 2 hybrids (17, 18, 19, 20, 21) was set up. After testing the known dimeric proteins (ZIP or MelR), this simple test provided evidence of the direct interaction and confirmed a previously proposed interaction between MelR and a created ankyrin protein ANK-N5C-281 (27). This in vivo method also suggested that nearly all Nbs bind to the cytoplasmic side of MelBSt, except for the Nb723:T18 fusion that did not form any colonies and had no readout (Fig. 3A). The conclusion was further confirmed by the [3H]melibiose active transport assay with intact cells when intracellularly co-expressing the soluble Nbs with the membrane protein MelBSt (Fig. 3B). Thus, the two-hybrid assay could provide valid information for intracellular Nb-membrane protein interactions. On the other hand, the results imply that the sidedness of MelBSt in proteoliposomes is predominantly inside-out orientated. This is consistent with the previous conclusion in MelBEc, where MelBEc in proteoliposomes was reported as uniformly inside-out oriented as determined by chemical modification (35) and the recent study with single-molecule force spectroscopy of MelBSt (36).
The group 1 members (714, 725, and 733) are structurally stable and caused full inhibition of Na+-coupled melibiose active transport and the downhill melibiose transport activities, and hence were prioritized for further analysis. All 3 Nbs are highly conserved with only 2 differences in the CDR-2/3 and one difference in the scaffold region; all bind MelBSt with a Kd value in a range of 0.52 to 1.61 μM and possess similar effects on MelBSt functions. The Nbs binding was largely inhibited by melibiose, and the melibiose binding to MelBSt was also inhibited by the Nbs, suggesting that this group of Nbs functions as negative allosteric modulators; thus, the anticooperativity implies that the melibiose-favored conformation conflicts with the Nb-favored conformation. It is likely that the state of the Nb-bound MelBSt largely differs from the available sugar-bound outward-facing structures solved by X-ray crystallography.
The Nb733-bound MelBSt retains binding to the coupling cation Na+ and the physiological regulator EIIAGlc. Interestingly, the observed non-cooperative binding of Nbs with Na+ and anti-cooperativity with melibiose are similar to what was observed for EIIAGlc (24, 28) (Table 3). The data further confirmed that MelBSt trapped by this group of Nbs retains a physiological conformation, likely a Na+-bound sugar conformation.
This study established a general in vivo assay to test in vivo protein-protein interactions and the binding sidedness to membrane proteins in E. coli and also identified a few potential useful Nbs for future structural determination of MelBSt. It is noteworthy that the critical coupling cations have not been resolved for any of the MFS transporters. The MelB Nbs could be a valuable tool to facilitate the structural determination of a Na+-bound MelBSt and deepen our understanding of the ion-coupled transported mechanisms.
Experimental procedures
Reagents
[1-3H]Melibiose (5.32 Ci/mmol) was custom synthesized by PerkinElmer, and unlabeled melibiose was purchased from Acros Organics (Thermo Fisher Scientific). MacConkey agar media (lactose-free) was purchased from Difco. Detergents UDM, DDM, and octyl-β-D-glucoside were purchased from Anatrace E. coli lipids (Extract Polar, 100600) were purchased from Avanti Polar Lipids, Inc. Oligodeoxynucleotides were synthesized by Integrated DNA Technologies. All other materials were reagent grade and obtained from commercial sources.
Strains
The genotype and source of E. coli strains used or created in this study are described in Supplementary Notes and listed in Tables S1 and S2 unless otherwise described specifically. Briefly, the commercial E. coli Stellar or XL1 Blue were used for plasmid construction. The E. coli DB3.1 strain was used to construct the ccdB-containing universal FX cloning vectors (37). E. coli DW2 (melB-lacY-) (38) was used for functional studies, and 3 cyaA- strains (DH5α, DW2, and T7 Express) were generated for the in vivo protein-protein interaction assay. E. coli BW25113 strain and plasmids pKD46, pKD4, and pCP20 (7) were used for gene deletion.
Plasmids construction
The plasmids used or created in this study are listed in Supplementary Notes and Table S1. The primers used for this study are listed in Table S2.
MelBSt protein expression and purification
Cell growth for the large-scale production of WT MelBSt was carried out using the pK95 ΔAH/MelBSt/CHis10 from E. coli DW2 cells (melA+melB, lacZY) as described (10, 39). Briefly, MelBSt purification from membranes by cobalt-affinity chromatography (Talon Superflow Metal Affinity Resin, Takara) after extraction by 1.5% UDM or 2% DDM. MelBSt protein was eluted with 250 mM imidazole in a buffer containing 50 mM NaPi, pH 7.5, 200 mM NaCl, 0.035% UDM or 0.01% DDM, and 10% glycerol, and dialyzed to change the buffer conditions accordingly.
MelBSt reconstitution
The MelBSt reconstitution into proteoliposomes was carried out by dilution using E. coli Extract Polar (Avanti) as described (13). Briefly, 46.25 mg of the lipids dissolved in 1.2% octyl-β-D-glucoside was mixed with 9.25 mg of the MelBSt in UDM at a ratio of 1:5 (mg:mg). After a 30 min incubation at room temperature, the mixture was subjected to a 74-fold dilution with a buffer of 20 mM NaPi, pH 7.5, and 150 mM NaCl, and further incubated for another 30 min with stirring. Proteoliposomes were harvested by ultracentrifugation at 28,000 rpm using Beckman rotor 45 Ti at 4 °C overnight. They were further washed by resuspending the pellets in 20 ml of 20 mM NaPi, pH 7.2, 150 mM NaCl buffer, followed by 3 cycles of freeze-thaw-sonication and collected by ultracentrifugation at 47,000 rpm on a Beckman rotor 70 Ti at 4 °C for 2 h. The pellets were re-suspended in the same buffer and subjected to another 3 cycles of freeze-thaw-sonication. The sonication was carried out in an ice-cold bath sonicator (Branson 2510) for 5 s 3 times. The samples were washed once with 24 ml of the same buffer and concentrated to a protein concentration of 8.5 mg/ml by ultracentrifugation, which yielded about 8.7 mg of MelBSt proteoliposomes.
Nbs generation and specific selection
The MelBSt proteoliposomes in 20 ml of 20 mM NaPi, pH 7.2, 150 mM NaCl buffer were used to raise Nbs as described (1). In brief, one llama (Lama glama) received 6 weekly injections of 100 μg of WT MelBSt-containing proteoliposomes. The Nb-encoding ORFs were amplified from total lymphocyte RNA and subcloned into the phage display/expression vector pMESy4.
Four defined conditions were used to select specific Nbs by phage display. The 4 buffers are (1) 20 mM KPi, pH 7.4 and 150 mM NaCl (2), 20 mM KPi, pH 7.4, 150 mM NaCl, and 10 mM melibiose (3), 20 mM KPi, pH 7.4 and 150 mM LiCl, and (4) 20 mM KPi, pH 7.4, 150 mM LiCl, and 10 mM melibiose, The buffer for coating MelBSt on a solid phase and for the Nbs selections is identical. A total of 344 individual colonies were randomly picked, and the Nbs were produced as soluble His- and Capture Select C-tagged proteins (MW 12–15 kDa) in the periplasm of E. coli. DNA sequencing analysis resulted in 6 groups (Table 1)
A bacterial two-hybrid assay
A bacterial two-hybrid assay was established, which is based on reconstituting CyaA activities from the T18/T25 fragments of B. pertussis CyaA toxin as described (18, 22). The cyaA gene product in E. coli solely responds to cAMP synthesis. Three E. coli strains, including DH5α, DW2 (melB-), and T7 Express, were subjected to cyaA gene deletion by one-step gene deletion method (7, 40, 41). A 2166 bp chromosomal DNA fragment coding for 722 amino acid residues (Leu51-Ser772) of CyaA of each strain was removed, and the resulting resistance cassette was also eliminated (Table S1). The deletion was confirmed by DNA sequencing analysis of the PCR product amplified from the chromosomal DNA of each strain, which revealed a short fragment (∼300 bp) instead of the 2166 bp fragment.
Expression vectors carrying T18 or T25 fragments were engineered to suit the FX Cloning method (27, 37). Resulting universal vectors carrying T18 or T25 fragments were used to generate 2 fusions with two target genes at the N-terminus of T18 or the C-terminus of T25 for FX Cloning, or at another polarity. Two known dimer proteins, including ZIP and the melibiose operon transcription activator (MelR), were selected for the positive control (Table S1). A previously engineered transcription inhibitor, which was based on ankyrin proteins (ANK-N5C) by a direct evolution method (27), with the MelR and the Nbs with MelBSt were subjected to test the potential intracellular interactions (Table S1).
The DH5α competent cells without the gene coding for the endogenous cyaA- strain were selected for the tests due to suitable fermentation signals. The cells were transformed by 2 compatible plasmids with different replication origins (pACYC and pCS19) (42) containing the 2 hybrids and plated onto the lactose-free MacConkey agar plate containing 30 mM maltose as the sole carbohydrate source, 100 mg/L ampicillin, 25 mg/L chloramphenicol, and 0.2 mM IPTG, and the plates were incubated in 30 °C for 7 days before photography. Red colonies grown on the MacConkey agar indicate positive fermentation due to the cAMP production derived from the interaction of 2 hybrids. Yellow colonies denote no fermentation and no cAMP production.
[1-3H]Melibiose transport assay and Western blot
E. coli DW2 cells (melA+, melB-, and lacZ-Y-) (39) transformed with the 2 compatible plasmids (pACYC and pCS19) with no or with MelBSt and Nbs, respectively, were grown in LB broth with 100 mg/L ampicillin and 25 mg/L chloramphenicol in a 37 °C shaker overnight. The overnight cultures were diluted by 5% to fresh LB broth with 0.5% glycerol, 100 mg/L ampicillin, 25 mg/L chloramphenicol, and 0.2 mM IPTG shaken at 30 °C for 5 h. The transport assay was performed at 20 mM Na+ and 0.4 mM [3H]melibiose (specific activity of 10 mCi/mmol) as described (26, 43). The cells were washed with 50 ml of 100 mM KPi, pH 7.5 3 times, followed by washing with the assay buffer (100 mM KPi, pH 7.5, 10 mM MgSO4). The cell pellets were resuspended with the assay buffer and adjusted to A420 = 10 (∼0.7 mg proteins/ml). The transport time courses were carried out by separating the cells from solutions at zero, 5 s, 10 s, 30 s, 1 m, 2 m, 5 m, 10 m, and 30 m by dilution and fast filtration. The filters were subjected to radioactivity measurements using a liquid scintillation counter.
MelBSt membrane expression was analyzed by Western blot. Cultured cells were washed with 50 mM NaPi (pH 7.5), resuspended with the same buffer, and broken by sonication. The unbroken cells or debris were removed by centrifugation, and the supernatant were subjected to ultracentrifugation at 62,000 rpm for 30 min in a Beckman rotor Ti 70. The membrane pellets were resuspended with 50 mM NaPi, pH 7.5, 200 mM NaCl, and 10% glycerol. After protein concentration determination, 50 μg of total membranes were loaded onto SDS-15%PAGE. Western blot analysis using anti-His antibody was carried out. MelBSt protein was detected using the SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific) by the ChemiDoc MP Imaging System (Bio-Rad).
Melibiose fermentation on MacConkey agar plates
E. coli DW2 cells (ΔmelBΔlacYZ) were transformed with 2 compatible plasmids with different replication origins (pACYC/MelBSt and pCS19/Nbs) and plated onto the lactose-free MacConkey agar plate containing 30 mM melibiose as the sole carbohydrate source, 100 mg/L ampicillin and plated on MacConkey agar plates containing 30 mM melibiose, 100 mg/L ampicillin, 25 mg/L chloramphenicol, and 0.2 mM IPTG. The plates were incubated at 37 °C for 18 h before photography. Magenta colonies, a normal melibiose fermentation; pink colonies, reduced fermentation; and yellow colonies, no melibiose fermentation.
Protein concentration assay
The Micro BCA Protein Assay (Pierce Biotechnology, Inc) was used to determine the protein concentration assay.
Nbs expression and purification
Cytoplasmic expression of Nbs was carried out using the expression plasmids p7xC3H (37) in the E. coli ArcticExpress (DE3) strain (Table S1). The cells were grown in LB broth with 0.5% glucose, and 50 mg/L kanamycin at 37 °C. Overnight cultures were inoculated at a 1:100 ratio into LB broth containing 0.5% glucose and 50 mg/L kanamycin and shaken at 37 °C; 0.5 mM IPTG was added when A600 equaled 0.8, and cells were then shaken at 12 °C overnight. Cells were washed once with 50 mM NaPi, pH 7.5, resuspended in 50 mM NaPi, pH 7.5, and 1 mM PMSF, and broken by passage through Emulsiflex twice. After ultracentrifugation at 70,409g for 30 min using Beckman rotor, type 45 Ti at 4 °C, the supernatants containing soluble Nbs were collected and subjected to cobalt-NTA (Talon resin)-affinity chromatography. The column was preequilibrated with 50 mM NaPi, pH 7.5, 500 mM NaCl, 10% glycerol, and 5 mM imidazole. After washing with the same buffer containing 45 mM imidazole, elution was performed using the same buffer containing 250 mM imidazole. Buffer exchange was performed to match the MelBSt solution by overnight dialysis. The protein concentration of Nbs was measured by the Micro BCA protein assay (Pierce Biotechnology, Inc).
EIIAGlc expression and purification
The overexpression of unphosphorylated EIIAGlc was performed in the E. coli T7 Express strain (New England Biolabs) as described (24).
Isothermal titration calorimetry
All ITC ligand-binding assays were performed with the TA Instruments (Nano ITC device) as described (12). In a typical experiment, the titrand (MelBSt) placed in the ITC sample cell was titrated with the specified titrant (placed in the syringe) by an incremental injection of 1.6- or 2 μL aliquots at an interval of 300 s at a constant stirring rate of 250 rpm (nano ITC). All samples were degassed using a TA Instruments Degassing Station (model 6326) for 15 min prior to the titration. Heat changes were collected at 25 °C, and data processing was performed with the NanoAnalyze (version 3.7.5 software) provided with the instrument. The normalized heat changes were subtracted from the heat of dilution elicited by the last few injections, where no further binding occurred, and the corrected heat changes were plotted against the molar ratio of titrant versus titrand. The values for the binding association constant (Ka) and enthalpy change (ΔH) were obtained by fitting the data using the one-site independent-binding model included in the NanoAnalyze software (version 3.7.5). The binding free energy (ΔG) = -RT ln Ka, where R is the gas constant (8.315 J/mol·K), and T is the absolute temperature; ΔG = ΔH - TΔS; the entropy change (-TΔS) = ΔG – ΔH; dissociation constant (Kd) = 1/Ka.
Data availability
Source data are available from the corresponding author.
Supporting information
This article contains supporting information (7, 17, 22, 24, 26, 27, 37, 38, 40, 41, 42).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We thank Eric R. Geertsma and Raimund Dutzler for their FX cloning tools, and Gerard Leblanc for a MelB-expressing vector and DW2 strain.
Author contributions
L. G. conceptualization; S. K., K. W., S. V., P. H., E. B. T., E. P., L. G. data curation; S. K., K. W., S. V., E. B. T., and L. G. formal analysis; L. G. funding acquisition; S. K., K. W., S. V., P. H., E. B. T., E. P., J. S., L. G. investigation; S. K., K. W., S. V., P. H., E. B. T., E. P., and L. G. methodology; L. G. project administration; L. G., K. B. K., and J. S. resources; L. G. supervision; L. G. validation; S. K., S. V., E. B. T., and L. G. visualization; L. G. writing—original draft; S. K., E. B. T., P. H., and L. G. writing—review and editing.
Funding and additional information
This work was supported by the National Institutes of Health Grants R01 GM095538 and R01 GM122759 to L. G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Karen Fleming
Supporting information
References
- 1.Pardon E., Laeremans T., Triest S., Rasmussen S.G., Wohlkonig A., Ruf A., et al. A general protocol for the generation of Nanobodies for structural biology. Nat. Protoc. 2014;9:674–693. doi: 10.1038/nprot.2014.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bao G., Tang M., Zhao J., Zhu X. Nanobody: a promising toolkit for molecular imaging and disease therapy. EJNMMI Res. 2021;11:6. doi: 10.1186/s13550-021-00750-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uchanski T., Pardon E., Steyaert J. Nanobodies to study protein conformational states. Curr. Opin. Struct. Biol. 2020;60:117–123. doi: 10.1016/j.sbi.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Ural-Blimke Y., Flayhan A., Strauss J., Rantos V., Bartels K., Nielsen R., et al. Structure of Prototypic peptide transporter DtpA from E. coli in complex with valganciclovir provides insights into drug binding of human PepT1. J. Am. Chem. Soc. 2019;141:2404–2412. doi: 10.1021/jacs.8b11343. [DOI] [PubMed] [Google Scholar]
- 5.Haffke M., Fehlmann D., Rummel G., Boivineau J., Duckely M., Gommermann N., et al. Structural basis of species-selective antagonist binding to the succinate receptor. Nature. 2019;574:581–585. doi: 10.1038/s41586-019-1663-8. [DOI] [PubMed] [Google Scholar]
- 6.Jiang X., Smirnova I., Kasho V., Wu J., Hirata K., Ke M., et al. Crystal structure of a LacY-nanobody complex in a periplasmic-open conformation. Proc. Natl. Acad. Sci. U. S. A. 2016;113:12420–12425. doi: 10.1073/pnas.1615414113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Datsenko K.A., Wanner B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saier M.H., Jr., Beatty J.T., Goffeau A., Harley K.T., Heijne W.H., Huang S.C., et al. The major facilitator superfamily. J. Mol. Microbiol. Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 9.Guan L. In: European Biophysical Societies. 2nd Ed. Roberts G., Watts A., editors. Springer; Berlin, Heidelberg: 2018. Na(+)/Melibiose Membrane Transport Protein, MelB. Encyclopedia of Biophysics. [Google Scholar]
- 10.Ethayathulla A.S., Yousef M.S., Amin A., Leblanc G., Kaback H.R., Guan L. Structure-based mechanism for Na(+)/melibiose symport by MelB. Nat. Commun. 2014;5:3009. doi: 10.1038/ncomms4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Markham K.J., Tikhonova E.B., Scarpa A.C., Hariharan P., Katsube S., Guan L. Complete cysteine-scanning mutagenesis of the Salmonella typhimurium melibiose permease. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hariharan P., Guan L. Thermodynamic cooperativity of cosubstrate binding and cation selectivity of Salmonella typhimurium MelB. J. Gen. Physiol. 2017;149:1029–1039. doi: 10.1085/jgp.201711788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hariharan P., Tikhonova E., Medeiros-Silva J., Jeucken A., Bogdanov M.V., Dowhan W., et al. Structural and functional characterization of protein-lipid interactions of the Salmonella typhimurium melibiose transporter MelB. BMC Biol. 2018;16:85. doi: 10.1186/s12915-018-0553-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan L., Hariharan P. X-ray crystallography reveals molecular recognition mechanism for sugar binding in a melibiose transporter MelB. Commun. Biol. 2021;4:931. doi: 10.1038/s42003-021-02462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yousef M., Guan L. A 3D structure model of the melibiose permease of Escherichia coli represents a distinctive fold for Na+ symporters. Proc. Natl. Acad. Sci. U. S. A. 2009;106:15291–15296. doi: 10.1073/pnas.0905516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granell M., Leon X., Leblanc G., Padros E., Lorenz-Fonfria V.A. Structural insights into the activation mechanism of melibiose permease by sodium binding. Proc. Natl. Acad. Sci. U. S. A. 2010;107:22078–22083. doi: 10.1073/pnas.1008649107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karimova G., Pidoux J., Ullmann A., Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U. S. A. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Battesti A., Bouveret E. The bacterial two-hybrid system based on adenylate cyclase reconstitution in Escherichia coli. Methods. 2012;58:325–334. doi: 10.1016/j.ymeth.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 19.Yang D.C., Peters N.T., Parzych K.R., Uehara T., Markovski M., Bernhardt T.G. An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc. Natl. Acad. Sci. U. S. A. 2011;108:E1052–1060. doi: 10.1073/pnas.1107780108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houot L., Fanni A., de Bentzmann S., Bordi C. A bacterial two-hybrid genome fragment library for deciphering regulatory networks of the opportunistic pathogen Pseudomonas aeruginosa. Microbiology. 2012;158:1964–1971. doi: 10.1099/mic.0.057059-0. [DOI] [PubMed] [Google Scholar]
- 21.Arede P., Milheirico C., de Lencastre H., Oliveira D.C. The anti-repressor MecR2 promotes the proteolysis of the mecA repressor and enables optimal expression of beta-lactam resistance in MRSA. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ladant D., Ullmann A. Bordatella pertussis adenylate cyclase: a toxin with multiple talents. Trends Microbiol. 1999;7:172–176. doi: 10.1016/s0966-842x(99)01468-7. [DOI] [PubMed] [Google Scholar]
- 23.Guan L. In: Encyclopedia of Biological Chemistry. 3rd Ed. IJJ, editor. Elsevier; Oxford: 2021. Glucose/sugar transport in bacteria; pp. 192–202. [Google Scholar]
- 24.Hariharan P., Guan L. Insights into the inhibitory mechanisms of the regulatory protein IIA(Glc) on melibiose permease activity. J. Biol. Chem. 2014;289:33012–33019. doi: 10.1074/jbc.M114.609255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hariharan P., Balasubramaniam D., Peterkofsky A., Kaback H.R., Guan L. Thermodynamic mechanism for inhibition of lactose permease by the phosphotransferase protein IIA(Glc) Proc. Natl. Acad. Sci. U. S. A. 2015;112:2407–2412. doi: 10.1073/pnas.1500891112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guan L., Nurva S., Ankeshwarapu S.P. Mechanism of melibiose/cation symport of the melibiose permease of Salmonella typhimurium. J. Biol. Chem. 2011;286:6367–6374. doi: 10.1074/jbc.M110.206227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tikhonova E.B., Ethayathulla A.S., Su Y., Hariharan P., Xie S., Guan L. A transcription blocker isolated from a designed repeat protein combinatorial library by in vivo functional screen. Sci. Rep. 2015;5:8070. doi: 10.1038/srep08070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hariharan P., Guan L. Cooperative binding ensures the obligatory melibiose/Na+ cotransport in MelB. J. Gen. Physiol. 2021;153 doi: 10.1085/jgp.202012710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen S.G., Choi H.J., Fung J.J., Pardon E., Casarosa P., Chae P.S., et al. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parker J.L., Deme J.C., Kuteyi G., Wu Z., Huo J., Goldman I.D., et al. Structural basis of antifolate recognition and transport by PCFT. Nature. 2021;595:130–134. doi: 10.1038/s41586-021-03579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan L., Kaback H.R. Lessons from lactose permease. Annu. Rev. Biophys. Biomol. Struct. 2006;35:67–91. doi: 10.1146/annurev.biophys.35.040405.102005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smirnova I., Kasho V., Kaback H.R. Lactose permease and the alternating access mechanism. Biochemistry. 2011;50:9684–9693. doi: 10.1021/bi2014294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan N. Structural biology of the major facilitator superfamily transporters. Annu. Rev. Biophys. 2015;44:257–283. doi: 10.1146/annurev-biophys-060414-033901. [DOI] [PubMed] [Google Scholar]
- 34.Drew D., North R.A., Nagarathinam K., Tanabe M. Structures and general transport mechanisms by the major facilitator superfamily (MFS) Chem. Rev. 2021;121:5289–5335. doi: 10.1021/acs.chemrev.0c00983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Lipp K., Sery N., Ganea C., Basquin C., Fendler K., Leblanc G. The inner interhelix loop 4-5 of the melibiose permease from Escherichia coli takes part in conformational changes after sugar binding. J. Biol. Chem. 2006;281:25882–25892. doi: 10.1074/jbc.M601259200. [DOI] [PubMed] [Google Scholar]
- 36.Blaimschein N., Hariharan P., Manioglu S., Guan L., Muller D.J. Substrate-binding guides individual melibiose permeases MelB to structurally soften and to destabilize cytoplasmic middle-loop C3. Structure. 2023;31:58–67.e54. doi: 10.1016/j.str.2022.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geertsma E.R., Dutzler R. A versatile and efficient high-throughput cloning tool for structural biology. Biochemistry. 2011;50:3272–3278. doi: 10.1021/bi200178z. [DOI] [PubMed] [Google Scholar]
- 38.Botfield M.C., Wilson T.H. Mutations that simultaneously alter both sugar and cation specificity in the melibiose carrier of Escherichia coli. J. Biol. Chem. 1988;263:12909–12915. [PubMed] [Google Scholar]
- 39.Pourcher T., Leclercq S., Brandolin G., Leblanc G. Melibiose permease of Escherichia coli: large scale purification and evidence that H(+), Na(+), and Li(+) sugar symport is catalyzed by a single polypeptide. Biochemistry. 1995;34:4412–4420. doi: 10.1021/bi00013a033. [DOI] [PubMed] [Google Scholar]
- 40.Kiraly O., Guan L., Szepessy E., Toth M., Kukor Z., Sahin-Toth M. Expression of human cationic trypsinogen with an authentic N terminus using intein-mediated splicing in aminopeptidase P deficient Escherichia coli. Protein Expr. Purif. 2006;48:104–111. doi: 10.1016/j.pep.2006.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiraly O., Guan L., Sahin-Toth M. Expression of recombinant proteins with uniform N-termini. Methods Mol. Biol. 2011;705:175–194. doi: 10.1007/978-1-61737-967-3_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spiess C., Beil A., Ehrmann M. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell. 1999;97:339–347. doi: 10.1016/s0092-8674(00)80743-6. [DOI] [PubMed] [Google Scholar]
- 43.Jakkula S.V., Guan L. Reduced Na(+) affinity increases turnover of Salmonella enterica serovar Typhimurium MelB. J. Bacteriol. 2012;194:5538–5544. doi: 10.1128/JB.01206-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source data are available from the corresponding author.