Abstract
HIV-1 replication in primary monocyte-derived macrophages (MDMs) is kinetically restricted at the reverse transcription step due to the low deoxynucleoside triphosphates (dNTP) pools established by host dNTPase, SAM and HD domain containing protein 1 (SAMHD1). Lentiviruses such as HIV-2 and some Simian immunodeficiency virus counteract this restriction using viral protein X (Vpx), which proteosomally degrades SAMHD1 and elevates intracellular dNTP pools. However, how dNTP pools increase after Vpx degrades SAMHD1 in nondividing MDMs where no active dNTP biosynthesis is expected to exists remains unclear. In this study, we monitored known dNTP biosynthesis machinery during primary human monocyte differentiation to MDMs and unexpectedly found MDMs actively express dNTP biosynthesis enzymes such as ribonucleotide reductase, thymidine kinase 1, and nucleoside-diphosphate kinase. During differentiation from monocytes the expression levels of several biosynthesis enzymes are upregulated, while there is an increase in inactivating SAMHD1 phosphorylation. Correspondingly, we observed significantly lower levels of dNTPs in monocytes compared to MDMs. Without dNTP biosynthesis availability, Vpx failed to elevate dNTPs in monocytes, despite SAMHD1 degradation. These extremely low monocyte dNTP concentrations, which cannot be elevated by Vpx, impaired HIV-1 reverse transcription in a biochemical simulation. Furthermore, Vpx failed to rescue the transduction efficiency of a HIV-1 GFP vector in monocytes. Collectively, these data suggest that MDMs harbor active dNTP biosynthesis and Vpx requires this dNTP biosynthesis to elevate dNTP levels to effectively counteract SAMHD1 and relieve the kinetic block to HIV-1 reverse transcription in MDMs.
Keywords: HIV-1, reverse transcription, macrophages, Vpx, SAMHD1, dNTP biosynthesis
Deoxynucleoside triphosphates (dNTPs), the molecular precursors and building blocks of DNA, are metabolically regulated in all living cells. Dividing cells harbor high dNTP pools to support novel chromosomal DNA synthesis during S phase (1, 2). Conversely, in nondividing cells, it is energetically favorable to maintain lower dNTP pools due to the lack of chromosomal DNA replication and dNTP consumption (3). In fact, dNTP concentrations in nondividing/terminally differentiated macrophages (20–40 nM) are ∼200 times lower than dividing/activated CD4+ T cells (1–16 μM) (3, 4). The dNTP concentration difference between these two HIV-1 target cell types is a metabolic outcome of the tight regulation between dNTP synthesis and dNTP hydrolysis. Intracellular dNTPs are produced in the cell by two different metabolic pathways: the de novo dNTP biosynthesis pathway or the salvage pathway. In the de novo pathway, ribonucleoside diphosphates are reduced by ribonucleotide reductase (RNR) to generate deoxyribonucleoside diphosphates, which are converted to dNTPs by nucleoside-diphosphate kinase (NDPK) (5, 6, 7). In contrast, a series of cellular deoxynucleoside and deoxynucleotide kinases such as thymidine kinase 1 (TK1) (8), deoxycytidine kinase (DCK)(9), and NDPK are responsible for mediating phosphorylation events to generate dNTPs from dNs in the salvage pathway. Many of these biosynthetic enzymes are upregulated at the G1/S phase transition to supply dNTPs for DNA replication (10, 11, 12). On the other hand, cellular dNTPs can be hydrolyzed into dN and triphosphate subcomponents by a dNTPase, SAM and HD domain containing protein 1 (SAMHD1) (13). SAMHD1 is inactivated by phosphorylation during the G1/S phase transition by cyclin-dependent kinase (CDK1)/cyclin A2 to reduce SAMHD1 activity and allow for dNTPs to accumulate for DNA synthesis (14, 15, 16, 17). SAMHD1 is then dephosphorylated at mitotic exit by phosphatase PP2A/B55α to reinstall dNTPase activity (18). Importantly, SAMHD1 abundantly exists in nondividing cells such as macrophages to maintain low dNTP levels. Overall, the balance of biosynthesis and hydrolysis to regulate intracellular dNTP pools is a necessity for cells. Failure to maintain the appropriate amount and balance of dNTPs is a hallmark of cancer cells (19, 20) and can support rapid DNA synthesis and mutagenesis (21, 22, 23, 24).
Retroviruses utilize intracellular dNTPs during reverse transcription to generate a DNA copy of their RNA genome. Lentiviruses are a unique genus of this family as they replicate in both the high dNTP environment of dividing cells and the low dNTP environment of nondividing cells (3, 25). HIV-1 replicates rapidly in activated CD4+ T-cells, whereas viral replication is kinetically restricted in macrophages (26). The low macrophage dNTP concentration established by host restriction factor, SAMHD1, falls below the Km of reverse transcriptase (RT) resulting in kinetically restricted reverse transcription (3, 13, 26, 27, 28). Indeed, proviral DNA synthesis during HIV-1 infection of activated CD4+ T-cells occurs in 12 to 16 h whereas reverse transcription requires up to 36 h during infection of macrophages (29). However, HIV-2 and some Simian immunodeficiency virus can replicate rapidly even in macrophages due to their accessory protein, viral protein X (Vpx) (30, 31). Indeed, Vpx targets SAMHD1 for proteasomal degradation (26, 32, 33), relieving restriction in macrophages by elevating the intracellular dNTP level above the Km of RT (28, 34, 35).
Interestingly, previous studies have reported that RNR inhibitors such as gemcitabine (35) and hydroxyurea (27) can limit Vpx-mediated dNTP pool elevation in macrophages. These findings would surprisingly imply the presence of RNR, a dNTP biosynthesis enzyme, in these nondividing macrophages that lack chromosomal DNA replication. Extensive investigations on RNR have been made mainly in dividing cells, particularly cancer cells, and various RNR inhibitors are clinically available for anticancer treatments (36). However, due to the low dNTP concentrations that we previously observed in macrophages, dNTP biosynthesis machinery has long been presumed to be unavailable in this nondividing cell type. Therefore, only the contribution of dNTP hydrolysis by SAMHD1 has been well characterized as a key mechanistic element of HIV-1 restriction in macrophages.
In this study, we first investigated the expression levels of several dNTP biosynthesis enzymes throughout differentiation of human primary monocytes into macrophages. Notably, we found dNTP biosynthesis enzymes present in macrophages and upregulated during the differentiation process. We also observed that monocytes harbor extremely low dNTP levels that cannot be elevated by Vpx due to the absence of dNTP biosynthesis machinery. Therefore, Vpx cannot rescue the block to HIV-1 reverse transcription in monocytes, which could contribute to the absence of productive lentivirus replication in monocytes. Collectively, our data support that Vpx-mediated dNTP elevation in macrophages is dependent on ongoing active dNTP biosynthesis machinery such as RNR.
Results
Ribonucleotide reductase is expressed in monocyte-derived macrophages
dNTP biosynthesis, which is closely tied with cell cycling, cell division, and chromosomal DNA replication, has been presumed to be absent in nondividing cell types where DNA replication does not occur. Therefore, it remained unclear how Vpx can elevate cellular dNTP levels in terminally differentiated/nondividing macrophages where dNTP biosynthesis is supposed to be absent and chromosomal DNA replication permanently lacks. However, we previously reported that RNR inhibitors such as gemcitabine (35) and hydroxyurea (27) block Vpx-mediated dNTP elevation in human primary monocyte-derived macrophages (MDMs). This unexpected observation was further confirmed in this study (Fig. 1, A and B), where we show that hydroxyurea and gemcitabine can completely counteract the Vpx-mediated dNTP elevation in MDMs. Therefore, these data strongly support the possibility that the key de novo dNTP biosynthesis enzyme, RNR, is present and functional in human primary nondividing MDMs even though nondividing cells have been long presumed to lack active dNTP biosynthesis.
Figure 1.
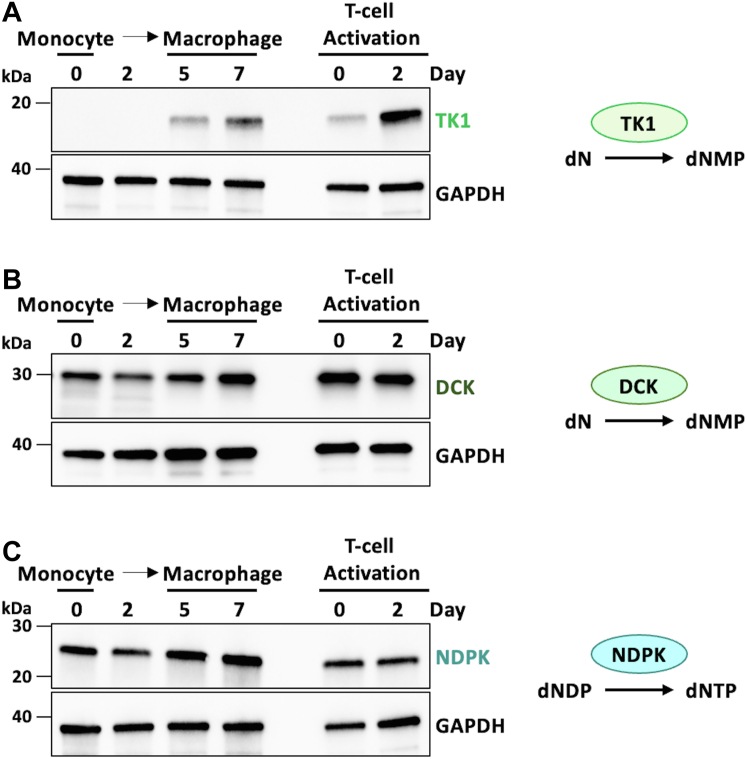
Effect of RNR inhibition on Vpx-mediated dNTP elevation in human primary macrophages and RNR expression profile during primary monocyte differentiation to macrophages. Primary human monocytes were isolated from five independent healthy donors, pooled in equal numbers, and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. CD4+ T-cells were isolated from the same donors and activated with PHA and IL-2. Fully differentiated macrophages were pretreated with indicated drug concentration and transduced using VLP Vpx+ or VLP Vpx-as a negative control. A, cellular SAMHD1 expression was evaluated using Western blot with GAPDH as a loading control. B, intracellular dATP levels were determined by RT-based dNTP assay. Data are the mean of three replicates from pooled primary cells, and error bars reflect standard deviation from the mean. p-values were determined using two-tailed, unpaired Welch’s t test to the no drug, VLP Vpx+ condition. C, samples were collected at indicated time points during primary monocyte differentiation into MDMs. Samples from the indicated time points during CD4+ T-cell activation were used as a positive cycling cell control. Cellular expression levels of the RNR M1 and RNR M2 subunits were evaluated using Western blot with anti-RNR M1 and anti-RNR M2 antibodies, respectively. GAPDH expression was used as a loading control. dNTP, deoxynucleoside triphosphates; GM-CSF, granulocyte-macrophage colony-stimulating factor; IL, interleukin; MDM, monocyte-derived macrophages; PHA, phytohaemagglutinin; RNR, ribonucleotide reductase; RT, reverse transcriptase; SAMHD1, SAM and HD domain containing protein 1; VLP, virus-like particle; Vpx, viral protein X.
Active RNR is a heterodimeric tetramer of two distinct subunits, RNR M1 and RNR M2 (5). In dividing cells, M1 is expressed throughout the cell cycle, while M2 is expressed during S phase to allow dNTPs to accumulate for DNA synthesis (10, 11). Therefore, to assess the expression of the functional RNR enzyme in macrophages, we differentiated human primary monocytes isolated and pooled from five healthy donors into macrophages using granulocyte-macrophage colony-stimulating factor (GM-CSF). We monitored the expression level of both RNR M1 and RNR M2 subunits at several time points during differentiation using Western blot (Fig. 1C). Nonactivated/nondividing (day 0) and activated/dividing (day 2) CD4+ T-cells (by interleukin 2 (IL-2)/phytohaemagglutinin [PHA]) isolated from the same donors were used as a positive cycling cell control and showed increased levels of RNR M1 and M2 subunits during the activation of cell cycling. Notably, we detected expression of both the RNR M1 and RNR M2 subunits at days 5 and 7 post GM-CSF treatment where the cells are attached and fully differentiated into macrophages. However, little to no M1 and M2 subunits were detected in freshly isolated human monocytes (day 0). Therefore, these data show that RNR expression is upregulated during monocyte differentiation to MDMs even though both monocytes and differentiated macrophages are nondividing cells without chromosomal DNA replication needs. Furthermore, these data support the possibility that Vpx-mediated dNTP elevation in MDMs requires active dNTP biosynthesis machinery such as RNR.
Macrophages, but not monocytes, express dNTP biosynthesis enzymes from the de novo and salvage pathways
Given terminally differentiated macrophages express the dNTP biosynthesis enzyme RNR, we next sought to assess the expression levels of other dNTP biosynthesis enzymes during monocyte differentiation to macrophages. For this, we collected lysates at several time points during primary monocyte differentiation to macrophages by GM-CSF and probed for dNTP biosynthesis enzymes using Western blot (Fig. 2). Here, we observed that TK1 and dCK, kinases involved in the dNTP salvage pathway, are both expressed in macrophages at higher levels, compared to monocytes (Fig. 2, A and B). NDPK, which is utilized in a final phosphorylation step to generate dNTPs by both the salvage pathway and the de novo dNTP biosynthesis pathway (7), is also expressed in both monocytes and macrophages (Fig. 2C). Monocytes likely need NDPK because NDPK also phosphorylates ribonucleotides for ribonucleoside triphosphate (rNTP) synthesis to support RNA synthesis and other cellular functions of rNTPs (ATP for energy carrying and ATP/GTP for kinase regulation) (37). TK1 expression, similar to RNR, is not present in monocytes and is significantly upregulated during differentiation into macrophages (Fig. 2A), whereas the dCK level only slightly increased during the differentiation (Fig. 2B). These data propose active dNTP biosynthesis in macrophages even though overall dNTP levels in this cell type are low. Monocytes, on the other hand, are missing key enzymes from both the de novo and salvage pathways such as RNR and TK1.
Figure 2.
Expression of dNTP biosynthesis enzymes throughout monocyte differentiation to macrophages. Primary human monocytes were isolated from five healthy independent donors and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. CD4+ T-cells isolated from these same donors were activated using PHA and IL-2 as a dividing cell control. Samples were collected for Western blot at indicated time points. Cellular expression levels of (A) thymidine kinase 1 (TK1), (B) deoxycytidine kinase (DCK), and (C) nucleoside diphosphate kinase (NDPK) were assessed using respective primary antibodies. GAPDH expression was evaluated using an anti-GAPDH antibody as a loading control. dNTP, deoxynucleoside triphosphates; GM-CSF, granulocyte-macrophage colony-stimulating factor; MDM, monocyte-derived macrophages; PHA, phytohaemagglutinin.
SAMHD1 in monocytes predominantly remains active/dephosphorylated, but SAMHD1 phosphorylation increases during differentiation to macrophages
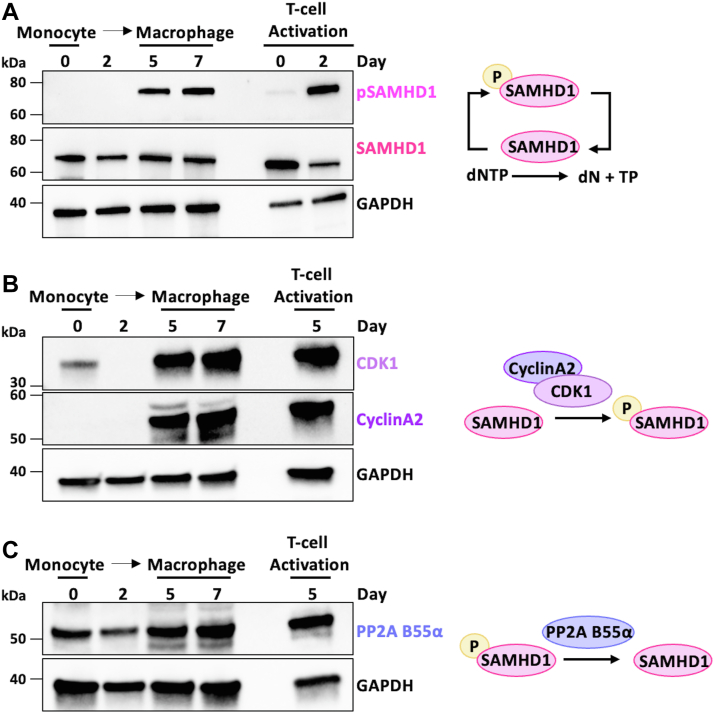
dNTP biosynthesis is counterbalanced by dNTP hydrolysis by host SAMHD1 (13, 27). The dNTPase activity of SAMHD1 is primarily regulated in dividing cells by phosphorylation at the C-T T672 residue that inactivates its dNTPase activity to supply sufficient dNTPs for chromosomal DNA replication. Therefore, we next monitored both total and phosphorylated/nonactive SAMHD1 levels throughout monocyte differentiation to macrophages as well as CD4+ T cell activation as a positive control. As shown in Figure 3A, we found SAMHD1 is abundantly present in both monocytes and macrophages, and this expression does not change during differentiation. Interestingly, there is a substantial population of pSAMHD1 in macrophage lysate, indicating a portion of the SAMHD1 present in macrophages is dNTPase inactive (38, 39). However, no pSAMHD1 was detected in monocyte lysate, suggesting SAMHD1 molecules in monocytes are predominantly active. This likely means that there is more potential for dNTP hydrolysis capacity in monocytes than in macrophages. Therefore, the macrophage dNTP metabolism environment can be characterized as having ongoing dNTP biosynthesis coexisting with dNTP hydrolysis whereas in monocytes only dNTP hydrolysis is completely present. Importantly, freshly isolated nonactivated CD4+ T cells, which are nondividing cells, also do not express the phosphorylated/nonactive form of SAMHD1 (Fig. 3A), which is consistent with our previous observation of the low dNTP levels in this type of T cell (40). However, SAMHD1 became phosphorylated during the IL-2/PHA mediated stimulation that leads to the activation of these cells into dividing cells, which helps the dividing cells maintain high levels of dNTPs to support chromosomal DNA replication.
Figure 3.
Expression of SAMHD1 and SAMHD1 dephosphorylation/phosphorylation enzymes throughout monocyte differentiation to macrophages. Primary human monocytes were isolated from five healthy independent donors and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. CD4+ T-cells isolated from these same donors were activated using PHA and IL-2 as a dividing cell control. Samples were collected for Western blot at indicated time points. Cellular expression levels of (A) T592 phosphorylated SAMHD1 (pSAMHD1) and total SAMHD1 (B) cyclin dependent kinase 1 (CDK1) and cyclin A2 (C) protein phosphatase2A B55α subunit (PP2A B55α) were assessed using respective primary antibodies. GAPDH expression was evaluated using an anti GAPDH antibody as a loading control. GM-CSF, granulocyte-macrophage colony-stimulating factor; PHA, phytohaemagglutinin; SAMHD1, SAM and HD domain containing protein 1.
After observing that pSAMHD1 levels increase throughout monocyte differentiation into macrophages, we next assessed levels of enzymes that regulate SAMHD1 phosphorylation and dephosphorylation during differentiation. As shown in Figure 3B, we observed increased amounts of the CDK1/cyclinA2 complex that mediates SAMHD1 phosphorylation (14) during the differentiation from monocytes to macrophages (Fig. 3B) (14), which is consistent with the significant SAMHD1 phosphorylation increase during the differentiation to macrophages (Fig. 3A). However, we also detected some increase of the PP2A B55α subunit, which is a specificity component of PP2A phosphatase that dephosphorylates SAMHD1 (18), throughout monocyte differentiation to macrophages (Fig. 3C). Overall, in monocytes, there is little to no capacity for the phosphorylation of SAMHD1 while dephosphorylation machinery is present in monocytes, accounting for the lack of pSAMHD1 in monocytes. On the other hand, in macrophages, phosphorylation and dephosphorylation of SAMHD1 are both occurring, resulting in a mixed population of pSAMHD1 and SAMHD1. However, it is still likely that macrophages express significant levels of active/dephosphorylated SAMHD1 protein as indicated by the strong expression of PP2A B55α (Fig. 3C), which is responsible for the low dNTP level and HIV restriction capability of macrophages.
Monocytes enter a G1/S phase-like state during differentiation to macrophages
Noncycling cells are thought to exist in G0, a phase that exists outside of the cell cycle (41). Even so, we have observed macrophages express TK1 (12), RNR M2 (10), and several other dNTP biosynthesis enzymes that are normally upregulated at the G1/S transition in cycling cells. Therefore, we investigated levels of G1/S phase transition markers throughout monocyte differentiation into macrophages (GM-CSF treatment). For this, we monitored the protein levels of E2F-1, a master transcription factor regulating genes required for cell cycle progression to S phase (42), and CDK2 that phosphorylates numerous downstream target proteins involved in the transition to S phase (43). As shown in Figure 4A, both E2F-1 and CDK2 are not expressed in monocytes, indicating that monocytes exist at G0-like status. However, these master cell cycle regulators are upregulated during differentiation, supporting the G1/S phase-like state status of macrophages.
Figure 4.
Cell cycle markers and cellular DNA synthesis during monocyte differentiation to macrophages. Primary human monocytes were isolated from (A) five and (B and C) three healthy independent donors and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. CD4+ T-cells isolated from these same donors and were activated using PHA and IL-2 as a dividing cell control. A, samples were collected for Western blot at indicated time points. Cellular expression levels of transcription factor E2F1 and cyclin-dependent kinase 2 (CDK2) were assessed using respective primary antibodies. GAPDH expression was evaluated using an anti-GAPDH antibody as a loading control. B, genomic DNA synthesis was evaluated using an EdU incorporation assay. Primary monocytes without GM-CSF exposure and fully differentiated MDMs were incubated with EdU for 2 h followed by EdU labeling with an iFluor 488 florescent azide. 293T cells were used as a dividing cell control. iFluor 488-labeled EdU incorporation was determined using flow cytometry. C, Mitochondrial DNA copy number was measured at indicated time points throughout CD4+ T-cell activation and monocyte differentiation using qPCR. Genomic DNA copy number was measured using qPCR within the same samples to normalize the mitochondrial DNA copy number. Data are the mean of three replicates and error bars reflect standard deviation from the mean. p-values were determined using two-tailed, unpaired Welch’s t test to the day 0 time point for T-cells or monocytes. GM-CSF, granulocyte-macrophage colony-stimulating factor; MDM, monocyte-derived macrophages; PHA, phytohaemagglutinin; qPCR, quantitative PCR.
Since macrophages express G1/S transition markers, we next assessed if macrophages were entering S phase by measuring genomic DNA synthesis, the hallmark of S phase (44). To do this, we employed an EdU assay to fluorescently label cells synthesizing DNA, followed by flow cytometry. While 27% of 293T cells, the positive cycling cell control, were synthesizing new DNA under our experimental conditions, we observed little to no DNA synthesis in both primary monocytes and macrophages (Fig. 4B). Therefore, similar to a previous report (45), we conclude that macrophages enter a “G1/S-like state” during differentiation but are not entering into S phase.
Next, we also investigated if dNTP biosynthesis machinery was being upregulated to support mitochondrial DNA synthesis during 7-days monocyte differentiation to macrophages. Here, we extracted total cellular DNA at several time points during monocyte differentiation or CD4+ T-cell activation as a positive cycling cell control. We then used quantitative PCR (qPCR) to measure mitochondrial DNA copy number normalized to nuclear DNA copy number. Here we observed an increase in mitochondrial DNA copy number during T-cell activation by day 5, but not during monocyte differentiation into macrophages even at 7 days post differentiation (Fig. 4C). Thus, the upregulation of dNTP biosynthesis enzymes during differentiation does not appear to function as a support for DNA synthesis, either genomic or mitochondrial.
Monocytes have extremely low dNTP levels that cannot be raised by Vpx
We hypothesized that macrophages have higher dNTP levels than monocytes due to the upregulation of dNTP biosynthesis machinery and increased SAMHD1 phosphorylation/inactivation during differentiation. To test this hypothesis, we directly measured dNTP levels in monocytes and macrophages using LC-MS/MS (46). Indeed, monocyte dNTP levels are significantly lower than the already low dNTP levels of macrophages (Fig. 5A).
Figure 5.
Effect of Vpx on dNTP levels in primary monocytes and macrophages. Primary human monocytes were isolated from three healthy independent donors and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. A, cellular dNTP levels of fully differentiated macrophages and monocytes without GM-CSF exposure were analyzed using LC-MS/MS. B, fully differentiated macrophages and monocytes without GM-CSF exposure were treated with VLPs Vpx+ for 24 h. Treatment with VLPs Vpx-was used as a negative control. dNTP levels were measured using LC-MS/MS. Data are the mean of three replicates and error bars reflect standard deviation from the mean. p-values were determined using two-tailed, unpaired Welch’s t test to (A) macrophages and (B) VLPs Vpx-treated macrophages or monocytes. dNTP, deoxynucleoside triphosphates; GM-CSF, granulocyte-macrophage colony-stimulating factor; VLPs, virus-like particles; Vpx, viral protein X.
Next, we tested whether Vpx, which elevates dNTP levels in macrophages (27), can also elevate dNTP levels in monocytes. Here, we predicted that Vpx would be unable to elevate dNTP pools in monocytes due to the absence of dNTP biosynthesis machinery. For this test, first, we treated monocytes and macrophages with vesicular stomatitis virus (VSV-G) psuedotyped virus-like particles (VLPs) Vpx+ or VLPs Vpx-, as a negative control, and confirmed SAMHD1 depletion in VLPs Vpx+ treated cells (Fig. S1A). Next, dNTP levels in these cells were measured using LC-MS/MS. As shown in Figure 5B, we observed an increase in dNTP level in macrophages treated with VLPs Vpx+ when compared to macrophages treated with VLPs Vpx-. In contrast, despite SAMHD1 depletion in monocytes treated with VLPs Vpx+, we observed no significant change in dNTP levels in these cells compared to monocytes treated with VLPs Vpx- (Fig. 5B). These data demonstrate that SAMHD1 depletion is not sufficient for Vpx-mediated dNTP elevation in monocytes and further support that Vpx-mediated dNTP elevation requires active dNTP biosynthesis as seen in macrophages.
dNTP concentrations found in monocytes block efficient reverse transcription
It has been reported that HIV-1 infection in monocytes is limited even though monocytes express the appropriate CD4 receptor and coreceptors (47, 48). Moreover, it has been proposed that the block to HIV-1 infection in monocytes occurs at a step post entry (47, 49). Therefore, we hypothesized that the extremely low dNTP pool of monocytes impairs the reverse transcription reaction, thus serving as a major replicative block in this cell type. To test this, we ran a biochemical simulation of HIV-1 reverse transcription in CD4+ T-cell, macrophage, and monocyte dNTP concentrations using a primer extension assay. First, we calculated the dNTP concentration of macrophages and monocytes using previously mentioned dNTP levels (Fig. 5A) and cell volume determined by confocal microscopy. The cell volumes of the monocytes and macrophages were found to be 1108 μM3 and 3063 μM3, respectively, comparable to our previous publication (3) (Table S1A). Therefore, the dNTP concentration of monocytes is up to 20-fold lower than macrophages, with the rate-limiting nucleotide being dATP (Fig. 6, A and B). For the biochemical simulation, a 5′ 32P-labeled 17-mer RNA primer annealed to a 40-mer DNA template was extended by purified HIV-1 RT protein under reaction conditions that contained dNTP concentrations present in activated CD4+ T cells (4 μM), macrophages treated with Vpx (500 nM), macrophages without Vpx (40 nM), and monocytes (2 nM) (Fig. 6C). In this primer extension assay, the full extension of the primer (P) by HIV-1 RT produces the 40-mer DNA product (F). We conducted the primer extension reactions at the dNTP concentrations found in activated CD4+ T cells with three different HIV-1 RT protein amounts (4×, 2× and 1×, Fig. 6C), which generated three different amounts of the fully extended product, validating that the reactions were in the linear range under our experimental conditions. When these reactions were repeated with the dNTP concentrations found in macrophages, there was a significant reduction of fully extended primer and the exacerbation of a pause site, indicative of replicative stress. However, this restricted DNA synthesis was rescued when the same reactions were conducted at the dNTP concentrations found in the Vpx-treated macrophages, which biochemically validates the anti-SAMHD1 function of Vpx. By contrast, in monocyte dNTP conditions we observed no fully extended primer and only the generation of new early pause sites. This biochemical simulation suggests that efficient reverse transcription cannot occur under monocyte dNTP concentrations, posing a significant block to HIV-1 infection in this cell type.
Figure 6.
HIV-1 reverse transcription biochemical simulation in monocyte dNTP concentrations.A, monocyte and macrophage dNTP concentrations were determined by using dNTP levels from Figure 5A and measured cell volumes (Table S1). B, dNTP concentration fold differences between monocytes and macrophages. C, a primer extension reaction using 5′ 32P-labeled 17-mer DNA primer (P) annealed to 40-mer template RNA, purified HIV-1 reverse transcriptase (RT), and dNTP concentrations found in primary activated CD4+ T-cells, macrophages treated with VLPs Vpx+, macrophages treated with VLPs Vpx-, and monocytes. A variety of RT was utilized to ensure the reactions were in the linear range. -: Negative control using water in place of dNTPs. +: positive control using 5 μM dNTPs and undiluted RT. P: 17-mer unextended primer. F: 40-mer fully extended primer. dNTP, deoxynucleoside triphosphates; VLP, virus-like particles; Vpx, viral protein X.
Vpx requires ongoing dNTP biosynthesis to accelerate reverse transcription and rescue HIV-1 transduction
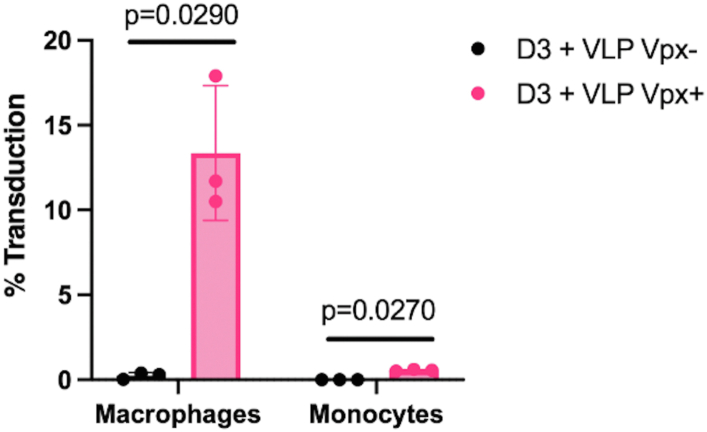
The low dNTP pool of macrophages restricts HIV-1 infection at the reverse transcription step (3, 13, 28). However, Vpx overcomes this restriction in macrophages by raising intracellular dNTP pools (26, 32). As Vpx cannot elevate intracellular dNTP levels in monocytes due to the absence of ongoing dNTP biosynthesis, we next tested the hypothesis that Vpx cannot relieve the block to reverse transcription and rescue HIV-1 infection in monocytes. For this test, we treated monocytes and macrophages with VSV-G pseudotyped VLPs Vpx+ or VLPs Vpx-for 24 h, and SAMHD1 depletion was confirmed with Western blot (Fig. S1B). Cells were then transduced with VSV-G pseudotyped D3-HIV-GFP vector that encodes the entire NL4-3 HIV-1 sequence except the env gene is deleted, and the nef gene is replaced with GFP. At 72 h post D3-HIV-GFP transduction, the cells were assessed for transduction efficiency using flow cytometry. As shown in Figure 7A, while Vpx was able to rescue D3-HIV-GFP transduction in macrophages with 13% of the population transduced, Vpx failed to rescue D3-HIV-GFP transduction in monocytes to more than 0.5%. Together, these data support that despite SAMHD1 depletion, Vpx cannot rescue HIV-1 infection in monocytes as it is unable to elevate intracellular dNTP pools to accelerate reverse transcription in the absence of ongoing dNTP biosynthesis.
Figure 7.
Effect of Vpx on HIV-1 vector transduction of monocytes and macrophages. Primary human monocytes were isolated from three healthy independent donors, pooled in equal numbers, and differentiated into monocyte-derived macrophages (MDMs) using GM-CSF. Fully differentiated macrophages and monocytes without GM-CSF exposure were treated with VLPs Vpx+ for 24 h. VLPs Vpx-treatment was used as a negative control. Cells were then transduced with a VSV-G pseudotyped and GFP labeled HIV-1 vector. Seventy-two hours later cells were harvested, and transduction efficiency was measured using flow cytometry. Data are the mean of three replicates from pooled cells and error bars reflect standard deviation from the mean. p-values were determined using two-tailed, unpaired Welch’s t test to VLPs Vpx-treated macrophages or monocytes. GM-CSF, granulocyte-macrophage colony-stimulating factor; VLPs, virus-like particles; Vpx, viral protein X; VSV, vesicular stomatitis virus.
Discussion
Unlike rNTPs that function in multiple biological processes such as RNA synthesis, energy carriers (ATP), and cell signaling regulation (ATP and GTP), cellular dNTPs serve one vital function, to support DNA synthesis. Therefore, dNTP biosynthesis is tightly regulated to the S phase of the cell cycle (1, 2, 50). The fidelity of this regulation is critical for proper cell replication as exemplified by cancer cells, which upregulate dNTP metabolism to support rapid and uncontrolled cell division (19, 20, 21, 51). This elevation in turn increases mutation synthesis during DNA replication and furthers genomic instability (22, 23). RNR has long been recognized as a cancer therapeutic target, with inhibitors such as gemcitabine used to treat pancreatic, bladder, and lung cancers in the clinic (36). Therefore, the regulation of dNTP biosynthesis in dividing cells and how this regulation is altered by cancer cells has been extensively studied. On the other hand, dNTP metabolism in nondividing cells such as macrophages has been presumed to be nonexistent due to their low dNTP levels and absence of chromosomal DNA synthesis for cell division. Although, with this assumption, it remained unclear how Vpx could rapidly elevate dNTP levels in macrophages, as in the absence of active dNTP biosynthesis, intracellular dNTP levels should remain low even after SAMHD1 depletion.
In this study, we confirmed the observation that inhibiting RNR activity counteracts Vpx-mediated dNTP elevation in primary MDMs (27, 35). Moreover, we found RNR and other dNTP biosynthesis enzymes including NDPK, TK1, and DCK are expressed in macrophages. In comparison, we observed little to no expression of RNR and TK1 in monocytes, the nondividing precursors of macrophages. As RNR and TK1 participate in early events in their respective biosynthesis pathways, their absence in monocytes likely impairs dNTP biosynthesis in this cell type regardless of the expression of enzymes that act later such as NDPK. Overall, we observed upregulation of dNTP biosynthesis enzymes during monocyte differentiation in macrophages. However, it is possible that in nondividing cells, the relative contributions from enzymes in the de novo synthesis and salvage pathways are different from what is observed in dividing cells.
While the expression levels of dNTP biosynthesis enzymes were upregulated during monocyte differentiation into macrophages, we observed an increase in the population of pSAMHD1 (enzymatically inactive form). Concurrently, there was an upregulation of CDK1/cyclinA2, the complex responsible for phosphorylating SAMHD1 (14), accounting for the increase in pSAMHD1 during differentiation. Furthermore, we observed G1/S phase transition markers E2F-1 and CDK2 in macrophages without evidence of DNA synthesis. This supports the idea that macrophages can enter a “G1/S transition-like state” during differentiation, agreeing with a previous report that proposed a G1-like state in macrophages (45). Mechanistically, existing in this “G1/S transition-like state” would allow for macrophages to upregulate dNTP biosynthesis and turn down dNTP hydrolysis, thus accumulating more dNTPs than monocytes. Although, macrophages have much lower dNTP levels than dividing cells due to the coexistence of dNTP biosynthesis with dNTP hydrolysis in macrophages (3).
Indeed, our data demonstrate that macrophages have higher dNTP levels than monocytes even though macrophage dNTP levels are still much lower than dNTP levels in activated CD4+ T cells (Fig. 8). While Vpx treatment of macrophages can elevate intracellular dNTP levels above the KM value of HIV-1 RT (Fig. 8), Vpx treatment of monocytes did not significantly elevate dNTP levels despite depletion of SAMHD1. This finding implies Vpx cannot elevate dNTP pools without ongoing dNTP biosynthesis. Also, no detection of the phosphorylated form of SAMHD1 in monocytes indicates that most of the SAMHD1 molecules are highly active and can constantly degrade dNTPs, which likely contributes to the extremely low dNTP levels observed in monocytes. These extremely low dNTP concentrations found in monocytes fall far below the KM of HIV-1 RT (Fig. 8) and therefore inhibited efficient reverse transcription in our biochemical simulation. Furthermore, this block to reverse transcription in monocytes cannot be relieved by Vpx due to the inability of Vpx to raise intracellular dNTP pools in this cell type. Therefore, Vpx fails to accelerate reverse transcription and rescue transduction efficiency by an HIV-1 vector in monocytes. Collectively these data suggest that Vpx requires ongoing active dNTP biosynthesis for effectively counteracting the anti-lentivirus activity of SAMHD1 in macrophages. More broadly, this study proposes the idea that nondividing cell subtypes may have differences in their dNTP metabolism and this in turn can impact HIV-1 infection. HIV-1 infection is also restricted by SAMHD1 and enhanced by Vpx in monocyte-derived dendritic cells and quiescent CD4+ T-cells, two nondividing cell types (26, 52). Based on the results of this study, it is possible these cell types also have some level of active dNTP biosynthesis potential that Vpx utilizes to enhance HIV-1 infection. Moreover, while the Vpx utilized in our study originated from SIVmac (53), we would expect that Vpx and Vpr proteins from other SAMHD1-counteracting lentiviruses function similarly to accelerate reverse transcription kinetics during replication.
Figure 8.
Comparison of dNTP levels in HIV-1 target cells to HIV-1 RT KMvalue. Bars represent reported average dNTP concentrations (nM) from CD4+ T-cells (3, 60), macrophages (3, 28), and monocytes treated with or without Vpx. The green box represents the KM (Michaelis–Menten steady state) value of HIV-1 reverse transcriptase (RT) (28). CD4+ T-cells have plentiful dNTP concentrations regardless of Vpx treatment. Macrophage dNTP levels fall below the KM value of HIV-1 RT, resulting in delayed reverse transcription in this cell type; however, this is relieved upon Vpx treatment. Monocyte dNTP levels are extremely low and cannot be elevated by Vpx due to the lack of ongoing dNTP biosynthesis. These extremely low dNTP concentrations fall far below the HIV-1 RT KM value resulting in a major block to reverse transcription regardless of Vpx treatment. dNTP, deoxynucleoside triphosphates; RT, reverse transcriptase; Vpx, viral protein X.
The finding that MDMs harbor active nucleotide biosynthesis has several therapeutic implications. For example, nucleoside reverse transcriptase inhibitors that are Food and Drug Administration authorized for the treatment and prevention of HIV-1 infection, such as lamivudine, emtricitabine, and tenofovir, rely on cellular nucleoside kinases to generate the active triphsophate form (54). Our results have demonstrated that nondividing macrophages harbor such enzymes affirming the potential of these therapeutics in treating HIV-1 infections in macrophages. Also, macrophage infection constitutes a unique part of the HIV-1 reservoir that persists in antiretroviral therapy treated patients (55, 56). This study has provided novel insight on the nucleotide metabolism environment of macrophages that can inform future intelligent design of HIV-1 pharmaceuticals.
Experimental procedures
Cell culture
293T cells were cultured in Dulbecco's modified Eagle's medium (Gibco) supplemented with 10% (v/v) fetal bovine serum (FBS) (Omega) and 1% (v/v) penicillin/streptomycin (Gibco). Primary human monocytes were isolated from peripheral blood buffy coats of three to five donors (New York Blood Center) as previously described (3). Briefly, peripheral blood mononuclear cells (PBMCs) were collected using Ficoll density gradients (Accu-Prep, Accurate Chemical & Scientific) in SepMate-50 conicals (Stemcell Technologies). PBMCs were incubated for 30 min with 150 μl of CD14 antibody–conjugated magnetic beads (Miltenyi), and monocytes were isolated using positive immunomagnetic selection. Resulting monocytes are pooled in equal numbers from each donor to mitigate individual variability.
Primary human CD4+ T cells were then isolated from monocyte-depleted PBMCs by positive selection using anti-CD4 antibody–conjugated magnetic beads (Miltenyi). Purified pooled human monocytes were differentiated into macrophages by culturing in RPMI (Gibco) supplemented with 10% (v/v) FBS (Omega), 1% (v/v) penicillin/streptomycin (Gibco), and 15 ng/ml GMC-SF (Miltenyi) for 5 days, and incubated for an additional 2 days in the absence of GMC-SF. Pooled CD4+ T cells were activated by culturing in RPMI (Gibco) supplemented with 10% (v/v) FBS (Omega), 1% (v/v) penicillin/streptomycin (Gibco), 5 μg/ml PHA (Sigma), and 5 ng/ml IL-2 (Miltenyi) for 2 days and were incubated for an additional 2 days without PHA.
Vectors
VLPs Vpx+ and Vpx-were generated as previously described (57). Eighty percent confluent T225 flasks (Falcon) of 293T cells were transfected with 40 μg of pSIV3+ or pSIV3+ ΔVpx (obtained from Dr Nathaniel Landau, New York University) (53) and 20 μg of pVSV-G using polyethyleneimine. Culture media was changed 16 h post transfection. The culture medium was then collected on days 2 and 3 post transfection and centrifuged (1500 rpm for 10 min) to clear cellular debris. VLPs were concentrated by ultracentrifugation (23,000 rpm, for 2 h, at 4 °C) over a 25% sucrose cushion (25% sucrose, 25 mM Tris–HCl, pH 7.5, 150 mM NaCl, and 5 mM EDTA). The resulting pellet was resuspended in serum-free RPMI (Gibco) and aliquoted. Aliquots were flash frozen and stored at −80 °C unless otherwise indicated.
D3-HIV-GFP production was modified from a previous protocol (21) to obtain a plasmid-free preparation. T225 flasks of 80% confluent 293T cells were given serum-free Dulbecco's modified Eagle's medium (Gibco). Cells were transfected with 30 μg pD3HIV-GFP, which encodes the full HIV-1 NL4-3 genome with an envelope gene deletion and the nef gene replaced by an enhanced GFP gene (3), and 5 μg pVSV-g using PolyJet (SignaGen Laboratories; SL100688). Five hours post transfection, the transfection media was replaced by culture media. Forty-eight hours post transfection culture media was harvested, centrifuged at 1500 rpm for 20 min, and filtered through a 0.45 μm filter to remove cell debris. Cleared media was ultracentrifuged (23,000 rpm for 2 h at 4 °C) and the pellet was resuspended in Hanks' Balanced Salt Solution (Gibco).
RNR inhibitor treatment
Primary macrophages were pretreated with either no drug, 1 mM hydroxyurea (MedChem Express), 2 mM hydroxyurea, 40 nM of gemcitabine (MedChem Express), or 100 nM of gemcitabine for 2 h before treatment with VLPs Vpx - or Vpx +. 24 h post VLP transduction macrophages were trypsinized with Trypsin–EDTA (0.25%), phenol red (Gibco) for counting or lysed in the plate for Western blot and dNTP measurements.
Western blot
Cells were lysed in ice cold radioimmunoprecipitation buffer (50 mM Tris–HCl, pH 8, 0.1% SDS, 150 mM NaCl, 0.25% deoxycholic acid, 1 mM EDTA, and 1% NP-40) supplemented with 1:500 (v/v) protease inhibitor cocktail (Sigma). Lysates were centrifuged (13,000 rpm for 10 min at 4 °C) and the cleared lysates were diluted with Laemmli buffer (Bio-Rad). Lysates were resolved on a 4 to 15% gradient SDS-PAGE gel (Bio-Rad), transferred onto a nitrocellulose membrane (Bio-Rad), and probed with the indicated primary antibodies and corresponding secondary antibodies. Proteins were visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and a Bio-Rad ChemiDoc MP Imaging System. Primary antibodies used in this study in the order of appearance are as follows: SAMHD1 (abcam; 67820), GAPDH (Cell Signaling Technology; 14C10), RNR M1 (abcam; 137114), RNR M2 (abcam; 172476), thymidine kinase 1 (abcam; 76495), dCK (abcam; 96599), nucleoside diphosphate kinase (abcam; 241162), p-SAMHD1 (Cell Signaling Technology; 89930S), PP2A B55α (Cell Signaling Technology; 4953T), CDK1 (abcam; 133327), cyclin A2 (abcam; 181591), E2F-1 (Cell Signaling Technology; 3742S), and CDK2 (abcam; 32147). Secondary antibodies used in this study are as follows: anti-mouse (Cytiva; NA931V) and anti-rabbit (Cytiva; NA934V).
dNTP extraction
Cells were rapidly lysed in the plate with ice cold 65% methanol, vortexed for 2 min, incubated at 95 °C for 3 min, and centrifuged at 14,000 rpm for 3 min. The supernatant was transferred to a clean Eppendorf tube and dried using a speed vac.
RT-based cellular dNTP measurement
Intracellular dNTP levels were measured by our HIV-1 RT-based dNTP assay as previously described (3). Dried dNTP extracts were resuspended in 20 μl of water and further diluted until the samples were within the linear range of the assay. 5′ 32P-end-labeled 18-mer DNA primer (5′-GTCCCTCTTCGGGCGCCA-3′; Integrated DNA Technologies) was annealed to four different 19-mer DNA templates (3′-CAGGGAGAAGCCCGCGGTX-5′; Integrated DNA Technologies), where X represents one of the four nucleotides. Two microliters of diluted extract was incubated with 200 fmol template/primer, 4 μl of purified RT (HIV-1 HXB2), 25 mM Tris–HCl, pH 8.0, 2 mM DTT, 100 mM KCl, 5 mM MgCl2, and 10 μM oligo (dT) in a 20 μl reaction at 37 °C for 5 min. Water or 0.5 mM dNTP mix replaced the diluted dNTP extract for a negative and positive control, respectively. Reactions were stopped by adding 10 μl of 40 mM EDTA and 99% (v/v) formamide and incubated at 95 °C for 2 min. Reactions were resolved on a 14% urea-PAGE gel (AmericanBio, Inc) and imaged using an Amersham Typhoon (Cytiva). ImageQuant TL (Cytiva) was used to quantify single-nucleotide extensions products which is used to determine the amount of dNTP present in the extract.
Edu assay
The EdU assay to measure genomic DNA synthesis was conducted using the EdU assay/EdU Staining Proliferation Kit (iFluor 488) (abcam; 219801) following the manufacturer’s protocol. Briefly, monocytes, macrophages, and positive control 293T cells were incubated with EdU for 2 h. Cells were fixed with 4% paraformaldehyde (PFA) for 15 min, permeabilized, and labeled with the iFluor 488 dye. Unstained cells and cells that were fixed prior to EdU incubation were used as a control for fluorescent background. Flow cytometry was conducted using a FACSymphony A5 (BD Biosciences) and analyzed using FlowJo software (BD Bioscience; https://www.bdbiosciences.com/en-us/products/software/flowjo-v10-software). Cell debris and doublets were excluded from iFluor 488 analysis. iFluor 488 gating was established on unstained cell populations.
Mitochondrial DNA copy number qPCR
Mitochondrial DNA copy number was calculated as previously published (58). Total DNA was extracted from activated CD4+ T-cells, monocytes, and macrophages at indicated time points using the Wizard SV Genomic DNA Purification System (Promega; A2360) following the manufacturer’s protocol. qPCR was performed in LightCycler 480 Multiwell Plate 96 plates (Roche; 04729692001) using a Roche LightCycler 480 Instrument. Each well contained a VIC-labeled assay for a mitochondrial DNA gene (ND1) (Thermo Fisher Scientific; Assay ID Hs02596873_s1) and a FAM-labeled assay for a nuclear gene (RPPH1) (Thermo Fisher Scientific; Assay ID Hs03297761_s1). Each biological replicate was run in triplicate. Cycle threshold (Ct) values were obtained from the LightCycler 480 Software (Roche) and Ct values across technical replicates were averaged. ΔCt was calculated by subtracting the mitochondrial Ct value from the nuclear Ct value. Relative mitochondrial DNA content was calculated as 2 ∗ 2ΔCt.
LC-MS/MS based dNTP measurement
To quantify the intracellular dNTPs, an ion pair chromatography-tandem mass spectrometry method was applied as previously published (46). Briefly, dNTPs were extracted from approximately six million cells per replicate. Dried dNTP extracts were resuspended in 100 μl of mobile phase A (2 mM of ammonium phosphate monobasic and 3 mM of hexylamine), and macrophage samples were further diluted five times. Samples were centrifuged at 12,000 rpm for 10 min and 45 μl of the resulting supernatant was mixed with 5 μl of 13C and 15N labeled dNTPs to serve as internal standards. Calibration curves were generated from standards by serial dilutions in mobile phase A (0.2–500 nM). Chromatographic separation and detection was performed on a Vanquish Flex System (Thermo Scientific Scientific) coupled with a TSQ Quantiva triple quadrupole mass spectrometer (Thermo Scientific Scientific). Samples were separated on a Kinetex EVO C18 column (100 × 2.1 mm, 2.6 μm) (Phenomenex). The gradient increased from 8% to 35% of mobile phase B (acetonitrile) in 5 min then returned to 8% mobile phase B. Thermo Xcalibur 3.0 software (https://www.thermofisher.com/order/catalog/product/OPTON-30965) was used for data collection and processing. Selected reaction monitoring in both positive and negative modes was used to detect the four dNTPs: dATP (492→136, pos), dGTP (508→152, pos), dCTP (468→112, pos), and TTP (481→158.9, neg).
VLP Vpx treatment of monocytes and macrophages
Freshly isolated monocytes were concentrated to 500,000 cells per 200 μl in a 48-well plate and treated with 50 μl freshly harvested VLP Vpx- or Vpx+ for 24-h. Fully differentiated macrophages in 24-well plates were treated with 25 μl freshly harvested VLP Vpx- or Vpx+ for 24-h. Approximately six million cells were used per replicate. Cells were washed thoroughly with saline before dNTP extraction and reserved wells were used for counting and Western blot. dNTP measurement was conducted using mass spectrometry.
Cell volume of monocytes and macrophages
Glass bottom chambered coverslips (ibidi; 80807) were treated with 0.1 mg/ml poly-D-lysine overnight, washed with PBS, and air dried. A total of 200,000 monocytes were loaded with CellTracker Green CMFDA Dye (Thermo Fischer Scientific; C7025) for 30 min. Cells were washed, resuspended in 200 μl of PBS, and plated onto the chambered coverslips. Cells were incubated for 2 h covered at room temperature to allow cells to settle onto the coverslip. Supernatant was gently aspirated, and cells were fixed to the coverslip using 4% PFA for 15 min. Cells were washed, coated in VECTASHIELD Antifade Mounting Medium (Vector Laboratories; H-1000–10), and stored at 4 °C until imaging. Macrophages were differentiated and cultured on the treated chamber coverslips. Fully differentiated macrophages were loaded with CellTracker Green CMFDA dye, fixed with 4% PFA, washed, covered with VECTASHIELD, and stored at 4 °C until imaging. Cells were imaged using a 60× objective on a Nikon spinning disk confocal microscope where Z-stacks were taken with 0.2 μM steps. Ten fields of vision were captured per sample. Volume for each cell in a field was determined using the surface tool in Imaris software (Oxford Instruments; Version 9.9.0; https://imaris.oxinst.com/support/imaris-release-notes/9-9-0). Touching objects were split morphologically and cells on the border of the image were excluded. Volume was calculated for over 200 cells per cell type. The median cell volume was obtained per field. Final volume is reported as a weighted average of median cell volume per field based on cell count per field.
Monocyte and macrophage dNTP concentration
Monocyte and macrophage dNTP concentration was calculated using the mean value from the measurement of each dNTP and the reported cell volumes.
Primer extension assay
The primer extension assay was modified from previous publications (3, 59). A 5′ 32P-labeled 17-mer DNA primer (5′-CGCGCCGAATTCCCGCT-3′, Integrated DNA Technologies) was annealed to a 40-mer template RNA (5′AAGCUUGGCUGCAGAAUAUUGCUAGC GGGAAUUCGGCGCG-3′, Integrated DNA Technologies) in the presence of a 3-fold excess template. Reactions (20 μl) contained 10 nM annealed primer/template, 25 mM Tris–HCl, pH 8.0, 2 mM DTT, 100 mM KCl, 5 mM MgCl2, 10 μM oligo (dT), indicated dNTP concentrations, and a variety of concentrations of HIV-1 RT (from HXB2). Reactions were incubated 37 °C for 5 min and stopped by adding 10 μl 40 mM EDTA and 99% (v/v) formamide followed by incubation at 95 °C for 2 min. This allows for multiple rounds of extension of the DNA primer. A variety of HIV-1 RT concentrations were used to ensure the reactions were conducted in the linear range. Reactions were resolved using a 14% urea-PAGE gel (AmericanBio, Inc) and imaged using an Amersham Typhoon (Cytiva).
HIV-vector and VLP Vpx treatment of monocytes and macrophages
Freshly isolated monocytes were concentrated to 500,000 cells per 200 μl in a 48-well plate and treated with 50 μl freshly harvested VLP Vpx- or Vpx+ for 24 h. Fully differentiated macrophages in 24-well plates were treated with 25 μl freshly harvested VLP Vpx- or Vpx+ for 24 h. After 24 h, monocytes and macrophages were transduced with 40 μl and 20 μl, respectively, of D3HIV-GFP. Cells not transduced with either vector were used as a negative control. After 72 h, cells were harvested for Western blot and flow cytometry. Western blot was used to measure SAMHD1 expression levels at the time of sample harvest. Flow cytometry was used to measure transduction efficiency by measuring GFP+ cell populations. For flow cytometry, cells were fixed with 4% PFA and assessed for GFP using a FACSymphony A5 (BD Biosciences). Cell debris and doublets were excluded from GFP analysis and GFP gating was established on the untransduced negative control cell populations. Samples were analyzed using FlowJo software (BD Bioscience).
Statistical analyses
All experiments in this study were conducted in triplicate (n = 3). Individual values are reported in each figure and the mean of these replicates is reflected by the bars on the graphs. Error bars in each figure represent standard deviation. All statistical analyses were conducted using two-tailed and unpaired Welch's t tests in Prism (Graphpad) and the resulting p-values are included in each figure. A p-value < 0.05 was considered to be statistically significant.
Data availability
All data generated and analyzed during this study are included in this chapter.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
This study was supported in part by the Emory Flow Cytometry Core (EFCC), one of the Emory Integrated Core Facilities (EICF) and is subsidized by the Emory University School of Medicine. Additional support was provided by the National Center for Georgia Clinical & Translational Science Alliance of the National Institutes of Health under Award Number UL1TR002378. Research reported in this publication was also supported in part by the Emory University Integrated Cellular Imaging Core and Children’s Healthcare of Atlanta.
Author contributions
N. E. B., S. T., and Y.-J. C. investigation; N. E. B., S. T., and Y.-J. C. data curation; N. E. B. and B. K. writing–original draft; N. E. B., S. T., R. F. S., and B. K. writing–review and editing; N. E. B. methodology; N. E. B., R. F. S., and B. K. funding acquisition; R. F. S. and B. K., conceptualization; B. K. supervision.
Funding and additional information
This study was supported by the NIH F31 AI157884 (to N. E. B.), AI136581 (to B. K.), AI162633 (to B. K.), CA254403 (to B. K/D. Y.), and MH116695 (to R. F. S.). The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institute of Health.
Reviewed by members of the JBC Editorial Board. Edited by Craig Cameron
Supporting information
References
- 1.Bjursell G., Skoog L. Control of nucleotide pools in mammalian cells. Antibiot. Chemother. (1971) 1980;28:78–85. doi: 10.1159/000386063. [DOI] [PubMed] [Google Scholar]
- 2.Cohen A., Barankiewicz J., Lederman H.M., Gelfand E.W. Purine and pyrimidine metabolism in human T lymphocytes. Regulation of deoxyribonucleotide metabolism. J. Biol. Chem. 1983;258:12334–12340. [PubMed] [Google Scholar]
- 3.Diamond T.L., Roshal M., Jamburuthugoda V.K., Reynolds H.M., Merriam A.R., Lee K.Y., et al. Macrophage tropism of HIV-1 depends on efficient cellular dNTP utilization by reverse transcriptase. J. Biol. Chem. 2004;279:51545–51553. doi: 10.1074/jbc.M408573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy E.M., Gavegnano C., Nguyen L., Slater R., Lucas A., Fromentin E., et al. Ribonucleoside triphosphates as substrate of human immunodeficiency virus type 1 reverse transcriptase in human macrophages. J. Biol. Chem. 2010;285:39380–39391. doi: 10.1074/jbc.M110.178582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greene B.L., Kang G., Cui C., Bennati M., Nocera D.G., Drennan C.L., et al. Ribonucleotide reductases: structure, chemistry, and metabolism suggest new therapeutic targets. Annu. Rev. Biochem. 2020;89:45–75. doi: 10.1146/annurev-biochem-013118-111843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu. Rev. Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- 7.Lacombe M.L., Wallet V., Troll H., Véron M. Functional cloning of a nucleoside diphosphate kinase from Dictyostelium discoideum. J. Biol. Chem. 1990;265:10012–10018. [PubMed] [Google Scholar]
- 8.Bello L.J. Regulation of thymidine kinase synthesis in human cells. Exp. Cell Res. 1974;89:263–274. doi: 10.1016/0014-4827(74)90790-3. [DOI] [PubMed] [Google Scholar]
- 9.Arnér E.S., Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol. Ther. 1995;67:155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- 10.Engström Y., Eriksson S., Jildevik I., Skog S., Thelander L., Tribukait B. Cell cycle-dependent expression of mammalian ribonucleotide reductase. Differential regulation of the two subunits. J. Biol. Chem. 1985;260:9114–9116. [PubMed] [Google Scholar]
- 11.Eriksson S., Gräslund A., Skog S., Thelander L., Tribukait B. Cell cycle-dependent regulation of mammalian ribonucleotide reductase. The S phase-correlated increase in subunit M2 is regulated by de novo protein synthesis. J. Biol. Chem. 1984;259:11695–11700. [PubMed] [Google Scholar]
- 12.Coppock D.L., Pardee A.B. Control of thymidine kinase mRNA during the cell cycle. Mol. Cell. Biol. 1987;7:2925–2932. doi: 10.1128/mcb.7.8.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstone D.C., Ennis-Adeniran V., Hedden J.J., Groom H.C., Rice G.I., Christodoulou E., et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480:379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 14.Cribier A., Descours B., Valadao A.L., Laguette N., Benkirane M. Phosphorylation of SAMHD1 by cyclin A2/CDK1 regulates its restriction activity toward HIV-1. Cell Rep. 2013;3:1036–1043. doi: 10.1016/j.celrep.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 15.White T.E., Brandariz-Nuñez A., Valle-Casuso J.C., Amie S., Nguyen L.A., Kim B., et al. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell Host Microbe. 2013;13:441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J., Hao C., DeLucia M., Swanson S., Florens L., Washburn M.P., et al. CyclinA2-Cyclin-dependent kinase regulates SAMHD1 protein Phosphohydrolase domain. J. Biol. Chem. 2015;290:13279–13292. doi: 10.1074/jbc.M115.646588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orris B., Huynh K.W., Ammirati M., Han S., Bolaños B., Carmody J., et al. Phosphorylation of SAMHD1 Thr592 increases C-terminal domain dynamics, tetramer dissociation and ssDNA binding kinetics. Nucleic Acids Res. 2022;50:7545–7559. doi: 10.1093/nar/gkac573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schott K., Fuchs N.V., Derua R., Mahboubi B., Schnellbächer E., Seifried J., et al. Dephosphorylation of the HIV-1 restriction factor SAMHD1 is mediated by PP2A-B55α holoenzymes during mitotic exit. Nat. Commun. 2018;9:2227. doi: 10.1038/s41467-018-04671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traut T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell. Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 20.Pavlova N.N., Thompson C.B. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonifati S., Daly M.B., St Gelais C., Kim S.H., Hollenbaugh J.A., Shepard C., et al. SAMHD1 controls cell cycle status, apoptosis and HIV-1 infection in monocytic THP-1 cells. Virology. 2016;495:92–100. doi: 10.1016/j.virol.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg G., Ullman B., Martin D.W., Jr. Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc. Natl. Acad. Sci. U. S. A. 1981;78:2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunkel T.A. DNA replication fidelity. J. Biol. Chem. 1992;267:18251–18254. [PubMed] [Google Scholar]
- 24.Kumar D., Abdulovic A.L., Viberg J., Nilsson A.K., Kunkel T.A., Chabes A. Mechanisms of mutagenesis in vivo due to imbalanced dNTP pools. Nucleic Acids Res. 2011;39:1360–1371. doi: 10.1093/nar/gkq829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamashita M., Emerman M. Retroviral infection of non-dividing cells: old and new perspectives. Virology. 2006;344:88–93. doi: 10.1016/j.virol.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 26.Laguette N., Sobhian B., Casartelli N., Ringeard M., Chable-Bessia C., Segeral E., et al. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature. 2011;474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahouassa H., Daddacha W., Hofmann H., Ayinde D., Logue E.C., Dragin L., et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 2012;13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lenzi G.M., Domaoal R.A., Kim D.H., Schinazi R.F., Kim B. Kinetic variations between reverse transcriptases of viral protein X coding and noncoding lentiviruses. Retrovirology. 2014;11:111. doi: 10.1186/s12977-014-0111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collin M., Gordon S. The kinetics of human immunodeficiency virus reverse transcription are slower in primary human macrophages than in a lymphoid cell line. Virology. 1994;200:114–120. doi: 10.1006/viro.1994.1169. [DOI] [PubMed] [Google Scholar]
- 30.Guyader M., Emerman M., Montagnier L., Peden K. VPX mutants of HIV-2 are infectious in established cell lines but display a severe defect in peripheral blood lymphocytes. EMBO J. 1989;8:1169–1175. doi: 10.1002/j.1460-2075.1989.tb03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu X.F., Yu Q.C., Essex M., Lee T.H. The vpx gene of simian immunodeficiency virus facilitates efficient viral replication in fresh lymphocytes and macrophage. J. Virol. 1991;65:5088–5091. doi: 10.1128/jvi.65.9.5088-5091.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrecka K., Hao C., Gierszewska M., Swanson S.K., Kesik-Brodacka M., Srivastava S., et al. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474:658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwefel D., Groom H.C., Boucherit V.C., Christodoulou E., Walker P.A., Stoye J.P., et al. Structural basis of lentiviral subversion of a cellular protein degradation pathway. Nature. 2014;505:234–238. doi: 10.1038/nature12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim B., Nguyen L.A., Daddacha W., Hollenbaugh J.A. Tight interplay among SAMHD1 protein level, cellular dNTP levels, and HIV-1 proviral DNA synthesis kinetics in human primary monocyte-derived macrophages. J. Biol. Chem. 2012;287:21570–21574. doi: 10.1074/jbc.C112.374843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hollenbaugh J.A., Tao S., Lenzi G.M., Ryu S., Kim D.H., Diaz-Griffero F., et al. dNTP pool modulation dynamics by SAMHD1 protein in monocyte-derived macrophages. Retrovirology. 2014;11:63. doi: 10.1186/s12977-014-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aye Y., Li M., Long M.J., Weiss R.S. Ribonucleotide reductase and cancer: biological mechanisms and targeted therapies. Oncogene. 2015;34:2011–2021. doi: 10.1038/onc.2014.155. [DOI] [PubMed] [Google Scholar]
- 37.Georgescauld F., Song Y., Dautant A. Structure, folding and stability of nucleoside diphosphate kinases. Int. J. Mol. Sci. 2020;21:6779. doi: 10.3390/ijms21186779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharya A., Wang Z., White T., Buffone C., Nguyen L.A., Shepard C.N., et al. Effects of T592 phosphomimetic mutations on tetramer stability and dNTPase activity of SAMHD1 can not explain the retroviral restriction defect. Sci. Rep. 2016;6 doi: 10.1038/srep31353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arnold L.H., Groom H.C., Kunzelmann S., Schwefel D., Caswell S.J., Ordonez P., et al. Phospho-dependent regulation of SAMHD1 oligomerisation couples catalysis and restriction. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldauf H.M., Pan X., Erikson E., Schmidt S., Daddacha W., Burggraf M., et al. SAMHD1 restricts HIV-1 infection in resting CD4(+) T cells. Nat. Med. 2012;18:1682–1687. doi: 10.1038/nm.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun D., Buttitta L. States of G(0) and the proliferation-quiescence decision in cells, tissues and during development. Int. J. Dev. Biol. 2017;61:357–366. doi: 10.1387/ijdb.160343LB. [DOI] [PubMed] [Google Scholar]
- 42.DeGregori J., Kowalik T., Nevins J.R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol. Cell. Biol. 1995;15:4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai L.H., Lees E., Faha B., Harlow E., Riabowol K. The cdk2 kinase is required for the G1-to-S transition in mammalian cells. Oncogene. 1993;8:1593–1602. [PubMed] [Google Scholar]
- 44.Limas J.C., Cook J.G. Preparation for DNA replication: the key to a successful S phase. FEBS Lett. 2019;593:2853–2867. doi: 10.1002/1873-3468.13619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mlcochova P., Sutherland K.A., Watters S.A., Bertoli C., de Bruin R.A., Rehwinkel J., et al. A G1-like state allows HIV-1 to bypass SAMHD1 restriction in macrophages. EMBO J. 2017;36:604–616. doi: 10.15252/embj.201696025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balachander S., Gombolay A.L., Yang T., Xu P., Newnam G., Keskin H., et al. Ribonucleotide incorporation in yeast genomic DNA shows preference for cytosine and guanosine preceded by deoxyadenosine. Nat. Commun. 2020;11:2447. doi: 10.1038/s41467-020-16152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sonza S., Maerz A., Uren S., Violo A., Hunter S., Boyle W., et al. Susceptibility of human monocytes to HIV type 1 infection in vitro is not dependent on their level of CD4 expression. AIDS Res. Hum. Retroviruses. 1995;11:769–776. doi: 10.1089/aid.1995.11.769. [DOI] [PubMed] [Google Scholar]
- 48.Lee B., Sharron M., Montaner L.J., Weissman D., Doms R.W. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neil S., Martin F., Ikeda Y., Collins M. Postentry restriction to human immunodeficiency virus-based vector transduction in human monocytes. J. Virol. 2001;75:5448–5456. doi: 10.1128/JVI.75.12.5448-5456.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rampazzo C., Miazzi C., Franzolin E., Pontarin G., Ferraro P., Frangini M., et al. Regulation by degradation, a cellular defense against deoxyribonucleotide pool imbalances. Mutat. Res. 2010;703:2–10. doi: 10.1016/j.mrgentox.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Kohnken R., Kodigepalli K.M., Wu L. Regulation of deoxynucleotide metabolism in cancer: novel mechanisms and therapeutic implications. Mol. Cancer. 2015;14:176. doi: 10.1186/s12943-015-0446-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Descours B., Cribier A., Chable-Bessia C., Ayinde D., Rice G., Crow Y., et al. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4(+) T-cells. Retrovirology. 2012;9:87. doi: 10.1186/1742-4690-9-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goujon C., Jarrosson-Wuillème L., Bernaud J., Rigal D., Darlix J.L., Cimarelli A. With a little help from a friend: increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIV(MAC) Gene Ther. 2006;13:991–994. doi: 10.1038/sj.gt.3302753. [DOI] [PubMed] [Google Scholar]
- 54.Hurwitz S.J., Schinazi R.F. Practical considerations for developing nucleoside reverse transcriptase inhibitors. Drug Discov. Today Technol. 2012;9:e183–e193. doi: 10.1016/j.ddtec.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong M.E., Jaworowski A., Hearps A.C. The HIV reservoir in monocytes and macrophages. Front. Immunol. 2019;10:1435. doi: 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gavegnano C., Schinazi R.F. Antiretroviral therapy in macrophages: implication for HIV eradication. Antivir. Chem. Chemother. 2009;20:63–78. doi: 10.3851/IMP1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hollenbaugh J.A., Gee P., Baker J., Daly M.B., Amie S.M., Tate J., et al. Host factor SAMHD1 restricts DNA viruses in non-dividing myeloid cells. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ashar F.N., Moes A., Moore A.Z., Grove M.L., Chaves P.H.M., Coresh J., et al. Association of mitochondrial DNA levels with frailty and all-cause mortality. J. Mol. Med. (Berl) 2015;93:177–186. doi: 10.1007/s00109-014-1233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenzi G.M., Domaoal R.A., Kim D.H., Schinazi R.F., Kim B. Mechanistic and kinetic differences between reverse transcriptases of vpx coding and non-coding lentiviruses. J. Biol. Chem. 2015;290:30078–30086. doi: 10.1074/jbc.M115.691576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bowen N.E., Temple J., Shepard C., Oo A., Arizaga F., Kapoor-Vazirani P., et al. Structural and functional characterization explains loss of dNTPase activity of the cancer-specific R366C/H mutant SAMHD1 proteins. J. Biol. Chem. 2021;297 doi: 10.1016/j.jbc.2021.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated and analyzed during this study are included in this chapter.