Abstract
Aims
Left ventricular scar is an arrhythmic substrate that may be missed by echocardiography and diagnosed only by cardiac magnetic resonance (CMR), which is a time-consuming and expensive imaging modality. Premature ventricular complexes (PVCs) with a right-bundle-branch-block (RBBB) pattern are independent predictors of late gadolinium enhancement (LGE) but their positive predictive value is low. We studied which electrocardiographic features of PVCs with an RBBB pattern are associated with a higher probability of the absence of an underlying LGE.
Methods
The study included 121 athletes (36 ± 16 years; 48.8% men) with monomorphic PVCs with an RBBB configuration and normal standard clinical investigations who underwent CMR. LGE was identified in 35 patients (29%), predominantly in those with PVCs with a superior/intermediate axis (SA-IntA) compared to inferior axis (IA) (38% vs. 10%, P = 0.002). Among patients with SA-IntA morphology, the contemporary presence of qR pattern in lead aVR and V1 was exclusively found in patients without LGE at CMR (51.0% vs. 0%, P < 0.0001). Among patients with IA, the absence of LGE correlated to a narrow ectopic QRS (145 ± 16 vs. 184 ± 27 msec, P < 0.001).
Conclusions
Among athletes with apparently idiopathic PVCs with a RBBB configuration, the presence of a concealed LGE at CMR was documented in 29% of cases, mostly in those with a SA-IntA. In our experience, the contemporary presence of qR pattern in lead aVR and V1 in PVCs with RBBB/SA-IntA morphology or, on the other hand, a relatively narrow QRS in PVCs with an IA, predicted absence of LGE.
Keywords: Premature ventricular complex, Right bundle branch block morphology, Cardiac magnetic resonance, Late gadolinium enhancement
Graphical Abstract
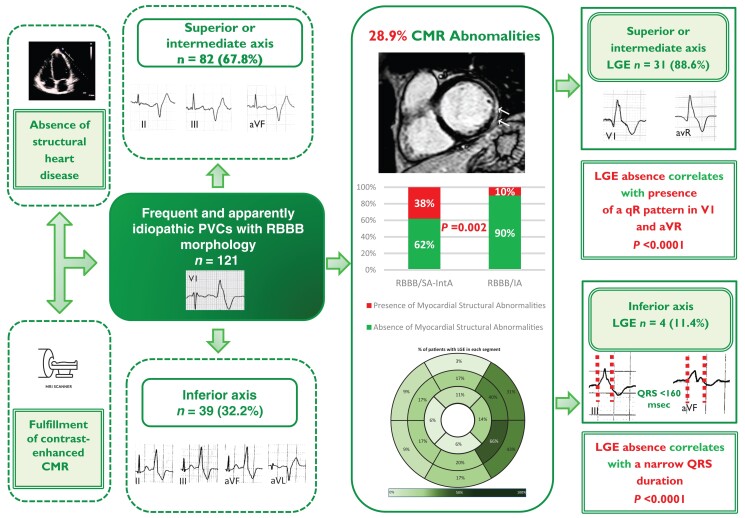
Graphical abstract.
CMR, cardiac magnetic resonance; IA, inferior axis; LGE, late gadolinium enhancement; PVCs, premature ventricular complexes; RBBB, right-bundle-branch-block; SA-IntA, superior/intermediate axis.
What’s new?
We analysed the electrocardiographic characteristics of the ectopic QRS in athletes with premature ventricular complexes (PVCs) with a right-bundle-branch-block (RBBB) configuration and apparently structurally normal heart (normal ECG and echocardiography) in order to predict the absence (or presence) of concealed late gadolinium enhancement (LGE) at cardiac magnetic resonance (CMR).
In our experience nearly one-third of athletes referred to CMR for RBBB-PVCs showed LGE at CMR, more often if the ectopic QRS showed superior/intermediate (SA-IntA) axis compared to an inferior axis (IA);
Predictors of the absence of LGE were a contemporary presence of a qR pattern in lead aVR and V1 in the subgroup of patients with RBBB/SA-IntA and a narrow QRS in the subgroup with RBBB/IA.
Evaluation of the ectopic QRS characteristics may help in selecting which patients with RBBB PVCs and normal echocardiography should not be referred to CMR.
Introduction
Premature ventricular complexes (PVCs) are found in up to 75% of healthy individuals and are generally considered benign in the absence of structural heart disease (SHD).1–5 However, PVCs can occasionally indicate an underlying SHD and may be associated with an increased risk of sudden cardiac death, particularly during sports activity.1 Clinical examination, investigation of the family history, 12-lead electrocardiogram (ECG), exercise stress testing, and echocardiography are basic clinical tools used to rule out a concealed SHD.6–8 Nevertheless, some arrhythmogenic substrates may still be missed. Cardiac magnetic resonance (CMR) has gained raising interest as part of clinical evaluation of patients with ventricular arrhythmias (VA), in order to allow myocardial tissue characterization.9–11 In fact, CMR can accurately identify myocardial abnormalities, such as late gadolinium enhancement (LGE), in a range between 16% and 26% of patients with an apparently structurally normal heart.6,9,12 Non-ischaemic myocardial fibrosis, which involves mid-mural and subepicardial myocardial layers and exhibits a ring-like pattern, is often associated with life-threatening arrhythmias.6,10,13–18
Since CMR cannot be proposed for systematic evaluation, the ECG plays a key role in the assessment of patients with idiopathic PVCs.19–21 Recent studies have highlighted that PVCs with right bundle branch block (RBBB) morphology, suggesting LV origin, are more often associated with LGE at CMR.22 However, the predictive value of this single parameter remains low.6,23–26
Therefore, we aimed to investigate if other electrocardiographic characteristics of the ectopic QRS complex correlate with the absence (or presence) of LGE in athletes with PVCs with a RBBB morphology and apparently normal heart.
Methods
Study population
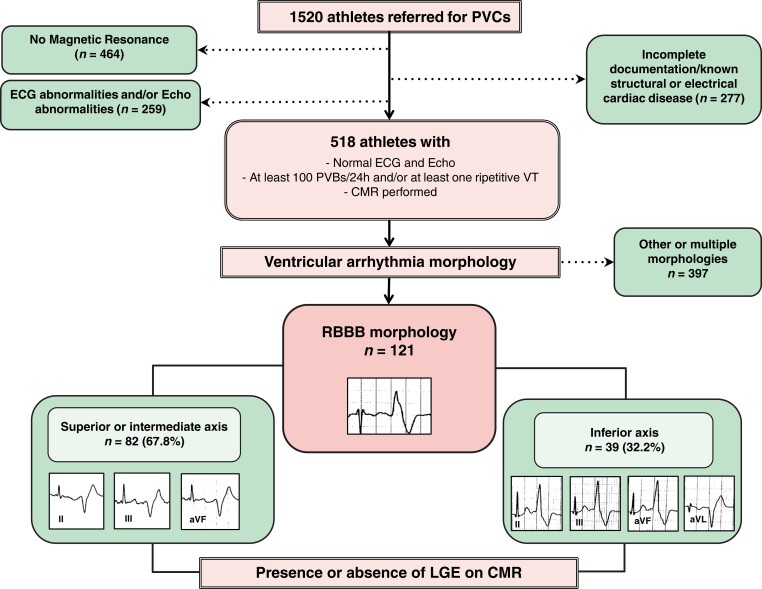
The study enrolled 121 consecutive athletes with frequent PVCs with RBBB morphology and structurally normal heart at routine diagnostic workup, recruited in two tertiary care hospitals (Rome and Padova), dedicated to the treatment of patients at risk of life-threatening VA. For the purpose of this study, an athlete is defined as an individual of young or adult age, either amateur or professional, who is engaged in regular exercise training and participates in official sports competition.27 The athletes were referred after identification of PVCs at preparticipation screening for further diagnostic work-up. Patients were included if they satisfied all following inclusion criteria: (i) presence of isolated monomorphic PVCs or ≥1 repetitive monomorphic VA (couplets, triplets, or non-sustained ventricular tachycardia) with an RBBB morphology on standard 12-lead ECG or exercise testing, (ii) recording of ≥ 100 isolated monomorphic PVCs or complex VA (couplets, triplets or non-sustained VA) with RBBB morphology at 24 h ECG Holter monitoring; (iii) normal 12-lead ECG, according to an international consensus panel of experts in sports cardiology and sports medicine,28 normal 2D trans-thoracic echocardiography, absence of coronary artery diseases evaluated by a maximal exercise stress test (in selected cases third level tests such as coronary computed angiography or coronary angiography were performed to exclude coronary artery diseases or an anomalous origin of coronary artery), and (iv) fulfilment of contrast-enhanced CMR as part of the diagnostic work-up (Figure 1). Patients were divided into two groups according to axis in frontal plane [RBBB-PVCs with a superior/intermediate (SA-IntA) axis and RBBB-PVCs with inferior axis (IA)] and subsequently, each group was subdivided according to the presence or absence of LGE.
Figure 1.
Summary of the study. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; PVCs, premature ventricular complexes; VT, ventricular tachycardia.
The study was approved by the Institutional Review Board (C.A.R.I.T.M.O. study) and was consistent with the ethical guidelines of the 2001 Declaration of Helsinki. All participants provided written informed consent before their inclusion in the study.
Electrocardiogram analysis
Standard 12-lead ECG was collected in all study participants and carefully analysed by two independent cardiologists (G.P. and A.S.); discrepancies were solved by consensus. The traces were recorded at 1 mV/cm and 25 mm/s paper speed. The PVCs morphology was classified as RBBB if the ectopic QRS complex was predominantly positive or isodiphasic in lead V1; an IA was recognized by a positive QRS complex in the inferior leads, while a SA was denoted by a negative QRS complex in inferior leads. An intermediate axis (IntA) was indicated by positive QRS complexes in both aVF and aVL. PVCs are supposed to have a fascicular origin (typical RBBB with left or right axis deviation and QRS duration ≤130 msec) and, as reported in previous studies, a good prognosis was excluded.29–31
Accordingly, to previous studies, the morphology analysis of the ectopic QRS focused on the following characteristics: (i) maximal QRS duration; (ii) QRS notching in one or more leads (QRS defined as a QRS complex deflection on the upstroke or downstroke of >0.05/mV that did not cross baseline occurring in at least two leads); (iii) lead I QRS duration ≥120 msec; (iv) QRS duration >160 msec in at least one lead; (v) intrinsicoid deflection time >80 msec in one or more leads, defined as the time between the QRS onset and the peak of the R-wave or the nadir of the Q-wave; (vi) initial slurring (pseudo-delta) in one or more leads, defined as the time between the QRS onset and earliest fast QRS deflection; and (vii) presence of Q wave in lead I, defined as an initial negative QRS deflection.32,33
Furthermore, we investigated innovative ECG characteristics: (i) qR morphology in lead V1, defined as an initial negative followed by a positive QRS deflection; (ii) qR morphology in lead aVR; (iii) qR morphology in leads V1 and aVR; (iv) QS morphology in lead aVR, defined as the absence of positive QRS deflections; and (v) QS morphology in leads V3-V6, defined as the absence of positive QRS deflections.
24-h ECG Holter monitoring
All patients were evaluated with a 24-h ECG monitoring and included in the study if presented with ≥100 isolated monomorphic PVCs or complex VA (couplets, triplets, or non-sustained VA) with RBBB morphology. Patients with PVCs with ≥2 morphologies that accounted for ≥10% of all PVCs were classified as polymorphic and excluded by the analysis. All recordings were reviewed by two experienced investigators (M.T. and C.C.).
Echocardiography
Two-dimensional trans-thoracic echocardiography was performed in all study participants by an investigator (E.F.) who was blind to ECG results and clinical status. Left ventricular end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) were calculated both in four- and two-chamber apical views using the modified Simpson method, and the two measurements were averaged.34 Left ventricular ejection fraction was then obtained with the formula: (LVEDV—LVESV)/LVEDV × 100.
Exercise stress test
Exercise stress test was performed with a bicycle, according to a standard protocol of 25–50 W increments every 2 min, and continued until maximal exercise intensity tolerated (regardless of heart rate). The onset of symptoms, electrocardiographic changes indicative of ischaemia and the behaviour of VA during exercise testing were recorded. Particularly, the persistence/appearance of isolated PVCs during exercise or exercise-induced repetitive VA was reported.
Cardiac magnetic resonance
All CMR studies were reviewed by two experienced physicians (E.F. and M.L.D.). In case of disagreement, a third expert was consulted (G.B.). Cardiac magnetic resonance was performed on a 1.5 T scanner (Philips Prodiva 1.5 Tesla, Eindhoven, The Netherlands and Magnetom Avanto, Siemens Healthineers, Erlangen, Germany) with ECG gating. After the acquisition of localizers, breath hold cine steady-state free precession (SSFP) sequences were used for functional analysis: long axis slices and a stack of contiguous short axis slices 8 mm thick encompassing the whole ventricles were used to quantify ventricle volumes, mass, and ejection fraction. Ten-to-fifteen minutes after an intravenous bolus of gadolinium-based contrast agent, LGE was acquired using segmented phase-sensitive gradient-echo inversion recovery sequences (T1-weighted). LGE imaging was carried out in the same orientation on cine SSFP sequences, and the inversion recovery time was adjusted on images to null normal myocardium. LGE was defined as ‘regions of scar, necrosis, and/or inflammation discriminated from normal tissue by prolonged retention of gadolinium-based contrast agents’. Furthermore, in all 17 segments (according to the American Heart Association/American College of Cardiology classification), the LGE pattern (subendocardial, intramyocardial, and subepicardial) and its distribution (stria or patchy) were assessed. Hyperenhanced areas were assessed using the full-with at half-maximum algorithm (hyperenhanced areas were defined as pixels with an signal intensity (SI) above a percentage of the maximal myocardial SI from the region of interest, with a threshold of 35%) and were manually adjusted and summed over the entire LV myocardium to calculate LGE extent expressed as absolute and relative to the LV myocardial mass.35 Isolated junctional LGE (i.e. at the insertion points of the right-ventricular free wall to the interventricular septum) was not considered abnormal as it is a common and non-pathologic finding in athletes.
Statistical analysis
Continuous and categorical variables were expressed as mean (±standard deviation) or median (25th–75th percentiles), according to distribution, which was assessed with the Shapiro–Wilk test. Categorical variables were expressed as n (%). Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Continuous data were compared using the Student t-test or the Mann–Whitney U test according to distribution. Univariate and multivariable binomial regression analysis was used to assess the clinical determinants of abnormal CMR. A P-value <0.05 was considered statistically significant. Data were analysed with SPSS® version 23 (IBM®).
Results
A total of 121 consecutive athletes with monomorphic RBBB-PVCs and apparently normal heart at routine diagnostic workup were included in this study. The mean age was 36 ± 16 years, 49% were males and 31 (26%) experienced cardiovascular symptoms. A family history of sudden cardiac death or cardiomyopathy was observed in two patients (2%), whereas eight patients (7%) had a family history of coronary artery diseases.
ECG findings
All patients showed normal electrocardiographic findings and no significant anomalies were recorded. A 12-lead ECG documentation of the PVCs, either at baseline or during exercise was available in all patients. In most of the patients (68%) a SA-IntA axis morphology was observed, whereas an IA morphology was noted in 39 patients (32%).
Cardiac magnetic resonance findings
In all patients showed a preserved left and right ventricular ejection fraction (59.2 ± 6.4% and 60.1 ± 8.1%, respectively) and normal biventricular volumes (left ventricle end-diastolic volume index and left ventricle end-systolic volume index: 85.7 ± 17.5 and 33.4 ± 10.3 mL/m2, respectively; right ventricle end-diastolic volume index and left ventricle end-systolic volume index: 82.4 ± 18.8 and 31.1 ± 9.5 mL/m2, respectively).
A total of 35 (28.9%) patients exhibited LGE. It was more frequently located in the mid-inferolateral wall (n = 8, 65.7%), followed by the basal segments of the infero-lateral (n = 15, 42.9%), and mid antero-lateral walls (n = 14, 40.0%). The most frequent LGE pattern was subepicardial and/or midmyocardial (n = 32, 91.4%), specifically midmyocardial in 9 (25.7%) patient, subepicardial in 11 (31.4%) patients, or both in 12 (34.3%) patients; whereas subendocardial/transmural (ischaemic) pattern was found in three patients (8.6%), in the absence of coronary artery disease on cardiac imaging. A stria was the most common LGE distribution (n = 32, 91.4%) revealed, followed by patchy (n = 3, 8.6%).
Predictors of absence of late gadolinium enhancement
The presence of LGE was identified more often in patients with PVCs with an SA-IntA compared to IA (38% vs. 10%, P = 0.002).
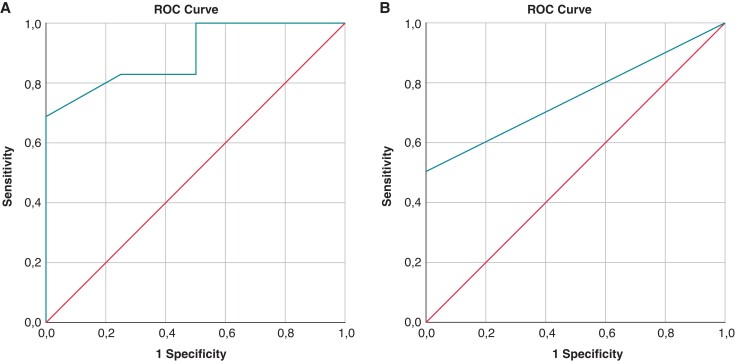
The ECG findings of PVCs with RBBB/IA morphology according to the presence of LGE are shown in detail in Table 1. In this subgroup, patients without LGE showed a shorter QRS duration of PVCs (145.1 ± 15.6 vs. 184.3 ± 26.9 msec, P < 0.001). A QRS duration <160 msec was able to correlate with the absence of LGE with a sensitivity and specificity of 63% and 100%, respectively (Figure 2, Panel A).
Table 1.
ECG findings and CMR in patients with RBBB IA PVCs
| Variable | RBBB IA |
P | |
|---|---|---|---|
| LGE + | LGE − | ||
| n = 4 | n = 35 | ||
| QRS, msec | 186.3 ± 24.9 | 144.2 ± 16.5 | 0.0001 |
| QRS notching in one or more leads, n (%) | 4 (100) | 24 (68.6) | 0.461 |
| QRS ≥ 120 msec in lead I, n (%) | 4 (100) | 15 (42.9) | 0.101 |
| Maximal QRS duration >160 msec, n (%) | 3 (75) | 13 (37.1) | 0.357 |
| Intrinsicoid deflection >80 msec, n (%) | 2 (50) | 16 (45.7) | 0.999 |
| Initial slurring (pseudo-delta), n (%) | 2 (50) | 15 (42.8) | 0.999 |
Values are mean ± SD unless specified.
Abbreviations: CMR, cardiac magnetic resonance; n, number of patients; LGE, late gadolinium enhancement; PVCs, premature ventricular complexes; RBBB, right bundle branch block.
Figure 2.
ROC curve analysis of QRS duration in PVCs with RBBB/IA morphology for the prediction of the absence of LGE at CMR (A). In Panel A, the area under the curve was 0.89 (0.77–1.00), P = 0.01. The cut-off point that best-identified the absence of LGE with a sensitivity of 63% and specificity of 100% was 160 msec. ROC curve analysis of the contemporary presence of qR morphology in lead aVR and V1 in PVCs with RBBB/SA-IntA morphology for the prediction of an absence of LGE at CMR (B). In Panel B, the area under the curve was 0.76 (0.65–0.86), P < 0.0001. A contemporary presence of qR morphology in lead aVR and V1 had a sensitivity of 51%, specificity of 100%, positive predictive value of 100%, and diagnostic accuracy of 69.5% in identifying a patient without the presence of LGE on CMR.
The ECG findings of PVCs with RBBB/SA-IntA morphology according to the presence of LGE are shown in detail in Table 2. Three ectopic QRS features were significantly more common in patients with normal CMR:
Table 2.
ECG findings and CMR in patients with RBBB superior or intermediate axis PVCs
| Variable | RBBB superior or intermediate axis |
P | |
|---|---|---|---|
| LGE + | LGE − | ||
| n = 31 | n = 51 | ||
| QRS, msec | 139.5 ± 19.9 | 145.4 ± 16.4 | 0.150 |
| QRS notching in one or more leads, n (%) | 11 (35.5) | 25 (49) | 0.259 |
| QRS ≥ 120 msec in lead I, n (%) | 19 (61.3) | 27 (52.9) | 0.499 |
| Maximal QRS duration >160 msec, n (%) | 10 (32.3) | 19 (37.3) | 0.812 |
| Intrinsicoid deflection >80 msec, n (%) | 8 (25.8) | 14 (27.5) | 0.925 |
| Initial slurring (pseudo-delta), n (%) | 7 (22.6) | 15 (29.4) | 0.610 |
| Q-wave in lead I, n (%) | 10 (32.3) | 8 (15.7) | 0.101 |
| qR morphology in lead V1, n (%) | 2 (6.4) | 27 (52.9) | <0.0001 |
| qR morphology in lead aVR, n (%) | 4 (12.9) | 40 (78.4) | <0.0001 |
| qR morphology in lead aVR and V1, n (%) | 0 (0) | 26 (51) | <0.0001 |
| QS morphology in lead aVR, n (%) | 0 (0) | 0 (0) | 1.000 |
Values are mean ± SD unless specified.
Abbreviations: CMR, cardiac magnetic resonance; n, number of patients; PVCs, premature ventricular complex; RBBB, right bundle branch block.
qR morphology in lead V1 (52.9% vs. 6.4%, P < 0.0001),
qR morphology in lead aVR (78.4% vs. 12.9%, P < 0.0001) and
contemporary presence of qR morphology in lead aVR and V1 (51.0% vs. 0%, P < 0.0001).
A contemporary presence of qR morphology in lead aVR and V1 had a sensitivity of 51%, specificity of 100%, positive predictive value of 100%, and diagnostic accuracy of 69.5% in identifying a patient without the presence of LGE on CMR (Figure2, Panel B).
Representative CMR findings and ECG characteristics of RBBB morphology SA-IntA patients are shown in Figures 3 and 4.
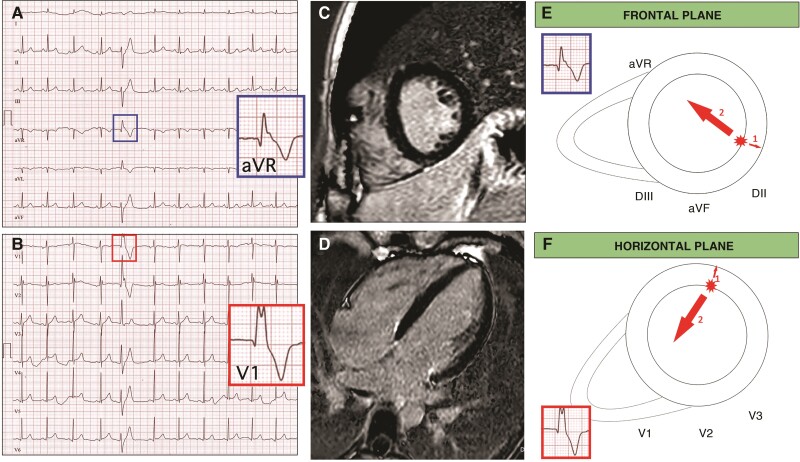
Figure 3.
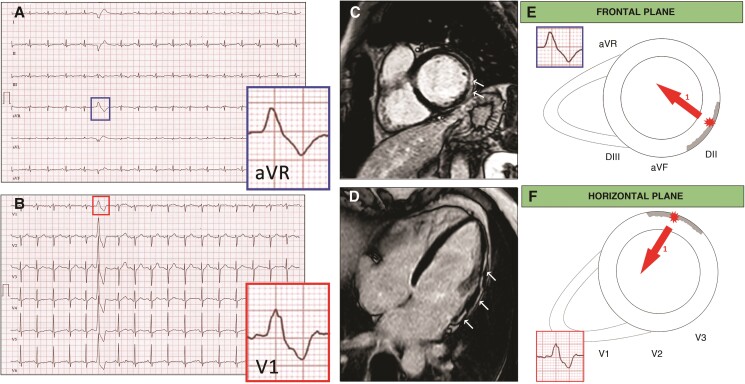
Representative case of a patient with frequent PVCs with RBBB morphology SA-IntA and absence of LGE on CMR. A 16-year-old athlete with no family history of heart diseases and no symptoms. Echocardiography was normal. A 12-lead ECG showed isolated premature ventricular beats with a right bundle branch block/superior axis morphology and distinguished by the presence of a qR pattern in both leads aVR and V1 (A, B). Cardiac magnetic resonance, short axis (C) and 2-chamber long axis (D) views demonstrated absence of LGE. Depolarization forces in frontal and transversal plane of PVCs RBBB/SA-IntA morphology. The main depolarization vector is directed away from its origin point. If PVCs originate from LV inferior and infero-lateral endocardial wall an initial depolarizing force is directed away from leads V1 and aVR, in an endo-epicardial activation sequence, which gives rise to a q wave (E, F, arrow 1). The volume arrow points in the main depolarization vector’s direction (E, F, arrow 2).
Figure 4.
Representative case of a patient with frequent PVCs with RBBB morphology SA-IntA and LGE on CMR. A 48-year-old athlete with no family history of heart diseases and no symptoms. Echocardiography was normal. A 12-lead ECG showed isolated premature ventricular beats, elicited by the exercise stress test, with a right bundle branch block/superior axis morphology and distinguished by the absence of a qR pattern in aVR and V1 (A, B). Cardiac magnetic resonance, short axis (C) and two-chamber long axis (D) views showed the presence of sub-epicardial LGE involving the basal and mid segments of the LV infero-lateral and antero-lateral walls. Depolarization forces in frontal and transversal plane of PVCs RBBB/SA-IntA morphology. PVCs that originate from infero-lateral wall at a meso and subepicardial level present a depolarization gradient predominantly directed towards leads V1 and aVR (E, F, arrow 1). Therefore, on standard 12-lead ECG, no q wave is recorded but an R wave with an intrinsicoid deflection presenting a slow ascent.
Discussion
The aim of the study was to analyse which electrocardiographic characteristics of the ectopic QRS complex correlate with the absence (or presence) of LGE in athletes with monomorphic PVCs with an RBBB morphology and apparently normal heart. The main findings were: (i) 29% of athletes referred to CMR for RBBB-PVCs showed an LGE at CMR; (ii) the LGE was identified predominantly in those with PVCs with SA-IntA compared to IA (38% vs. 10%, P = 0.002); (iii) among patients with SA-IntA, a contemporary presence of a qR pattern in V1 and aVR has been observed only in patients without LGE; (iv) among patients with IA, the absence of LGE correlated to a shorter duration of ectopic QRS (145.1 ± 15.6 vs. 184.3 ± 26.9msec, P < 0.001).
Idiopathic vs. structural heart disease -related ventricular arrhythmias
Premature ventricular complexes are widely diffused in the general population and are usually considered a benign and transient phenomenon,1–3 when an underlying pathological structural disease is excluded by common diagnostic work-up.7
The ECG analysis of the ectopic beat is able to identify the anatomical site of PVCs origin with good accuracy and in many cases the corresponding location of abnormal findings at CMR.23 The most common form of idiopathic and benign PVCs shows a left-bundle-branch-block (LBBB)/IA morphology, suggesting a right ventricular outflow tract (RVOT) origin. However, also in patients with RVOT PVCs, CMR can identify concealed abnormalities of uncertain significance2,36,37 or even overt SHD, mainly arrhythmogenic cardiomyopathy.37 Previous studies found that the analysis of the electrocardiographic features of the ectopic QRS complex may help to differentiate patients with early phase arrhythmogenic cardiomyopathy from those with RVOT PVCs unrelated to an underlying SHD.32,33,38
Another substrate of PVCs is the so-called isolated LGE, defined as the presence of LGE on CMR in the absence of features of specific heart disease (e.g. coronary artery disease or pathological LV hypertrophy). The detection of a non-ischaemic LV scar cannot be considered a benign sign but deserves proper clinical attention since it can be the substrate of life-threatening VA, especially in athletes. Cardiovascular evaluation of athletes before participation in competitive sports offers the possibility to identify athletes at risk of sudden cardiac death (SCD). As reported in a recent study, the incidence of sport-related cardiac arrest during long-term follow-up was low, due to pre-participation screening.39 However, economic cost could be considered a barrier to implementing screening programme, although the benefits in terms of public health. Since LGE may be missed by standard clinical investigations, diagnosis requires CMR imaging, which is time-consuming and expensive. Considering the economic implications, second-line investigations should be performed as a key test only for the evaluation of athletes with frequent PVCs and high-risk morphological features. Several characteristics have been associated with LGE in athletes with apparently normal heart, and one of the most important is the RBBB ectopic QRS configuration, suggesting LV origin.6,23–26 However, even among athletes referred for this type of VA, the majority show a normal CMR. No previous study focused on the predictive value for LGE of other electrocardiographic features of the ectopic QRS complexes among athletes with RBBB PVCs.
Prevalence of left ventricular scar in patients with frequent right-bundle-branch-block premature ventricular complexes
In our study sample of athletes with monomorphic PVCs with a RBBB configuration and normal standard clinical investigations, nearly one-third showed LGE on CMR, in line with previous observations.6,23–26 In particular, a much higher prevalence of LGE was observed in those with a RBBB/SA-IntA configuration than in those with an RBBB/IA. Muser et al.6 found concealed myocardial abnormalities detectable at CMR in 16% of patients with frequent PVCs and a negative diagnostic workup; RBBB pattern was the clinically dominant PVCs morphology associated with LGE at CMR compared with an LBBB pattern (51% vs. 5%; P < 0.01) and RBBB/SA pattern was related to higher evidence of scar at CMR compared with RBBB/IA morphology (70% vs. 36%). Also, in the study by Nucifora et al.,24 the RBBB/SA pattern was significantly related to the presence of LV scar. In a previous investigation including athletes from our centres referred for apparently idiopathic VA, the prevalence of LGE was much higher (17%) in those with monomorphic RBBB/SA-IntA PVCs compared to those with RBBB/IA (3%).26
Premature ventricular complexes with a right-bundle-branch-block /inferior axis configuration
Idiopathic PVCs with RBBB/IA morphology have been reported to originate from the antero-lateral portion of the mitral annulus in close proximity to the mitral-aortic continuity,40 rather than a posterior or posteroseptal focus. PVCs that arise from this region present as monophasic R waves in all the precordial leads, with a RBBB pattern, while the polarity of the QRS complex was positive in all inferior leads and negative in leads I and aVL. The early precordial transition and a concordant positive QRS pattern could be explained by the site of origin of PVCs in the posterior portion of the LV, while the R pattern in lead V1 by the anterior direction of the ventricular force.41 A distinct R wave with RBBB morphology, although not as prominent as mitral annular or left ventricular origin VA, could be also noted with arrhythmia arising from the left coronary cusp, localized posteriorly and leftward and thus further away from V1. However, only PVCs originating from endocardial or septal focus, not usually involved by LGE, show a narrow ectopic QRS, as noted in our subgroup of patients with RBBB/IA morphology.
Premature ventricular complexes with a right-bundle-branch-block/superior axis configuration
The higher prevalence of LV scar, observed in patients with RBBB/SA-IntA PVCs and apparently structural normal heart could be easily explained by the fact that isolated LGE predominantly affects the posterior and/or lateral LV wall. In patients with PVCs and RBBB/SA-IntA morphology, a qR pattern in V1 and aVR has been observed only in patients without LV scar at CMR. An ECG pattern of qR in V1 and aVR may be frequently observed in arrhythmias originating from papillary muscles, especially from the anterolateral one.42–46 It could be explained by an endo-epicardial activation sequence, which gives rise to a q wave in leads V1 and aVR, as represented in Figure 3, Panel E and Panel F. On the contrary, PVCs that originate from infero-lateral wall at a mid- and subepicardial level present a depolarization gradient predominantly directed towards leads V1 and aVR.47 Therefore, on standard 12-lead ECG, no q wave is recorded in leads V1 and aVR but an R wave with an intrinsicoid deflection presents a slow ascent (Figure 4, Panel E and Panel F). Although in this case the presence of an endocardial scar cannot be excluded (sometimes this feature is observed in patients with papillary muscles VA, especially if associated with mitral valve prolapse, or in patients with ischaemic heart disease), a qR pattern in aVR is not consistent with an origin from the epicardial/midmyocardial LV wall, which is instead more often affected by isolated LGE.
Clinical implication
The exclusion of SHD is the mainstay in determining prognosis in patients with frequent PVCs. The interpretation of 12-lead electrogram represents a cornerstone for the diagnostic work up in patients with the suspicion of cardiomyopathy. A careful evaluation of the ECG features can identify ‘red flags’ useful for insinuating clinical suspicion and for appropriately directing subsequent diagnostic and therapeutic decisions. The ability to identify SHD has evolved over time with advances in medical technology and scientific knowledge. CMR is nowadays the gold standard method for the study of cardiac structure and function as well as for tissue characterization. However, CMR has some well-known limitations: it is expensive and not yet widely available; claustrophobic patients and those with metal inserts may be unable to undergo the examination. Therefore, the evaluation of a patient with PVCs in order to select those to refer to CMR must necessarily be multi-parametric. In this setting, morphological pattern of the ectopic QRS could be a key feature for risk stratification. The recognition of specific ECG features strongly associated with the absence of LGE may be useful to identify subjects who most likely have idiopathic VA, potentially not requiring further investigation. In our population, simple electrocardiographic features such as QRS pattern in aVR and V1 and QRS duration predicted the absence of an underlying LGE. Therefore, it seems attractive to prioritize the early detection of myocardial abnormalities and effective management of cardiovascular diseases at risk of SCD, identifying patients to be referred for further investigation. Similarly, it is appealing to restrict economic costs related to the prescription of costly and time-consuming examinations in patients who do not exhibit ‘red flags’.
Study limitations
First, this study is limited by the relatively small number of athletes enrolled. However, we applied rigorous selection criteria including the presence of monomorphic RBBB PVCs and a negative standard clinical workup. Second, the study involved tertiary care hospitals dedicated to the treatment of patients at risk of life-threatening VA. This side may have influenced the characteristics of patients referred to CMR and, for this reason, the prevalence of LGE in our study sample may not be representative of the general population of athletes with RBBB PVCs. A third limitation may arise from not including a large number of patients who were unable to undergo CMR, because of patient preference or claustrophobia.
Conclusion
In conclusion, in athletes with PVCs with a RBBB morphology, normal standard clinical investigations including ECG and echocardiography do not rule out the presence of an LGE, which may be the substrate for life-threatening VA. Although definite diagnosis requires CMR, this imaging modality should be reserved for high risk cases. An ectopic QRS morphology with RBBB/SA-IntA configuration and a qR pattern in aVR and V1 was strongly associated with the absence of an underlying LGE. These findings may help identify the subset of athletes with apparently idiopathic PVCs that should not be referred to CMR, although further prospective studies with longer follow-up and greater number of patients are still required.
Contributor Information
Leonardo Calò, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Germana Panattoni, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Mario Tatangelo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Giulia Brunetti, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Italy.
Francesca Graziano, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Italy.
Luca Monzo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy; Université de Lorraine INSERM, Centre d’ Investigations Cliniques Plurithématique, Nancy, France.
Maria Ludovica Danza, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Elisa Fedele, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Domenico Grieco, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Cinzia Crescenzi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Marco Rebecchi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Alessandra Stazi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Edoardo Bressi, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Ermenegildo De Ruvo, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Paolo Golia, Division of Cardiology, Policlinico Casilino, Via Casilina 1049, 00169, Rome, Italy.
Fiorenzo Gaita, Division of Cardiology, Department of Medical Sciences, AOU Città della Salute e della Scienza Hospital, University of Turin, Italy.
Domenico Corrado, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Italy.
Alessandro Zorzi, Department of Cardiac, Thoracic and Vascular Sciences and Public Health, University of Padova, Italy.
Funding
None declared.
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Authors’ contributions
Prof Leonardo Calò and Dr Germana Panattoni conceived, designed the paper, performed the analysis and interpretation of data, and drafted the manuscript. Dr Mario Tatangelo, Dr Alessandra Stazi, Dr Maria Ludovica Danza, Dr Elisa Fedele, Dr Giulia Brunetti, Dr Edoardo Bressi, and Dr Cinzia Crescenzi contributed to the acquisition, analysis, and interpretation of data. Dr Ermenegildo De Ruvo, Dr Paolo Golia, Dr Alessandro Zorzi, and Dr Luca Monzo critically revised the manuscript. All gave their final approval of the version to be published and agreed to be accountable for all aspects of the work ensuring integrity and accuracy.
References
- 1. Kennedy HL, Whitlock JA, Sprague MK, Kennedy LJ, Buckingham TA, Goldberg RJ. Long-term follow-up of asymptomatic healthy subjects with frequent and complex ventricular ectopy. N Engl J Med 1985;312:193–7. [DOI] [PubMed] [Google Scholar]
- 2. Gaita F, Giustetto C, Di Donna P, Richiardi E, Libero L, Brusin MCet al. Long-term follow-up of right ventricular monomorphic extrasystoles. J Am Coll Cardiol 2001;38:364–70. [DOI] [PubMed] [Google Scholar]
- 3. Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli Set al. Long-term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol 2002;40:446–52. [DOI] [PubMed] [Google Scholar]
- 4. Niwano S, Wakisaka Y, Niwano H, Fukaya H, Kurokawa S, Kiryu Met al. Prognostic significance of frequent premature ventricular contractions originating from the ventricular outflow tract in patients with normal left ventricular function. Heart 2009;95:1230–7. [DOI] [PubMed] [Google Scholar]
- 5. Scorza R, Jonsson M, Friberg L, Rosenqvist M, Frykman V. Prognostic implication of premature ventricular contractions in patients without structural heart disease. Europace 2023;25:517–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muser D, Santangeli P, Castro SA, Casado Arroyo R, Maeda S, Benhayon DAet al. Risk stratification of patients with apparently idiopathic premature ventricular contractions: a multicenter international CMR registry. JACC Clin Electrophysiol 2020;6:722–35. [DOI] [PubMed] [Google Scholar]
- 7. Corrado D, Drezner JA, D’Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med 2020;54:1142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brunetti G, Graziano F, Cavigli L, Cipriani A, D’Ascenzi F, Bauce Bet al. Reproducibility of ventricular arrhythmias at exercise testing for prediction of non-ischaemic left ventricular scar in athletes. Eur J Prev Cardiol 2023;30:107–16. [DOI] [PubMed] [Google Scholar]
- 9. Andreini D, Dello Russo A, Pontone G, Mushtaq S, Conte E, Perchinunno Met al. CMR For identifying the substrate of ventricular arrhythmia in patients with normal echocardiography. JACC Cardiovasc Imaging 2020;13:410–21. [DOI] [PubMed] [Google Scholar]
- 10. Zorzi A, De Lazzari M, Mastella G, Niero A, Trovato D, Cipriani Aet al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc 2018;7:e009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Könemann H, Dagres N, Merino JL, Sticherling C, Zeppenfeld K, Tfelt-Hansen Jet al. Spotlight on the 2022 ESC guideline management of ventricular arrhythmias and prevention of sudden cardiac death: 10 novel key aspects. Europace 2023;25:euad091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hennig A, Salel M, Sacher F, Camaioni C, Sridi S, Denis Aet al. High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace 2018;20:f179–f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. di Gioia CR, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis Eet al. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol 2016;58:78–89. [DOI] [PubMed] [Google Scholar]
- 14. Disertori M, Rigoni M, Pace N, Casolo G, Masè M, Gonzini Let al. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: a meta-analysis. JACC Cardiovasc Imaging 2016;9:1046–55. [DOI] [PubMed] [Google Scholar]
- 15. Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JAet al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC Heart Fail 2017;5:28–38. [DOI] [PubMed] [Google Scholar]
- 16. Becker MAJ, Cornel JH, van de Ven PM, van Rossum AC, Allaart CP, Germans T. The prognostic value of late gadolinium-enhanced cardiac magnetic resonance imaging in nonischemic dilated cardiomyopathy: a review and meta-analysis. JACC Cardiovasc Imaging 2018;11:1274–84. [DOI] [PubMed] [Google Scholar]
- 17. Muser D, Nucifora G, Pieroni M, Castro SA, Casado Arroyo R, Maeda Set al. Prognostic value of nonischemic ringlike left ventricular scar in patients with apparently idiopathic nonsustained ventricular arrhythmias. Circulation 2021;143:1359–73. [DOI] [PubMed] [Google Scholar]
- 18. Laredo M, Tovia-Brodie O, Milman A, Michowitz Y, Roudijk RW, Peretto Get al. Electrocardiographic findings in patients with arrhythmogenic cardiomyopathy and right bundle branch block ventricular tachycardia. Europace 2023;25:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Josephson ME, Horowitz LN, Waxman HL, Cain ME, Spielman SR, Greenspan AMet al. Sustained ventricular tachycardia: role of the 12-lead electrocardiogram in localizing site of origin. Circulation 1981;64:257–72. [DOI] [PubMed] [Google Scholar]
- 20. Park KM, Kim YH, Marchlinski FE. Using the surface electrocardiogram to localize the origin of idiopathic ventricular tachycardia. Pacing Clin Electrophysiol 2012;35:1516–27. [DOI] [PubMed] [Google Scholar]
- 21. Oloriz T, Wellens HJ, Santagostino G, Trevisi N, Silberbauer J, Peretto Get al. The value of the 12-lead electrocardiogram in localizing the scar in non-ischaemic cardiomyopathy. Europace 2016;18:1850–9. [DOI] [PubMed] [Google Scholar]
- 22. Belhassen B, Laredo M, Roudijk RW, Peretto G, Zahavi G, Sen-Chowdhry Set al. The prevalence of left and right bundle branch block morphology ventricular tachycardia amongst patients with arrhythmogenic cardiomyopathy and sustained ventricular tachycardia: insights from the European survey on arrhythmogenic cardiomyopathy. Europace 2022;24:285–95. [DOI] [PubMed] [Google Scholar]
- 23. Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann Aet al. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol 2017;28:1316–23. [DOI] [PubMed] [Google Scholar]
- 24. Nucifora G, Muser D, Masci PG, Barison A, Rebellato L, Piccoli Get al. Prevalence and prognostic value of concealed structural abnormalities in patients with apparently idiopathic ventricular arrhythmias of left versus right ventricular origin: a magnetic resonance imaging study. Circ Arrhythm Electrophysiol 2014;7:456–62. [DOI] [PubMed] [Google Scholar]
- 25. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero Aet al. Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crescenzi C, Zorzi A, Vessella T, Martino A, Panattoni G, Cipriani Aet al. Predictors of left ventricular scar using cardiac magnetic resonance in athletes with apparently idiopathic ventricular arrhythmias. J Am Heart Assoc 2021;10:e018206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli Set al. 2020 ESC guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J 2021;42:17–96. [DOI] [PubMed] [Google Scholar]
- 28. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JMet al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J 2018;39:1466–80. [DOI] [PubMed] [Google Scholar]
- 29. Belhassen B, Shapira I, Pelleg A, Copperman I, Kauli N, Laniado S. Idiopathic recurrent sustained ventricular tachycardia responsive to verapamil: an ECG-electrophysiologic entity. Am Heart J 1984;108:1034–7. [DOI] [PubMed] [Google Scholar]
- 30. Li S, Wang Z, Shan Z, Shi X, Liang M, Liang Yet al. Surface electrocardiography characteristics and radiofrequency catheter ablation of idiopathic ventricular arrhythmias originating from the left infero-septal papillary muscles: differences from those originating from the left posterior fascicle. Europace 2018;20:1028–34. [DOI] [PubMed] [Google Scholar]
- 31. Di Florio A, Fusi C, Anselmi F, Cavigli L, Focardi M, Cameli Met al. Clinical management of young competitive athletes with premature ventricular beats: a prospective cohort study. Int J Cardiol 2021;330:59–64. [DOI] [PubMed] [Google Scholar]
- 32. Hoffmayer KS, Bhave PD, Marcus GM, James CA, Tichnell C, Chopra Net al. An electrocardiographic scoring system for distinguishing right ventricular outflow tract arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy from idiopathic ventricular tachycardia. Heart Rhythm 2013;10:477–82. [DOI] [PubMed] [Google Scholar]
- 33. Novak J, Zorzi A, Castelletti S, Pantasis A, Rigato I, Corrado Det al. Electrocardiographic differentiation of idiopathic right ventricular outflow tract ectopy from early arrhythmogenic right ventricular cardiomyopathy. Europace 2017;19:622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande Let al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 35. Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MGet al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Proclemer A, Basadonna PT, Slavich GA, Miani D, Fresco C, Fioretti PM. Cardiac magnetic resonance imaging findings in patients with right ventricular outflow tract premature contractions. Eur Heart J 1997;18:2002–10. [DOI] [PubMed] [Google Scholar]
- 37. Aquaro GD, Pingitore A, Strata E, Di Bella G, Molinaro S, Lombardi M. Cardiac magnetic resonance predicts outcome in patients with premature ventricular complexes of left bundle branch block morphology. J Am Coll Cardiol 2010;56:1235–43. [DOI] [PubMed] [Google Scholar]
- 38. Bastiaenen R, Pantazis A, Gonna H, Chis-Ster I, Castelletti S, Batchvarov VNet al. The ventricular ectopic QRS interval (VEQSI): diagnosis of arrhythmogenic right ventricular cardiomyopathy in patients with incomplete disease expression. Heart Rhythm 2016;13:1504–12. [DOI] [PubMed] [Google Scholar]
- 39. Sarto P, Zorzi A, Merlo L, Vessella T, Pegoraro C, Giorgiano Fet al. Value of screening for the risk of sudden cardiac death in young competitive athletes. Eur Heart J 2023;44:1084–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J, Hoff PI, Rossvoll O, De Bortoli A, Solheim E, Sun Let al. Ventricular arrhythmias originating from the aortomitral continuity: an uncommon variant of left ventricular outflow tract tachycardia. Europace 2012;14:388–95. [DOI] [PubMed] [Google Scholar]
- 41. Tada H, Ito S, Naito S, Kurosaki K, Kubota S, Sugiyasu Aet al. Idiopathic ventricular arrhythmia arising from the mitral annulus: a distinct subgroup of idiopathic ventricular arrhythmias. J Am Coll Cardiol 2005;45:877–86. [DOI] [PubMed] [Google Scholar]
- 42. Good E, Desjardins B, Jongnarangsin K, Oral H, Chugh A, Ebinger Met al. Ventricular arrhythmias originating from a papillary muscle in patients without prior infarction: a comparison with fascicular arrhythmias. Heart Rhythm 2008;5:1530–7. [DOI] [PubMed] [Google Scholar]
- 43. Yamada T, Doppalapudi H, McElderry HT, Okada T, Murakami Y, Inden Yet al. Idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: prevalence, electrocardiographic and electrophysiological characteristics, and results of the radiofrequency catheter ablation. J Cardiovasc Electrophysiol 2010;21:62–9. [DOI] [PubMed] [Google Scholar]
- 44. Al'Aref SJ, Ip JE, Markowitz SM, Liu CF, Thomas G, Frenkel Det al. Differentiation of papillary muscle from fascicular and mitral annular ventricular arrhythmias in patients with and without structural heart disease. Circ Arrhythm Electrophysiol 2015;8:616–24. [DOI] [PubMed] [Google Scholar]
- 45. Chang YT, Lin YJ, Chung FP, Lo LW, Hu YF, Chang SLet al. Ablation of ventricular arrhythmia originating at the papillary muscle using an automatic pacemapping module. Heart Rhythm 2016;13:1431–40. [DOI] [PubMed] [Google Scholar]
- 46. Lin AN, Shirai Y, Liang JJ, Chen S, Kochar A, Hyman MCet al. Strategies for catheter ablation of left ventricular papillary muscle arrhythmias: an institutional experience. JACC Clin Electrophysiol 2020;6:1381–92. [DOI] [PubMed] [Google Scholar]
- 47. Asirvatham SJ. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol 2009;20:955–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.