Abstract
Background
Seborrheic keratoses (SK) is a benign epithelial skin tumor and plasma exeresis is a new technique.
Aims
To compare the efficacy and safety of plasma exeresis and cryotherapy for treating SK.
Methods
This study is a randomized controlled trial (RCT). One side of each patient was randomly treated with plasma exeresis (peak‐to‐peak voltage of 3.44 kV and a frequency of 62.5 kHz) and the other side with cryotherapy.
Results
Thirty‐five males were enrolled. At week 3, 37.1 % (N = 13) of lesions treated by plasma exeresis were clear, which was higher than those treated by cryotherapy 17.1% (N = 6). However, this difference was not significant (p‐value: 0.06). At week 6, 16 (57.1 %) out of 28 remaining lesions, treated by plasma exeresis were clear, which was significantly higher (p‐value: 0.005) than those completely cleared by cryotherapy in 6 out of 29 remaining lesions (20.7%).
The mean physician assessment scale score was significantly reduced in both groups in the second follow‐up (plasma group first follow‐up 0.91 ± 0.89 vs. second follow‐up 0.5 ± 0.64 and p‐value: 0.0031; cryo group first follow‐up 1.4 ± 0.84 vs. second follow‐up 1.1 ± 0.72 and p‐value: 0.0002).
Regarding side effects, no significant difference was seen (p = 0.438). The most common complications in the plasma and cryotherapy groups were erythema (10/19, 52.63%) and hypo pigmentation (5/13, 38.46%).
Conclusions
Both cryotherapy and plasma exeresis are effective. We observed a significantly higher cleared lesions treated with plasma exeresis in 6 weeks and after two sessions.
Keywords: plasma exeresis seborrheic keratoses
1. INTRODUCTION
Seborrheic keratoses (SK) is one of the most common benign epithelial skin tumors and is generally observed in the elderly. SK presents with oval, slightly raised, light‐brown to black, sharply demarcated papules or plaques showing a verrucous, “stuck‐on” appearance. SKs develop in hair‐bearing areas and the predilection sites are the head, neck, trunk, and extremities. SK may be due to skin aging, ultraviolet exposure, genetic predisposition, and chronic mechanical skin irritation. Lesions have a tendency to develop over the years and spontaneous regression is rare. 1 , 2 , 3 , 4 , 5
Since SK is generally benign and asymptomatic, treatment is not routinely prescribed for medical reasons. However, the majority of patients seek treatment for cosmetic and aesthetic concerns. Moreover, lesions may become inflamed and irritated following trauma or secondary infection. In some cases, histopathological examination is performed to rule out malignancy.
There are various options for the treatment of SKs including minor surgical techniques, topical, and laser‐based options. The use of liquid nitrogen cryotherapy is among the commonly used procedures and previous studies have shown the effectiveness of this modality. 6 , 7 , 8
Plasma exeresis is a new minimally invasive technique that has recently gained attention in recent years. The plasma exeresis medical devices generate plasma energy by ionizing atmospheric gas between the device and the skin. 9 , 10 , 11 The resulting plasma exeresis sublimates the surface layers and transfers the stored heat energy to the skin surface. Recent studies have shown the efficacy of this technology for the treatment of various dermatologic indications including perioral wrinkles, acne, and nonsurgical blepharoplasty. 9 , 10 , 12 , 13
The aim of the current study is to compare the efficacy and safety of plasma exeresis and cryotherapy for the treatment of SK.
2. MATERIALS AND METHODS
The current study is a randomized controlled trial (RCT) (IRCT code: IRCT2020092904882N1) conducted between the years of 2021–2022. Patients aged more than 18 years old having at least four typical SKs with 2–3 mm thicknesses and sized between 5 and 15 mm were enrolled. Each patient had at least two SKs on each side. Lesions located on intertriginous areas, pedunculated, or covered with hair were excluded from our study. Furthermore, we excluded patients with lesions located on the eyelids or around the eyes (less than 5 mm distant). Before enrolment, all of our patients gave informed consent.
Each side was randomly assigned to be treated either with cryotherapy or plasma exeresis based on block randomization method. One side was treated with plasma exeresis (peak‐to‐peak voltage of 3.44 kV and a frequency of 62.5 kHz). The other side was treated with cryotherapy using a cotton swab dipped into liquid nitrogen for two freeze cycles of 15 s and a peripheral rim of 1 mm.
The present work used the plasma exeresis system from Plasma Fanavar Jam company (Plasma Beuty 100) (Figure 1). The electrical properties of the plasma device were tested and demonstrated using an oscilloscope and a high‐voltage probe (Tekterorix, P6015A, 1:1000). The voltage changes are sinusoidal. The device has a peak‐to‐peak voltage of 3.44 kV and a frequency of 62.5 kHz.
FIGURE 1.

(A&B) Plasma device.
After the first cycle, we monitored patients regarding local skin adverse reactions for at least 20 min. The patients were evaluated at baseline then every 3 weeks up to 6 weeks. The first and second follow‐up visits were 3 and 6 weeks after the procedure.
Two dermatologists blindly assessed patients’ photographs in three visits with 3‐week intervals (0, 3, 6 weeks) using a physical assessment scale (PAS) as follows: 14
Score 0: complete clearance of the SK
Score 1: partial clearance of the SK
Score 2: thin remained lesion with less than 1 mm thickness
Score 3: deep remained lesion with more than 1 mm thickness
In each follow‐up visit comparing the two groups, lesions scored 0 were considered as cleared and those scored 1, 2, and 3 as noncleared.
Regarding safety assessment, we evaluated patients for adverse effects, namely, erythema, edema, crusting, xerosis, vesicles, necrosis, ulcers, postinflammatory hyperpigmentation, postinflammatory hypopigmentation, atrophy, and scar. We also asked patients for pruritus and burning sensation at the site of procedure. Side effects were scored for each treated lesions as follows:
0: None
1: Mild
2: Moderate
3: Severe
We analyzed data using IBM SPSS version 22. Descriptive analyses, independent T‐test, and two‐way ANOVA test were used. A p‐value less than 0.05 was considered significant.
3. RESULTS
In the current study, a total number of 35 male patients with a mean age of 66.51 ± 11.04 years were enrolled. The majority of patients had Fitzpatrick skin type III (20, 57.1%) and the most common location of the lesions were forehead 30 (42.85%), temple 23 (32.85%), and neck 6 (8.57%), respectively (Table 1).
TABLE 1.
Characteristics of patients.
| Gender | Male | 35 |
| Female | 0 | |
| Age | 66.51 ± 11.04 years | |
| Age groups | 30–50 years | 3 (8.6%) |
| 50–70 years | 20 (57.1%) | |
| 70–90 years | 12 (34.3%) | |
| Fitzpatrick skin type | I | 0 |
| II | 6 (17.1%) | |
| III | 20 (57.1%) | |
| IV | 8 (22.9%) | |
| V | 1 (2.9%) | |
| Location of lesions | Forehead | 30 (42.85%) |
| Temple | 23 (32.85%) | |
| Neck | 6 (8.57%) | |
| Chick | 3 (4.2%) | |
| Scalp | 4 (5.71%) | |
| Back | 2 (2.85%) | |
| Chest | 2 (2.85%) | |
3.1. First follow‐up
In the plasma group, we observed complete clearance of SKs in 13 (37.1%), partial clearance in 14 (40%), thin remnant in 6 (17.1%), and thick remnant in 2 (5.7%) lesions.
In the cryotherapy group, we noted complete clearance of the SKs in 6 (17.1%), partial clearance in 11 (31.4%), thin remaining lesion in 16 (45.7%), and thin remnant in 2 (5.7%) cases.
Based on the definition of cleared (score 0) and noncleared (score 1–3) SKs, 13 (37.1 %) lesions treated by plasma exeresis were clear, which was higher than those treated by cryotherapy (N = 6, 17.1%). However, this difference was not significant (p‐value: 0.06) (Figure 2).
FIGURE 2.

(A) The percentage of lesions cleared at week 3 and (B) the percentage of lesions cleared at week 6.
3.2. Second follow‐up
At week 6, 28 out of 35 patients were evaluated in the plasma group. We observed complete clearance in 16 (57.1%), partial clearance in 10 (35.7%), and thin remaining lesion in 2 (7.1%) patients. Notably, none of the patients had remaining lesion more than 2 mm thick. In the cryotherapy group, 29 out of 35 cases were assessed. We noted complete clearance of the SKs in 6 (20.7%), partial clearance in 14 (48.3%), and thin remaining lesion in 9 (31%) cases. None of the patients had thick remaining lesions.
Based on the definition of cleared (score 0) and noncleared (score 1–3) SK, 16/28 (57.1 %) of lesions treated by plasma exeresis were cleared, significantly higher (p‐value: 0.005) than those completely cleared by cryotherapy 6/29 (20.7%) (Figure 2).
3.3. Physician assessment scale (PAS) score
The mean physician assessment scale (PAS) score was significantly reduced in both groups in the second follow‐up compared to the first follow‐up (plasma group first follow‐up 0.91 ± 0.89 vs. second follow‐up 0.5 ± 0.64 and p‐value: 0.0031; cryotherapy group first follow‐up 1.4 ± 0.84 vs. second follow‐up 1.1 ± 0.72 and p‐value: 0.0002) (Figure 3). The pictures of patients are shown in Figures 4 and 5.
FIGURE 3.

Scoring of patients in the first and second follow‐ups.
FIGURE 4.
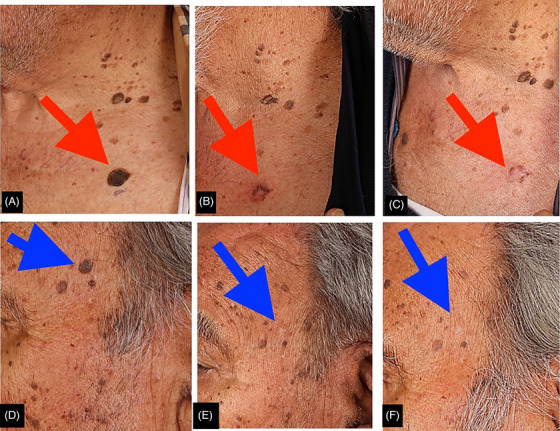
Cryotherapy at baseline (A&D), the first follow‐up (B&E), and the second follow‐up visits (C&F).
FIGURE 5.

Plasma therapy at baseline (A&C), in the first follow‐up (B&D), and in the second follow‐up visits.
3.4. Side effects
In the plasma group, 19 complications were observed in 16 patients, and in the cryotherapy group, 13 complications in 12 patients. Comparing the number of patients who had complications in each group, no significant difference was seen in the number of patients (p = 0.438). The most common complications observed in the plasma and cryotherapy groups were erythema (10/19, 52.63%) and hypopigmentation (5/13, 38.46%), respectively. (Figure 6).
FIGURE 6.

Side effects observed in patients.
4. DISCUSSION
In this study, we observed that both cryotherapy and plasma exeresis are effective in treating SKs. We also noted that the mean PSA score was significantly reduced in both groups in the second follow‐up compared to the first follow‐up. Notably, in the second follow‐up (after two sessions of treatment), the number of completely cleared lesions was significantly higher with plasma versus cryotherapy (p‐value: 0.005). In this study, no major side effects were noted. The most common adverse effect in the plasma exeresis group was erythema and the most frequent side effect in those who were treated with cryotherapy was hypopigmentation.
There are various treatment options for SKs. Destructive therapies and minor surgical techniques such as cryotherapy, shave dissection, and electrodesiccation and curettage can be used. Furthermore, numerous topical regimens, namely, nitric–zinc solutions, trichloroacetic (TCA), tazarotene 0.1%, topical dobesilate, 5% imiquimod cream, and 3% diclofenac gel have also been reported to be effective. Available data suggest that ablative lasers (including CO2 and erbium:YAG (Er:YAG)) and nonablative lasers (including potassium‐titanyl phosphate (KTP) and neodymium‐doped:YAG (Nd:YAG)) are effective in treating SKs. 14
Plasma is known as the fourth physical state of nature and its composition includes ions, electrons, electric emissions, thermal emissions, radicals, and bioactive molecules. It has a variety of different types according to the electrodes’ placement, the intensity of electrical potential, and environmental conditions. Generally, plasma is classified into thermal and nonthermal plasma. Regarding thermal plasma, high pressure and temperature are needed, whereas in nonthermal plasma, atmospheric pressure at low temperature and low energy are required. 15 , 16
In plasma, the atoms are excited and due to the presence of free electron and ions, plasma have been extensively used in medical science with various types including plasma exeresis, cold plasma jet, and floating electrode–dielectric barrier discharge (FE‐DBD). Plasma exeresis is created as a result of the high potential difference between the electrode and skin. Despite the high temperature of this type of plasma, the controlled form creates microburn on the skin. The plasma exeresis medical devices ionize the atmospheric gas between the device and the skin, sublimate the surface layers, and uniformly heat it. 9 , 10 , 11
Plasma exeresis is an innovative, minimally invasive technique that has been gaining attention in dermatology. Plasma has been widely used in treating moles, telangiectasia, stretch marks, acne scars, skin rejuvenation, and skin tightening. 12 , 13
In the literature, some authors refer to plasma exeresis as direct current (DC) electrofulgaration. Both plasma exeresis and electrofulguration (electrosurgery) affect the skin by spark formation between the tip of the device and the tissue. However, there are some differences between these two modalities.
Based on Sotiris et al.’s study, 10 plasma exeresis is an aesthetic procedure that works by ionizing the gases (air) in the space between the instrument's tip and the tissue being treated. It uses the voltage difference to generate a small electric arc and does not work if kept in direct contact with the tissue being treated. Based on Ferreira et al., sequences of sparks discharges are created using a DC voltage, in a constant distance of 2 mm between the tip of the device and conductively interconnected skin of the patient. Air that contains free electrons at the point of discharge must absorb a great amount of energy that leads to air breakthrough, which stops being an insulator and starts to lead an electric current. The air is ionized and it becomes plasma.
In contrast, electrofulguration uses high‐frequency alternating electrical current forming a spark between the tip of the electrode and the tissue. Hereby, no ionized gas is formed. Alternating current (AC) fulguration produced by electrocautery device has a wide beam with treatment area of 1 cm2, which is larger than plasma exeresis with a small spark flow area of 1 mm2. Therefore, plasma exeresis is more effective and precise to remove lesions without damaging surrounding tissue and creating undesirable results.
In a study done by Ghasemi et al., investigating the effect of plasma exeresis on skin parameters on rat models, it was concluded that plasma exeresis improves skin thickness, density, and elasticity, most likely due to increased keratinocytes and fibroblasts. 9 In one study conducted by Rossi et al., the efficacy of using plasma was evaluated in 250 patients with benign skin lesions (dermal nevi, fibroma, keratosis, xanthelasma), nonsurgical blepharoplasty, wrinkles (perioral, glabellar, neck, and preauricular regions), active acne, and scarring (postacneic and posttraumatic). The results revealed that plasma exeresis is extremely reliable and safe, without having any permanent side effects. 11
In the present study, we observed promising results following the use of plasma exeresis in treating SKs. The treatment was generally well tolerated, except for having erythema as the most common adverse event. The limitations of our study included small sample size and short duration of follow‐ups. Furthermore, not all patients presented for the second follow‐up. Another limitation was that the procedure was operator‐dependent; to solve this issue, only one person performed all the procedures. Larger studies with bigger sample size and studies comparing the efficacy of plasma exeresis with other methods such as electrocauterization are recommended in the future.
5. CONCLUSION
In this study, we found that both cryotherapy and plasma exeresis are effective treatment options for SK. However, we observed higher number of cleared lesions treated with plasma exeresis in 6 weeks and after two treatment sessions. The most common complications observed in the plasma and cryotherapy groups were erythema and hypo pigmentation, respectively. No serious adverse event was observed. Based on the results of our study, we believe that plasma exeresis can be a good treatment option with promising results for treating SKs with acceptable side effects.
CONFLICT OF INTEREST STATEMENT
None to declare.
FUNDING INFORMATION
We received no funding for this project.
INFORMED CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Noorbakhsh M, Kalantari Y, Ghasemi E, et al. Comparing the efficacy of plasma exeresis and cryotherapy for the treatment of seborrheic keratosis: A randomized controlled trial. Skin Res Technol. 2023;29:e13429. 10.1111/srt.13429
DATA AVAILABILITY STATEMENT
Not Applicable.
REFERENCES
- 1. Sun MD, Halpern AC. Advances in the etiology, detection, and clinical management of seborrheic keratoses. Dermatology. 2022;238(2):205‐217. [DOI] [PubMed] [Google Scholar]
- 2. Wollina U. Recent advances in managing and understanding seborrheic keratosis. F1000Res. 2019;8:F1000 Faculty Rev‐1520. doi: 10.12688/f1000research.18983.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Greco MJ, Bhutta BS. Seborrheic Keratosis. In: StatPearls [Internet]. StatPearls Publishing; 2022. PMID: 31424869. [PubMed] [Google Scholar]
- 4. Kalegowda D, Rajegowda HM, Rajendra BS, Madegowda SB. Topical trichloroacetic acid versus cryotherapy in the treatment of seborrheic keratoses: a prospective comparative study. J Pak Assoc Dermatol. 2022;32(1):1‐8. [Google Scholar]
- 5. Natarelli N, Krenitsky A, Hennessy K, Moore S, Grichnik J. Efficacy and safety of topical treatments for seborrheic keratoses: a systematic review. J Dermatol Treat. 2022;34(1):1‐2. [DOI] [PubMed] [Google Scholar]
- 6. Calik J, Migdal M, Zawada T, Bove T. Treatment of seborrheic keratosis by high frequency focused ultrasound ‐ an early experience with 11 consecutive cases. Clin Cosmet Investig Dermatol. 2022;15:145‐156. doi: 10.2147/CCID.S348106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wollina U. Seborrheic keratoses ‐ the most common benign skin tumor of humans. Clinical presentation and an update on pathogenesis and treatment options. Open Access Maced J Med Sci. 2018;6(11):2270‐2275. doi: 10.3889/oamjms.2018.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herron MD, Bowen AR, Krueger GG. Seborrheic keratoses: a study comparing the standard cryosurgery with topical calcipotriene, topical tazarotene, and topical imiquimod. Int J Dermatol. 2004;43(4):300‐302. doi: 10.1111/j.1365-4632.2004.02282.x [DOI] [PubMed] [Google Scholar]
- 9. Ghasemi E, Nilforoushzadeh MA, Khani M, et al. The quantitative investigation of spark plasma on skin parameters with skin elasticity, thickness, density, and biometric characteristics. Sci Rep. 2023;13(1):7738. doi: 10.1038/s41598-023-34425-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sotiris TG, Nittari G, Sagaro GG, Amenta F. A Descriptive study on the applications of plasma exeresis in dermatology. J Clin Aesthet Dermatol. 2021;14(3):E58‐E62. Epub 2021 Mar 1. PMID: 33841619; PMCID: PMC8021407. [PMC free article] [PubMed] [Google Scholar]
- 11. Rossi E, Farnetani F, Pellacani G. Applications of plasma exeresis in dermatology. Hi‐Tech Dermo. 2016;2:17‐22. [Google Scholar]
- 12. Gao J, Wang L, Xia C, et al. Cold atmospheric plasma promotes different types of superficial skin erosion wounds healing. Int Wound J. 2019;16(5):1103‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nejat F, Nabavi NS, Nejat MA, Aghamollaei H, Jadidi K. Safety evaluation of the plasma on ocular surface tissue: an animal study and histopathological findings. Clinical Plasma Medicine. 2019;14:100084. [Google Scholar]
- 14. Baumann LS, Blauvelt A, Draelos ZD, et al. Safety and efficacy of hydrogen peroxide topical solution, 40% (w/w), in patients with seborrheic keratoses: results from 2 identical, randomized, double‐blind, placebo‐controlled, phase 3 studies (A‐101‐SEBK‐301/302). J Am Acad Dermatol. 2018;79(5):869‐877. doi: 10.1016/j.jaad.2018.05.044. Epub 2018 Jun 1. Erratum in: J Am Acad Dermatol. 2021 Aug;85(2):531. PMID: 29864467. [DOI] [PubMed] [Google Scholar]
- 15. Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non‐invasive effects of non‐thermal plasma in pressure ulcer treatment. Sci Rep. 2018;8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Honarvar Z, Farhoodi M, Khani MR, et al. Application of cold plasma to develop carboxymethyl cellulose‐coated polypropylene films containing essential oil. Carbohydr Polym. 2017;176:1‐10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.


