Abstract
Aims
The DELIVER study demonstrates a significant improvement in cardiovascular death or hospitalization for heart failure among heart failure with mildly reduced ejection fraction (HFmrEF) or heart failure with preserved ejection fraction (HFpEF).Cost‐utility of the adjunct use of dapagliflozin to standard therapy among patients with HFpEF or HFmrEF remains unclear.
Methods and results
A five‐state Markov mode was constructed to project health and clinical outcomes of the adjunct use of dapagliflozin to standard therapy among 65‐year‐old patients with HFpEF or HFmrEF. A cost‐utility analysis was performed based on the DELIVER study and national statistical database. The cost and utility was inflated to 2022 by the usual discount rate of 5%. The primary outcomes were total cost and quality‐adjusted life‐years (QALYs) per patients as well as the incremental cost‐effectiveness ratio. Sensitivity analyses were also applied. Over a 15 year lifetime horizon, the average cost per patient was $7245.77 and $5407.55 in the dapagliflozin group and the standard group, along with an incremental cost of $1838.22. The average QALYs per patient was 6.00 QALYs and 5.84 QALYs in the dapagliflozin group and the standard group, along with an incremental QALYs of 0.15 QALYs, resulting in the incremental cost‐effectiveness ratio of $11 865.33/QALY, which was below the willingness‐to‐pay (WTP) of $12 652.5/QALY. The univariate sensitivity analysis indicated the cardiovascular death in both group was the most sensitive variable. Probability sensitivity analysis revealed that when the WTP thresholds were $12 652.5/QALY and $37 957.5/QALY, the probabilities of being cost‐effective with dapagliflozin as an add‐on were 54.6% and 71.6%, respectively.
Conclusions
From a public healthcare system perspective, the adjunct use of dapagliflozin to standard therapy among patients with HFpEF or HFmrEF generated advantages in cost‐effectiveness in China at a WTP of $12 652.5/QALY, which promoted the rational use of dapagliflozin for heart failure.
Keywords: Dapagliflozin, Cost‐utility analysis, Heart failure with preserved or mildly reduced ejection fraction, China
Introduction
Heart failure (HF) is the main cause of morbidity and mortality all over the world. Recent years have witnessed great success in the therapy of HF, but mortality and morbidity are still high, indicating that important pathogenic mechanisms remains unsolved. 1 , 2 , 3 HF is divided into heart failure with mildly reduced ejection fraction (HFmrEF), heart failure with reduced ejection fraction (HFrEF), and heart failure with preserved ejection fraction (HFpEF). HFmrEF and HFpEF consist of the majority of HF nowadays and are related to a number of death, disability, and healthcare costs, which is a public health problem with a great effect on the quality of life of HF patients that causes functional limitations. 3 More than 10 million patients were suffering HF in China, and more than 80% of in‐hospital patients have worsening HF events along with about 25% of readmission within 30 days and 30% of death within 1 year, which were associated with considerable clinical and economic burden in China. 3 Inpatient hospitalization expense was the main driver of total costs in HF. 4 Medical resource use and costs were little higher for patients recently hospitalized for HF compared with patients with stable HF. 5 It was estimated that the expense of hospitalization for HF in China has increased to about 26.3 billion US dollars. 6 Therefore, the reduction of the times of hospitalizations and worsening HF events will decrease the economic burden of the healthcare system.
Progress has been achieved in the standard therapy for HFrEF. Four drugs including angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers or angiotensin receptor neprilysin inhibitors, beta‐blockers, mineralocorticoid receptor antagonists, and sodium–glucose transport protein 2 inhibitors (SGLT2is) were regarded as class I recommendation in clinical practice and guidelines for HFrEF. 7 SGLT2is were proved to reduce the risk of hospitalization for HF and/or cardiovascular (CV) death among HFrEF patients in EMPEROR‐Reduced (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure and a Reduced Ejection Fraction) and DAPA‐HF (the Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure) study. 8 , 9 However, evidence‐based therapies for HFmrEF and HFpEF have been lacking so far. EMPEROR‐Preserved (The Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction) and DELIVER studies (Dapagliflozin Evaluation to Improve the Lives of Patients with Preserved Ejection Fraction Heart Failure) have indicated a significant improvement in CV death or hospitalization for HF among HF patients with left ventricular ejection fraction (LVEF >40%). 10 , 11 These findings have important implications for HFpEF and HFmrEF.
Although dapagliflozin has shown great benefits in HFpEF and HFmrEF patients, the economic burden of medical therapy should still be considered for China payers and healthcare decision makers. Pharmacoeconomic evaluation could solve the problem about the cost and utility of the add‐on dapagliflozin treatment for HFpEF and HFmrEF patients. Currently, many cost‐utility analyses focused on HFrEF or HF as a homogeneous group, and there was a lack of evidence on the cost‐utility analysis of dapagliflozin in HFpEF and HFmrEF in China. Thus, the objective of this study was to estimate the cost‐utility of the add‐on dapagliflozin treatment for HFpEF and HFmrEF patients to addresses a previous research gap.
Method
Overview
The HFpEF and HFmrEF disease simulation model was performed by Microsoft Excel 2010 to evaluate the cost effectiveness of the adjunct use of dapagliflozin to standard therapy among patients with HFpEF or HFmrEF (LVEF >40%), whose characteristics was consistent with the DELIVER study that enrolled HF patients who had a LEVF of >40% or had a previous LVEF of ≤40% but had a LEVF of >40% at the time of enrolment, which differed from the EMPEROR‐Preserved study. 11 The dapagliflozin group in simulated cohorts consisted of populations who received dapagliflozin (10 mg daily) as an add‐on to standard therapy for HFpEF and HFmrEF. The standard group received placebo and standard therapy. The starting age of simulated cohorts in the model was 65 years old based on the mean age of HF patients from the real world evidence. 12 The cost and utilities were discounted at 5.0% annually based on ‘The Guidelines of Pharmacoeconomic Evaluations of China (2020)’. 13
Model structure
A multistate Markov model based on our previous cost‐utility analysis of HF patients was constructed including five mutually independent states [New York Heart Association (NYHA) function class I, II, III, and IV and death (CV death and non‐CV death)] (Figure 1 ). 14 The model could predict the occurrence of CV death, non‐CV death, as well as admission and readmission for HF and estimated cost and utilities. The patients with HFpEF or HFmrEF would move between different NYHA functions classes when each cycle was over. A 3 month cycle was applied in the model in the DELIVER study in that patients recently hospitalized for HF could increase the risk of additional hospitalization for HF during the shorter period, which was about 90 days. 15 A half‐cycle correction was used to prevent the overestimate of expected survival. 13
Figure 1.
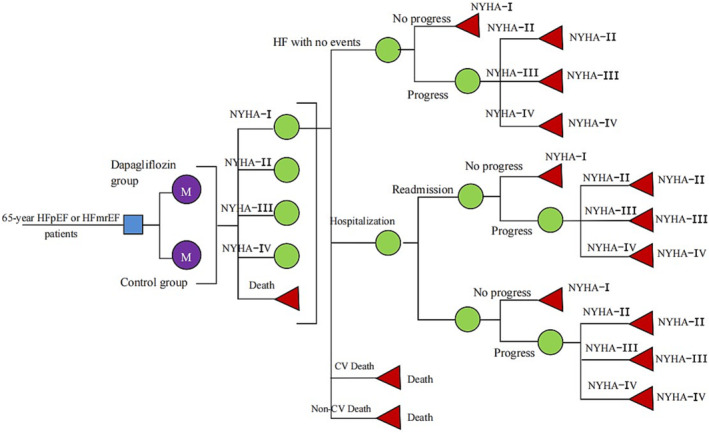
A multistate Markov model for heart failure. CV, cardiovascular; HF, heart failure; HFmrEF, heart failure with mildly reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; NYHA, New York Heart Association.
Clinical event probabilities
In this model, all patients with HFpEF or HFmrEF treated with standard therapy with or without dapagliflozin (10 mg once daily) were assumed to have stable state at the beginning. The efficacy of drug and the incidence of clinical events in the model did not change with ages. The NYHA function class distribution in the first cycle was consistent with the DELIVER study. The model inputs on CV death and hospitalization for HF were derived from 2.3 year follow‐up data of the DELIVER study. The rate of CV death was 7.4% in the dapagliflozin group and 8.3% in the standard group, and the rate of hospitalization for HF was 10.5% in the dapagliflozin group and 13.3% in the standard group, respectively. Considering that non‐CV death was not the main clinical outcome of the DELIVER study, age‐dependent non‐CV death was based on the data from the Chinese Center for Disease Control and Prevention by removing CV death. 16 The readmission for HF was based on the data from the I‐PRESERVE study. 17 We converted these data into 3 month probabilities by the formula r = −1/t ln(S), P = 1‐e^(−r*T) (S is the rate, t is the time, P is the clinical event probabilities) (Table 1 ). 18 The DELIVER study has demonstrated that adding dapagliflozin to standard therapy could improve NYHA function classes in HFrEF and HFmrEF patients and reduce the occurrence of deterioration, but the specific transition probabilities for movement between NYHA function classes under the treatment of dapagliflozin remained unclear. 19 The 3 month transition probability matrix between NYHA function classes was derived from published literature (Table 2 ). 20
Table 1.
Selected model inputs
| Variables | Value | Range | Distribution | Reference |
|---|---|---|---|---|
| Event probabilities | ||||
| CV death | ||||
| Standard group | 0.00937 | 0.00844–0.01031 | Beta | 11 |
| Dapagliflozin group | 0.00832 | 0.00749–0.00915 | Beta | 11 |
| Hospitalization for heart failure | ||||
| Standard group | 0.01539 | 0.01385–0.01693 | Beta | 11 |
| Dapagliflozin group | 0.01199 | 0.01079–0.01318 | Beta | 11 |
| Readmission for heart failure | 0.417 | 0.3753–0.4587 | Beta | 17 |
| Probability of non‐CV mortality by age | ||||
| 65–69 years | 0.2430% | 16 | ||
| 70–74 years | 0.3042% | 16 | ||
| 75–79 years | 0.4185% | 16 | ||
| Utility | ||||
| NYHA I | 0.825 | 0.790–0.860 | Beta | 24 |
| NYHA II | 0.780 | 0.750–0.810 | Beta | 24 |
| NYHA III | 0.650 | 0.610–0.690 | Beta | 24 |
| NYHA IV | 0.585 | 0.510–0.660 | Beta | 24 |
| Hospitalization and readmission | −0.1 | −0.13 to −0.08 | Beta | 20 |
| Cost | ||||
| Standard therapy | $131.96 | $131.96–310.83 | Gamma | 24 |
| Dapagliflozin | $61.31 | $49.05–85.86 | Gamma | Local data |
| Hospitalization and readmission | $1783.39 | $1029.73–3336.39 | Gamma | 22 |
| Discounted rate | 5% | 0–8% | 13 | |
CV, cardiovascular; NYHA, New York Heart Association.
Table 2.
New York Heart Association classification transition probabilities per cycle (3 months)
| To | I | II | III | IV | Distribution |
|---|---|---|---|---|---|
| From | |||||
| I | 0.977 | 0.019 | 0.004 | 0 | Dirichlet |
| II | 0.008 | 0.981 | 0.010 | 0.001 | Dirichlet |
| III | 0 | 0.034 | 0.960 | 0.006 | Dirichlet |
| IV | 0 | 0 | 0.055 | 0.945 | Dirichlet |
Healthcare resource use and cost inputs
As our model was performed from a China healthcare system perspective, only direct medical costs were considered, including the costs of hospitalization for HF, standard therapy and dapagliflozin. The cost and utility was inflated to 2022 by the usual discount rate of 5% and all costs of this study were converted into US dollars at an exchange rate of 1 $ = 6.4 RMB. 13 , 21 A one‐time cost per hospitalization for HF derived from the China Health Statistics Yearbook 2021, which was also applied for readmission for HF. 22 Although HFpEF and HFmrEF therapy lacked specific drugs, many patients in the real world received diuretics, SAC/VALs or angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta‐blockers and mineralocorticoid receptor antagonists. These 3 month cost of drugs were calculated based on published literature. 23 Considering that a part of patients in the DELIVER study received SAC/VAL, so we calculated the range of standard therapy for the sensitivity analysis. The price of dapagliflozin was obtained from the latest national negotiation in 2022 (Table 1 ).
Utilities
To calculate the quality‐adjusted life‐years (QALYs) for patients with HFpEF and HFmrEF, we multiplied the time length in each health state by the utilities in each health state. A utility of 0 usually represented death and a utility of 1 represented perfect health. The health utility of different NYHA function classes was derived from a real‐world evidence in China. 24 Given that one‐time hospitalization could reduce the life quality of HFpEF and HFmrEF patients, so each hospitalization for HF each cycle would reduce the utility value by 0.1 (Table 2 ). 20
Health and clinical outcomes
Health and clinical outcomes were compared between both groups including the number of hospitalization for HF and CV deaths per 1000 simulated patients, the average survival time, total cost, and QALYs per patients as well as the incremental cost‐effectiveness ratio (ICER), which was calculated by the incremental difference in outcomes. The ICER was applied to estimate the magnitude of the increased costs per QALY in health decision. As recommended by the relevant guidelines: ICER < 1‐time gross domestic product (GDP) per capita, the increased cost per QALY is completely worth it and very cost‐effective; 1‐time GDP per capita < ICER < 3‐time GDP per capita, the increased cost per QALY is acceptable and cost‐effective; ICER > 3‐time GDP per capita, the increased cost per QALY is not worth it and not cost‐effective. 13 The willingness‐to‐pay (WTP) thresholds of $12 652.5/QALY and $37 687.5/QALY, which was associated with the one‐time and three‐time GDP per capita of China in 2021 was applied to assess the cost‐utility of a intervention (dapagliflozin). An incremental cost per QALY was below the WTP, indicating adding dapagliflozin to standard therapy was considered cost effective. 25 , 26
Sensitivity analyses
Uncertainty of quantitative parameters was evaluated by one‐way and probability sensitivity analyses (PSA). One‐way sensitivity analysis was applied to assess the uncertainty of the model by changing each parameter within a reasonable range according to the recommendations of the guidelines of pharmacoeconomic evaluations of China (2020). 13 Some specified range such 95% confidence intervals could derive from the published literature. For probability and cost without specified range, the assuming range of ±10% and ±20% respectively was applied, and the range of annual discount rate was from 0% to 8% (Table 1 ). The results were presented as a tornado diagram.
PSA assessed the uncertainty of the model in the form of Monte Carlo simulation, using 1000‐time simulated results to assess how the simultaneous uncertainties about model parameter across their specified distributions might affect outcomes. The results were represented by cost‐effectiveness‐acceptability curves and scatter diagram.
Scenario analyses were performed for model input assumptions, including time horizon, the cost of dapagliflozin, and hospitalizations for HF. We explored the cost‐utility of the adjunct use of dapagliflozin to standard therapy among HF patients across the range of LVEF based on the DAPA‐HF and DELIVER studies (≤30%, >30 and ≤37%, >37 and ≤44%, >44 and ≤51%, >51 and ≤60%, >60%). 27 We also explore the cost‐utility of dapagliflozin among HFpEF or HFmrEF patients according to age and frailty index (FI). 28 , 29
Results
Model validation
When the model was operated for 2.3 years, the CV mortality and the rate of hospitalization for HF was 8.28% and 10.3% in the dapagliflozin group, and the CV mortality and the rate of hospitalization for HF was 9.1% and 13.2% in the standard group. The average survival time of the two groups was 15 and 13.75 years, respectively, indicating that our model was reliable.
Base case analysis
In the base case over a 15 year lifetime horizon, the dapagliflozin group we predicted in our model had 527 hospitalizations for HF and 496 CV deaths each 1000 simulated patients, compared with 658 hospitalizations for HF and 527 CV deaths each 1000 patients in the standard group, along with 131 lower hospitalizations for HF and 31 lower CV deaths in the dapagliflozin group. The average cost per patient was $7245.77 and $5407.55 in the dapagliflozin group and the standard group, along with an incremental cost of $1838.22. The average QALYs per patient was 6.00 QALYs and 5.84 QALYs in the dapagliflozin group and the standard group, along with an incremental QALYs of 0.15 QALYs, resulting in the ICER of $11 865.33/QALY, which was below the WTP of $12 652.5/QALY (Table 3 ).
Table 3.
The results from base‐case analysis
| Total cost ($) | Total life years (QALY) | Incremental cost ($) | Incremental life years (QALY) | ICER ($ per QALY) | |
|---|---|---|---|---|---|
| Dapagliflozin group | 7245.77 | 6.00 | 1838.22 | 0.15 | 11 865.33 |
| Control group | 5407.55 | 5.84 |
QALY, quality‐adjusted life‐year.
Univariate sensitivity analyses
The outcomes of univariate sensitivity analysis represented as a tornado diagram (Figure 2 ) indicated that the CV death in both group was the most sensitive variable, which was more than three‐time WTP of $37 957.5/QALY; other variables had a little impact on the ICER, which was all lower than one‐time GDP of $12 652.5/QALY.
Figure 2.
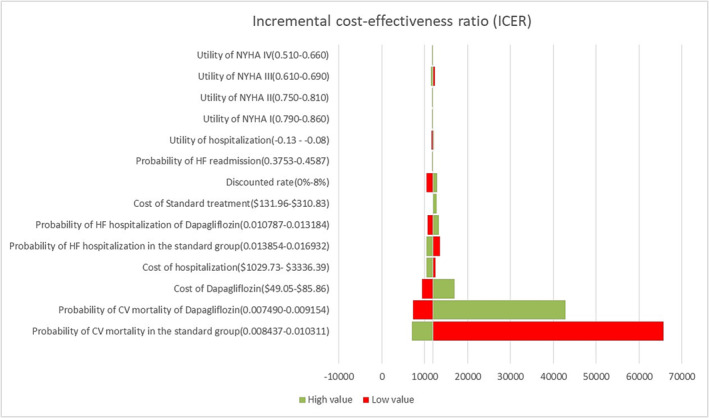
Tornado diagram showing the resulting ICERs across the values of the parameters. Red corresponds to the lower limit while green corresponds to the upper limit of the parameter. CV, cardiovascular; HF, heart failure; NYHA, New York Heart Association.
Probability sensitivity analysis
Most red spots were located in the upper‐right quadrant, demonstrating that dapagliflozin group was likely to produce a higher expense but gained a higher QALY (Figure 3 ). PSA showed that 54.6% of simulated population treated with dapagliflozin and standard therapy was cost‐effective at a WTP threshold $12 652.5/QALY, but 71.6% of simulated population treated with dapagliflozin and standard therapy was cost‐effective at a WTP threshold $37 957.5/QALY (Figure 4 ).
Figure 3.
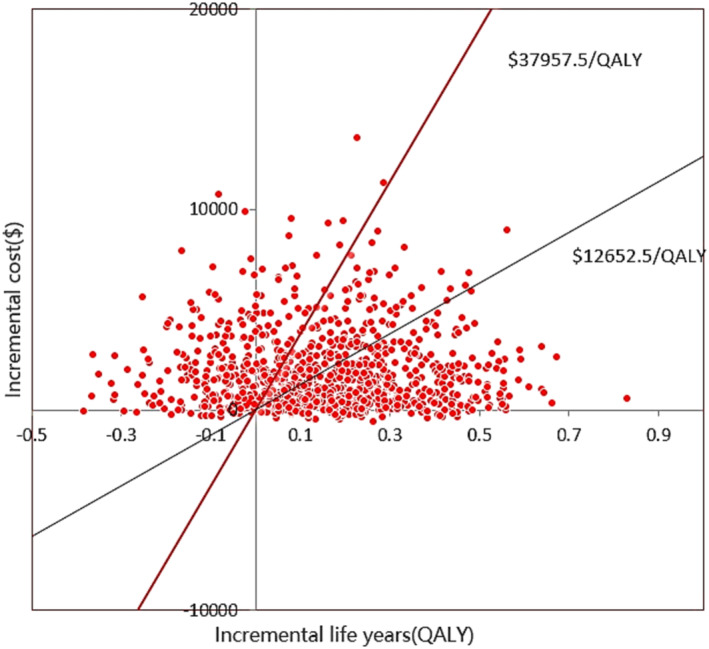
Scatter plot showing the incremental costs and the incremental quality‐adjusted life‐year for a thousand simulations. QALY, quality‐adjusted life‐year.
Figure 4.
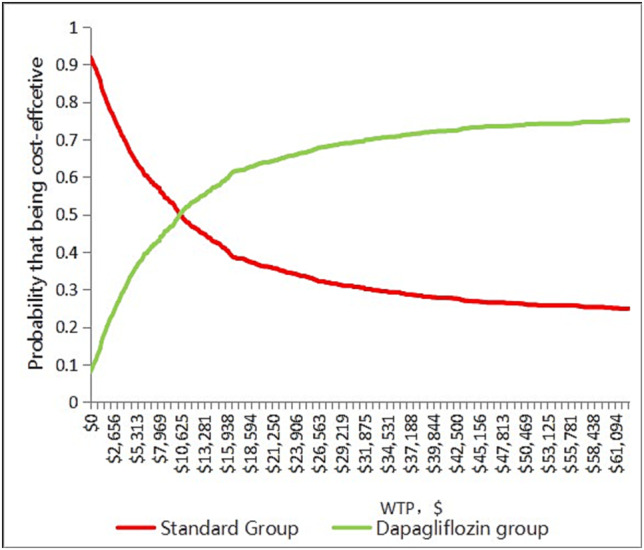
Cost‐effectiveness acceptability curve showing the maximum willingness to pay and the corresponding probability of cost‐effectiveness for dapagliflozin group and standard group. WTP, willingness‐to‐pay.
Scenario analysis
Different prices of dapagliflozin (reduced by 20%, 40%, and 60%) incurred different ICERs ($9305.654/QALY vs. $6745.982/QALY vs. $4186.31/QALY).The cost per QALY gained from the dapagliflozin group ranged from $11 865.33 to $27 599.35 approximately over 5 and 15 year time horizons, respectively. The cost per QALY gained from the dapagliflozin group changed from $10 440.18/QALY to $12 556.94/QALY over different level of hospital. The add‐on dapagliflozin treatment for HF was cost‐effective except HF patients with a LVEF of >51 and ≤60%. HFpEF or HFmrEF patients over 65 years was more cost‐effective than that under 65 years. HFpEF or HFmrEF patients with a higher FI (≥0.311) contributed to more pharmacoeconomic benefits (Table 4 and Data S1 ).
Table 4.
The result of scenario analyses presented as ICER
| Scenario | ICER ($ per QALY) |
|---|---|
| Price for dapagliflozin | |
| Reduced by 20% | 9305.65 |
| Reduced by 40% | 6745.98 |
| Reduced by 60% | 4186.31 |
| Time horizon | |
| 5 years | 27 599.35 |
| 10 years | 15 591.11 |
| 15 years | 11 865.33 |
| Hospital level | |
| Town hospital | 12 556.94 |
| County hospital | 12 372.18 |
| Municipal hospital | 11 865.33 |
| Provincial hospital | 11 712.85 |
| Ministerial hospital | 10 440.18 |
| LVEF | |
| ≤30% | 4882.72 |
| >30 and ≤37% | 10 294.79 |
| >37 and ≤44% | 6105.52 |
| >44 and ≤51% | 11 550.49 |
| >51 and ≤60% | 64 243.96 |
| >60% | 4646.05 |
| Pooled cohort | 7540.01 |
| Age | |
| <55 years old | −69 232.60 |
| 55–64 years old | −15 836.80 |
| 65–74 years old | 8440.93 |
| ≥75 years | 7612.60 |
| Frailty index (FI) | |
| FI ≤ 0.210 (not frail) | 12 861.62 |
| FI 0.211–0.310 (more frail) | −14 1465.00 |
| FI ≥ 0.311 (most frail) | 41 75.17 |
ICER, incremental cost‐effectiveness ratio; LVEF, left ventricular ejection fraction; QALY, quality‐adjusted life‐year.
Discussion
This study was a cost‐utility analysis of the adjunct use of dapagliflozin to standard therapy among patients with HFpEF or HFmrEF. The related data derived from DELIVER study and national statistical database. The findings in our study showed add‐on dapagliflozin was considered to be a cost‐effective option for patients with HFpEF or HFmrEF. The cost per QALY gained was $11 865.33, which was lower than one‐time GDP of $12 652.5/QALY. The cost‐effectiveness was consistently maintained throughout a serious sensitivity analysis. We provided insights for decision‐making in healthcare from simulated results.
As SGLT2i showed the benefits with sufficient statistical efficacy in patients with HFpEF or HFmrEF which opened a new era for the treatment of HFpEF and HFmrEF. 10 , 11 Acting through neurohumoral pathways showed their greatest benefit in patients with HFrEF, but showed attenuated benefit in patients with a LVEF of >40%. 30 SGLT2is tended to not act through neurohumoral pathways and no gradient in their effect associated with LVEF was estimated. The magnitude of the effect of empagliflozin on patients with a LVEF <65% was similar but that on patients with a LVEF ≥65% was attenuated, and the effect of dapagliflozin did not differ by LVEF. 27 , 31 What is more, promising evidence indicated the benefits of dapagliflozin in normal patients were consistent with frailer patients and recently hospitalized patients which broke traditional thoughts. 29 , 32 Overall, the benefits of dapagliflozin in patients with HF, including in patients with a LVEF (≥60%), in patients who received dapagliflozin during or soon after hospitalization, and in patients with a previous LVEF of ≤40% but has improved to 40% were conserved. Meanwhile, the add‐on dapagliflozin treatment for HF was cost‐effective except for HF patients with a LVEF of >51 and ≤60%. HFpEF or HFmrEF patients with a higher FI (≥0.311) contributed to more pharmacoeconomic benefits based on the scenario analysis. Considering that the mean age of HF patients in China was 65 years and treatment with dapagliflozin was expected to extend survival time by up to 2–2.5 years among 65‐year‐old patients with HFpEF or HFmrEF, 3 , 12 it was reasonable that the add‐on dapagliflozin treatment for HFpEF or HFmrEF patients over 65 years was more cost‐effective. A series of scenario analysis could provide the promising evidence for the subgroup of HFpEF or HFmrEF patients who could get more pharmacoeconomic benefits according to LVEF, age, and frailty.
In the sensitivity analysis, CV mortality in both group was the most sensitive variable in cost effectiveness, followed by the cost of dapagliflozin and hospitalization for HF, and other variables cause a little changes on the model. The finding was expected in our outcomes, because dapagliflozin could not reduce CV mortality (hazard ratio, 0.88; 95% CI, 0.74 to 1.05) in patients with HFpEF or HFmrEF, 11 but comparing results from absolute risk differences could provide an alternative picture of treatment benefit. From scenario analysis, the lower price of dapagliflozin and the higher the cost of hospitalization could incur more pharmacoeconomic benefits. There were over 10 million patients suffering HF in China 4 ; according to our model, dapagliflozin could reduce 131 hospitalizations for HF and 31 CV deaths each 1000 simulated patients a year, dapagliflozin could save $2.5 billion a year and 31 thousand life a year. Generally, dapagliflozin not only brought more pharmacoeconomic benefits but also improved life and health, which was also the significance of our study.
To our best knowledge, we are the first to study the cost‐utility of the add‐on dapagliflozin treatment for HFpEF and HFmrEF. Chinese HFpEF patients with higher comorbidities were associated with high risk of hospitalization for HF and CV death within 1 year. 33 Previous studies have focused on HFrEF or HF as a homogeneous group. A number of cost‐utility studies on adding SGLT2is to standard therapy for HFrEF have been performed. Dapagliflozin in HFrEF have generated advantages in cost‐effectiveness in China, United States, Australia, Egypt, and Asia‐Pacific region. 34 , 35 , 36 , 37 Cost‐utility analyses of the add‐on empagliflozin in HFrEF had also been proved to be cost‐effective in China, Asia‐Pacific region, and Thailand. 38 , 39 , 40 The adjunct use of SGLT2is to standard therapy among HFrEF patients in China was a cost‐effective option. However, there was little evidence on the cost‐utility of the add‐on SGLT2is treatment for HFpEF and HFmrEF. The cost per QALY gained was $11 809 in Thailand with a lower WTP ($4773.27/QALY), which empagliflozin was not a cost‐effective add‐on treatment for patients with HFpEF. 38 We previously reported the pharmacoeconomics advantage of empagliflozin in HFrEF and HFpEF in China. 41 Additionally, several studies have shown that dapagliflozin was cost‐effective in the treatment of diabetes 42 , 43 Therefore, adding dapagliflozin to standard therapy was a cost‐effective choice for HFpEF and HFmrEF patients with diabetes.
Our study has several advantages. One is our Markov model different from many previous cost‐effectiveness analyses in HF that adopted a three‐state Markov model (stable, hospitalization, and death), 44 and different NYHA function classes had different cost and utilities especially NYHA class III and NYHA class IV. Clinical events in our model including non‐CV death, CV death, hospitalization for HF, and readmission for HF better reflected the clinical pathway of patients with HFpEF or HFmrEF. The other was some related data including non‐CV death, cost, and utility derived from studies on Chinses HF populations, which was consistent with the local healthcare setting. To a certain extent, the adoption of the local data was more reasonable for the practical scenarios of the Chinese HF population.
Our results should be explained within the disadvantages of our analysis. First, hospitalization for non‐HF that could not be directly acquired from the DELIVER study trial was not enrolled in our model. Second, the data on CV death and hospitalization for HF derived from the DELIVER study remained unchanged over a lifetime horizon, leading to uncertainty of long‐term extrapolation which has been validated through a serious sensitivity analysis. Third, an assumption was proposed that patients with HFpEF or HFmrEF in the model could tolerate recommended dose without the adverse reaction events but the DELIVER study showed that the most common adverse reaction events including major hypoglycaemic events and volume depletion were not significantly different. Fourth, owing to a lack of relevant data, we did not take it into account that adding dapagliflozin to standard therapy could improve NYHA function classes in HFrEF and HFmrEF patients and reduce the occurrence of deterioration, and our results may be more reliable when considering the condition. Fifth, this was a mathematical model combined with national conditions in China, and the generalisability of our findings should be limited to settings or contexts similar to those of this study.
Conclusions
From a public healthcare system perspective, the adjunct use of dapagliflozin to standard therapy among patients with HFpEF or HFmrEF generated advantages in cost‐effectiveness in China at a WTP of $12 652.5/QALY. The clinical decision on whether to use dapagliflozin should be based on shared decision‐making that reflects therapy associated with benefits, risks, costs, and patient preferences. However, more cost‐effectiveness analyses based on real world evidence of populations in China need to be validated.
Conflict of interest
The authors declare that they have no competing interests.
Funding
The authors declare that they have no funding.
Supporting information
Data S1. Supporting Information.
Acknowledgement
We thank Rui Luo for advice on data analysis.
Tang, Y. , and Sang, H. (2023) Cost‐utility analysis of add‐on dapagliflozin in heart failure with preserved or mildly reduced ejection fraction. ESC Heart Failure, 10: 2524–2533. 10.1002/ehf2.14426.
References
- 1. Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure‐associated hospitalizations in the United States. J Am Coll Cardiol. 2013; 61: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gheorghiade M, Greene SJ, Butler J, Filippatos G, Lam CS, Maggioni AP, Ponikowski P, Shah SJ, Solomon SD, Kraigher‐Krainer E, Samano ET, Muller K, Roessig L, Pieske B. Effect of vericiguat, a soluble guanylate cyclase stimulator, on natriuretic peptide levels in patients with worsening chronic heart failure and reduced ejection fraction: the SOCRATES‐REDUCED randomized trial. JAMA (Russkoe izd). 2015; 314: 2251–2262. [DOI] [PubMed] [Google Scholar]
- 3. Vaduganathan M, Claggett BL, Jhund P, de Boer RA, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam C, Martinez F, Shah SJ, Desai AS, Lindholm D, Petersson M, Langkilde AM, McMurray J, Solomon SD. Estimated long‐term benefit of dapagliflozin in patients with heart failure. J Am Coll Cardiol. 2022; 80: 1775–1784. [DOI] [PubMed] [Google Scholar]
- 4. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray J, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler J, Djatche LM, Sawhney B, Chakladar S, Yang L, Brady JE, Yang M. Clinical and economic burden of chronic heart failure and reduced ejection fraction following a worsening heart failure event. Adv Ther. 2020; 37: 4015–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cook C, Cole G, Asaria P, Jabbour R, Francis DP. The annual global economic burden of heart failure. Int J Cardiol. 2014; 171: 368–376. [DOI] [PubMed] [Google Scholar]
- 7. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland J, Coats A, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam C, Lyon AR, McMurray J, Mebazaa A, Mindham R, Muneretto C, Francesco PM, Price S, Rosano G, Ruschitzka F, Kathrine SA. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 8. Colombo G, Casella R, Cazzaniga A, Casiraghi C. Dapagliflozin in patients with heart failure and reduced ejection fraction. Intern Emerg Med. 2020; 15: 515–517. [DOI] [PubMed] [Google Scholar]
- 9. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Bohm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez JJ, Kaul S, Brunner‐La RH, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020; 383: 1413–1424. [DOI] [PubMed] [Google Scholar]
- 10. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Bohm M, Brunner‐La RH, Choi DJ, Chopra V, Chuquiure‐Valenzuela E, Giannetti N, Gomez‐Mesa JE, Janssens S, Januzzi JL, Gonzalez‐Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Pina IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam C, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. 2021; 385: 1451–1461. [DOI] [PubMed] [Google Scholar]
- 11. Solomon SD, McMurray J, Claggett B, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam C, Martinez F, Shah SJ, Desai AS, Jhund PS, Belohlavek J, Chiang CE, Borleffs C, Comin‐Colet J, Dobreanu D, Drozdz J, Fang JC, Alcocer‐Gamba MA, Al HW, Han Y, Cabrera HJ, Janssens SP, Katova T, Kitakaze M, Merkely B, O'Meara E, Saraiva J, Tereshchenko SN, Thierer J, Vaduganathan M, Vardeny O, Verma S, Pham VN, Wilderang U, Zaozerska N, Bachus E, Lindholm D, Petersson M, Langkilde AM. Dapagliflozin in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2022; 387: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 12. Li L, Liu R, Jiang C, Du X, Huffman MD, Lam C, Patel A, Hillis GS, Anderson CS, Ma C, Zhao X, Wang X, Li L, Dong J. Assessing the evidence‐practice gap for heart failure in China: the heart failure registry of patient outcomes (HERO) study design and baseline characteristics. Eur J Heart Fail. 2020; 22: 646–660. [DOI] [PubMed] [Google Scholar]
- 13. Liu G. The guideline of pharmacoeconomic evaluation of China: 2020, 1st ed. Peking: China Market Press; 2020. p 27. [Google Scholar]
- 14. Jiang Y, Zheng R, Sang H. Cost‐effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front Pharmacol. 2021; 12: 733681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeVore AD, Hammill BG, Sharma PP, Qualls LG, Mentz RJ, Waltman JK, Fonarow GC, Curtis LH, Hernandez AF. In‐hospital worsening heart failure and associations with mortality, readmission, and healthcare utilization. J Am Heart Assoc. 2014; 3: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Center for Chronic and Noncommunicable Disease Control and Prevention; Chinese Center for Disease Control and Prevention . China Mortality Surveillance Dataset 2018. Beijing: China Science and Technology Press; 2019. p 17–82. [Google Scholar]
- 17. Carson PE, Anand IS, Win S, Rector T, Haass M, Lopez‐Sendon J, Miller A, Teerlink JR, White M, McKelvie RS, Komajda M, Zile MR, McMurray JJ, Massie B. The hospitalization burden and post‐hospitalization mortality risk in heart failure with preserved ejection fraction: results from the I‐PRESERVE trial (Irbesartan in Heart Failure and Preserved Ejection Fraction). JACC: Heart Fail. 2015; 3: 429–441. [DOI] [PubMed] [Google Scholar]
- 18. Park SK, Hong SH, Kim H, Kim S, Lee EK. Cost‐utility analysis of sacubitril/valsartan use compared with standard care in chronic heart failure patients with reduced ejection fraction in South Korea. Clin Ther. 2019; 41: 1066–1079. [DOI] [PubMed] [Google Scholar]
- 19. Ostrominski JW, Vaduganathan M, Claggett BL, de Boer RA, Desai AS, Dobreanu D, Hernandez AF, Inzucchi SE, Jhund PS, Kosiborod M, Lam C, Langkilde AM, Lindholm D, Martinez FA, O'Meara E, Petersson M, Shah SJ, Thierer J, McMurray J, Solomon SD. Dapagliflozin and New York Heart Association functional class in heart failure with mildly reduced or preserved ejection fraction: the DELIVER trial. Eur J Heart Fail. 2022; 24: 1892–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. King JB, Shah RU, Bress AP, Nelson RE, Bellows BK. Cost‐effectiveness of sacubitril‐valsartan combination therapy compared with enalapril for the treatment of heart failure with reduced ejection fraction. JACC‐Heart Fail. 2016; 4: 392–402. [DOI] [PubMed] [Google Scholar]
- 21. Bank of China . Foreign Exchange Rates, 2021.
- 22. Ma XW. China Health Statistics Yearbook 2020. Beijing: China Union Medical University Press; 2021. p 117–177. [Google Scholar]
- 23. Huang J, Yin H, Zhang M, Ni Q, Xuan J. Understanding the economic burden of heart failure in China: impact on disease management and resource utilization. J Med Econ. 2017; 20: 549–553. [DOI] [PubMed] [Google Scholar]
- 24. Zhu S, Zhang M, Ni Q, Sun Q, Xuan J. Indirect, direct non‐medical cost and QoL by New York Heart Association (NYHA) classification in Chinese heart failure patients. Value Health. 2017; 20: A268. [Google Scholar]
- 25. National Bureau of statistics of the people's Republic of China . China Health Statistics Yearbook 2021. Beijing: Peking Union Medical College Press. [Google Scholar]
- 26. Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost‐effectiveness analysis in health‐care resource allocation decision‐making: how are cost‐effectiveness thresholds expected to emerge? Value Health. 2004; 7: 518–528. [DOI] [PubMed] [Google Scholar]
- 27. Jhund PS, Kondo T, Butt JH, Docherty KF, Claggett BL, Desai AS, Vaduganathan M, Gasparyan SB, Bengtsson O, Lindholm D, Petersson M, Langkilde AM, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Kober L, Lam C, Martinez FA, Sabatine MS, Shah SJ, Solomon SD, McMurray J. Dapagliflozin across the range of ejection fraction in patients with heart failure: a patient‐level, pooled meta‐analysis of DAPA‐HF and DELIVER. Nat Med. 2022; 28: 1956–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peikert A, Martinez FA, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam C, Shah SJ, Katova T, Merkely B, Vardeny O, Wilderang U, Lindholm D, Petersson M, Langkilde AM, McMurray J, Solomon SD. Efficacy and safety of dapagliflozin in heart failure with mildly reduced or preserved ejection fraction according to age: the DELIVER trial. Circ Heart Fail. 2022; 15: e10080. [DOI] [PubMed] [Google Scholar]
- 29. Butt JH, Jhund PS, Belohlavek J, de Boer RA, Chiang CE, Desai AS, Drozdz J, Hernandez AF, Inzucchi SE, Katova T, Kitakaze M, Kosiborod MN, Lam C, Maria LA, Lindholm D, Bachus E, Martinez F, Merkely B, Petersson M, Saraiva J, Shah SJ, Vaduganathan M, Vardeny O, Wilderang U, Claggett BL, Solomon SD, McMurray J. Efficacy and safety of dapagliflozin according to frailty in patients with heart failure: a prespecified analysis of the DELIVER trial. Circulation. 2022; 146: 1210–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Solomon SD, Claggett B, Lewis EF, Desai A, Anand I, Sweitzer NK, O'Meara E, Shah SJ, McKinlay S, Fleg JL, Sopko G, Pitt B, Pfeffer MA. Influence of ejection fraction on outcomes and efficacy of spironolactone in patients with heart failure with preserved ejection fraction. Eur Heart J. 2016; 37: 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Butler J, Packer M, Filippatos G, Ferreira JP, Zeller C, Schnee J, Brueckmann M, Pocock SJ, Zannad F, Anker SD. Effect of empagliflozin in patients with heart failure across the spectrum of left ventricular ejection fraction. Eur Heart J. 2022; 43: 416–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cunningham JW, Vaduganathan M, Claggett BL, Kulac IJ, Desai AS, Jhund PS, de Boer RA, DeMets D, Hernandez AF, Inzucchi SE, Kosiborod MN, Lam C, Martinez F, Shah SJ, McGrath MM, O'Meara E, Wilderang U, Lindholm D, Petersson M, Langkilde AM, McMurray J, Solomon SD. Dapagliflozin in patients recently hospitalized with heart failure and mildly reduced or preserved ejection fraction. J Am Coll Cardiol. 2022; 80: 1302–1310. [DOI] [PubMed] [Google Scholar]
- 33. Cai A, Qiu W, Zhou Y, Feng Y, Chen J, Xia S, Li W, Liao Y, Li X, Zhou J, Wang H, Jin W, Zhang Q, Sun Z, Chen M, Wang J, Kong H, Zhang Y, Dong W, Bai L, Xu D, Yuan J, Liu C, Jiang M, Xu Y, Li L, Dong Y, Yang J. Clinical characteristics and 1‐year outcomes in hospitalized patients with heart failure with preserved ejection fraction: results from the China cardiovascular association database‐heart failure center registry. Eur J Heart Fail. 2022; 24: 2048–2062. [DOI] [PubMed] [Google Scholar]
- 34. Abdelhamid M, Elsisi GH, Seyam A, Shafie A, Kirollos M, Emad S, Mansy S, Sobhy M. Dapagliflozin cost‐effectiveness analysis in heart failure patients in Egypt. J Med Econ. 2022; 25: 450–456. [DOI] [PubMed] [Google Scholar]
- 35. Savira F, Wang BH, Kompa AR, Ademi Z, Owen AJ, Zoungas S, Tonkin A, Liew D, Zomer E. Cost‐effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol. 2021; 28: 975–982. [DOI] [PubMed] [Google Scholar]
- 36. McEwan P, Darlington O, McMurray J, Jhund PS, Docherty KF, Bohm M, Petrie MC, Bergenheim K, Qin L. Cost‐effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: a multinational health‐economic analysis of DAPA‐HF. Eur J Heart Fail. 2020; 22: 2147–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao Y, Zhang R, An T, Zhao X, Zhang J. Cost‐effectiveness of adding dapagliflozin to standard treatment for heart failure with reduced ejection fraction patients in China. ESC HEART FAIL. 2020; 7: 3582–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Krittayaphong R, Permsuwan U. Cost‐utility analysis of combination empagliflozin and standard treatment versus standard treatment alone in Thai heart failure patients with reduced or preserved ejection fraction. Am J Cardiovasc Drugs. 2022; 22: 577–590. [DOI] [PubMed] [Google Scholar]
- 39. Lin X, Lin M, Liu M, Huang W, Nie X, Chen Z, Zheng B. Cost‐effectiveness of empagliflozin as a treatment for heart failure with reduced ejection fraction: an analysis from the Chinese healthcare perspective. J Thorac Dis. 2022; 14: 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liao CT, Yang CT, Kuo FH, Lee MC, Chang WT, Tang HJ, Hua YM, Chang HY, Chen ZC, Strong C, Ou HT, Toh HS. Cost‐effectiveness evaluation of add‐on empagliflozin in patients with heart failure and a reduced ejection fraction from the healthcare system's perspective in the Asia‐Pacific region. Front Cardiovasc Med. 2021; 8: 750381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tang Y, Sang H. Cost‐utility analysis of empagliflozin in heart failure patients with reduced and preserved ejection fraction in China. Front Pharmacol. 2022; 13: 1030642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cai X, Shi L, Yang W, Gu S, Chen Y, Nie L, Ji L. Cost‐effectiveness analysis of dapagliflozin treatment versus metformin treatment in Chinese population with type 2 diabetes. J Med Econ. 2019; 22: 336–343. [DOI] [PubMed] [Google Scholar]
- 43. Gu S, Mu Y, Zhai S, Zeng Y, Zhen X, Dong H. Cost‐effectiveness of dapagliflozin versus acarbose as a monotherapy in type 2 diabetes in China. PLoS ONE. 2016; 11: e165629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McMurray J, Trueman D, Hancock E, Cowie MR, Briggs A, Taylor M, Mumby‐Croft J, Woodcock F, Lacey M, Haroun R, Deschaseaux C. Cost‐effectiveness of sacubitril/valsartan in the treatment of heart failure with reduced ejection fraction. Heart. 2018; 104: 1006–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information.


