Abstract
Aims
Tafamidis improves prognosis in patients with transthyretin amyloid cardiomyopathy (ATTR‐CM). However, real‐world data on the therapeutic effect of tafamidis are lacking. This study aimed to evaluate the clinical course, outcomes, and effectivity monitoring of the therapeutic effect of tafamidis in patients with ATTR‐CM.
Methods and results
This is a single‐centre, retrospective observational study. We evaluated the clinical characteristics and outcomes in 125 consecutive patients with wild‐type ATTR‐CM (ATTRwt‐CM) treated with tafamidis (treatment group) and 55 untreated patients (treatment‐naïve group). We monitored the therapeutic effect of tafamidis for 12 months by evaluating serial cardiac biomarker and imaging findings. The treatment group had significantly more favourable outcome in all‐cause mortality and hospitalization due to heart failure than the treatment‐naïve group in both the entire cohort (P < 0.01) and the propensity score‐matched cohort (P < 0.05). Kaplan–Meier survival curves showed that tafamidis treatment significantly reduced all‐cause mortality (P = 0.03, log‐rank test), with the curves diverging after approximately 18 months of treatment in the propensity score‐matched cohort. On inverse probability of treatment weighting analysis, tafamidis treatment showed a reduced all‐cause mortality [hazard ratio (HR), 0.31; 95% confidence interval (CI), 0.11–0.93; P = 0.04]. High‐sensitivity cardiac troponin T (hs‐cTnT) > 0.05 ng/mL, B‐type natriuretic peptide (BNP) > 250 pg/mL, and estimated glomerular filtration rate (eGFR) < 45 mL/min/1.73 m2 scored 1 point each. Multivariate logistic regression analysis revealed that a high score (2–3 points) was a significantly poor prognostic factor of composite clinical outcomes, including all‐cause death and hospitalization for heart failure (HR, 1.55; 95% CI, 1.22–1.98; P < 0.01) for patients in the treatment group. After 12 months of tafamidis treatment, hs‐cTnT levels decreased significantly [0.054 (0.036–0.082) vs. 0.044 (0.033–0.076); P = 0.002], with no significant changes in BNP levels, echocardiographic parameters, native T1 value, and extracellular volume fraction on cardiac magnetic resonance imaging.
Conclusions
The prognosis of patients with ATTRwt‐CM treated with tafamidis was more favourable than that of untreated patients. Patient stratification combined with biomarkers (hs‐cTnT, BNP, and eGFR) predicted clinical outcomes. hs‐cTnT may be a useful biomarker for evaluating the therapeutic effect of tafamidis.
Keywords: Transthyretin amyloid cardiomyopathy, Tafamidis, Prognosis, Biomarkers, Imaging
Introduction
Transthyretin amyloid cardiomyopathy (ATTR‐CM) is a progressive, restrictive cardiomyopathy characterized by insoluble transthyretin (TTR) amyloid fibrils deposited in the extracellular space of the myocardium. 1 , 2 , 3 ATTR‐CM is classified as hereditary (ATTRv) or wild‐type (ATTRwt‐CM), depending on the presence or absence of transthyretin (TTR) gene mutations, respectively. ATTR‐CM has been considered rare and untreatable, resulting in a lack of disease‐specific treatment. The restrictive physiology, atrial arrhythmias, and conduction defects brought about by ATTR‐CM cause heart failure. ATTR‐CM treatment has been limited to standard heart failure treatment for a long time, resulting in a poor clinical outcome with a median survival of 3–5 years. 4 , 5 , 6
Recently, ATTR‐CM has gained recognition as a hidden cause of heart failure with preserved ejection fraction in the elderly. 7 Furthermore, a non‐invasive diagnostic algorithm using bone scintigraphy has been established, 8 and emerging therapies (TTR stabilizer and TTR gene silencer) have become available that offer potential benefits. 9 , 10 Recently, serial evaluation of imaging parameters has been introduced to monitor the therapeutic effects of disease‐modifying therapies on the progression of cardiac amyloid deposition. 11 , 12
Tafamidis is a TTR stabilizer that binds to the thyroxine‐binding site of TTR and inhibits the dissociation of tetramers into monomers. The Transthyretin Amyloidosis Cardiomyopathy Clinical Trial (ATTR‐ACT) showed reductions in all‐cause mortality, cardiovascular‐related hospitalizations, and a slower decline in quality of life and functional capacity in the tafamidis treatment group compared with placebo. 9 Following this, the Japanese Ministry of Health, Labour and Welfare approved tafamidis for the treatment of ATTR‐CM in Japan on 26 March 2019. However, real‐world data on the therapeutic effect of tafamidis are lacking.
In this study, we evaluated the clinical characteristics and outcomes of tafamidis treatment in patients with ATTRwt‐CM. The therapeutic effect of tafamidis was monitored for 12 months by evaluating serial cardiac biomarker and imaging findings and compared with those in treatment‐naïve patients at our referral centre.
Methods
Patient selection criteria
This is a single‐centre, retrospective observational study involving patients who were diagnosed with ATTRwt‐CM between January 2002 and September 2022 at our institute and patients with ATTRwt‐CM referred from other hospitals for possible tafamidis treatment. We excluded (i) participants in clinical trials of novel ATTRwt‐CM treatments, (ii) patients with preclinical ATTRwt‐CM who did not meet the criteria for tafamidis administration in Japan, 13 (iii) patients who were waiting for the certification of grant recipient approval for patients with intractable diseases, and (iv) patients who were prescribed tafamidis from other hospital. The tafamidis treatment group comprised patients who received tafamidis for ATTRwt‐CM treatment, and the tafamidis‐naïve group comprised patients who did not receive tafamidis.
Ethical considerations
The study conforms to the principles outlined in the Declaration of Helsinki and was approved by the Institutional Review Board and Ethics Committee of our university (Approval No. 1590). The requirement for informed consent was waived because of the low risk of this retrospective study and the inability to obtain consent directly from all participants. We extensively promoted this study protocol at our university hospital and on our website and allowed patients to withdraw from the study.
Collection of clinical data and follow‐up
Data regarding demographic characteristics, comorbidities, laboratory results, electrocardiography, echocardiography, and medication were obtained at the start of tafamidis treatment for the treatment group and at diagnosis among patients who died or were lost to follow‐up before tafamidis approval or evaluation of the indications for tafamidis treatment for the treatment‐naïve group. The observation period began after tafamidis administration in the treatment group and at the time of diagnosis or evaluation of the indications for tafamidis treatment in the treatment‐naïve group. Tafamidis recipients were followed up every 6 or 12 months at our institution for cardiovascular and adverse events. The primary endpoint was all‐cause death, and the secondary endpoint was hospitalization due to heart failure. Mortality and hospitalization due to heart failure were identified through medical records and confirmed using questionnaires and direct contact via telephonic interviews with the attending physician, patient, or, if deceased, a family member. All deaths were reviewed, and the cause was classified as cardiovascular or non‐cardiovascular. Cardiovascular death was defined as death due to worsening heart failure, cardiovascular events, or sudden death. Hospitalization for heart failure was defined as unexpected hospitalization for heart failure. Patients in whom tafamidis treatment was discontinued were followed up until the all‐cause death occurred or until the last visit.
Indications for tafamidis treatment in patients with wild‐type transthyretin amyloid cardiomyopathy
The Ministry of Health, Labour and Welfare provided a statement regarding the appropriate use of tafamidis in Japan, for which all items must be present. 13
We considered the following cases to be unsuitable for tafamidis treatment: (i) New York Heart Association (NYHA) class IV, (ii) severe renal dysfunction [estimated glomerular filtration rate (eGFR) < 25 mL/min/1.73 m2], (iii) frequent hospitalization for worsening heart failure requiring inotropes (≥2 per year), (iv) incomprehension of the purpose of tafamidis treatment, (v) severe frailty (clinical frailty scale score > 7: completely dependent for personal care), and (vi) expected life expectancy < 1 year (e.g. advanced cancer). Indications for tafamidis treatment and appropriate treatment strategies were discussed with the heart team considering the patients' clinical conditions, wishes, and social backgrounds.
Diagnosis of wild‐type transthyretin amyloid cardiomyopathy
Amyloid deposition was diagnosed based on Congo red staining and apple‐green birefringence using cross‐polarized light microscopy. We performed immunohistochemical staining using antibodies against TTR to confirm TTR amyloid deposition. We diagnosed ATTRwt based on the absence of TTR gene mutations.
Transthyretin amyloid cardiomyopathy was diagnosed by (i) the presence of TTR deposits in the myocardium; (ii) the presence of TTR deposits in extracardiac tissues, such as subcutaneous tissue or the gastrointestinal tract, with positive findings on 99mTc‐labelled pyrophosphate (99mTc‐PYP) scintigraphy; or (iii) positive findings on 99mTc‐PYP scintigraphy without confirmation of pathological TTR deposition and exclusion of light‐chain amyloidosis using serum and urine protein electrophoresis (immunofixation electrophoresis) and serum‐free light‐chain assay (Freelite, The Binding Site Group Ltd., UK).
Biomarker and imaging analysis
Serum high‐sensitivity cardiac troponin T (hs‐cTnT) levels were measured using the Elecsys 2010 Troponin T HS kit (Roche Diagnostics, Indianapolis, IN, USA). hs‐cTnT values were missing in patients diagnosed before the approval of the high‐sensitivity assay. Plasma B‐type natriuretic peptide (BNP) levels were measured using the MI02 Shionogi BNP kit (Abbott Japan, Matsudo, Japan). The glomerular filtration rate was calculated using the Modification of Diet in Renal Disease modified for Japanese patients. The biomarker cut‐off values were >0.05 ng/mL for hs‐cTnT, >250 pg/mL for BNP, and <45 mL/min/1.73 m2 for eGFR, based on a staging system proposed by a previous study using biomarkers. 14 We calculated the biomarker scores by adding 1 point if hs‐cTnT and BNP levels increased to above the cut‐off values or if the eGFR decreased to below the cut‐off value, thereby dividing the patients into a low (0–1 point)‐score or high (2–3 points)‐score group.
Chamber size and wall thickness were measured using transthoracic echocardiography. The left ventricular ejection fraction was calculated using the modified Simpson's method. All echocardiographic images were stored in Digital Imaging and Communications in Medicine format on an echo management information system and retrieved later for offline global longitudinal strain (GLS) analysis.
Serial evaluation of extracellular volume fraction (ECV) and native T1 value obtained by cardiac magnetic resonance imaging (CMR) was conducted on a limited number of patients to determine the treatment effect of tafamidis.
Statistical analyses
Normally distributed parameters are expressed as mean ± standard deviation, whereas non‐normally distributed parameters are expressed as medians with interquartile ranges. Categorical values are presented as numbers (percentages) and compared using the chi‐square test or Fisher's exact test. Differences between the groups were examined using Student's t‐test or the Mann–Whitney U test for unpaired data or the paired t‐test or Wilcoxon's signed‐rank test for paired data. Kaplan–Meier curves for all‐cause mortality and hospitalization for heart failure were compared between the treatment and treatment‐naïve groups. Univariate Cox hazards analyses were performed to identify parameters that were significantly related to all‐cause mortality and the composite clinical outcomes (all‐cause mortality or hospitalization for heart failure). Multivariate Cox hazards analysis was performed using the forced‐entry method. Biomarkers and risk scores were not entered into the multivariate analysis simultaneously due to their strong intercorrelation. In addition, to reduce the effect of treatment selection bias and possible confounders, we performed adjustments for significant differences in patient baseline characteristics using propensity score matching. The predicted probability of receiving tafamidis was calculated by applying a logistic regression model, using all clinically relevant variables: age, sex, hs‐cTnT, BNP, eGFR, and haemoglobin. A participant of the tafamidis treatment group was matched with one participant in the tafamidis‐naïve group using nearest‐neighbour matching within a calliper width of 0.20 standard deviation without replacement. A comparison of the baseline characteristics between the treatment and treatment‐naïve groups in the entire and matched populations was performed using the standardized difference, whereby an absolute standardized difference above 0.10 represents meaningful imbalance. In addition, to confirm the robustness of the results, inverse probability of treatment weighting with the same predicted probability used in the propensity score matching was performed as sensitivity analysis for the same outcome in patients with ATTRwt‐CM. A two‐tailed value of P < 0.05 was considered significant. All statistical analyses were performed using IBM SPSS Statistics for Windows (Version 19.0; IBM Corp., Armonk, NY, USA) and R (Version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Study population
Figure 1 shows the flowchart for participant selection. A total of 192 consecutive patients with ATTRwt‐CM were diagnosed at our department between January 2002 and September 2022; a further 20 patients were referred by another hospital. We excluded 32 patients according to the aforementioned criteria. Finally, 125 patients were included in the tafamidis treatment group. Our institution participated in ATTR‐ACT (Registration No. NCT01994889) 9 and a long‐term extension study (Registration No. NCT02791230) 15 ; therefore, this study included 13 patients who were prescribed tafamidis before its approval in Japan.
Figure 1.
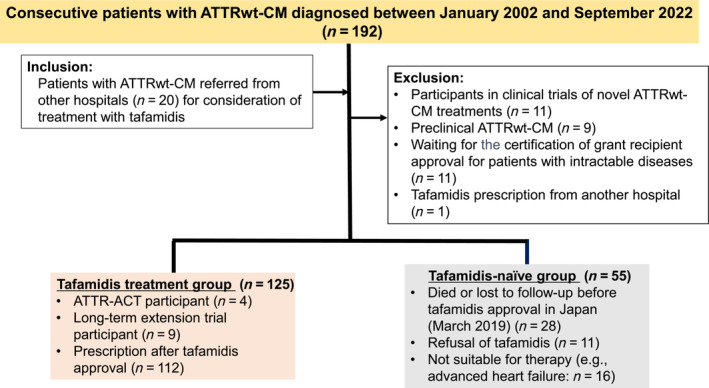
Flowchart for participant selection. ATTR‐ACT, Transthyretin Amyloidosis Cardiomyopathy Clinical Trial; ATTRwt‐CM, wild‐type transthyretin amyloid cardiomyopathy.
Among the ATTR‐ACT participants, three received placebos, two received tafamidis meglumine 80 mg, and two received tafamidis meglumine 20 mg from the first half of 2015. Tafamidis meglumine 20 mg was increased to tafamidis free acid 61 mg, and tafamidis meglumine 80 mg was changed to tafamidis free acid 61 mg on enrolment in the long‐term extension study from January 2019. Nine long‐term extension study participants received tafamidis free acid 61 mg from December 2018. After approval of tafamidis for the treatment of ATTR‐CM in Japan, tafamidis free acid 61 mg was changed to tafamidis meglumine 80 mg.
In total, 55 patients with ATTRwt‐CM were not treated with tafamidis (treatment‐naïve group). Among them, 28 patients died or were lost to follow‐up before tafamidis was approved, including 3 ATTR‐ACT participants who received placebo, 11 who refused treatment because of medical costs or advanced age, and 16 who were considered unsuitable for therapy.
Patient characteristics
Among the 125 patients in the treatment group, 36 patients (29%) were diagnosed before tafamidis approval, and the median duration from diagnosis to tafamidis initiation was 22.6 (range, 3–60) months in these patients. In the treatment‐naïve group (n = 55), 40 (73%) were diagnosed before tafamidis approval, 28 (51%) died or were lost to follow‐up before tafamidis approval in Japan, and the median duration from diagnosis to consideration for tafamidis treatment was 19 (range, 2–67) months. The remaining patients were diagnosed after tafamidis approval.
The baseline characteristics of the patients in each group are summarized in Table 1 . Patients in the treatment‐naïve group were significantly older; had higher hs‐cTnT, BNP, and creatinine levels; and had lower haemoglobin levels than those in the treatment group. On biomarker staging, significantly fewer patients in the high‐score group were observed in the treatment group than in the treatment‐naïve group (37% vs. 63%; P = 0.002).
Table 1.
Baseline characteristics of patients in each group
| Entire cohort (n = 180) | Propensity score‐matched cohort (n = 66) | ||||||
|---|---|---|---|---|---|---|---|
| Treatment group (n = 125) | Treatment‐naïve group (n = 55) | P value | Treatment group (n = 33) | Treatment‐naïve group (n = 33) | P value | SMD | |
| Age, years | 75.6 ± 5.3 | 78.9 ± 6.4 | <0.01 | 77.3 ± 6.8 | 77.0 ± 5.6 | 0.86 | 0.04 |
| Male, n (%) | 110 (88%) | 48 (87%) | 0.89 | 30 (91%) | 27 (82%) | 0.48 | 0.27 |
| Atrial fibrillation, n (%) | 69 (55%) | 29 (53%) | 0.76 | 17 (52%) | 15 (46%) | 0.81 | |
| Prior HF hospitalizations, n (%) | 44 (35%) | 25 (46%) | 0.19 | 12 (36%) | 15 (46%) | 0.62 | |
| NYHA class, n (%) | |||||||
| I | 16 (13%) | 7 (13%) | 5 (15%) | 5 (15%) | |||
| II | 68 (54%) | 25 (46%) | 16 (49%) | 18 (55%) | |||
| III | 41 (33%) | 22 (40%) | 12 (36%) | 9 (27%) | |||
| IV | 0 (0%) | 1 (2%) | 0 (0%) | 1 (3%) | |||
| NYHA class ≥ III, n (%) | 41 (33%) | 23 (42%) | 0.24 | 12 (36%) | 10 (30%) | 0.60 | |
| Blood testing | |||||||
| hs‐cTnT, ng/mL |
0.055 (0.037–0.082) (n = 124) |
0.069 (0.041–0.112) (n = 49) |
0.03 |
0.067 (0.043–0.102) (n = 33) |
0.052 (0.036–0.091) (n = 33) |
0.27 | 0.18 |
| hs‐cTnT > 0.05 ng/mL, n/total (%) | 68/124 (55%) | 33/49 (67%) | 0.13 | 24/33 (73%) | 18/33 (69%) | 0.13 | |
| BNP, pg/mL | 187 (112–282) | 329 (211–475) | <0.01 | 246 (208–469) | 274 (157–427) | 0.82 | 0.09 |
| BNP > 250 pg/mL, n (%) | 38 (30%) | 37 (67%) | <0.01 | 16 (49%) | 19 (58%) | 0.46 | |
| Sodium, mEq/L | 139.5 ± 2.6 | 139.6 ± 2.7 | 0.76 | 139.3 ± 2.6 | 139.7 ± 2.7 | 0.52 | |
| Creatinine, mg/dL | 1.09 ± 0.30 | 1.24 ± 0.71 | 0.049 | 1.08 ± 0.22 | 1.10 ± 0.31 | 0.70 | |
| eGFR, mL/min/1.73 m2 | 52.7 ± 14.0 | 48.6 ± 16.6 | 0.09 | 52.1 ± 11.2 | 50.9 ± 13.4 | 0.70 | 0.10 |
| eGFR < 45 mL/min/1.73 m2, n (%) | 37 (30%) | 24 (44%) | 0.07 | 9 (27%) | 11 (33%) | 0.59 | |
| Haemoglobin, g/dL | 13.9 ± 1.8 | 12.9 ± 1.7 | 0.001 | 13.7 ± 1.9 | 13.2 ± 1.7 | 0.21 | 0.31 |
| Risk score (0/1/2/3) | 43/35/30/16 (n = 124) | 6/12/19/12 (n = 49) | 7/10/9/7 (n = 33) | 6/11/11/5 (n = 33) | |||
| High‐score group (risk score ≥ 2), n/total (%) | 46/124 (37%) | 31/49 (63%) | 0.002 | 16/33 (49%) | 16/33 (63%) | 1.00 | |
| Echocardiogram parameters | |||||||
| LVDd, mm | 40.9 ± 5.6 | 43.0 ± 7.7 | 0.048 | 42.8 ± 8.4 | 41.8 ± 4.6 | 0.53 | |
| LVDs, mm | 32.0 ± 6.5 | 32.1 ± 7.3 | 0.96 | 31.6 ± 7.5 | 33.0 ± 5.9 | 0.39 | |
| IVSd, mm | 15.8 ± 2.3 | 16.1 ± 3.0 | 0.44 | 16.2 ± 2.2 | 16.3 ± 3.3 | 0.90 | |
| LVPWd, mm | 16.1 ± 2.7 | 15.9 ± 3.0 | 0.66 | 16.2 ± 1.7 | 16.2 ± 3.5 | 0.94 | |
| LVEF, % | 50.4 ± 10.2 | 50.5 ± 10.7 | 0.92 | 48.9 ± 10.2 | 51.6 ± 10.8 | 0.29 | |
| LV‐GLS (%) | −8.5 ± 2.8 | −8.9 ± 3.0 | 0.41 | −8.7 ± 2.7 | −9.4 ± 3.4 | 0.43 | |
| Apical LS (%) | −12.7 ± 4.3 | −13.6 ± 5.2 | 0.26 | −13.4 ± 4.8 | −14.2 ± 5.4 | 0.50 | |
| Middle LS (%) | −6.9 ± 3.9 | −7.2 ± 3.7 | 0.60 | −7.0 ± 4.5 | −7.3 ± 4.4 | 0.79 | |
| Basal LS (%) | −4.8 ± 2.7 | −4.5 ± 2.7 | 0.58 | −4.3 ± 2.0 | −5.0 ± 3.1 | 0.33 | |
| CMR parameters | |||||||
| ECV (%) | 57.3 ± 12.9 (n = 76) | 54.0 ± 14.8 (n = 13) | 0.42 | 61.3 ± 14.1 (n = 15) | 49.9 ± 11.2 (n = 10) | 0.04 | |
| Native T1 value (ms) | 1426 ± 54 (n = 82) | 1424 ± 50 (n = 14) | 0.87 | 1429 ± 39 (n = 16) | 1421 ± 53 (n = 11) | 0.74 | |
| Medications | |||||||
| RAS‐I, n (%) | 64 (51%) | 30 (55%) | 0.68 | 15 (46%) | 17 (52%) | 0.62 | |
| MRA, n (%) | 43 (34%) | 21 (38%) | 0.63 | 13 (39%) | 11 (33%) | 0.61 | |
| Beta‐blockers, n (%) | 27 (22%) | 17 (31%) | 0.18 | 7 (21%) | 10 (30%) | 0.40 | |
| Diuretics, n (%) | 97 (78%) | 49 (89%) | 0.07 | 27 (82%) | 29 (88%) | 0.49 | |
Note: Data are expressed as the median (interquartile range), mean ± standard deviation, or n (%).
Abbreviations: BNP, B‐type natriuretic peptide; CMR, cardiac magnetic resonance imaging; ECV, extracellular volume fraction; eGFR, estimated glomerular filtration rate; HF, heart failure; hs‐cTnT, high‐sensitivity cardiac troponin T; IVSd, interventricular septum diameter; LS, longitudinal strain; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LVPWd, left ventricular posterior wall diameter; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; RAS‐I, renin‐angiotensin system inhibitors; SMD, standardized mean difference.
Adverse events and clinical outcomes
The tafamidis meglumine dose was reduced from 80 to 20 mg in one patient because of gastrointestinal symptoms. The remaining patients received tafamidis meglumine 80 mg. Nineteen patients (15%) discontinued treatment because of death (n = 9), worsening heart failure (n = 2), gastrointestinal symptoms (n = 3), itching (n = 1), difficulty in taking medicine (n = 1), advanced pancreatic cancer (n = 1), intracerebral haemorrhage (n = 1), and concerns about high medical costs (n = 1).
Table 2 presents the clinical outcomes in each group. During the median follow‐up period of 21 (range, 0–90) months, 10 (8%) patients died and 21 (17%) were hospitalized for heart failure in the treatment group. In the treatment‐naïve group, during the median follow‐up period of 27 (range, 2–146) months, 40 patients (73%) died and 37 (67%) were hospitalized for heart failure.
Table 2.
Clinical outcomes in patients with wild‐type transthyretin amyloid cardiomyopathy in each group
| Entire cohort (n = 180) | Propensity score‐matched cohort (n = 66) | |||
|---|---|---|---|---|
| Treatment group (n = 125) | Treatment‐naïve group (n = 55) | Treatment group (n = 33) | Treatment‐naïve group (n = 33) | |
| Primary endpoint: All‐cause death | 10 (8%) | 40 (73%) | 6 (18%) | 22 (67%) |
| Cardiovascular death | 9 | 35 | 6 | 19 |
| Non‐cardiovascular death | 1 | 5 | 0 | 3 |
| Secondary endpoint: Heart failure hospitalization | 21 (17%) | 37 (67%) | 10 (30%) | 22 (67%) |
| Median (IQR) follow‐up duration (months) | 21 (10–31) | 27 (16–48) | 35 (24–40) | 40 (20–56) |
Abbreviation: IQR, interquartile range.
Kaplan–Meier analyses illustrated the period of overall survival and period without hospitalization for heart failure. The treatment group showed significantly more favourable clinical outcomes than the treatment‐naïve group (P < 0.001, log‐rank test; Figure 2 A,B ). The 12 and 24 month survival rates for the treatment group were 93% and 86%, respectively.
Figure 2.
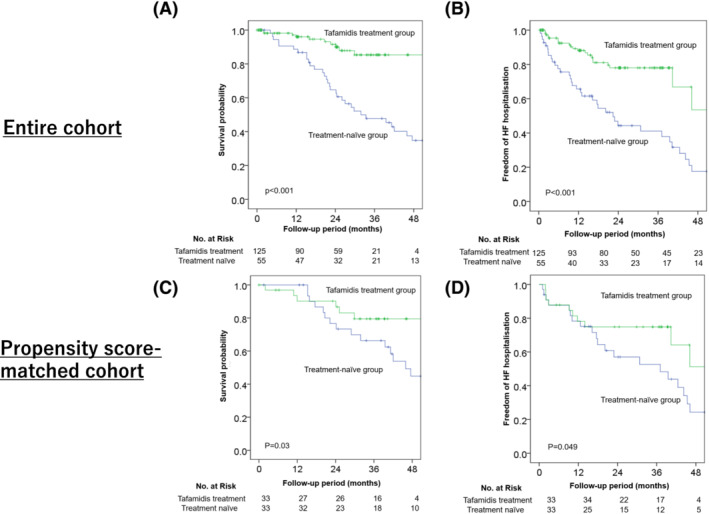
Comparison of all‐cause mortality (A, C) and hospitalization for heart failure (HF) (B, D) between the treatment and treatment‐naïve groups in the entire and propensity score‐matched cohorts. Kaplan–Meier analyses show that the treatment group has a significantly more favourable clinical outcome than the treatment‐naïve group in the entire and propensity score‐matched cohorts.
Propensity score‐matched analysis
A propensity score‐matched cohort comprising 66 patients with ATTRwt‐CM receiving treatment with or without tafamidis was constructed (Table 1 ). Table 2 shows the clinical outcomes. Kaplan–Meier survival curves showed that tafamidis treatment significantly reduced all‐cause mortality, with the curves diverging after approximately 18 months of treatment (P = 0.03, log‐rank test; Figure 2 C ). According to the univariate Cox regression analysis, all‐cause mortality was significantly lower in the tafamidis group than in the treatment‐naïve group [hazard ratio (HR), 0.37; 95% confidence interval (CI), 0.15–0.92; P = 0.03]. On inverse probability of treatment weighting analysis, tafamidis treatment reduced all‐cause mortality (HR, 0.31; 95% CI, 0.10–0.93; P = 0.04) in patients with ATTRwt‐CM.
Similarly, Kaplan–Meier survival curves showed that tafamidis significantly reduced hospitalization for heart failure (P = 0.049, log‐rank test; Figure 2 D ). According to the univariate Cox regression analysis, heart failure hospitalization rate was lower in the tafamidis group than in the treatment‐naïve group (HR, 0.48; 95% CI, 0.22–1.01; P = 0.054).
Prognostic factors in patients undergoing treatment
To identify the prognostic factors in the patients receiving tafamidis, composite clinical outcomes were evaluated (all‐cause death or hospitalization for heart failure). Univariate Cox proportional hazards analysis showed that NYHA functional class ≥ III, prior heart failure hospitalization, hs‐cTnT > 0.05 ng/mL, BNP > 250 pg/mL, eGFR < 45 mL/min/1.73 m2, being in the high‐score group, and impaired GLS were significant poor prognostic factors (P < 0.05). According to the multivariate Cox proportional hazards analysis, prior heart failure hospitalization (HR, 4.75; 95% CI, 1.39–16.22; P = 0.01) and being in the high‐score group (HR, 1.55; 95% CI, 1.22–1.98; P < 0.01) were significant poor prognostic factors (Table 3 ).
Table 3.
Univariate and multivariate Cox hazards analyses of predictors for composite clinical outcomes in the treatment group
| Variables | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age (years) | 1.07 | 0.99–1.16 | 0.10 | 1.08 | 0.99–1.18 | 0.07 |
| Female (yes) | 2.15 | 0.49–9.43 | 0.31 | |||
| NYHA class ≥ III | 4.81 | 1.85–12.54 | <0.01 | 2.31 | 0.66–8.11 | 0.19 |
| Atrial fibrillation (yes) | 1.24 | 0.53–2.90 | 0.63 | |||
| Prior HF hospitalizations | 6.08 | 2.34–15.84 | <0.01 | 4.75 | 1.39–16.22 | 0.01 |
| hs‐cTnT > 0.05 ng/mL | 1.30 | 1.09–1.56 | 0.004 | |||
| BNP > 250 pg/mL | 3.27 | 1.39–7.69 | 0.007 | |||
| eGFR < 45 mL/min/1.73 m 2 | 3.47 | 1.49–8.06 | 0.004 | |||
| High‐score group (risk score ≥ 2) | 1.33 | 1.13–1.56 | <0.01 | 1.55 | 1.22–1.98 | <0.01 |
| Haemoglobin (mg/dL) | 0.93 | 0.73–1.18 | 0.55 | |||
| LVEF (%) | 0.97 | 0.93–1.01 | 0.08 | |||
| Absolute GLS (%) | 0.80 | 0.67–0.95 | 0.01 | 0.33 | 0.73–1.11 | 0.33 |
Note: Bold indicates significant factors.
Abbreviations: BNP, B‐type natriuretic peptide; CI, confidence interval; eGFR, estimated glomerular filtration rate; GLS, global longitudinal strain; HF, heart failure; HR, hazard ratio; hs‐cTnT, high‐sensitivity cardiac troponin T; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Comparison of clinical data before and after 12 months with or without treatment
Table 4 shows the changes in biomarker and imaging parameters in patients with (n = 85) and without (n = 33) treatment for whom clinical data could be obtained after 12 months. Among the treatment group, hs‐cTnT levels were significantly reduced [0.054 (0.036–0.082) ng/mL vs. 0.044 (0.033–0.076) ng/mL; P = 0.002], whereas no significant differences were noted in the BNP levels, and eGFRs declined significantly. Echocardiography did not confirm any improvement in left ventricular hypertrophy and GLS. Serial CMR was performed in only 15 patients before and after treatment. The median time lags from the first CMR to treatment initiation and to the second CMR were 4 and 14 months, respectively. No significant differences were found in the native T1 values (normal values in our institution: 1200–1260 ms; 1414 ± 54 vs. 1421 ± 64 ms; P = 0.44) and ECV (52.2 ± 11.2% vs. 53.2 ± 11.7%; P = 0.44) in the first and second CMR, respectively.
Table 4.
Comparison of serial biomarker and imaging parameters in the treatment and treatment‐naïve groups
| Treatment group (n = 85) | Treatment‐naïve group (n = 33) | |||||
|---|---|---|---|---|---|---|
| Baseline | After 12 months | P value | Baseline | After 12 months | P value | |
| Biomarkers | ||||||
| hs‐cTnT, ng/mL | 0.054 (0.036–0.082) a | 0.044 (0.033–0.076) a | 0.002 | 0.050 (0.036–0.082) b | 0.068 (0.051–0.081) b | 0.15 |
| BNP, pg/mL | 210 (114–295) | 175 (120–306) | 0.59 | 329 (223–486) | 329 (168–546) | 0.68 |
| eGFR, mL/min/1.73 m2 | 53.0 ± 13.8 | 50.4 ± 14.5 | 0.001 | 47.8 ± 13.9 | 41.3 ± 15.1 | 0.004 |
| Echocardiogram parameters | ||||||
| LVDd, mm | 41.3 ± 5.0 | 41.6 ± 5.0 | 0.31 | 43.7 ± 7.3 c | 42.0 ± 6.8 c | 0.03 |
| LVDs, mm | 32.1 ± 6.2 | 32.6 ± 6.3 | 0.20 | 32.0 ± 7.2 c | 31.1 ± 6.8 c | 0.12 |
| IVSd, mm | 15.9 ± 2.3 | 15.7 ± 2.1 | 0.32 | 15.3 ± 2.2 c | 16.2 ± 2.0 c | 0.04 |
| LVPWd, mm | 16.0 ± 2.7 | 16.2 ± 2.8 | 0.45 | 15.3 ± 2.3 c | 15.9 ± 2.6 c | 0.16 |
| LVEF, % | 50.3 ± 10.6 | 50.1 ± 10.9 | 0.78 | 52.3 ± 9.8 c | 51.0 ± 10.1 c | 0.27 |
| LV‐GLS (%) | −8.3 ± 2.7 | −8.4 ± 2.8 | 0.76 | −9.8 ± 2.8 c | −9.3 ± 2.5 c | 0.33 |
| Apical LS (%) | −12.3 ± 4.1 | −13.0 ± 4.4 | 0.20 | −14.3 ± 4.7 c | −12.2 ± 6.2 c | 0.09 |
| Middle LS (%) | −6.9 ± 3.6 | −6.9 ± 2.7 | 0.95 | −8.8 ± 3.0 c | −8.7 ± 3.0 c | 0.90 |
| Basal LS (%) | −4.7 ± 2.7 | −4.3 ± 2.9 | 0.13 | −4.7 ± 2.7 c | −5.1 ± 2.7 c | 0.35 |
| CMR parameters | ||||||
| Native T1 value (ms) | 1414 ± 54 d | 1421 ± 64 d | 0.44 | No data | No data | |
| ECV (%) | 52.2 ± 11.2 d | 53.2 ± 11.7 d | 0.44 | No data | No data | |
Abbreviations: BNP, B‐type natriuretic peptide; CMR, cardiac magnetic resonance imaging; ECV, extracellular volume fraction; eGFR, estimated glomerular filtration rate; hs‐cTnT, high‐sensitivity cardiac troponin T; IVSd, interventricular septum diameter; LS, longitudinal strain; LVDd, left ventricular diastolic diameter; LVDs, left ventricular systolic diameter; LVEF, left ventricular ejection fraction; LV‐GLS, left ventricular global longitudinal strain; LVPWd, left ventricular posterior wall diameter.
n = 81.
n = 19.
n = 31.
n = 15.
Evaluation of serial biomarkers and echocardiographic parameters in the treatment‐naïve group at baseline and 12 months later revealed significant differences in the eGFR, left ventricular diastolic diameter, and interventricular septum diameter. hs‐cTnT levels tended to increase, whereas apical longitudinal strain tended to worsen.
The NYHA functional classes in the treatment and treatment‐naïve groups at baseline and after 12 months were as follows:
treatment group: NYHA class I/II/III/IV, 12/44/29/0 (baseline) changed to 11/43/29/2 (after 12 months); and
treatment‐naïve group: NYHA class I/II/III/IV, 9/11/7/0 (baseline) changed to 6/8/13/0 (after 12 months).
The rate of worsening of the NYHA functional class, defined as a deterioration of one or more in the NYHA functional class, was significantly lower in the treatment group (n = 11; 13%) than in the treatment‐naïve group (n = 8; 30%) (P = 0.04).
Discussion
Our study retrospectively evaluated the single‐centre experience of tafamidis treatment in 125 consecutive patients with ATTRwt‐CM in Japan. The clinical outcomes of patients with ATTRwt‐CM on tafamidis were favourable compared with those of patients not on tafamidis (in both the entire and propensity score‐matched cohorts). A simple staging system was useful in predicting the clinical outcomes in treated patients. After 12 months of treatment, hs‐cTnT levels decreased significantly in the treatment group. Our data provided a real‐world clinical experience with a relatively large number of patients, giving an insight into the routine practice of tafamidis treatment.
Compared with the baseline characteristics of the tafamidis‐treated patients with ATTRwt‐CM who were registered in ATTR‐ACT, 16 the age of the patients in our treatment group was similar (75.5 ± 6.7 vs. 75.6 ± 5.3 years old) and NYHA functional class ≥ III was more frequent in our study (24% vs. 33%). Compared with the tafamidis‐treated ATTR‐ACT participants, 9 there were more patients (i) on diuretics (66% vs. 78%), (ii) with less left ventricular hypertrophy (interventricular septum diameter: 16.7 ± 3.8 vs. 15.8 ± 2.3 mm), and (iii) with a more impaired GLS (−9.3 ± 3.5% vs. −8.5 ± 2.8%) among our study patients. We did not evaluate N‐terminal pro‐BNP levels and 6 min walk test distance; therefore, changes in natriuretic peptide levels and functional capacity could not be assessed.
The prognosis of patients on tafamidis was more favourable than that of patients in the treatment‐naïve group in the early‐phase analysis (<18 months), which could be attributed to the differences in baseline characteristics—severity of heart failure, frailty, and comorbidities. In this study, the survival rates of the patients were similar to those of patients with ATTRwt‐CM on tafamidis therapy according to further analyses of ATTR‐ACT 16 : The 12 and 24 month survival rates were 95% and 91%, respectively. This suggests that the prognosis of the treatment group in our clinical practice was consistent with that in the clinical trial. In our propensity score‐matched cohort, the treatment group showed a more favourable prognosis than the treatment‐naïve group after adjustment for confounding factors. Interestingly, a survival benefit was seen after 18 months in the Kaplan–Meier curves, which was similar to the results of the all‐cause mortality in ATTR‐ACT 9 and sub‐analyses on ATTRwt‐CM. 16 This suggests that the indications for tafamidis treatment have to be considered in patients with a life expectancy of >18 months.
Ochi et al. 17 reported the early experience of tafamidis in patients with ATTRwt‐CM in Kochi University (n = 38). Compared with our patients, their patients were older (78.4 ± 5.9 vs. 75.6 ± 5.3 years old) and had higher rates of prior heart failure hospitalization (53% vs. 35%) and NYHA functional class ≤ II (92% vs. 67%). However, the baseline BNP and hs‐cTnT levels were similar [BNP: 231 (164–353) vs. 206 (114–292) pg/mL; hs‐cTnT: 0.057 (0.038–0.072) vs. 0.057 (0.037–0.083) ng/mL]. The 1 year survival rate was favourable in both studies (92% vs. 96%). No deterioration in BNP levels and echocardiographic parameters was noted with short‐term tafamidis treatment, consistent with our results. These findings suggested that tafamidis prevents the progression of heart failure and cardiac involvement in Japanese patients with ATTRwt‐CM.
The indications for tafamidis use and methods for evaluating ATTR‐CM progression are important considerations. In ATTR‐ACT, the NYHA classification was used for the evaluation of heart failure severity, which is often difficult to estimate in ATTR‐CM because of comorbidities such as orthopaedic diseases, frailty, and respiratory dysfunction in the elderly. 18 Being objective and reproducible, biomarkers are useful tools to evaluate and identify the clinical stages that demonstrate ATTR‐CM progression and prognosis. 19 , 20 A simple staging system, which combines hs‐cTnT, BNP, and eGFR, was reported to be useful for predicting the prognosis in Japanese patients with ATTRwt‐CM. 14 Multivariate analysis showed that this staging system, not NYHA class ≥ III alone, was a significant predictor of prognosis in tafamidis‐treated patients with ATTRwt‐CM. This system can objectively evaluate patient suitability for this treatment. Future prospective external cohort studies are needed to verify the usefulness of this system.
Recently, CMR has been reported to be a useful method for monitoring the progression of amyloid load and the therapeutic effect of disease‐modifying therapies in ATTR‐CM 11 , 12 , 21 , 22 : No significant changes were noted in the native T1 values and ECV in patients treated with tafamidis, although untreated patients showed increased ECV. As CMR is mainly performed in specialized facilities, it is not applicable to routine clinical practice. Additionally, it is difficult to confirm the therapeutic effect if significant parametric changes are not observed during the course of treatment. Therefore, biomarkers would provide a relatively simpler and more reproducible method to determine therapeutic effects. 23
Our data showed that hs‐cTnT levels significantly decreased in the treatment group and increased in the treatment‐naïve group, suggesting its utility in assessing the effectiveness of treatment. In ATTR‐ACT, the change in the least squares mean (standard error) from baseline to Month 30 in troponin I concentrations for pooled tafamidis (80 and 20 mg doses together) compared with placebo was −0.09 (0.023) (P = 0.0002). This suggests that tafamidis may suppress the exacerbation of myocardial damage. 24 Although the exact mechanism responsible for cardiac troponin elevation in patients with cardiac amyloidosis remains unclear, various reasons have been proposed, including myocardial ischaemia, increased wall stress, coronary microvascular dysfunction, and cytotoxic effects of pre‐amyloidotic oligomers. 25 , 26 , 27 , 28 , 29 These mechanisms are enhanced in cardiac amyloidosis and boost cardiac troponin release through myocardial necrosis, apoptosis, and degradation. Tafamidis may suppress additional accommodation of TTR amyloid fibrils in the myocardium, thus relieving the above‐mentioned mechanisms and ongoing myocardial damage.
This study has some limitations. First, being a retrospective, single‐centre study with a short treatment duration, the long‐term effects on cardiac structure and function could not be assessed. Nonetheless, to the best of our knowledge, this is the only real‐world, large‐scale study on the use of tafamidis to treat ATTRwt‐CM. Second, serial evaluation of cardiac biomarkers, imaging parameters (particularly CMR), and NYHA functional class was conducted only in few patients, and serial data regarding objective indicators, such as the distance walked on the 6 min walk test (functional capacity) and the Kansas City Cardiomyopathy Questionnaire—Overall Summary score (quality of life), were unavailable. Large studies are warranted to clarify a robust strategy for monitoring the therapeutic effect of disease‐modifying therapies. Third, the propensity score‐matched cohort size was small and potential confounding factors and relevant selection bias may exist even after adjustment by propensity score‐matched analysis. As the calliper width was set at 0.20 for propensity score matching, this method may be less suitable for comparison of heterogeneous groups. Finally, the selected biomarker cut‐off values, utility of our staging system, and prognostic value of tafamidis‐induced changes in hs‐cTnT levels were not validated using large‐scale external data among tafamidis‐treated patients with ATTRwt‐CM.
In conclusion, the prognosis of tafamidis‐treated patients with ATTRwt‐CM was favourable compared with that of untreated patients. Patient stratification combined with biomarkers predicted favourable prognosis in patients treated with tafamidis. hs‐cTnT levels may be a useful biomarker for evaluating the therapeutic effects of tafamidis. Larger studies with a longer follow‐up are required to elucidate the utility of serial measurements of biomarkers for monitoring the therapeutic effect of disease‐modifying therapy in patients with ATTR‐CM and the association with changes in imaging parameters and functional capacity.
Conflict of interest
S.T., H.U., M.U., and K.T. have received consulting fees or honoraria and remuneration for lectures from Pfizer Japan Inc. The remaining authors have no conflicts of interest to declare.
Funding
This work was supported by JSPS KAKENHI (grant number 21K08131).
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Takashio, S. , Morioka, M. , Ishii, M. , Morikawa, K. , Hirakawa, K. , Hanatani, S. , Oike, F. , Usuku, H. , Kidoh, M. , Oda, S. , Yamamoto, E. , Matsushita, K. , Ueda, M. , and Tsujita, K. (2023) Clinical characteristics, outcome, and therapeutic effect of tafamidis in wild‐type transthyretin amyloid cardiomyopathy. ESC Heart Failure, 10: 2319–2329. 10.1002/ehf2.14380.
References
- 1. Izumiya Y, Takashio S, Oda S, Yamashita Y, Tsujita K. Recent advances in diagnosis and treatment of cardiac amyloidosis. J Cardiol. 2018; 71: 135–143. [DOI] [PubMed] [Google Scholar]
- 2. Maurer MS, Elliott P, Comenzo R, Semigran M, Rapezzi C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017; 135: 1357–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia‐Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio AL, Damy T, Eriksson U, Fontana M. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2021; 42: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamada T, Takashio S, Arima Y, Nishi M, Morioka M, Hirakawa K, Hanatani S, Fujisue K, Yamanaga K, Kanazawa H, Sueta D. Clinical characteristics and natural history of wild‐type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2020; 7: 2829–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lane T, Fontana M, Martinez‐Naharro A, Quarta CC, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019; 140: 16–26. [DOI] [PubMed] [Google Scholar]
- 6. González‐López E, Gagliardi C, Dominguez F, Quarta CC, de Haro‐Del Moral FJ, Milandri A, Salas C, Cinelli M, Cobo‐Marcos M, Lorenzini M, Lara‐Pezzi E. Clinical characteristics of wild‐type transthyretin cardiac amyloidosis: disproving myths. Eur Heart J. 2017; 38: 1895–1904. [DOI] [PubMed] [Google Scholar]
- 7. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐Del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 8. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016; 133: 2404–2412. [DOI] [PubMed] [Google Scholar]
- 9. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington‐Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. 2018; 379: 1007–1016. [DOI] [PubMed] [Google Scholar]
- 10. Solomon SD, Adams D, Kristen A, Grogan M, González‐Duarte A, Maurer MS, Merlini G, Damy T, Slama MS, Brannagan TH III, Dispenzieri A. Effects of patisiran, an RNA interference therapeutic, on cardiac parameters in patients with hereditary transthyretin‐mediated amyloidosis. Circulation. 2019; 139: 431–443. [DOI] [PubMed] [Google Scholar]
- 11. Rettl R, Mann C, Duca F, Dachs TM, Binder C, Ligios LC, Schrutka L, Dalos D, Koschutnik M, Donà C, Kammerlander A. Tafamidis treatment delays structural and functional changes of the left ventricle in patients with transthyretin amyloid cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2022; 23: 767–780. [DOI] [PubMed] [Google Scholar]
- 12. Fontana M, Martinez‐Naharro A, Chacko L, Rowczenio D, Gilbertson JA, Whelan CJ, Strehina S, Lane T, Moon J, Hutt DF, Kellman P. Reduction in CMR derived extracellular volume with patisiran indicates cardiac amyloid regression. JACC Cardiovasc Imaging. 2021; 14: 189–199. [DOI] [PubMed] [Google Scholar]
- 13. Endo J, Sano M, Izumiya Y, Tsujita K, Nakamura K, Tahara N, Kuwahara K, Inomata T, Ueda M, Sekijima Y, Ando Y. A statement on the appropriate administration of tafamidis in patients with transthyretin cardiac amyloidosis. Circ J. 2019; 84: 15–17. [DOI] [PubMed] [Google Scholar]
- 14. Nakashima N, Takashio S, Morioka M, Nishi M, Yamada T, Hirakawa K, Ishii M, Tabata N, Yamanaga K, Fujisue K. A simple staging system using biomarkers for wild‐type transthyretin amyloid cardiomyopathy in Japan. ESC Heart Fail. 2022; 9: 1731–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Elliott P, Drachman BM, Gottlieb SS, Hoffman JE, Hummel SL, Lenihan DJ, Ebede B, Gundapaneni B, Li B, Sultan MB, Shah SJ. Long‐term survival with tafamidis in patients with transthyretin amyloid cardiomyopathy. Circ Heart Fail. 2022; 15: e008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rapezzi C, Elliott P, Damy T, Nativi‐Nicolau J, Berk JL, Velazquez EJ, Boman K, Gundapaneni B, Patterson TA, Schwartz JH, Sultan MB. Efficacy of tafamidis in patients with hereditary and wild‐type transthyretin amyloid cardiomyopathy: further analyses from ATTR‐ACT. JACC Heart Fail. 2021; 9: 115–123. [DOI] [PubMed] [Google Scholar]
- 17. Ochi Y, Kubo T, Baba Y, Sugiura K, Miyagawa K, Noguchi T, Hirota T, Hamada T, Yamasaki N, Kitaoka H. Early experience of tafamidis treatment in Japanese patients with wild‐type transthyretin cardiac amyloidosis from the Kochi amyloidosis cohort. Circ J. 2022; 86: 1121–1128. [DOI] [PubMed] [Google Scholar]
- 18. Bennett JA, Riegel B, Bittner V, Nichols J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung. 2002; 31: 262–270. [DOI] [PubMed] [Google Scholar]
- 19. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 20. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 21. Duca F, Kammerlander AA, Panzenböck A, Binder C, Aschauer S, Loewe C, Agis H, Kain R, Hengstenberg C, Bonderman D, Mascherbauer J. Cardiac magnetic resonance T1 mapping in cardiac amyloidosis. JACC Cardiovasc Imaging. 2018; 11: 1924–1926. [DOI] [PubMed] [Google Scholar]
- 22. Shintani Y, Okada A, Morita Y, Hamatani Y, Amano M, Takahama H, Amaki M, Hasegawa T, Ohta‐Ogo K, Kanzaki H, Ishibashi‐Ueda H. Monitoring treatment response to tafamidis by serial native T1 and extracellular volume in transthyretin amyloid cardiomyopathy. ESC Heart Fail. 2019; 6: 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morioka M, Takashio S, Nakashima N, Nishi M, Fujiyama A, Hirakawa K, Hanatani S, Usuku H, Yamamoto E, Kidoh M, Oda S. Correlation between cardiac images, biomarkers, and amyloid load in wild‐type transthyretin amyloid cardiomyopathy. J Am Heart Assoc. 2022; 11: e024717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Damy T, Garcia‐Pavia P, Hanna M, Judge DP, Merlini G, Gundapaneni B, Patterson TA, Riley S, Schwartz JH, Sultan MB, Witteles R. Efficacy and safety of tafamidis doses in the Tafamidis in Transthyretin Cardiomyopathy Clinical Trial (ATTR‐ACT) and long‐term extension study. Eur J Heart Fail. 2021; 23: 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kociol RD, Pang PS, Gheorghiade M, Fonarow GC, O'Connor CM, Felker GM. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J Am Coll Cardiol. 2010; 56: 1071–1078. [DOI] [PubMed] [Google Scholar]
- 26. Takashio S, Yamamuro M, Izumiya Y, Sugiyama S, Kojima S, Yamamoto E, Tsujita K, Tanaka T, Tayama S, Kaikita K, Hokimoto S. Coronary microvascular dysfunction and diastolic load correlate with cardiac troponin T release measured by a highly sensitive assay in patients with nonischemic heart failure. J Am Coll Cardiol. 2013; 62: 632–640. [DOI] [PubMed] [Google Scholar]
- 27. Dorbala S, Vangala D, Bruyere J, Quarta C, Kruger J, Padera R, Foster C, Hanley M, Di Carli MF, Falk R. Coronary microvascular dysfunction is related to abnormalities in myocardial structure and function in cardiac amyloidosis. JACC Heart Fail. 2014; 2: 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ogawa H, Mizuno Y, Ohkawara S, Tsujita K, Ando Y, Yoshinaga M, Yasue H. Cardiac amyloidosis presenting as microvascular angina. Angiology. 2001; 52: 273–278. [DOI] [PubMed] [Google Scholar]
- 29. Dasari AK, Hughes RM, Wi S, Hung I, Gan Z, Kelly JW, Lim KH. Transthyretin aggregation pathway toward the formation of distinct cytotoxic oligomers. Sci Rep. 2019; 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]


