Abstract
Aims
Despite strong recommendations, outpatient cardiac rehabilitation is underused in chronic heart failure (CHF) patients. Possible barriers are frailty, accessibility, and rural living, which may be overcome by telerehabilitation. We designed a randomized, controlled trial to evaluate the feasibility of a 3‐month real‐time, home‐based telerehabilitation, high‐intensity exercise programme for CHF patients who are either unable or unwilling to participate in standard outpatient cardiac rehabilitation and to explore outcomes of self‐efficacy and physical fitness at 3 months post‐intervention.
Methods and results
CHF patients with reduced (≤40%), mildly reduced (41–49%), or preserved ejection fraction (≥50%) (n = 61) were randomized 1:1 to telerehabilitation or control in a prospective controlled trial. The telerehabilitation group (n = 31) received real‐time, home‐based, high‐intensity exercise for 3 months. Inclusion criteria were (i) ≥18 years, (ii) New York Heart Association class II‐III, stable on optimized medical therapy for >4 weeks, and (iii) N‐terminal pro‐brain natriuretic peptide >300 ng/L. All participants participated in a 2‐day ‘Living with heart failure’ course. No other intervention beyond standard care was provided for controls. Outcome measures were adherence, adverse events, self‐reported outcome measures, the general perceived self‐efficacy scale, peak oxygen uptake (VO2peak) and a 6‐min walk test (6MWT). The mean age was 67.6 (11.3) years, and 18% were women. Most of the telerehabilitation group (80%) was adherent or partly adherent. No adverse events were reported during supervised exercise. Ninety‐six per cent (26/27) reported that they felt safe during real‐time, home‐based telerehabilitation, high‐intensity exercise, and 96% (24/25) reported that, after the home‐based supervised telerehabilitation, they were motivated to participate in further exercise training. More than half the population (15/26) reported minor technical issues with the videoconferencing software. 6MWT distance increased significantly in the telerehabilitation group (19 m, P = 0.02), whereas a significant decrease in VO2peak (−0.72 mL/kg/min, P = 0.03) was observed in the control group. There were no significant differences between the groups in general perceived self‐efficacy scale, VO2peak, and 6MWT distance after intervention or at 3 months post‐intervention.
Conclusions
Home‐based telerehabilitation was feasible in chronic heart failure patients inaccessible for outpatient cardiac rehabilitation. Most participants were adherent when given more time and felt safe exercising at home under supervision, and no adverse events occurred. The trial suggests that telerehabilitation can increase the use of cardiac rehabilitation, but the clinical benefit of telerehabilitation must be evaluated in larger trials.
Keywords: Cardiopulmonary exercise test, Exercise training, Heart failure, Physical activity, VO2
Introduction
The prevalence of chronic heart failure (CHF) is high and increasing, currently affecting around 26 million people worldwide. 1 This is likely to be due to a combination of the increasing age of the general population and improved treatment for CHF patients. 2 CHF carries a high mortality rate and imposes reduced quality of life with frequent hospitalizations. 3 , 4 Consequently, the diagnosis has major implications for the individual patients, their families and society. 1 , 5 High costs are directly associated with medical treatment, and indirectly to a loss of productivity and other attributable costs. 1 , 6 The improvement of health, quality of life and longevity of CHF patients is therefore beneficial for both individual patients and society.
A hallmark of CHF is the inability of the heart to supply vital organs with sufficient blood flow, and this usually becomes evident during exercise. 7 Nevertheless, the heart's capacity can be improved by exercise in both healthy individuals and patients with CHF, 8 , 9 , 10 and cardiac rehabilitation (CR) is an important component of CHF treatment. By improving exercise capacity and quality of life, and by reducing hospitalizations, CR has obtained class 1 recommendation (level of evidence A) according to current guidelines. 11 , 12 High‐intensity interval training has been proven to be safe in patients with CHF, and some trials have shown greater improvement in different cardiovascular parameters, compared with exercise with moderate intensity. 13
Despite strong recommendations, many patients with CHF do not take part in CR programmes and remain physically inactive. 14 , 15 There are several barriers to CR participation, including a high proportion of old and frail patients, limited budgets, rural living, and long distances to outpatient rehabilitation. 16 , 17 Novel approaches to increase adherence to physical activity in patients with CHF are warranted. 18 Telerehabilitation may offer patients an opportunity to participate despite the barriers to regular outpatient CR. 19 Evidence of the feasibility of telerehabilitation for CHF patients is, however, still scarce. 20 , 21 , 22 , 23 , 24
The aim of this trial was to investigate the feasibility of a 3‐month real‐time, home‐based telerehabilitation, high‐intensity exercise programme for CHF patients inaccessible for outpatient cardiac rehabilitation and to explore outcomes of self‐efficacy and physical fitness at the 3 months post‐intervention follow‐up. We hypothesized that telerehabilitation was feasible and that it would benefit self‐efficacy and measures of physical fitness in the telerehabilitation group more than the control group.
Methods
Trial design
In this prospective, randomized controlled trial, CHF patients were randomized 1:1 to either a telerehabilitation or a control group with a 3‐month intervention period. Randomization was stratified by age (</≥60 years old) and left ventricular ejection fraction (</≥40%). The randomization procedure was administered by the Unit for Applied Clinical Research at the Norwegian University of Science and Technology (NTNU) in Trondheim, Norway, to ensure impartiality.
The current trial was based on data on feasibility, self‐efficacy, and physical fitness from the ITISHOPE4HF trial collected at baseline, directly after the intervention (‘end of intervention period’), and at 3‐month post‐intervention follow‐up. ‘End of intervention period’ was defined as completion of 24 exercise sessions (or last session for those not completing 24 sessions) for the telerehabilitation group (equals 3 months if participants met two times per week for 12 weeks) and 3 months for the control group. The main goal of the ITISHOPE4HF trial was to determine whether home‐based telerehabilitation could increase physical activity in CHF patients. The trial was approved by the Regional Committee for Medical and Health Research Ethics in Norway (2016/1597) and registered at ClinicalTrials.gov (NCT03183323). The participants volunteered after receiving oral and written information and provided a written, informed consent form in accordance with the Declaration of Helsinki.
Participants
A total of 61 participants of both genders who were unable or unwilling to participate in standard outpatient CR programmes were recruited from two heart failure outpatient clinics in Central Norway. CHF patients with reduced (≤40%), mildly reduced (41–49%), or preserved (≥50%) ejection fraction according to the 2016 and 2021 ESC guidelines for heart failure 11 , 12 were eligible if (i) their age was ≥18 years, (ii) they were in New York Heart Association (NYHA) functional class II‐III, stable on optimized medical therapy for >4 weeks, and (iii) N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) was >300 ng/L. Exclusion criteria were any of (i) participation in a CR programme within 6 months of enrolment, (ii) presence of reversible CHF (such as severe valvular disease, not revascularized coronary disease, uncontrolled hypertension, or untreated arrhythmias), (iii) severe or very severe pulmonary disease (e.g., COPD GOLD III‐IV), or (iv) inability to exercise safely at home for any reason.
Trial procedures
All participants underwent baseline assessments and participated in a 2‐day ‘Living with heart failure’ course prior to randomization. The baseline assessment included cardiopulmonary exercise testing (CPET), a 6‐min walk test (6MWT), the general perceived self‐efficacy scale questionnaire (GSES), the short physical performance battery (SPPB), and self‐reported outcome measures concerning satisfaction, safety, motivation, and technical challenges. All participants also underwent a clinical examination and a medical interview by an experienced physician and an echocardiography by an experienced sonographer or cardiologist. The supervising cardiologist was consulted in cases where symptoms or findings could indicate elevated risk during exercise. The same assessments were repeated both at the end of the intervention period and at the 3‐month post‐intervention follow‐up, with the exception of CPET and SPPB, which were not performed at the end of the intervention period. All data were collected between June 2017 and June 2020. All participants attending the trial were provided with a tablet computer (Apple iPad Air2®) for free.
Intervention
2‐day ‘Living with heart failure’ course
At baseline, all participants attended a 2‐day ‘Living with heart failure’ course which provided education about heart failure pathology, disease management, lifestyle modifications and exercise physiology in heart failure, and guideline‐based recommendations for exercise training. 11 , 12 The course was conducted by an experienced physician (>10 years of experience in internal medicine), a consultant cardiologist (>10 years of experience with exercise in heart failure) and an experienced physical therapist specialized in cardiac rehabilitation. Family members were welcome to join the course, but this was not mandatory for participation. To ensure the safety of home‐based exercise, participants underwent a maximal exercise test with 12‐lead ECG monitoring. Participants in the telerehabilitation group were educated in the use of the tablet computer, the online videoconferencing software used for the live group‐based exercises, and the pre‐recorded videos with instructions for home‐based self‐administered exercise.
Telerehabilitation group
The telerehabilitation group received real‐time, home‐based high‐intensity exercise intervention, guided remotely by an experienced physical therapist specialized in cardiac rehabilitation via online videoconferencing software, twice a week for a period of 3 months or equivalent (24 exercise sessions). In cases where intervention was delayed due to illness the intervention period was prolonged up to the planned 24 sessions. High‐intensity exercise was defined as 85–95% of HRmax. Participants were given extra time to achieve the prescribed number of exercise sessions if their participation was prevented by illness or injury. The online videoconferencing software enabled two‐way audio‐visual communication, which allowed the participants to communicate with and see each other during sessions, as well as group interaction.
Each session lasted for 60 min and started with a 20‐min warm‐up period, followed by 4 bouts of 4 min of high‐intensity intervals, with exercises involving large muscle groups, for example, deep squats, fast walking or running on the spot, side‐steps on the spot, and a combination of arm and leg movements. A video featuring the exercises used is included as Data S1. Participants were instructed and verbally encouraged to work at an intensity that caused them to breathe heavily and to maintain the high‐intensity effort during intervals, equivalent to Borg scale above 15. The intervals were interspersed with a 3‐min recovery‐period with exercises of lower intensity focusing on upper and lower body strength, as well as balance. Each session ended with a 15‐min calm‐down period. The only additional equipment needed for the exercises was a resistance band. To improve safety beyond the comprehensive evaluation that was conducted prior to randomization, all exercises were performed in front of the tablet computer/TV set‐up so that the participants could be monitored and talked to. Addresses and telephone numbers for all participants were made available, enabling the physical therapist to immediately call for professional medical help in the event of an emergency.
The tablet computer was either used alone or connected to the participant's TV for two‐way audiovisual communication, with supervision projected on the TV and real‐time recording of the patient by the tablet. Participants were provided with instructions or assistance to connect the tablet computer to their home Wi‐Fi network. A 4G mobile network SIM card was provided for participants without wireless internet facilities at home.
Participants in the telerehabilitation group were encouraged to perform additional exercise sessions in order to meet current guideline recommendations, 12 and they had access to pre‐recorded exercise session videos with exercises equivalent to the real‐time group‐based exercises. This gave the participants more flexibility to adapt the telerehabilitation programme into their daily life routines. After the end of the real‐time intervention period, the participants were encouraged to use the pre‐recorded videos for the continuation of their training.
Control group
Participants in the control group were encouraged to exercise according to current guidelines for patients with CHF 12 and underwent the exact same follow‐up assessment as the telerehabilitation group. The participants randomized to controls were not contacted beyond the planned follow‐up visits at the end of the intervention period or 3‐month post intervention, and they did not report on the number of exercise sessions performed.
Outcomes
The primary outcome measure of the current trial was the feasibility of the telerehabilitation exercise intervention in terms of adherence to protocol, occurrence of adverse events and self‐reported satisfaction, safety, motivation, and technical challenges (telerehabilitation group) during the intervention period. Secondary outcome measures were changes in self‐efficacy measured by the GSES (telerehabilitation vs. control group), physical fitness measured by the 6MWT and peak oxygen uptake (telerehabilitation vs. control group), and functional performance measured by the SPPB (telerehabilitation vs. control group) from baseline to the 3‐month post‐intervention follow‐up.
Adherence to exercise
The participants' adherence was categorised as: ‘adherent’ when attending ≥80% of the 24 sessions within 4 months; ‘partially adherent’ when attending 21–79% of the sessions within 4 months; and ‘non‐adherent’ when attending ≤20% of the sessions within 4 months, as previously described by Conraads et al. 17 In our per‐protocol analyses, an 80% participation rate within 4 months was used as a cut‐off for high adherence, in line with Conraads et al. 17 The supervising physical therapist registered the attendance of each participant. Participants were encouraged to provide notification in the event of absence, and enquiries were made if participants did not attend.
Many participants had atrial fibrillation, and we therefore chose not to use heart rate monitors during the supervised exercise sessions. To verify the probability that high‐intensity was reached, a subgroup of eight participants wore a heart rate monitor during the final week of the telerehabilitation period and used it during two supervised group‐based exercise sessions and two unsupervised sessions. Intensity zones were calculated according to estimated maximal heart rate (HRmax) during CPET at baseline, and high‐intensity was defined as 85–95% of HRmax. For the other participants, the intensity was monitored by the supervising physiotherapist aiming for heavy breathing and maintenance of the high‐intensity effort during the intervals.
Adverse events
Exercise session‐related adverse events (AEs) were defined as any unwanted or unfavourable medical event occurring during exercise sessions, and the occurrence of any such event was registered by the supervising physical therapist. At each study visit, an experienced physician specifically interviewed the participants regarding any clinical events. Hospital records were consulted in order to verify reported events, to discover any unreported events, and to ensure the complete registration of AEs and serious AEs (SAEs). All events were categorized separately by an experienced cardiologist and an experienced nephrologist, according to good clinical practice guidelines for clinical trials.
Self‐reported satisfaction, safety, motivation, and technical challenges
A short questionnaire about satisfaction, feeling of safety during exercise, motivation to exercise and technical challenges related to the telerehabilitation equipment was completed by participants in the telerehabilitation group after the intervention period. The questionnaire consisted of five items, with two free‐text spaces for general feedback on the project and technical issues related to the equipment. The five items had four response options: ‘not at all’, ‘small degree’, ‘moderate degree’, and ‘largely/all the time/very much/very often’ (Data S1).
Self‐efficacy
The GSES 25 consists of 10 items, with four graded alternative answers that are scored from 1 to 4, where 1 refers to ‘not at all true’ and 4 refers to ‘exactly true’. The cumulative score was reported. A high score indicates better perceived self‐efficacy in daily life and stressful situations. The questionnaire had previously been translated into Norwegian. 26 GSES was assessed at baseline, at the end of the intervention period, and at the 3‐month post‐intervention follow‐up.
Cardiopulmonary exercise testing
CPET was performed on a treadmill (Woodway USA Inc., Waukesha, WI, USA), using a direct (breath‐to‐breath) ergospirometry system (Vyntus CPX, Erich Jaeger GmbH, Hoechberg, Germany) with 12‐lead ECG monitoring (Custo‐Med, Promed, Dublin, Ireland). All tests were performed blinded to group assignment by experienced personnel, and under the surveillance of physicians whereof one of two was blinded. Gas calibration using high‐precision gas with known concentrations of 16.00% ± 0.04% O2 and 5.00% ± 0.1% CO2 (Riessner‐Gase GmbH & Co., Lichtenfels, Germany) was performed before every fourth tests. Calibration against ambient air and automatic volume calibration at 2 and 0.2 L/s were performed prior to every test. Peak O2 uptake was defined as the highest oxygen uptake, averaged over 30 s (three 10 s measurements) and presented in mL/kg/min. Blood pressure was measured at rest, every 2 min during the test, and in the recovery phase (SunTech Tango M2, SunTech Medical, Inc., NC, USA).
After an initial warm‐up at an individually adjusted speed, participants underwent a 3‐min steady state work economy measurement, before peak oxygen uptake was measured in an individualized ramp protocol until exhaustion. Opening speed was adjusted according to the Borg scale at the end of the steady state work economy measurement. The individually adjusted speed was held constant, and workload was added every minute by increasing the inclination by 2–3%. CPET was performed by an experienced physical therapist specialized in cardiac rehabilitation and exercise physiology and by an specially trained, experienced physician, at baseline and at 3‐month post‐intervention follow‐up.
6‐min walk test
The 6MWT was performed as previously described. 27 Total walking distance was measured and recorded to the nearest metre. The Borg scale score 28 and pulse rate were collected at the end of the test. Participants used walking aids if needed. Because of the reduced health status of many participants, the test was only performed once at each visit for all participants. The minimal clinically important difference for the 6MWT was considered to be 14 to 30.5 m in adults with pathology. 29 The 6MWT was supervised by unblinded study personnel, a physical therapist specialized in rehabilitation or an exercise physiologist. 6MWT was performed at baseline, at the end of the intervention period, and at 3‐month post‐intervention follow‐up.
Short physical performance battery
SPPB 30 was used to assess functional performance. This comprises three parts. Each part is scored from 0 to 4, and the maximum total score is 12 points. Assessment was conducted according to present standards by study personnel unblinded to group allocation. SPPB was performed by a physical therapist specialized in rehabilitation and exercise physiology at baseline and at 3‐month post‐intervention follow‐up.
Sample size
The sample size was calculated for the main trial based on longitudinal changes in physical activity levels over a period of 2 years. Although data were scarce, we estimated that the controls would be physically active at moderate intensity for 15 min/day, and that a clinically important change would be 15 min/day at moderate intensity. Using Sample Power (SPSS Sample Power, IBM®), 20 patients were needed in each group to detect a clinically important difference between the groups (SD 15 min), with power 90% and a two‐way significance level of 0.05. The estimated dropout rate was assumed to be 30%, and the estimated placebo effect was 5 min/day. Accordingly, 30–35 participants were planned for inclusion in each group. Based on experience, we expected the sample to provide adequate power for evaluation of the prespecified aims of the trial.
Statistical methods
Descriptive data are presented as mean (SD) and count (%). Continuous variables that were not normally distributed are presented as median (inter‐quartile range, IQR). Linear mixed models were used, with normally distributed continuous outcome variables as dependent variables and participants as random effect. Time and group, and the interaction of the two variables, were used as covariates. No systematic effect of group at baseline was assumed, and the normality of residuals was explored by Q‐Q plots. Log‐transformed data did not affect any results, so analyses of untransformed data are reported. The SPPB was analysed using mixed logistic models. Primary and secondary outcomes were analysed as intension‐to‐treat. Per‐protocol analyses using linear mixed models were performed post hoc, to explore the impact of the telerehabilitation intervention on the 6MWT. Per‐protocol adherence was set to an 80% participation rate for 24 exercise sessions during a period of 4 months. The level of significance was set to 0.05. All analyses were performed by an experienced statistician using R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Between June 2017 and April 2019, 231 participants with CHF from the two participating outpatient heart failure clinics were assessed for eligibility. Of these, 61 participants were randomized and included in the baseline analyses. Figure 1 shows recruitment, randomization, drop‐out, and follow‐up at two time‐points. Baseline characteristics were similar in both groups (Table 1 ), even small numerical differences were seen for body mass index and NT‐proBNP (both being non‐statistically different between groups).
Figure 1.
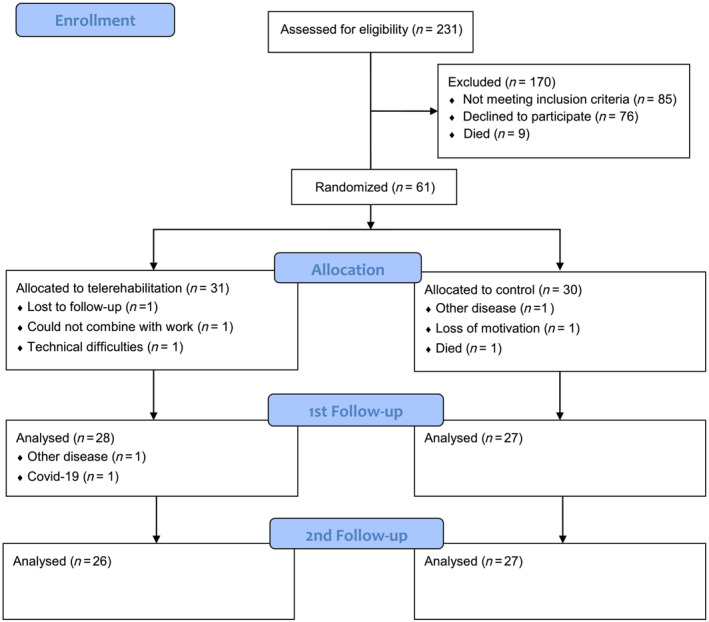
Flowchart of participants through the trial. 1st follow‐up: after the end of the intervention period; 2nd follow‐up: 3‐month post‐intervention.
Table 1.
Participant characteristics and demographics at baseline
| Telerehabilitation group (n = 31) | Control group (n = 30) | Total (n = 61) | |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 23 (74.2) | 27 (90.0) | 50 (82.0) |
| Female, n (%) | 8 (25.8) | 3 (10.0) | 11 (18.0) |
| Age, years | 67.6 ± 10.9 | 67.7 ± 11.9 | 67.6 ± 11.3 |
| Marital status | |||
| Unmarried, n (%) | 3 (9.7) | 7 (23.3) | 10 (16.4) |
| Married, n (%) | 19 (61.3) | 16 (53.3) | 35 (57.4) |
| Divorced, n (%) | 3 (9.7) | 4 (13.3) | 7 (11.5) |
| Cohabitant, n (%) | 2 (6.5) | 1 (3.3) | 3 (4.9) |
| Widow/widower, n (%) | 4 (12.9) | 2 (6.7) | 6 (9.8) |
| Education | |||
| Primary school, n (%) | 2 (6.5) | 5 (16.7) | 7 (11.5) |
| High school, n (%) | 17 (54.8) | 11 (36.7) | 28 (45.9) |
| University undergraduate, n (%) | 11 (35.5) | 11 (36.7) | 22 (36.1) |
| University graduate, n (%) | 1 (3.2) | 3 (10.0) | 4 (6.6) |
| Level of dependency | |||
| Employed or able to work, n (%) | 4 (12.9) | 4 (13.3) | 8 (13.1) |
| Independent ADL, n (%) | 27 (87.1) | 26 (86.7) | 53 (86.9) |
| Systolic blood pressure, mmHg | 120.4 ± 20.5 | 117.1 ± 18.9 | 118.8 ± 19.7 |
| Diastolic blood pressure, mmHg | 71.3 ± 12.3 | 72.5 ± 13.3 | 71.9 ± 12.7 |
| Anthropometrics | |||
| Body mass index (kg/m2) | 27.6 ± 4.7 | 28.9 ± 5.1 | 28.2 ± 4.9 |
| Body surface area (m2) | 2.0 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 |
| Waist‐to‐hip ratio | 1.01 ± 0.09 | 1.05 ± 0.07 | 1.03 ± 0.08 |
| Heart failure characteristics | |||
| Ischemic aetiology, n (%) | 15 (48) | 19 (63) | 34 (56) |
| Non‐ischaemic aetiology, n (%) | 16 (52) | 11 (37) | 27 (44) |
| Reduced ejection fraction, n (%) | 21 (68) | 23 (77) | 44 (72) |
| Mildly reduced ejection fraction, n (%) | 6 (19) | 6 (20) | 12 (20) |
| Preserved ejection fraction, n (%) | 4 (13) | 1 (3) | 5 (8) |
| NYHA class II, n (%) | 24 (77.4) | 26 (86.7) | 50 (82.0) |
| NYHA class III, n (%) | 7 (22.6) | 4 (13.3) | 11 (18.0) |
| Left ventricular ejection fraction (%) | 36.4 (12.1) | 32.4 (11) | 34.4 (11.6) |
| NT‐proBNP, ng/L | 1,501 (IQR 3305) | 1,640 (IQR 3942) | 1,555 (IQR 3578) |
| Co‐morbidity and risk factors | |||
| Peripheral artery disease, n (%) | 3 (9.7) | 4 (13.3) | 7 (11.5) |
| Diabetes, n (%) | 7 (22.6) | 10 (33.3) | 17 (27.9) |
| Hypertension, n (%) | 16 (51.6) | 17 (56.7) | 33 (54.1) |
| Heart rhythm | |||
| Atrial fibrillation/flutter, n (%) | 16 (51.6) | 13 (43.3) | 28 (45.9) |
| Sinus rhythm, n (%) | 11 (35.5) | 14 (46.7) | 25 (41.0) |
| Other, n (%) | 4 (12.9) | 3 (10.0) | 7 (11.5) |
| COPD (GOLD 1 or 2), n (%) | 2 (6.5) | 6 (20.0) | 8 (13.1) |
| Implanted pacemaker, n (%) | 2 (6.5) | 3 (10.0) | 5 (8.2) |
| Implanted CRT and/or ICD, n (%) | 3 (9.7) | 6 (20.0) | 9 (14.8) |
| Current smoker, n (%) | 4 (12.9) | 4 (13.3) | 8 (13.1) |
| Former smoker, n (%) | 15 (48.4) | 21 (70.0) | 36 (59.0) |
| Medication | |||
| Statins, n (%) | 21 (67.7) | 20 (66.7) | 41 (67.2) |
| Β‐blockers, n (%) | 30 (96.8) | 26 (86.7) | 56 (91.8) |
| Diuretics, n (%) | 27 (87.1) | 21 (70.0) | 48 (78.7) |
| ACE inhibitors or ARB, n (%) | 27 (87.1) | 28 (93.3) | 55 (90.2) |
| Angiotensin II receptor blocker, n (%) | 17 (55) | 11 (37) | 28 (46) |
| Aldosterone antagonist, n (%) | 13 (41.9) | 11 (36.7) | 24 (39.3) |
| ARNi, n (%) | 3 (9.7) | 3 (10.0) | 6 (9.8) |
| Ivabradine (%) | 0 (0) | 0 (0) | 0 (0) |
| SGLT2‐inhibitators (%) | 0 (0) | 0 (0) | 0 (0) |
| Quality of life | |||
| General perceived self‐efficacy scale | 29.8 ± 3.8 | 31.0 ± 4.9 | 30.4 ± 4.4 |
| Physical performance | |||
| VO2peak, mL/kg/min | 17.5 ± 4.4 | 17.9 ± 4.8 | 17.7 ± 4.6 |
| VO2peak, L/min | 1.47 ± 0.44 | 1.60 ± 0.59 | 1.53 ± 0.52 |
| 6‐min walk test distance, m | 470 ± 123 | 461 ± 118 | 465 ± 119 |
| Short physical performance battery | 11.0 (IQR 4.0) | 10.5 (IQR 2.3) | 11.0 (IQR 3.0) |
Descriptive data presented as mean ± SD, median (IQR) or count (%).
ACE inhibitors, angiotensin‐converting enzyme inhibitors; ADL, activities of daily living; ARB, angiotensin II receptor blocker; ARNi, angiotensin receptor‐neprilysin inhibitor; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter‐defibrillator; SGLT2 inhibitors, sodium‐glucose cotransporter 2 inhibitors; VO2peak, peak oxygen uptake; reduced ejection fraction (≤40%); mildly reduced ejection fraction (41–49%); preserved ejection fraction (≥50%).
Adherence to exercise
Details of adherence to the telerehabilitation protocol are presented in Table 2 . Of the 31 participants in the telerehabilitation group, 25 (80.6%) were categorized as adherent (≥80% attendance) or partly adherent (20–80% attendance) to the exercise protocol. Three of the six participants categorized as non‐adherent were dropouts. The other three participants had other health‐related issues that prevented them from exercising and/or experienced major technical problems. Regardless of the time spent, 80.6% of the participants completed ≥80% of the exercises (median number of exercise sessions 25 (IQR 21–28)). The corresponding number of months used for the exercise intervention for the telerehabilitation group was a median of 5 (IQR 4.5–7.0).
Table 2.
Adherence in the telerehabilitation group
| Telerehabilitation group | Exercise sessions | N (%) |
|---|---|---|
| Total | 13.6 (8.1) | 31 (100) |
| Adherent | 21.3 (2.5) | 12 (38.7) |
| Partially adherent | 12.6 (3.0) | 13 (41.9) |
| Non‐adherent | 0.3 (0.8) | 6 (19.4) |
Exercise sessions reported as mean (SD). Adherent ≥80% of 24 sessions, partially adherent 21–79%, non‐adherent ≤20% (within 4 months).
The eight participants wearing heart rate monitors during the remotely guided real‐time, home‐based telerehabilitation, exercise sessions reached an intensity of between 80% and 95% of peak heart rate (HRpeak) during the four bouts of 4‐min high‐intensity intervals with 81% (SD 16), 86% (SD 16), 86% (SD 15), and 88% (SD 16) of HRpeak during intervals 1–4, respectively.
Adverse events
No exercise session‐related adverse events were reported during the real‐time, home‐based exercise sessions that were supervised via videoconferencing. One participant reported an incident of hypoglycaemia after an unsupervised exercise session. From baseline to the 3‐month post‐intervention follow‐up, two participants were responsible for 41% of the 22 AEs/SAEs in the telerehabilitation group, and two participants were responsible for 69% of the 13 AEs/SAEs in the control group.
Self‐reported factors of satisfaction, safety, motivation, and technical challenges
In total, 96% (26/27) of the participants felt safe ‘largely all the time’ (23/27) or ‘to a moderate degree’ (3/27) during the real‐time, home‐based exercise sessions supervised by videoconferencing. A total of 96% (24/25) of the participants were ‘very much’ motivated (11/25) or motivated ‘to a moderate degree’ (13/25) to continue exercising on their own after the intervention period. Finally, 59% (16/27) of the participants experienced technical challenges related to the real‐time videoconferencing either ‘very often’ (5/27) or ‘to a moderate degree’ (11/27).
Self‐efficacy
The results from the GSES questionnaire are presented in Table 3 . There were no statistically significant differences between groups in the GSES scores at the end of the intervention period and at 3‐month post‐intervention follow‐up. No statistically significant change from baseline was seen in either group.
Table 3.
Self‐efficacy and physical fitness
| Variable | Baseline | End of intervention period | 3‐month post intervention follow‐up | Mean difference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Telerehabilitation | Control | Telerehabilitation | Control | End of intervention period | P | 3‐month post intervention follow‐up | P | ||
| General perceived self‐efficacy scale | 30.45 (29.33, 31.58) | 31.15 (29.67, 32.64) | 31.13 (29.64, 32.62) | 30.38 (28.85, 31.91) | 30.84 (29.35, 32.33) | 0.02 (−1.85, 1.89) | 0.983 | −0.46 (−2.35, 1.44) | 0.638 |
| VO2peak (mL/kg/min) | 17.57 (16.39, 18.75) | ‐ | ‐ | 17.62 (16.33, 18.91) | 16.85* (15.58, 18.11) | ‐ | ‐ | 0.78 (−0.14, 1.70) | 0.097 |
| VO2peak (L/min) | 1.52 (1.39, 1.66) | ‐ | ‐ | 1.51 (1.37, 1.66) | 1.48 (1.34, 1.62) | ‐ | ‐ | 0.03 (−0.06, 0.12) | 0.474 |
| 6MWT (m) | 464.62 (435.61, 493.64) | 483.71* (452.43, 515.00) | 479.88 (448.44, 511.32) | 473.78 (442.12, 505.44) | 466.50 (434.81, 498.20) | 3.84 (−18.88, 26.55) | 0.741 | 7.27 (−16.30, 30.85) | 0.545 |
Data from the linear mixed model are presented as mean (95% confidence intervals) and effect estimate (mean difference) (95% confidence intervals).
VO2peak, peak oxygen uptake; 6MWT, 6‐min walk test.
Significant change within group.
Cardiopulmonary exercise testing
The results for VO2peak from CPET at baseline and 3‐month post‐intervention follow‐up are presented in Table 3 . A statistically significant decrease of −0.72 mL/kg/min (95% CI −1.36, −0.08) in VO2peak was observed in the control group from baseline to 3‐month post‐intervention follow‐up, P = 0.03. There were no statistically significant differences between groups at 3‐month post‐intervention follow‐up.
6‐min walk test
The results from the 6MWT at baseline, at the end of the intervention period and at the 3‐month post‐intervention follow‐up are presented in Table 3 . From baseline to the end of the intervention period, the telerehabilitation group significantly increased their walking distance by 19.1 m (95% CI 3.0, 35.1), P = 0.02, while the control group increased their distance by 15.3 m (95% CI –1.1, 31.6), P = 0.07. At the 3‐month post‐intervention follow‐up, the corresponding increase from baseline was 9.2 m (95% CI –7.6, 25.9), P = 0.29, for the telerehabilitation group and 1.9 m (95% CI 15.0, 18.7), P = 0.83 for the control group. There were no statistically significant differences between groups at the end of the intervention period or at 3‐month post‐intervention follow‐up.
Short physical performance battery
There was no statistically significant difference in the SPPB total scores between the groups at the 3‐month post‐intervention follow‐up, where the mean difference was 0.66 (95% CI 0.20, 1.97), P = 0.50.
Per‐protocol analyses
Per‐protocol analyses for the 6MWT showed an increase of 29.6 m (95% CI 7.6, 51.7) in the adherent telerehabilitation group and 15.3 m (95% CI 0.6, 30.0) in the control group from baseline to the end of the intervention period with no statistically significant difference between the groups (mean difference 14.4 m (95% CI –12.2, 40.9), P = 0.29). At the 3‐month post‐intervention follow‐up, the change from baseline was 14.3 m (95% CI –9.4, 37.9) in the adherent telerehabilitation group and 1.9 m (95% CI –13.3, 17.0) in the control group, with no statistically significant difference between the groups (mean difference 12.4 m (95% CI –15.7, 40.5), P = 0.39).
Discussion
This is the first study to assess the feasibility of telerehabilitation in CHF patients inaccessible for outpatient cardiac rehabilitation. We found that a large proportion of participants were adherent or partially adherent to the exercise protocol in our trial. Importantly, the participants felt safe, and no adverse events were registered during the real‐time, supervised, high‐intensity exercise training at home. The participants were relatively old, and about half of them experienced some technical issues with the equipment that was used for videoconferencing. However, most participants managed both the physical and technical demands, and were motivated for further exercise training. Together, this adds to the knowledge base by showing that telerehabilitation may help more patients to receive treatment, in accordance with the guidelines.
Most participants completed 24 exercise sessions, but the median time taken to pass the 80% cut‐off was 5 months. According to the prespecified criteria for adherence of attending ≥80% of 24 exercise sessions within a 4‐month period, 39% of the telerehabilitation group were defined as adherent to the protocol. Following these criteria, adherence was lower than in some studies of outpatient and home‐based CR programmes. 21 , 24 , 31 To our knowledge, only one trial 21 has implemented real‐time, home‐based group exercise by videoconferencing in a heart failure population, and this reported an adherence of 71%. This trial is equivalent to ours, although we included participants who were either unable or unwilling to participate in standard outpatient CR. By including partially adherent participants in our trial, the proportion reached 80%, and, considering that most participants exercised 25 times over 5 months, the overall adherence was high and in line with other studies. 21 , 24 , 31 Different definitions of adherence and inclusion criteria across trials make comparisons difficult. Most participants were unable to attend every prespecified twice‐weekly exercise session and used a longer time to complete the CR, mainly due to ailments or hospitalizations caused by CHF or co‐morbidities. Most participants had co‐morbidities, which has been associated with lower participation rates in CR programmes. 17 Participation rates also decrease after 70 years of age and decrease even more after the age of 80. 32 Even though the mean age in our trial is below 70 years, 28 (46%) and 7 (11%) of our participants were older than 70 and 80 years, respectively. Female participants were underrepresented, and the main reason is probably the lower proportion of women with heart failure with reduced ejection fraction. Based on our data we found no indication that women were less motivated or frightened by the technological aspects of telerehabilitation.
Previous studies have shown that elderly patients experience barriers in the adoption of new technologies such as tablet computers, 33 and more than half the participants reported having technical issues at some point. These technical issues were often connectivity problems, yet most of the participants were motivated for further exercise. Importantly, most felt safe during the real‐time, home‐based group‐exercises conducted via videoconferencing.
This trial was not powered to evaluate safety. However, only one patient experienced an AE in close relation to the exercise. This was a 73‐year‐old woman with diabetes, who experienced hypoglycaemia after a self‐administered exercise session via pre‐recorded video. As 28% of the participants in this trial had diabetes mellitus (mostly type II), and the prevalence in CHF in general is high, the risk of exercise‐associated hypoglycaemia should be emphasized to patients and caregivers.
There was no change in the participants' self‐efficacy, as assessed by GSES, in either group. Self‐efficacy is believed to affect a person's ability to engage in and maintain healthy behaviour and to cope with stress, and the participants scored within the range of normal values for their age. 34
The low impact on physical fitness in the telerehabilitation group may partly be a result of discontinuity in the telerehabilitation protocol. The 12 participants completing 24 exercises within the 4‐month period had the greatest benefit from the telerehabilitation intervention, as shown by the per‐protocol analyses. Variations in the course of the disease or the co‐morbidities may have influenced not only the continuity in the telerehabilitation protocol, but also the schedule for the follow‐up visits and the assessments themselves. The telerehabilitation group significantly increased the walking distance (by 6MWT) from baseline to the 3‐month follow‐up by 19 m, which is considered to be clinically significant in adults with pathology. 29 Using the 6MWT, the control group increased their walking distance by 15 m, which is also within the range of clinical significance. 29 As the included participants were either unable or unwilling to participate in outpatient CR, both suboptimal adherence to the intervention and supranormal adherence to guidelines in controls could be expected. The control group had a significant decrease in peak oxygen uptake, while the telerehabilitation group managed to maintain oxygen uptake levels until the 3‐month post‐intervention follow‐up. Peak oxygen uptake was not measured directly after the end of the intervention period, as the main purpose was to study the long‐term effects of telerehabilitation on physical activity, and the effect of high‐intensity interval training on oxygen uptake is already well‐established. 15
Unlike other trials, we included participants who were either unable or unwilling to participate in standard outpatient CR programmes. Together with the wide inclusion criteria, this may have led to the inclusion of less mobile CHF patients than in other trials. However, the success of the inclusion of participants who refuse participation in outpatient CR, and the completion of the telerehabilitation programme (although using more time), is promising, with regard to reducing the underusage of CR. Furthermore, telerehabilitation have been shown to more cost‐effective than outpatient cardiac rehabilitation. 35 However, we have not available data to support this finding.
Strengths and limitations
A major strength of this trial is the novel approach of including participants who are unable or unwilling to participate in standard outpatient CR programmes. This subgroup of the CHF population is often excluded from randomized controlled trials and structured CR programmes. 16 This may have led to a less motivated and less adherent heart failure population in our trial, compared with other studies, and the generalizability of the trial may be limited due to the inclusion of this selected group of patients.
Another strength is the follow‐up conducted 3 months after the end of the intervention period to observe whether the effects of the telerehabilitation intervention were sustained after termination of the organized exercise sessions. We recognize that any conclusions about safety of real‐time, home‐based telerehabilitation for CHF patients based on our trial will be limited by the sample size and the length of the intervention.
Even so, our results are promising, showing no severe complications or adverse events during exercise.
The number of self‐administered exercise sessions was self‐reported, which means that the total training volume for both groups is uncertain. A cross‐over effect of being part of a trial is also anticipated, as those individuals who consent to participate tend to be more motivated to exercise than the average population. Several exercise sessions were interrupted by internet connectivity issues during the trial period, and stable internet environments are crucial for the satisfactory performance of this type of exercise. No measurement of O2 saturation was performed during the various test phases of the 6MWT, as this was not standard operating procedure at our hospital for patients without chronic obstructive pulmonary disease.
We are aware that some recommend intervals at moderate intensity ahead of high‐intensity exercise training. 36 There is, however, substantial evidence that high‐intensity interval training has been carried out safely and successfully in heart failure patients, both in clinical settings and as home‐based supervised exercise, without initializing moderate‐intensity exercise preparation. 15 , 37 , 38 Nevertheless, the inclusion of a supplementary control group exercising at moderate intensity would have strengthened the trial.
Clinical relevance
The present trial provides promising results regarding the uptake of CR in CHF patients by telerehabilitation, with a positive effect on physical performance when participants are adherent to the protocol. The participants found exercising in their own home, with real‐time supervision via videoconferencing software, to be safe and motivational, despite the occurrence of some technical issues. Unlike many other studies that have recruited a selected group of motivated CHF patients, we included CHF patients who were either unable or unwilling to participate in outpatient cardiac rehabilitation, and we showed that it is possible to engage this population in exercise‐based cardiac rehabilitation facilitated by telemedicine. Analyses of the long‐term effects of the telerehabilitation intervention and cost utility were not performed, and this should be explored in further research.
Conclusions
Telerehabilitation was feasible in CHF patients inaccessible for outpatient CR. The participants were highly adherent when given more time, and felt safe during real‐time, high‐intensity exercise in their homes, and reported a high level of satisfaction and motivation for further exercise. Although there were no significant differences between groups regarding self‐efficacy and physical fitness, per‐protocol analyses showed significant improvements in physical fitness achieved by telerehabilitation. The use of tele‐rehabilitation and videoconferencing to provide CR may enable more patients to exercise at home, and reduce the underusage of CR in heart failure.
Funding
This work was supported by funds from the Liaison Committee of Central Norway Regional Health Authority, the Norwegian University of Science and Technology, and the National Association for Heart and Lung Diseases.
Conflict of interest
None declared.
Supporting information
Data S1. Questions Telerehabilitation group.
Acknowledgements
Thanks to Vibeke Løckra at St. Olavs University Hospital, Trondheim, Norway, for conducting the live group‐based exercise sessions by videoconferencing; Bjørg Liabakk at St. Olavs University Hospital, Trondheim, Norway, for support during CPET testing; and Berit Marianne Bjelkåsen at the Unit of Applied Clinical Research, Institute of Cancer and Molecular Medicine, Norwegian University of Science and Technology, Trondheim, Norway, for administering the randomization procedure.
Lundgren, K. M. , Langlo, K. A. R. , Salvesen, Ø. , Zanaboni, P. , Cittanti, E. , Mo, R. , Ellingsen, Ø. , Dalen, H. , and Aksetøy, I.‐L. A. (2023) Feasibility of telerehabilitation for heart failure patients inaccessible for outpatient rehabilitation. ESC Heart Failure, 10: 2406–2417. 10.1002/ehf2.14405.
The work was performed at St. Olavs University Hospital, Trondheim, Norway, and at the Norwegian University of Science and Technology, Trondheim, Norway.
Trial registration: ClinicalTrials.gov: NCT03183323, https://clinicaltrials.gov/ct2/show/NCT03183323.
References
- 1. Ponikowski P, Anker SD, AlHabib KF, Cowie MR, Force TL, Hu S, Jaarsma T, Krum H, Rastogi V, Rohde LE, Samal UC, Shimokawa H, Budi Siswanto B, Sliwa K, Filippatos G. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014; 1: 4–25. [DOI] [PubMed] [Google Scholar]
- 2. Conrad N, Judge A, Tran J, Mohseni H, Hedgecott D, Crespillo AP, Allison M, Hemingway H, Cleland JG, McMurray JJV, Rahimi K. Temporal trends and patterns in heart failure incidence: a population‐based study of 4 million individuals. Lancet. 2018; 391: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsao CW, Lyass A, Enserro D, Larson MG, Ho JE, Kizer JR, Gottdiener JS, Psaty BM, Vasan RS. Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC Heart Fail. 2018; 6: 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor CJ, Ordóñez‐Mena JM, Roalfe AK, Lay‐Flurrie S, Jones NR, Marshall T, Hobbs FDR. Trends in survival after a diagnosis of heart failure in the United Kingdom 2000‐2017: population based cohort study. BMJ. 2019; 364: l223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McMurray JJ, Stewart S. Epidemiology, aetiology, and prognosis of heart failure. Heart. 2000; 83: 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Łyszczarz B. Indirect costs and public finance consequences of heart failure in Poland, 2012‐2015. BMC Public Health. 2018; 18: 1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovasc Pathol. 2012; 21: 365–371. [DOI] [PubMed] [Google Scholar]
- 8. Ellingsen Ø, Halle M, Conraads V, Støylen A, Dalen H, Delagardelle C, Larsen AI, Hole T, Mezzani A, Van Craenenbroeck EM, Videm V, Beckers P, Christle JW, Winzer E, Mangner N, Woitek F, Höllriegel R, Pressler A, Monk‐Hansen T, Snoer M, Feiereisen P, Valborgland T, Kjekshus J, Hambrecht R, Gielen S, Karlsen T, Prescott E, Linke A. High‐intensity interval training in patients with heart failure with reduced ejection fraction. Circulation. 2017; 135: 839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA. 2009; 301: 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mueller S, Winzer EB, Duvinage A, Gevaert AB, Edelmann F, Haller B, Pieske‐Kraigher E, Beckers P, Bobenko A, Hommel J, Van de Heyning CM, Esefeld K, von Korn P, Christle JW, Haykowsky MJ, Linke A, Wisløff U, Adams V, Pieske B, van Craenenbroeck EM, Halle M. Effect of high‐intensity interval training, moderate continuous training, or guideline‐based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021; 325: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M , Document R . 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016; 18: 891–975. [DOI] [PubMed] [Google Scholar]
- 13. Wisløff U, Støylen A, Loennechen JP, Bruvold M, Rognmo Ø, Haram PM, Tjønna AE, Helgerud J, Slørdahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen Ø, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007; 115: 3086–3094. [DOI] [PubMed] [Google Scholar]
- 14. Abreu A, Pesah E, Supervia M, Turk‐Adawi K, Bjarnason‐Wehrens B, Lopez‐Jimenez F, Ambrosetti M, Andersen K, Giga V, Vulic D, Vataman E, Gaita D, Cliff J, Kouidi E, Yagci I, Simon A, Hautala A, Tamuleviciute‐Prasciene E, Kemps H, Eysymontt Z, Farsky S, Hayward J, Prescott E, Dawkes S, Pavy B, Kiessling A, Sovova E, Grace SL. Cardiac rehabilitation availability and delivery in Europe: how does it differ by region and compare with other high‐income countries?: endorsed by the European Association of Preventive Cardiology. Eur J Prev Cardiol. 2019; 26: 1131–1146. [DOI] [PubMed] [Google Scholar]
- 15. Gomes Neto M, Duraes AR, Conceicao LSR, Saquetto MB, Ellingsen O, Carvalho VO. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: a systematic review and meta‐analysis. Int J Cardiol. 2018; 261: 134–141. [DOI] [PubMed] [Google Scholar]
- 16. Richter D, Guasti L, Walker D, Lambrinou E, Lionis C, Abreu A, Savelieva I, Fumagalli S, Bo M, Rocca B, Jensen MT, Pierard L, Sudano I, Aboyans V, Asteggiano R. Frailty in cardiology: definition, assessment and clinical implications for general cardiology. A consensus document of the Council for Cardiology Practice (CCP), Acute Cardiovascular Care Association (ACCA), Association of Cardiovascular Nursing and Allied Professions (ACNAP), European Association of Preventive Cardiology (EAPC), European Heart Rhythm Association (EHRA), Council on Valvular Heart Diseases (VHD), Council on Hypertension (CHT), Council of Cardio‐Oncology (CCO), Working Group (WG) Aorta and Peripheral Vascular Diseases, WG e‐Cardiology, WG Thrombosis, of the European Society of Cardiology, European Primary Care Cardiology Society (EPCCS). Eur J Prev Cardiol. 2021; 29: 216–227. [DOI] [PubMed] [Google Scholar]
- 17. Conraads VM, Deaton C, Piotrowicz E, Santaularia N, Tierney S, Piepoli MF, Pieske B, Schmid JP, Dickstein K, Ponikowski PP, Jaarsma T. Adherence of heart failure patients to exercise: barriers and possible solutions: a position statement of the Study Group on Exercise Training in Heart Failure of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2012; 14: 451–458. [DOI] [PubMed] [Google Scholar]
- 18. Taylor RS, Long L, Mordi IR, Madsen MT, Davies EJ, Dalal H, Rees K, Singh SJ, Gluud C, Zwisler AD. Exercise‐based rehabilitation for heart failure: Cochrane systematic review, meta‐analysis, and trial sequential analysis. JACC Heart Fail. 2019; 7: 691–705. [DOI] [PubMed] [Google Scholar]
- 19. Passantino A, Dalla Vecchia LA, Corrà U, Scalvini S, Pistono M, Bussotti M, Gambarin FI, Scrutinio D, La Rovere MT. The future of exercise‐based cardiac rehabilitation for patients with heart failure. Front Cardiovasc Med. 2021; 8: 709898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piotrowicz E, Piepoli MF, Jaarsma T, Lambrinou E, Coats AJ, Schmid JP, Corrà U, Agostoni P, Dickstein K, Seferović PM, Adamopoulos S, Ponikowski PP. Telerehabilitation in heart failure patients: the evidence and the pitfalls. Int J Cardiol. 2016; 220: 408–413. [DOI] [PubMed] [Google Scholar]
- 21. Hwang R, Bruning J, Morris NR, Mandrusiak A, Russell T. Home‐based telerehabilitation is not inferior to a centre‐based program in patients with chronic heart failure: a randomised trial. J Physiother. 2017; 63: 101–107. [DOI] [PubMed] [Google Scholar]
- 22. Frederix I, Vanhees L, Dendale P, Goetschalckx K. A review of telerehabilitation for cardiac patients. J Telemed Telecare. 2015; 21: 45–53. [DOI] [PubMed] [Google Scholar]
- 23. Piotrowicz E, Pencina MJ, Opolski G, Zareba W, Banach M, Kowalik I, Orzechowski P, Szalewska D, Pluta S, Glówczynska R, Irzmanski R, Oreziak A, Kalarus Z, Lewicka E, Cacko A, Mierzynska A, Piotrowicz R. Effects of a 9‐week hybrid comprehensive telerehabilitation program on long‐term outcomes in patients with heart failure: the telerehabilitation in heart failure patients (TELEREH‐HF) randomized clinical trial. JAMA Cardiol. 2020; 5: 300–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Skov Schacksen C, Henneberg NC, Muthulingam JA, Morimoto Y, Sawa R, Saitoh M, Morisawa T, Kagiyama N, Takahashi T, Kasai T, Daida H, Refsgaard J, Hollingdal M, Dinesen B. Effects of Telerehabilitation interventions on heart failure management (2015‐2020): scoping review. JMIR Rehabil Assist Technol. 2021; 8: e29714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schwarzer R, Jerusalem M. Generalized Self‐Efficacy Scale. In Weinman J., Wright S. C., eds. Measures in Health Psychology: A User's Portfolio Causal and Control Beliefs. Windsor: NFER‐NELSON; 1995. p 35–37. [Google Scholar]
- 26. Røysamb E. Adolescent risk making: behaviour patterns and the role of emotions and cognitions. University of Oslo; 1997. [Google Scholar]
- 27. Holland AE, Spruit MA, Troosters T, Puhan MA, Pepin V, Saey D, McCormack MC, Carlin BW, Sciurba FC, Pitta F, Wanger J, MacIntyre N, Kaminsky DA, Culver BH, Revill SM, Hernandes NA, Andrianopoulos V, Camillo CA, Mitchell KE, Lee AL, Hill CJ, Singh SJ. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014; 44: 1428–1446. [DOI] [PubMed] [Google Scholar]
- 28. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14: 377–381. [PubMed] [Google Scholar]
- 29. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6‐minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017; 23: 377–381. [DOI] [PubMed] [Google Scholar]
- 30. Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self‐reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994; 49: M85–M94. [DOI] [PubMed] [Google Scholar]
- 31. Anderson L, Sharp GA, Norton RJ, Dalal H, Dean SG, Jolly K, Cowie A, Zawada A, Taylor RS. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database Syst Rev. 2017; 6: Cd007130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruano‐Ravina A, Pena‐Gil C, Abu‐Assi E, Raposeiras S, van 't Hof A, Meindersma E, Bossano Prescott EI, González‐Juanatey JR. Participation and adherence to cardiac rehabilitation programs. A systematic review. Int J Cardiol. 2016; 223: 436–443. [DOI] [PubMed] [Google Scholar]
- 33. Cajita MI, Hodgson NA, Lam KW, Yoo S, Han HR. Facilitators of and barriers to mHealth adoption in older adults with heart failure. Comput Inform Nurs. 2018; 36: 376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luszczynska A, Scholz U, Schwarzer R. The general self‐efficacy scale: multicultural validation studies. J Psychol. 2005; 139: 439–457. [DOI] [PubMed] [Google Scholar]
- 35. Hwang R, Morris NR, Mandrusiak A, Bruning J, Peters R, Korczyk D, Russell T. Cost‐utility analysis of home‐based telerehabilitation compared with centre‐based rehabilitation in patients with heart failure. Heart Lung Circ. 2019; 28: 1795–1803. [DOI] [PubMed] [Google Scholar]
- 36. Pina IL, Apstein CS, Balady GJ, Belardinelli R, Chaitman BR, Duscha BD, Fletcher BJ, Fleg JL, Myers JN, Sullivan MJ, American Heart Association Committee on exercise r, prevention . Exercise and heart failure: a statement from the American Heart Association Committee on exercise, rehabilitation, and prevention. Circulation. 2003; 107: 1210–1225. [DOI] [PubMed] [Google Scholar]
- 37. Rognmo O, Moholdt T, Bakken H, Hole T, Molstad P, Myhr NE, Grimsmo J, Wisloff U. Cardiovascular risk of high‐ versus moderate‐intensity aerobic exercise in coronary heart disease patients. Circulation. 2012; 126: 1436–1440. [DOI] [PubMed] [Google Scholar]
- 38. Aamot IL, Forbord SH, Gustad K, Lockra V, Stensen A, Berg AT, Dalen H, Karlsen T, Stoylen A. Home‐based versus hospital‐based high‐intensity interval training in cardiac rehabilitation: a randomized study. Eur J Prev Cardiol. 2014; 21: 1070–1078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Questions Telerehabilitation group.


