Abstract
While technological advances in the field of continuous‐flow left ventricular assist device (CF‐LVAD) are constantly being made, CF‐LVAD recipients are still subjected to a relatively high rate of LVAD‐related adverse events, with post‐LVAD gastrointestinal bleeding (GIB) being the most common one. GIB is associated with a significant impairment in quality of life, multiple hospital admissions, blood transfusion requirements and possibly death. Furthermore, of those patients who bled once, many will experience recurrent GIB events, which further aggravates their discomfort. While some medical and endoscopic treatment options are available, evidence regarding their benefit remains largely equivocal, with all related studies based on data from registries rather than clinical trials. Although having a major impact on LVAD recipients, an effective and validated pre‐implant screening options to predict GIB events post‐implant are scarce. This review focuses on the aetiology, incidence, risk factors, treatment options and the effect of new generation devices on post‐LVAD GIB.
Keywords: Arteriovenous malformations, Continuous‐flow left ventricular assist device, Gastrointestinal bleeding, Heartmate 3
Introduction
The field of durable mechanical circulatory support has progressed substantially over the past 5 years. The introduction of the newer generation continuous‐flow left ventricular assist devices (CF‐LVADs) have contributed significantly to patient survival and salvaged numerous patients with advanced HF who were not eligible for heart transplant or could not wait for one. 1 While historically LVADs were implanted mainly as a bridge to transplant indication, 81.1% of LVADs were implanted as destination therapy in 2021. 2 HeartMate 3™ (HM3), a fully magnetically levitated centrifugal pump, is the newest device available and has been shown to further improve outcomes of this patient population. 2 , 3
Improved survival, quality of life, and functional capacity are realized despite a substantial burden of LVAD complications, including thromboembolic (TE) events, infections and non‐surgical bleeding events, particularly gastrointestinal bleeding (GIB). While the incidence of TE events has dropped significantly with the improved haemocompatibility pump profile of the HeartMate 3, non‐surgical bleeding, mainly GIB, continues to be a common occurring event post‐LVAD implant. 4 In this review, we discuss the incidence, aetiology, risk factors, treatment, and prevention of GIB in LVAD patients.
Incidence and outcomes
While the prevalence of GIB in LVAD patients varies significantly between different studies, depending on device type and year of study, 1 , 3 , 5 , 6 latest INTERMACS annual report estimates the incidence of post‐LVAD GIB at 18%, 23% and 27% at 1, 2, and 3 years post‐LVAD implantation, respectively, in the years 2017–2021. 2
Although GIB events can occur at any time after LVAD implantation, the risk is higher in the early post‐implant period (within 2 years of implantation). 2 , 7 Moreover, patients admitted for GIB once, have a 20–30% risk for another bleeding episode resulting in hospitalization. 8 Recurrent and debilitating GIB in CF‐LVAD patients affect quality of life and may negatively impact their heart transplant candidacy. Although with frequent GIB episodes patients can be up listed to higher UNOS listing status, when these bleeding episodes require transfusions, some patients will develop anti‐human leukocyte antigens (HLA) antibodies, which may undermine their chance of getting transplanted in a timely manner. 9
GIB in LVAD patients may originate in any part of the gut. Thirty‐three per cent of culprit lesions will be found in the upper gastrointestinal (GI) tract and 25% in the lower GI tract; however, in 42% of cases, no source of bleeding can be clearly identified. 10 Of those cases with an identifiable culprit, over 50% result from arteriovenous malformations (AVMs), most in the upper GI tract. 11 Other causes may include Dieulafoy lesions, peptic ulcer disease, bowel ischaemia, radiation proctitis, neoplasia, diverticulosis, or haemorrhoids. 12
GIB in LVAD patients is associated with more frequent hospitalizations, TE events, and healthcare‐related costs. 13 , 14 , 15 , 16 While some studies found a relationship between mortality and GIB in CF‐LVAD patients, 10 , 17 others have not. 13 , 18
Pathophysiology
As post‐LVAD GIB has rarely been reported in patients who were supported by the old generation pulsatile assist devices, it is the continuous flow nature of the pump that is thought to be responsible for this phenomenon. 19 Furthermore, the removal of LVAD for the purpose of orthotopic heart transplant was shown to dramatically reduce the rate of such events. 9 , 20 Multiple mechanisms have been described in relation to GIB in CF‐LVAD patients. The most common and accepted ones have been related to anticoagulant and antiplatelet therapies, an acquired von Willebrand syndrome, and AVM formation. 21 , 22 , 23
Use of anticoagulant and antiplatelet medications
The chronic use of anticoagulant and antiplatelet medications contributes to higher risk for bleeding events, GIB among those. However, patients with CF‐LVAD have higher bleeding rates than do patients exposed to these agents in other settings (e.g. mechanical valves). 24 Moreover, many CF‐LVAD patients with GIB present with subtherapeutic or normal international normalized ratio (INR) at the time of bleeding. This suggests that medication therapy is not the sole or even the most dominant factor leading to these events.
Acquired von Willebrand syndrome
The acquired von Willebrand syndrome was initially described by Heyde et al. and Warkentin et al. in patients with aortic stenosis 25 , 26 and was found later to also be a fundamental component in the mechanism of GIB in CF‐LVAD patients as well. 21 , 22 von Willebrand factor (vWF) is a high molecular weight multimer, which binds factor VIII and serves as an important modulator in haemostasis. In patients with acquired von Willebrand syndrome, shear stress on blood elements travelling through the rapidly spinning CF‐LVAD rotor results in massive degradation of this vWF into inactive low molecular weight fragments, making the patient subjected to bleeding events, most commonly from AVMs located in the GI tract.
Arteriovenous malformation formation due to lack of pulsatility
As the incidence of GIB is significantly higher in CF‐LVAD compared with earlier generation pulsatile LVADs, 19 the lack of pulsatility has been investigated as a possible contributor. As blood flows continuously through CF‐LVADS, pulsatility is usually markedly reduced, with any remaining minimal pulsatility through residual native cardiac function. As a result, the aortic valve is less frequently opened, what leads in turn to a further reduction in pulse pressure. This low pulse pressure is thought to result in GI hypoperfusion, vasodilatation and hypoxia, which trigger the release of angiogenic factors, 13 , 23 , 27 which are thought to play a role in CF‐LVAD related GIB. A recent study by Patel et al. reported a higher prevalence of AVMs in patients with HFrEF in general, with an even higher incidence in those with a higher NYHA class. This observation promotes the theory that those angiogenic factors do not necessarily promote new AVMs formation after LVAD implantation but rather increase the tendency to bleed in pre‐formed AVMs. 28
Risk stratification
Multiple studies have tried to establish risk factors associated with GIB post‐LVAD implantation. However, these studies have all been relatively small, and their findings have not always been reproducible. 10 , 29 , 30 , 31 , 32 , 33 , 34 Factors found to be associated with GIB in patients with CF‐LVAD are listed in Table 1 . Yin et al. recently published the Utah score 17 for predicting the probability of GIB in patients with CF‐LVAD. Three hundred fifty‐one patients implanted between 2004 and 2017 were included, of which 120 (34%) had GIB after a median follow up of 196 days. Independent predictors of GIB were age >54, history of previous bleeding, coronary artery disease, chronic kidney disease, mean pulmonary artery pressure <18 mmHg, and fasting glucose >107 mg/dL. Using those predictors, a score was created, and patients were stratified into low, medium, or high risk for GIB. High risk patients according to this score had an 83% probability of GI bleeding at 3 years. Currently, the Utah score is the only available model to predict GIB in CF‐LVAD, although it has not been validated in other studies. While this score performed very well in the index study, it is based on data from a modest number of patients at two centres in same state and pertains only to older generation axial and centrifugal pumps, which are either much less frequently or no longer implanted. A robust and updated model from a comprehensive national registry of newer generation CF‐LVAD's that could predict a patient's likelihood to bleed, based on preoperative patient characteristics, could have important implications on patient selection for LVAD and subsequent management.
Table 1.
Risk factors for gastrointestinal bleeding in patients with continuous‐flow left ventricular assist device
| Increased right atrial pressure |
| Low pulse pressure |
| Elevated blood urea nitrogen |
| Chronic obstructive pulmonary disease |
| Prior percutaneous coronary intervention |
| Coronary artery disease |
| Older age |
| Prior gastrointestinal abnormalities |
| Right heart failure |
| Tricuspid regurgitation or need for tricuspid valve surgery at time of left ventricular assist device |
| History of previous bleeding |
| Mean pulmonary artery pressure <18 mmHg |
| Increased fasting glucose |
| Chronic kidney disease |
| Phosphodiesterase‐5 inhibitors |
| Heartware left ventricular assist device (vs. Heartmate II) |
Diagnosis and management
Any history of melena, haematochezia, haematemesis, or coffee ground vomiting should prompt a quick evaluation for GIB. In some cases, the initial suspicion might arise from a decrease in serum haemoglobin accompanied by a positive faecal occult blood test, without obvious signs of bleeding. Because of the complex nature of these events, admitting the patient for evaluation is warranted. Most AVM‐related GIB events would resolve spontaneously within 4 days, and on average, one bleeding episode will require the administration of four packed red blood cell units. 35 GIB in LVAD patients might be a complex event, with many challenges imposed on the provider. These events should be treated by experienced providers and managed with a multidisciplinary approach. Currently, there is not a widely accepted, standardized protocol for managing GIB in LVAD recipients, and practice varies among different institutions.
Endoscopic evaluation and management
While endoscopic evaluation is the most common method of investigation, it sometimes fails to reveal a clear source. The diagnostic yield of conventional endoscopic evaluation in this patient population is estimated at 60%, with upper endoscopy having the highest yield. 36 , 37 Patients with haemodynamic instability should be resuscitated promptly in the intensive care unit prior to endoscopic evaluation.
Endoscopic diagnostic and treatment options include oesophagogastric duodenoscopy (EGD), colonoscopy, push enteroscopy, double‐balloon enteroscopy, and video capsule endoscopy (VCE). In most cases, the initial procedures in the evaluation will be an EGD, a colonoscopy, or both, depending on patient presentation. Coffee ground vomiting and haematemesis are strongly suggestive of an upper GIB source, while haematochezia is strongly suggestive of a lower one. Although melena suggests an upper GI origin in most cases, it can also result from a ‘high’ lower GI origin. If EGD and colonoscopy have not yielded a source, VCE would be the next step in most cases. While EGD allows visualization of the proximal upper GI tract only (up to first part of jejunum), VCE holds the ability to visualize the entire small intestine and identify AVMs/polyps in that region. Although VCE is the only endoscopic modality that cannot be deployed therapeutically, it is an important diagnostic tool in obscure GIB. Once a source of bleeding had been recognized, it could be treated locally by thermal coagulation therapy or haemostatic clips. 38 In case of a deeper upper GI source, a push enteroscopy or a double balloon enteroscopy might be warranted, in order to reach the bleeding source. As mentioned before, in about 40% of cases, there will be no identified source of the bleed. In cases of an active and ongoing GIB, a tagged RBC scan (if bleeding rate ≥0.1–0.5 mL/min) and angiography (if bleeding rate ≥0.5 mL/min) may be considered. 39 Figure 1 summarizes the diagnostic and therapeutic approach to GIB in patients with CF‐LVAD.
Figure 1.
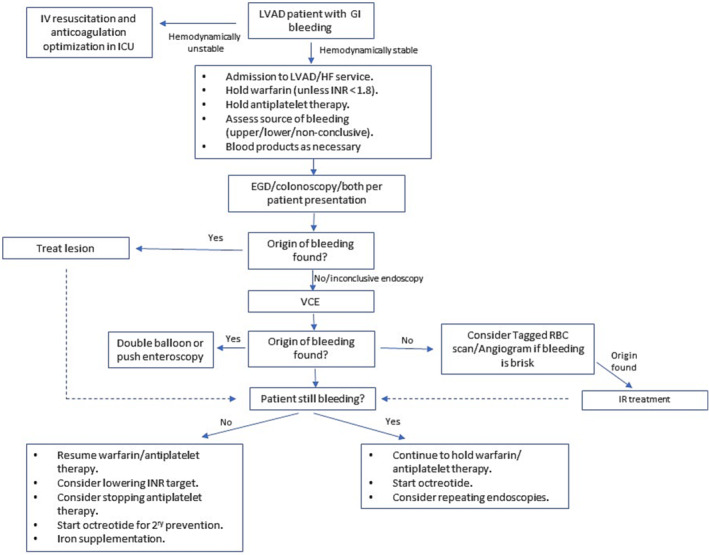
Suggested algorithm for the management of gastrointestinal bleeding in patients with CF‐LVAD. EGD, oesophagogastric duodenoscopy; GI, gastrointestinal; HF, heart failure; LVAD, left ventricular assist device; IR, interventional radiology; VCE, video capsule endoscopy.
Medical management
Systemic medical therapy has become an eminent part of treatment and prevention strategies. Possible non‐invasive strategies include rotor speed reduction, withholding anticoagulant and antiplatelet therapy, and administration of several medications. Currently, all data regarding medical therapy in this patient population are derived from observational studies, with no randomized clinical trials performed.
Reducing or holding anticoagulation and anti‐platelet medications
Anticoagulation and antiplatelet therapy, most often warfarin and aspirin, are standard of care in patients with CF‐LVAD. Lowering or holding the dose of anticoagulation therapy and holding antiplatelet therapy for severe or recurrent bleeding are common first steps in the treatment of these patients. However, these approaches tip the balance towards the thrombotic side and might result in a devastating TE event. Furthermore, clinical scenarios in which newly prescribed medications such as antibiotics for driveline infection and amiodarone for arrhythmias are common in LVAD patients and may increase the risk of drug interactions with warfarin. Currently, there are no widely accepted guidelines to guide providers on how to manage this issue, and a personalized approach is usually taken, with great variability between different centres.
The literature regarding the safety of reduced antithrombotic prophylaxis in this population is conflicting. The TRACE study evaluated the option of reduced therapy both in Europe and in the United States. In the European arm, 101 patients with HM2 were treated with warfarin only and no antiplatelet therapy, with a target INR of 2.3–2.5. Ninety‐two per cent of those patients were treated with this regimen based on a centre standard of care or due to physician preference and not due to past bleeding events. At 2 years, freedom from bleeding, ischaemic stroke, haemorrhagic stroke, and pump thrombosis after initiation of warfarin only therapy was 81% ± 6%, 96% ± 2%, 94% ± 3%, and 94% ± 3%, respectively. Given the combination of a relatively low risk of bleeding and stroke/pump thrombosis, the authors concluded that this strategy may help to reduce the incidence of major bleeding without increasing the risk of TE events. 40 In the US arm, 100 patients with HM2 were treated with either warfarin only (38%), aspirin only (28%), or no anti‐thrombotic agent at all (34%). The main difference from the European study population was that in the US arm, 82% of patients were treated with a reduced regimen because of past bleeding events. Freedom from ischaemic stroke at 1 year was 93.8% ± 2.5%, and freedom from device thrombosis was 92.7% ± 2.7%. Bleeding event occurred in 52% of patients. Although TE event rate was overall low, it was higher than observed in the European arm and in previously described cohorts on a full antithrombotic regimen. The authors concluded that reduced therapy is achievable but might increase the risk of TE events. 41 A study by Stulak et al. demonstrated an increased risk of TE events in CF‐LVAD patients who had an episode of GIB. Although the exact mechanism was not known, the authors concluded that it is likely that a reduction in anticoagulant and antiplatelet management might have contributed to this risk. 15 While clear evidence regarding the efficacy of reducing anticoagulant therapy in older generation CF‐LVADs is lacking, recent papers examining this question in HM3 patients revealed more favourable results. In an observational single‐centre study by Lim et al., stopping aspirin in 53 out of 80 HM3 recipients resulted in lower GIB rates, while no significant difference in either mortality or TE events was observed. 42 An additional retrospective single‐centre study by Marshall et al. also reported a lower bleeding rate but similar TE event rate in patients treated with a reduced antithrombotic therapy (defined as the discontinuation of aspirin, warfarin, or both), compared with those on standard antithrombotic regimen. 43
In summary, patients admitted for GIB and have an INR above their target should be treated with holding warfarin until bleeding stops and INR returns to advisable value. Reversal agents to counter the effect of warfarin may increase the risk of experiencing TE events and therefore should be administered with great caution, according to the specific clinical circumstances. In patients who bleed with an INR in the usual therapeutic range (2.0–3.0), lowering the target INR and/or holding antiplatelet therapy may be considered.
Medications for primary prophylaxis
Angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers
In addition to the multiple mechanisms by which angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers (ACEI/ARB) exert their beneficial effect on patients with HF, they have also been known to downregulate the action of transforming growth factor β (TGF‐β), vascular endothelial growth factor (VEGF), and angiopoietin‐2. A few studies investigated the hypothesis that downregulation of these factors might reduce angiogenesis and thus reduce the occurrence and bleeding from AVMs. In 2017, Houston et al. published the first study to suggest a reduction in GIB rate in CF‐LVAD patients receiving an ACEI or ARB. In this retrospective analysis of 131 patients with a HeartWare ventricular assist device or HM2, the rate of GIB in those patients who were not treated with ACEI/ARB was significantly higher (48%) compared with those who were treated (24%). Logistic regression hazards model demonstrated that treatment with ACEI or ARB therapy resulted in an odds ratio of 0.29 for GIB. 44 In 2019, Converse et al. validated this observation, by reviewing 111 patients with HM2 and demonstrating a 57% reduction in GIB in those patients who were treated with ACEI or ARB and a 63% reduction in the risk of AVM‐related GIB. They were also able to demonstrate that when the mean daily post‐operative lisinopril‐equivalent ACE inhibitor/ARB dose was >5 mg, the risk of major GIB decreased in a dose‐threshold manner. 45 Neither the Houston nor the Converse study adjusted for propensity to receive an ACE inhibitor or ARB. Brinkley et al. published an analysis from the ISHLT IMACS registry, in which 11,494 patients with CF‐LVAD were reviewed with regards to ACEI/ARB and mineralocorticoid receptor antagonist therapy, using propensity score matching to adjust for differences in baseline characteristics related to the use of ACEI/ARB. In this analysis, ACEI/ARB treatment was associated with a significantly reduced GIB rate (HR of 0.88) and reduced mortality (HR of 0.72). 46 However, not all studies were able to establish a clear relation between GIB and ACEI/ARB therapy. In a recent analysis from INTERMACS by Jennings et al., ACEI/ARB did not demonstrate a protective effect against GIB. 47 Vukelic et al. similarly could not demonstrate a clear relation between ACEI/ARB and GIB in CF‐LVAD patients. 48 Three recent meta‐analysis examined the relation between ACEI/ARB treatment and GIB in LVAD patents. Two of them concluded that this treatment did mitigate GIB (odds ratio 0.35 [0.22–0.56] and 0.58 [0.34–0.98]), 49 , 50 while the other did not (hazard ratio 0.46 [0.16–1.3]. 51 In summary, although evidence is equivocal, it appears that ACEI/ARB might provide certain protection from GIB events, and their use seems reasonable. As none of the mentioned studies, including those in the meta‐analyses were randomized clinical trials, a definitive judgement as to benefits of ACEI/ARB with respect to GIB cannot be made.
Digoxin
Digoxin inhibits hypoxia‐inducible factor 1‐alpha (HIF‐1α), a transcription factor that up‐regulates VEGF and angiopoietin‐2, thus reducing angiogenesis in the GI tract. Vukelic et al. demonstrated a two‐fold reduction in GIB events in LVAD patients treated with digoxin (16% vs. 33%) in 199 CF‐LVAD patients, especially if the bleeding was presumed to be of AVM origin. 48 Later, El‐Rafei et al. analysed 649 patients with CF‐LVAD (12% with HM3) and found that digoxin was associated with a 34% less GIB. Furthermore, the GIB rate was even lower when the analysis was confined to patients with AVM‐related GIB. 52 Digoxin has a relatively narrow therapeutic window, and thus, drug levels should be monitored.
Omega‐3 fatty acids
Omega‐3 fatty acids possesses anti‐inflammatory and antiangiogenic properties. In a paper published by Imamura et al., 30 patients with CF‐LVAD were treated with 4 g/day of omega‐3s for approximately 1 year. When compared with the patients who were not treated with omega‐3s, those who were had a significantly higher chance for being free of GIB (97% vs. 73%) at 1 year. 53 The mechanism of action remains obscure but may be related to reductions in tumour necrosis factor‐alpha, with subsequent suppression of angiopoietin‐2 expression. Omega‐3 fatty acids are not known to have significant side effects, except for occasional dyspepsia.
Proton pump inhibitors
Hickman et al. retrospectively examined the effect of proton pump inhibitors (PPIs) on 30‐day bleeding rate in patients who underwent an LVAD implantation and demonstrated a reduced HR for GIB of 0.18 in patients who were treated with PPI. 54 While the benefits in the immediate post‐operative period are evident, there is no clear evidence that PPIs reduce the rate of GIB in LVAD patients beyond this period.
Haemodynamic adjustments
Increased right heart pressure has been reported as a risk factor for GIB in this patient population. 30 , 55 Although there is no clear evidence that haemodynamic optimization in patients with elevated right sided filling pressures genuinely reduces GIB, it is considered a reasonable measure to take.
As mentioned earlier, one possible mechanism for the increased rate of GIB in CF‐LVAD compared with older generation pulsatile devices is the lack of pulsatility. Pump speed reduction results in an increased left ventricular volume and pressure, which leads to a more powerful ventricular contraction and opening of the aortic valve, thus creating a pulse. Several studies have examined this hypothesis. Wever‐Pinzon et al. evaluated the proportion of pulsatility in patients with HM2 LVAD and reported more GIB in patients with lower PI (i.e. less pulsatility). 13 Muthiah et al. demonstrated recovery of vWF profile after improving pulsatility (evaluated by aortic valve opening on echocardiography) in patients with HeartWare ventricular assist device. 56 However, this practice is not performed routinely in contemporary practice (unless the left ventricle is underfilled), as speed reduction might impair LV unloading, which in turn might result in HF exacerbation and thrombotic events. Furthermore, a benefit of this manipulation on the newer generation HM3 LVAD has not been demonstrated. Patients with refractory GIB who do not respond to endoscopic or conventional medical therapy might be candidates for this approach. 36
Medications for secondary prophylaxis
Octreotide
Octreotide, a somatostatin analogue, reduces splanchnic blood flow and gastric acid secretion in the upper GI tract and has been used to treat AVM‐related GIB in non‐LVAD patients. 57 It can be administered via injection, either as a short or a long‐acting formulation. In recent years, several studies have examined the efficacy and safety of this agent in CF‐LVAD recipients. A multicentre study by Shah et al. evaluated 51 HM2 patients who were treated with octreotide after a first GIB event and found those patients to have a significantly reduced risk of rebleeding compared with a matched historical control cohort. 58 Juricek et al. evaluated 30 CF‐LVAD patients with recurrent GIB and demonstrated a significantly reduced frequency of GIB following treatment with octreotide. 59 In a similar study by Wilson et al., 32 CF‐LVAD patients with recurrent GIB had fewer GIB events with octreotide (4.3 vs. 0.9 events/year). 60 Although octreotide use can be associated with adverse events (mainly GI symptoms), those events are mostly of mild nature and would not typically result in drug discontinuation. 61 Furthermore, in the above‐mentioned studies, there were no adverse events reported. 59 , 60 To date, octreotide has not been studied for primary prevention of GIB in LVAD patients.
Thalidomide
Thalidomide is an anti‐angiogenic, orally administer medication that may prevent formation and bleeding from AVMs by suppression of VEGF. Anecdotal reports have demonstrated reduced rates of GIB when thalidomide has been used for secondary prevention in LVAD patients with recurrent and intractable GIB. 62 , 63 , 64 , 65 Thalidomide however has a relatively high rate of side effects, including birth defects, TE events, neuropathy, and liver failure, and thus should be considered only in patients with recurrent and refractory GIB that cannot be treated with other modalities. The risk of TE event is particularly relevant in CF‐LVAD patients, especially with older generation devices. Hence, thalidomide is not recommended in CF‐LVAD patients with a history of pump thrombosis, and antithrombotic therapy should not be stopped in a CF‐LVAD patient treated with thalidomide. 63
Oestrogen based hormonal therapy
Oestrogen‐based hormonal therapy has been used for secondary prophylaxis of bleeding AVMs in non‐LVAD patients, 66 , 67 possibly via improving vascular endothelium integrity. However, a multicentred, placebo‐controlled, randomized controlled trial in 72 non‐LVAD patients failed to show a significant reduction in bleeding with oestrogen. 68 Data regarding oestrogen‐based hormonal therapy in LVAD patients consist only of case reports. 69 Furthermore, the possible risk of TE events raises major concerns in this patient population.
Danazol
Danazol is a synthetic steroid with anti‐estrogenic properties that reduces endometrial bleeding in women. 70 By possibly inhibiting endothelial permeability, danazol was examined as a possible mitigator in LVAD patients with recurrent GIB in two studies. Schettle et al. observed fewer hospitalizations and transfusion needed following initiation of danazol in 19 CF‐LVAD patients with recurrent GIB. 71 In a similar retrospective single‐arm study of 30 LVAD recipients with recurrent GIB, Mathur et al. reported shorter hospital length of stay and fewer blood units transfused during subsequent hospitalizations for GIB. 72 Given the limited evidence for its benefit, and because of concern for possible liver injury, danazol should not be used routinely, and further studies should continue to evaluate its efficacy.
Doxycycline
Doxycycline is a widely used antibacterial medication. In an in vitro study on eight healthy humans conducted by Bartoli et al., doxycycline significantly inhibited the action of ADAMTS‐13, which serves as a vWF protease, leading to reduced vWF degradation and enhanced function. 73 In a later study by the same author, blood samples were collected from 31 CF‐LVAD patients. Blood samples from LVAD patients who had GIB from presumed AVMs had more vWF degradation fragments compared with LVAD patients who bled from a non‐AVM origin. The authors conclude that a theoretical basis exists for possible use of doxycycline in GIB in CF‐LVAD patients. 74 However, at present, there is no clinical evidence supporting the use of doxycycline for prevention of recurrent bleeding in LVAD patients.
Desmopressin
Desmopressin is a vasopressin analogue that is currently used in patients with bleeding diathesis such as haemophilia A, type 1 von Willebrand disease, or uraemic bleeding. 75 Desmopressin exerts its haemostatic effect by inducing vWF formation and thus had been suggested to potentially be beneficial in CF‐LVAD patients. A case report by Hollis et al. in a HM2 patient with refractory GIB found that inhaled desmopressin (150 μg nasal inhalation three times per week) substantially reduced hospital admissions and transfusion requirements. 76 Further studies are needed.
Bevacizumab
Bevacizumab (Avastin) is a humanized monoclonal antibody against VEGF that confers potent anti‐angiogenic properties. Asleh et al. recently reported a pilot study of IV Bevacizumab in five CF‐LVAD patients with recurrent GIB and demonstrated a significant reduction in blood product requirements, hospital admissions and endoscopies. No major adverse effects were noted. 77 This promising option warrants further investigation.
Table 2 summarizes available medical treatments for GIB in CF‐LVAD patients, along with mechanism of action, level of evidence, and main side effects.
Table 2.
Medical therapy in continuous‐flow left ventricular assist device patients with gastrointestinal bleeding
| Medication | Proposed mechanism | Strength of evidence | Possible side effects (main) |
|---|---|---|---|
| Primary prevention | |||
| ACEI/ARB | TGF‐β, VEGF and angiopoietin down regulation |
++ Registry‐based studies and meta‐analyses |
Angioedema, cough, AKI, and hyperkalaemia |
| Omega‐3 fatty acids | Inhibition of TNF‐α, with subsequent suppression of angiopoietin‐2 expression |
+/− One registry‐based study |
Dyspepsia |
| Digoxin | Inhibition of HIF‐1α |
+ Two registry‐based studies |
Bradyarrhythmias, abdominal pain, diarrhoea, skin rash, and visual disturbances |
| Proton pump inhibitors | Inhibition of gastric acid secretion |
+/− One retrospective study of 30‐day perioperative period only. |
Diarrhoea, vomiting, and vitamin malabsorption |
| Secondary prevention | |||
| Octreotide | Reducing splanchnic blood flow and reducing gastric acid secretion |
++ Registry based studies |
Biliary abnormalities, abdominal complaints, bradycardia, and hyperglycaemia |
| Thalidomide | Suppression of VEGF |
+ Registry based studies |
TE events, fetal toxicity, neuropathy, and liver failure |
| Danazol | Unknown |
+/− Case reports |
Liver injury, acne, weight gain, and vaginal symptoms |
| Oestrogen‐based hormonal therapy | Improving vascular endothelium integrity |
+/− Case reports |
TE events |
| Doxycycline | ADAMTS‐13 inhibition |
+/− Case reports |
Fetal toxicity, skin hyperpigmentation, oesophageal injury, and photosensitivity |
| Desmopressin | vWF formation induction |
+/− Case reports |
Headache, dry mouth, dyspepsia, and nausea |
| Bevacizumab | VEGF inhibition |
+/− Case report |
Epistaxis, taste alteration, headache, haemorrhage, hypertension, arthralgia, and headache |
ACEI/ARB, angiotensin‐converting enzyme inhibitor/angiotensin receptor blockers; AKI, acute kidney injury; HIF‐1α, hypoxia‐inducible factor 1‐alpha; TE, thromboembolic; TGF‐β, transforming growth factor β; TNF‐α, tumour necrosis factor‐alpha; VEGF, vascular endothelial growth factor; ADAMTS13, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13; vWF, von Willebrand factor.
The era of HeartMate 3™ (HM3)
In 2017, the US Food and Drug administration approved the implantation of HM3 LVAD in patients with advanced HF, based on the favourable results of the MOMENTUM 3 trial. 78 In 2019, Mehra et al. published the final report of the MOMENTUM 3 trial, which demonstrated superiority of HM3 over HM II for the primary outcome of freedom from disabling stroke and need for reoperation to replace or remove a malfunctioning device. 3 The use of HM3 has increased significantly in the last few years, and in 2021, HM3 represented 92.7% of LVADs implanted in the United States, according to the latest INTERMACS report. 2
As a fully magnetically levitated centrifugal pump, HM3 is designed to reduce shear stress on blood elements and avert pump thrombosis. It has wide blood flow passages and thus reduces friction between blood elements and pump surfaces. This pump is also programmed for periodic cycling of rapid changes in rotor speed and thus creates an artificial pulse. This fixed pulse is thought to reduce stasis in the pump. 78 , 79 Although early reports could not demonstrate a clear reduction of GIB with HM3 compared with HM II, the final report of MOMENTUM3 trial did show a significant reduction in GIB events in patients with HM3 (24.5% of patients with HM3 vs. 30.9% of patients with HM II). 3 This reduction in GIB events might be related to several factors—first, the reduction in shear stress is thought to ameliorate the degradation of high‐molecular‐weight multimers of von Willebrand factor. 21 Second, the artificial pulse is thought to reduce the tendency to form new AVM in GI tract of LVAD patients. 13 Third, because patients with HM3 tend to have less thrombotic events, physician behaviour might also be related to this GIB reduction, possibly by prescribing lower doses of anticoagulant and antiplatelet medications. Nonetheless, there is still a significant residual risk of GIB in patients with HM3, and therefore, the need for better strategies to reduce GIB remains.
Better information on the risks and benefits of withholding antiplatelet therapy in HM3 recipients will soon be forthcoming. Recognizing the much lower risks of pump thrombosis and ischaemic stroke with the HM3 than with earlier continuous flow devices, the ARIES study (NCT04069156) randomized 628 patients newly implanted with a HM3 to either warfarin plus aspirin arm or warfarin with placebo. Results are expected in autumn 2023.
Gaps in knowledge
Although common, there are still many aspects of post‐LVAD GIB that remain obscure. First, we still lack the fundamental understanding of the complex pathomechanism of this phenomenon, which in turn limits our ability to properly prevent or treat these events. Next, while multiple treatment options exist, most studies regarding the efficacy of such treatment options are still largely equivocal, with no randomized trials available. Next, most data regarding post‐LVAD GIB stem from old generation devices, which are mostly unavailable today, and therefore, novel studies evaluating this phenomenon in patients with the newer generation and less thrombogenic HM3 LVAD are of necessity. Last, a tool to stratify the risk for developing post‐LVAD GIB according to pre‐implant characteristics could have an impact on the stratification process before LVAD implantation, the need for possible primary prophylactic therapy, and the intensity of anticoagulation a specific patient requires.
Conclusions and future directions
GIB remains a significant limitation of durable‐CF LVADs, resulting in frequent rehospitalization and impairing recipients' healthcare‐related quality of life. GIB in patients with CF‐LVAD is a complex phenomenon related to a combination of host, pump, and medical factors. While technological efforts in pump technology have directly and indirectly contributed to reduced GIB, we as providers should aspire for a better understanding of the mechanisms of GIB and investigate novel treatment options. Future directions should focus on obtaining more robust evidence regarding the available medical treatment options for secondary prophylaxis of recurrent GIB. Given the lower incidence of pump thrombosis and TE events with the HM3 (relative to older generation pumps), future studies should explore primary prevention strategies for GIB, particularly if these could be targeted to patients at highest risk for GIB. The development of a stratifying tool for the risk of post‐LVAD GIB according to pre‐implant characteristics could have important implications on these patients.
Conflict of interest
None declared.
Hammer, Y. , Bitar, A. , and Aaronson, K. D. (2023) Gastrointestinal bleeding on continuous‐flow left ventricular assist device therapy. ESC Heart Failure, 10: 2214–2224. 10.1002/ehf2.14433.
References
- 1. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The Society of Thoracic Surgeons Intermacs Database Annual Report: evolving indications, outcomes, and scientific partnerships. J Heart Lung Transplant. 2019; 38: 114–126. [DOI] [PubMed] [Google Scholar]
- 2. Yuzefpolskaya M, Schroeder SE, Houston BA, Robinson MR, Gosev I, Reyentovich A, Koehl D, Cantor R, Jorde UP, Kirklin JK, Pagani FD, D’Alessandro DA. The Society of Thoracic Surgeons Intermacs 2022 Annual Report: focus on the 2018 Heart Transplant Allocation System. Ann Thorac Surg. 2023; 115: 311–327. [DOI] [PubMed] [Google Scholar]
- 3. Mehra MR, Uriel N, Naka Y, Cleveland JC Jr, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J, Ewald GA, Itoh A, Raval NY, Silvestry SC, Cogswell R, John R, Bhimaraj A, Bruckner BA, Lowes BD, Um JY, Jeevanandam V, Sayer G, Mangi AA, Molina EJ, Sheikh F, Aaronson K, Pagani FD, Cotts WG, Tatooles AJ, Babu A, Chomsky D, Katz JN, Tessmann PB, Dean D, Krishnamoorthy A, Chuang J, Topuria I, Sood P, Goldstein DJ, MOMENTUM 3 Investigators . A fully magnetically levitated left ventricular assist device ‐ final report. N Engl J Med. 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 4. Mehra MR, Crandall DL, Gustafsson F, Jorde UP, Katz JN, Netuka I, Uriel N, Connors JM, Sood P, Heatley G, Pagani FD. Aspirin and left ventricular assist devices: rationale and design for the international randomized, placebo‐controlled, non‐inferiority ARIES HM3 trial. Eur J Heart Fail. 2021; 23: 1226–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kataria R, Jorde UP. Gastrointestinal bleeding during continuous‐flow left ventricular assist device support: state of the field. Cardiol Rev. 2019; 27: 8–13. [DOI] [PubMed] [Google Scholar]
- 6. Draper KV, Huang RJ, Gerson LB. GI bleeding in patients with continuous‐flow left ventricular assist devices: a systematic review and meta‐analysis. Gastrointest Endosc. 2014; 80: 435–446.e1. [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal A, Pant R, Kumar S, Sharma P, Gallagher C, Tatooles AJ, Pappas PS, Bhat G. Incidence and management of gastrointestinal bleeding with continuous flow assist devices. Ann Thorac Surg. 2012; 93: 1534–1540. [DOI] [PubMed] [Google Scholar]
- 8. Stern B, Maheshwari P, Gorrepati VS, Bethards D, Chintanaboina J, Boehmer J, Clarke K. Initial endoscopic intervention is not associated with reduced risk of recurrent gastrointestinal bleeding in left ventricular assist device patients. Ann Gastroenterol. 2021; 34: 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel SR, Oh KT, Ogriki T, Sims D, Shin JJ, Madan S, Saeed O, Goldstein DJ, Jorde UP. Cessation of continuous flow left ventricular assist device‐related gastrointestinal bleeding after heart transplantation. ASAIO J. 2018; 64: 191–195. [DOI] [PubMed] [Google Scholar]
- 10. Thohan V, Shi Y, Rappelt M, Yousefzai R, Sulemanjee NZ, Hastings TE, Cheema OM, Downey F, Crouch JD. The association between novel clinical factors and gastrointestinal bleeding among patients supported with continuous‐flow left ventricular assist device therapy. J Card Surg. 2019; 34: 453–462. [DOI] [PubMed] [Google Scholar]
- 11. Marsano J, Desai J, Chang S, Chau M, Pochapin M, Gurvits GE. Characteristics of gastrointestinal bleeding after placement of continuous‐flow left ventricular assist device: a case series. Dig Dis Sci. 2015; 60: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 12. Gurvits GE, Fradkov E. Bleeding with the artificial heart: gastrointestinal hemorrhage in CF‐LVAD patients. World J Gastroenterol. 2017; 23: 3945–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wever‐Pinzon O, Selzman CH, Drakos SG, Saidi A, Stoddard GJ, Gilbert EM, Labedi M, Reid BB, Davis ES, Kfoury AG, Li DY, Stehlik J, Bader F. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous‐flow left ventricular assist device HeartMate II. Circ Heart Fail. 2013; 6: 517–526. [DOI] [PubMed] [Google Scholar]
- 14. Marasco SF, Summerhayes R, Quayle M, McGiffin D, Luthe M. Cost comparison of heart transplant vs. left ventricular assist device therapy at one year. Clin Transplant. 2016; 30: 598–605. [DOI] [PubMed] [Google Scholar]
- 15. Stulak JM, Lee D, Haft JW, Romano MA, Cowger JA, Park SJ, Aaronson KD, Pagani FD. Gastrointestinal bleeding and subsequent risk of thromboembolic events during support with a left ventricular assist device. J Heart Lung Transplant. 2014; 33: 60–64. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez RE, Singh SK, Hoang DT, Ali SW, Elayda MAA, Mallidi HR, Frazier OH, Meyers DE. Present‐day hospital readmissions after left ventricular assist device implantation: a large single‐center study. Tex Heart Inst J. 2015; 42: 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yin MY, Ruckel S, Kfoury AG, McKellar SH, Taleb I, Gilbert EM, Nativi‐Nicolau J, Stehlik J, Reid BB, Koliopoulou A, Stoddard GJ, Fang JC, Drakos SG, Selzman CH, Wever‐Pinzon O. Novel model to predict gastrointestinal bleeding during left ventricular assist device support. Circ Heart Fail. 2018; 11: e005267. [DOI] [PubMed] [Google Scholar]
- 18. Li F, Hinton A, Chen A, Mehta NK, Eldika S, Zhang C, Hussan H, Conwell DL, Krishna SG. Left ventricular assist devices impact hospital resource utilization without affecting patient mortality in gastrointestinal bleeding. Dig Dis Sci. 2017; 62: 150–160. [DOI] [PubMed] [Google Scholar]
- 19. Cushing K, Kushnir V. Gastrointestinal bleeding following LVAD placement from top to bottom. Dig Dis Sci. 2016; 61: 1440–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD, HeartMate II Clinical Investigators . Pre‐operative risk factors of bleeding and stroke during left ventricular assist device support: an analysis of more than 900 HeartMate II outpatients. J Am Coll Cardiol. 2014; 63: 880–888. [DOI] [PubMed] [Google Scholar]
- 21. Geisen U, Heilmann C, Beyersdorf F, Benk C, Berchtold‐Herz M, Schlensak C, Budde U, Zieger B. Non‐surgical bleeding in patients with ventricular assist devices could be explained by acquired von Willebrand disease. Eur J Cardiothorac Surg. 2008; 33: 679–684. [DOI] [PubMed] [Google Scholar]
- 22. Uriel N, Pak SW, Jorde UP, Jude B, Susen S, Vincentelli A, Ennezat PV, Cappleman S, Naka Y, Mancini D. Acquired von Willebrand syndrome after continuous‐flow mechanical device support contributes to a high prevalence of bleeding during long‐term support and at the time of transplantation. J Am Coll Cardiol. 2010; 56: 1207–1213. [DOI] [PubMed] [Google Scholar]
- 23. Demirozu ZT, Radovancevic R, Hochman LF, Gregoric ID, Letsou GV, Kar B, Bogaev RC, Frazier OH. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011; 30: 849–853. [DOI] [PubMed] [Google Scholar]
- 24. Huda SA, Kahlown S, Jilani MH, Chaudhuri D. Management of life‐threatening bleeding in patients with mechanical heart valves. Cureus. 2021; 13: e15619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pate GE, Chandavimol M, Naiman SC, Webb JG. Heyde's syndrome: a review. J Heart Valve Dis. 2004; 13: 701–712. [PubMed] [Google Scholar]
- 26. Warkentin TE, Moore JC, Morgan DG. Aortic stenosis and bleeding gastrointestinal angiodysplasia: is acquired von Willebrand's disease the link? Lancet. 1992; 340: 35–37. [DOI] [PubMed] [Google Scholar]
- 27. Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial‐flow left ventricular assist devices. Circ Heart Fail. 2011; 4: 779–784. [DOI] [PubMed] [Google Scholar]
- 28. Patel SR, Vukelic S, Chinnadurai T, Madan S, Sibinga N, Kwah J, Saeed O, Goldstein DJ, Jorde UP. Gastrointestinal angiodysplasia in heart failure and during CF LVAD support. J Heart Lung Transplant. 2022; 41: 129–132. [DOI] [PubMed] [Google Scholar]
- 29. Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk factors and outcomes of gastrointestinal bleeding in left ventricular assist device recipients. Am J Cardiol. 2016; 117: 240–244. [DOI] [PubMed] [Google Scholar]
- 30. Joly JM, el‐Dabh A, Kirklin JK, Marshell R, Smith MG, Acharya D, Rajapreyar IN, Tallaj JA, Tresler M, Pamboukian SV. High right atrial pressure and low pulse pressure predict gastrointestinal bleeding in patients with left ventricular assist device. J Card Fail. 2018; 24: 487–493. [DOI] [PubMed] [Google Scholar]
- 31. Welden CV, Truss W, McGwin G, Weber F, Peter S. Clinical predictors for repeat hospitalizations in left ventricular assist device (LVAD) patients with gastrointestinal bleeding. Gastroenterology Res. 2018; 11: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gonuguntla K, Patil S, Rojulpote C, Cowden RG, Nasir M, Karambelkar P, Buch T, Aujla A, Bhattaru A, Borja ZE, Schulman P. A population based analysis of trends, risk factors and outcomes associated with gastrointestinal bleeding in patients with left ventricular assist devices. Am J Cardiovasc Dis. 2020; 10: 247–257. [PMC free article] [PubMed] [Google Scholar]
- 33. Kawabori M, Kurihara C, Critsinelis AC, Sugiura T, Kaku Y, Civitello AB, Rosengart TK, Morgan JA. Gastrointestinal bleeding after HeartMate II or HVAD implantation: incidence, location, etiology, and effect on survival. ASAIO J. 2020; 66: 283–290. [DOI] [PubMed] [Google Scholar]
- 34. Jakstaite AM, Luedike P, Schmack B, Pizanis N, Riebisch M, Weymann A, Kamler M, Ruhparwar A, Rassaf T, Papathanasiou M. Increased bleeding risk with phosphodiesterase‐5 inhibitors after left ventricular assist device implantation. ESC Heart Fail. 2021; 8: 2419–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ahsan I, Faraz A, Mehmood A, Ullah W, Ghani AR. Clinical approach to manage gastrointestinal bleeding with a left ventricular assist device (LVAD). Cureus. 2019; 11: e6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berg D, Lebovics E, Kai M, Spielvogel D. The predicament of gastrointestinal bleeding in patients with a continuous‐flow left ventricular assist device: pathophysiology, evaluation, and management. Cardiol Rev. 2019; 27: 222–229. [DOI] [PubMed] [Google Scholar]
- 37. Joseph Elmunzer B, Padhya KT, Lewis JJ, Rangnekar AS, Saini SD, Eswaran SL, Scheiman JM, Pagani FD, Haft JW, Waljee AK. Endoscopic findings and clinical outcomes in ventricular assist device recipients with gastrointestinal bleeding. Dig Dis Sci. 2011; 56: 3241–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence‐based approach based on meta‐analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009; 7: 33–47 quiz 1–2. [DOI] [PubMed] [Google Scholar]
- 39. Guha A, Eshelbrenner CL, Richards DM, Monsour HP Jr. Gastrointestinal bleeding after continuous‐flow left ventricular device implantation: review of pathophysiology and management. Methodist Debakey Cardiovasc J. 2015; 11: 24–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Netuka I, Litzler PY, Berchtold‐Herz M, Flecher E, Zimpfer D, Damme L, Sundareswaran KS, Farrar DJ, Schmitto JD, EU TRACE Investigators . Outcomes in HeartMate II patients with no antiplatelet therapy: 2‐year results from the European TRACE study. Ann Thorac Surg. 2017; 103: 1262–1268. [DOI] [PubMed] [Google Scholar]
- 41. Katz JN, Adamson RM, John R, Tatooles A, Sundareswaran K, Kallel F, Farrar DJ, Jorde UP, TRACE study . Safety of reduced anti‐thrombotic strategies in HeartMate II patients: a one‐year analysis of the US‐TRACE study. J Heart Lung Transplant. 2015; 34: 1542–1548. [DOI] [PubMed] [Google Scholar]
- 42. Lim HS, Ranasinghe A, Chue C, Mascaro J. Two‐year outcome of warfarin monotherapy in HeartMate 3 left ventricular assist device: a single‐center experience. J Heart Lung Transplant. 2020; 39: 1149–1151. [DOI] [PubMed] [Google Scholar]
- 43. Marshall D, Sanchez J, Yuzefpolskaya M, Sayer GT, Takeda K, Naka Y, Colombo PC, Uriel N, Topkara VK. Safety of reduced anti‐thrombotic strategy in patients with HeartMate 3 left ventricular assist device. J Heart Lung Transplant. 2021; 40: 237–240. [DOI] [PubMed] [Google Scholar]
- 44. Houston BA, Schneider AL, Vaishnav J, Cromwell DM, Miller PE, Faridi KF, Shah A, Sciortino C, Whitman G, Tedford RJ, Stevens GR, Judge DP, Russell SD, Rouf R. Angiotensin II antagonism is associated with reduced risk for gastrointestinal bleeding caused by arteriovenous malformations in patients with left ventricular assist devices. J Heart Lung Transplant. 2017; 36: 380–385. [DOI] [PubMed] [Google Scholar]
- 45. Converse MP, Sobhanian M, Taber DJ, Houston BA, Meadows HB, Uber WE. Effect of angiotensin II inhibitors on gastrointestinal bleeding in patients with left ventricular assist devices. J Am Coll Cardiol. 2019; 73: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 46. Brinkley DM Jr, Wang L, Yu C, Grandin EW, Kiernan MS. Impact of renin‐angiotensin‐aldosterone system inhibition on morbidity and mortality during long‐term continuous‐flow left ventricular assist device support: an IMACS report. J Heart Lung Transplant. 2021; 40: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jennings DL, Truby LK, Littlefield AJ, Ciolek AM, Marshall D, Jain R, Topkara VK. Impact of heart failure drug therapy on rates of gastrointestinal bleeding in LVAD recipients: an INTERMACS analysis. Int J Artif Organs. 2021; 44: 3913988211013366. [DOI] [PubMed] [Google Scholar]
- 48. Vukelic S, Vlismas PP, Patel SR, Xue X, Shitole SG, Saeed O, Sims DB, Chinnadurai T, Shin JJ, Forest SJ, Goldstein DJ, Jorde UP. Digoxin is associated with a decreased incidence of angiodysplasia‐related gastrointestinal bleeding in patients with continuous‐flow left ventricular assist devices. Circ Heart Fail. 2018; 11: e004899. [DOI] [PubMed] [Google Scholar]
- 49. Kittipibul V, Vutthikraivit W, Kewcharoen J, Rattanawong P, Tantrachoti P, Putthapiban P, Nair N. Angiotensin II antagonists and gastrointestinal bleeding in left ventricular assist devices: a systematic review and meta‐analysis. Int J Artif Organs. 2021; 44: 215–220. [DOI] [PubMed] [Google Scholar]
- 50. Mahmoud A, Taha Y, Meece LE, Bavry AA, Ahmed MM. Effect of angiotensin II antagonism on gastrointestinal bleeding in patients with left ventricular assist devices: a systematic review and meta‐analysis. ASAIO J. 2022; 68: 1470–1474. [DOI] [PubMed] [Google Scholar]
- 51. Rai D, Tariq R, Tahir MW, Chowdhury M, Wahab A, Kharsa A, Bandyopadhyay D, Feitell SC, Parikh V, Aronow WS, Lanier GM, Levine E, Fonarow GC, Kaul V. Primary and secondary prevention strategies for gastrointestinal bleeding in patients with left ventricular assist device: a systematic review and network meta‐analysis. Curr Probl Cardiol. 2021; 46: 100835. [DOI] [PubMed] [Google Scholar]
- 52. el Rafei A, Trachtenberg BH, Schultz J, John R, Estep JD, Araujo‐Gutierrez R, Suarez TEE, Goodwin K, Cogswell R. Association between digoxin use and gastrointestinal bleeding in contemporary continuous flow left ventricular assist device support. J Heart Lung Transplant. 2021; 40: 671–676. [DOI] [PubMed] [Google Scholar]
- 53. Imamura T, Nguyen A, Rodgers D, Kim G, Raikhelkar J, Sarswat N, Kalantari S, Smith B, Chung B, Narang N, Juricek C, Burkhoff D, Song T, Ota T, Jeevanandam V, Sayer G, Uriel N. Omega‐3 therapy is associated with reduced gastrointestinal bleeding in patients with continuous‐flow left ventricular assist device. Circ Heart Fail. 2018; 11: e005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hickman AW, Lonardo NW, Mone MC, Presson AP, Zhang C, Barton RG, Selzman CH, Drakos SG, Koliopoulou A, McKellar SH. Acid suppression to prevent gastrointestinal bleeding in patients with ventricular assist devices. J Surg Res. 2019; 234: 96–102. [DOI] [PubMed] [Google Scholar]
- 55. Liebo M, Newman J, Yu M, Hussain Z, Malik S, Lowes B, Joyce C, Zolty R, Basha HI, Heroux A, McGee E, Um JY, Raichlin E. Preoperative right heart dysfunction and gastrointestinal bleeding in patients with left ventricular assist devices. ASAIO J. 2021; 67: 324–331. [DOI] [PubMed] [Google Scholar]
- 56. Muthiah K, Connor D, Ly K, Gardiner EE, Andrews RK, Qiao J, Rutgers D, Robson D, Low J, Jarvis S, Macdonald P, Dhital K, Jansz P, Joseph J, Hayward CS. Longitudinal changes in hemostatic parameters and reduced pulsatility contribute to non‐surgical bleeding in patients with centrifugal continuous‐flow left ventricular assist devices. J Heart Lung Transplant. 2016; 35: 743–751. [DOI] [PubMed] [Google Scholar]
- 57. Johansson C, Aly A. Stimulation of gastric mucus output by somatostatin in man. Eur J Clin Invest. 1982; 12: 37–39. [DOI] [PubMed] [Google Scholar]
- 58. Shah KB, Gunda S, Emani S, Kanwar MK, Uriel N, Colombo PC, Uber PA, Sears ML, Chuang J, Farrar DJ, Brophy DF, Smallfield GB. Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail. 2017; 10. [DOI] [PubMed] [Google Scholar]
- 59. Juricek C, Imamura T, Nguyen A, Chung B, Rodgers D, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Burkhoff D, Sayer G, Jeevanandam V, Uriel N. Long‐acting octreotide reduces the recurrence of gastrointestinal bleeding in patients with a continuous‐flow left ventricular assist device. J Card Fail. 2018; 24: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wilson TJ, Baran DA, Herre JM, Cameron CM, Yehya A, Ingemi AI. Gastrointestinal bleeding rates in left ventricular assist device population reduced with octreotide utilization. ASAIO J. 2021; 67: 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Molina TL, Krisl JC, Donahue KR, Varnado S. Gastrointestinal bleeding in left ventricular assist device: octreotide and other treatment modalities. ASAIO J. 2018; 64: 433–439. [DOI] [PubMed] [Google Scholar]
- 62. Draper K, Kale P, Martin B, Kelly Cordero R, Ha R, Banerjee D. Thalidomide for treatment of gastrointestinal angiodysplasia in patients with left ventricular assist devices: case series and treatment protocol. J Heart Lung Transplant. 2015; 34: 132–134. [DOI] [PubMed] [Google Scholar]
- 63. Sieg AC, Moretz JD, Horn E, Jennings DL. Pharmacotherapeutic management of gastrointestinal bleeding in patients with continuous‐flow left ventricular assist devices. Pharmacotherapy. 2017; 37: 1432–1448. [DOI] [PubMed] [Google Scholar]
- 64. Seng BJJ, Teo LLY, Chan LL, Sim DKL, Kerk KL, Soon JL, Tan TE, Sivathasan C, Lim CP. Novel use of low‐dose thalidomide in refractory gastrointestinal bleeding in left ventricular assist device patients. Int J Artif Organs. 2017; 40: 636–640. [DOI] [PubMed] [Google Scholar]
- 65. Namdaran P, Zikos TA, Pan JY, Banerjee D. Thalidomide use reduces risk of refractory gastrointestinal bleeding in patients with continuous flow left ventricular assist devices. ASAIO J. 2020; 66: 645–651. [DOI] [PubMed] [Google Scholar]
- 66. Barkin JS, Ross BS. Medical therapy for chronic gastrointestinal bleeding of obscure origin. Am J Gastroenterol. 1998; 93: 1250–1254. [DOI] [PubMed] [Google Scholar]
- 67. van Cutsem E, Rutgeerts P, Vantrappen G. Treatment of bleeding gastrointestinal vascular malformations with oestrogen‐progesterone. Lancet. 1990; 335: 953–955. [DOI] [PubMed] [Google Scholar]
- 68. Junquera F, Feu F, Papo M, Videla S, Armengol JR, Bordas JM, Saperas E, Piqué JM, Malagelada JR. A multicenter, randomized, clinical trial of hormonal therapy in the prevention of rebleeding from gastrointestinal angiodysplasia. Gastroenterology. 2001; 121: 1073–1079. [DOI] [PubMed] [Google Scholar]
- 69. Gutsche JT, Atluri P, Augoustides JG. Treatment of ventricular assist‐device‐associated gastrointestinal bleeding with hormonal therapy. J Cardiothorac Vasc Anesth. 2013; 27: 939–943. [DOI] [PubMed] [Google Scholar]
- 70. Beaumont H, Augood C, Duckitt K, Lethaby A. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007; 2007: CD001017. [DOI] [PubMed] [Google Scholar]
- 71. Schettle SD, Pruthi RK, Pereira NL. Continuous‐flow left ventricular assist devices and gastrointestinal bleeding: potential role of danazol. J Heart Lung Transplant. 2014; 33: 549–550. [DOI] [PubMed] [Google Scholar]
- 72. Mathur P, Marino D, Bittner K, Bartell N, Taylor C, Aranez JL, Alexis J, Carlson B, Chen L, Ott L, Kothari TH, Kaul V, Kothari S. Su1395 initiation of danazol in left ventricular assist device patients with recurrent Gi bleeding leads to shorter hospital stays and fewer blood transfusions. Gastrointest Endosc. 2019; 89: AB361–AB362. [Google Scholar]
- 73. Bartoli CR, Kang J, Restle DJ, Zhang DM, Shabahang C, Acker MA, Atluri P. Inhibition of ADAMTS‐13 by doxycycline reduces von Willebrand factor degradation during supraphysiological shear stress: therapeutic implications for left ventricular assist device‐associated bleeding. JACC Heart Fail. 2015; 3: 860–869. [DOI] [PubMed] [Google Scholar]
- 74. Bartoli CR, Zhang DM, Hennessy‐Strahs S, Kang J, Restle DJ, Bermudez C, Atluri P, Acker MA. Clinical and in vitro evidence that left ventricular assist device‐induced von Willebrand factor degradation alters angiogenesis. Circ Heart Fail. 2018; 11: e004638. [DOI] [PubMed] [Google Scholar]
- 75. James PD, Connell NT, Ameer B, di Paola J, Eikenboom J, Giraud N, Haberichter S, Jacobs‐Pratt V, Konkle B, McLintock C, McRae S, R. Montgomery R, O’Donnell JS, Scappe N, Sidonio R Jr, Flood VH, Husainat N, Kalot MA, Mustafa RA. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021; 5: 280–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hollis IB, Chen SL, Chang PP, Katz JN. Inhaled desmopressin for refractory gastrointestinal bleeding in a patient with a HeartMate II left ventricular assist device. ASAIO J. 2017; 63: e47–e49. [DOI] [PubMed] [Google Scholar]
- 77. Asleh R, Albitar HAH, Schettle SD, Kushwaha SS, Pereira NL, Behfar A, Stulak JM, Rodeheffer RJ, Iyer VN. Intravenous bevacizumab as a novel treatment for refractory left ventricular assist device‐related gastrointestinal bleeding. J Heart Lung Transplant. 2020; 39: 492–495. [DOI] [PubMed] [Google Scholar]
- 78. Mehra MR, Naka Y, Uriel N, Goldstein DJ, Cleveland JC Jr, Colombo PC, Walsh MN, Milano CA, Patel CB, Jorde UP, Pagani FD, Aaronson KD, Dean DA, McCants K, Itoh A, Ewald GA, Horstmanshof D, Long JW, Salerno C, MOMENTUM 3 Investigators . A fully magnetically levitated circulatory pump for advanced heart failure. N Engl J Med. 2017; 376: 440–450. [DOI] [PubMed] [Google Scholar]
- 79. Heatley G, Sood P, Goldstein D, Uriel N, Cleveland J, Middlebrook D, Mehra MR, MOMENTUM 3 Investigators . Clinical trial design and rationale of the multicenter study of MagLev technology in patients undergoing mechanical circulatory support therapy with HeartMate 3 (MOMENTUM 3) investigational device exemption clinical study protocol. J Heart Lung Transplant. 2016; 35: 528–536. [DOI] [PubMed] [Google Scholar]


