Abstract
Aims
Comorbidities play a significant role towards the pathophysiology of heart failure with preserved ejection fraction (HFpEF), characterized by abnormal macrovascular function and altered ventricular–vascular coupling. However, our understanding of the role of comorbidities and arterial stiffness in HFpEF remains incomplete. We hypothesized that HFpEF is preceded by a cumulative rise in arterial stiffness as cardiovascular comorbidities accumulate, beyond that associated with ageing.
Methods and results
Arterial stiffness was assessed using pulse wave velocity (PWV) in five groups: Group A, healthy volunteers (n = 21); Group B, patients with hypertension (n = 21); Group C, hypertension and diabetes mellitus (n = 20); Group D, HFpEF (n = 21); and Group E, HF with reduced ejection fraction (HFrEF) (n = 11). All patients were aged 70 and above. Mean PWV increased from Groups A to D (PWV 10.2, 12.2, 13.0, and 13.7 m/s, respectively) as vascular comorbidities accumulated independent of age, renal function, haemoglobin, obesity (body mass index), smoking status, and hypercholesterolaemia. HFpEF exhibited the highest PWV and HFrEF displayed near‐normal levels (13.7 vs. 10 m/s, P = 0.003). PWV was inversely related to peak oxygen consumption (r = −0.304, P = 0.03) and positively correlated with left ventricular filling pressures (E/e′) on echocardiography (r = −0.307, P = 0.014).
Conclusions
This study adds further support to the concept of HFpEF as a disease of the vasculature, underlined by an increasing arterial stiffness that is driven by vascular ageing and accumulating vascular comorbidities, for example, hypertension and diabetes. Reflecting a pulsatile arterial afterload associated with diastolic dysfunction and exercise capacity, PWV may provide a clinically relevant tool to identify at‐risk intermediate phenotypes (e.g. pre‐HFpEF) before overt HFpEF occurs.
Keywords: Heart failure with preserved ejection fraction, Arterial stiffness, Pulse wave velocity, Ventricular–arterial coupling
Introduction
Despite the breakthrough in the treatment of heart failure with preserved ejection fraction (HFpEF) with sodium‐glucose cotransporter‐2 (SGLT2) inhibitors, 1 our understanding of its pathophysiology remains incomplete. Exactly how SGLT2 inhibitors confer prognostic benefits to the HFpEF population is not fully elucidated. One theory suggests that they restore the balance between left ventricular (LV) function and arterial resistance (i.e. ventricular–arterial coupling) by ameliorating systemic arterial stiffness. 2 , 3 This supports the concept of HFpEF as a disease of the vasculature, characterized by augmented aortic stiffness and unfavourable late‐systolic afterload on the ventricle. 4 , 5 , 6 This results in maladaptive ventricular remodelling, elevated LV end‐diastolic pressure (LVEDP), and exercise intolerance, marking the onset of HFpEF. 7 , 8 , 9 A conceptual state of ‘myocardial fatigue’ is hypothesized, in which the energy‐deprived myocardium becomes functionally impaired. 10 With unceasing arterial afterload mismatch, a transition to irreversible myocardial damage may develop. 11 , 12 This link between HFpEF and arterial stiffness is well documented, 8 , 9 , 13 , 14 but little data exist regarding the early stages of arterial stiffness that precede HFpEF and its impact on LVEDP induced by amassing vascular comorbidities, for example, diabetes mellitus (DM) and hypertension.
HFpEF is not simply a collection of comorbidities. 15 We hypothesized that HFpEF is preceded by a cumulative rise in arterial stiffness and LVEDP as cardiovascular comorbidities accumulate, beyond that associated with ageing. Furthermore, to highlight its distinct pathological process from HF with reduced ejection fraction (HFrEF), which is characterized by neurohormonal overactivation, we anticipated that arterial stiffness peaks in HFpEF and abates in HFrEF. To our knowledge, no studies have collectively investigated this process. Although endothelial dysfunction is often cited as the precursor link between HFpEF and comorbidities, this cellular observation lacks clinical validation. We aimed to provide a more clinically relevant and mechanistic approach by using carotid–femoral pulse wave velocity (cf‐PWV) as a gold‐standard measure of large arterial stiffness. 16 This may help determine the usefulness of arterial stiffness as a biomarker in identifying intermediate phenotypes at risk of HFpEF, aligning with our previously posited theory of pre‐HFpEF: an incipient stage before HFpEF. 17 Finally, to provide insight on exercise intolerance, we explored the relationship between cf‐PWV and aerobic capacity, expressed as peak oxygen consumption (VO2 peak) during cardiopulmonary exercise testing (CPET).
Methods
Study design
IDENTIFY‐HF was a single‐centre prospective study in which five pre‐defined groups were enrolled for the evaluation of arterial compliance and microvascular function as primary and secondary measures, respectively, in parallel with echocardiographic parameters of LV diastolic function and exercise capacity. The recruitment of participants and the study design have been detailed previously. 18 IDENTIFY‐HF (NCT03186833) was approved by the West Midlands and Black Country Research Ethics Committee (17/WM/0039) and conducted at the University Hospitals Coventry and Warwickshire NHS Trust in accord with the Declaration of Helsinki. All subjects provided written informed consent before participation.
Study population
All participants were aged ≥70 years and categorized into the following five groups: Group A (n = 21), healthy volunteers with a resting blood pressure (BP) < 140/90 mmHg and no history of DM according to WHO criteria 19 ; Group B (n = 21), individuals with hypertension only (defined as BP ≥ 140/90 mmHg on at least two clinic visits or ambulatory BP monitoring and on current anti‐hypertensives); Group C (n = 20), individuals with hypertension and DM; Group D (n = 21), patients with HFpEF [LV ejection fraction (LVEF) ≥ 50%]; and Group E (n = 11), patients with HFrEF (LVEF < 40%). Groups A–C had no evidence of HF based on the European Society of Cardiology (ESC) HF diagnostic criteria. 20 Healthy volunteers were recruited through social media and posters, whereas Groups B–E were recruited from outpatient medical and HF clinics.
Study procedure
To ensure reliable vascular measurements, all study subjects were asked to omit their morning medications, fast for 12 h, and abstain from caffeine, tobacco, and alcohol for the preceding 24 h. Vascular function studies were performed in a quiet, temperature‐controlled room (21–23°C) after resting for at least 10 min in the supine position. Baseline blood tests, transthoracic echocardiography, PWV, and laser Doppler flowmetry (LDF) were assessed successively and followed by CPET, when possible. Comparisons were made between Groups A and D and separately between Groups D and E. Estimated glomerular filtration rate was calculated using the Modification of Diet in Renal Disease formula, which used the serum creatinine values obtained from the participant's blood test at their first research visit.
Pulse waveform analysis
Arterial tonometry was adapted from an expert consensus document on the measurement of aortic stiffness using cf‐PWV. 16 A hand‐held micromanometer‐tipped transcutaneous probe (SPC‐301; Millar Instruments, USA) coupled with the SphygmoCor™ system (SphygmoCor BPAS; PWV Medical, Australia) was gently placed over the ipsilateral carotid and femoral arteries at the point of maximal pulsation. Pulse waves were gated to simultaneous electrocardiography. Distance between the two recording sites was measured in a straight line above the body surface. Cf‐PWV was computed as the ratio of this distance and time differential between the onset of flow at the two points. Measurements were triplicated, ensuring values differed by no more than 0.5 m/s, before an average PWV (m/s) was taken. A high PWV indicates raised arterial stiffness, signifying a rapid return of peripherally reflected waves to the heart during mid‐to‐late systole. Aortic augmentation index (AIx), another surrogate marker of systemic arterial stiffness and LV systolic loading, 21 , 22 , 23 was calculated as a ratio between augmented pressure (AP) and central pulse pressure (cPP), expressed as a percentage. AP was calculated as the difference between the first inflection point and the second (P2) maximum peak of the central arterial waveform, whereas cPP was estimated as the difference between P2 and the foot of the wave. 22
Laser Doppler flowmetry
LDF, an index of microvascular function expressed in perfusion units, was measured as previously described. 24 Briefly, the LDF probe was kept constant over the thenar region before and during the reactive hyperaemia manoeuvre at room temperature (22°C). This was performed by a single user to avoid inter‐observer variability. Laser Doppler signals were continuously registered on a computer software (Perisoft Data Acquisition; Perimed Inc). Resting basal flow was averaged over 6 min of stable recordings. Forearm blood flow was then impeded with a pneumatic cuff inflated to 50 mmHg above the systolic BP (SBP) for 3 min. The signal obtained during complete arterial occlusion was taken as the biological zero. Peak hyperaemia was defined as the highest flow signal after release of arterial occlusion. Post‐occlusive reactive area of hyperaemia (PORH) was defined as the area under the curve during hyperaemia. Linear regression was used to estimate the slope from time of release to the maximum upstroke value.
Cardiopulmonary exercise test
All participants were invited for CPET on a cycle ergometer at 70 rpm using a ramp protocol adapted from existing guidelines. 25 This was conducted by a trained exercise physiologist blinded to previous vascular measurements. Breath‐by‐breath metabolic gas exchange and minute ventilation were measured and used to derive respiratory exchange ratio (RER) and VO2 peak (mL/kg/min), which was averaged over the final 30 s of the test. Termination was symptom driven. Peak exercise test was defined by RER ≥ 1.1.
Study power
Assuming 80% power and 5% α‐value (two‐tailed hypothesis), sample sizes of n = 11 for HFrEF group and n = 21 for the other groups were required to detect a difference in cf‐PWV among the five groups using the overall F‐test in a one‐way ANOVA and for pairwise comparisons between healthy volunteers and other groups. Group means (8.6 m/s for control, 8.9 m/s for HFrEF, 11.3 m/s for HFpEF, and 9.95 m/s for hypertension and diabetes groups) and standard deviation (SD) of 1.7 m/s were based on previous studies. 24 , 26
Statistical analysis
Continuous data were summarized as mean ± SD or median (interquartile range) depending on normality. Categorical data, expressed as numbers (percentages), were compared using χ2 tests. As appropriate, ANOVA and Kruskal–Wallis tests were used to compare continuous demographic data (e.g. age) and for unadjusted analysis of outcome measurements. Analysis of covariance, specifically a general linear model adjusting for potential confounders [age, body mass index (BMI), and renal function], was used to compare mean outcomes among the five groups with post hoc Bonferroni correction for multiple comparisons. For pairwise comparison, mean differences with 95% confidence intervals (CIs) were reported. Pearson's correlation was used to assess for correlations between estimated LVEDP, VO2 peak, and PWV. P < 0.05 was considered statistically significant. All data were analysed on IBM SPSS v26 software.
Results
Baseline characteristics
Baseline characteristics of study subjects for the five groups are summarized in Table 1 . Mean ages for the five groups were unequal (P = 0.001), with mean ages being greater in HF participants (HFpEF, 81 ± 5.7; HFrEF, 79 ± 6.8 years) than healthy volunteers (74 ± 3.5 years). Although there was no significant difference in mean BMI across the five groups (P = 0.206), the proportion of patients with obesity (BMI ≥ 30) differed significantly (P = 0.021). Groups C–E had a significantly higher proportion of obese individuals compared with the control group. In the HFpEF group, 4/21 (19.0%) male and 5/21 (23.8%) female patients were found to be obese. Expectedly, atrial fibrillation (AF) was most prevalent in the HFpEF group, of which 2/21 (9.5%) were thin (BMI < 25) female patients with AF. No current smokers were identified in both HF groups compared with 9.5% in Groups B and C. A progressive reduction in renal function was observed from Groups B to E, reaching a nadir in the HF population (Group B, 91.4 ± 19.8; Group C, 66.8 ± 22.1; HFpEF, 54 ± 23; HFrEF, 58.9 ± 17.6 mL/min/1.73 m2), compared with controls (92.7 ± 22.2 mL/min/1.73 m2, P < 0.001). Baseline HF medications were optimized in all patients with HFrEF, with every patient receiving a form of renin‐angiotensin‐aldosterone‐system inhibitor, including sacubitril/valsartan. However, due to concerns regarding hyperkalaemia, only 36% could tolerate mineralocorticoid antagonists. Of note, only one patient with HFrEF did not tolerate beta‐blockers. In patients with hypertension (Groups B and C), approximately 55–60% received angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, whereas the remaining received calcium channel blockers (CCBs), in line with UK national hypertension guidelines in patients older than 55 years. Around 10% in these groups were prescribed loop diuretics by their general practitioners to manage ankle oedema for reasons other than HF, such as a side effect of CCBs.
Table 1.
Baseline characteristics of patients from Groups A to E
| Patient groups | ||||||
|---|---|---|---|---|---|---|
| Baseline variables |
Group A Control (n = 21) |
Group B HTN (n = 21) |
Group C HTN + DM (n = 20) |
Group D HFpEF (n = 21) |
Group E HFrEF (n = 11) |
P‐value |
| Age (years) | 74 ± 3.5 | 75 ± 3.8 | 76 ± 4.7 | 81 ± 5.7 | 79 ± 6.8 | 0.001 |
| Male sex, no. (%) | 9 (42.9) | 10 (47.6) | 14 (70.0) | 8 (38.1) | 7 (63.6) | 0.347 |
| Systolic BP (mmHg) | 124.9 ± 11.4 | 151.1 ± 25.4 | 139.2 ± 26.7 | 124 ± 19.9 | 115 ± 13.7 | 0.001 |
| Diastolic BP (mmHg) | 75.5 ± 11 | 84.7 ± 13.8 | 75.2 ± 11 | 66 ± 12.1 | 70.3 ± 9.5 | <0.001 |
| Body mass index (kg/m2) | 24.6 ± 3.5 | 27.4 ± 4.2 | 30.9 ± 6.6 | 29.5 ± 4.1 | 29.7 ± 4.3 | 0.206 |
| Obesity (BMI ≥ 30) | 1 (4.8) | 5 (23.8) | 9 (45.0) | 9 (42.9) | 5 (45.5) | 0.021 |
| Functional class, n (%) | ||||||
| NYHA class 1 | — | — | — | 4 (19) | 1 (9) | |
| NYHA class 2 | — | — | — | 12 (57) | 7 (64) | — |
| NYHA class 3 | — | — | — | 5 (24) | 3 (27) | |
| NYHA class 4 | — | — | — | 0 | 0 | |
| Comorbidities, no. (%) | ||||||
| Diabetes mellitus | 0 | 0 | 20 (100) | 7 (33.3) | 3 (27.3) | — |
| Treated hypertension | 0 | 21 (100) | 20 (100) | 17 (81) | 8 (72.7) | — |
| Ischaemic heart disease | 0 | 2 (9.5) | 6 (30) | 2 (9.5) | 4 (36) | 0.007 |
| Hypercholesterolaemia | 0 | 5 (23.8) | 9 (45) | 8 (38.1) | 7 (63.6) | 0.002 |
| Atrial fibrillation | 0 | 0 | 0 | 14 (66.7) | 5 (45.5) | <0.001 |
| Current smoker | 1 (4.7) | 2 (9.5) | 2 (9.5) | 0 | 0 | 0.277 |
| Echocardiography | ||||||
| LVEF | 66.1 ± 5.4 | 65 ± 3.1 | 60.8 ± 6.1 | 62.9 ± 7.4 | 27.2 ± 13.4 | <0.001 |
| Average E/e′ | 8.5 ± 1.1 | 9.5 ± 2.2 | 12.9 ± 4.8 | 18.6 ± 3.5 | 21.4 ± 1.9 | <0.001 |
| Indexed LV mass (g/m2) | 97 ± 24.7 | 87 ± 39.8 | 96 ± 24.2 | 127 ± 33.6 | 131 ± 24.5 | 0.001 |
| Laboratories | ||||||
| SCr (mmol/L) | 79.8 ± 18.3 | 79.6 ± 16.4 | 112 ± 41.1 | 133 ± 46.8 | 119 ± 32.0 | <0.001 |
| eGFR (mL/min/1.73 m2) | 92.7 ± 22.2 | 91.4 ± 19.8 | 66.8 ± 22.1 | 54 ± 23 | 58.9 ± 17.6 | <0.001 |
| Haemoglobin (g/L) | 139 ± 11.7 | 140.1 ± 11.5 | 126 ± 16.7 | 119.7 ± 13.4 | 132.2 ± 18.6 | <0.001 |
| NT‐proBNP (pg/mL) | 27.5 ± 47.6 | 25.9 ± 23.7 | 53.5 ± 55.2 | 269.3 ± 158.2 | 259.3 ± 158 | <0.001 |
| Medications, no. (%) | ||||||
| DHP‐CCB | 0 | 14 (66.7) | 10 (50) | 7 (33.3) | 1 (9.1) | — |
| Beta‐blockers | 0 | 4 (19.0) | 7 (35.0) | 8 (38.1) | 10 (90.9) | — |
| Thiazide loop diuretic | 0 | 7 (33.3) | 6 (30) | 1 (4.8) | 1 (9.1) | — |
| Loop diuretic | 0 | 2 (9.5) | 2 (10) | 18 (85.7) | 10 (90.9) | — |
| ACE‐I | 0 | 9 (42.9) | 4 (20) | 5 (23.8) | 5 (45.5) | — |
| ARB | 0 | 5 (23.8) | 7 (35) | 5 (23.8) | 1 (9.1) | — |
| Spironolactone | 0 | 2 (9.5) | 1 (5) | 4 (19.0) | 4 (36.4) | — |
| Sacubitril/valsartan | 0 | 0 | 0 | 0 | 5 (45.5) | — |
ACE‐I, angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; BP, blood pressure; DHP‐CCB, dihydropyridine calcium channel blocker; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HTN, hypertension; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; NYHA, New York Heart Association; SCr, serum creatinine.
+/− values are means ± standard deviation.
Haemodynamics and echocardiography
Average SBP was unequal across the five groups (P = 0.001) with hypertensive subjects with or without DM (Group B, 151.1 ± 25.4; Group C, 139.2 ± 26.7 mmHg), exhibiting greater SBP than that of healthy volunteers (Table 1 ). Although both HF groups had a high prevalence of hypertension, average SBP was within the normal range (HFpEF, 124 ± 19.9; HFrEF, 115 ± 13.7 mmHg), reflecting either an advanced stage of HF associated with decapitated hypertension or a greater use of renin‐angiotensin‐aldosterone‐system inhibitors. Average LVEF was severely reduced in HFrEF (27.2 ± 13.4%) compared with HFpEF (62.9 ± 7.4%). Subjects with HFpEF and HFrEF exhibited markedly raised estimated LVEDP (average E/e′ 18.6 ± 3.5 in HFpEF; E/e′ 21.4 ± 1.9 in HFrEF) associated with greater indexed LV masses, compared with the other groups (Table 1 ).
Pulse wave analysis
There was a consecutive rise in cf‐PWV across Groups A–D as vascular comorbidities accumulated by definition of groups, peaking in HFpEF (Group A, 10.19 ± 2.02; Group B, 12.20 ± 2.39; Group C, 13.05 ± 2.70; Group D, 13.36 ± 2.92 m/s) before returning to near‐normal levels in HFrEF (10 ± 0.9 ms) (Figure 1 and Table 2 ). After adjusting for age, obesity, smoking, hypercholesterolaemia, anaemia, and renal function, mean differences in cf‐PWV between control and each of Groups B–D remained statistically significant (Table 3 ). The largest adjusted mean difference in cf‐PWV was observed between Group C (hypertension and diabetes) and HFrEF [cf‐PWV 3.17 (1.40, 4.93), P < 0.001], followed by HFpEF vs. HFrEF [cf‐PWV 2.82 (1.03, 4.61), P = 0.002] and Group C vs. control [cf‐PWV 2.25 (1.15, 4.65), P = 0.001]. Augmentation index was elevated in subjects with hypertension (35 ± 8%), hypertension and diabetes (32 ± 11%), followed by HFpEF (29 ± 11%), indicating increased late‐systolic reflective waves from a stiff arterial vasculature.
Figure 1.
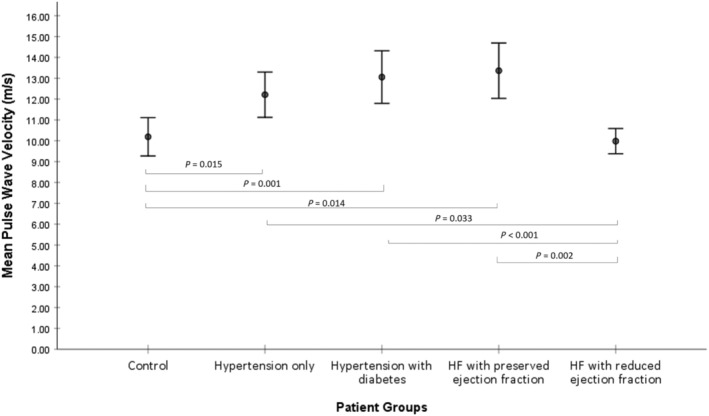
Mean pulse wave velocity for all groups with 95% confidence intervals and pairwise P‐values from linear model results in Table 2 adjusted for age and estimated glomerular filtration rate.
Table 2.
Vascular function data of patients from Groups A to E
| Patient groups | ||||||
|---|---|---|---|---|---|---|
|
Group A Control (n = 21) |
Group B Hypertension (n = 21) |
Group C Hypertension + diabetes (n = 20) |
Group D HFpEF (n = 21) |
Group E HFrEF (n = 11) |
Unadjusted P‐value † | |
| Arterial tonometry a | ||||||
| Aortic pulse pressure (mmHg) | 49 ± 15.3 (43–57) | 64 ± 21 (54–73) | 63 ± 31 (48–77) | 57 ± 20 (48–67) | 44 ± 14 (35–53) | 0.053 |
| Pulse wave analysis (m/s) | 10.19 ± 2.02 (9.27–11.11) | 12.20 ± 2.39 (11.12–13.29) | 13.05 ± 2.70 (11.79–14.31) | 13.36 ± 2.92 (12.03–14.68) | 10.00 ± 0.90 (11.37–12.49) | <0.001 |
| Augmentation index (%) | 25 ± 7 (22–28) | 35 ± 8 (32–39) | 32 ± 11 (27–37) | 29 ± 11 (25–35) | 21 ± 10 (15–28) | 0.001 |
| Laser flowmetry b | ||||||
| Basal blood flow | 43 (55) | 40 (35) | 76 (98) | 44 (68) | 39 (118) | 0.61 |
| Peak blood flow | 154 (90) | 126 (129) | 207 (185) | 153 (203) | 118 (137) | 0.46 |
| Slope | 37 (58) | 31 (48) | 41 (28) | 26 (63) | 19 (41) | 0.55 |
| Area of hyperaemia (PORH) | 6323 (12 074) | 6538 (13 426) | 4658 (7356) | 6087 (8938) | 4471 (5929) | 0.48 |
| Time to peak hyperaemia (s) a | 3.14 ± 0.37 (2.95–3.32) | 3.25 ± 0.1 (3.2–3.31) | 3.56 ± 0.95 (2.96–4.16) | 3.29 ± 0.03 (3.27–3.31) | 3.38 ± 0.005 (3.28–3.29) | 0.19 |
HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PORH, post‐occlusive reactive area of hyperaemia.
Means ± standard deviation (95% confidence intervals).
Median (interquartile range).
P < 0.001 for group comparisons unadjusted for age and renal function.
Table 3.
Comparison of groups with pulse wave velocity after adjusting for age, renal function, body mass index, haemoglobin, smoking status, and hypercholesterolaemia
| Predictors of PWV | Multivariate regression analysis (beta, 95% CI, P‐value) |
|---|---|
| Age (years) | 0.23 (0.13, 0.34), <0.001 |
| Renal function (eGFR) | 0.02 (−0.005, 0.04), 0.126 |
| Body mass index | 0.14 (0.03, 0.24), 0.012 |
| Haemoglobin | 0.008 (−0.03, 0.04), 0.670 |
| Current smoker | 0.75 (−1.37, 2.87), 0.485 |
| Hypercholesterolaemia | −0.72 (−1.93, 0.49), 0.241 |
| Group comparison | Mean difference in pulse wave velocity (95% CI), P‐value |
|---|---|
| Comparisons with control | |
| Hypertension without diabetes mellitus | 1.85 (0.37, 3.33), 0.015* |
| Hypertension with diabetes mellitus | 2.25 (1.15, 4.65), 0.001* |
| HFpEF | 1.87 (0.54, 4.57), 0.014* |
| HFrEF | −0.80 (−2.31, 1.78), 0.80 |
| Comparisons with hypertension group | |
| Hypertension with diabetes mellitus | 1.05 (−0.59, 2.69), 0.206 |
| HFpEF | 0.28 (−1.20, 2.62), 0.461 |
| HFrEF | −2.11 (−4.05, −0.18), 0.033* |
| Comparison with hypertension + diabetes | |
| HFpEF | −0.34 (−1.94, 1.26), 0.67 |
| HFrEF | −3.17 (−4.93, −1.40), <0.001* |
| Comparison with HFpEF | |
| HFrEF | −2.82 (−4.61, −1.03), 0.002* |
CI, confidence interval; eGFR, estimated glomerular filtration rate; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PWV, pulse wave velocity.
Statistically significantly calculated by analysis of covariance general linear model after adjustment of above predictor variables. By Bonferroni's correction, a pairwise comparison between two groups is statistically significant if P < 0.005 (0.05/10).
Microvascular function by laser Doppler flowmetry
There was a suggestion that microvascular dysfunction was most prominent in subjects with hypertension and diabetes (Group C). This group exhibited numerically higher basal and peak blood flow, delayed peak to hyperaemia, and lowest area of PORH (i.e. reduced global flow and impaired vasodilatory response) among the five groups (Table 2 ). However, there were no statistically significant differences in these parameters between the groups. It is important to note the marked intra‐group variability in most of these parameters as reflected by their wide interquartile range. Area under the curve for PORH can be affected by red blood cell concentration (e.g. reduced in anaemia); however, PORH was similar between HFpEF and control Group A despite significant differences in baseline haemoglobin.
Exercise capacity
Out of a total of 74 participants, 23 participants were unable to complete CPET due to either participant refusal or orthopaedic impairment to exercise. CPET data were obtained in 51 participants, all of which reached an average RER ≥ 1.1 to mark peak exercise effort. A consecutive decrease in exercise capacity was observed across Groups A through to E (Table 4 ). Average VO2 peak was significantly different between the five groups (P = 0.003) with HFpEF subjects achieving a very low average VO2 peak (10.5 ± 2.6 mL/kg/min) followed by HFrEF (11.2 ± 2.3 mL/kg/min) compared with normal values seen in the healthy controls (19.4 ± 6.4 mL/kg/min). Both HF groups had an average percentage predicted VO2 peak of approximately 62%, indicating moderate to severely impaired exercise tolerance secondary to HF.
Table 4.
Cardiopulmonary exercise testing (CPET) data from Groups A to E
| Patient groups | ||||||
|---|---|---|---|---|---|---|
| CPET parameters |
Group A Control (n = 16) |
Group B Hypertension (n = 15) |
Group C Hypertension + diabetes (n = 7) |
Group D HFpEF (n = 6) |
Group E HFrEF (n = 7) |
P‐value |
| Peak VO2 (mL/kg/min) a | 19.4 ± 6.4 (16–22.8) | 15.5 ± 3.3 (13.7–17.3) | 14.6 ± 8.8 (6.4–22.7) | 10.5 ± 2.6 (7.7–13.2) | 11.2 ± 2.3 (9–13.3) | 0.003 |
| Peak VO2, % predicted b | 91.5 (86.3–107.8) | 78 (73.5–82.5) | 72 (59–78) | 62.5 (53.5–81.2) | 62 (61–75) | <0.001 |
| Peak exercise RER a | 1.20 ± 0.14 (1.12–1.26) | 1.24 ± 0.15 (1.15–1.32) | 1.23 ± 0.11 (1.12–1.33) | 1.11 ± 0.07 (1.04–1.18) | 1.12 ± 0.11 (0.94–1.3) | 0.24 |
HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; RER, respiratory exchange ratio; VO2, oxygen consumption.
Mean ± standard deviation (95% confidence interval); peak VO2 was log‐transformed before comparison with one‐way ANOVA.
Median (interquartile range).
Relationship between pulse wave velocity, estimated left ventricular end‐diastolic pressure, and exercise capacity
Finally, we determined whether raised arterial stiffness was associated with exercise intolerance and raised LVEDP, both of which are hallmark signs of HFpEF. As shown in Figure 2 , there was a moderate negative correlation between cf‐PWV and VO2 peak (Groups A–E, r = −0.304, 95% CI −0.603, −0.032, P = 0.03; if Group E excluded, r = −0.426, 95% CI −0.739, −0.15, P = 0.004), suggesting that the higher the arterial stiffness, the lower the exercise capacity. There was a moderate positive correlation between cf‐PWV and estimated LVEDP based on E/e′ (r = 0.307, 95% CI 0.068, 0.567, P = 0.014).
Figure 2.
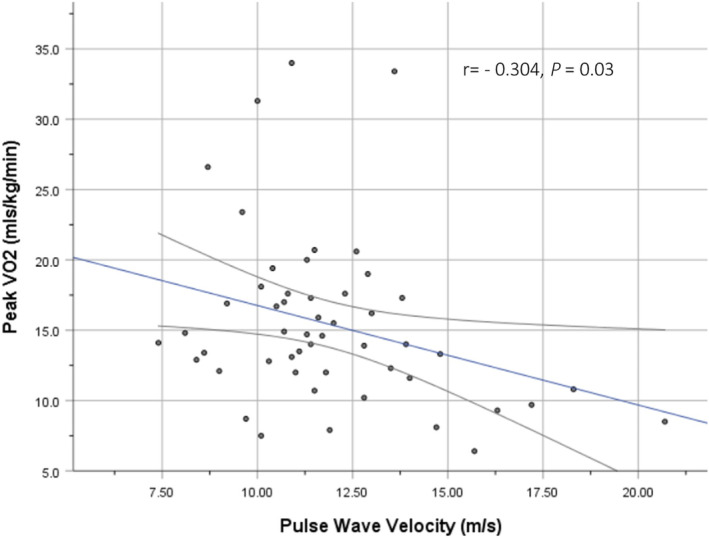
Relationship between pulse wave velocity and peak VO2.
Discussion
This study aids towards the understanding of the pathophysiology of HFpEF by investigating its relationship with arterial stiffness, diastolic dysfunction, and exercise capacity across five groups of individuals. Our findings were as follows: (i) cf‐PWV increased from Groups A to D as vascular comorbidities of diabetes and hypertension accumulated independent of age, renal function, BMI, and other risk factors for arterial stiffness; (ii) HFpEF exhibited the highest cf‐PWV and HFrEF displayed near‐normal levels; and (iii) arterial stiffness was inversely related to exercise capacity on CPET and positively correlated with LV filling pressures on echocardiography.
Arterial stiffness and the continuum of heart failure with preserved ejection fraction
By studying at earlier time points in the disease process, early changes in arterial compliance, LV remodelling, and LV diastolic dysfunction (LVDD) can be appreciated. It is well recognized that arterial stiffening is a common feature of ageing, 27 , 28 hypertension, 6 , 29 , 30 diabetes, 31 , 32 and chronic kidney disease (CKD) 33 , 34 and an independent predictor of cardiovascular events in HFpEF. 9 , 24 , 26 , 35 , 36 By investigating this collectively, the potential additive effects of such comorbidities on the ventricular–vascular stiffness that underlie HFpEF can be appreciated, as reflected in modern international guidelines. Whereas the latest ESC 2021 HF guideline categorizes HF by LVEF cut‐offs, 37 the American Heart Association equivalent recognizes HF as a clinical continuum, defining stages A and B as individuals at risk of HF with evidence of structural heart disease in the latter. 38 This spectrum is reflected in our study groups. Group C (hypertension and DM) is akin to stage B, wherein asymptomatic individuals exhibited rising arterial stiffness, estimated LVEDP (E/e′ ≥ 13), and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) (two‐fold greater than healthy controls) towards but below the threshold that marked the onset of HFpEF (Group D). Pathophysiologically, if the effects of comorbidities remain unaddressed, it may only be a matter of time for the high arterial stiffness to exert enough hydraulic afterload on a maladaptively remodelled LV for Group C to progress to HFpEF. 8 , 23 , 35 In support of this, PWV‐based arterial stiffness was found to be independently associated with the degree of LV hypertrophy (LVH), diastolic dysfunction, and NT‐proBNP irrespective of BP, BMI, and renal function. 13 , 27 , 39 , 40 Furthermore, individuals with HF with mid‐range ejection fraction were found to have greater cf‐PWV than HFrEF, but similar to HFpEF, adding support to the concept of HF as a continuous spectrum with varying degrees of ventricular–arterial stiffness. 41 Together, these findings corroborate with our previous observations that LVDD transitions through stages from pre‐clinical LVDD, pre‐HFpEF, to HFpEF, 17 , 42 plausibly driven by an increasing arterial stiffness from rising age and comorbidity burden.
Effects of comorbidities on arterial stiffness
Arterial stiffness progresses rapidly with duration and worsens with amalgamating comorbidities, changes that may be small individually but cumulatively significant. 4 , 27 , 36 For example, even though cf‐PWV did not substantially increase from Groups B to C (P = 0.21) or from Groups C to D (P = 0.67) (Table 3 ), the small incremental rises (Figure 1 ) can have significant impact on ventricular–arterial coupling and LVDD, particularly in comorbid patients. Indeed, a difference in cf‐PWV of 2–3 m/s was found to be equivalent to several decades of vascular ageing. 31 Studies have shown that diabetic subjects with HFpEF had significantly more pronounced arterial stiffness, LVH, and HF symptoms than HFpEF alone 31 and that the effect of diabetes on cf‐PWV was greater among individuals with CKD than those without CKD. 33 Pathophysiologically, as comorbidities accumulate, elastic arteries become stiffer from elastin fragmentation, medial calcification, and collagen deposition. 34 , 43 Reflected pressure waves consequently arrive earlier to the heart, imposing excessive pulsatile workload on the LV with increased myocardial oxygen demand. This in turn drives a process of LVH, myocardial energetic inefficiency, and cardiac dysfunction. 5 , 11 , 12 , 44 Although SGLT2 inhibitors may play a role in mediating this process, optimizing comorbidities remains a vital means of halting the progression to and of HFpEF. 3 , 6
Significance of vascular ageing
It is also important not to overlook age as another important driver in the development of HFpEF because advanced age, particularly in women, has been shown to contribute significantly to arterial and diastolic stiffness. 45 This was appreciated in our study. First, Group D reflects the typical profile of HFpEF, in which individuals were predominantly older and more likely to be women. 37 , 38 Second, whereas the rise in cf‐PWV from Groups A to D remained statistically significant after adjustment for age (and other risk factors), the relationship between cf‐PWV and VO2 peak (r = −0.304) and with E/e′ (r = 0.307) was lost after correction for age. In a study assessing the effects of age and gender on arterial stiffness and LVDD, a similar strength correlation was found between PWV and E/e′ (r = 0.26), which was only seen in elderly women and not in younger women or men. 28 It may be speculated that comorbidities and female gender predispose the individual to HFpEF, whereas the additional effects of increasing age on ventricular–vascular stiffness may accelerate and eventually precipitate the development of HFpEF.
Arterial stiffness in heart failure with reduced ejection fraction
One major difference in the pathophysiology between HFrEF and HFpEF relates to changes in the pulsatile arterial haemodynamics. It has been suggested that whereas HFpEF is primarily associated with high vascular afterload, HFrEF exhibits lower pulse wave analysis‐derived measures of arterial stiffness (PWV, cPP, and AIx) due to failure of the mechanical pump, whereby a significantly reduced stroke volume leads to truncated ejection duration and arterial flow. 23 , 41 This may explain why Group E (HFrEF), with an average LVEF of 27%, displayed levels of cf‐PWV similar to those of healthy controls (10 ± 0.9 vs. 10.19 ± 2.02 m/s, P = 0.56). Strictly speaking, arterial stiffness was increased in Groups A and E, albeit slightly, because cf‐PWV > 10 m/s is considered pathologic and predictive of major adverse cardiovascular events. 16 , 46 In the case of HFrEF, the marginal rise in cf‐PWV, despite the reduced arterial flow from mechanical pump failure, may be attributed to coexisting cardiovascular risk factors and advanced age. A prospective cohort study adjusted for these risk factors and found that the association between cf‐PWV and adverse cardiac events in HFrEF was no longer significant, suggesting that the poorer outcome was likely driven by cardiovascular risk factors rather than the elevated cf‐PWV itself. 47
It is also important to note that brachial BP does not reflect arterial stiffness. Both HF groups had similarly normal brachial BP, which could be attributed to optimal anti‐hypertensive therapy over a longer duration. However, cf‐PWV in HFpEF was on average 2.68 m/s greater than that in HFrEF (P = 0.003). This could not be explained by age, BMI, or renal function because neither of these variables varied significantly between the two. Patients with identical BP can have substantially different afterload patterns due to the complex and dynamic interplay in the mechanical properties of the heart and arterial tree. 23 In other words, HFpEF patients can still have significantly raised arterial stiffness in the face of normotension.
Exercise capacity
High arterial stiffness may contribute to the pathophysiology of exercise intolerance, a hallmark of HFpEF. 7 A statistically significant negative correlation was observed between cf‐PWV and VO2 peak (r = −0.304, P = 0.03), suggesting that the greater the arterial stiffness, the lower the exercise capacity (Figure 2 ). Its strength of correlation was likely attenuated by including individuals from Group E who had near‐normal cf‐PWV values but significantly reduced VO2 peak. By excluding Group E, the strength of the same correlation was moderate (r = −0.426, P = 0.004). A relatively small number of patients who completed the CPET were included in the analysis. Nonetheless, our results are consistent with other studies that have shown an independent association between cf‐PWV and VO2 peak in hypertensive patients (r = −0.512, P = 0.003), 48 HFpEF (r = −0.34, P < 0.05), 49 and HFrEF (r = −0.39, P = 0.0007). 50 This high resting arterial stiffness will rise steeply during exercise, leading to a dramatic increase in proximal arterial load on the LV. 49 The imbalance in ventricular–arterial coupling widens further and exercise intolerance ensues. 7
Microvascular dysfunction
The secondary measure of LDF‐based microvascular dysfunction was not found to be significantly different between the groups. Likewise, other studies have not been able to consistently demonstrate endothelial dysfunction in HFpEF clinically. 26 , 51 Even the assessment of endothelial‐dependent vasodilatory response, for example, to acetylcholine, was not found to be useful in differentiating pathophysiological mechanisms between HFpEF and HFrEF because both groups had equally impaired vasodilatory responses. 26 However, a larger sample size was required given the large variability for each parameter in all five groups.
Limitations
This study should be interpreted within the context of its limitations. The cross‐sectional nature cannot establish a cause‐and‐effect relationship between arterial stiffness and HFpEF; however, valuable insight was provided on the role of comorbidities in HFpEF by examining across various patient phenotypes. Although this study adjusted for certain confounding variables known to affect arterial stiffness, for example, BMI and age, the available data on smoking and hypercholesterolaemia were limited to binary categorical values. A more nuanced analysis could have been achieved by incorporating continuous variables such as smoking pack years and serum lipid levels. Although the study size ensured 80% power for its primary outcome measure, a larger sample would be required to adequately assess for between‐group differences in microvascular function and CPET. Invasive assessment of ventricular–arterial elastance may offer more accurate values, but the potential use of peri‐procedural analgesia ± anaesthesia can confound normal physiology. Instead, we used a gold‐standard non‐invasive measure of arterial stiffness, in accordance with expert consensus. 16 Finally, although the HFpEF population was not age and gender matched, which can introduce confounding, deliberate matching patients would have resulted in an atypical HFpEF population (i.e. younger with male predominance), which can impact on the generalizability of our findings.
Conclusions
This study adds further support to the concept of HFpEF as a disease of the vasculature, underlined by an increasing arterial stiffness that is driven by vascular ageing and accumulating non‐cardiac comorbidities of hypertension and diabetes. Reflecting a pulsatile arterial afterload associated with diastolic dysfunction and exercise capacity, PWV may provide a clinically relevant tool to identify at‐risk intermediate phenotypes (e.g. pre‐HFpEF) before overt HFpEF occurs. After all, prevention is preferred over controlling the disease process, despite the promising benefits of SGLT2 inhibitors in HFpEF.
Conflict of interest
None declared.
Funding
This study was funded by a research grant from the West Midlands Clinical Research Network, National Institute of Health Research, UK and sponsored by the Research, Development & Innovation department of the University Hospitals Coventry & Warwickshire NHS Trust (RDI, UHCW), UK.
Ali, D. , Tran, P. , Ennis, S. , Powell, R. , McGuire, S. , McGregor, G. , Kimani, P. K. , Weickert, M. O. , Miller, M. A. , Cappuccio, F. P. , and Banerjee, P. (2023) Rising arterial stiffness with accumulating comorbidities associates with heart failure with preserved ejection fraction. ESC Heart Failure, 10: 2487–2498. 10.1002/ehf2.14422.
References
- 1. Packer M, Butler J, Zannad F, Filippatos G, Ferreira JP, Pocock SJ, Carson P, Anand I, Doehner W, Haass M, Komajda M, Miller A, Pehrson S, Teerlink JR, Schnaidt S, Zeller C, Schnee JM, Anker SD, for the EMPEROR‐Preserved Trial Study Group . Effect of empagliflozin on worsening heart failure events in patients with heart failure and a preserved ejection fraction: the EMPEROR‐Preserved trial. Circulation. 2021; 144: 1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bosch A, Ott C, Jung S, Striepe K, Karg MV, Kannenkeril D, Dienemann T, Schmieder RE. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol. 2019; 18: 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co‐transporter 2 (SGLT2) inhibitors: a state‐of‐the‐art review. JACC Basic Transl Sci. 2020; 5: 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mohammed SF, Borlaug BA, Roger VL, Mirzoyev SA, Rodeheffer RJ, Chirinos JA, Redfield MM. Comorbidity and ventricular and vascular structure and function in heart failure with preserved ejection fraction: a community‐based study. Circ Heart Fail. 2012; 5: 710–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffman JI, Buckberg GD. The myocardial oxygen supply:demand index revisited. J Am Heart Assoc. 2014; 3: e000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lyle MA, Brozovich FV. HFpEF, a disease of the vasculature: a closer look at the other half. Mayo Clin Proc. 2018; 93: 1305–1314. [DOI] [PubMed] [Google Scholar]
- 7. Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001; 38: 796–802. [DOI] [PubMed] [Google Scholar]
- 8. Ooi H, Chung W, Biolo A. Arterial stiffness and vascular load in heart failure. Congest Heart Fail. 2008; 14: 31–36. [DOI] [PubMed] [Google Scholar]
- 9. Weber T. The role of arterial stiffness and central hemodynamics in heart failure. Int J Heart Fail. 2020; 2: 209–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tran P, Maddock H, Banerjee P. Myocardial fatigue: a mechano‐energetic concept in heart failure. Curr Cardiol Rep. 2022; 24: 711–730. [DOI] [PubMed] [Google Scholar]
- 11. Tran P, Joshi M, Banerjee P. Concept of myocardial fatigue in reversible severe left ventricular systolic dysfunction from afterload mismatch: a case series. Eur Heart J ‐ Case Rep. 2021; 5: ytab089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Banerjee P. Heart failure: a story of damage, fatigue and injury? Open Heart. 2017; 4: e000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Desai AS, Mitchell GF, Fang JC, Creager MA. Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. J Card Fail. 2009; 15: 658–664. [DOI] [PubMed] [Google Scholar]
- 14. Reddy YNV, Andersen MJ, Obokata M, Koepp KE, Kane GC, Melenovsky V, Olson TP, Borlaug BA. Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2017; 70: 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Little WC, Zile MR. HFpEF: cardiovascular abnormalities not just comorbidities. Circ Heart Fail. 2012; 5: 669–671. [DOI] [PubMed] [Google Scholar]
- 16. Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid‐femoral pulse wave velocity. J Hypertens. 2012; 30: 445–448. [DOI] [PubMed] [Google Scholar]
- 17. Banerjee P, Motiwala A, Mustafa HM, Gani MA, Fourali S, Ali D. Does left ventricular diastolic dysfunction progress through stages? Insights from a community heart failure study. Int J Cardiol. 2016; 221: 850–854. [DOI] [PubMed] [Google Scholar]
- 18. Ali D, Callan N, Ennis S, Powell R, McGuire S, McGregor G, Weickert MO, Miller MA, Cappuccio FP, Banerjee P. Heart failure with preserved ejection fraction (HFpEF) pathophysiology study (IDENTIFY‐HF): does increased arterial stiffness associate with HFpEF, in addition to ageing and vascular effects of comorbidities? Rationale and design. BMJ Open. 2019; 9: e027984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Geneva WHO . Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia. Geneva: World Health Organization; 2006. Available at https://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf Accessed on 23/11/2021. [Google Scholar]
- 20. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 21. Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005; 18: 3S–10S. [DOI] [PubMed] [Google Scholar]
- 22. Nürnberger J, Keflioglu‐Scheiber A, Opazo Saez AM, Wenzel RR, Philipp T, Schäfers RF. Augmentation index is associated with cardiovascular risk. J Hypertens. 2002; 20: 2407–2414. [DOI] [PubMed] [Google Scholar]
- 23. Weber T, O'Rourke MF, Ammer M, Kvas E, Punzengruber C, Eber B. Arterial stiffness and arterial wave reflections are associated with systolic and diastolic function in patients with normal ejection fraction. Am J Hypertens. 2008; 21: 1194–1202. [DOI] [PubMed] [Google Scholar]
- 24. Maréchaux S, Samson R, van Belle E, Breyne J, de Monte J, Dédrie C, Chebai N, Menet A, Banfi C, Bouabdallaoui N, le Jemtel TH, Ennezat PV. Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. J Card Fail. 2016; 22: 3–11. [DOI] [PubMed] [Google Scholar]
- 25. Society AT , Physicians ACoC . ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003; 167: 211–277. [DOI] [PubMed] [Google Scholar]
- 26. Balmain S, Padmanabhan N, Ferrell WR, Morton JJ, McMurray JJ. Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. Eur J Heart Fail. 2007; 9: 865–871. [DOI] [PubMed] [Google Scholar]
- 27. Cauwenberghs N, Knez J, D'hooge J, Thijs L, Yang WY, Wei FF, et al. Longitudinal changes in LV structure and diastolic function in relation to arterial properties in general population. JACC Cardiovasc Imaging. 2017; 10: 1307–1316. [DOI] [PubMed] [Google Scholar]
- 28. Kim HL, Lim WH, Seo JB, Chung WY, Kim SH, Kim MA, Zo JH. Association between arterial stiffness and left ventricular diastolic function in relation to gender and age. Medicine (Baltimore). 2017; 96: e5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chirinos JA, Segers P, Duprez DA, Brumback L, Bluemke DA, Zamani P, Kronmal R, Vaidya D, Ouyang P, Townsend RR, Jacobs DR Jr. Late systolic central hypertension as a predictor of incident heart failure: the Multi‐Ethnic Study of Atherosclerosis. J Am Heart Assoc. 2015; 4: e001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, Laurent Ś. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002; 39: 10–15. [DOI] [PubMed] [Google Scholar]
- 31. Chirinos JA, Bhattacharya P, Kumar A, Proto E, Konda P, Segers P, Akers SR, Townsend RR, Zamani P. Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. J Am Heart Assoc. 2019; 8: e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Prenner SB, Chirinos JA. Arterial stiffness in diabetes mellitus. Atherosclerosis. 2015; 238: 370–379. [DOI] [PubMed] [Google Scholar]
- 33. Townsend RR, Anderson AH, Chirinos JA, Feldman HI, Grunwald JE, Nessel L, Roy J, Weir MR, Wright JT Jr, Bansal N, Hsu CY, Appel LJ, Go AS, He J, Kusek JW, Lash JP, Ojo A, Rahman M. Association of pulse wave velocity with chronic kidney disease progression and mortality: findings from the CRIC study (Chronic Renal Insufficiency Cohort). Hypertension. 2018; 71: 1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Feola M, Testa M, Ferreri C, Rosso G, Rossi A, Ruocco G. The analysis of arterial stiffness in heart failure patients in comparison with healthy subjects and patients with cardiovascular risk factors. J Clin Med. 2019; 8: 1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003; 107: 714–720. [DOI] [PubMed] [Google Scholar]
- 36. Chow B, Rabkin SW. The relationship between arterial stiffness and heart failure with preserved ejection fraction: a systemic meta‐analysis. Heart Fail Rev. 2015; 20: 291–303. [DOI] [PubMed] [Google Scholar]
- 37. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 38. Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B, American College of Cardiology , American Heart Association Task Force on Practice Guidelines , American College of Chest Physicians , International Society for Heart and Lung Transplantation , Heart Rhythm Society . ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005; 112: e154–e235. [DOI] [PubMed] [Google Scholar]
- 39. Tokitsu T, Yamamoto E, Oike F, Hirata Y, Tsujita K, Yamamuro M, Kaikita K, Hokimoto S. Clinical significance of brachial‐ankle pulse‐wave velocity in patients with heart failure with preserved left ventricular ejection fraction. J Hypertens. 2018; 36: 560–568. [DOI] [PubMed] [Google Scholar]
- 40. Mika M, Kanzaki H, Hasegawa T, Fukuda H, Amaki M, Kim J, Asakura M, Asanuma H, Nishimura M, Kitakaze M. Arterial stiffening is a crucial factor for left ventricular diastolic dysfunction in a community‐based normotensive population. Int J Cardiol Hypertens. 2020; 6: 100038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang WM, Sung SH, Yu WC, Cheng HM, Huang CJ, Guo CY, Lu DY, Lee CW, Chen CH. Perturbations of pulsatile hemodynamics and clinical outcomes in patients with acute heart failure and reduced, mid‐range or preserved ejection fraction. PLoS ONE. 2019; 14: e0220183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tran P, Obukofe R, Thompson W, Desai N, Kader NA, Banerjee P. 151 The pre‐HFpEF entity: a window of opportunity to prevent and halt the progression to HF with preserved ejection fraction (HFpEF). Heart. 2021; 107: A115. [Google Scholar]
- 43. Towler DA. Arteriosclerotic calcification: a serpi(n)ginous path to cardiovascular health? Circ Res. 2015; 117: 744–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail. 2010; 3: 149–156. [DOI] [PubMed] [Google Scholar]
- 45. Redfield MM, Jacobsen SJ, Borlaug BA, Rodeheffer RJ, Kass DA. Age‐ and gender‐related ventricular‐vascular stiffening: a community‐based study. Circulation. 2005; 112: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010; 121: 505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pandey A, Khan H, Newman AB, Lakatta EG, Forman DE, Butler J, Berry JD. Arterial stiffness and risk of overall heart failure, heart failure with preserved ejection fraction, and heart failure with reduced ejection fraction: the Health ABC Study (Health, Aging, and Body Composition). Hypertension. 2017; 69: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kallistratos E, Tsinivizov P, Kalogeris A, Poulimenos LE, Latsou D, Andriopoulou M, Dimaki N, Kouremenos N, Koukouzeli A, Chamodraka EF, Pittaras A, Makris T, Vlahakos D, Manolis AJ. Correlation of pulse wave velocity with functional capacity in healthy subjects and well controlled hypertensive patients. J Hypertens. 2019; 37: e41. [Google Scholar]
- 49. Fujiwara K, Shimada K, Nishitani‐Yokoyama M, Kunimoto M, Matsubara T, Matsumori R, Abulimiti A, Aikawa T, Ouchi S, Shimizu M, Fukao K, Miyazaki T, Honzawa A, Yamada M, Saitoh M, Morisawa T, Takahashi T, Daida H, Minamino T. Arterial stiffness index and exercise tolerance in patients undergoing cardiac rehabilitation. Int Heart J. 2021; 62: 230–237. [DOI] [PubMed] [Google Scholar]
- 50. Bonapace S, Rossi A, Cicoira M, Franceschini L, Golia G, Zanolla L, Marino P, Zardini P. Aortic distensibility independently affects exercise tolerance in patients with dilated cardiomyopathy. Circulation. 2003; 107: 1603–1608. [DOI] [PubMed] [Google Scholar]
- 51. Shantsila E, Wrigley B, Shantsila A, Tapp LD, Blann AD, Gill PS, Lip GYH. Ethnic differences in macrovascular and microvascular function in systolic heart failure. Circ Heart Fail. 2011; 4: 754–762. [DOI] [PubMed] [Google Scholar]


