Abstract
Aims
We sought to investigate the relationship between circulating tissue plasminogen activator (t‐PA) level and long‐term outcomes in stable coronary artery disease patients with or without aortic valve sclerosis (AVSc).
Methods and results
Serum levels of t‐PA were determined in 347 consecutive stable angina patients with (n = 183) or without (n = 164) AVSc. Outcomes were prospectively recorded as planned clinic evaluations every 6 months up to 7 years. The primary endpoint was a composite of cardiovascular death and rehospitalization due to heart failure. The secondary endpoint included all‐cause mortality, cardiovascular death, and rehospitalization due to heart failure. Serum t‐PA was significantly higher in AVSc than in non‐AVSc patients (2131.22 pg/mL vs. 1495.85 pg/mL, P < 0.001). For patients with AVSc, those with t‐PA level above the median (>1840.68 pg/mL) were more likely to meet the primary and secondary endpoints (all P < 0.001). After adjusting for potential confounding factors, serum t‐PA level remained significantly predictive for each endpoint in the Cox proportional hazard models. The prognostic value of t‐PA was good, with an AUC‐ROC of 0.753 (P < 0.001). The combination of t‐PA with traditional risk factors improved the risk reclassification of AVSc patients, with a net reclassification index of 0.857 and an integrated discrimination improvement of 0.217 (all P < 0.001). However, for patients without AVSc, both primary and secondary endpoints were similar, irrespective of t‐PA levels.
Conclusions
Elevated circulating t‐PA confers an increased risk for poor long‐term clinical outcomes in stable coronary artery disease patients with AVSc.
Keywords: Aortic valve sclerosis, Coronary artery disease, Prognosis, Tissue plasminogen activator
Introduction
Aortic valve sclerosis (AVSc), non‐uniform thickening and calcification of aortic valve leaflet in the absence of ventricular outflow obstruction, 1 is increasingly prevalent and usually considered an age‐related degenerative process followed by progressive valve motion abnormality. 2 Robust evidence has demonstrated that AVSc shares strikingly similar pathophysiology with atherosclerosis. 3 , 4 Several traditional risk factors for coronary artery disease (CAD), such as dyslipidemia and hypertension, also affect the incidence of AVSc. 5 , 6 Besides, the development of AVSc involves chronic inflammatory reactions characterized by endothelial damage, deposition of lipoproteins, infiltration of inflammatory cells, and oxidative stress, which directly results in extracellular matrix degradation and calcification, akin to atherosclerosis as well. 7 Additionally, AVSc predicts all‐cause and cardiovascular mortality. 8
Tissue plasminogen activator (t‐PA), a glycoprotein produced by vascular endothelial cells, induces clot dissolution by converting plasminogen to plasmin, 9 and acts as an in vivo marker of endothelial cell injury. Circulating t‐PA has been shown to correlate with the presence and severity of CAD 10 and plays an important role in plaque instability through destabilizing the fibrous caps of atheromatous plaques 11 and initiating a proteolytic cascade of matrix degradation of the plaque. 4 , 12 Previously, we observed that circulating t‐PA provided good diagnostic performance as a biomarker of AVCs. 4 However, the prognostic value of t‐PA in CAD patients, especially for those with AVSc, remains not well understood. In this study, we sought to examine the relationship between serum t‐PA and long‐term clinical outcomes in stable CAD patients with and without AVSc.
Methods
Patient population
A total of 805 patients diagnosed as CAD by coronary angiography in the Department of Cardiovascular Medicine, Shanghai Ruijin Hospital, from September 2011 to June 2012 due to typical anginal symptoms and/or electrocardiographic ST‐T wave changes were consecutively recruited. Among them, 571 patients underwent standard transthoracic echocardiography and Doppler flow imaging, according to the recommendations of the American Society of Echocardiography during hospitalization, and were included in the screening procedure for the current study. Patients with acute coronary syndrome or stroke (n = 62), cancer (n = 10), infectious disease (n = 5), severe renal impairment [estimated glomerular filtration rate (eGFR) < 15 mL/min/1.73 m2 or requiring haemodialysis] (n = 11), anticoagulation treatment (n = 26), hypertrophic or dilated cardiomyopathy (n = 17), atrial fibrillation (n = 30), pulmonary heart disease (n = 6), haematological (n = 3), or rheumatic disease (n = 3) were excluded. According to the echo result, we also excluded patients with valve stenosis (n = 12), congenital aortic valve malformation (n = 9), previous aortic valve replacement (n = 8), and congenital heart disease (n = 11). Furthermore, 11 patients refused to participate in the study. The remaining 347 patients were included in the final analysis. AVSc and non‐AVCs were identified in 183 and 164 patients, respectively (Figure 1 ).
Figure 1.
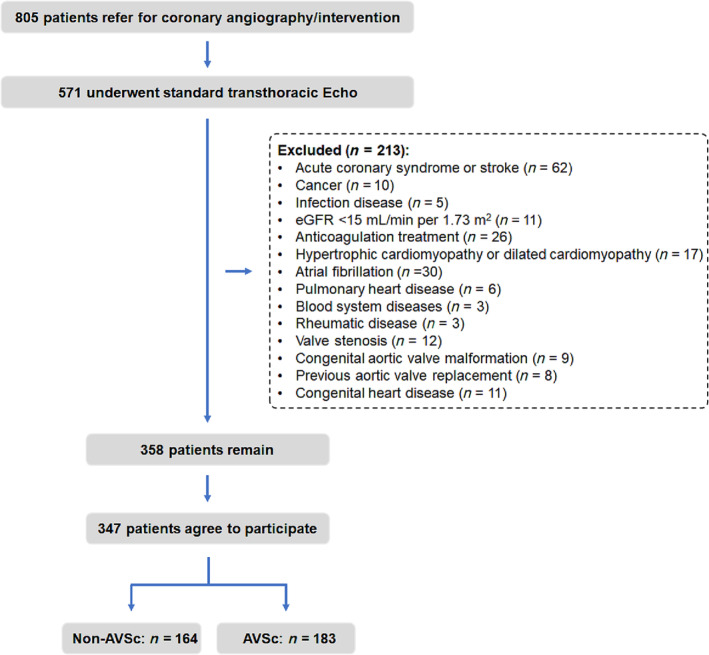
Flowchart of patient enrollment.
The study protocol was approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, and written informed consent was obtained from all patients.
Definitions and measurements
AVSc was defined as non‐transparent aortic valve leaflets with focal areas of mild thickening or increased echogenicity without motive restriction and a peak flow velocity across the aortic valve <2.0 m/s (Figure 2 ). 13 CAD was diagnosed when there was ≥50% luminal diameter stenosis in at least one major epicardial coronary artery, according to the lesion classification scheme of the American College of Cardiology/American Heart Association. 14 Renal function was evaluated by eGFR, which was calculated using the Modification of Diet in Renal Disease equation. 15 Serum level of t‐PA was measured in the fasting venous blood samples using an ELISA (Tissue Plasminogen Activator (t‐PA) Human SimpleStep ELISA Kit, Abcam, Cat No. ab190812) according to the product instructions.
Figure 2.
Typical echo images of non‐AVSc (left) and AVSc (right).
Outcomes
Patients were followed until death or the last visit. Outcomes were prospectively recorded as planned clinic evaluations every 6 months up to 7 years after enrollment. The primary endpoint was a composite of cardiovascular death or rehospitalization due to heart failure, and the second endpoint included cardiovascular death, rehospitalization due to heart failure, and all‐cause mortality. Heart failure was diagnosed based on clinical symptoms with an elevation of plasma N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). Death was identified primarily by the review of medical records and direct contact with the patient's family. Cardiovascular death included sudden cardiac death or death caused by acute myocardial infarction. Rehospitalization due to heart failure was confirmed.
Statistical analysis
Continuous variables are expressed as the mean and standard deviation when normally distributed and the median with interquartile interval when not normally distributed. Categorical data are summarized as proportions and frequencies. Continuous and categorical variables were compared by independent t‐test and nonparametric or χ 2 test, respectively. Kaplan–Meier (KM) curves were constructed to predict the outcome for each endpoint (primary endpoint, cardiovascular death, rehospitalization, and all‐cause mortality). Log‐rank test was used to compare survival situations among groups. Cox proportional hazard analysis was used to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) and assess whether t‐PA could predict the prognosis of patients using log‐transformed t‐PA levels. Multivariate analyses were performed to evaluate the association of t‐PA with the composite primary outcome, cardiovascular death, rehospitalization, and all‐cause mortality. The results were first adjusted for age and sex and then for the full model, including age, sex, risk factors for CAD, alcohol consumption, glycosylated haemoglobin (HbA1c), lipid profiles including triglyceride (TG), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), and high‐density lipoprotein (HDL‐C), r‐glutamine transaminase (r‐GT), eGFR, and drug usage. Moreover, each of the analyses was performed separately in CAD patients with and without AVSc.
All statistical analyses were performed by SPSS software (version 19.0) or R software (version 4.0.2). Statistical significance was set at two‐tailed, and P values <0.05 were considered statistically significant.
Results
Baseline clinical characteristics
Compared with non‐AVSc patients, those with AVSc were older and had a higher proportion of males and hypertension (Table 1 , all P < 0.05). In addition, serum levels of TG, HDL‐C and eGFR were lower, but the Hb1Ac level was higher in patients with AVSc (all P < 0.05). However, the two groups did not differ with respect to the percentage of diabetes, smokers, body mass index (BMI), and fast blood glucose. The proportion of medications with statins, beta‐blocker, and antiplatelet agents was higher in the AVSc group (Table 1 ). Moreover, seven patients developed aortic valve stenosis, and their t‐PA level was higher compared with those who did not develop valve stenosis (3085.68 pg/mL vs. 2133.01 pg/mL, P = 0.006). Further analysis showed that in non‐AVSc group, patients with high t‐PA level were older and had greater BMI compared with those with low t‐PA level. In AVSc group, patients with high t‐PA level had higher rGT and lower HDL and eGFR (Table S1 ).
Table 1.
Baseline characteristics of CAD patients with or without AVSc
| AVSc | Non‐AVSc | P value | |
|---|---|---|---|
| (n = 183) | (n = 164) | ||
| Age, years | 74.00 (66.00, 80.00) | 61.00 (56.00, 68.00) | <0.001 |
| Male, sex | 117 (63.93%) | 85 (51.83%) | 0.022 |
| Smoking | 57 (31.15%) | 41(25.00%) | 0.204 |
| Alcohol use | 21 (11.5%) | 17 (10.37%) | 0.741 |
| Hypertension | 144 (78.69%) | 109 (66.46%) | 0.011 |
| DM | 73 (39.89%) | 51 (31.10%) | 0.088 |
| Fasting blood‐glucose | 5.02 (4.56, 5.95) | 4.92 (4.50, 5.68) | 0.451 |
| Hb1Ac% | 6.00 (5.70, 6.80) | 5.90 (5.60, 6.40) | 0.029 |
| TG mmol/L | 1.30 (0.98, 2.01) | 1.47 (1.17, 2.07) | 0.034 |
| TC | 3.85 (3.13, 4.53) | 3.95 (3.36, 4.62) | 0.086 |
| HDL | 1.05 (0.91, 1.23) | 1.10 (0.96, 1.29) | 0.045 |
| LDL mmol/L | 2.25 (1.74, 2.99) | 2.32 (1.72, 2.89) | 0.956 |
| rGT mmol/L | 21.00 (15.00, 29.00) | 19.00 (12.25, 30.00) | 0.295 |
| eGFR mL/min/1.73 m2 | 72.51 ± 19.24 | 87.06 ± 17.46 | <0.001 |
| T‐PA pg/mL | 2131.22 (1415.44, 2690.03) | 1495.85 (1043.11, 2145.82) | <0.001 |
| Aspirin usage | 156 (85.25%) | 108 (65.85%) | <0.001 |
| ACEI/ARB | 121 (66.12%) | 106 (64.63%) | 0.771 |
| Beta‐blocker | 139 (75.96%) | 104 (63.41%) | 0.011 |
| Statin | 156 (85.25%) | 121 (73.78%) | 0.008 |
ACEI, angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; rGT, γ‐glutamyl transferase; HDL, high density lipoprotein; LDL, low density lipoprotein; TG, triglyceride; TC, total cholesterol; T‐PA, tissue plasminogen activator.
Serum tissue plasminogen activator (t‐PA) and outcome in aortic valve sclerosis (AVSc) and non‐AVSc patients
For patients with AVSc, those with higher t‐PA levels in serum (above the median value 1840.68 pg/mL) were more likely to meet primary (P < 0.001, Figure 3 A,B ) and secondary endpoints (P < 0.001, Figure 3 C,D ). Higher t‐PA levels conferred an increased risk of the primary endpoint (HR = 2.934, P < 0.001), cardiovascular death (HR = 3.712, P < 0.001), rehospitalization due to heart failure (HR = 3.220, P < 0.001), and all‐cause mortality (HR = 3.561, P < 0.001) (Figure 4 ). After adjusting for traditional confounding factors, including age, sex, BMI, smoking, alcohol consumption, history of diabetes or hypertension, levels of HbA1c, TG, TC, HDL, LDL, rGT, eGFR, and use of aspirin, angiotensin‐converting enzyme inhibitor (ACEI) /angiotensin receptor blocker (ARB), beta‐blocker or statins, serum t‐PA still remained significantly predictive for the primary endpoint (HR = 2.920, P < 0.001), cardiovascular death (HR = 3.237, P < 0.001), rehospitalization due to heart failure (HR = 3.284, P < 0.001), and all‐cause mortality (HR = 3.111, P < 0.001) (Figure 4 ). The C‐statistic analyses revealed an increased predictive value for the primary endpoint when considering t‐PA levels (AUC‐ROC = 0.846, P < 0.001) (Table 2 ). The addition of serum t‐PA also improved the risk reclassification of AVSc patients, with a net reclassification index (NRI) of 0.857 and an integrated discrimination improvement (IDI) of 0.217 (both P < 0.001, Table 2 ). In fact, this value of t‐PA level was associated with a six‐fold increased risk for the primary endpoint (HR = 6.218 [3.168–12.204], P < 0.001) and five‐ to seven‐fold increased risk for secondary endpoints (cardiovascular death, HR = 6.973 [2.460–19.764], P < 0.001; rehospitalization due to heart failure, HR = 5.650 [2.841–11.235], P < 0.001; all‐cause mortality, HR = 7.626 [2.705–21.498], P < 0.001) (Table 3 ). The AUC‐ROC analysis showed that an optimal cutoff of t‐PA 1818.71 pg/mL best predicted the primary end point in these patients. The number of patients with t‐PA levels above and below this value was 170 and 177, respectively. Furthermore, there was a significant difference in event rates stratified according to the t‐PA cut‐off value (Figure 5 A,B ).
Figure 3.
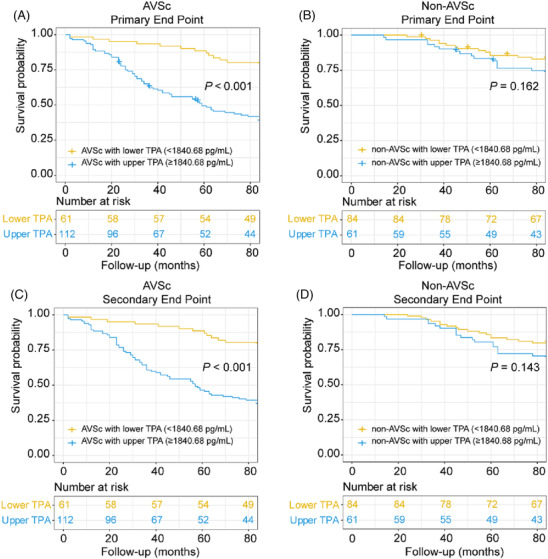
Prediction of primary and secondary outcomes according to median t‐PA level. Primary outcomes of AVSc (A) and non‐AVSc (B), and secondary outcomes of AVSc (C) and non‐AVSc (D).
Figure 4.
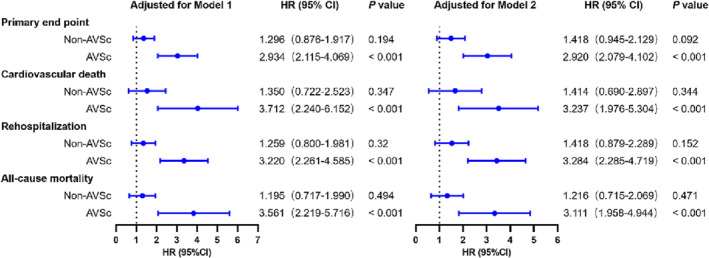
Forest plot of t‐PA as a predictor for endpoints in CAD patients with or without AVSc. T‐PA level was analysed as a log‐transformed continuous variable. Model 1: adjusted for age and sex, Model 2: adjusted for age and sex, and then for the whole model, including age, sex, risk factors for coronary artery disease, alcohol consumption, HbA1c, lipid profiles, r‐GT, eGFR, and drug usage.
Table 2.
C‐statistic analysis of t‐PA as predictors for primary endpoint
| Total cohort (n = 347) | AVSc (n = 183) | Non‐AVSc (n = 164) | |
|---|---|---|---|
| AUC‐ROC (T‐PA only) | 0.709 (0.650–0.768) | 0.753 (0.682–0.823) | 0.561 (0.442–0.681) |
| P < 0.001 | P < 0.001 | 0.301 | |
| AUC‐ROC (Traditional factors only) | 0.657 (0.595–0.719) | 0.679 (0.599–0.759) | 0.581 (0.466–0.697) |
| P < 0.001 | P < 0.001 | 0.171 | |
| AUC‐ROC (Traditional factors + t‐PA) | 0.750 (0.693–0.806) | 0.846 (0.789–0.904) | 0.581 (0.466–0.697) |
| P < 0.001 | P < 0.001 | 0.171 | |
| NRI | 0.493 (0.272–0.714) | 0.857 (0.590–1.124) | 0.165 (−0.236–0.566) |
| P < 0.001 | P < 0.001 | 0.419 | |
| IDI | 0.096 (0.064–0.128) | 0.217 (0.156–0.277) | 0.027 (−0.006–0.061) |
| P < 0.001 | P < 0.001 | 0.106 |
Traditional factors included age, sex, BMI, smoking, alcohol use, history of DM, history of hypertension, HbA1c, TG, TC, HDL, LDL, rGT, eGFR, aspirin use, ACEI/ARB use, beta‐blocker use, and statin use.
Table 3.
Multivariate Cox proportional hazard models for t‐PA as a predictor of endpoints in CAD patients with or without AVSc
| AVSc | Non‐AVSc | |||
|---|---|---|---|---|
| t‐PA level | HR (CI) | P value | HR (CI) | P value |
| Primary endpoint | ||||
| <1818.71 pg/mL | 1 | 1 | 1 | 1 |
| ≥1818.71 pg/mL | 6.218 [3.168–12.204] | <0.001 | 2.073 [0.972–4.423] | 0.059 |
| Cardiovascular death | ||||
| <1818.71 pg/mL | 1 | 1 | 1 | 1 |
| ≥1818.71 pg/mL | 6.973 [2.460–19.764] | <0.001 | 2.734 [0.823–9.081] | 0.101 |
| Rehospitalization due to heart failure | ||||
| <1818.71 pg/mL | 1 | 1 | 1 | 1 |
| ≥1818.71 pg/mL | 5.650 [2.841–11.235] | <0.001 | 1.833 [0.719–4.676] | 0.205 |
| All‐cause mortality | ||||
| <1818.71 pg/mL | 1 | 1 | 1 | 1 |
| ≥1818.71 pg/mL | 7.626 [2.705–21.498] | <0.001 | 2.096 [0.751–5.847] | 0.157 |
In Model 2, traditional factors as in Table 2 were adjusted.
Figure 5.
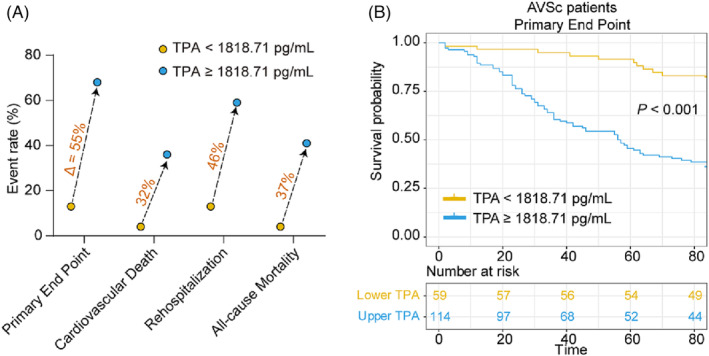
Event rates and Kaplan–Meier curve analysis according to estimated t‐PA cut‐off value in patients with AVSc. (A) Rates of the primary endpoint, cardiovascular death, rehospitalization, and all‐cause mortality for patients below and above t‐PA cut‐off value (1818.71 pg/mL) in AUC‐ROC analysis. (B) Kaplan–Meier curves for primary endpoint for all patients according to the cut‐off value of t‐PA level.
In contrast, both primary and secondary endpoints were similar for non‐AVSc patients, irrespective of t‐PA levels (Figures 3 C,D and 4 ). Moreover, serum t‐PA did not provide additive value in risk prediction for these patients (Tables 2 and 3 ).
Discussion
Our results indicated that elevated circulating t‐PA level was significantly associated with long‐term adverse outcomes in stable CAD patients with AVSc.
Previous studies showed that t‐PA secretion was positively related to the severity of myocardial ischaemia 16 , 17 , 18 and coronary stenosis. 19 , 20 Besides, circulating t‐PA served as a reliable predictor of subsequent myocardial infarction 21 , 22 and a risk factor for cardiac arrhythmia after thrombolytic therapy. 23 , 24 , 25 The present study is the first to show that t‐PA level in serum was associated with adverse outcomes for CAD patients with AVSc. Interestingly, a significant association between t‐PA level and adverse outcomes was not observed in CAD patients without AVSc, suggesting that t‐PA may have potential relationships with the pathological manifestation of AVSc.
T‐PA has been reported to directly regulate cytokine signalling through non‐enzymatic mechanisms, thus promoting inflammatory responses in fibrinolytic systems. 26 Several studies have shown that t‐PA could damage the endothelial cell barrier by inhibiting the Sonic Hedgehog (Shh) pathway 27 or induction of C‐C motif chemokine ligand 2 (CCL2). 28 Although thrombolytic therapy for acute ischaemic stroke is applicable, t‐PA‐induced damages to human microvascular endothelial cells still occur. 29 As endothelial‐to‐mesenchymal transition was influential in the pathological process of AVSc, 30 , 31 t‐PA‐mediated endothelial dysfunction might be a possible trigger of AVSc. Overall, these observations support a notion that elevated t‐PA might be responsible for the deterioration of endothelial cells, leading to poor prognosis of CAD patients with AVSc.
It is well recognized that t‐PA activates matrix metalloproteinases (MMPs), 32 including MMP‐2, 33 MMP‐8, 34 and MMP‐9, 35 , 36 which are associated with the progression of ventricular remodelling, 37 , 38 valvular disease, 39 and heart failure. 40 The expression of MMP‐9 was significantly decreased after t‐PA knockout with focal cerebral ischaemia. In human microvascular endothelial cells, the expression of MMP‐9 was up‐regulated when adding exogenous recombinant t‐PA. Moreover, the development of AVSc involves phenotypic changes of aortic valve interstitial cells through the osteogenic pathway, substantiating the role of interstitial components in the pathological progression of AVSc. 41 , 42 Therefore, it was postulated that high circulating t‐PA might induce up‐regulation of MMPs expression, leading to remodelling of the local extracellular matrix in the aortic valve and accelerating subsequent aortic valve fibrosis. Nevertheless, the detailed mechanisms of how t‐PA influences the development of AVSc still require more investigations.
Our study showed that in patients without AVSc, both primary and secondary endpoints were similar, irrespective of t‐PA levels. The reason for this might be multifactorial. AVSc was reported to predict all‐cause and cardiovascular mortality. 8 Moreover, the development of AVSc shares certain similar mechanism of atherosclerosis, such as endothelial damage, deposition of lipoproteins, and calcification, 7 which suggests that AVSc itself might be a marker of more widespread arterial disease.
There are some limitations in our study. First, we only enrolled patients with CAD in our study, and the sample size was relatively small. Further studies with a large cohort of patients with versatile backgrounds, including those without CAD, are warranted to prove our results. Second, the diagnosis of AVSc was mainly based on transthoracic echocardiography. Cardiac computed tomography could provide a quantitative assessment. Last, despite careful adjustment for major known confounding factors, unspecified elements may also interfere with our results.
Taken together, this study demonstrates circulating t‐PA level as an independent factor for the prognosis of stable CAD patients with AVSc. The findings may provide insight to physicians who are involved in decision‐making for these patients, and further large‐scale, prospective studies are warranted to confirm our results.
Funding
This work was funded by the Programs of National Natural Science Foundation of China (82100381 and 82000378), the Shanghai Key Clinical Specialty Project (shslczdzk06202) and the Top‐Level Clinical Discipline Project of Shanghai Pudong District Grant (PWYgf 2021‐01).
Conflict of interest
None.
Supporting information
Table S1. Baseline characteristics between patients with high or low t‐PA levels in non‐AVSc and non‐AVSc groups.
Lin, B. , Shen, Y. , Zhang, P. , Shen, Y. , Gu, Y. , He, X. , Li, J. , Yang, K. , Shen, W. , Zhang, Q. , Xin, Y. , and Liu, Y. (2023) Prognostic role of tissue plasminogen activator in coronary artery disease with or without aortic valve sclerosis. ESC Heart Failure, 10: 2541–2549. 10.1002/ehf2.14420.
Bowen Lin, Ying Shen, and Pengfei Zhang contributed equally to this study.
Contributor Information
Yuanfeng Xin, Email: xinyuanfeng@hotmail.com.
Yehong Liu, Email: flshch@163.com.
References
- 1. Freeman RV, Otto CM. Spectrum of calcific aortic valve disease: pathogenesis, disease progression, and treatment strategies. Circulation. 2005; 111: 3316–3326. [DOI] [PubMed] [Google Scholar]
- 2. Liu Y, Gu Y, Shen Y, Lin B, Li Y, He X, Zhang Y, Lu L, Shen W, Zhang Q, Yang K. Association between serum leptin level and calcific aortic valve disease. J Am Heart Assoc. 2019; 8: e012495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu YH, Liu Y, Xin YF, Zhang Q, Ding ML. Identification of key genes involved in calcific aortic valve disease based on integrated bioinformatics analysis. Exp Biol Med (Maywood). 2022: 15353702221118088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen Z, Shen Y, Xue Q, Lin BW, He XY, Zhang YB, Yang Y, Shen WF, Liu YH, Yang K. Clinical relevance of plasma endogenous tissue‐plasminogen activator and aortic valve sclerosis: performance as a diagnostic biomarker. Front Cardiovasc Med. 2020; 7: 584998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chandra HR, Goldstein JA, Choudhary N, O'Neill CS, George PB, Gangasani SR, Cronin L, Marcovitz PA, Hauser AM, O'Neill WW. Adverse outcome in aortic sclerosis is associated with coronary artery disease and inflammation. J Am Coll Cardiol. 2004; 43: 169–175. [DOI] [PubMed] [Google Scholar]
- 6. Owens DS, Budoff MJ, Katz R, Takasu J, Shavelle DM, Carr JJ, Heckbert SR, Otto CM, Probstfield JL, Kronmal RA, O'Brien KD. Aortic valve calcium independently predicts coronary and cardiovascular events in a primary prevention population. JACC Cardiovasc Imaging. 2012; 5: 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossi A, Faggiano P, Amado AE, Cicoira M, Bonapace S, Franceschini L, Dini Lloyd F, Ghio S, Agricola E, Temporelli PL, Vassanelli C. Aortic valve sclerosis is a marker of atherosclerosis independently of traditional clinical risk factors. Analysis in 712 patients without ischemic heart disease. Int J Cardiol. 2012; 158: 163–164. [DOI] [PubMed] [Google Scholar]
- 8. Volzke H, Haring R, Lorbeer R, Wallaschofski H, Reffelmann T, Empen K, Rettig R, John U, Felix SB, Dorr M. Heart valve sclerosis predicts all‐cause and cardiovascular mortality. Atherosclerosis. 2010; 209: 606–610. [DOI] [PubMed] [Google Scholar]
- 9. Kooistra T, Schrauwen Y, Arts J, Emeis JJ. Regulation of endothelial cell t‐PA synthesis and release. Int J Hematol. 1994; 59: 233–255. [PubMed] [Google Scholar]
- 10. Segarra A, Chacon P, Martinez‐Eyarre C, Argelaguer X, Vila J, Ruiz P, Fort J, Bartolome J, Camps J, Moliner E, Pelegri A, Marco F, Olmos A, Piera L. Circulating levels of plasminogen activator inhibitor type‐1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: biochemical correlations and role as independent predictors of coronary artery stenosis. J Am Soc Nephrol. 2001; 12: 1255–1263. [DOI] [PubMed] [Google Scholar]
- 11. Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997; 17: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 12. Clowes AW, Clowes MM, Au YP, Reidy MA, Belin D. Smooth muscle cells express urokinase during mitogenesis and tissue‐type plasminogen activator during migration in injured rat carotid artery. Circ Res. 1990; 67: 61–67. [DOI] [PubMed] [Google Scholar]
- 13. Milin AC, Vorobiof G, Aksoy O, Ardehali R. Insights into aortic sclerosis and its relationship with coronary artery disease. J Am Heart Assoc. 2014; 3: e001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA Guideline Focused Update on Duration of Dual Antiplatelet Therapy in Patients With Coronary Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: An Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non‐ST‐Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation. 2016; 134: e123–e155. [DOI] [PubMed] [Google Scholar]
- 15. Ellis SG, Vandormael MG, Cowley MJ, DiSciascio G, Deligonul U, Topol EJ, Bulle TM. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation. 1990; 82: 1193–1202. [DOI] [PubMed] [Google Scholar]
- 16. Winnerkvist A, Wiman B, Valen G, Vaage J. Release of tissue plasminogen activator during reperfusion after different times of ischaemia in isolated, perfused rat hearts. Thromb Res. 1996; 82: 533–542. [DOI] [PubMed] [Google Scholar]
- 17. Aspelin T, Eriksen M, Lindgaard AK, Lyberg T, Ilebekk A. Cardiac fibrinolytic capacity is markedly increased after brief periods of local myocardial ischemia, but declines following successive periods in anesthetized pigs. J Thromb Haemost. 2005; 3: 1947–1954. [DOI] [PubMed] [Google Scholar]
- 18. Osterlund B, Andersson B, Häggmark S, Jern C, Johansson G, Seeman‐Lodding H, Biber B. Myocardial ischemia induces coronary t‐PA release in the pig. Acta Anaesthesiol Scand. 2002; 46: 271–278. [DOI] [PubMed] [Google Scholar]
- 19. Steins MB, Padró T, Li CX, Mesters RM, Ostermann H, Hammel D, Scheld HH, Berdel WE, Kienast J. Overexpression of tissue‐type plasminogen activator in atherosclerotic human coronary arteries. Atherosclerosis. 1999; 145: 173–180. [DOI] [PubMed] [Google Scholar]
- 20. Olofsson BO, Dahlén G, Nilsson TK. Evidence for increased levels of plasminogen activator inhibitor and tissue plasminogen activator in plasma of patients with angiographically verified coronary artery disease. Eur Heart J. 1989; 10: 77–82. [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Vaughan DE, Stampfer MJ, Manson JE, Hennekens CH. Endogenous tissue‐type plasminogen activator and risk of myocardial infarction. Lancet. 1993; 341: 1165–1168. [DOI] [PubMed] [Google Scholar]
- 22. Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995; 332: 635–641. [DOI] [PubMed] [Google Scholar]
- 23. Linnik W, Tintinalli JE, Ramos R. Associated reactions during and immediately after rtPA infusion. Ann Emerg Med. 1989; 18: 234–239. [DOI] [PubMed] [Google Scholar]
- 24. Mehta JL, Nichols WW, Saldeen TG, Chandna VK, Nicolini FA, Lawson DL, ter Riet MF. Superoxide dismutase decreases reperfusion arrhythmias and preserves myocardial function during thrombolysis with tissue plasminogen activator. J Cardiovasc Pharmacol. 1990; 16: 112–120. [DOI] [PubMed] [Google Scholar]
- 25. Schaefer U, Machida T, Vorlova S, Strickland S, Levi R. The plasminogen activator system modulates sympathetic nerve function. J Exp Med. 2006; 203: 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Heissig B, Salama Y, Takahashi S, Osada T, Hattori K. The multifaceted role of plasminogen in inflammation. Cell Signal. 2020; 75: 109761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gong P, Li M, Zou C, Tian Q, Xu Z. Tissue plasminogen activator causes brain microvascular endothelial cell injury after oxygen glucose deprivation by inhibiting sonic hedgehog signaling. Neurochem Res. 2019; 44: 441–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mao L, Li P, Zhu W, Cai W, Liu Z, Wang Y, Luo W, Stetler RA, Leak RK, Yu W, Gao Y, Chen J, Chen G, Hu X. Regulatory T cells ameliorate tissue plasminogen activator‐induced brain haemorrhage after stroke. Brain. 2017; 140: 1914–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ishiguro M, Mishiro K, Fujiwara Y, Chen H, Izuta H, Tsuruma K, Shimazawa M, Yoshimura S, Satoh M, Iwama T, Hara H. Phosphodiesterase‐III inhibitor prevents hemorrhagic transformation induced by focal cerebral ischemia in mice treated with tPA. PLoS ONE. 2010; 5: e15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Valerio V, Myasoedova VA, Moschetta D, Porro B, Perrucci GL, Cavalca V, Cavallotti L, Songia P, Poggio P. Impact of oxidative stress and protein S‐Glutathionylation in aortic valve sclerosis patients with overt atherosclerosis. J Clin Med. 2019; 8: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sverdlov AL, Ngo DT, Chan WP, Chirkov YY, Gersh BJ, McNeil JJ, Horowitz JD. Determinants of aortic sclerosis progression: implications regarding impairment of nitric oxide signalling and potential therapeutics. Eur Heart J. 2012; 33: 2419–2425. [DOI] [PubMed] [Google Scholar]
- 32. Berger DH. Plasmin/plasminogen system in colorectal cancer. World J Surg. 2002; 26: 767–771. [DOI] [PubMed] [Google Scholar]
- 33. Wang M, Lakatta EG. Altered regulation of matrix metalloproteinase‐2 in aortic remodeling during aging. Hypertension. 2002; 39: 865–873. [DOI] [PubMed] [Google Scholar]
- 34. Pirilä E, Ramamurthy NS, Sorsa T, Salo T, Hietanen J, Maisi P. Gelatinase a (MMP‐2), collagenase‐2 (MMP‐8), and laminin‐5 gamma2‐chain expression in murine inflammatory bowel disease (ulcerative colitis). Dig Dis Sci. 2003; 48: 93–98. [DOI] [PubMed] [Google Scholar]
- 35. Golab P, Kielbus M, Bielewicz J, Kurzepa J. The effect of recombinant tissue plasminogen activator on MMP‐2 and MMP‐9 activities in vitro. Neurol Res. 2015; 37: 9–13. [DOI] [PubMed] [Google Scholar]
- 36. Wang X, Lee SR, Arai K, Lee SR, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor‐mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003; 9: 1313–1317. [DOI] [PubMed] [Google Scholar]
- 37. Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Récalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Lévy B, Bonecchi R, Locati M, Combadière C, Silvestre JS. The chemokine decoy receptor D6 prevents excessive inflammation and adverse ventricular remodeling after myocardial infarction. Arterioscler Thromb Vasc Biol. 2012; 32: 2206–2213. [DOI] [PubMed] [Google Scholar]
- 38. Lindsey ML, Zamilpa R. Temporal and spatial expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases following myocardial infarction. Cardiovasc Ther. 2012; 30: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jung JJ, Razavian M, Challa AA, Nie L, Golestani R, Zhang J, Ye Y, Russell KS, Robinson SP, Heistad DD, Sadeghi MM. Multimodality and molecular imaging of matrix metalloproteinase activation in calcific aortic valve disease. J Nucl Med. 2015; 56: 933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moshal KS, Rodriguez WE, Sen U, Tyagi SC. Targeted deletion of MMP‐9 attenuates myocardial contractile dysfunction in heart failure. Physiol Res. 2008; 57: 379–384. [DOI] [PubMed] [Google Scholar]
- 41. Cheek JD, Wirrig EE, Alfieri CM, James JF, Yutzey KE. Differential activation of valvulogenic, chondrogenic, and osteogenic pathways in mouse models of myxomatous and calcific aortic valve disease. J Mol Cell Cardiol. 2012; 52: 689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weiss RM, Miller JD, Heistad DD. Fibrocalcific aortic valve disease: opportunity to understand disease mechanisms using mouse models. Circ Res. 2013; 113: 209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics between patients with high or low t‐PA levels in non‐AVSc and non‐AVSc groups.


