Abstract
Aims
We sought to investigate the outcomes of heart transplant patients supported with Impella 5.5 temporary mechanical circulatory support.
Methods and results
Patient demographics, perioperative data, hospital timeline, and haemodynamic parameters were followed during initial admission, Impella support, and post‐transplant period. Vasoactive‐inotropic score, primary graft failure, and complications were recorded. Between March 2020 and March 2021, 16 advanced heart failure patients underwent Impella 5.5 temporary left ventricular assist device support through axillary approach. Subsequently, all these patients had heart transplantation. All patients were either ambulatory or chair bound during their temporary mechanical circulatory support until heart transplantation. Patients were kept on Impella support median of 19 days (3–31) with the median lactate dehydrogenase level of 220 (149–430). All Impella devices were removed during heart transplantation. During Impella support, patients had improved renal function with median creatinine serum level of 1.55 mg/dL decreased to 1.25 (P = 0.007), pulmonary artery pulsatility index scores increased from 2.56 (0.86–10) to 4.2 (1.3–10) (P = 0.048), and right ventricular function improved (P = 0.003). Patients maintained improved renal function and favourable haemodynamics after their heart transplantation as well. All patients survived without any significant morbidity after their heart transplantation.
Conclusions
Impella 5.5 temporary left ventricular assist device optimizes care of heart transplant recipients providing superior haemodynamic support, mobility, improved renal function, pulmonary haemodynamics, and right ventricular function. Utilizing Impella 5.5 as a direct bridging strategy to heart transplantation resulted in excellent outcomes.
Keywords: Impella, Heart transplant, VADs, Heart failure, MCS
Introduction
Heart transplantation (HTx) remains the sole effective therapy for selected patients with advanced heart failure (HF) refractory to medical therapy, with excellent short‐ and long‐term outcomes of an estimated survival at 1 and 10 years of 85% and 50%, respectively. 1 Donor organ shortage leading to a deterioration of patients on the transplant waiting list has led to the emergence of the concept of bridging with mechanical circulatory support (MCS) to stabilize the patients and improve their quality of life while waiting for donor hearts. From 2009 to 2016, 42.9% of patients were bridged with MCS prior to HTx compared with 39.8% on inotropes. 2
The last two decades saw a forward leap in the development of MCS and the left ventricular assist devices (LVADs), whether in the duration of support (durable vs. temporary) or in the implemented strategy as a bridge to transplant, decision, recovery, or destination therapy. One of the remarkable advancements in the technology was the introduction of the miniaturized catheter‐based LVADs, namely, the Impella® (Abiomed, Danvers, MA, USA). The Impella micropumps are a group of miniaturized catheter‐based LVADs, inserted percutaneously or surgically into the left ventricle (LV) across the aortic valve. First approved in the United States by the Food and Drug Administration (FDA) in 2008 (the Impella 2.5), different models were put in use later (Impella CP, LD, 5.0, and 5.5). The most common indications for implanting the device are the treatment of acute cardiogenic shock due to acute myocardial infarction or acute cardiomyopathy, post‐cardiotomy circulatory support and to provide left ventricular support during high‐risk percutaneous coronary intervention (PCI). 3 , 4
The Impella also has a role in advanced HF therapy. Impella 5.0/LD was reported to be used as a bridge to durable LVAD 5 , 6 , 7 or as a bridge to decision vs. transplant. 6 , 8 , 9 In this article, we present our experience with the newly approved Impella 5.5 for clinical use as a direct bridge to transplant, investigating the potential haemodynamic benefits, the duration of the hospital course, and the observed complications.
Methods
Data source
The study was approved by the Mayo Clinic Institutional Review Board (IRB# 20‐010592) with a waiver for patient consent. Data were collected through our electronic medical record (EMR) chart review.
Statistical analysis
The analysis was performed using the SPSS Statistics Version 22.0 (IBM Corp, Armonk, NY, USA). The continuous variables were reported as median (minimum–maximum) values, whereas the categorical variables were reported as frequency (percentage). Due to the low number of the study cohort, a non‐parametric related samples median test (Wilcoxon's signed rank‐sum test) was used to compare medians. A P value < 0.05 was considered statistically significant.
Study population
Adult patients (≥18 years) on the heart transplant waiting list (or deemed eligible to be listed) who were bridged with Impella 5.5 as a direct bridge to transplant between 1 March 2020 and 31 March 2021 were included. Patients who required multi‐organ transplants were excluded.
The device and operative details
The Impella 5.5 is a catheter‐based micro‐axial continuous‐flow LVAD, approved by the FDA in 2019. Insertion requires a surgical cutdown in the axillary or the femoral artery, sewing an 8 mm vascular graft into the artery, and passing the catheter into the LV through the aortic valve under fluoroscopic and echocardiographic guidance. The device provides flows up to 5.5 L/min by continuously draining blood from the LV and ejecting it into the ascending aorta. The Impella unloads the LV, decreases myocardial oxygen demand, and increases the mean arterial pressure and cardiac output, thus improving the coronary flow and distal organ perfusion. In addition, the Impella decreases the pulmonary wedge pressure with a secondary reduction in the right ventricular (RV) afterload.
Device explantation at the time of transplant can be done by exploring the axillary cutdown, pushing the Impella catheter into the LV to avoid cross‐clamping the catheter, and then cutting the catheter with an explanted heart. The remaining Impella chord can be retrieved later through the graft, which is then clamped, obliterated with metallic clips, and sewed with poly‐propylene suture.
Patient selection, characteristics, variables, and outcomes
Patients with INTERMACS profiles 1–3 were selected for the device insertion, provided no contraindication such as previous mechanical aortic valve implantation, LV thrombus, arrhythmic storm, or axillary artery diameter < 5.5 mm. Sixteen patients were included in the study from 1 March 2020 to 31 March 2021. Patient characteristics such as age, gender, and causes of HF were recorded. Survival until HTx and 30 days, 90 days, and 1 year post‐transplant outcomes were noted. During this study period, all patients who underwent axillary Impella 5.5 insertion survived to HTx. The hospital timeline was evaluated as days in hospital prior to Impella insertion, days of support on Impella until HTx, post‐transplant intensive care unit (ICU) stay, time till extubation post‐transplant, and total hospital stay. The functional status of the patients during the Impella support, after discharge, and the need for additional MCS during hospital stay were observed. Variables were grouped as follows. (i) Biochemical markers included creatinine, glomerular filtration rate (GFR), lactate, total bilirubin, and haemolysis indicators as lactate dehydrogenase (LDH) (highest and lowest recorded values during Impella support), haemoglobin, and platelet count (lowest recorded during Impella support). Blood products were utilized and anticoagulation was also recorded. (ii) Haemodynamic parameters included pulmonary artery pressure (PAP), pulmonary capillary wedge pressure (PCWP), right atrial (RA) pressure, pulmonary artery pulsatility index (PAPI), pulmonary vascular resistance (PVR), and RV function. Pressor requirements were quantified using the vasoactive‐inotropic score (VIS) (1–10 minimal requirements, 11–30 moderate requirements, and >30 high requirements). Complications were categorized as post‐Impella and post‐transplant complications.
Results
Patients' characteristics and hospital timeline
Patients' characteristics and hospital timeline are presented in Table 1 . Sixteen patients were supported with Impella 5.5 with the intent of bridge to transplant. Ten patients (62.5%) were listed for transplantation before the Impella implant, whereas six patients (37.5%) were listed afterwards. Two patients (12%) were supported with additional MCS pre‐Impella, one (6%) with intra‐aortic balloon pump (IABP) and one (6%) with Impella CP. Two patients (12%) needed an additional MCS during or after Impella insertion. One patient (6%) needed a veno‐arterial extracorporeal membrane oxygenator (VA‐ECMO); this was a planned ECPELLA due to severe biventricular HF and metabolic cardiogenic shock; he was managed on ECPELLA support and upgraded United Network for Organ Sharing (UNOS) listing to Status 1 until his transplant. The other patient (6%) needed an RV assist device (RVAD) due to RV failure with Protek Duo and was maintained on both Impella and RVAD support until transplantation. Fourteen patients (88%) had a full ambulatory status during the Impella support, whereas two patients (12%) were chair bound. After discharge, 14 patients (88%) were able to go home, and 2 patients (12%) were discharged to rehab.
Table 1.
Patient characteristics and hospital timeline
|
Parameter N = 16 |
Median (range) n (%) |
|---|---|
| Age | 57.5 (31–73) |
| Male gender | 14 (88%) |
| Cause of heart failure |
NIDCM (10, 62.5%) IDCM (5, 31.3%) Restrictive cardiomyopathy (1, 6.3%) |
| Impella support (days) | 19 (3–31) |
| Days in hospital pre‐Impella | 7 (1–40) |
| Hospital stay (days) | 42.5 (16–70) |
| ICU stay after HTx (h) | 96 (48–181) |
| Extubation after HTx (h) | 14.5 (6–24) |
| Functional status during Impella support |
Ambulatory, 14 (88%) Chair bound, 2 (12%) |
| Patient disposition after discharge |
Home or self‐care, 14 (88%) Rehab, 2 (12%) |
|
TMCS pre‐Impella N = 2 |
IABP, 1 (6%) Impella CP, 1 (6%) |
|
TMCS during Impella support N = 2 |
VA‐ECMO, 1 (6%) Protek Duo, 1 (6%) |
HTx, heart transplantation; IABP, intra‐aortic balloon pump; ICU, intensive care unit; IDCM, ischaemic dilated cardiomyopathy; NIDCM, non‐ischaemic dilated cardiomyopathy; TMCS, temporary mechanical circulatory support; VA‐ECMO, veno‐arterial extracorporeal membrane oxygenator.
Operative details
Operative details are presented in Table 2 . All patients had their Impella implanted through right axillary cutdown, with an 8 mm diameter 30 cm long woven Gelweave graft sewn to the axillary artery. During the HTx, 15 patients (94%) had their Impella cut with an explanted heart after pushing it into the LV while the Impella chord was pulled later on through the axillary graft, and 1 patient (6%) has his Impella removed through the axillary graft. All patients had their axillary graft surgically obliterated during HTx.
Table 2.
Operative details
|
N (%) Median (range) |
|
|---|---|
| Method of Impella removal | Cut with the heart, 15 (94%) |
| Axillary wound exploration | 16 (100%) |
| Donor heart ischaemic time (min) | 223.5 (41–255) |
| Pump time (min) | 170.5 (124–258) |
| Prior sternotomy | 4 (25%) |
The median time for donor heart ischaemia was 223.5 (41–225) min, one patient (6%) received a donation after circulatory death (DCD) heart donor with an Organ Care System (OCS), and the rest of the recipients received their hearts (94%) from brain‐dead donors (BDDs).
Biochemical markers, haemolysis indicators, blood product utilization, and anticoagulation
Biochemical markers, haemolysis indicators, blood product utilization, and anticoagulation are presented in Tables 3 and 4 . Eight patients (50%) required blood transfusion during Impella support. All patients received both purge and systemic anticoagulation, 12 patients (75%) were anticoagulated with heparin, whereas 4 patients (25%) received bivalirudin. One patient (6%) received tissue plasminogen activator (tPA) for high purge pressures due to possible pump thrombosis, which was resolved after the second tPA administration without requiring pump exchange (Table 4 ).
Table 3.
Biochemical markers
| Marker |
Pre‐Impella Median (range) |
Post‐Impella Median (range) |
P value Pre‐ and post‐Impella |
Post‐transplant Median (range) |
P value Pre‐Impella and post‐transplant |
|---|---|---|---|---|---|
| Creatinine | 1.55 (1–2.6) | 1.25 (0.6–1.8) | 0.007 | 1.1 (0.5–2.5) | 0.015 |
| GFR | 51.5 (24–90) | 58.5 (38–90) | 0.020 | 67 (27–90) | 0.031 |
| Total bilirubin | 0.6 (0.2–3.4) | 0.45 (0.1–4.1) | 0.068 | 0.6 (0.1–3.5) | 0.635 |
| Lactate | 1 (0.6–2.4) | 1 (0.5–1.5) | 0.245 |
1.95 (0.7–4.6) at 24 h 1.1 (0.6–2) at 72 h |
0.013 0.694 |
| LDH post‐Impella (lowest) | — | 220 (149–430) | — | — | — |
| LDH post‐Impella (highest) | — | 322 (240–830) | — | — | — |
| Haemoglobin during Impella support | — | 9.85 (7.9–12.8) | — | — | — |
| Lowest platelet # during Impella support | — | 125.5 (66–230) | — | — | — |
GFR, glomerular filtration rate; LDH, lactate dehydrogenase.
Table 4.
Blood products utilization and anticoagulation
| N = 16 |
N patients (%) Median (range) |
P value a |
|---|---|---|
| Blood transfusion during Impella |
8 (50%) # units, 2 (1–4) |
<0.001 |
| Blood transfusion during HTx |
16 (100%) # units, 4 (2–10) |
— |
| Blood transfusion after HTx |
2 (12%) # units, 1.5 (1–2) |
N/A |
| Platelets transfusion during Impella | None | — |
| Purge anticoagulation |
Heparin, 12 (75%) Bivalirudin, 4 (25%) |
— |
| Systemic anticoagulation |
Heparin, 12 (75%) Bivalirudin, 4 (25%) |
— |
| tPA for high purge pressure | 1 (6%) | — |
HTx, heart transplantation; N/A, not applicable; tPA, tissue plasminogen activator.
P value calculated for blood transfusion during Impella and transplant.
Haemodynamics, pressor requirements, and Impella parameters
Haemodynamics, pressor requirements, and Impella parameters are presented in Table 5 . Results were reported during each therapy phase. Cardiac indices post‐Impella and post‐transplant were 2.7 L/min/m2 (2.1–3.7) and 3.1 L/min/m2 (2.4–4.4), respectively. RV dysfunction was scaled from normal (1) to severe (4), recorded for pre‐Impella and post‐Impella therapy, and significant improvement in mean RV function is noted (Figure 1 ). We did not compare other echocardiographic parameters like RV ejection fraction or tricuspid annular plane systolic excursion (TAPSE) to assess RV function due to a lack of data and limited echo views after Impella placement, but visual RV function assessment was collected and graded.
Table 5.
Haemodynamics and pressor requirements
| Parameter |
Pre‐Impella Median (range) |
Post‐Impella Median (range) |
Post‐transplant Median (range) |
P value Pre‐ and post‐Impella |
P value Pre‐Impella and post‐transplant |
|---|---|---|---|---|---|
| PA systolic, mmHg | 45.5 (35–75) | 36 (26–51) | 37 (23–46) | 0.001 | 0.004 |
| PA diastolic, mmHg | 23.5 (17–37) | 17 (11–33) | 16 (8–20) | 0.002 | 0.001 |
| PCWP, mmHg | 21 (15–32) | 15.5 (11–33) | — | 0.011 | — |
| RA pressure, mmHg | 8.5 (3–22) | 3.5 (2–12) | 7 (2–16) | 0.018 | 0.608 |
| PAPI | 2.56 (0.86–10) | 4.2 (1.3–10) | 3.45 (1.1–10) | 0.048 | 0.367 |
| PVR, dynes‐s/cm5 | 228 (80–576) | 115 (50–292) | 99 (70–142) | 0.006 | 0.001 |
| Pressors, % | 15 (94%) | 14 (88%) | 16 (100%) | 1 | 1 |
| Vasoactive‐inotropic score | 5 (2.5–17.5) | 5 (2.5–10) |
5 (2–9) at 24 h 4 (1–6) at 72 h |
0.064 | 0.455 0.030 |
| Impella power | — | 6 (4–8) | — | — | — |
| Impella flow | — | 3.85 (2.1–4.6) | — | — | — |
PA, pulmonary artery; PAPI, pulmonary artery pulsatility index; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RA, right atrial.
Figure 1.
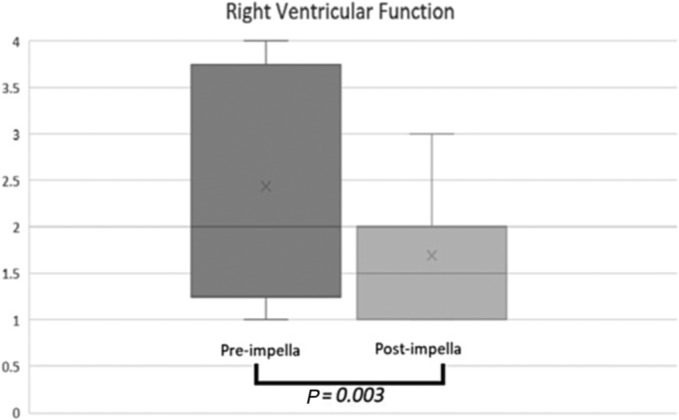
Right ventricular function presented on box‐plot graph before and after Impella placement; x mark represents mean right ventricular function (pre‐Impella 2.44 vs. post‐Impella 1.69, P = 0.003), and y axis scaled 1 (normal function) to 4 (severe dysfunction).
Pressor requirements were quantified using the VIS; values 0–10 are quantified as minimal, 11–30 as moderate, and >30 as high pressor requirements. Fifteen patients (94%) required pressors pre‐Impella with a median VIS of 5 (2.5–17.5), whereas 14 patients (88%) required pressors post‐Impella with VIS of 5 (2.5–10) (P = 0.064). After transplant, all patients required pressors with VIS at 24 h of 5 (2–9) and at 72 h of 4 (1–6) (P = 0.03). The median Impella flow during the support period was 3.85 L/min (2.1–4.6), whereas the power was 6 (4–8).
Final outcomes and complications
Final outcomes and complications are presented in Table 6 . All 16 patients survived to HTx, with a median waiting time after device insertion of 19 days (3–31). Thirty days, 90 days, and 1 year of survival after HTx was 100%. Complications were uncommon in general. During the Impella support, one patient had device dislodgement from the LV requiring reinsertion in the operating room (OR), one had a resolved mild stroke, and two had gastrointestinal (GI) bleeding that required endoscopic interventions. Post‐HTx complications were noted for one patient who had a minor stroke without residual deficit and for another who had axillary artery dissection that required stenting. None of the patients required dialysis at any point, and no axillary nerve or aortic valve injuries were observed.
Table 6.
Complications and post‐transplant survival
| Post‐Impella complication | Frequency # | Post‐transplant complication | Frequency # |
|---|---|---|---|
| Nerve injury | 0 | Stroke (mild) | 1 |
| Bleeding requiring axillary exploration | 0 | Dialysis in hospital | 0 |
| Impella site infection | 0 | Dialysis after discharge | 0 |
| Distal embolization | 0 | Arm embolization | 0 |
| Aortic valve injury | 0 | Axillary site infection | 0 |
| Device dislodgement a | 1 | Sternal wound infection | 0 |
| Device malfunction a | 0 | Open chest | 0 |
| Axillary artery dissection or pseudoaneurysm | 0 | Need for pacemaker | 0 |
| Device‐related ventricular arrhythmia | 0 | Need for MCS | 0 |
| Stroke (mild) | 1 | Axillary artery dissection | 1 |
| GI bleeding | 2 | Mortality 30 days, 90 days, and 1 year | 0 |
GI, gastrointestinal; MCS, mechanical circulatory support.
Required operating‐room reinsertion.
Discussion
The new UNOS allocation system was introduced in October 2018, with the primary aim of addressing waitlist mortality, acuity of the patients, and inequality in geographic locations. 10 Under the previous allocation system, stable LVAD patients would receive the same prioritization as the non‐dischargeable patients on temporary MCS (TMCS), despite the fact that LVAD patients have seen improved survival and a lower rate of complications over the last few years. 11 Under the new allocation system, patients on TMCS would receive prioritization to Status 1 or 2, which led to increased transplant rates. Since its implementation, the percentage of patients transplanted while on TMCS has risen from 10% to 41%. 12
Waitlist mortality, time to transplant, and post‐transplant survival
In our report, we investigated the use of the newly approved Impella 5.5 as a direct bridge to transplant. We indicated placing Impella 5.5 in advanced HF patients to improve their transplant status in the hospital, improve their haemodynamics and multi‐organ function, decrease inotropic support, and increase their physical activities while listed. All patients survived from device insertion until HTx and 30 days, 90 days, and 1 year after, with median Impella support days of 19 (3–31). Yin et al. used data from the International Society for Heart and Lung Transplantation between 2005 and 2016 to stratify outcomes of HTx according to the type of MCS. 13 Patients on ECMO had the lowest 1 year survival of 71.2% to be followed by patients with percutaneous LVAD (including the Impella and Tandem heart) with 79.9%. Hall et al. reported 87% first‐year‐HTx survival for patients who were bridged with Impella 5.0. 14 Cogswell reported 90 days of survival of 87.6% under the new allocation system with 83% of the transplants occurring in Status 1, 2, or 3. 12 Based on our experience, early‐term survival is encouraging with Impella 5.5 support, but further evaluation would be necessary.
Because the new allocation system prioritizes patients with TMCS as Status 1 or 2 over stable LVAD patients with Status 4, an increase in waitlist time is expected for durable LVAD patients. This fact clearly shifted patients and referred cardiologists' preference away from durable LVADs as a bridge to transplant. Brown et al. investigated the optimal timing to transplant patients with durable LVAD between 2009 and 2014 using fee‐for‐service Medicare patients. 15 In their report, 45% (n = 2639) were bridged to transplant (BTT) with durable LVADs. LVAD patients were bridged for an average of 346.6 ± 288.1 days and a median of 265 days. In addition, one interesting subgroup was the patients who received a transplant within 31 days of LVAD implantation, which showed a 50% increase in the risk of death during the LVAD support and a 30 day survival rate of 81.5% after transplant, citing that this early period after LVAD implantation is not an optimal time for HTx. In comparison, our study showed a median transplant waiting time of 19 (3–31) days, with 100% 30 day survival, which can put the Impella 5.5 BTT strategy in a superior stand in comparison with the durable LVAD bridge by decreasing waitlist mortality and improving early survival.
Optimization of pulmonary haemodynamics and right ventricular function
Secondary pulmonary hypertension (PHTN) is frequently encountered in HF patients undergoing transplant evaluation; up to one‐third of evaluated patients have either reversible or fixed PHTN. 16 LVADs are reported to lower mean PAP and improve RV function. 17 , 18 Our results showed significant improvement in pulmonary haemodynamics and RV function between pre‐ and post‐Impella therapies (Table 5 and Figure 1 ). Median pulmonary artery (PA) systolic pressure decreased from 45 to 36 mmHg, and PVR significantly improved. PAPI score, which is an indicator of RV reserve and a predictor of adverse events in HF patients or the need for RVAD support after LVAD placement, 19 , 20 also showed significant improvement. These results indicate that the Impella 5.5 provides adequate unloading of the LV, leading to significant improvement in pulmonary haemodynamics and RV function despite the short duration of the therapy. This improvement in PAP and RV function was also observed after HTx. As a smaller micropump, it was encouraging to see comparative haemodynamic improvements in TMCS and newly transplanted hearts.
Biochemical markers, haemolysis, anticoagulation, and blood products transfusion
Renal dysfunction can be seen in up to two‐thirds of hospitalized advanced HF patients. 21 Main aetiologies are decreased renal perfusion due to low cardiac output, venous congestion due to RV dysfunction, and increased activation of the renin–angiotensin system. We noticed a significant improvement in GFR and serum creatinine after Impella support. This improvement was observed in the post‐transplant period as well. None of the patients required dialysis during Impella support or after HTx.
Multiple studies have demonstrated recovery of renal function after LVAD implantation, 22 , 23 although this recovery could be in the short term. Several reports showed a slow progressive decline in renal function several months after the LVAD implant. 24 , 25 This observation may put the Impella device in an advantageous position over LVADs, because the duration of the Impella therapy is short, thus providing the initial renal recovery and avoiding possible long‐term decline. TMCS is associated with a certain degree of haemolysis in general, and the Impella device is no exception, with an incidence range from 7.5% up to 62.5%. 26 , 27 The INTERMACS defines haemolysis as an increased LDH level of 2.5 times the upper normal or plasma free haemoglobin (PFH) of >20 mg/dL measured 72 h after MCS implantation, in addition to clinical and laboratory signs of haemoglobinuria, renal impairment, anaemia, and hyperbilirubinaemia. We noticed that the Impella 5.5 was having a safer haemolysis margin, providing lower LDH levels, less requirement for blood transfusion, and no platelet transfusion. This was also reflected in renal recovery by avoiding further injury to the kidneys during a longer duration of MCS therapy. Our experience overall showed that the Impella 5.5 had a lower risk of haemolysis in comparison with previous models and a low tendency for inlet thrombus formation despite using it for a few weeks.
Low incidence of complications and better patient conditioning for surgery
Complications were infrequent during Impella therapy. One patient had their Impella cannula dislodged from the ventricle, needing reposition in the OR. Simple repositioning can be done at the bedside with transthoracic echocardiography (TTE) guidance or fluoroscopy if the cannula is still inside the ventricle; when the cannula is expelled to the ascending aorta, bedside fluoroscopy or TEE could be attempted but, if not successful, axillary wound exploration will be necessary. 28 Two patients (12%) had GI bleeding that was managed with endoscopy and holding anticoagulation. None of the patients had aortic valve injury, bleeding, or infection at the site of insertion. In the post‐Impella therapy, phase complications were also infrequent. We had one patient (6%) having a minor stroke without the residual deficit and one patient having an axillary artery dissection diagnosed a few days after the transplant; the dissection required surgery and stenting.
Durable LVAD recipients are expected to spend more time on the waitlist; thus, the incidence of complications would increase. Up to 60% of LVAD recipients will have one or more adverse events within 6 months, 29 Chauhan et al. 30 reported up to 30% re‐exploration rate for surgical bleeding after durable LVAD implantation, 22.4% incidence of device‐related infection, 10% for device thrombosis, and 10% for device malfunction, in addition to a stroke rate for durable VADs of ~10%. 31 Therefore, we argue that the complication profile for the Impella 5.5 is lower than the durable VAD. Temporary Impella support through an axillary approach also provides a limited surgical wound away from the sternum and less inflammation compared with a durable VAD surgery. This can explain the superiority of smaller VADs for early extubation, better pain management, absence of adhesions in the mediastinum, and gentle explantation process during HTx. The majority of our Impella patients participated in active physical therapy 32 (Figure 2 ). The transplant procedure itself ran a less complicated course in comparison with an LVAD explant surgery; all patients had their chest closed during HTx. The median cardiopulmonary bypass (CPB) time was 170.5 (124–258) min, and the extubation time was 14.5 (6–24) h.
Figure 2.
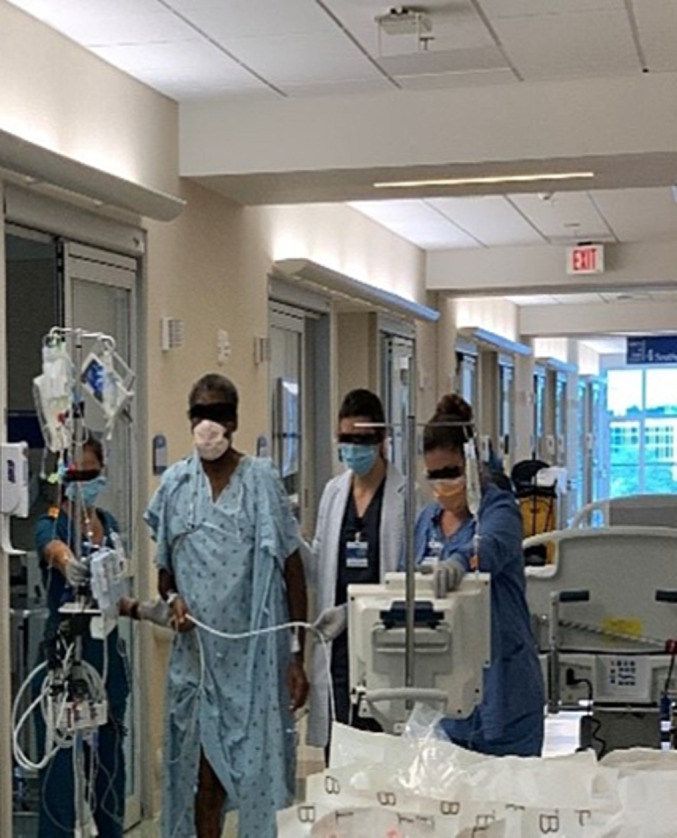
Patient bridged with Impella 5.5 ambulating in the intensive care unit while waiting for transplant.
In conclusion, we believe the new generation Impella 5.5 would serve as a safe bridging option for HTx, providing better organ function preservation and improvement. Patients would have great mobility and would experience superior haemodynamics on device therapy.
Limitations
We presented a small number of patients enrolled in this study, and the monocentric nature of the study lacked a comparison group. Once we adopted Impella 5.5 as a bridge to HTx, we did not have many LVAD patients bridged or got transplanted during the study time period. A multicentre prospective study would accomplish a larger cohort number and help us to understand the better comparison between different types of TMCS or LVADs with satisfactory statistics. Also, our patient population presented here is dominated by Status 3 patients escalating to Status 2 with Impella 5.5 therapy relatively stable on inotropes, and only a fewer patients in refractory cardiogenic shock were presented. A cohort dominated by refractory cardiogenic shock with multi‐organ dysfunction would lead to different outcomes. Finally, there was an interobserver variability of qualitative echocardiographic assessment and a lack of quantitative data on right heart function.
Conflict of interest
No conflict of interests.
Funding
The study did not receive any funding.
Haddad, O. , Sareyyupoglu, B. , Goswami, R. M. , Bitargil, M. , Patel, P. C. , Jacob, S. , El‐Sayed Ahmed, M. M. , Leoni Moreno, J. C. , Yip, D. S. , Landolfo, K. , and Pham, S. M. (2023) Short‐term outcomes of heart transplant patients bridged with Impella 5.5 ventricular assist device. ESC Heart Failure, 10: 2298–2306. 10.1002/ehf2.14391.
References
- 1. Previato M, Osto E, Kerkhof PLM, Parry G, Tona F. Heart transplantation survival and sex‐related differences. Adv Exp Med Biol. 2018; 1065: 379–388. [DOI] [PubMed] [Google Scholar]
- 2. Lund LH, Khush KK, Cherikh WS, Goldfarb S, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Chambers DC, Yusen RD, Stehlik J, International Society for Heart and Lung Transplantation . The Registry of the International Society for Heart and Lung Transplantation: thirty‐fourth adult heart transplantation report—2017; focus theme: allograft ischemic time. J Heart Lung Transplant. 2017; 36: 1037–1046. [DOI] [PubMed] [Google Scholar]
- 3. Glazier JJ, Kaki A. The Impella device: historical background, clinical applications and future directions. Int J Angiol. 2019; 28: 118–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patel SM, Lipinski J, al‐Kindi SG, Patel T, Saric P, Li J, Nadeem F, Ladas T, Alaiti A, Phillips A, Medalion B, Deo S, Elgudin Y, Costa MA, Osman MN, Attizzani GF, Oliveira GH, Sareyyupoglu B, Bezerra HG. Simultaneous venoarterial extracorporeal membrane oxygenation and percutaneous left ventricular decompression therapy with Impella is associated with improved outcomes in refractory cardiogenic shock. ASAIO J. 2019; 65: 21–28. [DOI] [PubMed] [Google Scholar]
- 5. Suradi H, Breall JA. Successful use of the Impella device in giant cell myocarditis as a bridge to permanent left ventricular mechanical support. Tex Heart Inst J. 2011; 38: 437–440. [PMC free article] [PubMed] [Google Scholar]
- 6. Lima B, Kale P, Gonzalez‐Stawinski GV, Kuiper JJ, Carey S, Hall SA. Effectiveness and safety of the Impella 5.0 as a bridge to cardiac transplantation or durable left ventricular assist device. Am J Cardiol. 2016; 117: 1622–1628. [DOI] [PubMed] [Google Scholar]
- 7. Doersch KM, Tong CW, Gongora E, Konda S, Sareyyupoglu B. Temporary left ventricular assist device through an axillary access is a promising approach to improve outcomes in refractory cardiogenic shock patients. ASAIO J. 2015; 61: 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seese L, Hickey G, Keebler ME, Mathier MA, Sultan I, Gleason TG, Toma C, Kilic A. Direct bridging to cardiac transplantation with the surgically implanted Impella 5.0 device. Clin Transplant. 2020; 34: e13818. [DOI] [PubMed] [Google Scholar]
- 9. Monteagudo‐Vela M, Panoulas V, García‐Saez D, de Robertis F, Stock U, Simon AR. Outcomes of heart transplantation in patients bridged with Impella 5.0: comparison with native chest transplanted patients without preoperative mechanical circulatory support. Artif Organs. 2021; 45: 254–262. [DOI] [PubMed] [Google Scholar]
- 10. Stevenson LW, Kormos RL, Young JB, Kirklin JK, Hunt SA. Major advantages and critical challenge for the proposed United States heart allocation system. J Heart Lung Transplant. 2016; 35: 547–549. [DOI] [PubMed] [Google Scholar]
- 11. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 12. Cogswell R, John R, Estep JD, Duval S, Tedford RJ, Pagani FD, Martin CM, Mehra MR. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant. 2020; 39: 1–4. [DOI] [PubMed] [Google Scholar]
- 13. Yin MY, Wever‐Pinzon O, Mehra MR, Selzman CH, Toll AE, Cherikh WS, Nativi‐Nicolau J, Fang JC, Kfoury AG, Gilbert EM, Kemeyou L, McKellar SH, Koliopoulou A, Vaduganathan M, Drakos SG, Stehlik J. Post‐transplant outcome in patients bridged to transplant with temporary mechanical circulatory support devices. J Heart Lung Transplant. 2019; 38: 858–869. [DOI] [PubMed] [Google Scholar]
- 14. Hall SA, Uriel N, Carey SA, Edens M, Gong G, Esposito M, O'Kelly R, Annamalai S, Aghili N, Adatya S, Kapur NK. Use of a percutaneous temporary circulatory support device as a bridge to decision during acute decompensation of advanced heart failure. J Heart Lung Transplant. 2018; 37: 100–106. [DOI] [PubMed] [Google Scholar]
- 15. Brown CR, Khurshan F, Chen Z, Groeneveld PW, McCarthy F, Acker M, Rame JE, Desai N. Optimal timing for heart transplantation in patients bridged with left ventricular assist devices: is timing of the essence? J Thorac Cardiovasc Surg. 2019; 157: 2315–2324.e4. [DOI] [PubMed] [Google Scholar]
- 16. Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999; 34: 1802–1806. [DOI] [PubMed] [Google Scholar]
- 17. Selim AM, Wadhwani L, Burdorf A, Raichlin E, Lowes B, Zolty R. Left ventricular assist devices in pulmonary hypertension group 2 with significantly elevated pulmonary vascular resistance: a bridge to cure. Heart Lung Circ. 2019; 28: 946–952. [DOI] [PubMed] [Google Scholar]
- 18. Haddad H, Elabbassi W, Moustafa S, Davies R, Mesana T, Hendry P, Masters R, Mussivand T. Left ventricular assist devices as bridge to heart transplantation in congestive heart failure with pulmonary hypertension. ASAIO J. 2005; 51: 456–460. [DOI] [PubMed] [Google Scholar]
- 19. Kochav SM, Flores RJ, Truby LK, Topkara VK. Prognostic impact of pulmonary artery pulsatility index (PAPi) in patients with advanced heart failure: insights from the ESCAPE trial. J Card Fail. 2018; 24: 453–459. [DOI] [PubMed] [Google Scholar]
- 20. Kang G, Ha R, Banerjee D. Pulmonary artery pulsatility index predicts right ventricular failure after left ventricular assist device implantation [published correction appears in J Heart Lung Transplant. 2017 Nov;36(11):1272]. J Heart Lung Transplant. 2016; 35: 67–73. [DOI] [PubMed] [Google Scholar]
- 21. Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004; 43: 61–67. [DOI] [PubMed] [Google Scholar]
- 22. Slaughter MS, Pagani FD, Rogers JG, Miller LW, Sun B, Russell SD, Starling RC, Chen L, Boyle AJ, Chillcott S, Adamson RM, Blood MS, Camacho MT, Idrissi KA, Petty M, Sobieski M, Wright S, Myers TJ, Farrar DJ. Clinical management of continuous‐flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010; 29: S1–S39. [DOI] [PubMed] [Google Scholar]
- 23. Kilic A, Chen CW, Gaffey AC, Wald JW, Acker MA, Atluri P. Preoperative renal dysfunction does not affect outcomes of left ventricular assist device implantation. J Thorac Cardiovasc Surg. 2018; 156: 1093–1101.e1. [DOI] [PubMed] [Google Scholar]
- 24. Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous‐flow left ventricular assist devices. J Am Coll Cardiol. 2012; 59: 26–36. [DOI] [PubMed] [Google Scholar]
- 25. Lok SI, Martina JR, Hesselink T, Rodermans BFM, Hulstein N, Winkens B, Klopping C, Kirkels JH, Doevendans PA, Ramjankhan F, de Weger RA, de Jonge N, Lahpor JR. Single‐centre experience of 85 patients with a continuous‐flow left ventricular assist device: clinical practice and outcome after extended support. Eur J Cardiothorac Surg. 2013; 44: e233–e238. [DOI] [PubMed] [Google Scholar]
- 26. Lauten A, Engström AE, Jung C, Empen K, Erne P, Cook S, Windecker S, Bergmann MW, Klingenberg R, Lüscher TF, Haude M, Rulands D, Butter C, Ullman B, Hellgren L, Modena MG, Pedrazzini G, Henriques JPS, Figulla HR, Ferrari M. Percutaneous left‐ventricular support with the Impella‐2.5‐assist device in acute cardiogenic shock: results of the Impella‐EUROSHOCK‐registry. Circ Heart Fail. 2013; 6: 23–30. [DOI] [PubMed] [Google Scholar]
- 27. Badiye AP, Hernandez GA, Novoa I, Chaparro SV. Incidence of hemolysis in patients with cardiogenic shock treated with Impella percutaneous left ventricular assist device. ASAIO J. 2016; 62: 11–14. [DOI] [PubMed] [Google Scholar]
- 28. Haddad O, Jacob S, Ung RL, Goswami RM, Patel PC, Pham SM, Sareyyupoglu B. Impella flow pump reinsertion after axillary graft thrombectomy: technical points in replacing axillary Impella. SAGE Open Med Case Rep. 2021; 9: 2050313X2110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wever‐Pinzon O, Drakos SG, Kfoury AG, Nativi JN, Gilbert EM, Everitt M, Alharethi R, Brunisholz K, Bader FM, Li DY, Selzman CH, Stehlik J. Morbidity and mortality in heart transplant candidates supported with mechanical circulatory support: is reappraisal of the current United Network for Organ Sharing thoracic organ allocation policy justified? Circulation. 2013; 127: 452–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chauhan D, Okoh AK, Fugar S, Karanam R, Baran D, Zucker M, Camacho M, Russo MJ. Impact of left‐ventricular assist device‐related complications on posttransplant graft survival. Ann Thorac Surg. 2017; 104: 1947–1952. [DOI] [PubMed] [Google Scholar]
- 31. Cornwell WK 3rd, Ambardekar AV, Tran T, Pal JD, Cava L, Lawley J, Tarumi T, Cornwell CL, Aaronson K. Stroke incidence and impact of continuous‐flow left ventricular assist devices on cerebrovascular physiology. Stroke. 2019; 50: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bitargil M, Pham S, Haddad O, Sareyyupoglu B. Single arterial access for Ecpella and jugular venous cannulation provides full mobility on a status 1 heart transplant recipient [published online ahead of print, 2022 Feb 23]. ESC Heart Fail. 2022; 9: 2003–2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


