Abstract
Aims
Whether sex affects selection for and outcomes after heart transplantation (HTx) remains unclear. We aimed to show sex differences in pre‐transplant characteristics and outcomes after HTx.
Methods and results
From 1995 to 2019, 49 200 HTx recipients were prospectively enrolled in the Organ Procurement and Transplantation Network. Logistic regression models were used to evaluate clinical characteristics by sex. Multivariable Cox regression models were fitted to assess sex differences in all‐cause mortality, cardiovascular mortality, graft failure, cardiac allograft vasculopathy (CAV), and malignancy. In 49 200 patients (median age 55 years, interquartile range 46–62; 24.6% women), 49 732 events occurred during a median follow‐up of 8.1 years. Men were older than women, had more often ischaemic cardiomyopathy (odds ratio [OR] 3.26, 95% confidence interval [CI] 3.11–3.42; P < 0.001), and a higher burden of cardiovascular risk factors, whereas women had less malignancies (OR 0.47, CI 0.44–0.51; P < 0.001). Men were more often treated in intensive care unit (OR 1.24, CI 1.12–1.37; P < 0.001) with a higher need for ventilatory (OR 1.24, CI 1.17–1.32; P < 0.001) or VAD (OR 1.53, CI 1.45–1.63; P < 0.001) support. After multivariable adjustment, men had a higher risk for CAV (hazard ratio [HR] 1.21, CI 1.13–1.29; P < 0.001) and malignancy (HR 1.80, CI 1.62–2.00; P < 0.001). There were no differences in all‐cause mortality, cardiovascular mortality, and graft failure between sexes.
Conclusions
In this US transplant registry, men and women differed in pre‐transplant characteristics. Male sex was independently associated with incident CAV and malignancy even after multivariable adjustment. Our results underline the need for better personalized post‐HTx management and care.
Keywords: Heart transplantation, Mortality, Outcomes, Sex differences, United Network for Organ Sharing (UNOS)
Introduction
Heart transplantation (HTx) is the preferred and curative treatment for end‐stage heart failure (HF) with good long‐term prognosis. 1 Despite recent advances in organ allocation, 2 pre‐operative therapy 3 and improvements in technical‐procedural aspects and postoperative care, long‐term survival after HTx remains still limited. 4 Although differences between sexes can be detected in different cardiovascular (CV) diseases leading to HF, 5 sex‐specific research in HTx often focussed on the impact of sex mismatch. 6 , 7 Previous studies showed inconsistent results regarding sex as an independent risk factor for survival after HTx. 4 , 8 , 9 , 10 , 11 Notably, data from the International Society of Heart and Lung Transplantation (ISHLT) reported a trend towards improved survival in women. 12
Graft failure (GF), cardiac allograft vasculopathy (CAV), and malignancy are the most common causes of morbidity and mortality after HTx. 13 , 14 , 15 , 16 GF represents one of the leading complications in the short‐term with an estimated incidence rate of about 2.5% in the first 3 years and mortality rates up to 85.5%. 17 , 18 , 19 CAV is major cause of late organ dysfunction, 15 contributing about 10% of long‐term mortality. 20 Malignancies are found in approximately 40% of patients 10 years after HTx 20 with earlier and more aggressive clinical course than in non‐transplanted patients with cancer. 16 , 21
Therefore, we aimed to investigate (i) sex differences in pre‐transplant clinical characteristics, (ii) sex differences in transplant‐related outcomes, and (iii) predictors of outcomes in patients after HTx.
Methods
Study population
Data were obtained from the Organ Procurement and Transplantation Network (OPTN) and its contractor United Network for Organ Sharing (UNOS). OPTN is the main source for the Scientific Registry of Transplant Recipients (SRTR), which includes comprehensive data on all organ donors, patients prior to and after HTx in the United States. 22
All adult patients undergoing a first HTx were included in this analysis. Follow‐up time was censored at 20 years. From 1995 to 2019, 49 200 HTx recipients were prospectively enrolled. Follow‐up data contained 44 821 patients. Due to the registry nature of the study, this project was exempt from the approval of an ethics committee. Analyses were based on OPTN data (30 June 2019).
Variables and outcomes
Age, ethnicity, and education were stored as sociodemographic variables. Medical history comprised aetiology of HF, co‐morbidities (dialysis, prior malignancy, and prior cardiac surgery), CV risk factors (blood pressure, body mass index [BMI], diabetes, and cigarette use), immunological risk factors (humane leukocyte antigen [HLA] mismatch) and blood group. Periprocedural characteristics were as follows: (i) on waiting list: UNOS waiting list status, days on waiting list, dialysis, treatment on intensive care unit (ICU), mechanical ventilation, transfusions, left and right ventricular assist device (VAD) as durable mechanical circulatory support devices; (ii) at time of transplant: inotropic therapy, extracorporeal membrane oxygenation (ECMO) as temporary mechanical circulatory support, and ischaemic time.
UNOS old status 1 was used to determine urgency status for HTx candidates listed prior to 1999. Since 2018, HTx candidates are listed using six urgency categories (UNOS status 1–6). The new adult UNOS status 1–3 was assigned to the previous category UNOS status 1A, the new adult UNOS status 4 to the status 1B and new adult UNOS status 5–6 to the previous category UNOS status 2.
Primary outcomes were all‐cause mortality, CV mortality, GF, CAV, and malignancy as reported by the participating centres. In OPTN, GF is defined as recipient death, organ replacement or mechanical circulatory support after surgery.
Statistical analysis
For continuous variables, median, interquartile range (IQR) as well as P‐values of Mann–Whitney test were reported. For categorical variables, absolute and relative frequencies as well as P‐values of χ 2 test were reported. Relative frequencies and test statistics were computed without missing values.
To evaluate clinical pre‐transplant characteristics associated with sex, a univariable logistic regression model was fitted with sex as predictor and the baseline characteristics as outcome. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each variable.
Survival analyses and incidence curves were performed using the Kaplan–Meier method. The P‐value for the difference between men and women in all‐cause mortality, CV mortality, and cumulative incidence curves for GF, CAV, and malignancy were calculated using the log‐rank test.
For adjusted analysis, stepwise multivariable Cox‐regression models were performed. Predictors were selected based on clinical experience. 13 , 14 , 15 , 23 The different Cox models were (1) unadjusted, (2) adjusted for age and ethnicity, and (3) adjusted for sociodemographic factors (age, ethnicity, and education [education higher than high school]), and CV risk factors (BMI, history of cigarette use, and diabetes), immunological variables (blood group, HLA mismatch >4 loci), and periprocedural characteristics as UNOS status, mechanical and circulatory (VAD or ECMO) and ventilatory support and need for dialysis. For malignancy, model 3 was also adjusted for prior malignancy.
To identify sex‐specific predictors of outcome, an interaction model based on the adjusted Cox regression model was fitted for the subgroups men vs. women. P‐value of interaction, hazard ratio (HR), and P‐values of effects by sex were reported.
Most actual waiting list/follow‐up data were used, and missing values were replaced by former information. All analyses were based on complete cases. A two‐tailed P‐value <0.05 was considered statistically significant. Statistical analyses were performed in R version 4.0.3. 24
Results
Baseline characteristics
Overall 49 200 HTx recipients enrolled from April 1995 to January 2019 in the OPTN were analysed for sex differences in waiting list characteristics after exclusion of 9882 patients aged <18 years or re‐transplantation (Figure 1 ). Detailed baseline characteristics for men and women are shown in Table 1 . Of the 49 200 HTx recipients (median age 55 years), 12 098 (24.6%) were women. At time of listing, women were younger (median age 53 years, IQR 41–60) compared with men (median age 56 years, IQR 48–62; P < 0.001).
Figure 1.
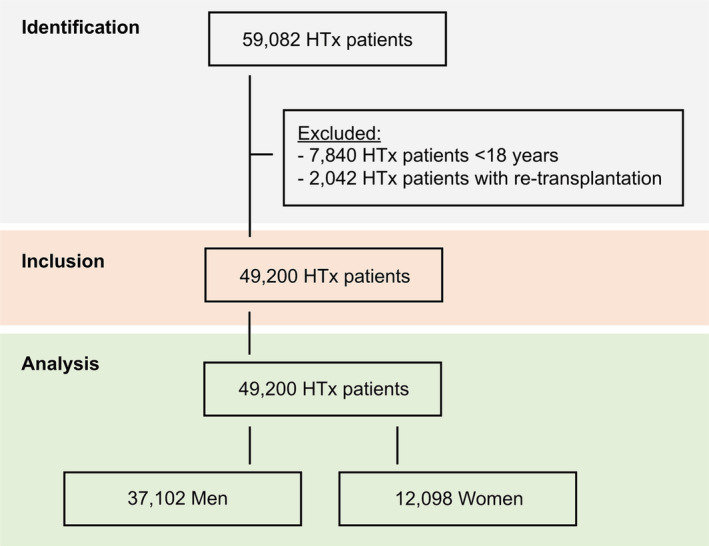
STROBE diagram of the study population. HTx, heart transplantation.
Table 1.
Baseline characteristics
| Missing | All (N = 49 200) | Men (N = 37 102) | Women (N = 12 098) | P‐value | |
|---|---|---|---|---|---|
| Recipient age at transplant | |||||
| Age (years) | 0 (0) | 55 (46, 62) | 56 (48, 62) | 53 (41, 60) | <0.001 |
| Aetiology of heart failure | |||||
| Dilated cardiomyopathy, n (%) | 0 (0) | 21 560 (43.8) | 15 321 (41.3) | 6239 (51.6) | <0.001 |
| Ischaemic cardiomyopathy, n (%) | 0 (0) | 20 575 (41.8) | 17 887 (48.2) | 2688 (22.2) | <0.001 |
| Congenital heart disease, n (%) | 0 (0) | 1338 (2.7) | 808 (2.2) | 530 (4.4) | 0.001 |
| Hypertrophic cardiomyopathy, n (%) | 0 (0) | 1014 (2.1) | 543 (1.5) | 471 (3.9) | <0.001 |
| Restrictive cardiomyopathy, n (%) | 0 (0) | 1178 (2.4) | 780 (2.1) | 398 (3.3) | <0.001 |
| Valvular cardiomyopathy, n (%) | 0 (0) | 950 (1.9) | 655 (1.8) | 295 (2.4) | <0.001 |
| Other cardiomyopathy, n (%) | 0 (0) | 2585 (5.3) | 1108 (3.0) | 1477 (12.2) | <0.001 |
| Cardiovascular risk factors | |||||
| Diabetes (type I and II), n (%) | 4599 (9.3) | 8072 (18.1) | 6404 (19.2) | 1668 (14.9) | <0.001 |
| Diabetes type II, n (%) | 4502 (9.2) | 7390 (16.5) | 5897 (17.6) | 1493 (13.3) | <0.001 |
| Diabetes type I, n (%) | 4502 (9.2) | 682 (1.5) | 507 (1.5) | 175 (1.6) | 0.76 |
| History of cigarette use, n (%) | 18 038 (36.7) | 14 696 (47.2) | 1851 (51.1) | 2845 (35.8) | 0.001 |
| BMI (kg/m2 ) | 512 (1.0) | 26.5 (23.4, 30.1) | 26.8 (23.8, 30.2) | 25.5 (22.0, 29.7) | <0.001 |
| Systolic BP (mmHg) | 47 452 (96.4) | 101 (89, 112) | 102 (90, 112) | 101 (89, 112) | 0.97 |
| Co‐morbidities | |||||
| Prior dialysis, n (%) | 1320 (2.7) | 1544 (3.2) | 1192 (3.3) | 352 (3.0) | 0.087 |
| Prior malignancy, n (%) | 791 (1.6) | 3063 (6.3) | 1852 (5.1) | 1211 (10.2) | <0.001 |
| Prior cardiac surgery, a n (%) | 18 414 (37.4) | 7986 (25.1) | 6407 (26.9) | 1579 (19.6) | <0.001 |
| Education | |||||
| Higher than high school, n (%) | 7524 (15.3) | 22 037 (52.9) | 16 686 (53.2) | 5351 (51.9) | 0.025 |
| Ethnicity | |||||
| White, n (%) | 3 (0) | 34 987 (71.1) | 27 195 (73.3) | 7792 (64.4) | <0.001 |
| African American, n (%) | 3 (0) | 8849 (18.0) | 5877 (15.8) | 2972 (24.6) | <0.001 |
| Hispanic, n (%) | 3 (0) | 3522 (7.2) | 2630 (7.1) | 892 (7.4) | 0.30 |
| Asian, n (%) | 3 (0) | 1314 (2.7) | 1018 (2.7) | 296 (2.4) | 0.084 |
| Multiracial, n (%) | 3 (0) | 229 (0.5) | 149 (0.4) | 80 (0.7) | <0.001 |
| American Indian/Alaska Native, n (%) | 3 (0) | 158 (0.3) | 119 (0.3) | 39 (0.3) | 1.00 |
| Native Hawaiian/other pacific islander, n (%) | 3 (0) | 138 (0.3) | 111 (0.3) | 27 (0.2) | 0.20 |
| Urgency status | |||||
| Old status 1, b n (%) | 10 (0) | 5357 (10.9) | 4271 (11.5) | 1086 (9.0) | <0.001 |
| 1A, n (%) | 10 (0) | 22 061 (44.8) | 17 185 (46.3) | 4876 (40.3) | <0.001 |
| 1B | 10 (0) | 14 346 (29.2) | 10 488 (28.3) | 3858 (31.9) | <0.001 |
| 2, n (%) | 10 (0) | 7346 (14.9) | 5110 (13.8) | 2236 (18.5) | <0.001 |
| Days on waiting list (days) | 10 (0) | 93 (28, 261) | 101 (31, 278) | 73 (22, 213) | <0.001 |
| Immunological status | |||||
| HLA mismatch (>4), n (%) | 6189 (12.6) | 25 468 (59.2) | 19 230 (59.3) | 6238 (58.9) | 0.49 |
| Blood group | |||||
| A, n (%) | 0 (0) | 20 474 (41.6) | 15 619 (42.1) | 4855 (40.1) | <0.001 |
| O, n (%) | 0 (0) | 19 060 (38.7) | 14 186 (38.2) | 4874 (40.3) | <0.001 |
| B, n (%) | 0 (0) | 7005 (14.2) | 5248 (14.1) | 1757 (14.5) | 0.31 |
| AB, n (%) | 0 (0) | 2661 (5.4) | 2049 (5.5) | 612 (5.1) | 0.053 |
| Periprocedural characteristics | |||||
| Candidate in ICU, n (%) | 40 177 (96.1) | 5463 (60.5) | 4356 (61.7) | 1107 (56.5) | <0.001 |
| Candidate on ventilator, n (%) | 40 177 (96.1) | 256 (2.8) | 194 (2.7) | 62 (3.2) | 0.36 |
| Episodes of ventilatory support since listing, n (%) | 9009 (18.3) | 7134 (17.8) | 5577 (18.5) | 1557 (15.4) | <0.001 |
| i.v. inotropes, n (%) | 0 (0) | 21 368 (43.4) | 15 934 (42.9) | 5434 (44.9) | <0.001 |
| Transfusions, n (%) | 3013 (6.1) | 9918 (21.5) | 7760 (22.3) | 2158 (18.9) | <0.001 |
| ECMO, n (%) | 0 (0) | 304 (0.6) | 220 (0.6) | 84 (0.7) | 0.24 |
| VAD, c n (%) | 0 (0) | 8825 (17.9) | 7187 (19.4) | 1638 (13.5) | <0.001 |
| Ischaemic time (h) | 2197 (4.5) | 3.1 (2.4, 3.8) | 3.1 (2.4, 3.8) | 3.1 (2.4, 3.8) | 0.099 |
BMI, body mass index; BP, blood pressure; DIA, diastolic; ECMO, extracorporeal membrane oxygenation; HLA, human leucite antigen; HTx, heart transplantation; ICU, intensive care unit; IQR, interquartile range; i.v., intravenous; SD, standard deviation; SYS, systolic; UNOS; United Network of Organ sharing; VAD, ventricular assist device.
Between listing and HTx.
UNOS old status 1 was used to determine medical urgency status for heart and heart‐lung candidates listed prior to 1999.
Any VAD until HTx.
Men were more likely to have ischaemic cardiomyopathy (men vs. women, 48.2% vs. 22.2%; P < 0.001). Dilated cardiomyopathy (41.3% vs. 51.6%; P < 0.001) and other aetiologies of advanced HF such as congenital heart disease, hypertrophic, restrictive, and valvular cardiomyopathy were more common in women than in men (Table 1 ). Women were more likely to have history of malignancy (5.1% vs. 10.2%; P < 0.001). Men had a higher burden of CV risk factors compared with women (e.g., history of cigarette use [51.1% vs. 35.8%; P < 0.001], diabetes [19.2% vs. 14.9%; P < 0.001]), had a longer time on waiting list compared with women (101 vs. 73 days; P < 0.001), although men were listed more often in UNOS status 1A (46.3% vs. 40.3%; P < 0.001) (Table 1 ). Men were treated more frequently in ICU (61.7% vs. 56.5%; P < 0.001) and needed more often ventilatory support (18.5% vs. 15.4%; P < 0.001) or VAD support (19.4% vs. 13.5%; P < 0.001), whereas the need for ECMO therapy was comparable between both sexes (0.6% vs. 0.7%; P = 0.24). Women received inotropes more often compared with men (42.9% vs. 44.9%; P < 0.001) (Table 1 ).
Clinical characteristics independently associated with sex
As compared with the baseline characteristics, results observed in the logistic regression analysis were similar (Figure 2 ). Of all variables which were tested, clinical characteristics independently associated with male sex were ischaemic cardiomyopathy (OR 3.26, CI 3.11–3.42; P < 0.001), a high burden of CV risk factors, treatment in ICU (OR 1.24, CI 1.12–1.37; P < 0.001) including the need for ventilatory support (OR 1.24, CI 1.17–1.32; P < 0.001), and VAD support (OR 1.53, CI 1.45–1.64; P < 0.001). Independent clinical factors associated with female sex were history of malignancy (OR 0.47, CI 0.44–0.51; P < 0.001) and use of inotropes (OR 0.92, CI 0.89–0.96; P < 0.001). There were no differences in ECMO therapy between both sexes (Figure 2 ).
Figure 2.
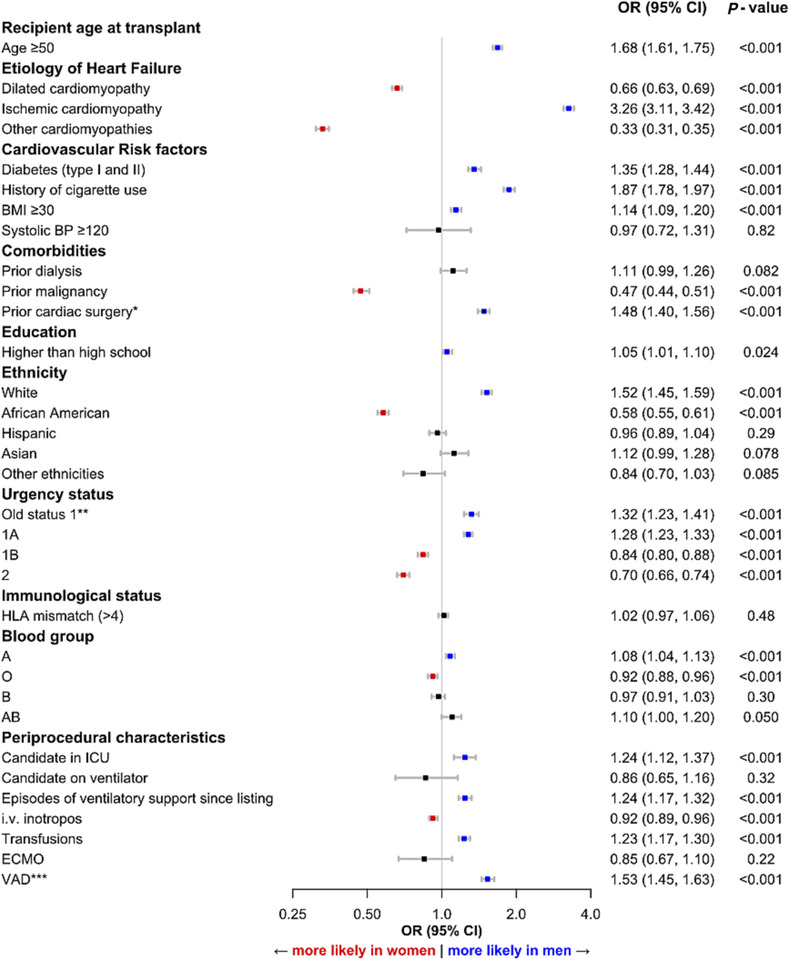
Clinical characteristics associated with sex among patients post heart transplantation (HTx). Odds ratios (ORs) and 95% confidence intervals (CIs) are depicted. *between listing and HTx, **UNOS old status 1 was used to determine medical urgency status for heart and heart‐lung candidates listed prior to 1999, ***Any VAD until HTx. BMI, body mass index; BP, blood pressure; ECMO, extracorporeal membrane oxygenation; HLA, human leucite antigen; ICU, intensive care unit; i.v., intravenous; UNOS, United Network of Organ Sharing; VAD, ventricular assist device.
Sex differences in outcomes
After a median follow‐up of 8.1 years, 49 732 events occurred. Unadjusted cox regression analysis showed a higher risk for men for all‐cause mortality (HR 1.04, 95% CI 1.01–1.08: P = 0.012), CAV (HR 1.18, 95% CI 1.13–1.23; P < 0.001) and for malignancy (HR 1.89, 95% CI 1.78–2.00; P < 0.001). Women had a higher risk for GF in the unadjusted model (HR 0.84, 95% CI 0.78–0.9; P < 0.001) (Figure S1 ).
After multivariable adjustment, there were no differences in all‐cause mortality between men and women (HR 0.97, 95% CI 0.91–1.03; P = 0.33), CV mortality (HR 1.05, CI 0.91–1.22; P = 0.49), and GF (HR 0.93, CI 0.83–1.03; P = 0.17) (Figure 3 , Figure 4 ). Men had a higher risk for CAV (HR 1.21, CI 1.13–1.29; P < 0.001) and malignancy (HR 1.80, CI 1.62–2.00; P < 0.001) (central figure, Figure 3 , Figure 4 ). Detailed characteristics regarding malignancies post transplantation were depicted in Table S1 .
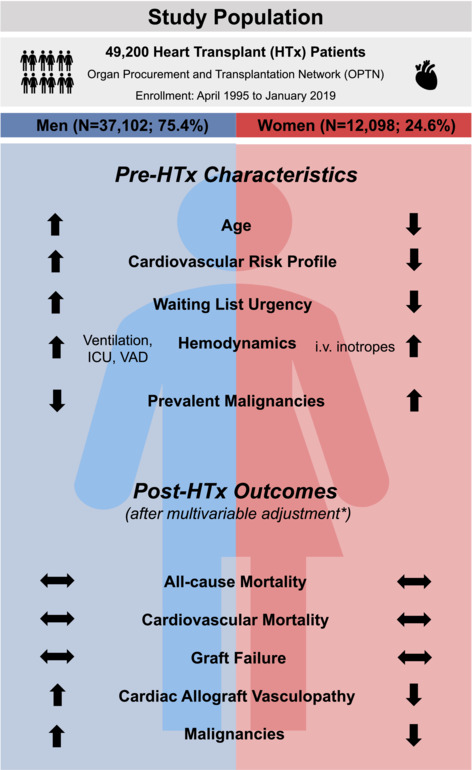
Central Figure. *The different Cox models were 1) adjusted for age and ethnicity and 2) adjusted for sociodemographic factors (age, ethnicity, education [education higher than high school]), and cardiovascular risk factors (BMI, history of cigarette use, diabetes), immunological variables (blood group, HLA mismatch >4 loci), and periprocedural characteristics as UNOS status, mechanical and circulatory (VAD or ECMO) and ventilatory support and need for dialysis. For malignancy, model 2 was also adjusted for prior malignancy. Abbreviations: HTx, heart transplantation; ICU, intensive care unit; i.v. intravenous; UNOS, United Network of Organ Sharing; VAD, ventricular assist device.
Figure 3.
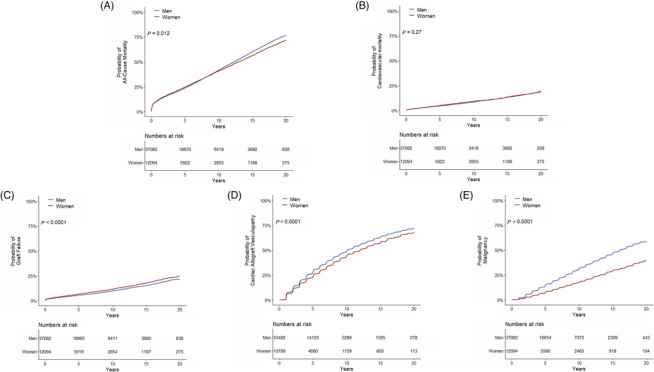
Cumulative incidence curves for all‐cause mortality (A), cardiovascular mortality (B), graft failure (C), cardiac allograft vasculopathy (D), and malignancy (E).
Figure 4.
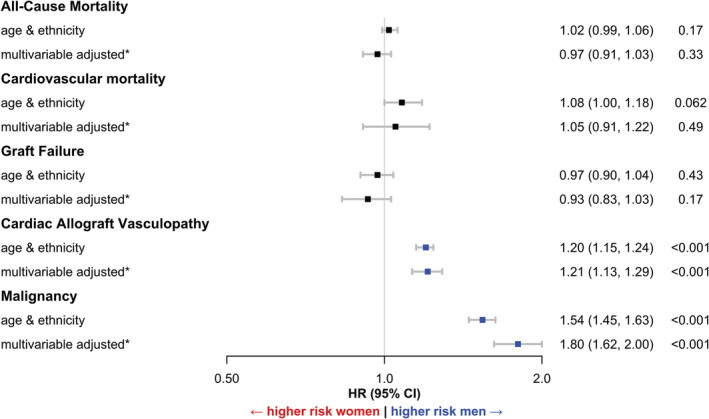
Cox regression models for men vs. women for all‐cause mortality, cardiovascular death, graft failure, cardiac allograft vasculopathy and malignancy. The different Cox models were adjusted (1) for age and ethnicity and (2) adjusted for * sociodemographic factors (age, ethnicity, education [education higher than high school]), and cardiovascular risk factors (BMI, history of cigarette use, diabetes), immunological variables (blood group, HLA mismatch >4 loci), and periprocedural characteristics as UNOS status, mechanical and circulatory (VAD or ECMO) and ventilatory support and need for dialysis. For malignancy, model 2 was also adjusted for prior malignancy. CAV, cardiac allograft vasculopathy; CV, cardiovascular; HR, hazard ratio; CI, confidence interval.
Sex interactions in outcome analyses
Men at higher age (HR men 1.01, CI 1.00–1.01, P < 0.001; HR women 1.00, CI 0.99–1.00, P = 0.025; P‐interaction<0.001), and women with prior dialysis (HR men 1.48, CI 1.29–1.70, P < 0.001; HR women 1.98, CI 1.57–2.49, P < 0.001; P‐interaction = 0.0036), and need for ventilatory support (HR men 1.03, CI 0.95–1.11, P = 0.48; HR women 1.2, CI 1.05–1.36, P = 0.0077; P‐interaction = 0.047) were at higher risk for all‐cause mortality (Figure S2 ). There were no differences in CV mortality between both sexes (Figure S3 ). Men on VAD support prior to HTx (HR men 1.15, CI 1.02–1.30, P = 0.022; HR women 0.85, 0.67–1.08, P = 0.19; P‐interaction = 0.023) were at higher risk for GF than women (Figure S4 ), while there were no sex interactions in CAV (Figure S5 ). Men at higher age had a higher risk of malignancy (HR women 1.07, CI 1.06–1.07, P < 0.001; HR men 1.05, CI 1.04–1.06, P < 0.001; P‐interaction = 0.0024) (Figure S6 ).
Discussion
This analysis of sex differences in all cardiac transplant patients in the United States in the last decade found substantial sex differences in pre‐transplant characteristics and post‐transplant outcomes. These include
Men were more often listed for HTx due to ischaemic cardiomyopathy and had a higher burden of CV risk factors. Women were more often listed with other aetiologies of advanced HF.
On waiting list, men were listed more urgently and needed more ventilatory and mechanical circulatory support, while women suffered more often from prior malignancy and had a higher need for inotropic therapy.
Risk for all‐cause mortality and CV mortality was comparable between men and women after multivariable adjustment.
Men had a higher risk for CAV and malignancy than women which could not be explained completely by pre‐ and periprocedural characteristics.
Sex differences in clinical characteristics
In our study, women accounted for less than one third of HTx recipients. This sex distribution has also been documented in previous UNOS‐ and non‐UNOS based studies 12 , 25 and did not change over the last years. 26 This sex disparity might be partly explained by differences in disease course, symptom patterns, delayed clinical presentation, and selection and/or referral bias of patients with advanced HF. 27 , 28 Sex differences for patients listed for HTx have been previously reported. 3 , 28 In line with prior studies, men were older, showed a worse CV risk profile and had, consecutively, a higher prevalence of ischaemic cardiomyopathy in our analysis. 11 , 25 While women had a lower waiting list urgency status (UNOS status IB and UNOS status II), men were more likely to be treated at ICU and had a higher need for mechanical ventilatory and circulatory support, which is consistent with current literature. 9 , 11 , 29 The higher severity of HF in men might be explained by higher age and a higher burden of co‐morbidities in the pre‐operative stage.
Sex differences in outcomes and risk predictors
Although there is evidence that women tend to have a better long‐term survival than men, 12 sex did not predict mortality in our study as it did in others. 11 Recent studies showed a complex relationship of risk factors and post HTx mortality. CV risk factors (e.g., diabetes and BMI), dialysis and ECMO therapy 4 or blood group O were reported to be associated with higher mortality. 29 In our study, we confirmed these findings by reporting a higher risk for all‐cause mortality in women with need for dialysis and ventilatory support.
It is known that GF represents one of the main causes for early post‐transplant mortality. 18 Within 30 days post‐transplant, 66% of deaths occur due to GF or multi‐organ dysfunction, with GF being assumed as the most frequent cause for the latter. 13 Data from single‐centre studies assume a higher incidence of acute rejection in female recipients, 30 , 31 a precursor or at least part of the GF syndrome. Previously known risk factors for GF as CV risk factors and previous ECMO therapy 14 , 17 could be confirmed by our data. We could additionally show that previous VAD therapy was associated with elevated risk for new‐onset GF in men.
CAV is the leading cause of late organ dysfunction. 12 , 15 In line with our findings, incidence of CAV was shown to be higher in men compared with women. 19 , 32 Age, sex, CV risk factors, immunological factors, and cellular rejection were related to new‐onset CAV. 15 We could not explain the higher incidence of CAV in men by clinical parameters as shown in the interaction analysis. Major differences in immunological response between sexes are known. 33 , 34 Immunological, metabolic, and hormonal causes, which were not investigated in this study, might also drive sex differences in CAV incidence.
Results from smaller registry‐based studies indicated that women suffer more often from malignancy than men before HTx. 35 In contrast, the incidence and mortality related to malignancy after HTx are increased for men over time. 23 , 36 , 37 These findings were in line with our results. Furthermore, we could show that male sex persisted as risk factor for post‐transplant malignancy even after further adjustment. Immunosuppressive therapy is assumed to be a leading cause for cancer development. 38 While we can only speculate on the reason for these sex disparities, higher cancer risk may result from differences in immune system or cancer susceptibility between men and women. On cellular level, differences in innate and adapted immune response are substantial with higher efficiency of antigen‐presentation 33 and higher activity of phagocytosis 34 in women, as well as oestrogen dependent promotion of interferons, chemokine ligands, and interleukins. 39 Desoxyribnoucleid acid damage repair mechanisms differ between sexes leading to higher genomic instability and higher amount of mutations in men. 40 Finally, sex differences in risk behaviour as exposure to noxae or sunlight might also influence higher malignancy rates in men.
Limitations
There are several limitations to be considered in this study. While the UNOS database captures an almost complete set of cardiac transplant patients in the United States, entry and validation in the SRTR database is partly imperfect based on its multi‐centre large‐scale registry character. Strategies to minimize this challenge are edit checks and validation of data at time of entry. Although the registry covers many variables, potential inaccuracy of data entry cannot be completely ruled out. Furthermore, definitions of variables in the UNOS reporting system could be interpreted differentially from individual centres and may influence the results. For example, the definition of GF is indistinctive in this dataset. Thus, we could not differ between primary GF and secondary GF as defined by the ISHLT consensus document. 13 Hence, misclassification or misinterpretation might have biased our results. In addition, due to the registry nature a high frequency of missing data e.g. panel reactive antibodies and immunosuppression are noted and were not included in the study. Importantly, no other classification for severity or phenotypes of acute HF is given in the OPTN database. Furthermore, types of inotropic agents are not presented due to the high number of missing data.
Conclusions
In this large‐scale transplant US registry, we found major differences in men and women in pre‐HTx characteristics. While sex did not predict all‐cause or CV mortality, male sex was identified as an independent predictor for CAV and malignancy even after multivariable adjustment. Our results highlight the need for an individualized clinical follow‐up and patient‐centred care in men and women.
Conflict of interest
None declared.
Conflict of interest statement
Dr. Fluschnik received grants from Biotronik and Medtronic, all outside this submitted work.
Dr. Schrage received funding from the German Research Foundation and the Else Kroener‐Fresenius‐Stiftung, and speakers fees from AstraZeneca, all outside this submitted work. Dr. Becher received funding from the German Research Foundation and received speakers fees from AstraZeneca and IngelheimBoehringer, all outside this submitted work. Dr. Bernhardt reported personal fees from Abbott, Abiomed, AstraZeneca, BerlinHeart, Medtronic, Novartis, all outside this submitted work. Dr. Blankenberg received speakers fee from Medtronic, Pfizer, Roche, Novartis, SiemensDiagnostics, all outside this submitted work. Dr. Reichenspurner received honoraria from Abiomed and Medtronic, all outside this submitted work. Dr. Kirchhof received research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Centre for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and received honoraria from several such companies in the past, but not in the last 3 years, all outside this submitted work. Dr. Kirchhof is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). Dr. Schnabel received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme [grant No 648131], from the European Union's Horizon 2020 research and innovation programme [grant No 847770] (AFFECT‐EU) and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103); German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031 L0239) (unrelated to the submitted work). Dr. Schnabel's Conflicts: Dr Schnabel received lecture fees and advisory board fees from BMS/Pfizer outside this work. Dr. Magnussen receives funding from the German Center for Cardiovascular Research (DZHK) within the Promotion of women scientist program, the Deutsche Stiftung fuer Herzforschung and the Dr. Rolf Schwiete Stiftung unrelated to the current work. Dr. Magnussen received speaker fees from several drug and device companies outside of this study.
Supporting information
Table S1. Detailed characteristics regarding malignancies post transplantation.
Figure S1. Cox regression models unadjusted and adjusted for men vs. women for all‐cause mortality, cardiovascular death, graft failure, cardiac allograft vasculopathy and malignancy.
Figure S2. Sex interaction analysis in all‐cause mortality.
Figure S3. Sex interaction analysis in cardiovascular mortality.
Figure S4. Sex interaction analysis in graft failure.
Figure S5. Sex interaction analysis in cardiac allograft vasculopathy.
Figure S6. Sex interaction analysis in malignancy.
Acknowledgements
This work was supported in part by Health Resources and Services Administration contract 234‐2005‐37011C. The content is the responsibility of the authors alone and does not necessarily reflect the view and polices of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Open Access funding enabled and organized by Projekt DEAL.
Kondziella, C. , Fluschnik, N. , Weimann, J. , Schrage, B. , Becher, P. M. , Memenga, F. , Bernhardt, A. M. , Blankenberg, S. , Reichenspurner, H. , Kirchhof, P. , Schnabel, R. B. , and Magnussen, C. (2023) Sex differences in clinical characteristics and outcomes in patients undergoing heart transplantation. ESC Heart Failure, 10: 2596–2606. 10.1002/ehf2.14413.
Christoph Kondziella and Nina Fluschnik contributed equally.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Defilippis EM, Truby LK, Clerkin KJ, Donald E, Sinnenberg L, Varshney AS, Cogswell R, Kittleson MM, Haythe JH, Givertz MM, Hsich EM, Agarwal R, Topkara VK, Farr M. Increased opportunities for transplantation for women in the new heart allocation system. J Card Fail. 2022; 28: 1149–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morris AA, Cole RT, Laskar SR, Kalogeropoulos A, Vega JD, Smith A, Butler J. Improved outcomes for women on the heart transplant wait list in the modern era. J Card Fail. 2015; 21: 555–560. [DOI] [PubMed] [Google Scholar]
- 4. Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, Sadavarte A, Singh TP, Zuckermann A, Stehlik J, International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐sixth adult heart transplantation report ‐ 2019; focus theme: Donor and recipient size match. J Heart Lung Transplant. 2019; 38: 1056–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsich EM. Sex differences in advanced heart failure therapies. Circulation. 2019; 139: 1080–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation registry. J Heart Lung Transplant. 2012; 31: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ayesta A, Urrutia G, Madrid E, Vernooij RWM, Vicent L, Martinez‐Selles M. Sex‐mismatch influence on survival after heart transplantation: a systematic review and meta‐analysis of observational studies. Clin Transplant. 2019; 33: e13737. [DOI] [PubMed] [Google Scholar]
- 8. Previato M, Osto E, Kerkhof PLM, Parry G, Tona F. Heart transplantation survival and sex‐related differences. Adv Exp Med Biol. 2018; 1065: 379–388. [DOI] [PubMed] [Google Scholar]
- 9. Moayedi Y, Fan CPS, Cherikh WS, Stehlik J, Teuteberg JJ, Ross HJ, Khush KK. Survival outcomes after heart transplantation: does recipient sex matter? Circ Heart Fail. 2019; 12: e006218. [DOI] [PubMed] [Google Scholar]
- 10. Kaczmarek I, Meiser B, Beiras‐Fernandez A, Guethoff S, Uberfuhr P, Angele M, Seeland U, Hagl C, Reichart B, Eifert S. Gender does matter: gender‐specific outcome analysis of 67,855 heart transplants. Thorac Cardiovasc Surg. 2013; 61: 29–36. [DOI] [PubMed] [Google Scholar]
- 11. Hsich EM, Blackstone EH, Thuita LW, McNamara DM, Rogers JG, Yancy CW, Goldberg LR, Valapour M, Xu G, Ishwaran H. Heart transplantation: an in‐depth survival analysis. JACC Heart Fail. 2020; 8: 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chambers DC, Cherikh WS, Goldfarb SB, Hayes D Jr, Kucheryavaya AY, Toll AE, Khush KK, Levvey BJ, Meiser B, Rossano JW, Stehlik J. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐fifth adult lung and heart‐lung transplant report‐2018; Focus theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018; 37: 1169–1183. [DOI] [PubMed] [Google Scholar]
- 13. Kobashigawa J, Zuckermann A, Macdonald P, Leprince P, Esmailian F, Luu M, Mancini D, Patel J, Razi R, Reichenspurner H, Russell S, Segovia J, Smedira N, Stehlik J, Wagner F, Consensus Conference participants . Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J Heart Lung Transplant. 2014; 33: 327–340. [DOI] [PubMed] [Google Scholar]
- 14. Lopez‐Sainz A, Barge‐Caballero E, Barge‐Caballero G, Couto‐Mallon D, Paniagua‐Martin MJ, Seoane‐Quiroga L, Iglesias‐Gil C, Herrera‐Noreña JM, Cuenca‐Castillo JJ, Vázquez‐Rodríguez JM, Crespo‐Leiro MG. Late graft failure in heart transplant recipients: incidence, risk factors and clinical outcomes. Eur J Heart Fail. 2018; 20: 385–394. [DOI] [PubMed] [Google Scholar]
- 15. Loupy A, Coutance G, Bonnet G, Van Keer J, Raynaud M, Aubert O, Bories MC, Racapé M, Yoo D, Duong Van Huyen JP, Bruneval P, Taupin JL, Lefaucheur C, Varnous S, Leprince P, Guillemain R, Empana JP, Levine R, Naesens M, Patel JK, Jouven X, Kobashigawa J. Identification and characterization of trajectories of cardiac allograft vasculopathy after heart transplantation: a population‐based study. Circulation. 2020; 141: 1954–1967. [DOI] [PubMed] [Google Scholar]
- 16. Lateef N, Abdul Basit K, Abbasi N, Kazmi SM, Ansari AB, Shah M. Malignancies after heart transplant. Exp Clin Transplant. 2016; 14: 12–16. [DOI] [PubMed] [Google Scholar]
- 17. Russo MJ, Iribarne A, Hong KN, Ramlawi B, Chen JM, Takayama H, Mancini DM, Naka Y. Factors associated with primary graft failure after heart transplantation. Transplantation. 2010; 90: 444–450. [DOI] [PubMed] [Google Scholar]
- 18. Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Goldfarb S, Levvey BJ, Meiser B, Rossano JW, Yusen RD, Stehlik J. The registry of the International Society for Heart and Lung Transplantation: thirty‐second official adult heart transplantation report—2015; focus theme: early graft failure. J Heart Lung Transplant. 2015; 34: 1244–1254. [DOI] [PubMed] [Google Scholar]
- 19. Taylor DO, Stehlik J, Edwards LB, Aurora P, Christie JD, Dobbels F, Kirk R, Kucheryavaya AY, Rahmel AO, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twenty‐sixth Official Adult Heart Transplant Report‐2009. J Heart Lung Transplant. 2009; 28: 1007–1022. [DOI] [PubMed] [Google Scholar]
- 20. Khush KK, Cherikh WS, Chambers DC, Goldfarb S, Hayes D Jr, Kucheryavaya AY, Levvey BJ, Meiser B, Rossano JW, Stehlik J, International Society for Heart and Lung Transplantation . The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty‐fifth Adult Heart Transplantation Report‐2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transplant. 2018; 37: 1155–1168. [DOI] [PubMed] [Google Scholar]
- 21. Giuliano K, Canner JK, Etchill E, Suarez‐Pierre A, Choi CW, Higgins RSD, Hsu S, Sharma K, Kilic A. High rates of de novo malignancy compromise post‐heart transplantation survival. J Card Surg. 2021; 36: 1401–1410. [DOI] [PubMed] [Google Scholar]
- 22. Leppke S, Leighton T, Zaun D, Chen SC, Skeans M, Israni AK, Snyder JJ, Kasiske BL. Scientific Registry of Transplant Recipients: collecting, analyzing, and reporting data on transplantation in the United States. Transplant Rev (Orlando). 2013; 27: 50–56. [DOI] [PubMed] [Google Scholar]
- 23. Doesch AO, Muller S, Konstandin M, Celik S, Kristen A, Frankenstein L, Ehlermann P, Sack FU, Katus HA, Dengler TJ. Malignancies after heart transplantation: incidence, risk factors, and effects of calcineurin inhibitor withdrawal. Transplant Proc. 2010; 42: 3694–3699. [DOI] [PubMed] [Google Scholar]
- 24. Therneau TMG, Grambsch PM. Modeling Survival Data: Extending the Cox Model; Statistics for biology and health. New York: Springer; 2000. [Google Scholar]
- 25. Garcia‐Cosio MD, Gonzalez‐Vilchez F, Lopez‐Vilella R, Barge‐Caballero E, Gomez‐Bueno M, Martinez‐Selles M, Arizón JM, Rangel Sousa D, González‐Costello J, Mirabet S, Pérez‐Villa F, Díaz‐Molina B, Rábago G, Portolés Ocampo A, de la Fuente‐Galán L, Garrido I, Delgado‐Jiménez JF. Gender differences in heart transplantation: twenty‐five year trends in the nationwide Spanish heart transplant registry. Clin Transplant. 2020; 34: e14096. [DOI] [PubMed] [Google Scholar]
- 26. Colvin M, Smith JM, Ahn Y, Skeans MA, Messick E, Goff R, Bradbrook K, Foutz J, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2019 annual data report: heart. Am J Transplant. 2021; 21: 356–440. [DOI] [PubMed] [Google Scholar]
- 27. Fluschnik N, Strangl F, Kondziella C, Gossling A, Becher PM, Schrage B, Schnabel RB, Bernadyn J, Bremer W, Grahn H, Bernhardt AM, Reichenspurner H, Rybczynski M, Blankenberg S, Kirchhof P, Magnussen C, Knappe D. Gender differences in characteristics and outcomes in heart failure patients referred for end‐stage treatment. ESC Heart Fail. 2021; 8: 5031–5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hsich EM, Blackstone EH, Thuita L, McNamara DM, Rogers JG, Ishwaran H, Schold JD. Sex differences in mortality based on united network for organ sharing status while awaiting heart transplantation. Circ Heart Fail. 2017; 10: e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ando M, Takeda K, Kurlansky PA, Garan AR, Topkara VK, Yuzefpolskaya M, Colombo PC, Farr M, Naka Y, Takayama H. Association between recipient blood type and heart transplantation outcomes in the United States. J Heart Lung Transplant. 2020; 39: 363–370. [DOI] [PubMed] [Google Scholar]
- 30. Esmore D, Keogh A, Spratt P, Jones B, Chang V. Heart transplantation in females. J Heart Lung Transplant. 1991; 10: 335–341. [PubMed] [Google Scholar]
- 31. Sharples LD, Caine N, Mullins P, Scott JP, Solis E, English TA, Large SR, Schofield PM, Wallwork J. Risk factor analysis for the major hazards following heart transplantation—rejection, infection, and coronary occlusive disease. Transplantation. 1991; 52: 244–252. [DOI] [PubMed] [Google Scholar]
- 32. Fluschnik N, Geelhoed B, Becher PM, Schrage B, Brunner FJ, Knappe D, Bernhardt AM, Blankenberg S, Kobashigawa J, Reichenspurner H, Schnabel RB, Magnussen C. Non‐immune risk predictors of cardiac allograft vasculopathy: results from the U.S. organ procurement and transplantation network. Int J Cardiol. 2021; 331: 57–62. [DOI] [PubMed] [Google Scholar]
- 33. Weinstein Y, Ran S, Segal S. Sex‐associated differences in the regulation of immune responses controlled by the MHC of the mouse. J Immunol. 1984; 132: 656–661. [PubMed] [Google Scholar]
- 34. Scotland RS, Stables MJ, Madalli S, Watson P, Gilroy DW. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood. 2011; 118: 5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ladowski SD, Abel M, Beatty L, Scatena M, Ladowski JS. Long‐term follow‐up of hearttransplant recipients with pre‐transplant malignancies. Tex Heart Inst J. 2006; 33: 27–30. [PMC free article] [PubMed] [Google Scholar]
- 36. Crespo‐Leiro MG, Alonso‐Pulpon L, Vazquez de Prada JA, Almenar L, Arizon JM, Brossa V, Delgado JF, Fernandez‐Yañez J, Manito N, Rábago G, Lage E, Roig E, Diaz‐Molina B, Pascual D, Muñiz J. Malignancy after heart transplantation: incidence, prognosis and risk factors. Am J Transplant. 2008; 8: 1031–1039. [DOI] [PubMed] [Google Scholar]
- 37. Jaamaa‐Holmberg S, Salmela B, Lemstrom K, Pukkala E, Lommi J. Cancer incidence and mortality after heart transplantation ‐ a population‐based national cohort study. Acta Oncol. 2019; 58: 859–863. [DOI] [PubMed] [Google Scholar]
- 38. Rinaldi M, Pellegrini C, D'Armini AM, Aiello M, Negri M, Arbustini E, Ippoliti G, Viganò M. Neoplastic disease after heart transplantation: single center experience. Eur J Cardiothorac Surg. 2001; 19: 696–701. [DOI] [PubMed] [Google Scholar]
- 39. Hewagama A, Patel D, Yarlagadda S, Strickland FM, Richardson BC. Stronger inflammatory/cytotoxic T‐cell response in women identified by microarray analysis. Genes Immun. 2009; 10: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fischer KE, Riddle NC. Sex differences in aging: genomic instability. J Gerontol A Biol Sci Med Sci. 2018; 73: 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Detailed characteristics regarding malignancies post transplantation.
Figure S1. Cox regression models unadjusted and adjusted for men vs. women for all‐cause mortality, cardiovascular death, graft failure, cardiac allograft vasculopathy and malignancy.
Figure S2. Sex interaction analysis in all‐cause mortality.
Figure S3. Sex interaction analysis in cardiovascular mortality.
Figure S4. Sex interaction analysis in graft failure.
Figure S5. Sex interaction analysis in cardiac allograft vasculopathy.
Figure S6. Sex interaction analysis in malignancy.


