Abstract
Adenoviruses (Ad) are a significant cause of acute infections in humans; however, replication-defective forms of this virus are currently under investigation for human gene therapy. Approximately 20 to 25% of all the gene therapy trials (phases I to III) conducted over the past 10 years involve the use of Ad gene delivery for treatment inherited or acquired diseases. At present, the most promising applications involve the use of Ad vectors to irradicate certain nonmetastatic tumors and to promote angiogenesis in order to alleviate cardiovascular disease. While specific problems of using Ad vectors remain to be overcome (as is true for almost all viral and nonviral delivery methods), a distinct advantage of Ad is the extensive knowledge of its macromolecular structure, genome organization, sequence, and mode of replication. Moreover, significant information has also been acquired on the interaction of Ad particles with distinct host cell receptors, events which strongly affect virus tropism. This review provides an overview of the structure and function of Ad attachment (coxsackievirus and Ad receptor [CAR]) and internalization (αv integrins) receptors and discusses their precise role in virus infection and gene delivery. Recent structure studies of integrin-Ad complexes by cryoelectron microscopy are also highlighted. Finally, unanswered questions arising from the current state of knowledge of Ad-receptor interactions are presented in the context of improving Ad vectors for future human gene therapy applications.
Peter Medawar, who was awarded the 1960 Nobel Prize for Medicine and Physiology, defined a virus as a piece of nucleic acid surrounded by bad news (66). While this is certainly true of many human viruses, adenovirus (Ad) has also contributed significantly to the benefit of mankind. Ad represent a large family of nonenveloped viruses containing a double-stranded DNA genome of approximately 36 kb (43, 72). Human Ad, of which there are 50 different viral serotypes, are associated with respiratory, ocular, and gastrointestinal diseases. Ad infections are usually self-limiting; however, they can cause fatal disseminated disease in immunocompromised individuals (41, 58, 61, 62, 86). Despite its association with human diseases, Ad has also served as a valuable tool that has been used to uncover a number of important molecular and cell biological processes such as RNA processing (7, 15) and cell cycle regulation (14, 105).
An accumulation of information on the structure, molecular, and cell biology of Ad over the past several decades has also allowed the development of Ad vectors for in vivo gene therapy (10, 23, 53, 106). Multiple phase I or phase II clinical trials involving replication-defective forms of Ad to treat acquired and inherited diseases are underway. Some of the most encouraging uses of Ad vectors are those based on “conditional” replicating viruses for treating head and neck tumors (8). While several problems of using Ad vectors for in vivo gene therapy remain to be overcome, Ad vectors have already proven useful for studying the structure and function of various gene products in vitro (98).
Ad also offers several advantages for studying direct interactions of the virus with host cell receptors. Ad particles are stable and can be produced at high titers in human epithelial cell lines. In addition, several of the important coat proteins of the virus can be produced as soluble recombinant proteins in bacterial or insect cell expression systems, thus allowing analysis of their functional properties independent of the intact virion. Ad capsids are approximately 900 Å in diameter and have icosahedral symmetry. The virion contains 240 copies of the major coat protein, known as the hexon. Each of the 12 vertices of Ad also contains a complex, known as the penton, which is composed of the 320-kDa penton base and the 182-kDa fiber protein (88, 90). The Ad fiber protein mediates attachment to cells via interaction with a 46-kDa cell receptor designated CAR (coxsackievirus and Ad receptor) (5, 95) (Fig. 1). The penton base is composed of five identical polypeptide subunits, while the fiber contains three identical proteins. CAR serves as the receptor for many but not all Ad serotypes (47, 76). At present, all Ad serotypes except those belonging to subgroup B recognize CAR. The normal cellular function of CAR, a member of the immunoglobulin superfamily, has not yet been elucidated. The cytoplasmic domain of CAR is not required for virus attachment or infection (100), suggesting that cell signaling through this receptor is not involved in viral entry.
FIG. 1.
Schematic illustration of the interaction of Ad2 with different cellular receptors involved in infection. High-affinity virus attachment is mediated by the interaction of the fiber capsid protein (white) with a 46-kDa receptor known as CAR. A second interaction of the penton base capsid protein (red) with αv integrins promotes virus internalization.
Efficient internalization of Ad into cells requires a second interaction with a separate cell receptor. The Ad penton base protein binds to integrins αvβ3 and αvβ5, and this promotes virus internalization (3, 104). In studies performed over 40 years ago (30, 71), the penton base was described as a virus-associated toxic factor which caused cells to detach from their substratum (the extracellular matrix) in culture. It is now known that the penton base is not toxic but instead has the capacity to interfere with integrin interactions with the extracellular matrix, thereby promoting cell detachment in vitro. Integrins are relatively large heterodimeric transmembrane proteins composed of an α subunit and a β subunit (50). There are over 20 different members of the integrin family, many of which recognize an arginine, glycine, aspartic acid (RGD) sequence in host extracellular matrix proteins such as vitronectin, fibronectin, and tenacin. Interactions of integrins with these host cell ligands serve a number of important host cell functions including cell attachment, migration, growth, and differentiation. These functions are also reflected in the role of integrins in several homeostatic processes including wound healing, osteogenesis, tumor metastasis, and perhaps certain neuronal functions (40, 50).
Although Ad is the first virus that was shown to use distinct cellular receptors for attachment (CAR) and internalization (αv integrins), more recent studies have demonstrated that several enveloped viruses including human immunodeficiency virus type 1 (32), herpes simplex virus type 1 (35), and adeno-associated virus (AAV) (92) use separate cell receptors for attachment and entry. Of these, only AAV and Ad appear to use αv integrins for entry. Other members of the integrin family also promote the entry of different viruses (see “Other viral pathogens that use integrins for infection” below), as well as certain bacteria.
IN VITRO AND IN VIVO EVIDENCE THAT αV INTEGRINS PROMOTE ADENOVIRUS CELL ENTRY
Several lines of evidence indicate that interactions of Ad with integrins promote virus internalization rather than attachment. Function-blocking antibodies directed against αv integrins or RGD-synthetic peptides inhibit virus endocytosis and infection but do not interfere with Ad attachment (104). Recombinant Ad fiber but not the penton base competes for attachment sites of viral particles (104). Moreover, the Ad2 fiber protein has an approximately 50-fold-higher binding affinity for CAR than the penton base has for αv integrins. Taken together, these earlier studies indicated that the fiber protein interaction with CAR facilitates virus attachment while the integrin binding to the penton base enhances virus uptake into cells. At present, it is not clear why CAR fails to promote virus internalization. The role of CAR in normal host cell functions, if any, also has not yet been established, and further knowledge of this may shed light on its role in virus infection.
Another indication of the important role of interactions of αv integrin with Ad is that the penton base proteins of multiple Ad serotypes from different virus subgroups contain a conserved RGD motif (60). Different Ads also use integrins for infection and/or bind to these receptors (59). Preincubation of virus particles with soluble recombinant αvβ5 integrin also inhibits Ad internalization and virus-mediated gene delivery in vitro (59).
One interesting feature of the Ad penton base is that the number of amino acid residues flanking the RGD motif varies among the different Ad serotypes. For example, sequence alignments have revealed that the Ad12 penton base contains approximately 20 residues (87) while that of Ad2/Ad5 has approximately 80 residues (64) (Fig. 2). An obvious question arising from these findings is whether variations in the RGD-flanking sequences influence integrin binding to the penton base. Studies such as those with phage display libraries (42) may be helpful for the identification of the precise amino acid residues in the penton base that influence Ad-αv integrin interactions. Ad12 virions are reported to be more susceptible to inhibition by anti-integrin antibodies than are Ad2 virions (3), suggesting that Ad12 has lower intrinsic binding to αv-integrins than does Ad2. This is supported by recent binding studies with soluble recombinant integrin αvβ5 (59). Our structural studies also showed that soluble integrin αvβ5 bound to Ad12 penton base has restricted mobility compared to integrins bound to the Ad2 penton base, which display a high degree of flexibility (14a). The flexibility of the RGD loop in fibronectin, an extracellular matrix protein, has also been postulated to play a role in integrin binding (2, 18).
FIG. 2.
An alignment of penton base sequences from different Ad serotypes. Identical amino acid residues, located primarily at the N and C termini of the proteins, are indicated by vertical lines. Gaps indicated by dotted lines were used to maximize the alignment. Note the conserved RGD sequence indicated in boldface type with asterisks. The Ad12 sequence was obtained from reference 87.
Although Ad from most subgroups use αv integrins for infection, it is interesting that the penton base of Ad from subgroup F, serotypes 40/41, lacks the conserved RGD motif (25). These enteric Ad which are quite difficult to propagate in vitro, may not interact with integrins. It will be of interest to determine whether they use an alternative cell entry pathway.
Given the conservation of the RGD integrin-binding motif among many different Ad serotypes, one might predict that introduction of a mutation in this sequence might negatively affect virus internalization and/or infection. In keeping with this prediction, a mutant Ad2 lacking the penton base RGD sequence was shown to have decreased infectivity in vitro (4). Interestingly, infection by the RGD mutant Ad was delayed but not completely abolished. These findings raise the possibility that virus infection can still occur in the absence of an integrin-penton base interaction. A likely scenario is that the fiber-CAR interaction is sufficient to allow virus entry, albeit at a significantly reduced internalization rate. An RGD mutant Ad bound to the surface of host cells via the CAR-fiber interaction but with a decreased capacity for internalization may be particularly vulnerable to attack in vivo by various components of the innate host defense system including proteolytic enzymes and complement (17). It remains to be determined whether the infectivity of such a mutant virus is significantly decreased in vivo.
The role of αv integrins in Ad infection and virus-mediated gene delivery has also been revealed in a number of other studies. Hematopoietic cells are notoriously difficult to infect with Ad2/Ad5. A major block in infection appears to be the relatively low abundance or absence of αv integrins on normal peripheral blood mononuclear cells. Upregulation of αv integrin expression on human monocytes by specific growth factors such as macrophage colony-stimulating factor or granulocyte-macrophage colony-stimulating factor (27) render these cells susceptible to Ad-mediated gene delivery (45). An interesting feature of Ad infection of monocytic cells is that these cells also express relatively low levels of CAR. Virus attachment to monocytes is mediated by the penton base interaction with integrin αmβ2, while particle uptake is dependent upon interactions with αv integrins (46). Integrin expression is also upregulated on human B lymphocytes following their infection and immortalization with Epstein-Barr virus, and this greatly increases their susceptibility to Ad-mediated gene delivery (48). Recently, multiple myeloma cells, but not normal bone marrow-derived B cells, were found to express αv integrins, and this allowed targeting by a therapeutic Ad vector (94).
Several approaches have also been explored in an effort to overcome the lack of cell integrins and/or CAR on certain cell types to promote Ad infection. For example, Wickham et al. (103) modified Ad2 particles with a bispecific monoclonal antibody which recognized a synthetic peptide sequence incorporated into the RGD domain of the penton base and a T-lymphocyte-specific antigen, CD3. This modified virus significantly increased transduction of T lymphocytes in vitro. A potential problem with this approach is that the Fc region of cell-targeting antibodies may also direct the virus to Fc receptor-bearing cells including dendritic cells, macrophages, and B cells in vivo. High-efficiency gene transfer with Ad-polylysine-DNA complexes has also been reported (22).
As noted above, Ad vectors are currently under evaluation as gene delivery vehicles for treating a number of genetic diseases including cystic fibrosis. In addition to the inflammatory reactions to the viral vector, another hindrance to gene delivery appears to be the lack of integrin expression on the human airway epithelium (36). Using a human bronchial xenograft model, Goldman and Wilson demonstrated that undifferentiated epithelial cells express high levels of αvβ5 integrins and are easily infected with recombinant Ad (36). Ad-mediated gene delivery could also be inhibited by function-blocking antibodies to αv integrins or by RGD peptides. In contrast to undifferentiated epithelial cells, pseudostratified epithelium containing columnar cells expresses little or no αv integrin and is relatively difficult to infect with Ad. More recent in vitro studies have indicated that integrins are expressed on the basolateral surface of poorly differentiated columnar epithelial cells but are present at very low levels or absent on the apical surface of these cells (73). The authors of this study failed to demonstrate a role for αv integrins in Ad-mediated gene delivery. Integrins may not be available for Ad interactions unless the regions of cell-cell contact (tight junctions) are disrupted to expose their basolateral surface.
Ad-mediated gene delivery is influenced not only by αv integrin expression but also by the presence of the fiber receptor, CAR. For example, a recent study of Ad gene delivery to a panel of human glioma cell lines indicated that Ad infection was dependent upon the level of CAR expression rather than on the presence of αv integrins (63). CAR expression also influences Ad-mediated gene delivery in the airway epithelium (107). To circumvent the lack of CAR on certain cell types, Dmitriev et al. (28) used a recombinant Ad vector in which an RGD sequence was inserted into an exposed loop on the fiber knob domain. This modified virus showed enhanced gene delivery to a number of transformed cell lines that lacked CAR but expressed αv integrins. It will be of interest to determine whether this approach will be useful for improving Ad in vivo gene therapy.
ROLE OF DYNAMIN, ACTIN, AND CELL SIGNALING IN ADENOVIRUS ENTRY
Some of the earliest studies of Ad cell entry involved transmission electron microscopy. These early studies suggested that Ad is internalized into host cell by clathrin-mediated endocytosis (12, 70). Biochemical studies have subsequently supported this finding (39, 96). A genetic approach was used to determine whether the infectious pathway of Ad involves dynamin, a 100-kDa cytosolic GTPase which regulates clathrin-mediated endocytosis (24). HeLa cells expressing a dominant negative form of dynamin 1 (K44A) failed to support efficient Ad internalization or gene delivery (73, 99). These findings are consistent with the notion that the primary entry pathway of Ad is via the clathrin-coated pit pathway. It should be noted that expression of mutant dynamin does not completely abolish virus entry or infection. This suggests the possibility that Ad entry also occurs via a clathrin-independent endocytic pathway (55), perhaps mediated by the fiber interaction with CAR.
A major question arising from studies of Ad entry is whether αv integrins promote virus entry via specific cell signaling events. A growing body of evidence indicates that integrin-mediated signaling regulates cell adhesion, cell growth, and cell migration (50, 69). Integrin clustering following ligand binding is frequently associated with the reorganization of actin and actin-associated proteins into focal adhesions which also contain a number of signaling molecules such as protein and lipid kinases that associate with the integrin β-subunit cytoplasmic domain (16, 68). It has also been demonstrated that the actin cytoskeleton and integrin signaling play a role in the cell entry of a number of pathogenic bacteria (29). Earlier studies indicated that disruption of cortical actin filaments by cytochalasins blocked Ad entry and infection (70). More recently, the actin cytoskeleton has been reported to play a role in clathrin-mediated endocytosis of enveloped viruses (37) as well as nonviral ligands (54).
A number of signaling molecules associate with integrins upon receptor ligation by various components of the extracellular matrix including the ERK1/ERK2 mitogen-activated protein (MAP) kinases (13, 80). These signaling molecules have been reported to be involved in αv integrin-mediated cell motility (52). Interestingly, the ERK1/ER2 MAP kinases appear to play little if any role in αv integrin-mediated Ad endocytosis. Instead, Ad internalization was shown to be regulated by a lipid kinase, phosphatidylinositol-3-OH kinase (PI3K) (57). The phospholipid products of PI3K are thought to act as second messengers for a number of important biological processes including cell cycle progression (33) and reorganization of the actin cytoskeleton (101). Ad penton base interaction with αv integrins was specifically shown to activate the p85 subunit of PI3K, and activation was shown to be required for efficient virus internalization and infection (57). Pharmacologic inhibitors or expression of dominant negative forms of PI3K but not ERK1/ER2 MAP kinases also inhibited Ad internalization and infection. Thus, cell motility and virus internalization mediated by the same integrin appear to involve distinct signaling pathways (Fig. 3). In further support of this concept, focal adhesion kinase, p125FAK, a signaling molecule known to function upstream of the ERK1/ER2 MAP kinases, is phosphorylated and activated upon adenovirus entry (57a) but this kinase is not required for virus uptake into cells or infection. Activation of the MAP kinase pathway, although not required for virus entry, may have other consequences for the host including the production of inflammatory cytokines (11).
FIG. 3.
Schematic diagram of the signaling events involved in Ad internalization and cell migration. The signaling molecules not selectively involved in αv integrin-mediated cell migration are shown in the boxed region on the left.
An obvious question arising from these studies is that of how PI3K promotes virus uptake. PI3K is known to be linked to both the Ras and Rho signaling cascades (16, 44). These small GTPases, which cycle between an inactive GDP-bound form and an active GTP-bound form, have the capacity to alter the host cell actin cytoskeleton (65). Recently, we demonstrated that the Rho family GTPases, Rac1, CDC42, and RhoA, rather than Ras, promote Ad endocytosis (56). Further studies indicated that the mechanism by which Rho GTPases enhance virus uptake is via reorganization of the actin cytoskeleton. Thus, Ad uptake into cells was inhibited by treatment with cytochalasin D and also by the expression of effector domain mutants of Rac or CDC42 that impair cytoskeletal function but not JNK/MAP kinase pathway activation. Further studies are necessary to identify the downstream effectors of Rho GTPases that promote actin polymerization and virus uptake. The precise role of the actin cytoskeleton in viral endocytosis also remains to be determined. At least two possible roles for actin have been considered. Polymerized actin filaments may assist dynamin-mediated endosome formation (78) or perhaps increase the half-life of signaling complexes involved in endocytosis.
Studies of Ad cell entry have led to another important question: what is the relevance of the Ad signaling pathway to the normal function of the αv integrins? In this regard, αvβ5 integrin has been reported to mediate the internalization of a conformationally altered form of vitronectin, the natural host ligand for this integrin receptor (67). A region of the β5 integrin subunit cytoplasmic tail containing the NPXY internalization motif has recently been implicated in the association of the integrin with clathrin-coated portions of the cell membrane (26). However, it is not yet clear whether the molecular events required for the internalization of the monovalent host ligand (vitronectin) by integrins are precisely the same as those required for the internalization of the multivalent viral ligand (penton base).
ROLE OF αV INTEGRINS IN ADENOVIRUS-MEDIATED ENDOSOME DISRUPTION AND GENE DELIVERY
Integrin-mediated internalization of prebound Ad particles occurs relatively rapidly (5 min) at 37°C (39, 104). Once fully enclosed in cell endosomes, Ad particles disrupt the endosomal membrane and escape into the cytoplasm, where they begin their journey toward the cell nucleus. Since vesicle rupture occurs relatively rapidly, within 15 min at 37°C (39), it is likely that escape occurs from the early endosome prior to endosome fusion with lysosomal vacuoles. At present, there is relatively little information on the precise mechanisms involved in virus penetration of cell endosomes. Several studies, based on the used of lysosomatropic agents which affect vaculor pH, suggest that virus penetration requires exposure to mildly acidic pH conditions (39). However, other studies have questioned the validity of these findings (75). Further studies on this problem are needed to resolve this controversy.
The ability of Ad to efficiently escape lysosomal degradation has been exploited for gene delivery applications. Intact Ad particles (19, 22) or components of the virus capsid that mediate virus penetration have been conjugated to plasmid DNA to deliver genes to cells in vitro (31). Ad particles mediate the release of small molecules such as [3H]choline from cells at a pH optimum of 6.0 (83, 85, 102) and induce the formation of ion channels in model bilayer lipid membranes (9, 79).
Over the past several decades, there has been an accumulation of evidence suggesting that the Ad penton base interaction with integrins plays a central role in Ad escape from the cell endosome. Earlier studies showed that the penton base played the major role in Ad-mediated delivery of a endotoxin-growth factor conjugate to cells (82), while more recent studies have reported that the isolated penton base from Ad3 can be used to deliver DNA into cells (31). At reduced pH, the penton base protein shows a high propensity to bind nonionic detergent (84). This raises the possibility that exposure to low pH induces a conformational change in the penton base protein which allows a physical interaction (insertion) with the endosomal membrane. Consistent with this view, previous studies showed that the penton base protein is released from viral particles at the stage of endosome release (39). In previous studies, we demonstrated that integrin αvβ5 rather than αvβ3 interaction with the Ad2 penton base selectively mediates Ad membrane permeabilization and gene delivery (102). The selective involvement of αvβ5 integrin in membrane permeabilization appears to be due to the ability of this receptor to bind to the virus protein at reduced pH compared to integrin αvβ3. It should be noted that αvβ5 interaction with the isolated Ad2 penton base alone does not induce membrane permeabilization (102), and thus it is likely that other viral and/or host cell factors participate in this process.
Recent work has indicated that the 23-kDa Ad cysteine protease also participates in virus penetration (20). A temperature-sensitive mutant Ad which contains a defective protease fails to escape cell endosomes (38). Treatment of wild-type Ad particles with protease inhibitors blocks infection as well as Ad-mediated gene delivery (20). Interestingly, interaction of the Ad with αv integrins was shown to be required for protease activation and escape from the cell endosome (38). Ad protein VI, a capsid protein located under the vertex region of the virus particle (90), is also degraded following protease activation, suggesting that protease activation may also be involved in viral DNA uncoating as well as penetration. Clearly, further studies are needed to define the precise molecular and cellular events associated with Ad penetration.
STRUCTURE OF A FUNCTION-BLOCKING MONOCLONAL ANTIBODY COMPLEXED WITH ADENOVIRUS
To gain further insights into the role of αv integrin-mediated Ad entry, structural studies have been performed to identify the sites of integrin interaction on virus particles. In the earliest studies, the three-dimensional structure of Ad2 particles at 35 Å resolution was revealed by cryo-electron microscopy (cryo-EM) and image reconstruction (88). Further refinements which improved the resolution to 25 Å (90) allowed the visualization of five protrusions, approximately 22 Å in diameter, on each penton base protein. These protrusions were proposed to contain the RGD sequence and additional flanking residues corresponding to the site of integrin binding. To confirm this possibility, we examined the structure of Ad2 complexed with Fab fragments of function-blocking penton base monoclonal antibody (designated Dav-1) (89). Peptide-mapping studies showed that the DAV-1 mAb recognized a linear epitope of 9 amino acids containing the integrin-binding motif, IRGDTFATR. This antibody also blocked penton base binding to cells. Somewhat surprisingly, Fab fragments of DAV-1 but not intact antibody molecules inhibited Ad infection, suggesting that Fab fragments but not whole antibodies were able to fully occupy each of the five RGD sites on the penton base protein. To investigate this possibility further, we used an automated biosensor to measure the kinetics and stoichiometry of antibody-Ad2 penton base interactions. We found that only two or three molecules of the intact DAV-1 monoclonal antibody could bind to the penton base protein whereas five Fab fragments could bind.
A cryo-EM structural study of the Ad2/DAV-1 Fab complex confirmed that the protrusions on the penton base contain the integrin-binding RGD sequence (89). It also revealed that the bound Fab fragments undergo a large range of motion since a large volume of weak, diffuse density was observed for the Fab (Fig. 4). Examination of the penton base in a reconstruction of Ad2 without bound antibody molecules showed a region of weak density at the top of the penton base protrusions, providing further evidence that the RGD peptide loop is quite mobile (Fig. 5). Cryo-EM studies performed with the isolated penton base of Ad3 also suggested that the RGD protrusions are flexible (81). Interestingly, the position of the protrusions was observed to shift slightly in the presence or absence of the central fiber protein. This latter observation was also shown for Ad2 when comparing the structures of wild-type and fiberless particles (97). This structural change does not significantly affect integrin-binding function, since Ad particles lacking the fiber protein appear to retain the ability to infect monocytic cells via an integrin-dependent pathway (97).
FIG. 4.
Cryo-EM reconstruction of the vertex region of the Ad2/DAV-1 Fab fragment. The viral capsid proteins are displayed in the same color scheme as in Fig. 5 and with the hexons in blue. (A) The reconstructed Fab density (magenta) is weak and diffuse. (B) A model showing the cryo-EM Ad2 vertex density together with five crystallographic Fab fragments filtered to 19-Å resolution (magenta). Each Fab fragment is shown in a different orientation to suggest mobility. The wire mesh corresponds to the total model density obtained from Fab fragments in eight distinct orientations. The scale bar is 100 Å. Reprinted from reference 89 with permission of the publisher.
FIG. 5.
Ad2 penton base (yellow and red) and fiber (green) from a cryo-EM reconstruction of the intact virus particle. Each of the five penton base protrusions has a region of weak density (red) at the top corresponding to the mobile RGD loop. Note that during the Ad reconstruction process, icosahedral (60-fold) symmetry was imposed. Regions of the virus that do not follow perfect icosahedral symmetry, such as the flexible fiber, are not fully reconstructed. The actual length of the fiber protein is approximately six times longer than is shown here. (A) Top view. (B) Side view. Bar, 25 Å. Reprinted from reference 89 with permission of the publisher.
STRUCTURE OF INTEGRIN αVβ5 BOUND TO ADENOVIRUS
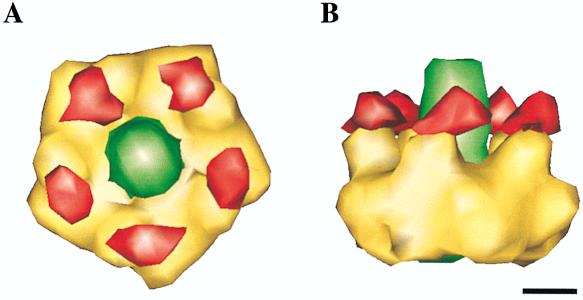
In recent cryo-EM structural studies, we examined the complex of Ad with soluble αvβ5 integrin molecules (59). Given the high degree of mobility of the RGD surface loop in Ad2, we also examined the structure of another serotype, Ad12, which has a much smaller RGD loop and therefore is more likely to provide detailed structural information (Fig. 6). The cryo-EM structures of Ad2 and Ad12 revealed a ring of integrin density above the penton base RGD loops of each virus serotype (14a). However, the integrin density in the Ad2 complex was found to be weak and diffuse whereas that of the Ad12 complex was compact and well defined, thus allowing a better visualization of the integrin structure. Cryo-EM visualization of Ad12 revealed five closely packed integrin molecules per penton base, a finding supported by kinetic analyses. In contrast, kinetic analyses indicated that only 1.7 DAV-1 monoclonal antibodies are capable of binding. Thus, the structure of Ad facilitates the interaction with cell integrins while restricting the binding of potentially neutralizing antibodies. Each integrin molecule was shown to consist of two discrete subdomains, a globular domain with an RGD-binding cleft of approximately 20 Å in diameter and a distal domain made up of extended, flexible tails (Fig. 7).
FIG. 6.
Cryo-EM reconstruction of the Ad12-αvβ5 complex. (A) Full virus-receptor complex, viewed along an icosahedral threefold axis. The penton base is shown in yellow, the fiber is shown in green, and the rest of the viral capsid is shown in blue. The integrin density is shown in red. (B) Enlarged view of the vertex region. The integrin appears to have two domains, a globular domain bound to the penton base and an extended tail domain farther from the viral surface. Bars, 100 Å. Reprinted from Chiu et al. (14a) with permission of the publisher.
FIG. 7.
The ring formed by five RGD-binding globular domains of integrin from the Ad12-αvβ5 integrin cryo-EM reconstruction, shown color coded by height from the viral surface. (A) Top view. Note that the bound integrin heterodimers form a continuous ring with close associations between adjacent globular domains. (B) Side view. Five columns of density (red) connect the integrin globular domains to the more flexible tail domains (not shown). (C) Bottom view. The arrows mark five clefts where the RGD-containing protrusions of the penton base bind. Bar, 100 Å. Reprinted from Chiu et al. (14a) with permission of the publisher.
These recent structural findings also suggest that the precise spatial arrangement of five RGD protrusions on the penton base promotes integrin-clustering and cell-signaling events required for virus internalization. Three lines of evidence support this concept. The first is that the Ad penton base protein, but not a monomeric RGD peptide (50-mer) derived from the penton base sequence, activates the p72syk kinase and promotes B-lymphoblastoid cell adhesion (91). The second is that the spacing of the integrin-binding sites on Ad is virtually identical to that of an unrelated virus, foot-and-mouth disease virus (FMDV) (1, 51) which also uses integrins for infection. Finally, the cryo-EM structure of the Ad12-integrin complex revealed substantial regions of contact between the integrin extracellular domains.
OTHER VIRAL PATHOGENS THAT USE INTEGRINS FOR INFECTION
At least two members of the Picornaviridae family of nonenveloped viruses use integrins for infection. These include FMDV (51) and coxsackievirus A9 (49, 77). For both of these viruses, the role of the integrin in infection appears to be at the level of virus attachment. Integrin α2β1 is a receptor for echovirus 1 (6), and this promotes viral attachment. Two coat proteins of rotavirus, a virus which is a major cause of acute gastrointestinal disease of humans, are also reported to interact with integrins α2β1, αxβ2, and α4β1, and this interaction may be required to complete the infectious process (21).
More recently, several other human viruses have been identified as using integrins for infection. The newly emerged hantaviruses, which are associated with severe pulmonary syndrome disease in humans, use β3-type integrins for cell entry (34); however, the stage at which the integrin acts has not yet been reported. AAV-2 has recently been reported to use αvβ5 integrin as a coreceptor for entry (92). Initial attachment of AAV-2 to cells is mediated by interaction with heparin sulfate proteoglycan (93) and/or fibroblast growth factor receptor 1 (74). Secondary interaction with integrin αvβ5 facilitates AAV-2 entry. Interestingly, the AVV-2 interaction with αvβ5 integrin appears to be RGD independent, since synthetic RGD peptides do not interfere with AAV-2–integrin interaction and the AAV-2 coat protein lacks an RGD sequence. It will be of interest to identify the non-RGD sequences involved in viral interaction with this integrin.
CONCLUSIONS AND FUTURE DIRECTIONS
It is a remarkable feat that an organism as small as Ad has provided such a wealth of knowledge of several important host cell processes. In this review, we have discussed the earliest interactions of Ad with its cellular receptors and focused on the role of the virus internalization receptors, integrins αvβ3 and αvβ5. While evidence has accumulated that integrins promote Ad entry both in vitro and in vivo, the precise mechanism by which virus uptake is accomplished is not completely understood. It is clear that Ad entry requires a signaling pathway involving PI3K, the Rho family of small GTPases, and polymerization of actin filaments. However, the specific role for these molecules in Ad entry remains to be determined. Even less well understood is how integrin αvβ5 facilitates the escape of viral particles from the cell endosome. Integrins are required but not sufficient for virus penetration, and therefore other viral or cellular molecules must be involved in the process. Other than the 23-kDa Ad cysteine protease, the identities of these molecules remain to be determined.
From a structural point of view, recent cryo-EM reconstructions of Ad particles in a complex with function-blocking antibodies or soluble integrins have provided some clues to how the architecture of Ad promotes receptor clustering while restricting antibody binding.
The current state of knowledge of Ad structure and biology has already led to significant improvements in first-generation viral vectors for gene therapy. Specifically, modifications of the virus fiber protein or the penton base have allowed an expansion of vector tropism in vitro. We anticipate that with an even greater understanding of the structural features of virus-receptor interactions as well as the precise cellular events involved in Ad entry and uncoating, it should be possible to increase the specificity of vector targeting in vivo. This may allow a reduction in the amount of vector needed to achieve a therapeutic response, thereby lessening the severity of the host immune response. Further progress in the area of cellular and molecular biology as well as virology and structural biology will have to come together to result in success in this endeavor.
ACKNOWLEDGMENTS
We thank present and former members of the Nemerow, Stewart, and Cheresh laboratories for their contributions during the course of these studies, and we thank Catalina Hope and Joan Gausepohl for assistance with the manuscript.
This work was supported in part by NIH grants EY11431, HL54352, AI42929 and RR00833.
Footnotes
Paper 12291-IMM from The Scripps Research Institute.
REFERENCES
- 1.Acharya R, Fry E, Stuart D, Fox G, Rowlands D, Brown F. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature. 1989;337:709–716. doi: 10.1038/337709a0. [DOI] [PubMed] [Google Scholar]
- 2.Akke M, Liu J, Cavanagh J, Erickson H P, Palmer A G., III Pervasive conformational fluctuations on microsecond time scales in a fibronectin type III domain. Nat Struct Biol. 1998;5:55–59. doi: 10.1038/nsb0198-55. [DOI] [PubMed] [Google Scholar]
- 3.Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bai M, Harfe B, Freimuth P. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J Virol. 1993;67:5198–5205. doi: 10.1128/jvi.67.9.5198-5205.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 6.Bergelson J M, Shepley M P, Chan B M C, Hemler M E, Finberg R W. Identification of the integrin VLA-2 as a receptor for echovirus I. Science. 1992;255:1718–1720. doi: 10.1126/science.1553561. [DOI] [PubMed] [Google Scholar]
- 7.Berget S M, Moore C, Sharp P A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc Natl Acad Sci USA. 1977;74:3171–3175. doi: 10.1073/pnas.74.8.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bischoff J R, Kirn D H, Williams A, Heise C, Horn S, Muna M, Ng L, Nye J A, Sampson-Johannes A, Fattaey A, McCormick F. An adenovirus mutant that replicates selectively in p53 deficient human tumor cells. Science. 1996;274:373–376. doi: 10.1126/science.274.5286.373. [DOI] [PubMed] [Google Scholar]
- 9.Blumenthal R, Seth P, Willingham M C, Pastan I. pH-dependent lysis of liposomes by adenovirus. Biochemistry. 1986;25:2231–2237. doi: 10.1021/bi00356a057. [DOI] [PubMed] [Google Scholar]
- 10.Brody S L, Crystal R G. Adenovirus-mediated in vivo gene therapy. Ann N Y Acad Sci. 1994;716:90–103. doi: 10.1111/j.1749-6632.1994.tb21705.x. [DOI] [PubMed] [Google Scholar]
- 11.Bruder J T, Kovesdi I. Adenovirus infection stimulates the Raf/MAPK signaling pathway and induces interleukin-8 expression. J Virol. 1997;71:398–404. doi: 10.1128/jvi.71.1.398-404.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chardonnet Y, Dales S. Early events in the interaction of adenoviruses with HeLa cells. I. Penetration of type 5 and intracellular release of the DNA genome. Virology. 1970;40:462–477. doi: 10.1016/0042-6822(70)90189-3. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Kinch M S, Lin T H, Burridge K, Juliano R L. Integrin-mediated cell adhesion activates mitogen-activated protein kinases. J Biol Chem. 1994;6:26602–26605. [PubMed] [Google Scholar]
- 14.Chinnadurai G. Adenovirus 2lp+locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983;33:759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- 14a.Chiu C Y, Mathias P, Nemerow G R, Stewart P L. Structure of adenovirus complexed with its internalization receptor, αvβ5 integrin. J Virol. 1999;73:6759–6768. doi: 10.1128/jvi.73.8.6759-6768.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow L T, Gelinas R E, Broker T R, Roberts R J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell. 1977;12:1–8. doi: 10.1016/0092-8674(77)90180-5. [DOI] [PubMed] [Google Scholar]
- 16.Clark E A, Brugge J S. Integrins and signal transduction pathways: The road taken. Science. 1995;268:233–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 17.Cooper N R. In J. Gallin and R. Snyderman (ed.), p. 281–315. Inflammation: basic principles and clinical correlates, 3rd ed. Lippincott-Raven, Philadelphia, Pa. 1999. Biology of the complement system. [Google Scholar]
- 18.Copie V, Tomita Y, Akiyama S K, Aota S, Yamada K M, Venable R M, Pastor R W. Solution structure and dynamics of linked cell attachment modules of mouse. J Mol Biol. 1998;277:663–682. doi: 10.1006/jmbi.1998.1616. [DOI] [PubMed] [Google Scholar]
- 19.Cotten M, Wagner E, Zatloukal K, Birnstiel M L. Chicken adenovirus (CELO virus) particles augment receptor-mediated DNA delivery to mammalian cells and yield exceptional levels of stable transformants. J Virol. 1993;67:3777–3785. doi: 10.1128/jvi.67.7.3777-3785.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotten M, Weber J M. The adenovirus protease is required for virus entry into host cells. Virology. 1995;213:494–502. doi: 10.1006/viro.1995.0022. [DOI] [PubMed] [Google Scholar]
- 21.Coulson B S, Londrigan S L, Lee D J. Rotavirus contains integrin ligand sequences and a disintegrin-like domain that are implicated in virus entry into cells. Proc Natl Acad Sci USA. 1997;94:5389–5394. doi: 10.1073/pnas.94.10.5389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curiel D T. High-efficiency gene transfer employing adenovirus-polylysine-DNA complexes. Nat Immun. 1994;13:141–164. [PubMed] [Google Scholar]
- 23.Dai Y, Schwarz E M, Gu D, Zhang W, Sarvetnick N, Verma I M. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc Natl Acad Sci USA. 1995;92:1401–1405. doi: 10.1073/pnas.92.5.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Damke H, Baba T, Warnock D E, Schmid S L. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol. 1994;127:915–934. doi: 10.1083/jcb.127.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davison A J, Telford E A R, Watson M S, McBride K, Mautner V. The DNA sequence of adenovirus Type 40. J Mol Biol. 1993;234:1308–1316. doi: 10.1006/jmbi.1993.1687. [DOI] [PubMed] [Google Scholar]
- 26.DeDeyne P G, O’Neill A, Resneck W G, Dmytrenko G M, Pumplin D W, Bloch R J. The vitronectin receptor associates with clathrin-coated membrane domains via the cytoplasmic domain of its beta5 subunit. J Cell Sci. 1998;111:2729–2740. doi: 10.1242/jcs.111.18.2729. [DOI] [PubMed] [Google Scholar]
- 27.De Nichilo M O, Burns G F. Granulocyte-macrophage and macrophage colony-stimulating factors differentially regulate αv integrin expression on cultured human macrophages. Proc Natl Acad Sci USA. 1993;90:2517–2521. doi: 10.1073/pnas.90.6.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 30.Everett S F, Ginsberg H S. A toxin-like material separable from type 5 adenovirus particles. Virology. 1958;6:770–771. doi: 10.1016/0042-6822(58)90123-5. [DOI] [PubMed] [Google Scholar]
- 31.Fender P, Ruigrok R W H, Gout E, Buffet S, Chroboczek J. Adenovirus dodecahedron, a new vector for human gene transfer. Nat Biotechnol. 1997;15:52–56. doi: 10.1038/nbt0197-52. [DOI] [PubMed] [Google Scholar]
- 32.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 33.Franke T F, Kaplan D R, Cantley L C. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 34.Gavrilovskaya I N, Shepley M, Shaw R, Ginsberg M H, Mackow E R. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc Natl Acad Sci USA. 1998;95:7074–7079. doi: 10.1073/pnas.95.12.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geraghty R J, Krummenacher C, Cohen G H, Eisenberg R J, Spear P G. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 36.Goldman M J, Wilson J M. Expression of αvβ5 integrin is necessary for efficient adenovirus-mediated gene transfer in the human airway. J Virol. 1995;69:5951–5958. doi: 10.1128/jvi.69.10.5951-5958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gottlieb T A, Ivanov I E, Adesnik M, Sabatini D D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993;120:695–709. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greber U F, Webster P, Helenius A, Weber J. The role of the adenovirus protease in virus entry into cells. EMBO J. 1996;15:1766–1777. [PMC free article] [PubMed] [Google Scholar]
- 39.Greber U F, Willetts M, Webster P, Helenius A. Stepwise dismantling of adenovirus 2 during entry into cells. Cell. 1993;75:477–486. doi: 10.1016/0092-8674(93)90382-z. [DOI] [PubMed] [Google Scholar]
- 40.Grotewiel M S, Beck C D O, Wu K-H, Zhu X-R, Davis R L. Integrin-mediated short-term memory in Drosophila. Nature. 1998;391:455–460. doi: 10.1038/35079. [DOI] [PubMed] [Google Scholar]
- 41.Hierholzer J C. Adenoviruses in the immunocompromised host. Clin Microbiol Rev. 1992;5:262–274. doi: 10.1128/cmr.5.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S S, Boulanger P. Protein ligands of the human adenovirus type 2 outer capsid identified by biopanning of a phage-displayed peptide library on separate domains of wild-type and mutant penton capsomers. EMBO J. 1995;14:4714–4727. doi: 10.1002/j.1460-2075.1995.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horwitz M S. Adenovirus. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2149–2171. [Google Scholar]
- 44.Hotchin N, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang S, Endo R I, Nemerow G R. Upregulation of integrins αvβ3 and αvβ5 on human monocytes and T lymphocytes facilitates adenovirus-mediated gene delivery. J Virol. 1995;69:2257–2263. doi: 10.1128/jvi.69.4.2257-2263.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang S, Kamata T, Takada Y, Ruggeri Z M, Nemerow G R. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J Virol. 1996;70:4502–4508. doi: 10.1128/jvi.70.7.4502-4508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang S, Reddy V, Dasgupta N, Nemerow G R. A single amino acid in the adenovirus type 37 fiber confers binding to human conjunctival cells. J Virol. 1999;73:1600–1698. doi: 10.1128/jvi.73.4.2798-2802.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang S, Stupack D G, Mathias P, Wang Y, Nemerow G. Growth arrest of Epstein-Barr virus immortalized B lymphocytes by adenovirus-delivered ribozymes. Proc Natl Acad Sci USA. 1997;94:8156–8161. doi: 10.1073/pnas.94.15.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hughes P J, Horsnell C, Hyypia T, Stanway G. The coxsackievirus A9 RGD motif is not essential for virus viability. J Virol. 1995;69:8035–8040. doi: 10.1128/jvi.69.12.8035-8040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hynes R O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 51.Jackson T, Sharma A, Ghazaleh R A, Blakemore W E, Ellard F M, Simmons D L, Newman J W, Stuart D I, King A M. Arginine-glycine-aspartic acid-specific binding by foot-and mouth disease viruses to the purified integrin αvβ3 in vitro. J Virol. 1997;71:8357–8361. doi: 10.1128/jvi.71.11.8357-8361.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klemke R L, Cai S, Giannini A L, Gallagher P J, de Lanerolle P, Cheresh D A. Regulation of cell motility by mitogen-activated protein kinase. J Cell Biol. 1997;137:481–492. doi: 10.1083/jcb.137.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knowles M R, Hohneker K W, Zhou Z, Olsen J C, Noah T L, Hu P C, Leigh M W, Engelhardt J F, Edwards L J, Jones K R, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. N Engl J Med. 1995;333:823–831. doi: 10.1056/NEJM199509283331302. [DOI] [PubMed] [Google Scholar]
- 54.Lamaze C, Fujimoto L M, Yin H L, Schmid S L. The actin cytoskeleton is required for receptor-mediated endocytosis in mammalian cells. J Biol Chem. 1997;272:20332–20335. doi: 10.1074/jbc.272.33.20332. [DOI] [PubMed] [Google Scholar]
- 55.Lamaze C, Schmid S L. The emergence of clathrin-independent pinocytic pathways. Curr Biol. 1995;7:573–580. doi: 10.1016/0955-0674(95)80015-8. [DOI] [PubMed] [Google Scholar]
- 56.Li E, Stupack D, Bokoch G, Nemerow G R. Adenovirus endocytosis requires reorganization of the actin cytoskeleton mediated by Rho family GTPases. J Virol. 1998;72:8806–8812. doi: 10.1128/jvi.72.11.8806-8812.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li E, Stupack D, Cheresh D, Klemke R, Nemerow G. Adenovirus endocytosis via αv integrins requires phosphoinositide-3-OH-kinase. J Virol. 1998;72:2055–2061. doi: 10.1128/jvi.72.3.2055-2061.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57a.Li, E., et al. Submitted for publication.
- 58.Londergan T A, Walzak M P. Hemorrhagic cystitis due to adenovirus infection following bone marrow transplantation. J Urol. 1994;151:1013–1014. doi: 10.1016/s0022-5347(17)35153-4. [DOI] [PubMed] [Google Scholar]
- 59.Mathias P, Galleno M, Nemerow G R. Interactions of soluble recombinant integrin αvβ5 with human adenoviruses. J Virol. 1998;72:8669–8675. doi: 10.1128/jvi.72.11.8669-8675.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mathias P, Wickham T J, Moore M, Nemerow G. Multiple adenovirus serotypes use αv integrins for infection. J Virol. 1994;68:6811–6814. doi: 10.1128/jvi.68.10.6811-6814.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGrath D, Falagas M E, Freeman R, Rohrer R, Fairchild R, Colbach C, Snydman D R. Adenovirus infection in adult orthotopic liver transplant recipients: Incidence and clinical significance. J Infect Dis. 1998;177:459–462. doi: 10.1086/517375. [DOI] [PubMed] [Google Scholar]
- 62.Michaels M G, Green M, Wald E R, Starzl T E. Adenovirus infection in pediatric liver transplant recipients. J Infect Dis. 1992;165:170–174. doi: 10.1093/infdis/165.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miller C R, Buchsbaum D J, Reynolds P N, Douglas J T, Gillespie G Y, Mayo M S, Raben D, Curiel D T. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998;58:5738–5748. [PubMed] [Google Scholar]
- 64.Neumann R, Chroboczek J, Jacrot B. Determination of the nucleotide sequence for the penton-base gene of human adenovirus type 5. Gene. 1988;69:153–157. doi: 10.1016/0378-1119(88)90389-7. [DOI] [PubMed] [Google Scholar]
- 65.Nobes C D, Hall A. Rho, Rac, and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 66.Oldstone M B A. Introduction to the principles of virology. In: Oldstone M B A, editor. Viruses, plagues, & history. New York, N.Y: Oxford University Press; 1998. pp. 8–15. [Google Scholar]
- 67.Panetti T S, McKeown-Longo P J. The αvβ5 integrin receptor regulates receptor-mediated endocytosis of vitronectin. J Biol Chem. 1993;268:11492–11495. [PubMed] [Google Scholar]
- 68.Parsons, J. T. 1996. Integrin-mediated signaling: regulation by protein tyrosine kinases and small GTP-binding proteins. Curr. Opin. Cell Biol. :146:152. [DOI] [PubMed]
- 69.Parsons J T, Schaller M D, Hildebrand J, Leu T-H, Richardson A, Otey C. Focal adhesion kinase: structure and signaling. J Cell Sci Suppl. 1994;18:109–113. doi: 10.1242/jcs.1994.supplement_18.16. [DOI] [PubMed] [Google Scholar]
- 70.Patterson S, Russell W C. Ultrastructural and immunofluorescence studies of early events in adenovirus-HeLa cell interactions. J Gen Virol. 1983;64:1091–1099. doi: 10.1099/0022-1317-64-5-1091. [DOI] [PubMed] [Google Scholar]
- 71.Pereira H G. A protein factor responsible for the early cytopathic effect of adenoviruses. Virology. 1958;6:601–611. doi: 10.1016/0042-6822(58)90109-0. [DOI] [PubMed] [Google Scholar]
- 72.Philipson L, Stewart P L, Burnett R M. The molecular repertoire of adenovirus I. Berlin, Germany: Springer-Verlagug; 1995. [Google Scholar]
- 73.Pickles R J, McCarty D, Matsui H, Hart P J, Randell S H, Boucher R C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qing K, Mah C, Hansen J, Zhou S, Dwarki V, Srivastava A. Human fibroblast growth factor receptor 1 is a co-receptor for infection by adeno-associated virus 2. Nat Med. 1999;5:71–77. doi: 10.1038/4758. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez E, Everitt E. Adenovirus uncoating and nuclear establishment are not affected by weak-base amines. J Virol. 1996;70:3470–3477. doi: 10.1128/jvi.70.6.3470-3477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roelvink P W, Lizonova A, Lee J G M, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roivaninen M, Hypiä T, Pirainen L, Kalkkinen N, Stanway G, Hovi T. RGD-dependent entry of coxsackievirus A9 into host cells and its bypass after cleavage of VP1 protein by intestinal proteases. J Virol. 1991;65:4735–4740. doi: 10.1128/jvi.65.9.4735-4740.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roos J, Kelly R B. Is dynamin really a ‘pinchase’. Trends Cell Biol. 1997;7:257–259. doi: 10.1016/S0962-8924(97)01068-4. [DOI] [PubMed] [Google Scholar]
- 79.Rosenkranz A A, Antonenko Y N, Smirnova O A, Yurov G K, Naroditsky B S, Sobolev A S. Avian adenovirus induces ion channels in model bilayer lipid membranes. Biochem Biophys Res Commun. 1997;236:750–753. doi: 10.1006/bbrc.1997.7040. [DOI] [PubMed] [Google Scholar]
- 80.Schlaepfer D D, Hanks S K, Hunter T, vanderGeer P. Integrin-Mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 81.Schoehn G, Fender P, Chroboczek J, Hewat E A. Adenovirus 3 penton dodecahedron exhibits structural changes of the base on fibre binding. EMBO J. 1996;15:6841–6846. [PMC free article] [PubMed] [Google Scholar]
- 82.Seth P, Fitzgerald D, Ginsberg H, Willingham M, Pastan I. Evidence that the penton base of adenovirus is involved in potentiation of toxicity of Pseudomonas exotoxin conjugated to epidermal growth factor. Mol Cell Biol. 1984;4:1528–1533. doi: 10.1128/mcb.4.8.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Seth P, Pastan I, Willingham M C. Adenovirus-dependent increase in cell membrane permeability. J Biol Chem. 1985;260:9598–9602. [PubMed] [Google Scholar]
- 84.Seth P, Wilingham M C, Pastan I. Binding of adenovirus and its external proteins to Triton X-114. J Biol Chem. 1985;260:14431–14434. [PubMed] [Google Scholar]
- 85.Seth P, Willingham M C, Pastan I. Adenovirus-dependent release of 51Cr from KB cells at an acidic pH. J Biol Chem. 1984;259:14350–14353. [PubMed] [Google Scholar]
- 86.Shields A F, Hackman R C, Fife K H, Corey L, Meyers J D. Adenovirus infections in patients undergoing bone-marrow transplantation. N Engl J Med. 1985;312:529–533. doi: 10.1056/NEJM198502283120901. [DOI] [PubMed] [Google Scholar]
- 87.Sprengel J, Schmitz B, Heuss-Neitzel D, Zock C, Doerfler W. Nucleotide sequence of human adenovirus type 12 DNA: comparative functional analysis. J Virol. 1994;68:379–389. doi: 10.1128/jvi.68.1.379-389.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stewart P L, Burnett R M, Cyrklaff M, Fuller S D. Image reconstruction reveals the complex molecular organization of adenovirus. Cell. 1991;67:145–154. doi: 10.1016/0092-8674(91)90578-m. [DOI] [PubMed] [Google Scholar]
- 89.Stewart P L, Chiu C Y, Huang S, Muir T, Zhao Y, Chait B, Mathias P, Nemerow G R. Cryo-EM visualization of an exposed RGD epitope on adenovirus that escapes antibody neutralization. EMBO J. 1997;16:1189–1198. doi: 10.1093/emboj/16.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Stewart P L, Fuller S D, Burnett R M. Difference imaging of adenovirus: bridging the resolution gap between X-ray crystallography and electron microscopy. EMBO J. 1993;12:2589–2599. doi: 10.1002/j.1460-2075.1993.tb05919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stupack D G, Li E, Kehler J A, Geahlen R L, Hahn K, Nemerow G R, Cheresh D A. Matrix valency regulates integrin-mediated lymphoid adhesion via Syk-kinase. J Cell Biol. 1999;144:777–787. doi: 10.1083/jcb.144.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Summerford C, Bartlett J S, Samulski R J. αvβ5 integrin: a coreceptor for adeno-associated virus type 2 infection. Nat Med. 1999;5:78–82. doi: 10.1038/4768. [DOI] [PubMed] [Google Scholar]
- 93.Summerford C, Samulski R J. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol. 1999;72:1438–1445. doi: 10.1128/jvi.72.2.1438-1445.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teoh G, Chen L, Urashima M, Tai Y-T, Celi L A, Chen D, Chauhan D, Ogata A, Finberg R W, Webb I J, Kufe D W, Anderson K C. Adenovirus vector-based purging of multiple myeloma cells. Blood. 1998;92:4591–4601. [PubMed] [Google Scholar]
- 95.Tomko R P, Xu R, Philipson L. HCAR and MAR: The human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA. 1997;94:3352–3356. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Varga M J, Weibull C, Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991;65:6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Von Seggern D J, Chiu C Y, Fleck S K, Stewart P L, Nemerow G R. A helper-independent adenovirus vector with E1, E3, and fiber deleted: structure and infectivity of fiberless particles. J Virol. 1999;73:1601–1608. doi: 10.1128/jvi.73.2.1601-1608.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Von Seggern D J, Nemerow G R. Adenoviral vectors for protein expression. In: Fernandez J, Hoeffler J, editors. Gene expression systems: using nature for the art of expression. San Diego, Calif: Academic Press, Inc.; 1999. pp. 111–156. [Google Scholar]
- 99.Wang K, Huang S, Kapoor-Munshi A, Nemerow G R. Adenovirus internalization and infection required dynamin. J Virol. 1998;72:3455–3458. doi: 10.1128/jvi.72.4.3455-3458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, Bergelson J M. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J Virol. 1999;73:2559–2562. doi: 10.1128/jvi.73.3.2559-2562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wennström S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of phosphoinositide 3-kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- 102.Wickham T J, Filardo E J, Cheresh D A, Nemerow G R. Integrin αvβ5 selectively promotes adenovirus mediated cell membrane permeabilization. J Cell Biol. 1994;127:257–264. doi: 10.1083/jcb.127.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wickham T J, Lee G M, Titus J A, Sconocchia G, Bakacs T, Kovesdi I, Segal D M. Targeted adenovirus-mediated gene delivery to T cells via CD3. J Virol. 1997;71:7663–7669. doi: 10.1128/jvi.71.10.7663-7669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 105.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 106.Zabner J, Couture L A, Gregory R J, Graham S M, Smith A E, Welsh M J. Adenovirus-mediated gene transfer transiently corrects the chloride transport defect in nasal epithelia of patients with cystic fibrosis. Cell. 1993;75:207–216. doi: 10.1016/0092-8674(93)80063-k. [DOI] [PubMed] [Google Scholar]
- 107.Zabner J, Freimuth P, Puga A, Fabrega A, Welsh M J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J Clin Investig. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]