It is generally accepted that iron is the most important micronutrient used by bacteria. With members of the family Lactobacillae being the only exceptions so far (3), this metal is essential for cellular metabolism, since it is needed as a cofactor for a large number of enzymes (96). However, this element is not easily available to microorganisms in aerobic environments. While in anaerobic conditions Fe2+ is soluble at physiological pH and cells obtain iron without much difficulty from the external medium, the ion becomes quickly converted to Fe3+ upon exposure to oxygen and forms insoluble hydroxides at neutral pH, making the available metal very scarce (20). In order to acquire iron from the extracellular medium, virtually all aerobic bacteria produce and secrete low-molecular-weight compounds termed siderophores (sideros phoros, iron carriers). These compounds chelate Fe3+ with high affinity and specificity (68). Subsequently, the cell recovers the ferrisiderophore complexes through specific outer membrane receptors (30). Some of these high-affinity systems of iron uptake are important virulence factors in bacteria infecting animal fluids and tissues because they can chelate the metal bound to host proteins (7, 36, 60, 71). Furthermore, because iron availability is generally growth limiting for bacteria thriving in an animal millieu, the lack of the metal is a major environmental signal to trigger expression of virulence determinants (60). However, an excess of iron is toxic because of its ability to catalyse Fenton reactions and formation of active species of oxygen. Iron uptake has to be, therefore, exquisitely regulated to maintain the intracellular concentration of the metal between desirable limits. Considering that excretion mechanisms for iron are not known in bacteria, microorganisms appear to control iron homeostasis, regulating its transport through the membrane (5, 21).
THE fur GENE AND THE Fur PROTEIN
A key breakthrough in the understanding of how bacteria regulate iron transport was the description by Hantke in 1981 (45) of an Escherichia coli mutant which behaved as if the expression of all known functions inhibited by iron (siderophore production and biosynthesis of distinct outer membrane proteins) were constitutively expressed. This mutant, which behaved like a Salmonella typhimurium mutant isolated sometime before (31), was named fur (for ferric uptake regulation), and its behavior clearly suggested that a metal-dependent repression was at the basis of the control exerted by iron on many, if not all, Fe-responsive genes. The fur gene was subsequently mapped (4), cloned (46), and sequenced (79), its protein product was purified (100), and some basic aspects of the regulation mechanism were elucidated (4, 45, 46).
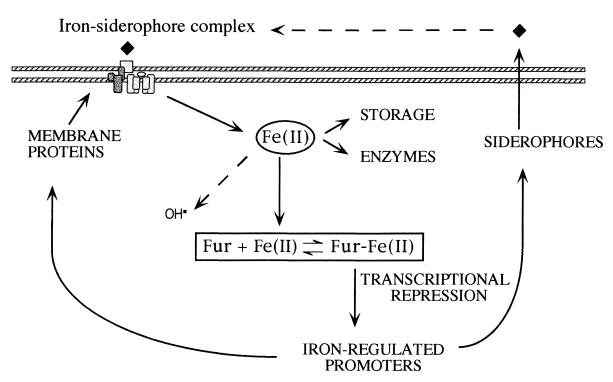
The Fur protein of E. coli is a 17-kDa polypeptide (6, 78) which acts as a transcriptional repressor of iron-regulated promoters by virtue of its Fe2+-dependent DNA binding activity (5, 25, 32, 33). Figure 1 shows the long-accepted model of Fur-mediated repression of metalloregulated genes. Under iron-rich conditions Fur binds the divalent ion, acquires a configuration able to bind target DNA sequences (generally known as Fur boxes or iron boxes, Fig. 2), and inhibits transcription from virtually all the genes and operons repressed by the metal. On the contrary, when iron is scarce, the equilibrium is displaced to release Fe2+, the RNA polymerase accesses cognate promoters, and the genes for the biosynthesis of siderophores and other iron-related functions are expressed (41, 55). In some cases (notably in Pseudomonas aeruginosa [58]) Fur may control expression of a sigma factor which, in turn, causes a discrete set of genes to be expressed. Homologues of the fur gene have been described for many Gram-negative bacteria, including several important human pathogens like Yersinia (82), Salmonella (31), Vibrio (59, 61, 90, 104), Pseudomonas (76, 95), Helicobacter pylori (8), Bordetella (14), Campylobacter (103), Acinetobacter baumannii (23), Legionella (51), Neisseria (9, 88, 89), and Haemophilus (17) and even for plant pathogens like Erwinia chrysanthemi (36). Fur-like proteins have been found also in Gram-positive bacteria, e.g., Bacillus subtilis (15) and Staphylococcus (49), and even in cyanobacteria (40). Most of these homologues are able to complement an E. coli fur mutant, suggesting that the molecular mechanisms that control transcriptional regulation by iron are shared by many microorganisms.
FIG. 1.
Regulation of iron transport in E. coli. This classical model of response to iron starvation still remains basically correct (4). It is based on the existence of two configurations of the Fur protein in an equilibrium which is displaced by Fe2+ towards the form competent for binding DNA and thus for repression of transcription. The lack of iron results in the derepression of an entire collection of genes for the biosynthesis and transport of siderophores and hence the activity of one or more high-affinity iron uptake systems. These scavenge the Fe3+ present in the medium and drive ferrisiderophore complexes through an elaborate transport scheme which includes not only specific outer membrane receptors but also periplasmic and inner membrane proteins. The metal is then reduced intracellularly to Fe2+. Transport of this chemical element in aerobically grown cells is subjected to a very fine tuning, since iron overload promotes generation of the highly reactive forms of oxygen.
FIG. 2.
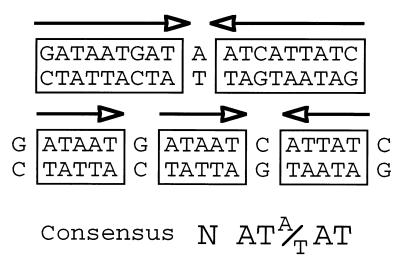
Alternative interpretations of the Fur box. The scheme shows different views of the 19-bp sequence bound by the repressor and generally known as the Fur box or the iron box in E. coli. The standard interpretation considers this box as a palindromic sequence composed of two 9-bp inverted repeats. However, it is also possible to conceive the same sequence as an array of three repeats of 6 bp (two directed and one inverted) of the invariable sequence NATA/TAT (22). Fur binding sites can then be assembled by combining multiple repeats in various orientations (see the text for explanation).
The Fur protein appears to be a dimer in solution regardless of the presence or absence of Fe2+ (19, 67, 69). On this basis, the protein has been proposed to have two different domains (19, 85). The C terminus is implicated in dimerization, whereas the N-terminal module accounts for the ability of the repressor to bind DNA (52, 85). Although several mutant alleles of fur have been described (12, 19, 43) and some protein variants have been purified, the constellation of amino acids that define the main metal binding site, as well as those implicated in dimerization or in interactions with DNA, remain to be elucidated. Furthermore, Fur is able to multimerize through protein-protein interactions (56), but the protein domains involved, perhaps different from those for dimerization, are unknown. In addition, Fur appears to be an abundant protein. Watnick et al. (98) found in Vibrio around 2,500 Fur molecules per cell during the logarithmic phase, which increased to 7,500 in the stationary stage. This relatively high amount of protein sharply contrasts with the generally low concentrations of other regulators. As discussed later, this might be connected not only to the large regulon controlled by the repressor but also to the unusual fashion with which this protein binds DNA (26, 34; see below).
BEYOND SIDEROPHORES: THE MANY ROLES OF Fur
In the last few years, the number of genes described as Fur-controlled have increased significantly for E. coli as well as for other bacteria (72, 84, 92). The large variety of genes controlled by this regulator has been revealed through the use of ingenious genetic and biochemical techniques. For instance, FURTA (Fur titration assays [84]), allow the detection of iron-regulated promoters from a cosmid or plasmid library. This method is based on the use of a chromosomal iron-regulated lacZ fusion to the fhuF gene. This fusion is exceptionally sensitive to small changes in iron concentration because of the weak affinity of the fhuF promoter for the Fur-Fe2+ repression complex. Introduction of a multicopy plasmid carrying Fur-binding sites into the test strain appears to deplete the intracellular Fur pool. This gives rise to the dissociation of the repressor from the fusion promoter, thereby allowing lacZ transcription. The screening of a plasmid gene bank from E. coli or S. typhimurium with this method led to the identification of numerous new Fur-controlled genes (84, 92; see below). On the other hand, the technique called SELEX (systematic evolution of ligands by exponential enrichment) has also been used successfully for isolating 16 novel Fur-dependent genes in P. aeruginosa (72). In this case, mixtures of chromosomal DNA fragments were passed through a filter bearing immobilized Fur protein, from which they were later extracted and amplified with the PCR. All newly found DNA sequences were protected by Fur in DNase I footprinting assays and, accordingly, were transcribed preferentially when cells faced iron starvation. In addition many of them bore similarity to siderophore receptors and alternative sigma factors (72).
An interesting counterpart of searching for iron-responsive promoters has been the identification of fur mutants in bacteria other than E. coli. In this respect, screening for manganese-resistant colonies has been a very effective method to pinpoint such mutants (47). This procedure is based on the observation that, at high concentrations, Mn2+ mimics Fe2+ and thus causes a lethal repression of the iron uptake systems (47). Cells that lack Fur escape such a repression and can thus be selected on plates. An overall picture of the complement of genes whose expression is affected by Fur in a given species can be drawn by comparing bidimensional protein electrophoresis patterns of the wild-type versus fur mutant variants. This approach has been successful in examining strains of various species such as Campylobacter jejuni (93), Vibrio cholerae (62), Yersinia pestis (83), Neisseria gonorrhoeae (89), and S. typhimurium (38). The combination of two-dimensional electrophoresis followed by reverse genetics for all these microorganisms has permitted identification of a large number of proteins and genes which are regulated or at least influenced by the Fur protein. More recently, the combined use of two-dimensional electrophoresis, mass spectrometry, and bioinformatic tools has allowed the identification of 10 iron-regulated proteins within the genome of Mycobacterium tuberculosis and their patterns of expression (102).
Inspection of the genes found to be regulated by Fur has revealed that this protein participates also in functions not directly related to iron metabolism. These include cellular processes as varied as the acid shock response (43), defense against oxygen radicals (70, 87), chemotaxis (54), metabolic pathways (47, 84), bioluminescence (65), swarming (66), and production of toxins and other virulence factors (60). In fact, as mentioned above, coupling expression of virulence factors to iron starvation makes sense given the lack of available metal that is predominant in host fluids and tissues. This seems to be the case for Shiga toxin in Shigella (60) for Shiga-like toxin I (60) and colicin V and hemolysin (37) in E. coli, and for exotoxin A in P. aeruginosa (76, 94). The same applies to the observed inhibition by iron of fimbrial expression in enterotoxigenic E. coli (54) and the involvement of Fur in virulence of plant pathogens such as E. chrysanthemi (36).
In other cases, the meaning of the iron regulation of some genes is more obscure. For instance, Fur appears to control flagellum assembly and chemotaxis through the regulation of one of its main transcriptional activators, FlhD (positive in the FURTA assay [84]). In this case, Fur seems to couple iron status to motility, so that expression of the flagella is inhibited when bacteria have an excess of the metal. This may prevent the departure of the cells towards other environments with less available iron (66, 84). Along the line, Fur-mediated repression of key metabolic genes such as purR or metJ (again positives in the FURTA assay [84]) might help the fine-tuning of cell metabolism for optimal growth conditions. Fur also represses some genes induced in response to oxidative stress like the Mn-dependent superoxide dismutase (MnSOD) gene, sodA, in E. coli (87) and P. aeruginosa (48) or the 8-hydroxyguanine endonuclease gene in E. coli (57). Since MnSOD is a key enzyme in defense against oxygen toxicity, the physiological significance of this negative regulation is unclear. Given that an excess of iron may lead to Fenton chemistry reactions and thus to oxidative stress, perhaps expression of MnSOD along with siderophore production prevents the damaging effects of a transient iron overload (91).
While these functions are not directly related to iron transport they can at least be understood mechanistically, considering Fur as a transcriptional repressor. However, a few intriguing cases have been reported in recent years in which Fur appears to act positively rather than negatively in the expression of certain promoters. The most remarkable instance is the expression of the acid tolerance response of S. typhimurium. This phenomenon requires Fur, the absence of which prevents expression of key acid shock proteins (43). The acid-sensing and the iron-sensing mechanisms mediated by Fur in this microorganism can be separated by mutations in the protein. A change in the H90 residue of the repressor makes Fur blind to iron (i.e., it becomes unable to repress cognate promoters), while it still maintains its function as mediator of the acid shock response (43). The proteins which act as indicators of such a response belong to the group of at least 34 polypeptides of S. typhimurium whose expression is affected by the loss of the Fur product (38). The existence of positively regulated genes in the E. coli Fur regulon has been also suggested (47, 70). The observation that the fur mutant grew poorly on succinate suggested that the protein activates the uptake and/or the catabolism of this carbon source (47). In addition, it has been proposed that expression of sodB, encoding an Fe-dependent superoxide dismutase (FeSOD), is controlled positively by Fur (70). Moreover, fur mutants have low levels of several iron-containing proteins such as fumarases A and B and aconitase A, thus raising the possibility that Fur is implicated in more activities involving iron metabolism in the cell (1). On the other hand, the operons for bacterioferritins (brfA-katA [64]) and catalase/peroxidase (brfB-aphA [73]) in P. aeruginosa are expressed in response to oxidative stress and to the presence of iron. Although cases where given genes or given products (38, 62, 83, 89, 93, 102) appear to be activated rather than repressed by Fur, the hard fact is that the function of the protein as a transcriptional activator has never been rigorously proven. Direct interactions between the Fur protein and the promoter regions of the iron-activated genes have never been shown. Therefore, so far Fur appears to be exclusively a repressor and its positive effects on certain promoters are likely to be the result of indirect rather than direct effects.
That Fur could have different roles in distinct species has been suggested by the observation that the gene is essential in Neisseria (9, 88), Pseudomonas (76, 95), Rhizobium (28), and Vibrio anguillarum (90) but not in E. coli (45), Bacillus (15), Yersinia (82), or Vibrio cholerae (62). On the other hand, the abundance of promoters that are regulated one way or another by this protein is reminiscent of the effects of global regulators such as Fis, integration host factor, HU, Lrp and H-NS, as has been already proposed for the Fur protein of V. anguillarum (18). These proteins control directly the output of only a few specific promoters, while they exert a direct or indirect effect on the performance of many others. Since iron influences so many processes in the cell, it is tempting to also consider Fur more like a global regulator which adjusts the entire metabolism to changes in environmental iron than like a very specific transcription factor for a few siderophore promoters. Being a general regulator, however, requires that binding sites are not limited to very specific sequences but can also be used for more relaxed and abundant DNA targets. How does this fit with what we know about the interactions of Fur with the promoters of metalloregulated genes?
OPENING THE IRON BOX IN E. COLI
As soon as Fur became available as a purified protein (100) it became possible to study in vitro the interaction between Fur-Fe2+ and a few iron-responsive promoters of E. coli (13, 25, 27, 42, 53, 87). The DNase I analyses of several Fur-binding sites allowed the early definition of a 19-bp consensus Fur box (i.e., the iron box; Fig. 2). Additional in vivo assays showed that this sequence, cloned downstream of a heterologous promoter, was sufficient to ensure that its transcriptional activity was regulated by iron (16). At a later point, sequence alignment of a collection of more than 30 iron-controlled promoters of various origins (12) confirmed that the sequence 5′GATAATGATAATCATTATC3′ was the functional target of the Fur protein. This, together with the apparent dimeric nature of the protein (19, 67, 69), suggested a mode of Fur-DNA interaction similar to that of classical bacterial repressors, in which a protein dimer recognizes a palindromic DNA sequence (77). But other results are not compatible with this notion. Hydroxyl radical footprinting of Fur within the promoter of the aerobactin (siderophore) operon (10, 24), revealed that protein binding to its target site gives rise to a distinct pattern of two protected and four nonprotected base pairs which is repeated three times along the primary binding site (26). On the other hand, many iron-regulated promoters appear to have not just one Fur box but multiple, sometimes overlapping, boxes (42, 53, 87), which is hardly compatible with the dimer-palindrome model (77). Furthermore, it appears that Fur wraps helically around the DNA, as indicated not only by the hydroxyl radical data (26) but also by direct electron microscopy observations (37, 56). How can all these observations be reconciled?
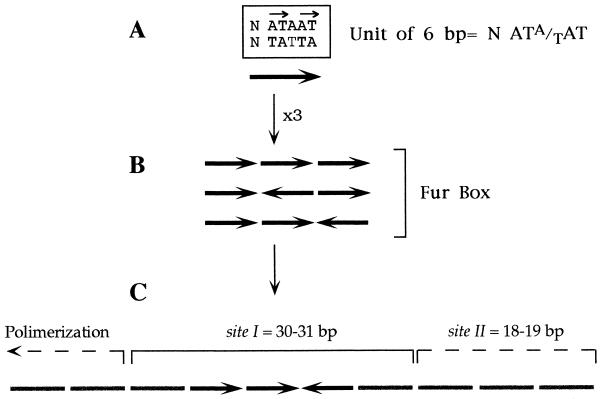
As shown in Fig. 2, the iron box motif can be interpreted as a palindrome formed by two 9-bp inverted repeats, 5′GATAATGAT3′, separated by one unmatched A. But interestingly, the same 19-bp sequence can be viewed as a combination of three adjacent repeats, 5′NATA/TAT3′. Since the pattern of Fur-DNA interaction revealed by hydroxyl radical footprinting of the aerobactin promoter (26) consists of a succession of equivalent protections with a periodicity of 6 bp, it became plausible that the consensus sequence could in fact be recognized by Fur as three repeats of 6 bp rather than as a 19-bp palindrome. A recent study suggests that this could in fact be the case (34). We have observed that while a minimum of three repeats is required to produce an effective Fur binding site, their relative orientation and their number may not be so important. The hexamer NATA/TAT appears to be the unit of interaction with Fur in a target site, although only the sum of DNA-protein and protein-protein interactions involving at least three units may endow the complex with enough strength and specificity to be fully functional (34; Fig. 3).
FIG. 3.
Rules that govern the generation of Fur binding sites. Active targets for the Fur-Fe2+ complex can be assembled by combining a minimum of three repeats of NATA/TAT. (A) The thymines present in the core sequence AT-AT of each repeat are directly engaged in interactions with the repressor (22). The presence of a central T residue in the upper or the lower strand probably determines the orientation of each minimal unit in the array. (B) The combination in any orientation of three repeats (i.e., three units of 6 bp each) gives rise to a functional Fur box. Those found in natural promoters generally adopt the configuration two direct/one inverted repeat. (C) The grouping of a Fur box with a number of adjacent hexamers with various degrees of similarity to NATA/TAT originate secondary sites (such as sites II found in a number of promoters). In come cases, extended protections caused by the polymerization of the protein can be observed along neighboring DNA segments with less sequence similarity.
These notions about the true nature of Fur boxes as an array of shorter sequence motifs could explain why the target DNA admits so many changes and why no individual bases essential for the interaction have been found in mutagenesis experiments (84). Fur-DNA interaction may therefore be quite different from the classical model of LacI, Cro, or catabolite gene activator protein, in which a dimeric (or tetrameric) protein binds a palindromic sequence (77). This type of interaction could provide the protein with the ability to behave both as a very specific repressor and as a more general regulator.
DO SIMILAR Fur-DNA INTERACTIONS APPLY TO OTHER BACTERIA?
As mentioned above, fur gene homologues have been found in a variety of gram-negative species. Since the cognate protein sequences exhibit a high degree of similarity, it is predictable that very closely related target sequences will also be found at iron-regulated promoters in microorganisms such as members of the family Enterobacteriaceae. In fact, this is the case in every single instance where the issue has been examined. Bacteria bearing Fur homologues systematically also possess Fur boxes in front of the fur gene or other iron-regulated genes (29, 35, 51, 88). In many cases, binding of such sequences by FurEC (the Fur protein from E. coli) has been observed in in vitro or in vivo assays. For instance, some Pseudomonas genes that are regulated by Fur are retarded in band-shift gels and protected in footprinting assays with the protein from E. coli (71, 74). Similarly, the fbpA gene of N. gonorrhoeae (encoding a periplasmic binding protein) is both regulated by FurEC in in vivo and protected from DNase I in vitro (9, 29). In vivo FURTA assays using E. coli as a host have highlighted the efficiency of DNA sequences of various origins in binding FurEC. These include the promoter regions of the pfrI and pupIR genes from Pseudomonas putida (95), the sfuA gene of Serratia marcescens, and the genes hemPR (heme uptake operon) and foxA/fcuA (extracellular ferrioxamin receptor) from Yersinia enterocolitica (84). But this is not limited to gram-negative bacteria. The genome of B. subtilis also contains perfect Fur boxes in the promoter regions of siderophore-related genes (15) whose functionality in binding FurEC in vivo has been proved in some cases (81). The recognition of the consensus Fur box of E. coli by the Fur protein of B. subtilis is consistent with the similarity of the DNA binding motifs of both repressors (15).
But what about interactions of Fur homologues with DNA sequences in their cognate hosts? Although the data is more scarce than for E. coli, the few instances where the issue has been examined do indicate that all Fur proteins bind the same consensus target DNA. Canonical iron boxes bind the Fur products of C. jejuni (63), P. aeruginosa (48, 58), Vibrio anguillarum (18), and V. cholerae (99), as revealed with in vitro band-shift assays. Furthermore, DNase I footprinting experiments of FurPA with a large collection of P. aeruginosa DNA fragments selected with SELEX have revealed that the general organization of the Fur boxes and the protected sequences in this microorganism are virtually identical to those described for E. coli (48, 72). The promoters examined contained one or two overlapping or tandemly organized Fur binding motifs with a consensus identical to that of E. coli. In addition, the protected sequences added up to the standard 30 to 38 bp protected by the protein which have been repeatedly observed in Fur sites of E. coli promoters. Likewise, the characterization of the interaction between Fur and the promoter of the iron transport fatDCBA operon in V. anguillarum has revealed several complexes in gel retardation assays and extended protected sequences in DNase I gels (18). This promoter is AT rich, but not all portions of the binding sequences showed a clear conservation of the consensus Fur box or the characteristic dyad symmetry. This is not unlike what has been described for the Fur polymerization region in the aerobactin promoter (25, 26). Most sequences of P. aeruginosa, V. anguillarum, and other bacteria targeted by their native Fur proteins could be reinterpreted as arrays of NATA/TAT sequences, as discussed above. The generalization of this concept requires, however, further investigation.
Although B. subtilis and Staphylococcus aureus do possess a distinct Fur-like homologue (15, 49), the protein known as the diphtheria toxin regulator (DtxR) of Corynebacterium diphtheriae constitutes the archetype of a class of iron-related regulators found in gram-positive bacteria (11). This class includes SirR of Staphylococcus epidermidis (50) and IdeR of M. tuberculosis (80). DtxR does not have much similarity to Fur (less than 20% identity at the amino acid level), and complementation between these proteins is weak or nonexistent (11). The crystal structure of DtxR has revealed that two dimeric repressor proteins are bound to opposite sides of the tox operator (101). Although the operator sequences bound by DtxR bear some similarity to the Fur box and the primary region protected from DNase I also spans 30 bp, it is plausible that DtxR and Fur have different DNA binding mechanisms (86, 97).
A FAMILY OF Fur-RELATED METALLOREGULATORY PROTEINS?
The type of regulation described for Fur may also be applicable to other metal-dependent repressors. In this respect, the Zur (zinc uptake regulator) protein, described thus far in E. coli (75), B. subtilis (39), and Listeria monocytogenes (22), is worth a comment. Similar to iron, Zn is an element that, depending on the concentration, can be an essential micronutrient or a potent toxin. The uptake of this metal is regulated by the Zur protein in combination with zinc, resembling the phenomenology already discussed for Fur. Furthermore, Zur and Fur have a significant sequence identity (24% in B. subtilis and 27% in E. coli [39, 75]). Nothing is known about the repression mechanism, but some Zur-binding sequences have been described for promoters of genes related to Zn uptake, and, intriguingly, they are similar to the Fur box (39). This may allow a degree of regulatory cross-talk but must also permit each repressor to recognize and regulate specifically their own set of genes. It will be interesting to analyze whether Zur uses the same molecular mechanisms as Fur to repress transcription and bind DNA. Some proteins initially found through genomic search and classified as Fur homologues are certainly more similar to Zur than to Fur (75) and perhaps could be reclassified as regulators of zinc uptake. Moreover, the fact that Fur has been recently defined as a Zn-metalloprotein containing one structural ion of Zn2+ per polypeptide makes the relation between these proteins even more complex (2).
Analysis of the growing number of the genomes of bacterial species which have been or are being completely sequenced, as well as direct experimental observations, have started to unveil the existence of an entire family proteins structurally related to Fur which control gene expression in response to different stress signals. Besides Fur and Zur, B. subtilis harbors one more related protein called PerR (15). The PerR regulon includes genes involved in the response to oxidative stress such as katA (encoding catalase A) and aphC (alkyl hydroperoxide reductase) as well as fur and perR. Although these genes can be repressed by either manganese or iron, the PerR-Fe2+ complex is the form that reacts with hydrogen peroxide to cause derepression (15). The Per boxes are thus associated with oxidative stress genes in several gram-positive bacteria rather than with iron transport. The Irr product of Bradyrhizobium japonicum also bears a considerable similarity (29% identity [44]) to the Fur protein of E. coli. The Irr protein regulates heme biosynthesis and appears to coordinate this pathway with iron homeostasis (which is in itself regulated by a genuine Fur homologue in this bacterium). In contrast to Fur, Irr is active under iron limitation. As in the case of Zur, some proteins described as Fur homologues could functionally be Irr-like regulators (44).
CONCLUSION
The abundance of the protein, the form of interaction with target DNA sequences, and the involvement of Fur in many cell functions indicate that this protein performs more like a general regulator than as a specific repressor. The reinterpretation of Fur binding sites with the NATA/TAT array model affords not only an explanation of many thus far unaccounted for results about the interaction of the protein with its target sequences but also provides a basis for understanding the differences observed in the responses of various genes to physiological iron status. The combination of repetitive sequence elements that allow cooperative binding of the Fur protein in extended promoter regions would explain how a relatively simple protein controls a complex regulon in a gradual fashion. The intracellular iron concentration and the variability and extension of the sequences targeted by the protein may cause an ample range of responses in each specific case. On this basis, some genes like fur undergo mild regulation or coregulation by iron, while others like the aerobactin operon in E. coli are subject to a strong repression/induction switch.
ACKNOWLEDGMENTS
The work of this Laboratory on metalloregulation is funded by grants ENV4-CT95-0141 (Environment) of the EU and grant BIO98-0808 of the Comisión Interministerial de Ciencia y Tecnología.
We are indebted to J. B. Neilands for constant inspiration of work on iron metabolism in microorganisms.
REFERENCES
- 1.Abdul-Tehrani H, Hudson A J, Chang Y S, Timms A R, Hawkins C, Williams J M, Harrison P M, Guest J R, Andrews S C. Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient. J Bacteriol. 1999;181:1415–1428. doi: 10.1128/jb.181.5.1415-1428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Althaus E W, Outten C E, Olson K E, Cao H, O’Halloran T V. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999;38:6559–6569. doi: 10.1021/bi982788s. [DOI] [PubMed] [Google Scholar]
- 3.Archibald F. Lactobacillus plantarum, an organism not requiring iron. FEMS Microbiol Lett. 1983;19:29–32. [Google Scholar]
- 4.Bagg A, Neilands J B. Mapping of a mutation affecting regulation of iron uptake systems in Escherichia coli K-12. J Bacteriol. 1985;161:450–453. doi: 10.1128/jb.161.1.450-453.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- 6.Bagg A, Neilands J B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden S W, Perry R D. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999;32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 8.Bereswill S, Lichte F, Vey T, Fassbinder F, Kist M. Cloning and characterization of the fur gene from Helicobacter pylori. FEMS Microbiol Lett. 1998;159:193–200. doi: 10.1111/j.1574-6968.1998.tb12860.x. [DOI] [PubMed] [Google Scholar]
- 9.Berish S A, Subbarao S, Chen C-Y, Trees D L, Morse S A. Identification and cloning of a Fur homologue from Neisseria gonorrhoeae. Infect Immun. 1993;61:4599–4606. doi: 10.1128/iai.61.11.4599-4606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bindereif A, Neilands J B. Promoter mapping and transcriptional regulation of the iron assimilation system of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1985;162:1039–1046. doi: 10.1128/jb.162.3.1039-1046.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd J, Oza M N, Murphy J R. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc Natl Acad Sci USA. 1990;87:5968–5972. doi: 10.1073/pnas.87.15.5968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun V, Schäffer S, Hantke K, Tröger W. Regulation of gene expression by iron. 41. Colloquium Mosbach 1990. The molecular basis of bacterial metabolism. Berlin, Germany: Springer-Verlag; 1990. [Google Scholar]
- 13.Brickman T J, Ozenberger B A, McIntosh M A. Regulation of divergent transcription from the iron-responsive fepB-entC promoter-operator regions in Escherichia coli. J Mol Biol. 1990;212:669–682. doi: 10.1016/0022-2836(90)90229-F. [DOI] [PubMed] [Google Scholar]
- 14.Brickman T J, Armstrong S K. Bordetella pertussis fur gene restores iron repressibility of siderophore and protein expression to deregulated Bordetella bronchiseptica mutants. J Bacteriol. 1995;177:268–270. doi: 10.1128/jb.177.1.268-270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–198. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 16.Calderwood S, Mekalanos J J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988;170:1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carson S D B, Thomas C E, Elkins C. Cloning and sequencing of a Haemophilus ducreyi fur homolog. Gene. 1996;176:125–129. doi: 10.1016/0378-1119(96)00236-3. [DOI] [PubMed] [Google Scholar]
- 18.Chai S, Welch T J, Crosa J H. Characterization of the interaction between Fur and the iron transport promoter of the virulence plasmid in Vibrio anguillarum. J Biol Chem. 1998;273:33841–33847. doi: 10.1074/jbc.273.50.33841. [DOI] [PubMed] [Google Scholar]
- 19.Coy M, Neilands J B. Structural dynamics and functional domains of the Fur protein. Biochemistry. 1991;30:8201–8210. doi: 10.1021/bi00247a016. [DOI] [PubMed] [Google Scholar]
- 20.Crichton R R. Inorganic biochemistry of iron metabolism. New York, N.Y: Ellis Horwood; 1991. [Google Scholar]
- 21.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalet K, Gouin E, Cenatiempo Y, Cossart P, Hechard Y. Characterization of a new operon encoding a Zur-like protein and an associated ABC zinc permease in Listeria monocytogenes. FEMS Microbiol Lett. 1999;174:111–116. doi: 10.1111/j.1574-6968.1999.tb13556.x. [DOI] [PubMed] [Google Scholar]
- 23.Daniel C, Haentjens S, Bissinger M C, Courcol R J. Characterization of the Acinetobacter baumannii Fur regulator: cloning and sequencing of the fur homolog gene. FEMS Microbiol Lett. 1999;170:199–209. doi: 10.1111/j.1574-6968.1999.tb13375.x. [DOI] [PubMed] [Google Scholar]
- 24.de Lorenzo V, Bindereif A, Paw B H, Neilands J B. Aerobactin biosynthesis and transport genes of plasmid ColV-K30 in Escherichia coli K-12. J Bacteriol. 1986;165:570–578. doi: 10.1128/jb.165.2.570-578.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Lorenzo V, Wee S, Herrero M, Neilands J B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Lorenzo V, Giovannini F, Herrero M, Neilands J B. Metal ion regulation of gene expression: Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988;203:875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 27.de Lorenzo V, Herrero M, Giovannini F, Neilands J B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988;173:537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- 28.de Luca N G, Wexler M, Pereira M J, Yeoman K H, Johnston A W. Is the fur gene of Rhizobium leguminosarum essential? FEMS Microbiol Lett. 1998;168:289–295. doi: 10.1016/s0378-1097(98)00454-6. [DOI] [PubMed] [Google Scholar]
- 29.Desai P J, Angerer A, Genco C A. Analysis of Fur binding to operator sequences within the Neisseria gonorrhoeae fbpA promoter. J Bacteriol. 1996;178:5020–5023. doi: 10.1128/jb.178.16.5020-5023.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Earhart C F. Uptake and metabolism of iron and molybdenum. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella, cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1075–1090. [Google Scholar]
- 31.Ernst J F, Bennett R L, Rothfield L I. Constitutive expression of the iron-enterochelin and ferrichrome uptake systems in a mutant strain of Salmonella typhimurium. J Bacteriol. 1978;135:928–934. doi: 10.1128/jb.135.3.928-934.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Escolar L, de Lorenzo V, Pérez-Martín J. Metalloregulation in vitro of the aerobactin promoter of Escherichia coli by the Fur (ferric uptake regulation) protein. Mol Microbiol. 1997;26:799–808. doi: 10.1046/j.1365-2958.1997.6211987.x. [DOI] [PubMed] [Google Scholar]
- 33.Escolar L, Pérez-Martín J, de Lorenzo V. Coordinated repression in vitro of the divergent fepA-fes promoters of Escherichia coli by the iron uptake regulation (Fur) protein. J Bacteriol. 1998;180:2579–2582. doi: 10.1128/jb.180.9.2579-2582.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escolar L, Pérez-Martín J, de Lorenzo V. Binding of the Fur (ferric uptake regulator) repressor of Escherichia coli to arrays of the GATAAT sequence. J Mol Biol. 1998;283:537–547. doi: 10.1006/jmbi.1998.2119. [DOI] [PubMed] [Google Scholar]
- 35.Fetherston J D, Bertolino V J, Perry R D. YbtP and YbtQ: two ABC transporters required for iron uptake in Yersinia pestis. Mol Microbiol. 1999;32:289–299. doi: 10.1046/j.1365-2958.1999.01348.x. [DOI] [PubMed] [Google Scholar]
- 36.Franza T, Sauvage C, Expert D. Iron regulation and pathogenicity in Erwinia chrysanthemi 3937: role of the Fur repressor protein. Mol Plant Microbe Interact. 1999;12:119–128. doi: 10.1094/MPMI.1999.12.2.119. [DOI] [PubMed] [Google Scholar]
- 37.Fréchon D, Le Cam E. Fur (ferric uptake regulation) protein interaction with target DNA: comparison of gel retardation, footprinting and electron microscopy analyses. Biochem Biophys Res Commun. 1994;201:346–355. doi: 10.1006/bbrc.1994.1708. [DOI] [PubMed] [Google Scholar]
- 38.Foster J W, Hall H K. Effect of Salmonella typhimurium ferric uptake regulator (fur) mutations on iron- and pH-regulated protein synthesis. J Bacteriol. 1992;174:4317–4323. doi: 10.1128/jb.174.13.4317-4323.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaballa A, Helmann J D. Identification of a zinc-specific metalloregulatory protein, Zur, controlling zinc transport operons in Bacillus subtilis. J Bacteriol. 1998;180:5815–5821. doi: 10.1128/jb.180.22.5815-5821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghassemian M, Straus N A. Fur regulates the expression of iron-stress genes in the cyanobacterium Synechococcus sp. strain PCC7942. Microbiology. 1996;142:1469–1476. doi: 10.1099/13500872-142-6-1469. [DOI] [PubMed] [Google Scholar]
- 41.Griggs D W, Tharp B B, Konisky J. Cloning and promoter identification of the iron-regulated cir gene of the Escherichia coli. J Bacteriol. 1987;169:5343–5352. doi: 10.1128/jb.169.12.5343-5352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griggs D W, Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of Fur protein to the promoters. J Bacteriol. 1989;171:1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hall H K, Foster J W. The role of Fur in the acid tolerance response of Salmonella typhimurium is physiologically and genetically separable from its role in iron acquisition. J Bacteriol. 1996;178:5683–5691. doi: 10.1128/jb.178.19.5683-5691.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamza I, Chauhan S, Hassett R, O’Brian M R. The bacterial irr protein is required for coordination of heme biosynthesis with iron availability. J Biol Chem. 1998;273:21669–21674. doi: 10.1074/jbc.273.34.21669. [DOI] [PubMed] [Google Scholar]
- 45.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 46.Hantke K. Cloning of the repressor protein gene of iron regulated system in E. coli K-12. Mol Gen Genet. 1984;197:337–341. doi: 10.1007/BF00330982. [DOI] [PubMed] [Google Scholar]
- 47.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K12: fur not only affects iron metabolism. Mol Gen Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 48.Hassett D J, Howell M L, Ochsner U A, Vasil M L, Johnson Z, Dean G E. An operon containing fumA and sodA encoding fumarase C and manganese superoxide dismutase is controlled by the ferric uptake regulator in Pseudomonas aeruginosa: fur mutants produce elevated alginate levels. J Bacteriol. 1997;179:1452–1459. doi: 10.1128/jb.179.5.1452-1459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heidrich C, Hantke K, Bierbaum G, Sahl H G. Identification and analysis of a gene encoding a Fur-like protein of Staphylococcus epidermidis. FEMS Microbiol Lett. 1996;140:253–259. doi: 10.1111/j.1574-6968.1996.tb08345.x. [DOI] [PubMed] [Google Scholar]
- 50.Hill P J, Cockayne A, Landers P, Morrissey J A, Sims C M, Williams P. SirR, a novel iron-dependent repressor in Staphylococcus epidermidis. Infect Immun. 1998;66:4123–4129. doi: 10.1128/iai.66.9.4123-4129.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinkey E K, Cianciotto N P. Cloning and sequencing of the Legionella pneumoniae fur gene. Gene. 1994;143:117–121. doi: 10.1016/0378-1119(94)90615-7. [DOI] [PubMed] [Google Scholar]
- 52.Holm L, Syer C, Ruterjans H, Schnarr M, Fogh R, Boelens R, Kaptein R. LexA repressor and iron uptake regulator from Escherichia coli: new members of the CAP-like DNA binding domain superfamily. Prot Eng. 1994;7:14449–14453. doi: 10.1093/protein/7.12.1449. [DOI] [PubMed] [Google Scholar]
- 53.Hunt M D, Pettis G S, McIntosh M A. Promoter and operator determinants for Fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin system. J Bacteriol. 1994;176:3944–3955. doi: 10.1128/jb.176.13.3944-3955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karjalainen T K, Evans D G, Evans D J, Graham D Y, Jr, Lee C H. Iron represses the expression of CFA/I fimbriae of enterotoxigenic E. coli. Microb Pathog. 1991;11:317–323. doi: 10.1016/0882-4010(91)90017-5. [DOI] [PubMed] [Google Scholar]
- 55.Klebba P E, McIntosh M A, Neilands J B. Kinetics of biosynthesis of iron-regulated membrane proteins in Escherichia coli. J Bacteriol. 1982;149:880–888. doi: 10.1128/jb.149.3.880-888.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le Cam E, Fréchon D, Barray M, Fourcade A, Delain E. Observation of binding and polymerization of Fur repressor onto operator-containing DNA with electron and atomic force microscopes. Proc Natl Acad Sci USA. 1994;91:11816–11820. doi: 10.1073/pnas.91.25.11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee H S, Lee Y S, Kim H S, Choy J Y, Hassan H M, Chung M H. Mechanism of regulation of 8-hydroxyguanine endonuclease by oxidative stress: roles of FNR, ArcA, and Fur. Free Radic Biol Med. 1998;24:1193–1201. doi: 10.1016/s0891-5849(97)00427-9. [DOI] [PubMed] [Google Scholar]
- 58.Leoni L, Ciervo A, Orsi N, Visca P. Iron-regulated transcription of the pvdA gene in Pseudomonas aeruginosa: effect of Fur and PvdS on promoter activity. J Bacteriol. 1996;178:2299–2313. doi: 10.1128/jb.178.8.2299-2313.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Litwin M, Boyko S A, Calderwood S B. Cloning, sequencing and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992;174:1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Litwin M, Calderwood S B. Role of iron in regulation of virulence genes. Clin Microbiol Rev. 1993;6:137–149. doi: 10.1128/cmr.6.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Litwin M, Calderwood S B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993;175:706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Litwin C M, Calderwood S B. Analysis of the complexity of gene regulation by Fur in Vibrio cholerae. J Bacteriol. 1994;176:240–248. doi: 10.1128/jb.176.1.240-248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Loong Chan V, Louie H, Bingham H L. Cloning and transcription regulation of the ferric uptake regulatory gene of Campylobacter jejuni TGH9011. Gene. 1995;164:25–31. doi: 10.1016/0378-1119(95)00477-n. [DOI] [PubMed] [Google Scholar]
- 64.Ma J, Oschsner U A, Klotz M G, Nanayakkara V K, Howell M L, Johnson Z, Posey J E, Vasil M L, Monaco J J, Hassett D J. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3730–3742. doi: 10.1128/jb.181.12.3730-3742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Makemson J C, Hastings J W. Iron represses bioluminescence in Vibrio harveyi. Curr Microbiol. 1982;7:181–186. [Google Scholar]
- 66.McCarter L, Silverman M. Iron regulation of swarmer cell differentiation of Vibrio parahaemolyticus. J Bacteriol. 1989;171:731–736. doi: 10.1128/jb.171.2.731-736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Michaud-Soret I, Adrait A, Jaquinod M, Forest E, Touati D, Latour J-M. Electrospray ionization mass spectroscopy analysis of the apo- and metal-substituted forms of the Fur protein. FEBS Lett. 1997;413:473–476. doi: 10.1016/s0014-5793(97)00963-0. [DOI] [PubMed] [Google Scholar]
- 68.Neilands J B. Siderophore systems of bacteria and fungi. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley & Sons, Inc.; 1989. pp. 141–163. [Google Scholar]
- 69.Neilands J B, Nakamura K. Detection, determination, isolation, characterization and regulation of microbial iron chelates. In: Winkelmann G, editor. Handbook of microbial iron chelates. Boca Raton, Fla: CRC Press; 1991. pp. 1–14. [Google Scholar]
- 70.Niederhoffer E C, Naranjo C M, Bradley K L, Fee J A. Control of Escherichia coli superoxide dismutase (sodA and sodB) genes by the ferric uptake regulation (fur) locus. J Bacteriol. 1990;172:1930–1938. doi: 10.1128/jb.172.4.1930-1938.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ochsner U A, Vasil A I, Vasil M L. Role of the ferric uptake regulator of Pseudomonas aeruginosa in the regulation of siderophores and exotoxin A expression: purification and activity on iron-regulated promoters. J Bacteriol. 1995;177:7194–7201. doi: 10.1128/jb.177.24.7194-7201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ochsner U A, Vasil M L. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ochsner U A, Johnson Z, Hassett D J, Vasil M L. Abstracts of the 99th General Meeting of the American Society for Microbiology 1999. Washington, D.C.: American Society for Microbiology; 1999. Characterization of a locus encoding a bacterioferritin and a peroxidase in Pseudomonas aeruginosa, abstr. K46; p. 409. [Google Scholar]
- 74.O’Sullivan D J, Dowling D N, de Lorenzo V, O’Gara F. Escherichia coli ferric uptake regulator (Fur) can mediate regulation of a pseudomonad iron-regulated promoter. FEMS Lett. 1994;117:327–332. doi: 10.1016/0378-1097(94)90579-7. [DOI] [PubMed] [Google Scholar]
- 75.Patzer S I, Hantke K. The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli. Mol Microbiol. 1998;28:1199–1210. doi: 10.1046/j.1365-2958.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 76.Prince R W, Cox C D, Vasil M L. Coordinate regulation of siderophore and exotoxin A production: molecular cloning and sequencing of the Pseudomonas aeruginosa fur gene. J Bacteriol. 1993;175:2589–2598. doi: 10.1128/jb.175.9.2589-2598.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ptashne M. A genetic switch. Cambridge, Mass: Cell Press and Blackwell Scientific Publications; 1992. [Google Scholar]
- 78.Saito I, Wormald M R, Williams R J P. Some structural features of the iron-uptake regulation protein. Eur J Biochem. 1991;197:29–38. doi: 10.1111/j.1432-1033.1991.tb15878.x. [DOI] [PubMed] [Google Scholar]
- 79.Schaffer S, Hantke K, Braun V. Nucleotide sequence of the iron regulatory gene fur. Mol Gen Genet. 1985;201:204–212. doi: 10.1007/BF00383321. [DOI] [PubMed] [Google Scholar]
- 80.Schmitt M P, Predich M, Doukhan L, Smith I, Holmes R K. Characterization of an iron-dependent regulatory protein (IdeR) of Mycobacterium tuberculosis as a functional homolog of the diphtheria toxin repressor (DtxR) from Corynebacterium diphtheriae. Infect Immun. 1995;63:4284–4289. doi: 10.1128/iai.63.11.4284-4289.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schneider R, Hantke K. Iron-hydroxamate uptake systems in Bacillus subtilis: identification of a lipoprotein as part of a binding protein-dependent transport system. Mol Microbiol. 1993;8:111–121. doi: 10.1111/j.1365-2958.1993.tb01208.x. [DOI] [PubMed] [Google Scholar]
- 82.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Staggs T M, Fetherston J D, Perry R D. Pleiotropic effects of a Yersinis pestis fur mutation. J Bacteriol. 1994;176:7614–7624. doi: 10.1128/jb.176.24.7614-7624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stojiljkovic I, Bäumer A J, Hantke K. Fur regulon in Gram-negative bacteria. J Mol Biol. 1994;236:531–545. doi: 10.1006/jmbi.1994.1163. [DOI] [PubMed] [Google Scholar]
- 85.Stojiljkovic I, Hantke K. Functional domains of the Escherichia coli ferric uptake regulator protein (Fur) Mol Gen Genet. 1995;247:199–205. doi: 10.1007/BF00705650. [DOI] [PubMed] [Google Scholar]
- 86.Tao X, Schiering N, Zeng H, Ringe D, Murphy J R. Iron, DtxR and the regulation of diphtheria toxin expression. Mol Microbiol. 1994;14:191–197. doi: 10.1111/j.1365-2958.1994.tb01280.x. [DOI] [PubMed] [Google Scholar]
- 87.Tardat B, Touati D. Iron and oxygen regulation of Escherichia coli MnSOD expression: competition between the global regulators Fur and ArcA for binding to DNA. Mol Microbiol. 1993;9:53–63. doi: 10.1111/j.1365-2958.1993.tb01668.x. [DOI] [PubMed] [Google Scholar]
- 88.Thomas C E, Sparling P F. Identification and cloning of a fur homologue from Neisseria meningitidis. Mol Microbiol. 1994;11:725–737. doi: 10.1111/j.1365-2958.1994.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 89.Thomas C E, Sparling P F. Isolation and analysis of a fur mutant of Neisseria gonorrhoeae. J Bacteriol. 1996;178:4224–4232. doi: 10.1128/jb.178.14.4224-4232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tolmasky M E, Wertheimer A M, Actis L A, Crosa J H. Characterization of the Vibrio anguillarum fur gen: role in regulation of expression of the FatA outer membrane protein and cathecols. J Bacteriol. 1994;176:213–220. doi: 10.1128/jb.176.1.213-220.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsolis R M, Bäumler A J, Stojiljkovic I, Heffron F. Fur regulon in Salmonella typhimurium: identification of new iron-regulated genes. J Bacteriol. 1995;177:4628–4637. doi: 10.1128/jb.177.16.4628-4637.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van Vliet A H M, Wooldridge K G, Ketley J M. Iron-responsive gene regulation in a Campylobacter jejuni fur mutant. J Bacteriol. 1998;180:5291–5298. doi: 10.1128/jb.180.20.5291-5298.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vasil M L, Ochsner U A, Johnson Z, Colmer J A, Hamood A N. The Fur-regulated gene encoding the alternative sigma factor PvdS is required for iron-dependent expression of the LysR-type regulator PtxR in Pseudomonas aeruginosa. J Bacteriol. 1998;180:6784–6788. doi: 10.1128/jb.180.24.6784-6788.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Venturi V, Ottevanger C, Bracke M, Weinsbeek P. Iron regulation of siderophore biosynthesis and transport in Pseudomonas putida WCS358: involvement of a transcriptional activator and of the Fur protein. Mol Microbiol. 1995;15:1081–1093. doi: 10.1111/j.1365-2958.1995.tb02283.x. [DOI] [PubMed] [Google Scholar]
- 96.Wackett L P, Orme-Johnson W H, Walsh C T. Transition metal enzymes in bacterial metabolism. In: Beveridge T J, Doyle R J, editors. Metal ions and bacteria. New York, N.Y: John Wiley & Sons, Inc.; 1989. pp. 165–206. [Google Scholar]
- 97.Wang G, Wylie G P, Twigg P D, Caspar D I, Murphy J R, Logan T M. Solution structure and peptide binding studies of the C-terminal src homology 3-like domain of the diphtheria toxin repressor protein. Proc Natl Acad Sci USA. 1999;96:6119–6124. doi: 10.1073/pnas.96.11.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watnick P I, Eto T, Takahashi H, Calderwood S B. Purification of Vibrio cholerae Fur and estimation of its intracellular abundance by antibody sandwich enzyme-linked immunosorbent assay. J Bacteriol. 1997;179:243–247. doi: 10.1128/jb.179.1.243-247.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Watnick P I, Butterton J R, Calderwood S B. The interaction of the Vibrio cholerae transcription factors, Fur and IrgB, with the overlapping promoters of two virulence genes, irgA and irgB. Gene. 1998;209:65–70. doi: 10.1016/s0378-1119(98)00018-3. [DOI] [PubMed] [Google Scholar]
- 100.Wee S, Neilands J B, Bittner M L, Hemming B C, Haymore B L, Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K-12. Biol Metals. 1988;1:62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- 101.White A, Ding X, vanderSpeck J C, Murphy J R, Ringe D. Structure of the metal-ion-activated diphtheria toxin repressor/tox operator complex. Nature. 1998;394:502–506. doi: 10.1038/28893. [DOI] [PubMed] [Google Scholar]
- 102.Wong D K, Lee B Y, Horwitz M A, Gibson B W. Identification of Fur, aconitase, and other proteins expressed by Mycobacterium tuberculosis under conditions of low and high concentrations of iron by combined two-dimensional gel electrophoresis and mass spectrometry. Infect Immun. 1999;67:327–336. doi: 10.1128/iai.67.1.327-336.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wooldridge K G, Williams P H, Ketley J M. Iron-responsive genetic regulation in Campylobacter jejuni: cloning and characterization of a fur homologue. J Bacteriol. 1994;176:5852–5856. doi: 10.1128/jb.176.18.5852-5856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yamamoto S, Funahashi T, Ikai H, Shinoda S. Cloning and sequencing of the Vibrio parahaemolyticus fur gene. Microbiol Immunol. 1997;41:737–740. doi: 10.1111/j.1348-0421.1997.tb01919.x. [DOI] [PubMed] [Google Scholar]