Key Points
Question
Among at-risk patients with hypertension or diabetes, what is the prevalence of albuminuria that is undetected?
Findings
In this cohort study of 192 108 adults with hypertension or diabetes having at least 2 outpatient health care visits during 2017 to 2018, the estimated prevalence of albuminuria was 17.5%; approximately two-thirds did not have urine albumin tested. Albuminuria testing was associated with higher prevalence of chronic kidney disease treatment.
Meaning
These results suggest that improving identification of albuminuria among at-risk individuals represents a substantial opportunity to optimize care delivery for reducing kidney disease progression and cardiovascular complications.
This cohort study estimates the level of albuminuria underdetection from lack of testing and evaluates its association with chronic kidney disease (CKD) treatment among patients with hypertension or diabetes.
Abstract
Importance
Albuminuria testing is crucial for guiding evidence-based treatments to mitigate chronic kidney disease (CKD) progression and cardiovascular morbidity, but it is widely underutilized among persons with or at risk for CKD.
Objective
To estimate the extent of albuminuria underdetection from lack of testing and evaluate its association with CKD treatment in a large US cohort of patients with hypertension or diabetes.
Design, Setting, and Participants
This cohort study examined adults with hypertension or diabetes, using data from the 2007 to 2018 National Health and Nutrition Examination Surveys (NHANES) and the Optum deidentified electronic health record (EHR) data set of diverse US health care organizations. Analyses were conducted from October 31, 2022, to May 19, 2023.
Main Outcomes and Measures
Using NHANES as a nationally representative sample, a logistic regression model was developed to estimate albuminuria (urine albumin-creatinine ratio ≥30 mg/g). This model was then applied to active outpatients in the EHR from January 1, 2017, to December 31, 2018. The prevalence of albuminuria among those with and without albuminuria testing during this period was estimated. A multivariable logistic regression was used to examine associations between having albuminuria testing and CKD therapies within the subsequent year (prescription for angiotensin-converting enzyme inhibitor [ACEi] or angiotensin II receptor blocker [ARB], prescription for sodium-glucose cotransporter 2 inhibitor [SGLT2i], and blood pressure control to less than 130/80 mm Hg or less than 140/90 mm Hg on the latest outpatient measure).
Results
The total EHR study population included 192 108 patients (mean [SD] age, 60.3 [15.1] years; 185 589 [96.6%] with hypertension; 50 507 [26.2%] with diabetes; mean [SD] eGFR, 84 [21] mL/min/1.73 m2). There were 33 629 patients (17.5%) who had albuminuria testing; of whom 11 525 (34.3%) had albuminuria. Among 158 479 patients who were untested, the estimated albuminuria prevalence rate was 13.4% (n = 21 231). Thus, only 35.2% (11 525 of 32 756) of the projected population with albuminuria had been tested. Albuminuria testing was associated with higher adjusted odds of receiving ACEi or ARB treatment (OR, 2.39 [95% CI, 2.32-2.46]), SGLT2i treatment (OR, 8.22 [95% CI, 7.56-8.94]), and having blood pressure controlled to less than 140/90 mm Hg (OR, 1.20 [95% CI, 1.16-1.23]).
Conclusions and Relevance
In this cohort study of patients with hypertension or diabetes, it was estimated that approximately two-thirds of patients with albuminuria were undetected due to lack of testing. These results suggest that improving detection of CKD with albuminuria testing represents a substantial opportunity to optimize care delivery for reducing CKD progression and cardiovascular complications.
Introduction
Chronic kidney disease (CKD) is a major health problem with a rising prevalence globally.1 In the US alone, approximately 37 million adults have CKD and face substantially increased risk for cardiovascular disease, hospitalizations, in-hospital complications, and early mortality.2 CKD is defined by either reduced filtration (estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2) or albuminuria (urine albumin-creatinine ratio [UACR] ≥30 mg/g). Persons with albuminuria and preserved eGFR compose as much as 40% of the population with CKD3 and have heightened risk for cardiovascular events, CKD progression, and mortality, independent of eGFR.4,5,6,7,8
Early detection of CKD among individuals at risk for CKD, such as those with hypertension or diabetes, is necessary to ensure optimal dissemination of disease-modifying CKD therapies, including angiotensin-converting enzyme inhibitor (ACEi), angiotensin II receptor blocker (ARB), and sodium-glucose cotransporter-2 inhibitor (SGLT2i).9 Although eGFR testing is obtained approximately 90% among persons with hypertension or diabetes, widespread underutilization of albuminuria testing is well described.10,11,12 Among persons with diabetes, for whom annual albuminuria testing has long been guideline-recommended,13 testing rates have consistently been approximately 50% or lower across a variety of settings.14,15,16 Albuminuria testing rates among persons with hypertension without diabetes are much lower at approximately 10%.10 Thus, underdetection of CKD likely contributes to the ongoing suboptimal dissemination of CKD therapies.16,17,18,19,20 Improving uptake of guideline-directed therapies, including ACEi or ARB, SGLT2i, and nonsteroidal mineralocorticoid antagonists among individuals with CKD and albuminuria represents a critical opportunity to curb CKD progression and related kidney and cardiovascular complications.3,21,22,23
The effect of underutilization of albuminuria testing on the low usage of cardiovascular and kidney therapies is unknown. However, determining the proportion of individuals with albuminuria who have not had urine albumin testing has been challenging to evaluate. National surveys can estimate the population prevalence of albuminuria, but they do not distinguish the proportion that has been tested by their clinicians. Although clinical data sources capture testing and positive results, albuminuria prevalence among the tested cannot be extrapolated to the untested population due to nonrandom testing.24
In this study, we aimed to estimate the extent of albuminuria underdetection in clinical health systems accounting for nonrandom testing using a 2-step approach.25 First, we developed a model to estimate albuminuria prevalence using nationally representative survey data; then, we applied this model to estimate the prevalence of albuminuria in health system populations without uniform testing. We also examined whether testing for albuminuria was associated with likelihood of receiving evidence-based treatments to reduce the risk of CKD progression or complications.
Methods
Study Population
Our main analysis used data from a 5% random sample of the Optum deidentified electronic health record (EHR) data set, which includes EHR data collected from health systems across the US. We included adults aged at least 18 years who had at least 2 outpatient visits from January 1, 2017, to December 31, 2018, and who had either hypertension or diabetes. As this study involved deidentified, preexisting data, the University of California, San Francisco institutional review board considered the study to be exempt from requirements for review and informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
For our preanalytic step of developing a model to estimate albuminuria, we used data from the 2007 to 2018 National Health and Nutrition Examination Surveys (NHANES).26 NHANES is a continuously conducted nationally representative survey that collects demographic, health questionnaire, physical examination, and laboratory data, including albuminuria testing. We chose a nationally representative population because a model would be susceptible to bias if it were developed only among a clinical population receiving albuminuria testing, as the individuals with testing would be unlikely to represent the population without testing. For the NHANES model development, we included adults aged at least 18 years who had either hypertension or diabetes and who reported having a routine site for health care. Hypertension was defined as self-reported high blood pressure (BP), systolic BP greater than or equal to 140 mm Hg or diastolic BP greater than or equal to 90 mm Hg, or current use of antihypertensive medications.27 Diabetes was defined as self-reported diabetes, hemoglobin A1c greater than 6.5%, or current use of diabetes medications (to convert hemoglobin A1c to proportion of total hemoglobin, multiply by 0.01).28 Analyses of NHANES were conducted taking into account survey weights to obtain nationally representative estimates.
For the EHR study population, we used validated algorithms from the Electronic Medical Records and Genomics (eMERGE) network for identifying hypertension and diabetes in EHR data.29 Hypertension was defined as having at least 2 of (1) elevated BP, defined as either 2 values of systolic BP greater than or equal to 140 mm Hg at least 6 months apart or 2 values of diastolic BP greater than or equal to 90 mm Hg at least 6 months apart; (2) International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes for hypertension on more than 1 date (eTable 1 in Supplement 1); or (3) at least 1 prescription for an antihypertensive medication. Diabetes was defined as having ICD-10 diagnosis codes for diabetes plus either (1) prescription for any antihyperglycemic medication, (2) hemoglobin A1c greater than or equal to 6.5%, or (3) random glucose greater than 200 mg/dL (to convert glucose to mmol/L, multiply by 0.0555). We excluded patients with end-stage kidney disease (defined as previous receipt of dialysis or kidney transplantation) or receiving hospice care.
Study Variables
Additional variables were defined as follows. For NHANES, variables collected to describe the study population included demographic characteristics (age, sex, race, and ethnicity), smoking status, insurance type, and comorbidities (heart failure, coronary artery disease, and cerebrovascular disease); these variables were self-reported. Each participant had up to 4 BP readings recorded; we used the mean of all readings to define BP for our analysis. Medication use was ascertained through review of medication containers by study staff.30 In the EHR cohort, demographic information, smoking status, and insurance type were obtained from the EHR of source health systems. Clinical variables were defined relative to an index date defined for each patient. For patients with UACR testing, the index date was defined as the date of their UACR test. Patients without UACR testing were randomly assigned a date from within 2017 to 2018 to serve as the index date. Baseline medication use was defined based on prescriptions within a 1-year period before the index date. BP and laboratory results were defined as the most recent available recorded values within the prior year.
Estimation Model for Albuminuria
We used NHANES to develop an estimation model for albuminuria, defined as UACR greater than or equal to 30 mg/g, which could be applied to untested patients in the EHR study population. We fit a logistic regression model with age, sex, race, ethnicity, systolic BP, eGFR, diabetes, coronary artery disease, and heart failure as independent variables based on their established associations with albuminuria.11,31 Continuous variables were modeled as spline terms to accommodate nonlinear associations. We computed C statistics to evaluate model discrimination, and we compared estimated with observed albuminuria prevalence to assess calibration. To check model transportability to different subpopulations with potentially different albuminuria testing rates, we assessed model performance stratified by diabetes, insurance type, and education level. Model performance was assessed in the subgroup of the EHR study population who had UACR testing. We did not recalibrate the model to optimize performance in the EHR-tested population because tested patients likely represented a group that is more likely to have albuminuria. Therefore, optimizing performance of our model within this tested group could inflate risk estimation among untested patients. However, as a sensitivity analysis, we repeated our main analysis using an albuminuria estimation model derived from the EHR-tested population instead of NHANES.
Estimated Prevalence of Albuminuria
For our primary analysis, we estimated the total prevalence of albuminuria in the EHR study population. Among tested patients, we used measured UACR greater than or equal to 30 mg/g to identify the proportion with albuminuria. For untested patients, we applied our estimation model to assign a probability of albuminuria to each patient, and the estimated prevalence of albuminuria was determined as the mean estimated probability of albuminuria. The estimated total prevalence of albuminuria was the combination of albuminuria prevalence among tested and untested populations. We similarly determined the estimated prevalence of albuminuria in subgroups based on age, race, ethnicity, hypertension status, and diabetes status. Given the strong association of diabetes with albuminuria testing, we also conducted stratified analyses by diabetes status.
Association of UACR Testing, Estimated Albuminuria Risk, and Albuminuria Treatment
To examine associations between UACR testing and CKD treatment, we restricted our analytic population to patients having at least 12 months of follow-up data after their index date. The treatments of interest were ACEi or ARB treatment, SGLT2i treatment, and BP control to either less than 130/80 mm Hg (as recommended by guidelines for CKD3) or a higher threshold of less than 140/90 mm Hg.32
We categorized estimated albuminuria risk into quintiles, and we examined the proportion of patients receiving each treatment, stratified by testing status and by quintile of estimated albuminuria risk. To assess whether receiving testing was associated with each treatment among patients having similar risk of albuminuria, we used multivariable logistic regression models for each treatment, with testing as the exposure of interest and adjustment for age, sex, race, ethnicity, insurance type, smoking status, eGFR, heart failure, coronary artery disease, and prior stroke.
Statistical Analysis
Two-sided P < .05 was considered statistically significant. All analyses were conducted using SAS software version 9.4 (SAS Institute) from October 31, 2022, to May 19, 2023.
Results
Study Population
Among the 192 108 patients included in the primary analysis study population, 105 591 (55.0%) were female; 21 001 (10.9%) were Black, 8153 (4.2%) were Hispanic, 152 655 (79.5%) were White, and 10 299 (5.4%) were other race or ethnicity; 185 589 (96.6%) had hypertension and 50 507 (26.2%) had diabetes, the mean (SD) age was 60.3 [15.1] years; and the mean (SD) eGFR was 84 (21) mL/min/1.73m2 (Table 1). eFigure 1 in Supplement 1 shows the derivation of the study population from the EHR data set. eTable 2 in Supplement 1 shows characteristics of included patients compared with those excluded due to missing eGFR. Among included patients, 33 629 (17.5%) had undergone UACR testing. When stratified by diabetes status (eTable 3 in Supplement 1), UACR testing was greater among patients with diabetes (52.3% [26 410 of 50 507]) compared with patients with hypertension only (5.1% [7219 of 141 601]).
Table 1. Study Population Characteristics of Patients With Hypertension or Diabetes, Overall and by Urine Albumin Testing Status.
| Characteristic | Patients, No. (%) | ||
|---|---|---|---|
| Overall (n = 192 108) | Tested (n = 33 629) | Untested (n = 158 479) | |
| Age, mean (SD), y | 60.3 (15.1) | 61.7 (13.6) | 60.0 (15.3) |
| Sex | |||
| Female | 105 591 (55.0) | 16 946 (50.4) | 88 645 (55.9) |
| Male | 86 517 (45.0) | 16 683 (49.6) | 69 834 (44.1) |
| Race and ethnicity | |||
| Black | 21 001 (10.9) | 4644 (13.8) | 16 357 (10.3) |
| Hispanic | 8153 (4.2) | 2100 (6.2) | 6053 (3.8) |
| White | 152 655 (79.5) | 24 801 (73.8) | 127 854 (80.1) |
| Othera | 10 299 (5.4) | 2084 (6.2) | 8215 (5.2) |
| Insurance | |||
| Commercial | 112 370 (58.5) | 18 576 (55.2) | 93 794 (59.2) |
| Medicaid | 12 925 (6.7) | 2152 (6.4) | 10 773 (6.8) |
| Medicare | 58 307 (30.4) | 11 371 (33.8) | 46 936 (29.6) |
| Uninsured | 3856 (2.0) | 828 (2.5) | 3028 (1.9) |
| Other/unknown | 4650 (2.4) | 702 (2.1) | 3948 (2.5) |
| Current smoker | 22 443 (11.7) | 4212 (12.5) | 21 907 (13.8) |
| eGFR, mean (SD), mL/min/1.73 m2 | 84 (21) | 82 (23) | 84 (21) |
| eGFR category, mL/min/1.73 m2 | |||
| ≥60 | 163 606 (85.2) | 27 283 (81.1) | 136 323 (86.0) |
| 45-59 | 18 709 (9.7) | 3982 (11.8) | 14 727 (9.3) |
| 30-44 | 7992 (4.2) | 1939 (5.8) | 6053 (3.8) |
| <30 | 1801 (0.9) | 425 (1.3) | 1376 (0.9) |
| Systolic BP, mean (SD), mm Hg | 131 (13) | 131 (12) | 131 (13) |
| Diastolic BP, mean (SD), mm Hg | 77 (8) | 76 (8) | 77 (9) |
| Heart failure | 13 307 (6.9) | 2472 (7.4) | 10 835 (6.8) |
| Coronary artery disease | 28 010 (14.6) | 5517 (16.4) | 22 493 (14.2) |
| Hypertension only | 141 601 (73.7) | 7219 (21.5) | 134 382 (84.8) |
| Diabetes only | 6519 (3.4) | 3070 (9.1) | 3449 (2.2) |
| Hypertension and diabetes | 43 988 (22.9) | 23 340 (69.4) | 20 648 (13.0) |
Abbreviations: BP, blood pressure; eGFR, estimated glomerular filtration rate.
Other race and ethnicity includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander.
Estimation Model for Albuminuria Using NHANES
The development population included 13 410 NHANES participants (eFigure 2 in Supplement 1). Mean (SD) age was 57.9 (14.9) years, mean eGFR was 87 (22) mL/min/1.73 m2, and the prevalence of diabetes was 29.3% (95% CI, 28.1%-30.4%) (eTable 4 in Supplement 1). Our estimation model for albuminuria demonstrated good discrimination: C statistics were 0.730 (95% CI, 0.719-0.740) in the NHANES population and 0.682 (95% CI, 0.676-0.688) when applied to the EHR subgroup with UACR testing. Calibration was excellent in the NHANES population including in subgroups of diabetes, insurance, and education levels, but among patients in the EHR data set with UACR testing, the model appeared to underestimate albuminuria prevalence by 8% to 15% (eFigure 3 in Supplement 1).
Prevalence of Albuminuria Among Patients in the EHR Data Set
Among tested patients, the true prevalence of albuminuria was 34.3% (11 525 of 33 629), while the estimated prevalence of albuminuria was 13.4% (21 231 of 158 479) among untested patients. Thus, the estimated prevalence of albuminuria in the overall study population was 17.1% (32 756 of 192 108), of whom only 35.2% (11 525 of 32 756) were detected by UACR testing (eTable 5 in Supplement 1). This sizable underdetection of individuals with albuminuria was consistently observed across all demographic and comorbidity subgroups (Figure 1). The lowest detection fraction was among patients with hypertension and no diabetes (10.4% [1742 of 16 681]). Consequently, among patients with undetected albuminuria, 70.4% (14 939 of 21 231) were those with hypertension and no diabetes. In contrast, 62.1% (9221 of 14 858) of prevalent albuminuria was detected among patients with hypertension and diabetes. In analyses stratified by diabetes status, we found that the fraction of albuminuria that was detected was uniformly higher for patients with diabetes vs without diabetes in every subgroup of sex, age, race and ethnicity, and eGFR (eTable 6 and eFigure 4 in Supplement 1).
Figure 1. Estimated Albuminuria Prevalence and Proportion Detected Among Patients With Hypertension and/or Diabetes (N = 192 108).
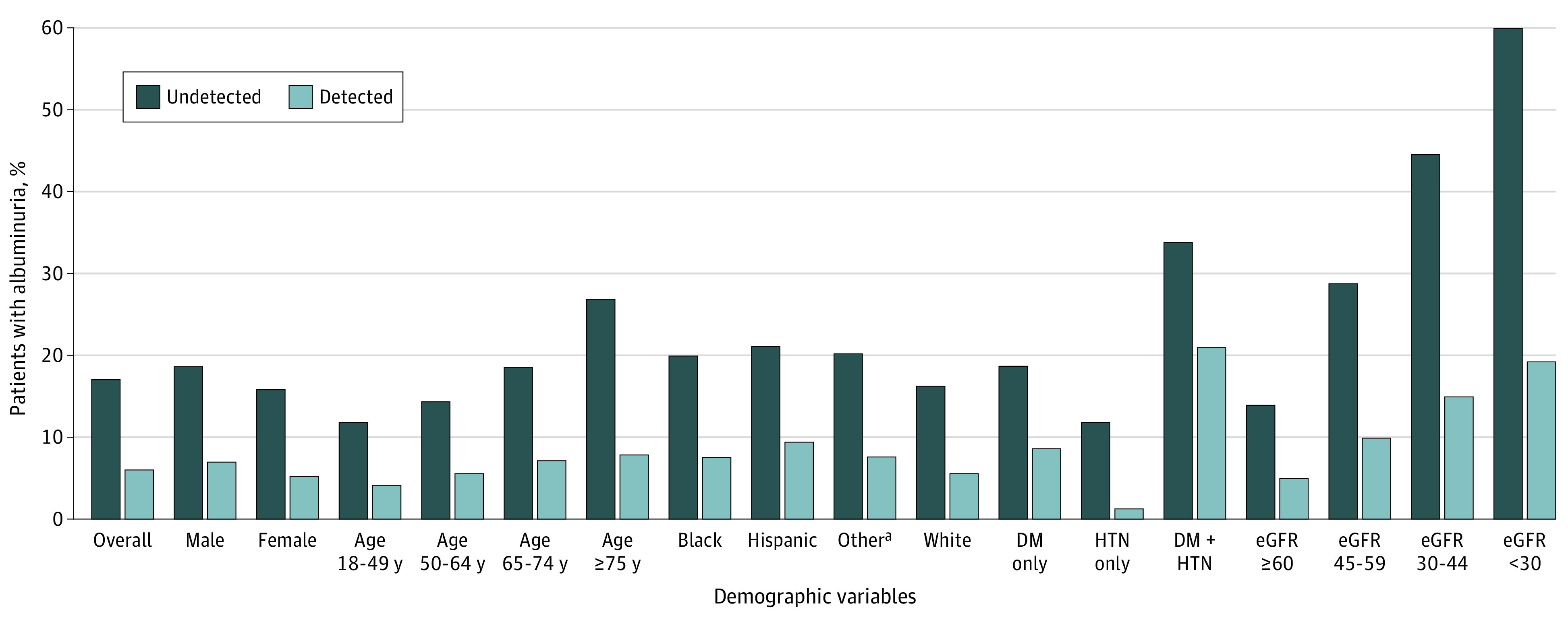
Detected albuminuria prevalence refers to tested patients having albuminuria ≥30 mg/g. Undetected albuminuria prevalence is the estimated prevalence of albuminuria ≥30 mg/g based on patient characteristics among the untested population. D indicates diabetes; eGFR, estimated glomerular filtration rate (measured in mL/min/1.73 m2); HTN, hypertension.
aOther race and ethnicity includes American Indian or Alaska Native, Asian, Native Hawaiian or Other Pacific Islander.
Albuminuria Testing by Estimated Risk of Albuminuria
When examining UACR testing rates by quintile of estimated albuminuria risk, higher risk of albuminuria was associated with progressively higher UACR testing rates (Table 2). The UACR testing rates ranged from 3.7% (95% CI, 3.5%-3.9%) among patients least likely to have albuminuria to 36.0% (95% CI, 35.5%-36.4%) among the patients most likely to have albuminuria. When examined by diabetes status, higher quintiles of estimated albuminuria risk were associated with modestly higher UACR testing in the population without diabetes, ranging from 3.4% (968 of 28 320) in quintile 1 to 7.1% (2000 of 28 320) in quintile 5. In the population with diabetes, UACR testing was similar across all quintiles of risk, ranging from 47.9% (4842 of 10 101) in quintile 5 to 54.4% (5494 of 10 102) in quintile 2.
Table 2. Albuminuria Testing by Quintiles of Estimated Albuminuria Risk Among Patients With Hypertension and/or Diabetes (N = 192 108).
| Group | Risk range, % | Patients, No. | ||
|---|---|---|---|---|
| Tested | In quintile | Proportion tested, % | ||
| Overall | ||||
| Quintile 1 | <13 | 1414 | 38 421 | 3.7 |
| Quintile 2 | 13 to <17 | 1835 | 38 422 | 4.8 |
| Quintile 3 | 17 to <21 | 4181 | 38 422 | 10.9 |
| Quintile 4 | 21 to <30 | 12 380 | 38 422 | 32.2 |
| Quintile 5 | ≥30 | 13 819 | 38 421 | 36.0 |
| No diabetes | ||||
| Quintile 1 | <7 | 968 | 28 320 | 3.4 |
| Quintile 2 | 7 to <9 | 1300 | 28 320 | 4.6 |
| Quintile 3 | 9 to <11 | 1380 | 28 321 | 4.9 |
| Quintile 4 | 11 to <17 | 1571 | 28 320 | 5.5 |
| Quintile 5 | ≥17 | 2000 | 28 320 | 7.1 |
| Diabetes | ||||
| Quintile 1 | <15 | 5361 | 10 101 | 53.1 |
| Quintile 2 | 15 to <18 | 5494 | 10 102 | 54.4 |
| Quintile 3 | 18 to <23 | 5458 | 10 101 | 54.0 |
| Quintile 4 | 23 to <32 | 5255 | 10 102 | 52.0 |
| Quintile 5 | ≥32 | 4842 | 10 101 | 47.9 |
Association of UACR Testing and Albuminuria Treatment
The analyses examining associations between UACR testing and albuminuria treatment included 149 554 patients (eFigure 1 and eTable 7 in Supplement 1). Figure 2 displays the proportions of patients receiving ACEi or ARB therapy, receiving SGLT2i therapy, and having BP controlled to less than 130/80 mm Hg or less than 140/90 mm Hg. Within each quintile of estimated albuminuria risk, tested patients were overall more likely to be receiving ACEi or ARB and SGLT2i therapy. In contrast, UACR testing did not consistently correlate with higher prevalence of BP control across quintiles of estimated albuminuria risk. In multivariable adjusted analyses, having UACR testing was associated with an approximate 2.4-fold odds of receiving ACEi or ARB treatment (odds ratio [OR], 2.39 [95% CI, 2.32-2.46]), an 8.2-fold odds of receiving SGLT2i therapy (OR, 8.22 [95% CI, 7.56-8.94]), as well as 1.2-fold odds of BP control to less than 140/90 mm Hg (OR, 1.20 [95% CI, 1.16-1.23]) (Table 3). When stratified by diabetes status, similar results were observed, although the association between UACR testing and blood pressure control to less than 130/80 mm Hg was no longer statistically significant for either nondiabetes or diabetes groups. Testing was associated with SGLT2i use in the nondiabetes group (OR, 11.55 [95% CI, 5.80-23.00]); this association was weaker but still statistically significant among the diabetes group (OR, 1.44 [95% CI, 1.32-1.57]).
Figure 2. Therapy Among Tested and Untested Patients by Quintile of Estimated Risk of Albuminuria (N = 149 554).
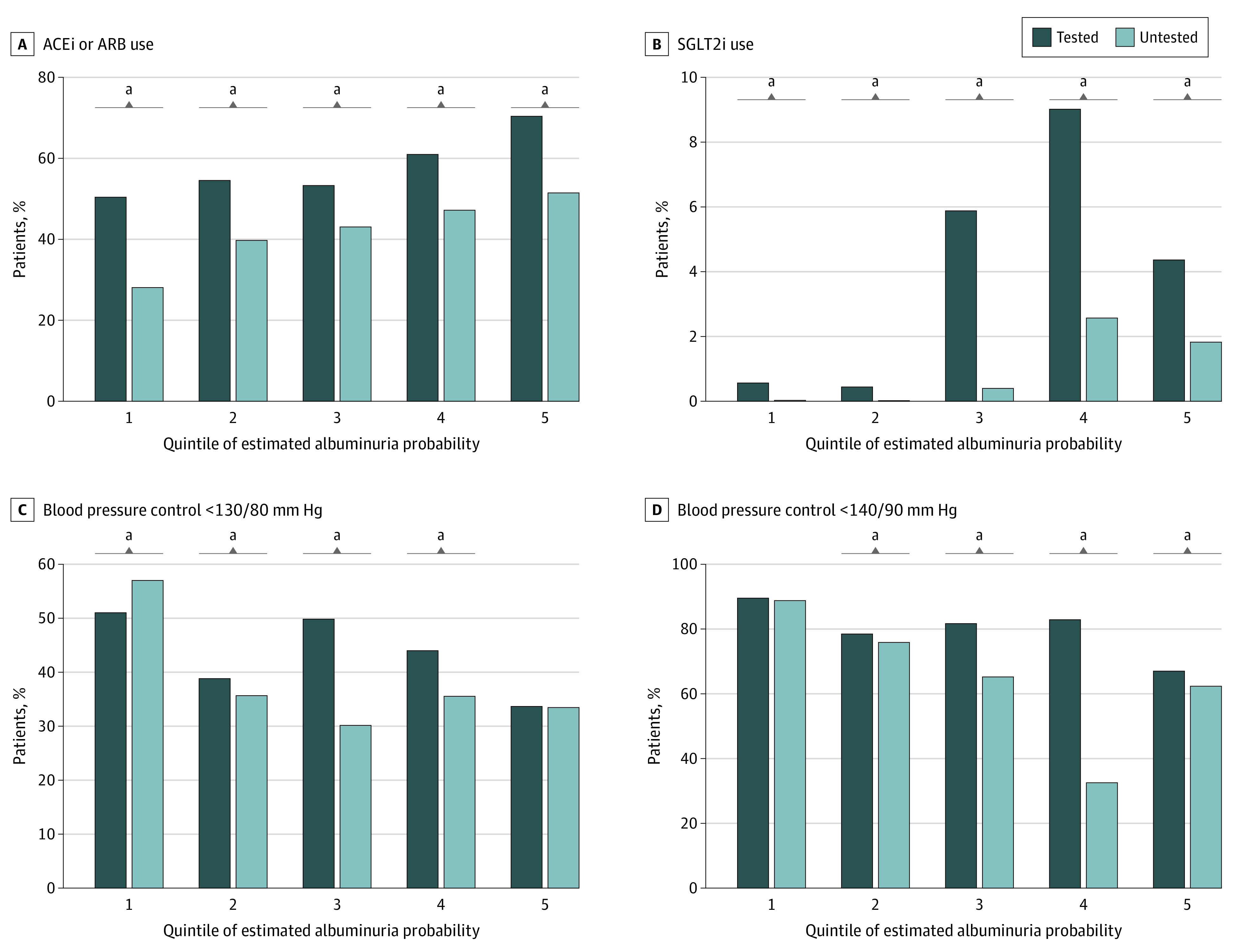
ACEi indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
aQuintile with a statistically significant difference between tested and untested groups. All comparisons of tested vs untested were significant to P < .001 except for the following: blood pressure less than 130/80 mm Hg for quintile 2 (P = .01), blood pressure less than 130/80 mm Hg for quintile 5 (P = .75), blood pressure less than 140/90 mm Hg for quintile 1 (P = .44), and blood pressure less than 140/90 mm Hg for quintile 2 (P = .02).
Table 3. Associations Between Urine Albumin-Creatinine Ratio Testing and Albuminuria Treatment (N = 149 554).
| Albuminuria therapy | Odds ratio (95% CI)a | P value |
|---|---|---|
| Overall | ||
| ACEi or ARB use | 2.39 (2.32-2.46) | <.001 |
| SGLT2i use | 8.22 (7.56-8.94) | <.001 |
| Blood pressure control <130/80 mm Hg | 1.07 (1.04-1.10) | <.001 |
| Blood pressure control <140/90 mm Hg | 1.20 (1.16-1.23) | <.001 |
| No diabetes | ||
| ACEi or ARB use | 2.00 (1.89-2.11) | <.001 |
| SGLT2i use | 11.55 (5.80-23.00) | <.001 |
| Blood pressure control <130/80 mm Hg | 0.98 (0.93-1.04) | .56 |
| Blood pressure control <140/90 mm Hg | 1.08 (1.02-1.15) | .01 |
| Diabetes | ||
| ACEi or ARB use | 1.76 (1.69-1.83) | <.001 |
| SGLT2i use | 1.44 (1.32-1.57) | <.001 |
| Blood pressure control <130/80 mm Hg | 1.02 (0.97-1.06) | .47 |
| Blood pressure control <140/90 mm Hg | 1.13 (1.08-1.19) | <.001 |
Abbreviations: ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; SGLT2i, sodium-glucose cotransporter 2 inhibitor.
All models are adjusted for age, sex, race/ethnicity, insurance type, smoking status, eGFR, heart failure, coronary artery disease, and prior stroke.
Sensitivity Analyses
When we derived an albuminuria estimation model using data from tested patients in the EHR instead of NHANES, the model was well-calibrated (eFigure 5 in Supplement 1). Using this model, we estimated that 23.2% (11525 of 49634) of persons with albuminuria had been detected by testing, and patients with hypertension only represented the group with the lowest detection rate of 5.7% (1742 of 30 522) (eTable 8 in Supplement 1).
Discussion
In this national cohort study of US adults at high risk for CKD, we observed that UACR testing was generally underutilized, with only about one-third of prevalent albuminuria having been detected via UACR testing. We found that the albuminuria detection rate was lowest for persons with hypertension and no diabetes, with only an estimated 10.4% receiving UACR testing among those with albuminuria in this subgroup. In adjusted analyses, lack of UACR testing was associated with lower odds of ACEi or ARB and SGLT2i utilization as well as lower rates of BP control. These results suggest that underutilization of UACR represents a major barrier to diagnosis of CKD and deployment of therapies to prevent CKD progression and the associated cardiovascular risk.
Consistent with prior studies, we found greater UACR testing among patients with diabetes compared with those with hypertension only.11,16 Higher UACR testing in persons with diabetes may relate to more consistent guideline recommendations and national quality metrics promoting annual albuminuria assessment for patients with diabetes.33,34 Even so, we found that only 52% of patients with diabetes had UACR testing, a figure similar to those reported in prior studies.14,15,16 Concerningly, although patients with diabetes and hypertension represented the group with the highest albuminuria detection fraction, we projected that only 62.1% had been detected, leaving a substantial fraction of patients with albuminuria who remained undetected by their clinicians. Given the crucial role of albuminuria for risk stratification and determining indicated treatments in CKD, our results reinforce the need for concerted efforts to increase the use of albuminuria testing for enhanced kidney and cardiovascular preventive care.
We found that patients with greater estimated risk of albuminuria were more likely to have UACR testing. Interestingly, this finding appeared to be driven by testing in patients without diabetes, as UACR testing among the study population with diabetes was fairly uniform with approximately 50% testing across estimated risk quintiles. Future work should investigate the reasons behind the variation in UACR testing patterns between patients with and without diabetes, as this could inform development of the optimal implementation strategies to improve testing and risk stratification in each population. Notably, patients with hypertension and no diabetes represented the group with the lowest testing rate (5.1%) and the lowest proportion of patients of patients with undetected albuminuria (10.4%). Given the low testing rate and the absolute size of the hypertension-only population, we estimated that approximately 70% of persons with undetected albuminuria were within this population. These results suggest that efforts to increase albuminuria testing should include hypertension as an indication for testing to best capture the population with currently undetected CKD.
We found that lack of UACR testing was associated with lower rates of ACEi or ARB use, SGLT2i use, and BP control. Due to residual confounding, we cannot conclude that UACR testing was directly driving care delivery. However, a prior study of a large integrated health system found that higher levels of albuminuria were associated with greater likelihood of ACEi or ARB initiation, supporting the concept that UACR testing does affect subsequent care.35
Strengths and Limitations
Strengths of our study included the large sample size and diverse study population. Our study had several limitations. First, the estimates for albuminuria prevalence and detection rates relied on estimated albuminuria, as measured albuminuria was not available for all patients in the EHR study population. However, with the model’s tendency to underestimate albuminuria risk when validated among the tested population, the resulting estimates for albuminuria prevalence are likely lower than the true values. Second, we did not examine alternative measures such as urine protein-creatinine ratio, 24-hour urine albumin or protein quantification, or urine dipstick protein. We focused on UACR testing because it is considered the criterion standard for urinary protein assessment, owing to laboratory assay standardization, validation in risk stratification, and role in guideline recommendations.36 In addition, EHR data are health system–specific and would not completely capture care in outside health systems. We examined associations between testing and guideline-recommended treatments but were unable to determine whether testing or test results clinically motivated these treatments; future studies could be designed prospectively to determine temporality of testing with treatment initiation. Another limitation was the substantial number of patients who were excluded due to missing eGFR for reasons that may be attributable to being a younger, healthier population. Additionally, we were unable to assess interventions focused on modifying health behaviors, such as smoking cessation or weight loss, which have been shown to improve albuminuria.37,38
Conclusions
In this national cohort study of US adults at risk for CKD, we estimated that approximately two-thirds of persons with albuminuria have not been identified by UACR testing. Early identification of albuminuria is increasingly crucial given the growing number of therapies, such as SGLT2i39,40,41,42 and nonsteroidal mineralocorticoid antagonists,43 that have been shown to slow the progression of CKD and prevent cardiovascular complications. More albuminuria testing is clearly needed among at-risk persons to ensure effective dissemination of these therapies and to fully realize their potential benefit.
eTable 1. Diagnosis Code List for Variable Definition
eTable 2. Characteristics of Included and Excluded Optum® EHR Study Population
eTable 3. Study Population Characteristics of Optum® EHR Patients by Diabetes Status
eTable 4. Population Characteristics of NHANES Participants with Hypertension or Diabetes, 2007-2018
eTable 5. Albuminuria Prevalence, Testing Rates, and Detection Rates
eTable 6. Albuminuria Prevalence, Testing, and Detection by Diabetes Status
eTable 7. Baseline Characteristics for Secondary Analysis Including Optum® EHR Patients Having ≥1 Year Follow-Up
eTable 8. Albuminuria Prevalence, Testing, and Detection, Using Optum® EHR-Derived Prediction Model
eFigure 1. Consort Diagram – Optum® EHR Study Population
eFigure 2. Consort Diagram – NHANES Study Population
eFigure 3. Calibration Plots for Albuminuria Prediction Model
eFigure 4. Estimated Albuminuria Prevalence and Proportion Detected by Diabetes Status (Optum® EHR; N = 192,108)
eFigure 5. Calibration Plot for Optum® Electronic Health Record Derived Albuminuria Prediction Model
Data Sharing Statement
References
- 1.Bikbov B, Purcell CA, Levey AS, et al. ; GBD Chronic Kidney Disease Collaboration . Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709-733. doi: 10.1016/S0140-6736(20)30045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Renal Data System . 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2020. [Google Scholar]
- 3.International Society of Nephrology, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Work Group . Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. Published 2013. Accessed June 26, 2023. https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
- 4.Matsushita K, van der Velde M, Astor BC, et al. ; Chronic Kidney Disease Prognosis Consortium . Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073-2081. doi: 10.1016/S0140-6736(10)60674-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astor BC, Matsushita K, Gansevoort RT, et al. ; Chronic Kidney Disease Prognosis Consortium . Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int. 2011;79(12):1331-1340. doi: 10.1038/ki.2010.550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsushita K, Coresh J, Sang Y, et al. ; CKD Prognosis Consortium . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3(7):514-525. doi: 10.1016/S2213-8587(15)00040-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemmelgarn BR, Manns BJ, Lloyd A, et al. ; Alberta Kidney Disease Network . Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423-429. doi: 10.1001/jama.2010.39 [DOI] [PubMed] [Google Scholar]
- 8.Hsu CY, Chinchilli VM, Coca S, et al. ; ASSESS-AKI Investigators . Post-Acute Kidney Injury Proteinuria and Subsequent Kidney Disease Progression: The Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Study. JAMA Intern Med. 2020;180(3):402-410. doi: 10.1001/jamainternmed.2019.6390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlipak MG, Tummalapalli SL, Boulware LE, et al. ; Conference Participants . The case for early identification and intervention of chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2021;99(1):34-47. doi: 10.1016/j.kint.2020.10.012 [DOI] [PubMed] [Google Scholar]
- 10.Alfego D, Ennis J, Gillespie B, et al. Chronic kidney disease testing among at-risk adults in the U.S. remains low: real-world evidence from a national laboratory database. Diabetes Care. 2021;44(9):2025-2032. doi: 10.2337/dc21-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin JI, Chang AR, Grams ME, et al. ; CKD Prognosis Consortium . Albuminuria testing in hypertension and diabetes: an individual-participant data meta-analysis in a global consortium. Hypertension. 2021;78(4):1042-1052. doi: 10.1161/HYPERTENSIONAHA.121.17323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stempniewicz N, Vassalotti JA, Cuddeback JK, et al. Chronic kidney disease testing among primary care patients with type 2 diabetes across 24 U.S. health care organizations. Diabetes Care. 2021;44(9):2000-2009. doi: 10.2337/dc20-2715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Diabetes Association . Summary of Revisions: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(suppl 1):S4-S6. doi: 10.2337/dc21-Srev [DOI] [PubMed] [Google Scholar]
- 14.Navaneethan SD, Akeroyd JM, Ramsey D, et al. Facility-level variations in kidney disease care among veterans with diabetes and CKD. Clin J Am Soc Nephrol. 2018;13(12):1842-1850. doi: 10.2215/CJN.03830318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J, Chu C, Guzman D, et al. Albuminuria testing by race and ethnicity among patients with hypertension with and without diabetes. Am J Nephrol. 2019;50(1):48-54. doi: 10.1159/000500706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu CD, Powe NR, McCulloch CE, et al. ; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Trends in chronic kidney disease care in the US by race and ethnicity, 2012-2019. JAMA Netw Open. 2021;4(9):e2127014. doi: 10.1001/jamanetworkopen.2021.27014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy DP, Drawz PE, Foley RN. Trends in angiotensin-converting enzyme inhibitor and angiotensin II receptor blocker use among those with impaired kidney function in the United States. J Am Soc Nephrol. 2019;30(7):1314-1321. doi: 10.1681/ASN.2018100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu CD, Powe NR, McCulloch CE, et al. ; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team . Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use among hypertensive US adults with albuminuria. Hypertension. 2021;77(1):94-102. doi: 10.1161/HYPERTENSIONAHA.120.16281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCoy IE, Han J, Montez-Rath ME, Chertow GM. Barriers to ACEI/ARB use in proteinuric chronic kidney disease: an observational study. Mayo Clin Proc. 2021;96(8):2114-2122. doi: 10.1016/j.mayocp.2020.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamprea-Montealegre JA, Madden E, Tummalapalli SL, et al. Prescription patterns of cardiovascular- and kidney-protective therapies among patients with type 2 diabetes and chronic kidney disease. Diabetes Care. 2022;45(12):2900-2906. doi: 10.2337/dc22-0614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheung AK, Chang TI, Cushman WC, et al. ; Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group . KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99(3S):S1-S87. doi: 10.1016/j.kint.2020.11.003 [DOI] [PubMed] [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115. doi: 10.1161/HYP.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 23.Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022;102(5S):S1-S127. doi: 10.1016/j.kint.2022.06.008 [DOI] [PubMed] [Google Scholar]
- 24.Mazhar F, Sjölander A, Fu EL, et al. Estimating the prevalence of chronic kidney disease while accounting for non-random testing with inverse probability weighting. Kidney Int. Published November 30, 2022. doi: 10.1016/j.kint.2022.10.027 [DOI] [PubMed] [Google Scholar]
- 25.Shahinian VB, Hedgeman E, Gillespie BW, et al. ; CDC CKD Surveillance System . Estimating prevalence of CKD stages 3-5 using health system data. Am J Kidney Dis. 2013;61(6):930-938. doi: 10.1053/j.ajkd.2013.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention (CDC) . National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Data. Accessed June 26, 2023. https://wwwn.cdc.gov/nchs/nhanes/
- 27.Crim MT, Yoon SS, Ortiz E, et al. National surveillance definitions for hypertension prevalence and control among adults. Circ Cardiovasc Qual Outcomes. 2012;5(3):343-351. doi: 10.1161/CIRCOUTCOMES.111.963439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.International Expert Committee . International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32(7):1327-1334. doi: 10.2337/dc09-9033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newton KM, Peissig PL, Kho AN, et al. Validation of electronic medical record-based phenotyping algorithms: results and lessons learned from the eMERGE network. J Am Med Inform Assoc. 2013;20(e1):e147-e154. doi: 10.1136/amiajnl-2012-000896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Center for Health Statistics . National Health and Nutrition Examination Survey 1988-2018 Data Documentation, Codebook, and Frequencies. Accessed April 14, 2020. https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/RXQ_DRUG.htm
- 31.Inker LA, Eneanya ND, Coresh J, et al. ; Chronic Kidney Disease Epidemiology Collaboration . New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749. doi: 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 [DOI] [PubMed] [Google Scholar]
- 33.American Diabetes Association . 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2019. Diabetes Care. 2019;42(suppl 1):S124-S138. doi: 10.2337/dc19-S011 [DOI] [PubMed] [Google Scholar]
- 34.National Committee for Quality Assurance . HEDIS. NCQA. Accessed February 2, 2020. https://www.ncqa.org/hedis/
- 35.Qiao Y, Shin JI, Chen TK, et al. Association of albuminuria levels with the prescription of renin-angiotensin system blockade. Hypertension. 2020;76(6):1762-1768. doi: 10.1161/HYPERTENSIONAHA.120.15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sumida K, Nadkarni GN, Grams ME, et al. ; Chronic Kidney Disease Prognosis Consortium . Conversion of urine protein-creatinine ratio or urine dipstick protein to urine albumin-creatinine ratio for use in chronic kidney disease screening and prognosis: an individual participant-based meta-analysis. Ann Intern Med. 2020;173(6):426-435. doi: 10.7326/M20-0529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schrauben SJ, Apple BJ, Chang AR. Modifiable lifestyle behaviors and CKD progression: a narrative review. Kidney360. 2022;3(4):752-778. doi: 10.34067/KID.0003122021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roehm B, Simoni J, Pruszynski J, Wesson DE. Cigarette smoking attenuates kidney protection by angiotensin-converting enzyme inhibition in nondiabetic chronic kidney disease. Am J Nephrol. 2017;46(4):260-267. doi: 10.1159/000481206 [DOI] [PubMed] [Google Scholar]
- 39.Perkovic V, Jardine MJ, Neal B, et al. ; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi: 10.1056/NEJMoa1811744 [DOI] [PubMed] [Google Scholar]
- 40.Neal B, Perkovic V, Mahaffey KW, et al. ; CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644-657. doi: 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 41.Wanner C, Inzucchi SE, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334. doi: 10.1056/NEJMoa1515920 [DOI] [PubMed] [Google Scholar]
- 42.Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. ; DAPA-CKD Trial Committees and Investigators . Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383(15):1436-1446. doi: 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 43.Bakris GL, Agarwal R, Anker SD, et al. ; FIDELIO-DKD Investigators . Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383(23):2219-2229. doi: 10.1056/NEJMoa2025845 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Diagnosis Code List for Variable Definition
eTable 2. Characteristics of Included and Excluded Optum® EHR Study Population
eTable 3. Study Population Characteristics of Optum® EHR Patients by Diabetes Status
eTable 4. Population Characteristics of NHANES Participants with Hypertension or Diabetes, 2007-2018
eTable 5. Albuminuria Prevalence, Testing Rates, and Detection Rates
eTable 6. Albuminuria Prevalence, Testing, and Detection by Diabetes Status
eTable 7. Baseline Characteristics for Secondary Analysis Including Optum® EHR Patients Having ≥1 Year Follow-Up
eTable 8. Albuminuria Prevalence, Testing, and Detection, Using Optum® EHR-Derived Prediction Model
eFigure 1. Consort Diagram – Optum® EHR Study Population
eFigure 2. Consort Diagram – NHANES Study Population
eFigure 3. Calibration Plots for Albuminuria Prediction Model
eFigure 4. Estimated Albuminuria Prevalence and Proportion Detected by Diabetes Status (Optum® EHR; N = 192,108)
eFigure 5. Calibration Plot for Optum® Electronic Health Record Derived Albuminuria Prediction Model
Data Sharing Statement


