Abstract
The existence of thousands of per- and polyfluoroalkyl substances (PFAS) and evidence that some cause adverse health effects has created immense need to better understand PFAS toxicity and to move beyond one-chemical-at-a-time approaches to hazard assessment for this chemical class. The zebrafish model enables rapid assessment of large libraries of PFAS, powerful comparison of compounds in a single in vivo system, and evaluation across life stages and generations, and has led to significant advances in PFAS research in recent years. The focus of this review is to assess contemporary findings regarding PFAS toxicokinetics, toxicity and apical adverse health outcomes, and potential modes of action using the zebrafish model. Much of the peer-reviewed literature has focused on a small subset of PFAS structural subclasses, such as the perfluoroalkyl sulfonic acids and perfluoroalkyl carboxylic acids. However, recent data on more diverse PFAS structures are enabling prioritization of compounds of concern. Structure-activity comparisons and the utilization of modeling and ‘omics technologies in zebrafish have greatly contributed to our understanding of the hazard potential for a growing number of PFAS and will surely inform our understanding and predictive capabilities for many more PFAS in the future.
Keywords: PFAS, developmental toxicity, toxicokinetics, mode of action, mechanisms
Per- and polyfluoroalkyl substances (PFAS) are a broad class of anthropogenic chemicals defined by carbon-hydrogen bonds replaced in part (polyfluorinated) or in full (perfluorinated) by carbon-fluorine bonds (Buck et al., 2011). More specifically, PFAS are any fluorinated substances consisting of at least one fully fluorinated methyl (-CF3) or methylene (-CF2-) group (Wang et al., 2021). The strength of the C-F bond imparts high chemical stability and oleophobic and hydrophobic properties that are highly beneficial for many industrial, manufacturing, and consumer product uses. Most recognizably, PFAS have been used as components in aqueous film-forming fire-fighting foams (AFFFs) and in coatings for textiles, cookware and food packaging; however, there are numerous other applications related to aerospace, electronics, the energy sector, and more (Gluge et al., 2020). The C-F bond is highly persistent in the environment, resisting degradation (Buck et al., 2011). Thus, PFAS are detected globally in drinking, surface, and groundwater (Kurwadkar et al., 2022), food (Domingo and Nadal, 2017; Pasecnaja et al., 2022), biota, and in human tissues (De Silva et al., 2021; Jian et al., 2018). The ubiquity of human exposure to PFAS paired with evidence that some cause adverse health effects has raised public concern internationally regarding the toxicity and continued use of this chemical class (Cousins et al., 2020).
A significant amount of toxicological data is available for a select few PFAS, such as perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS). PFOA and PFOS are 2 legacy PFAS (ie, long-chain perfluoroalkyl acids that have been largely phased out of production in many developed countries due to growing evidence of persistence and health effects) with 7- and 8-fully-fluorinated-carbon chains, respectively. They induce a range of adverse health effects, such as low birth weights, liver, kidney, and thyroid disease, and immune dysfunction, evidenced by both epidemiological and animal studies (Fenton et al., 2021). Widespread public and government concern prompted major PFAS manufacturers in the United States (U.S.) to initiate phase-outs of PFOS between 2000 and 2002 and of PFOA between 2006 and 2015 (USEPA, 2022b). Such phase-outs have generally decreased the concentrations of these long-chain PFAS in human biomatrices, most often measured in blood and serum (Land et al., 2018). However, declining concentrations over the past 2 decades are not universal, depending on chemical and region (Fan et al., 2022), and organ-specific trends are not as well-understood. Additionally, there is evidence of steady or increasing concentrations of legacy PFAS in biota, with implications for human dietary exposure (Barbo et al., 2023; Schultes et al., 2020). Phase outs have also led to the adoption of alternative, often shorter-chain PFAS typically with limited toxicity data (Ateia et al., 2019; Brendel et al., 2018). The U.S. Environmental Protection Agency (EPA) recently announced proposed drinking water regulation for 6 PFAS (ie, PFOS, PFOA, perfluorohexane sulfonic acid [PFHxS], perfluorobutane sulfonic acid [PFBS], perfluorononanoic acid [PFNA], and GenX) (USEPA, 2023), and total PFAS concentrations in drinking water are regulated in the European Union (EU, 2022). While perfluoroalkyl carboxylic acids (PFCAs; eg, PFOA) and sulfonic acids (PFSAs; eg, PFOS) have been the most frequently studied and have the most evidence to support such regulation, PFAS are a diverse chemical class with many emerging contaminants (Table 1). A more complete scientific understanding of PFAS toxicity will not happen without comprehensively examining a much broader representation of PFAS structural diversity.
Table 1.
PFAS subclasses and structures
| Subclass | Representative Structure | Compounds Discussed in This Review |
|---|---|---|
| Perfluoroalkyl Carboxylic Acids (PFCAs) |
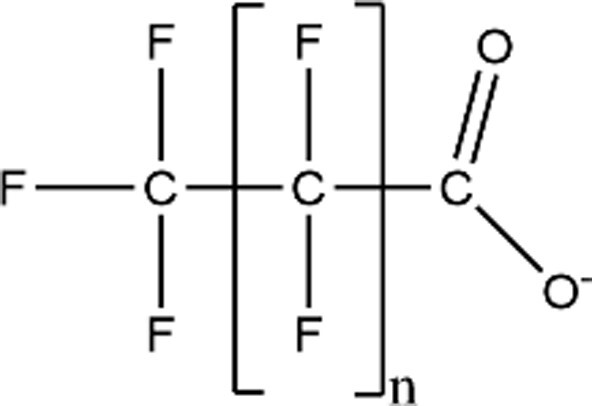
|
PFBA (n = 2; CAS: 375-22-4) |
| PFPeA (n = 3; CAS: 2706-90-3) | ||
| PFHxA (n = 4; CAS: 307-24-4) | ||
| PFHpA (n = 5; CAS: 375-85-9) | ||
| PFOA (n = 6; CAS: 335-67-1) | ||
| PFNA (n = 7; CAS: 375-95-1) | ||
| PFDA (n = 8; CAS: 335-76-2) | ||
| PFUnDA (n = 9; CAS: 2058-94-8) | ||
| PFDoA (n = 10; CAS: 307-55-1) | ||
| Perfluoroalkyl Sulfonic Acids (PFSAs) |
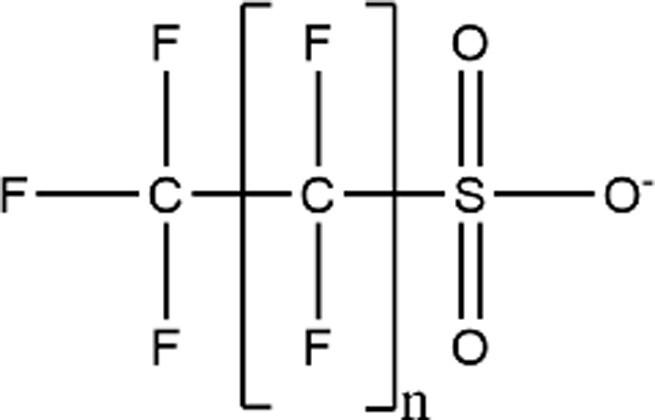
|
PFHxS (n = 5; CAS: 355-46-4) |
| PFOS (n = 7; CAS: 1763-23-1) | ||
| Cl-PFOSa (CAS: not available) | ||
| Per/poly-fluoroethers Carboxylic Acids (PFECAs) |
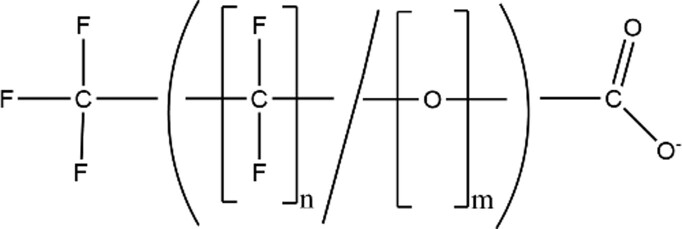
|
GenXa (CAS: 13252-13-6) |
| PFMOBA (n = 5, m = 1; CAS: 863090-89-5) | ||
| PFDMMOBAa (CAS: 801212-59-9) | ||
| PFO2HpA (n = 3, m = 2; CAS: 151772-58-6) | ||
| ADONAa (CAS: 958445-44-8) | ||
| PFO3TDA (n = 8, m = 3; CAS: 330562-41-9) | ||
| PFO3DA (n = 5, m = 3; CAS: 151772-59-7) | ||
| PFO3OA (n = 3, m = 3; CAS: 39492-89-2) | ||
| PFO4DA (n = 4, m = 4; CAS: 39492-90-5) | ||
| PFO5DoDA (n = 5, m = 5; CAS: 39492-91-6) | ||
| Per/poly-fluoroether Sulfonic Acids (PFESAs) |
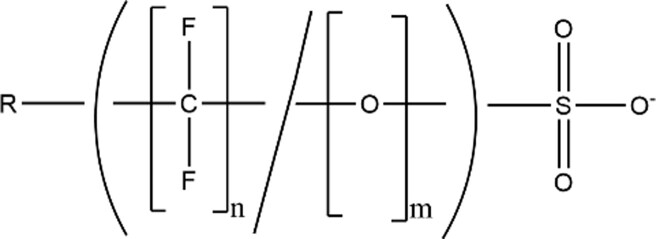
|
6:2 Cl-PFESA (n = 8, m = 1, R = Cl; CAS: 73606-19-6) |
| 8:2 Cl-PFESA (n = 10, m = 1, R = Cl; CAS: 83329-89-9) | ||
| PFESA 1a (Nafion Byproduct 1; CAS: 29311-67-9) | ||
| Perfluoroalkyl Sulfonyl Fluoride |
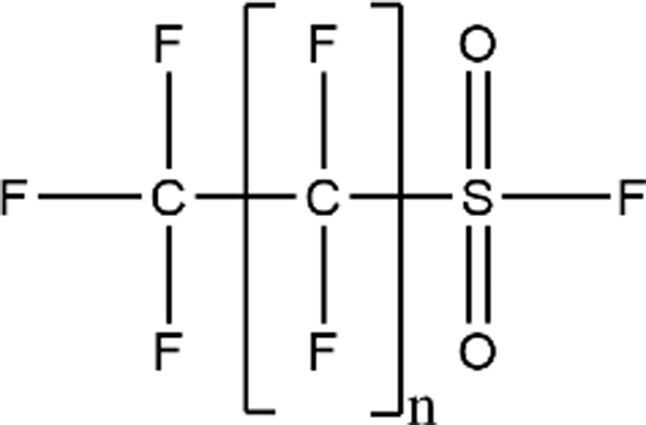
|
PFOS-F (n = 7; CAS: 307-35-7) |
| Sulfonamides (FASAs) |
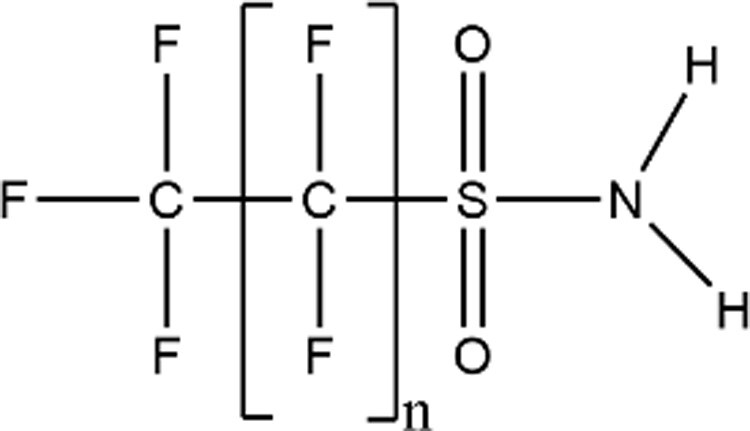
|
FBSA (n = 3; CAS: 30334-69-1) |
| FOSA (n = 7; CAS: 754-91-6) | ||
| Perfluoroalkyl Phosphinic Acids (PFPiAs) |
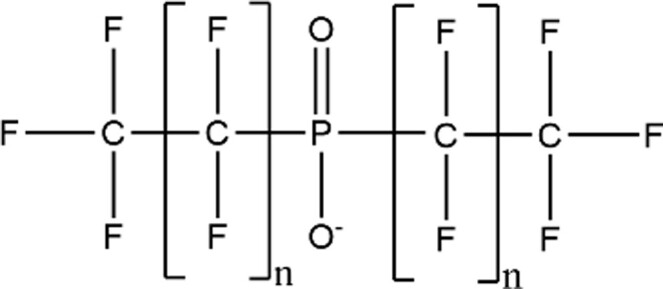
|
8:8 PFPiA (n = 7; CAS: 500776-69-2) |
| Perfluoroalkyl Phosphonic Acids |
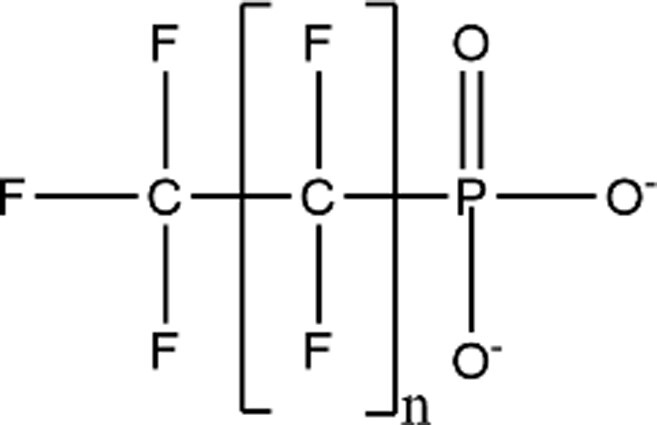
|
– |
| Perfluoroalkyl Carboxamides |
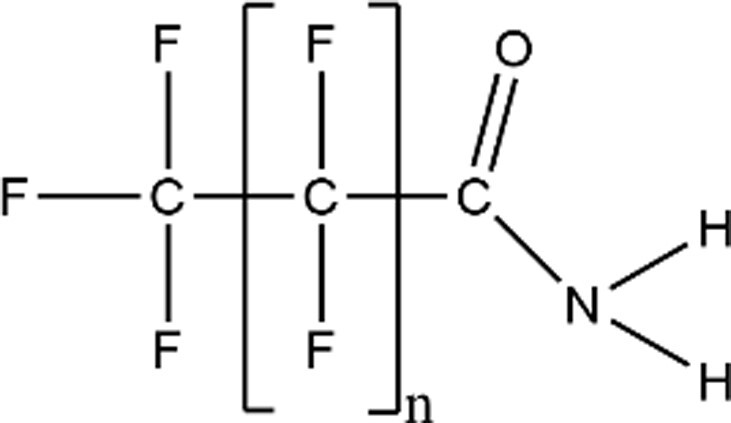
|
– |
| Fluorotelomer Sulfonic Acids (FTSAs) |
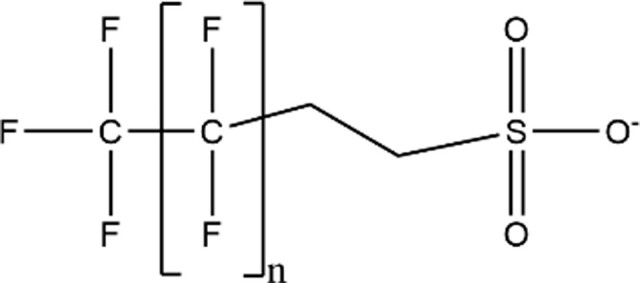
|
4:2 FTSA (n = 3; CAS: 757124-72-4) |
| 6:2 FTSA (n = 5; CAS: 27619-97-2) | ||
| Fluorotelomer Sulfonamide (FTSAm) |
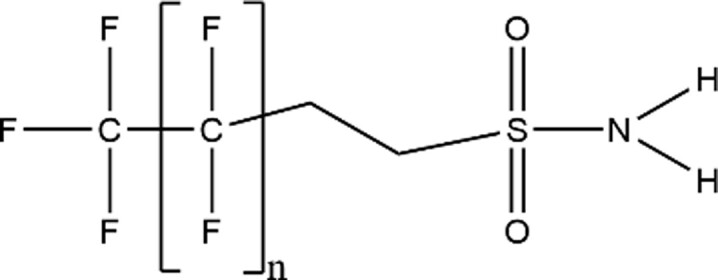
|
6:2 FTSAm (n = 5; CAS: not provided) |
| Fluorotelomer Sulfonamide Alkylbetaine (FTAB) |
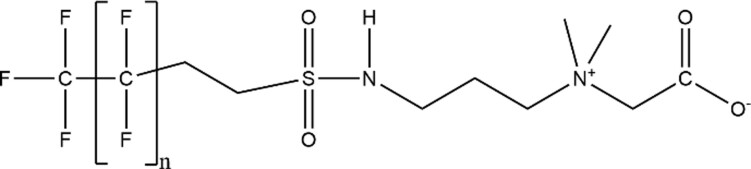
|
6:2 FTAB (n = 5; CAS: 34455-29-3) |
| Fluorotelomer Sulfonamide Alkylamine (FTAA) |
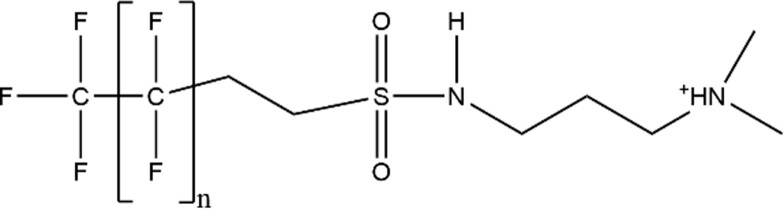
|
6:2 FTAA (n = 5; CAS: 34455–22–6) |
| Fluorotelomer Alochol (FTOHs) |
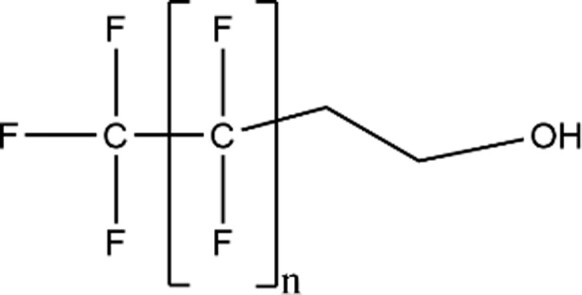
|
6:2 FTOH (n = 5; CAS: 647-42-7) |
| Phosphate Esters |
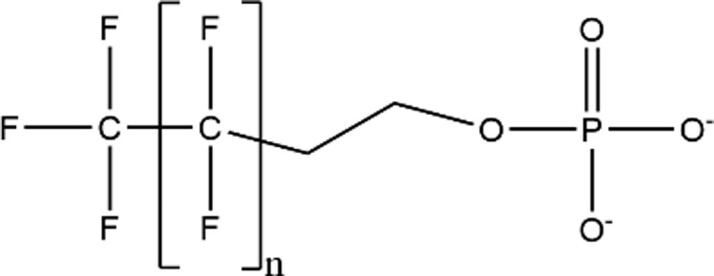
|
– |
| Carboxyesters |
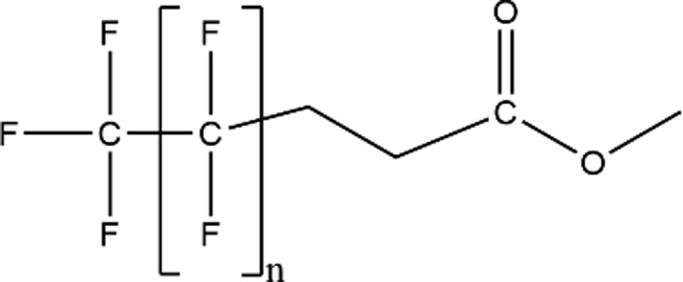
|
– |
| Other |
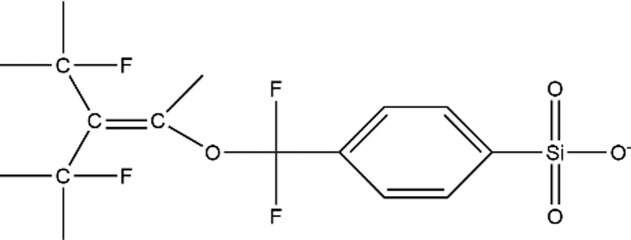
|
OBS (CAS: 70829-87-7) |
Specified structures and CAS numbers are representative. Additional CAS numbers for a given PFAS (eg, for a salt form of the compound) may have been used in the referenced studies.
Not represented by representative structure due to additional R group or branching.
The PFAS chemical class consists of thousands of compounds. In 2018, the Organization for Economic Co-operation and Development (OECD) identified 4730 PFAS with unique CAS numbers (OECD, 2018), and more than 12 000 PFAS structures have been identified by the U.S. EPA (USEPA, 2022a). A one-chemical-at-a-time approach to all PFAS toxicity assessment and regulation based on mammalian testing is not feasible, given the low-throughput nature of this testing and the large number of PFAS without toxicity data. In vitro and in silico models are increasingly important but are limited in biological complexity. Therefore, we need an in vivo model capable of assessing PFAS for potential human health hazards. The zebrafish is a widely used alternative model that can rapidly assess the toxicity of these chemicals. The zebrafish is robust and can bridge the gap between in vitro and higher vertebrate models; it is amenable to high-throughput developmental toxicity testing and intergenerational assessments. Embryos develop rapidly and transparently, are genetically tractable, and manifest simple assayable behaviors as early as 24 h post fertilization (hpf). Thus, zebrafish can be leveraged as a whole animal biosensor, to quickly rank PFAS hazard potential, prioritize compounds of high concern for additional testing, and characterize toxicity. Assessing a broad diversity of PFAS in a single system offers a powerful platform that eliminates the challenges of cross-species extrapolation and enables direct comparisons between compounds. This review addresses PFAS structure-activity relationships pertaining to toxicokinetics and toxicity, apical adverse health outcomes, and potential modes of action using the zebrafish model. Throughout this review, all waterborne exposure concentrations have been converted to molar units to facilitate comparisons between studies (Supplementary Table 1). To facilitate readability, chemical abbreviations are used throughout the review, and full chemical names can be found in Table 2.
Table 2.
Chemical abbreviations
| 4:2 FTSA—4:2 fluorotelomer sulfonic acid |
| 6:2 Cl-PFESA—perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) |
| 6:2 FTAA—fluorotelomer sulfonamide alkylamine |
| 6:2 FTAB—fluorotelomer sulfonamide alkylbetaine |
| 6:2 FTSA—6:2 fluorotelomer sulfonic acid |
| 6:2 FTSAm—6:2 fluorotelomer sulfonamide |
| 8:2 Cl-PFESA—2-[(8-Chloro-1,1,2,2,3,3,4,4,5,5,6,6,7,7,8,8-hexadecafluorooctyl)oxy]-1,1,2,2-tetrafluoroethanesulfonic acid |
| 8:8 PFPiA—8:8 perfluoroalkyl phosphinic acid |
| ADONA—4,8-dioxa-3H-perfluorononanoate |
| Cl-PFOS—1-chloro-perfluorooctane sulfonic acid |
| F-53B—perfluoro(2-((6-chlorohexyl)oxy)ethanesulfonic acid) |
| FBSA—perfluorobutane sulfonamide |
| FOSA—perfluorooctane sulfonamide |
| GenX—perfluoro-2-methyl-3-oxahexanoic acid |
| OBS—perfluorous nonenoxybenzenesulfonate |
| PFBS—perfluorobutane sulfonic acid |
| PFDA—perfluorodecanoic acid |
| PFDMMOBA—4-(heptafluoroisopropoxy)hexafluorobutanoic acid |
| PFDoA—perfluorododecanoic acid |
| PFESA 1—Perfluoro-3,6-dioxa-4-methyl-7-octene-1-sulfonic acid |
| PFHxA—perfluorohexanoic acid |
| PFHxS—perfluorohexane sulfonic acid |
| PFMOBA—perfluoro (4-methoxy butanoic) acid |
| PFNA—perfluorononanoic acid |
| PFO2HpA—perfluoro-3,6-dioxaheptanoic acid |
| PFO3DA—perfluoro-3,6,9-trioxadecanoic acid |
| PFO3OA—perfluoro (3,5,7-trioxaoctanoic) acid |
| PFO3TDA—perfluoro-3,6,9-trioxatridecanoic acid |
| PFO4DA—perfluoro (3,5,7,9-tetraoxadecanoic) acid |
| PFO5DoDA—perfluoro (3,5,7,9,11-pentaoxadodecanoic) acid |
| PFOA—perfluorooctanoic acid |
| PFOS—perfluorooctane sulfonic acid |
| PFOS-F—perfluorooctanesulfonyl fluoride |
| PFPeA—perfluoropentanoic acid |
Exposure and toxicokinetics
The trope that all PFAS are “forever chemicals” stems from the incredible strength of the C-F bond. Perfluoroalkyl acids, like PFOS and PFOA, are highly resistant to degradation and metabolism, and considered terminal degradation products (Buck et al., 2011). Half-lives in humans exposed to highly contaminated drinking water have recently been estimated between 1.05–3.4 years for PFOS and 1.77–3.9 years for PFOA, typically measured in serum (Li et al., 2018; Worley et al., 2017; Xu et al., 2020; Li et al., 2019). However, growing evidence shows that resistance to degradation in the environment and metabolism in vivo is not characteristic of all PFAS; many compounds are considered precursors that may be broken down into terminal products. A better understanding of PFAS absorption, distribution, metabolism, and excretion (ADME) is essential to understand the hazards posed by this chemical class (Tal and Vogs, 2021) and has been the focus of numerous recent studies.
Developmental zebrafish
Bioconcentration
Recent studies in developmental zebrafish have related structural features to bioconcentration factor (BCF), a cumulative measure of the competing uptake and elimination rates that encompasses biotransformation of the parent compound (Arnot and Gobas, 2006). Developmental zebrafish exposure studies addressed in this and following sections typically occur aqueously from 1 to 5 days post fertilization (dpf), a window that encompasses key events such as neurogenesis, cardiovascular, hepatic (3 dpf) and gill development (Villeneuve et al., 2014). The dynamic nature of this life stage is beneficial for interrogation of chemical perturbation and is important to consider when interpreting developmental exposure data.
Among PFAS with similar chain length, the highest bioconcentration potential is ranked as follows: compounds with sulfonamide head groups (Han et al., 2021; Rericha et al., 2022a) > sulfonic acids > fluorotelomer sulfonic acids (Menger et al., 2020; Rericha et al., 2022a) > carboxylic acids (Gaballah et al., 2020; Han et al., 2021; Menger et al., 2020; Rericha et al., 2022a; Vogs et al., 2019) and emerging fluoroethers, such as GenX (Gaballah et al., 2020; Satbhai et al., 2022). For instance, embryonic exposure to a mixture of 9 PFAS (2–3.8 µM of each) yielded BCF values for PFHxS, 6:2 FTSA, and PFPeA of 100, 35, and 0.9, respectively (Menger et al., 2020). For PFAS with the same head group, bioconcentration increased with increasing fluorinated chain length (Gaballah et al., 2020; Han et al., 2021; Menger et al., 2020). For those with carboxylic acid groups, BCF values of 0.9, 3.0, 18, 100, and 610 were observed for the 4-fluorinated-carbon compound (PFPeA) through the 8-fluorinated-carbon (PFNA) (Menger et al., 2020). Similarly, sulfonic acid PFAS exhibited BCF values of 230 and 2600 for the 6- and 8-fluorinated-carbon compounds PFHxS and PFOS, respectively, following approximately 0.8 µM exposures (Vogs et al., 2019). GenX was consistently reported to have a low BCF relative to other PFAS and was rapidly eliminated (Gaballah et al., 2020; Satbhai et al., 2022).
Interestingly, an inverse relationship between BCF and exposure concentration across a variety of PFAS has recurred across studies (Gaballah et al., 2020; Han et al., 2021; Satbhai et al., 2022; Vogs et al., 2019). For example, PFOS exhibited a BCF of 1348 at 1.8 µM, but a BCF of 684 at 3.1 µM (Gaballah et al., 2020). This trend may be attributed to the saturation of substrate binding sites that facilitate uptake. Considering other properties, no trends have been identified between PFAS BCF and L-FABP (liver-fatty acid binding protein) binding affinity, with only a weak positive association to Log Kow. Instead, retention time in an HPLC C18 analytical column, which approximated hydrophobicity, positively correlated with BCF (Han et al., 2021). While exposure paradigms varied somewhat between studies, BCF trends related to carboxylic acid or sulfonic acid head group and fluorinated chain length largely agree.
In addition to PFCAs and PFSAs, lesser-studied emerging alternatives, such as perfluoropolyether carboxylic acids (PFECAs) also bioconcentrate. Internal PFECA concentrations increased with an increasing number of OCF2 moieties. PFO5DoDA, with 5 OCF2 moieties and a total of 6 fluorinated carbons, bioconcentrated more readily than homologs with 4 or 3 ether moieties and more than PFOA after exposure to nominal concentrations of 90, 106, 128, and 97 µM, respectively (Wang et al., 2020). Han et al. also reported another PFECA, PFO3TDA, as particularly bioaccumulative (Han et al., 2021). Relatively high BCF values for PFECAs suggest some polyether alternatives may not be less bioaccumulative than legacy PFAS. Functional head group type, chain length, and ether moieties clearly potentiate bioconcentration.
Uptake, elimination, and metabolism
Similar to BCF trends, PFSAs (eg, PFHxS and PFOS) induced higher uptake rates than PFCAs (eg, PFBA and PFOA), up to approximately 10 times higher (Vogs et al., 2019). Following exposures to PFOS, PFHxS, and PFOA, but not the shorter-chain PFBA, uptake was biphasic with slower initial uptake that increased after hatching (48 hpf) from the chorion, a protective acellular envelope. Some studies remove the zebrafish chorion prior to exposure, whereas others leave it intact. Vogs et al. concluded that a 2-compartment toxicokinetic model was the best fit, accounting for the chorion as a significant barrier that led to slower initial perfluoroalkyl acid uptake, particularly for higher concentrations. Uptake rates were more dependent on carbon chain length and functional head group than were elimination rates (Vogs et al., 2019). Comparing toxicokinetics of PFOS and its alternative OBS after approximately 0.02 µM exposures from 72 to 120 hpf, uptake rate constants were similar whereas the elimination rate constant was lower for PFOS, leading to a higher PFOS BCF value (Zou et al., 2021). Metabolism also plays a key role in bioconcentration. Studies largely support a lack of larval metabolism of perfluoroalkyl acids, like PFOS and PFOA (Vogs et al., 2019), but rapid interrogation of in vivo metabolism of a 74 PFAS library has demonstrated metabolism of other diverse PFAS structures (Han et al., 2021). Hydrolysis is a major metabolism pathway particularly for PFAS with carboxamide, sulfonamide, and carboxyester functional groups. Perfluoroalkyl carboxamides were the most rapidly metabolized into terminal PFCAs. Fluorotelomer alcohols (FTOHs) with 1–2 hydrocarbons were predominately metabolized via β-oxidation and Phase II reactions, whereas those with more than 2 hydrocarbons were mostly metabolized via taurine conjugation (Han et al., 2021). Metabolism of numerous structurally diverse PFAS into a variety of transformation products highlights the need for additional studies of this nature and magnitude, along with reverse toxicokinetic models to apply findings to human risk assessment (Tal and Vogs, 2021).
Adult zebrafish
Adult zebrafish are more amenable to the measurement of ADME endpoints, such as tissue distribution, given their larger size and fully developed organ systems. As in developing zebrafish, PFAS uptake rates in adults increased with chain length and were greater for PFSAs than PFCAs (Wen et al., 2019), whereas elimination rate constants were less affected by these structural features (Wen et al., 2017). Uptake rates for PFCAs and PFSAs were highest in the liver and blood, followed by gill, ovary, then brain and muscle, with similar findings for tissue concentrations and BCF values (Wen et al., 2017). Longer-chain compounds accumulated to a greater extent in the blood, and shorter-chains in the liver (Wen et al., 2019). Short-chain PFCAs and PFSAs (mixture of 5 at 0.027–0.047 µM each) reached a steady state in approximately 5 days, whereas co-exposure with long-chains (mixture with 6 at 0.016–0.024 µM each), delayed the short-chain steady state to 14–21 days, which suggests that long-chain PFAS compete for transporters and binding sites (Wen et al., 2017).
Other lesser studied PFAS, 6:2 FTAB and 6:2 FTAA, exhibited differences in ADME in adult zebrafish exposed to 65% and 35% mixtures (0.057/0.034 µM and 0.57/0.34 µM, respectively) for 180 days (Shi et al., 2019). 6:2 FTAB was undetectable in adult tissue and offspring. 6:2 FTAB was rapidly metabolized into 6:2 FTAA, which accumulated in a sex-dependent manner and composed approximately 92% of all quantified PFAS. Metabolic products of 6:2 FTAA, 6:2 FTSAm, and 6:2 FTSA, contributed to 2.8–8.5% of quantified PFAS. Concentration-dependent maternal transfer of chemical to embryos occurred following 6:2 FTAA exposure, and concentrations in F1 embryos of parent compound and metabolites combined were as high as 25% that in F0 adult tissues (Shi et al., 2019). Earlier studies reported maternal transfer rates of 10% following PFOS exposure (Sharpe et al., 2010). Recent adult zebrafish studies were not sufficiently numerous to establish trends in BCF, but did provide evidence of tissue-specificity, metabolism, and maternal transfer.
Toxicity: adverse apical outcomes
Developmental zebrafish
PFOS has been the most often studied PFAS in zebrafish developmental studies, with extensive evidence that it elicits adverse apical outcomes. PFOS increased mortality in zebrafish by 5 dpf after developmental exposure as low as 14 µM (Martinez et al., 2019) and caused 100% mortality by 5.5 dpf (9.3 µM) (Shi et al., 2008). When considering all affected morphological features, a median benchmark dose lower confidence limit (BMDL) was 4.7 µM at 5 dpf (Martinez et al., 2019). Spinal deformities, altered head-trunk angles, and body axis curvature were among the most pronounced malformations and have been observed from PFOS exposures as low as 1 µM (Shi et al., 2008), 1.9 µM (Martinez et al., 2019), and 20 µM (Lee et al., 2021). Other common morphological effects of developmental exposure include decreased body length (Jantzen et al., 2016a; Martinez et al., 2019; Wu et al., 2022) or relative liver size (Wang et al., 2022), reduction in swim bladder inflation (Martinez et al., 2019; Shi et al., 2008), yolk sac edema (Lee et al., 2021; Martinez et al., 2019; Shi et al., 2008), pericardial edema (Lee et al., 2021), reduced eye-snout distance (Martinez et al., 2019), epiboly deformities (Shi et al., 2008), and altered heart rate (Shi et al., 2008). While some noted normal hatch rates after developmental exposure to PFOS up to 186 µM (Martinez et al., 2019), others noted dose-dependent lower hatch rates and hatch delay relative to controls (Shi et al., 2008). One study reported no behavior changes associated with 32 µM PFOS exposures from 1 to 5 or 1 to 15 dpf (Sant et al., 2021), whereas others demonstrated an array of photomotor behavior effects. Within the range of 0.02–20 µM, PFOS caused hyperactive locomotor activity at 5 dpf in the light, and also at 14 dpf, with mixed hyperactivity and hypoactivity in the dark and a decreased incidence and duration of swimming bursts (Jantzen et al., 2016a; Lee et al., 2021, 2022; Wu et al., 2022). Lower PFOS concentrations of 0.002–0.02 µM (Liu et al., 2022) and 0.5–5 nM (Haimbaugh et al., 2022) induced hypoactive behavior in the F0 generation that manifested as hyperactivity in the F1 generation (Haimbaugh et al., 2022). The current literature provides ample evidence of PFOS-induced morphological and behavioral effects in developing zebrafish.
C-F chain length
Assessment of PFOS and PFOA homologs showed that increased PFAS toxicity was correlated with increasing C-F chain length, up to 8 fluorinated carbons. Gaballah et al. reported no effects of PFBS exposure, but abnormal body axis phenotypes and failed swim bladder inflation were associated with exposures to PFOS, PFHpS, PFHxS, and PFPeS (descending from 8 to 5 fluorinated carbons), with respective EC50 values of 28.2, 168.1, 227.9, and 48.8 µM. Inclusion of the high EC50 for PFPeS in the analysis resulted in no linear correlation between EC50 and chain length for the PFSAs; otherwise, a clear trend in toxicity based on C-F chain length was evident. Menger et al. (2020) observed a similar trend as PFHxS caused malformation and mortality in exposures as low as 12 µM, and PFOS as low as 4.3 µM. In a PFCA homologous series consisting of compounds with 3- to 12-fluorinated-carbon chains, only PFOA and PFNA (7 and 8 fluorinated carbons) induced malformations and mortality at as low as 16.4 and 74.8 µM, respectively. Besides PFDoA (11 fluorinated carbons), all PFCA homologs caused abnormal larval behavior (Rericha et al., 2021), though PFDoA (0.4–10 µM) has otherwise been associated with delayed decrease in swimming activity and slower swimming speeds (Guo et al., 2018). PFOS and PFHxS (8 and 6 fluorinated carbons) both reduced swimming distance in the dark and induced a greater startle response (Menger et al., 2020) and increased swimming distance regardless of the light phase (Gaballah et al., 2020). Studies generally agree on the presence of abnormal morphology and behavior following exposure to the majority of assessed PFCAs and PFSAs, with C-F chain length influencing effects (Gaballah et al., 2020; Liu et al., 2022; Menger et al., 2020; Wasel et al., 2021).
Functional groups
PFAS with sulfonic acid functional head groups elicited greater toxicity than those with carboxylic acid head groups in developing zebrafish. While all PFSAs evaluated by Gaballah et al. elicited morphological effects, PFNA was the only PFCA that caused malformations and lethality. PFPeA, PFHxA, PFHpA, and PFOA did not induce morphological effects, though exposures to most caused altered larval behavior (Gaballah et al., 2020; Menger et al., 2020). PFOA and PFNA consistently altered locomotor activity in exposures as low as 0.2 µM (Jantzen et al., 2016a; Menger et al., 2020) and 0.06 µM (Yu et al., 2022).The concentration that caused mortality in 50% of the exposed zebrafish (LC50) for PFOA was 725 µM, and it caused malformations (ie, bent tail, yolk sac, and pericardial edema) at exposure concentrations as low as 48 µM (Gaballah et al., 2020; Menger et al., 2020; Pecquet et al., 2020); however PFSAs induced effects at lower concentrations. Differences in toxicity values between studies are likely related to slight variations in exposure paradigms, including whether exposure solutions were buffered to neutralize pH which can greatly influence observed toxicity (Wasel et al., 2021). Overall, while PFCAs have the potential to induce morphological effects, they often do so at higher concentrations relative to PFSAs.
Lesser-studied PFAS subclasses, including fluoroethers, phosphinic acids, and those with fluorotelomer chemistries (Table 1), have been the focus of several recent studies. Emerging fluoroethers ADONA and PFESA 1 did not induce toxicity when tested between 4.4 and 80.0 µM (Gaballah et al., 2020). After early developmental exposure for 24 h to a variety of perfluoropolyether carboxylic acids, PFO3TDA elicited the lowest LC50 of 38 µM, followed by PFO3DA (202 µM), PFOA (232 µM), PFDMMOBA (248 µM), GenX (383 µM), PFO2HpA (441 µM), and PFMOBA (499 µM) (Gebreab et al., 2020). PFOA and PFECAs both reduced interocular distance and increased listing incidence or impaired righting behavior. These findings suggest that some PFECAs may not be safer alternatives to PFOA (Gebreab et al., 2020). LC50 was inversely correlated with alkyl chain length, but there was no correlation with substitution of CF2 by ether groups in PFECAs (Gebreab et al., 2020). The uninflated swim bladder is a frequently identified malformation following PFOA and PFECA exposures, as well as yolk sac edema for PFOA and PFo4DA or spinal deformity for PFO3OA and PFO5DoDA. EC50 rankings were PFO5DoDA < PFO4DA < PFOA < PFO3OA, with EC50 values for uninflated swim bladders of 52, 310, 606, and 3932 µM, respectively. Toxicity increased with an increase in backbone OCF2 moieties (Wang et al., 2020). When tested between 0.01 and 5.79 µM, 8:8 PFPiA, a phosphinic acid, did not affect larval survival, hatch rate, or cause malformations; however, 0.34–5.79 µM exposures reduced swimming activity at 120 hpf during the dark photoperiod (Kim et al., 2020). 6:2 FTSA did not cause malformations but altered behavior at 180 µM (Menger et al., 2020). Beyond PFCAs and PFSAs, it is difficult to determine toxicity trends among other PFAS subclasses based on the available smaller-scale studies, ie, those that evaluated 1–8 compounds. A shift towards assessments of larger PFAS libraries is necessary.
Toward rapid testing of all PFAS
PFAS toxicity research is driven by the thousands of PFAS on the global market and the urgent need to understand which structures are hazardous and why (OECD, 2018). Because testing in zebrafish is an efficient approach to examine large numbers of structurally diverse PFAS, 2 immediate goals emerged: (1) to establish basic structure-bioactivity relationships for as many PFAS as can be tested in zebrafish, and (2) prioritize thousands of PFAS down to just those structures for which bioactivity merits closer scrutiny in rodent testing. After all, it is doubtful that a PFAS that is developmentally inactive in zebrafish would be found to present a hazard liability in higher vertebrates. The current limiting factor for such high-throughput testing is the number of analytically validated PFAS available for procurement.
The largest PFAS zebrafish developmental toxicity assessment to date was of 139 structures. It identified 49 (35%) as bioactive in at least one morphology or behavior endpoint (Truong et al., 2022). Among the 139 PFAS, 31 induced aberrant morphology, 11 altered embryonic behavior at 24 hpf, and 25 altered larval behavior at 120 hpf. PFDA was the most potent, with a modeled benchmark dose to elicit 10% of any morphological effect (BMD10) of 0.22 µM. Among commonly studied PFAS identified as bioactive by Truong et al., potency rankings were PFDA > FOSA > PFOS > PFHpS > PFHxS > PFOS-F. Even with a 139 PFAS dataset, the authors concluded it was insufficient to establish broadly applicable structure-activity relationships. Analyses revealed limited correlation between bioactivity and physiochemical properties, such as LogP, mass, and number of fluorinated carbons. However, there was an association between bioactivity and specific chemotypes, including sulfonyl, sulfenamide, sulfonamide, and alkyl hydroxide chemotypes (Truong et al., 2022). In another recent study, high prevalence of altered larval behavior (36%) following developmental exposure to a diverse set of 58 PFAS at a single concentration of approximately 0.1 µM was associated with PFCAs, PFSAs, and PFAS with fluorotelomer carboxylic acids, ether/polyether carboxylic acids, phosphinic acids, phosphate esters, and phosphinic acids (Rericha et al., 2021). Clearly, diverse PFAS spanning structural subclasses exhibit bioactivity.
Notably, multiple studies identified FOSA, which contains a sulfonamide head group and an 8-fluorinated-carbon chain, as particularly bioactive. It was the sole compound to alter morphology, and embryonic and larval behavior in the 139 PFAS study (Truong et al., 2022). FOSA was also the most potent developmental toxicant in 2 other high-content screening studies that evaluated 38 (Dasgupta et al., 2020) and 74 PFAS (Han et al., 2021). Following earlier exposures beginning at 0.75 hpf, FOSA induced significant delays to epiboly (25 µM), and 0.75–24 hpf exposures (0.78 µM) decreased liver area and increased yolk sac neutral lipids by 128 hpf (Dasgupta et al., 2020). A shorter sulfonamide PFAS homolog—perfluorobutane sulfonamide (FBSA)—was identified as the most toxic in a 4-fluorinated-carbon homologous series tested from 1 to 100 µM. While PFPeA, PFBS, and 4:2 FTSA induced abnormal larval behavior, FBSA was the only one to elicit abnormal morphology (Rericha et al., 2022a). FOSA and other sulfonamide PFAS have been shown to bioconcentrate to a greater extent than other PFAS (Han et al., 2021; Rericha et al., 2022a), which may explain their higher toxicity. Some have argued that toxicokinetics explains the majority of variability in toxicity between PFAS, and that once within the organism, nonunique toxic mechanisms are operant, specifically for perfluoroalkyl acids (Vogs et al., 2019). However, Han et al. suggested that FOSA may have a unique mode of action as it is more toxic than other compounds with similar or even slightly higher BCF values. Ultimately, apical organism-level endpoints cannot discern whether PFAS induce toxicity via similar or different modes of action. Such inquiries require ‘omics and mechanistic investigation.
Adult zebrafish
Adult zebrafish studies enable the interrogation of organ-level, sex-specific, intergenerational effects, and considerably more complex behavior endpoints. PFOS, F-53B, and OBS 1 µM exposures for 28 days caused vacuolation in the liver and an increased liver somatic index induced by F-53B (Huang et al., 2022a). Following a developmental aqueous exposure (2 nM) to PFOA that was immediately followed by dietary exposure to 8 picomolar PFOA, fish were significantly smaller in weight and length, with no effect on survival (Jantzen et al., 2017). Similarly, exposures to 6:2 FTAB and 6:2 FTAA mixtures (0.057/0.034 µM or 0.57/0.34 µM, respectively) for 180 days decreased body weight and K-factor, a concentration-dependent effect in both sexes. It also decreased the average number of eggs produced and increased the incidence of malformations (uninflated swim bladder, pericardial and yolk sac edemas, and bent spines) and mortality in the developing F1 generation (Shi et al., 2019). Altered behavior following PFAS exposure is also frequently observed; a study of sublethal exposure from 3 to 120 hpf to 2.0 µM PFOS, PFOA, and PFNA found no change in adult body length or weight but various altered adult behaviors. PFNA elicited broad effects on behavior in male fish, including reduced distance traveled, increased thigmotaxis, and increased aggressive behavior, and they spent more time in light arenas in a light/dark assay than controls. Conversely, PFOS-exposed males showed reduced aggression and PFOA-exposed females spent less time in the lighted arenas relative to controls (Jantzen et al., 2016b). A dietary exposure to PFHxA, extending from the juvenile into the subadult life stage, found no effect on body weight and length but altered behavior in the F0 and subsequent generations (Rericha et al., 2022b). Altered behavior across generations was observed following developmental exposures to PFOS and PFOA, and was associated with altered gene expression patterns that were exacerbated in successive generations (Haimbaugh et al., 2022). Aqueous exposures to 0.27–0.83 µM PFBS for 1 week altered embryo development, specifically nutrient loading. Spawning immediately after exposure yielded embryo concentrations of 99–253 pg/embryo, with PFAS concentration dropping below detection in embryos after the 4th or 5th spawning, all of which averaged 1.71 days apart. PFBS loading in embryos led to decreased survival, though there was no difference in the number of embryos produced by the F0 (Annunziato et al., 2022). In adult zebrafish, exposure to a variety of PFAS, primarily PFCAs and PFSAs, largely did not induce significant organism-level malformations in F0 populations but did affect fecundity, behavior, and development of the next generation.
Toxicity: modes of action and mechanisms
Developmental zebrafish
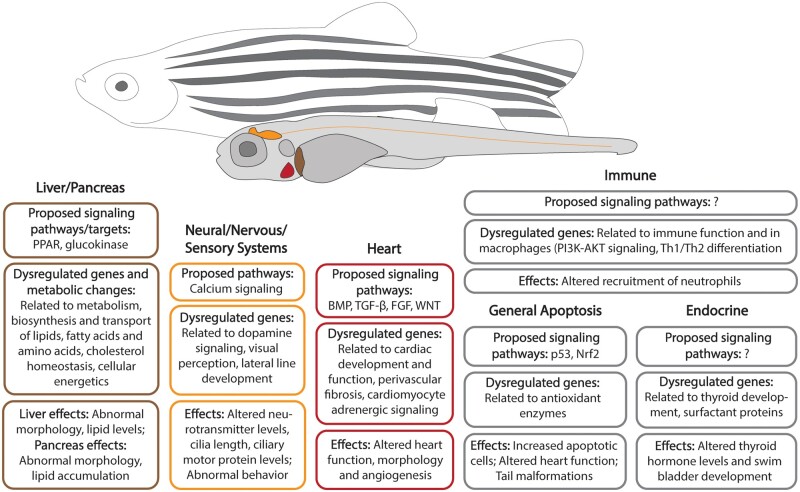
Recent strides towards elucidating the mechanisms of PFAS toxicity in zebrafish have predominately employed transcriptomics (Table 3). ‘Omics endpoints are more sensitive and specific than macroscopic morphological effects and provide insight into the modes of action by which PFAS induce toxicity (Annunziato et al., 2019; Gebreab et al., 2020; Martinez et al., 2019; Tu et al., 2019). While the field is making progress towards understanding the mechanistic pathways leading to adverse effects of PFAS exposures by employing ‘omics technologies, in silico molecular docking studies, and systems biology approaches, more work is needed to determine molecular initiating events that drive toxicity (Figure 1).
Table 3.
Summary of disrupted systems and pathways based on transcriptomic studies
| Summarized Major Systems and Functions | Representative Disrupted Pathways | Chemical | References |
|---|---|---|---|
| Nervous system development, neurological function | Neurodevelopment, neuron projection regeneration, notochord development, dopamine biosynthesis, dopamine neuron function | PFOS | (Dong et al., 2021; Huang et al., 2021; Lee et al., 2021) |
| PFOS | (Wu et al., 2022) | ||
| PFOA | (Yu et al., 2021) | ||
| OBS | (Huang et al., 2021) | ||
| PFDoA | (Guo et al., 2018) | ||
| – | FBSA | (Rericha et al., 2022a) | |
| Sensory system development | Visual perception | PFOS | (Dong et al., 2021) |
| Lipid synthesis, transport, and metabolism | Lipid metabolism, fatty acid synthesis, β-oxidation, cholesterol transport, peroxisome proliferator-activated receptor (PPAR) signaling, energy expenditure | PFOS | (Cheng et al., 2016; Christou et al., 2020; Dong et al., 2021; Haimbaugh et al., 2022; Martinez et al., 2019; Mylroie et al., 2021; Tu et al., 2019; Wang et al., 2022; Yi et al., 2019; Zhu et al., 2021) (F2 generation) |
| Cl-PFOS | (Yi et al., 2019) | ||
| PFBS | (Gong et al., 2022; Sant et al., 2019) | ||
| F-53B | (Wang et al., 2022; Yi et al., 2019) | ||
| 8:2 Cl-PFESA | (Yi et al., 2019) | ||
| PFNA | (Gong et al., 2022) | ||
| FBSA | (Rericha et al., 2022a) | ||
| – | OBS | (Wang et al., 2022) | |
| FOSA | (Dasgupta et al., 2020) | ||
| Immune response | Chemokine activity, proteases implicated to antigen presentation, inflammatory response | PFOS | (Diaz et al., 2021; Guo et al., 2019; Huang et al., 2022b; Lee et al., 2021; Martinez et al., 2019) |
| PFOA | (Diaz et al., 2021) | ||
| PKCO signaling in T lymphocytes, B cell receptor signaling, role of NEAT in regulation of the immune response | PFOS | (Liu et al., 2022) | |
| PFOA | (Liu et al., 2022) | ||
| FOSA | (Liu et al., 2022) | ||
| Immune cell function and trafficking | PFOA | (Haimbaugh et al., 2022) (F2 generation) | |
| – | F-53B | (Huang et al., 2022b) | |
| OBS | (Huang et al., 2022b) | ||
| PFBS | (Tang et al., 2020) (F1 eggs) | ||
| 6:2 FTAB | (Shi et al., 2018) | ||
| FBSA | (Rericha et al., 2022a) | ||
| Cellular signaling | – | PFOS | (Lee et al., 2021) |
| Liver development | Hepatotoxicity | PFOS | (Lee et al., 2021) |
| FOSA | (Dasgupta et al., 2020) | ||
| Oxidative stress | Nrf2 signaling | PFOS | (Lee et al., 2021; Sant et al., 2018) |
| – | F-53B | (Wu et al., 2019 | |
| Endocrine system | Steroid synthesis | PFOS | (Haimbaugh et al., 2022) (F2 generation) |
| PFBS | (Sant et al., 2019) | ||
| Estrogen receptor signaling | PFOA | (Haimbaugh et al., 2022) (F1 generation) | |
| – | PFOA | (Wang et al., 2020) | |
| PFO5DoDA | (Wang et al., 2020) | ||
| PFO4DA | (Wang et al., 2020) | ||
| PFO3OA | (Wang et al., 2020) | ||
| Cardiac development and function | Cardiac hypertrophy signaling | PFOS | (Liu et al., 2022) |
| PFOA | (Liu et al., 2022) | ||
| FOSA | (Liu et al., 2022) | ||
| Cardiac muscle contraction | PFBS | (Gong et al., 2022) | |
| Wnt/β-catenin pathway | F-53B | (Shi et al., 2017b) | |
| Erythrocyte-related | 2-(Perfluorohexyl) ethanoic acid | (Shi et al., 2017a) |
Figure 1.
Representative organs and systems, biological pathways, and endpoints affected by PFAS exposures in zebrafish.
Metabolic pathways and energetics
Key events related to metabolic pathways and altered energetics are frequently identified as characteristic of PFAS toxicity. PFOS and F-53B exposures as low as 0.46 and 0.024 µM, respectively, increased oxidative energy expenditure of 2 dpf embryos and reduced feed intake, whereas PFOS and OBS exposures as low as 4.6 and 0.06 µM reduced embryo oxygen consumption rate (Tu et al., 2019). The liver and pancreas have been identified as target organs of toxicity. Hepatic toxicity in the form of steatosis, vacuolation, increased total cholesterol and triglyceride levels, and decreased levels of low-density lipoprotein at 7 dpf were induced by 1 µM exposures to PFOS, Cl-PFOS, 6:2 Cl-PFESA (F-53B), or 8:2 Cl-PFESA (Yi et al., 2019). While Sant et al. found no change in cholesterol, triglycerides, glucose, or unsaturated fatty acid concentrations at 4 dpf following PFOS exposure at 16 or 32 µM, they did see an increase in saturated fatty acids. PFOS exposure up to 15 dpf caused aberrant pancreatic islet morphologies and lipid accumulation (Sant et al., 2021). PFBS exposure at 16 and 32 µM also decreased pancreas length and increased the incidence of severely hypomorphic and fragmented islets at 4 dpf (Sant et al., 2019). Lipid dysregulation, as well as altered liver and pancreas morphology, are strong evidence of altered energetics following PFAS exposures.
Transcriptomic studies have further revealed enrichment of biological pathways involved in lipid metabolism and energetics following exposure to PFBS (Sant et al., 2019), PFOS (Christou et al., 2020; Haimbaugh et al., 2022; Zhu et al., 2021), chlorinated PFOS homologs (Yi et al., 2019), FOSA (Dasgupta et al., 2020), and FBSA (Rericha et al., 2022a) (Table 3). PFOS, Cl-PFOS, and 6:2 Cl-PFESA (F-53B) (1 µM), all of which have 8 fluorinated carbons, had comparable disruptive potency on lipid metabolism responsive genes, increasing expression of genes involved with fatty acid β-oxidation (Supplementary Table 2). 8:2 Cl-PFESA (1 µM) exhibited the opposite trend, and both suppressed genes involved in fatty acid export (Yi et al., 2019). At 72 hpf, following exposure to 1 µM PFOS, pathways involving biosynthesis of fatty acids and cholesterol homeostasis and regulation were among the most significantly enriched biological pathways (Dong et al., 2021). Following exposure to PFOS at similar concentrations (0.046–4.6 µM), and also following F-53B (0.026–2.6 µM), or OBS (0.064–6.4 µM), transcript abundance at 96 hpf related to central regulation of energy expenditure, glucose metabolism, and lipid metabolism was decreased (Tu et al., 2019). OBS uniquely induced dose-dependent decrease in GK protein abundance, and PFOS reduced NPY protein abundance (Tu et al., 2019). Zhu et al. (2021) proposed that PFOS-induced metabolic reprogramming and increased fatty acid uptake may contribute to tumor cell proliferation. Several studies point to peroxisome proliferator-activated receptors (PPARs) as a modulator of PFAS toxicity due to their prevalent role in lipid metabolism. Developmental PFOS exposure led to decreased PPAR gene expression at 96 hpf (pparaa and pparg at 32 µM, and pparg at 16 µM) (Sant et al., 2021). PFNA and PFBS (20 µM) exposures also enriched PPAR signaling at 120 hpf (Gong et al., 2022). In another study PFBS exposure did not alter expression of PPAR genes at 96 hpf but affected numerous PPAR targets (Sant et al., 2019). Similarly, no PPAR genes were differentially expressed after FBSA exposure, but PPARa was predicted to regulate 36 differentially expressed genes (DEGs) (Rericha et al., 2022a).
Metabolic profiling has also identified PFAS targets involved in lipid metabolism and energetics. The metabolic effects of GenX (303 µM) and PFO3TDA (17.8 µM) exposures were similar to PFOA (120.8 µM) in that after exposure from 72 to 96 hpf they were related to carbohydrate, lipid and amino acid metabolism, and associated cellular energetics. Reduced molar ratio of branched-chain to aromatic amino acids relative to controls was a metabolic indication of liver damage, also significantly correlated with LC50. Furthermore, PFO3TDA led to decreased trimethylamine N-oxide, a proposed biomarker of metabolic syndrome. PFOA and PFO3TDA decreased carnitine, which is essential for fatty acid transport and related to β-oxidation, and decreased cholesterol, and PFO3TDA exposure decreased fatty acid levels. Metabolic changes also suggested mitochondrial dysfunction and possibly PPAR-mediated effects (Gebreab et al., 2020). PFOS exposures at 2 µM from 48 to 120 hpf were not associated with altered lipid metabolism despite increased yolk sac area at 120 hpf; PFOS-affected pathways were related to amino acid, purine, carbon, and 2-oxocarboxylic acid metabolism (Ortiz-Villanueva et al., 2018). An integrated biomolecule network analysis based on transcriptomic, proteomic, and metabolomic data from developmental PFOS exposures (0.1–20 µM) identified clusters of affected pathways related to proteasome-linked actin-binding protein and amino acid metabolisms, lipid metabolism and cell proliferation and apoptosis, and phospholipid-translocating ATPase activity mechanisms (Lee et al., 2021).
Two molecular docking studies provided insight into potential biological targets. Molecular docking revealed that 8:2 Cl-PFESA exhibited higher PPAR antagonism than PFOS and other chlorinated homologs (binding energy −7.6 kcal/mol for PPARα and −8.8 kal/mol for PPARβ) (Yi et al., 2019). The order of binding affinities to glucokinase (zfGK) for PFOS and several alternatives were: F-53B (−7.8 kcal/mol) > PFOS > OBS. (Tu et al., 2019).
Apoptosis and oxidative stress
Apoptosis is a recurring endpoint in zebrafish PFAS studies that is often linked to oxidative stress. In a recent study of the PFOS alternative 6:2 FTAB, developmental exposure to 70.1 µM significantly increased apoptotic cells detected in the tail area at 96 hpf (Shi et al., 2018). An earlier study reported an increase in apoptotic cells, especially around the heart and tail after 10 µM PFOS exposure, and suggested that apoptosis may help explain observations of altered heart rates and tail malformations (Shi et al., 2008). Similar findings of increased apoptotic cells in the heart region at 72 hpf and decreased heart rate at 48 hpf following 2–30 hpf PFOA exposures as low as 0.06 µM were noted (Yu et al., 2022). The abundance of p53 and bax transcripts at 96 hpf was increased in a dose-dependent manner, up to 5.3-fold after exposure to 10 µM PFOS (Shi et al., 2008). Heightened expression of p53 (1.5-fold) and bax (1.54-fold), apaf1 (1.33-fold), and mdm2 (1.48-fold), and increased activity of at least 138% for caspase-3, -8, and -9 was reported following exposure to 70.1 µM 6:2 FTAB. Shi et al. suggested that PFAS-induced cell apoptosis may occur via activation of p53 and may then be caspase-dependent, ultimately leading to tail malformations as a result of the cell death, though they also noted that apoptosis may be induced by reactive oxygen species (ROS) (Du et al., 2017; Shi et al., 2018).
Exposure to 6:2 FTAB (Shi et al., 2018), PFOS (Du et al., 2017; Wu et al., 2022), and F-53B (Wu et al., 2019) increased ROS levels in larvae. At 96 hpf, early exposure to 35 and 70 µM 6:2 FTAB increased malondialdehyde (MDA) levels, another indicator of potential oxidative damage, along with catalase (CAT) enzyme activity by 3.0-fold (70 µM); glutathione peroxidase (GPx) activity decreased (Shi et al., 2018). PFOS exposure (3.0 µM) increased MDA, SOD, and Gpx, and decreased CAT activity in one study (Du et al., 2017) but had only a significant decrease in MDA levels in another study 24 h after a 72–120 hpf exposure (10 µM) (Zou et al., 2021). At 120 hpf, all antioxidant enzyme activities decreased after 0.35 µM F-53B exposure, except for GPx. Along with changed activity levels, gene expression of antioxidant enzymes (Supplementary Table 2) was reduced and expression of nrf2 was heightened. After western blot and molecular docking analysis, the authors proposed F-53B inhibits PI3K, an upstream Nrf2 activator, leading to decreased protein expression of Nrf2 through the PI3K/Akt pathway (Wu et al., 2019). PFOS and OBS (10 µM) exposures also lead to decreased Nrf2 expression after a 24 h depuration period (Zou et al., 2021). Metabolomic evidence of oxidative stress induced by PFOA (121 µM), GenX (303 µM), and PFO3TDA (17.8 µM) exposures included increased taurine levels and decreased glutathione levels, both associated with heightened response to oxidative stress and with the Nrf2 pathway (Gebreab et al., 2020). Disruption of embryogenesis by PFOS may be in part modulated through the Nrf family of genes and their capacity to induce antioxidant response. The incidence of pericardial edema, reduced growth, and apoptosis was ameliorated in mutant nrf2afh318−/− zebrafish exposed to PFOS, though mutants exhibited increased yolk utilization. There is evidence of other compensatory antioxidant responses despite Nrf2a deficiency such as dose-dependent increased expression of pparg and its targets apoa1a and fabp1b1 in mutants. In silico evidence of Nrf family-PPAR-response elements crosstalk led the authors to suggest that PPARƴ may play an important role; ultimately, there may be multiple mediators of oxidative stress response to PFOS (Sant et al., 2018).
Cardiac system
Altered heart rate and incidence of pericardial edema have been observed following exposures to several PFAS, along with altered erythrocyte numbers and disrupted angiogenesis. PFAS exposures hindered cardiac function, leading to decreased heart rate and lower stroke volumes and output (Liu et al., 2022) and histological heart malformations (PFNA, PFBS) (Gong et al., 2022). Exposure to 37.2 µM PFOS or its alternative OBS (31.9 µM) shortened intersegmental vessel length and enhanced dorsal aorta vessel formation in Tg(fli1: eGFP) zebrafish, a vascular reporter line. OBS exposure also decreased formation of dorsal longitudinal anastomotic vessels, whereas PFOS decreased posterior cardinal vein formation (Huang et al., 2021). FOSA exposure between 0.002 and 0.2 µM led to dose-dependent elongation of the heart in the cardiomyocyte reporter line Tg(myl7: eGFP), ie, increased sinus venosus and bulbus arteriosis lengths, expanded pericardial sac area, and caused irregular heart beat and cardiac output at 72 hpf (Chen et al., 2022). At this same timepoint, developmental exposure to 11–32 µM 2-(Perfluorohexyl)ethanoic acid (CAS: 53826-12-3) and 2.6–21.0 µM F-53B decreased numbers of erythrocytes and decreased heart rate. 2-(Perfluorohexyl)ethanoic acid exposure altered expression of erythrocyte-related genes, and F-53B affected the Wnt/β-catenin pathway, critical for cardiac development (Table 3) (Shi et al., 2017a,b).
In fact, numerous studies employed transcriptomics to identify altered gene expression and biological pathways related to cardiac development and function, and specifically related to WNT signaling. Single-cell RNA sequencing following 0.06 µM PFOA exposure revealed that the highest number of DEGs were found in heart cells. Affected biological functions included gas transport, muscle cell differentiation, actin cytoskeleton, adrenergic signaling in cardiomyocytes, lysosome, autophagy, and apoptosis (Yu et al., 2022). PFOS, PFOA, and FOSA exposures all altered biological pathways related to cardiac hypertrophy signaling at 120 hpf within the range of 0.002–0.02 µM, and further analysis revealed miR-16-5p as an activated upstream regulator among all the chemicals (Liu et al., 2022). At 120 hpf, PFOS exposure to 1 µM decreased expression of genes related to muscle fiber regeneration and hematopoietic processes (Dong et al., 2021). By 120 hpf, exposure to 0.002 and 0.02 µM FOSA caused dysregulation of genes with predicted functions related to dysfunction of the heart, fibrosis left ventricular dysfunction, and perivascular fibrosis. BMP, TGF-β, and FGF signaling pathways mediated affected processes. WNT signaling was activated through the WNT/β-catenin, WNT/Ca2+, and WNT/Rho pathways. Following 0.02 µM exposure, there was also significant enrichment of the aryl hydrocarbon receptor (AHR) pathway, an important regulator of xenobiotic metabolism and more (Chen et al., 2022). Though WNT and AHR signaling pathways have been proposed, additional investigation is needed to determine the mechanisms of cardiac toxicity.
Neural/nervous/sensory systems
Dysregulation of the brain and nervous system development, and probable motor function, are commonly observed following PFAS exposures, identified using transcriptomics (Annunziato et al., 2019; Dong et al., 2021; Huang et al., 2021; Kim et al., 2020; Rericha et al., 2022a; Wu et al., 2022) and metabolomics (Gebreab et al., 2020). PFOA exposure (2.4 µM) promoted the proliferation of BrdU/HuC/D positive cells in the preoptic area of the hypothalamus at 48 hpf and disrupted the expression of genes essential to dopamine neuron development and function. Concentrations as low as 0.24 µM altered gene expression that suggests perturbation of dopamine neurotransmission signaling and homeostasis (Wu et al., 2022; Yu et al., 2021). PFOS (0.6–6 µM) similarly affected gene expression related to dopamine synthesis, corroborated by measured increases in protein levels and brain dopamine content (Wu et al., 2022). KEGG pathway analysis of DEGs after 3.83 µM PFOS exposure revealed enrichment of extracellular matrix-receptor interaction, cardiac muscle contraction, and calcium signaling (Christou et al., 2020). Christou et al. investigated effects on calcium signaling related to ryanodine receptors (RyR) through co-exposures with caffeine or dantrolene. Dantrolene decreased swimming speed in controls but had no effect upon co-exposure with PFOS; perhaps PFOS has a higher affinity to RyR binding sites than dantrolene. PFOS altered endogenous neurotransmitter levels after exposure at 10 and 20 µM, and altered several genes associated with seizures (Lee et al., 2022). Across multiple studies, bdnf expression was decreased by exposure to PFOS and OBS (Huang et al., 2021), PFHxA, PFHxS, and 6:2 FTOH (Annunziato et al., 2019). 8:8 PFPiA exposure decreased mRNA expression of neuronal genes elavl3 and tuba1 (Kim et al., 2020) and a similar effect was observed after PFHxA exposure, along with decreased protein levels (Guo et al., 2021). Integrated multi-omics analyses following 0.1–20 µM PFOS exposure identified network clusters of pathways related to Ca2+ binding-related, producing resource substances of neurotransmission, and Ca2+-regulated muscle contraction (Lee et al., 2021). Behavior effects following PFDoA (10 µM) exposure coincided with decreased ACh content, AChE activity, increased dopamine levels, and altered gene expression related to nervous system development (Guo et al., 2018).
Dysregulation of genes related to the sensory and motor systems has also been reported. Visual perception was among the most highly enriched biological processes at 72 hpf following 1 µM PFOS exposure (Dong et al., 2021). The lateral line development gene ap1s1 was induced in a dose-dependent manner following PFHxA exposure at 2.0 (3.63-fold) and 20.0 (13.2-fold) µM, and was induced by 20 µM 6:2 FTOH exposure, whereas 0.2 µM PFHxS decreased expression (Annunziato et al., 2019). Related to muscle development, tgfb1a expression increased after PFHxA and 6:2 FTOH exposures as low as 0.2 µM. Length of cilia, an important organelle for signal transduction, in the retinal neuroepithelium was reduced by PFOS (37.2 µM) and OBS (47.9 µM) exposures. Expression of genes related to cilia in the dynein arm family was increased, whereas that of genes in the kinesin and tubulin families was decreased. Abundance of ciliary motor proteins was decreased, and molecular docking revealed potential for PFAS-protein interaction; for instance, PFOS exhibited a binding affinity of −9.4 kcal/mol towards DYNC1H1 (Huang et al., 2021). Overall, PFAS exposures altered development and function of the brain and sensory systems through several key events. Integrated multi-omics analyses identified widespread dysregulation of calcium signaling, though the driving mechanisms remain uncertain.
Endocrine system
Investigation of PFAS as endocrine disrupting compounds has predominately revealed effects related to the thyroid system. PFOA and alternative PFECAs lowered T3 and T4 levels at 5 dpf in a dose-dependent manner, at as low as 22.5 µM following PFO5DoDA developmental exposure. PFO5DoDA, PFO3OA, and PFOA exposures also increased abundance of transcripts related to TH synthesis (dio2) and regulation (crh) at 5 dpf. These PFAS and PFO4DA increased expression of genes involved in TH metabolism (ugt1ab, st1, and st5) and downregulated st4, whereas only PFOA increased tshβ and ttr transcript abundance. Increased expression of metabolizing genes likely increased TH clearance, leading to the decreased TH levels. T3 and T4 supplementation partially rescued PFOA- and PFECA-induced swim bladder malformations and yolk sac edemas. Decreased expression of outer mesothelial markers anxa5 and hprt1l at 5 dpf following PFOA (1207.5 µM) and PFo5DoDA (22.5–90.1 µM) exposures suggested swim bladder malformation was linked to mesothelium development (Wang et al., 2020). After 4.5–6 dpf exposures to PFOA (11 µM) and PFBA (640 µM), larvae exhibited decreasing swim bladder surface area, the same effect observed after T3 exposure, also increasing expression of tpo and 2 genes encoding surfactant proteins, sp-a and sp-c at 6 dpf. PFOA also heightened expression of tshB and trA, whereas PFBA increased ttf-1. Following 4.5–28 dpf exposures, PFOA and PFBA increased percentage of fish without anterior swim bladders, but heightened expression of sp-a and sp-c only persisted in PFOA-exposed groups. The shorter chain PFBA (3 fluorinated carbons) had similar phenotype to PFOA (7 fluorinated carbons), but at 28 times higher concentrations (Godfrey et al., 2017). 8:8 PFPiA (5.79 µM) also increased expression of genes related to thyroid hormones, including crhb, dio3a, and tshr (Kim et al., 2020). PFOS exposure as low as 0.2 µM increased expression of thyroid development marker genes hhex and pax8, and also reduced expression of sex hormone regulating genes cyp19a and cyp19b, all at 96 hpf (Shi et al., 2008).
Immune system
While immunotoxicity is a frequently noted effect of PFAS exposure, only a couple of recent studies have investigated effects in developmental zebrafish. RNA-seq studies have identified immune-related biological pathways associated with PFAS-induced gene expression changes (Haimbaugh et al., 2022; Liu et al., 2022; Rericha et al., 2022a) (Supplementary Table 2). Additionally, PFOS (200 nM) exposure exacerbated chemically induced inflammation (pooled larvae analysis), specifically expression of il17a, tnfa, and il1b at 120 hpf, and increased neutrophil recruitment to the intestine (Diaz et al., 2021). Exposure to 1.2 or 12 µM PFOA decreased the number of neutrophils at inflicted wound sites at 48 hpf by 1.4- and 1.8-fold, respectively (Pecquet et al., 2020). Exposure to 6:2 FTAB at 8.8 µM led to dose-dependent decreased expression of ccl1 at 96 hpf, whereas 17.5 µM increased expression of il1β, il8, and tnfa, and 70 µM heightened cxcl-c1c and ifn expression at 96 hpf (Shi et al., 2018). Single-cell RNA sequencing after 0.06 µM PFOA exposure showed that macrophages were among the most affected cell populations, exhibiting altered fatty acid metabolism, PI3K-AKT signaling, and Th1 and Th2 differentiation (Yu et al., 2022). Limited data on key events related to immunotoxicity warrant additional studies in zebrafish.
Adult and intergenerational
Studies conducted in adult zebrafish corroborate affected systems and biological pathways identified in developmental studies and provide insight into intergenerational and sex-specific effects. Adult exposures caused lipid dysregulation, oxidative stress, and immunotoxicity. Chronic exposure to 0.5 µM PFOS for 5 months caused liver steatosis and altered serum lipid profiles, with more frequent and severe effects observed in males. Male zebrafish exhibited lower hepatosomatic index, reduced ATP concentration and altered expression of nuclear receptors and genes related to fatty acid oxidation in the liver (Cheng et al., 2016). PFOS, F-53B, and OBS (1 µM for 21 days) exposures affected lipid metabolism-related pathways, specifically pertaining to PPAR signaling (Wang et al., 2022). Expression of genes related to lipid transport and synthesis, and fatty acid β-oxidation was decreased, including pparƴ after PFOS and F-53B. Molecular docking demonstrated similar binding affinities to zfPPARƴ of −8.5 and −9.1, respectively (Wang et al., 2022). PFOS, F-53B, and OBS exposures also repressed expression of pro-inflammatory cytokines, increased renal insterstitium in the head kidney, reduced expression of immune-related genes in the liver and altered expression in the intestine, shortened average intestine villus height, and caused dysbiosis of intestinal microbial communities(Huang et al., 2022b; Wang et al., 2022). Morphological and transcriptomic evidence of immunotoxicity in the spleen was observed after exposure to 0.12–1.2 µM PFOA (Zhong et al., 2020). Molecular docking revealed the 3 PFAS bound to zfNF-κB, with the highest affinity of −7.4 kcal/mol for F-53B, which likely mediated immune-related responses (Huang et al., 2022b). In adults developmentally exposed to PFNA, PFOA, or PFOS (0.02–2 µM), alterations in slco2b1, slco1d1, tgfb1a, and bdnf expression were chemical- and sex-dependent, and several persisted from the juvenile life stage into adulthood (Jantzen et al., 2016b).
Adult exposures also provided insight into intergenerational effects. Exposures to 0.033 or 0.33 µM PFBS disrupted maternal transcript transfer to F1 eggs, resulting in differentially enriched pathways related to immune function, along with adheren junctions, protein dimerization, and DNA packaging processes (Tang et al., 2020). Nrf2a was again suggested as a key pathway involved in effects on energy balance. F0 PFBS exposures to 0.27–0.83 µM disrupted lipid homeostasis and altered nutrient profiles and fatty acid composition in F1 embryos, particularly in nrf2afh318/fh318 mutants (Annunziato et al., 2022). Following a 2 nM PFOA developmental aqueous exposure combined with a 4 month 8 pM dietary exposure up to 6 months old, F0 adults exhibited decreased expression of organic anion transporters slco2b1, slco4a1, slco3a1, increased slco1d1, and decreased tgfb1a. F1 offspring exhibited significant developmental delay and an increase in ap1s1 expression, related to protein cargo sorting and vesicular trafficking (Jantzen et al., 2017). Evidence of disrupted biological processes intergenerationally warrants particular scrutiny when assessing the hazard potential of these compounds.
Conclusions and future directions
A significant amount of data on PFAS ADME and toxicity in zebrafish exists for what is still a relatively small number of PFAS, including the PFCAs, PFSAs, and PFECAs. Trends generally show greater bioconcentration and toxicity for longer chain compounds and those with sulfonamide and sulfonic acid functional groups. While some studies have tested PFAS from lesser-studied subclasses and larger libraries, evaluation of many more PFAS is needed to sufficiently interrogate structure-activity relationships, differential ADME, and modes of action. Knowledge of PFAS modes of action is growing, and the recent zebrafish literature indicates affected biological pathways and measured key events related to lipid metabolism and energetics, oxidative stress, the nervous system, as well as the cardiac, endocrine, and immune systems. Studies have highlighted a few mediating receptors, such as Nrf2a, PPAR, and AHR. Additional pharmacokinetic modeling (Khazaee and Ng, 2018), modeling to inform development of quantitative Adverse Outcome Pathways (Warner et al., 2022), functional, ‘omics and integrated multi-omics analyses are needed. Large-scale chemical assessment using the throughput advantages of zebrafish will be essential to evaluate more PFAS in a vertebrate system. While PFAS mixture toxicity was outside the scope of this review, mixture studies are essential to determine PFAS hazard potential, as exposures often occur as complex environmental mixtures. Comparison of PFAS sensitivity among species, aquatic and otherwise, was beyond the present scope but is an important consideration for translational and risk assessment purposes that others have begun to address (Savoca and Pace, 2021; Wasel et al., 2021).
This review has identified several important considerations for future PFAS testing in zebrafish. GenX, a PFECA, has been shown to degrade in dimethyl sulfoxide, a commonly used solvent for toxicity testing (Gaballah et al., 2020). Compatibility between chemicals and solvents to ensure stock solution integrity is essential. Similarly, shifts in pH alone can cause toxicity for developing embryos and confound observed PFAS toxicity and comparison between studies if exposure solutions are not buffered. Also essential is the need for analytically characterized PFAS standards so that investigators know that the label compound is responsible for the observed bioactivity rather than an industrial contaminant or a degradation product. The chorion status of embryos can also significantly influence PFAS toxicity. While simple automated removal of the chorion en masse has been available to the zebrafish community for over a decade, it is still not a common practice. Laboratories that do not practice the technique should be cognizant of its potential for false negative estimations of PFAS bioactivity. As the scientific community and international regulatory agencies work to determine how PFAS should be regulated, we must clearly establish a broader foundation of data for structurally diverse PFAS so that what we learn from hundreds of PFAS structures can be leveraged to predict the hazard potential of thousands more.
Supplementary Material
Contributor Information
Yvonne Rericha, Environmental & Molecular Toxicology Department, College of Agricultural Sciences, Oregon State University, Corvallis, Oregon, USA; Sinnhuber Aquatic Research Laboratory, Oregon State University, Corvallis, Oregon, USA.
Michael T Simonich, Environmental & Molecular Toxicology Department, College of Agricultural Sciences, Oregon State University, Corvallis, Oregon, USA; Sinnhuber Aquatic Research Laboratory, Oregon State University, Corvallis, Oregon, USA.
Lisa Truong, Environmental & Molecular Toxicology Department, College of Agricultural Sciences, Oregon State University, Corvallis, Oregon, USA; Sinnhuber Aquatic Research Laboratory, Oregon State University, Corvallis, Oregon, USA.
Robyn L Tanguay, Environmental & Molecular Toxicology Department, College of Agricultural Sciences, Oregon State University, Corvallis, Oregon, USA; Sinnhuber Aquatic Research Laboratory, Oregon State University, Corvallis, Oregon, USA.
Supplementary data
Supplementary data are available at Toxicological Sciences online.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research was funded by the U.S. Environmental Protection Agency (grant number 83948101) and the National Institutes of Health (P30 ES030287, P42 ES016465, and T32 ES007060).
References
- Annunziato K. M., Jantzen C. E., Gronske M. C., Cooper K. R. (2019). Subtle morphometric, behavioral and gene expression effects in larval zebrafish exposed to PFHxA, PFHxS and 6:2 FTOH. Aquat. Toxicol. 208, 126–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato K. M., Marin M., Liang W. L., Conlin S. M., Qi W. P., Doherty J., Lee J., Clark J. M., Park Y., Timme-Laragy A. R. (2022). The nrf2a pathway impacts zebrafish offspring development with maternal preconception exposure to perfluorobutanesulfonic acid. Chemosphere 287, 132121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnot J. A., Gobas F. A. P. C. (2006). A review of bioconcentration factor (BCF) and bioaccumulation factor (BAF) assessments for organic chemicals in aquatic organisms. Environ. Rev. 14, 257–297. [Google Scholar]
- Ateia M., Maroli A., Tharayil N., Karanfil T. (2019). The overlooked short- and ultrashort-chain poly- and perfluorinated substances: A review. Chemosphere 220, 866–882. [DOI] [PubMed] [Google Scholar]
- Barbo N., Stoiber T., Naidenko O. V., Andrews D. Q. (2023). Locally caught freshwater fish across the United States are likely a significant source of exposure to PFOS and other perfluorinated compounds. Environ. Res. 220, 115165. [DOI] [PubMed] [Google Scholar]
- Brendel S., Fetter E., Staude C., Vierke L., Biegel-Engler A. (2018). Short-chain perfluoroalkyl acids: Environmental concerns and a regulatory strategy under reach. Environ. Sci. Eur. 30, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck R. C., Franklin J., Berger U., Conder J. M., Cousins I. T., de Voogt P., Jensen A. A., Kannan K., Mabury S. A., van Leeuwen S. P. (2011). Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 7, 513–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Qiu W., Yang X., Chen F., Chen J., Tang L., Zhong H., Magnuson J. T., Zheng C., Xu E. G. (2022). Perfluorooctane sulfonamide (PFOSA) induces cardiotoxicity via aryl hydrocarbon receptor activation in zebrafish. Environ. Sci. Technol. 56, 8438–8448. [DOI] [PubMed] [Google Scholar]
- Cheng J., Lv S., Nie S., Liu J., Tong S., Kang N., Xiao Y., Dong Q., Huang C., Yang D. (2016). Chronic perfluorooctane sulfonate (PFOS) exposure induces hepatic steatosis in zebrafish. Aquat. Toxicol. 176, 45–52. [DOI] [PubMed] [Google Scholar]
- Christou M., Fraser T. W. K., Berg V., Ropstad E., Kamstra J. H. (2020). Calcium signaling as a possible mechanism behind increased locomotor response in zebrafish larvae exposed to a human relevant persistent organic pollutant mixture or PFOS. Environ. Res. 187, 109702. [DOI] [PubMed] [Google Scholar]
- Cousins I. T., DeWitt J. C., Glüge J., Goldenman G., Herzke D., Lohmann R., Ng C. A., Scheringer M., Wang Z. (2020). The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 22, 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S., Reddam A., Liu Z., Liu J., Volz D. C. (2020). High-content screening in zebrafish identifies perfluorooctanesulfonamide as a potent developmental toxicant. Environ. Pollut. 256, 113550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A. O., Armitage J. M., Bruton T. A., Dassuncao C., Heiger-Bernays W., Hu X. C., Karrman A., Kelly B., Ng C., Robuck A., et al. (2021). PFAS exposure pathways for humans and wildlife: A synthesis of current knowledge and key gaps in understanding. Environ. Toxicol. Chem. 40, 631–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz O. E., , SoriniC., , MoralesR. A., , LuoX., , FredeA., , KraisA. M., , ChávezM. N., , WincentE., , DasS., and , Villablanca E. J. (2021). Perfluorooctanesulfonic acid modulates barrier function and systemic T-cell homeostasis during intestinal inflammation. Dis. Model. Mech. 14, dmm049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo J. L., Nadal M. (2017). Per- and polyfluoroalkyl substances (PFASs) in food and human dietary intake: A review of the recent scientific literature. J. Agric. Food Chem. 65, 533–543. [DOI] [PubMed] [Google Scholar]
- Dong G., Zhang R., Huang H., Lu C., Xia Y., Wang X., Du G. (2021). Exploration of the developmental toxicity of TCS and PFOS to zebrafish embryos by whole-genome gene expression analyses. Environ. Sci. Pollut. Res. Int. 28, 56032–56042. [DOI] [PubMed] [Google Scholar]
- Du J., Cai J., Wang S., You H. (2017). Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZNO nanoparticles. Int. J. Occup. Med. Environ. Health 30, 213–229. [DOI] [PubMed] [Google Scholar]
- EU. (2022). Directive (EU) 2020/2184 of the European Parliament and the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. Off J Eur Union. 63, 26–53. [Google Scholar]
- Fan X., Tang S., Wang Y., Fan W., Ben Y., Naidu R., Dong Z. (2022). Global exposure to per- and polyfluoroalkyl substances and associated burden of low birthweight. Environ. Sci. Technol. 56, 4282–4294. [DOI] [PubMed] [Google Scholar]
- Fenton S. E., Ducatman A., Boobis A., DeWitt J. C., Lau C., Ng C., Smith J. S., Roberts S. M. (2021). Per- and polyfluoroalkyl substance toxicity and human health review: Current state of knowledge and strategies for informing future research. Environ. Toxicol. Chem. 40, 606–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaballah S., Swank A., Sobus J. R., Howey X. M., Schmid J., Catron T., McCord J., Hines E., Strynar M., Tal T. (2020). Evaluation of developmental toxicity, developmental neurotoxicity, and tissue dose in zebrafish exposed to GENX and other PFAS. Environ. Health Perspect. 128, 47005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreab K. Y., Eeza M. N. H., Bai T., Zuberi Z., Matysik J., O’Shea K. E., Alia A., Berry J. P. (2020). Comparative toxicometabolomics of perfluorooctanoic acid (PFOA) and next-generation perfluoroalkyl substances. Environ. Pollut. 265, 114928. [DOI] [PubMed] [Google Scholar]
- Gluge J., Scheringer M., Cousins I. T., DeWitt J. C., Goldenman G., Herzke D., Lohmann R., Ng C. A., Trier X., Wang Z. (2020). An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 22, 2345–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A., Hooser B., Abdelmoneim A., Horzmann K. A., Freemanc J. L., Sepulveda M. S. (2017). Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish. Aquat. Toxicol. 193, 228–235. [DOI] [PubMed] [Google Scholar]
- Gong H., Du J., Xu J., Yang Y., Lu H., Xiao H. (2022). Perfluorononanoate and perfluorobutane sulfonate induce cardiotoxic effects in zebrafish. Environ. Toxicol. Chem. 41, 2527–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., , WuP., , CaoJ., , LuoY., , ChenJ., , WangG., , GuoW., , WangT., and , He X. (2019). The PFOS disturbed immunomodulatory functions via nuclear Factor-κB signaling in liver of zebrafish (Danio rerio). Fish Shellfish Immunol. 91, 87–98. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang S., Liu X., Lu S., Wu Q., Xie P. (2021). Evaluation of the acute toxicity and neurodevelopmental inhibition of perfluorohexanoic acid (PFHxA) in zebrafish embryos. Ecotoxicol. Environ. Saf. 225, 112733. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhang S., Lu S., Zheng B., Xie P., Chen J., Li G., Liu C., Wu Q., Cheng H., et al. (2018). Perfluorododecanoic acid exposure induced developmental neurotoxicity in zebrafish embryos. Environ. Pollut. 241, 1018–1026. [DOI] [PubMed] [Google Scholar]
- Haimbaugh A., Wu C. C., Akemann C., Meyer D. N., Connell M., Abdi M., Khalaf A., Johnson D., Baker T. R. (2022). Multi- and transgenerational effects of developmental exposure to environmental levels of PFAS and PFAS mixture in zebrafish (Danio rerio). Toxics 10, 334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J., Gu W., Barrett H., Yang D., Tang S., Sun J., Liu J., Krause H. M., Houck K. A., Peng H. (2021). A roadmap to the structure-related metabolism pathways of per- and polyfluoroalkyl substances in the early life stages of zebrafish (Danio rerio). Environ. Health Perspect. 129, 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Liu Y., Wang Q., Yi J., Lai H., Sun L., Mennigen J. A., Tu W. (2022a). Concentration-dependent toxicokinetics of novel PFOS alternatives and their chronic combined toxicity in adult zebrafish. Sci. Total Environ. 839, 156388. [DOI] [PubMed] [Google Scholar]
- Huang J., Sun L., Mennigen J. A., Liu Y., Liu S., Zhang M., Wang Q., Tu W. (2021). Developmental toxicity of the novel PFOS alternative OBS in developing zebrafish: An emphasis on cilia disruption. J. Hazard. Mater. 409, 124491. [DOI] [PubMed] [Google Scholar]
- Huang J., Wang Q., Liu S., Lai H., Tu W. (2022b). Comparative chronic toxicities of PFOS and its novel alternatives on the immune system associated with intestinal microbiota dysbiosis in adult zebrafish. J. Hazard. Mater. 425, 127950. [DOI] [PubMed] [Google Scholar]
- Jantzen C. E., Annunziato K. A., Bugel S. M., Cooper K. R. (2016a). PFOS, PFNA, and PFOA sub-lethal exposure to embryonic zebrafish have different toxicity profiles in terms of morphometrics, behavior and gene expression. Aquat. Toxicol. 175, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen C. E., Annunziato K. M., Cooper K. R. (2016b). Behavioral, morphometric, and gene expression effects in adult zebrafish (Danio rerio) embryonically exposed to PFOA, PFOS, and PFNA. Aquat. Toxicol. 180, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen C. E., Toor F., Annunziato K. A., Cooper K. R. (2017). Effects of chronic perfluorooctanoic acid (PFOA) at low concentration on morphometrics, gene expression, and fecundity in zebrafish (Danio rerio). Reprod. Toxicol. 69, 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian J. M., Chen D., Han F. J., Guo Y., Zeng L., Lu X., Wang F. (2018). A short review on human exposure to and tissue distribution of per- and polyfluoroalkyl substances (PFASs). Sci. Total Environ. 636, 1058–1069. [DOI] [PubMed] [Google Scholar]
- Khazaee M., Ng C. A. (2018). Evaluating parameter availability for physiologically based pharmacokinetic (PBPK) modeling of perfluorooctanoic acid (PFOA) in zebrafish. Environ. Sci. Process. Impacts 20, 105–119. [DOI] [PubMed] [Google Scholar]
- Kim S., Stroski K. M., Killeen G., Smitherman C., Simcik M. F., Brooks B. W. (2020). 8:8 Perfluoroalkyl phosphinic acid affects neurobehavioral development, thyroid disruption, and DNA methylation in developing zebrafish. Sci. Total Environ. 736, 139600. [DOI] [PubMed] [Google Scholar]
- Kurwadkar S., Dane J., Kanel S. R., Nadagouda M. N., Cawdrey R. W., Ambade B., Struckhoff G. C., Wilkin R. (2022). Per- and polyfluoroalkyl substances in water and wastewater: A critical review of their global occurrence and distribution. Sci. Total Environ. 809, 151003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land M., de Wit C. A., Bignert A., Cousins I. T., Herzke D., Johansson J. H., Martin J. W. (2018). What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ. Evid. 7, 4. [Google Scholar]
- Lee H., Sung E. J., Seo S., Min E. K., Lee J. Y., Shim I., Kim P., Kim T. Y., Lee S., Kim K. T. (2021). Integrated multi-omics analysis reveals the underlying molecular mechanism for developmental neurotoxicity of perfluorooctanesulfonic acid in zebrafish. Environ. Int. 157, 106802. [DOI] [PubMed] [Google Scholar]
- Lee H., Tran C. M., Jeong S., Kim S. S., Bae M. A., Kim K. T. (2022). Seizurogenic effect of perfluorooctane sulfonate in zebrafish larvae. Neurotoxicology 93, 257–264. [DOI] [PubMed] [Google Scholar]
- Li Y., Fletcher T., Mucs D., Scott K., Lindh C. H., Tallving P., Jakobsson K. (2018). Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 75, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xu Y., Scott K., Lindh C., Jakobsson K., Fletcher T. (2019). Half-lives of PFOA, PFPeS, PFHxS, PFHpS and PFOS after end of exposure to contaminated drinking water. Environ. Epidemiol. 3, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Liu S., Qiu W., Magnuson J. T., Liu Z., Yang G., Chen H., Li Y., Xu X., Zheng C. (2022). Cardiotoxicity of PFOA, PFOS, and PFOSA in early life stage zebrafish: Molecular changes to behavioral-level response. Sustainable Horizons 3, 100027. [Google Scholar]
- Martinez R., Navarro-Martin L., Luccarelli C., Codina A. E., Raldua D., Barata C., Tauler R., Pina B. (2019). Unravelling the mechanisms of PFOS toxicity by combining morphological and transcriptomic analyses in zebrafish embryos. Sci. Total Environ. 674, 462–471. [DOI] [PubMed] [Google Scholar]
- Menger F., Pohl J., Ahrens L., Carlsson G., Orn S. (2020). Behavioural effects and bioconcentration of per- and polyfluoroalkyl substances (PFASs) in zebrafish (Danio rerio) embryos. Chemosphere 245, 125573. [DOI] [PubMed] [Google Scholar]
- Mylroie J. E., , WilbanksM. S., , KimbleA. N., , ToK. T., , CoxC. S., , McLeodS. J., , GustK. A., , MooreD. W., , PerkinsE. J., and , Garcia-Reyero N. (2021). Perfluorooctanesulfonic acid-induced toxicity on zebrafish embryos in the presence or absence of the chorion. Environ. Toxicol. Chem. 40, 780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OECD. (2018). Toward a new comprehensive global database of per- and polyfluoroalkyl substances (PFASs): Summary report on updating the OECD 2007 list of per- and polyfluoroalkyl substances (PFASs). OECD Environment, Health and Safety Publications, Series on Risk Management No. 39. Organisation for Economic Co-operation and Development, Paris.
- Ortiz-Villanueva E., Jaumot J., Martinez R., Navarro-Martin L., Pina B., Tauler R. (2018). Assessment of endocrine disruptors effects on zebrafish (Danio rerio) embryos by untargeted LC-HRMS metabolomic analysis. Sci. Total Environ. 635, 156–166. [DOI] [PubMed] [Google Scholar]
- Pasecnaja E., Bartkevics V., Zacs D. (2022). Occurrence of selected per- and polyfluorinated alkyl substances (PFASs) in food available on the European market – A review on levels and human exposure assessment. Chemosphere 287, 132378. [DOI] [PubMed] [Google Scholar]
- Pecquet A. M., Maier A., Kasper S., Sumanas S., Yadav J. (2020). Exposure to perfluorooctanoic acid (PFOA) decreases neutrophil migration response to injury in zebrafish embryos. BMC Res. Notes 13, 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rericha Y., Cao D., Truong L., Simonich M., Field J. A., Tanguay R. L. (2021). Behavior effects of structurally diverse per- and polyfluoroalkyl substances in zebrafish. Chem. Res. Toxicol. 34, 1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rericha Y., Cao D., Truong L., Simonich M. T., Field J. A., Tanguay R. L. (2022a). Sulfonamide functional head on short-chain perfluorinated substance drives developmental toxicity. Iscience 25, 103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rericha Y., Truong L., Leong C., Cao D., Field J. A., Tanguay R. L. (2022b). Dietary perfluorohexanoic acid (PFHxA) exposures in juvenile zebrafish produce subtle behavioral effects across generations. Toxics 10, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant K. E., Annunziato K., Conlin S., Teicher G., Chen P., Venezia O., Downes G. B., Park Y., Timme-Laragy A. R. (2021). Developmental exposures to perfluorooctanesulfonic acid (PFOS) impact embryonic nutrition, pancreatic morphology, and adiposity in the zebrafish, Danio rerio. Environ. Pollut. 275, 116644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant K. E., Sinno P. P., Jacobs H. M., Timme-Laragy A. R. (2018). Nrf2a modulates the embryonic antioxidant response to perfluorooctanesulfonic acid (PFOS) in the zebrafish, Danio rerio. Aquat. Toxicol. 198, 92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sant K. E., Venezia O. L., Sinno P. P., Timme-Laragy A. R. (2019). Perfluorobutanesulfonic acid disrupts pancreatic organogenesis and regulation of lipid metabolism in the zebrafish, Danio rerio. Toxicol. Sci. 167, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satbhai K., Vogs C., Crago J. (2022). Comparative toxicokinetics and toxicity of PFOA and its replacement GENX in the early stages of zebrafish. Chemosphere 308, 136131. [DOI] [PubMed] [Google Scholar]
- Savoca D., Pace A. (2021). Bioaccumulation, biodistribution, toxicology and biomonitoring of organofluorine compounds in aquatic organisms. Int. J. Mol. Sci. 22, 6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultes L., Sandblom O., Broeg K., Bignert A., Benskin J. P. (2020). Temporal trends (1981–2013) of per- and polyfluoroalkyl substances and total fluorine in baltic cod (Gadus morhua). Environ. Toxicol. Chem. 39, 300–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe R. L., Benskin J. P., Laarman A. H., MacLeod S. L., Martin J. W., Wong C. S., Goss G. G. (2010). Perfluorooctane sulfonate toxicity, isomer-specific accumulation, and maternal transfer in zebrafish (Danio rerio) and rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 29, 1957–1966. [DOI] [PubMed] [Google Scholar]
- Shi G., Cui Q., Pan Y., Sheng N., Guo Y., Dai J. (2017a). 6:2 Fluorotelomer carboxylic acid (6:2 FTCA) exposure induces developmental toxicity and inhibits the formation of erythrocytes during zebrafish embryogenesis. Aquat. Toxicol. 190, 53–61. [DOI] [PubMed] [Google Scholar]
- Shi G., Cui Q., Pan Y., Sheng N., Sun S., Guo Y., Dai J. (2017b). 6:2 Chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos. Aquat. Toxicol. 185, 67–75. [DOI] [PubMed] [Google Scholar]
- Shi G., Cui Q., Zhang H., Cui R., Guo Y., Dai J. (2019). Accumulation, biotransformation, and endocrine disruption effects of fluorotelomer surfactant mixtures on zebrafish. Chem. Res. Toxicol. 32, 1432–1440. [DOI] [PubMed] [Google Scholar]
- Shi G., Xie Y., Guo Y., Dai J. (2018). 6:2 Fluorotelomer sulfonamide alkylbetaine (6:2 FTAB), a novel perfluorooctane sulfonate alternative, induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 195, 24–32. [DOI] [PubMed] [Google Scholar]
- Shi X., Du Y., Lam P. K., Wu R. S., Zhou B. (2008). Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol. Appl. Pharmacol. 230, 23–32. [DOI] [PubMed] [Google Scholar]
- Tal T., Vogs C. (2021). Invited perspective: PFAS bioconcentration and biotransformation in early life stage zebrafish and its implications for human health protection. Environ. Health Perspect. 129, 71304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L., Song S., Hu C., Liu M., Lam P. K. S., Zhou B., Lam J. C. W., Chen L. (2020). Parental exposure to perfluorobutane sulfonate disturbs the transfer of maternal transcripts and offspring embryonic development in zebrafish. Chemosphere 256, 127169. [DOI] [PubMed] [Google Scholar]
- Truong L., Rericha Y., Thunga P., Marvel S., Wallis D., Simonich M. T., Field J. A., Cao D., Reif D. M., Tanguay R. L. (2022). Systematic developmental toxicity assessment of a structurally diverse library of PFAS in zebrafish. J. Hazard. Mater. 431, 128615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W., Martinez R., Navarro-Martin L., Kostyniuk D. J., Hum C., Huang J., Deng M., Jin Y., Chan H. M., Mennigen J. A. (2019). Bioconcentration and metabolic effects of emerging PFOS alternatives in developing zebrafish. Environ. Sci. Technol. 53, 13427–13439. [DOI] [PubMed] [Google Scholar]
- USEPA. (2022a). Comptox chemicals dashboard, PFAS master list of PFAS substances. https://comptox.epa.gov/dashboard/chemical-lists/PFASMASTER. Accessed July 1, 2022.
- USEPA. (2022b). Interim drinking water health advisory: Perfluorooctanoic acid (PFOA) CASRN 335-67-1. EPA Document Number: EPA/822/R-22/003. U.S. Environmental Protection Agency Office of Water (4304T), Office of Science and Technology, Health and Ecological Criteria Division, Washington, DC.
- USEPA. (2023). Per- and polyfluoroalkyl substances (PFAS)- proposed PFAS national primary drinking water regulation. https://www.epa.gov/sdwa/and-polyfluoroalkyl-substances-pfas. Accessed March 16, 2023.
- Villeneuve D., Volz D. C., Embry M. R., Ankley G. T., Belanger S. E., Leonard M., Schirmer K., Tanguay R., Truong L., Wehmas L. (2014). Investigating alternatives to the fish early-life stage test: A strategy for discovering and annotating adverse outcome pathways for early fish development. Environ. Toxicol. Chem. 33, 158–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogs C., Johanson G., Naslund M., Wulff S., Sjodin M., Hellstrandh M., Lindberg J., Wincent E. (2019). Toxicokinetics of perfluorinated alkyl acids influences their toxic potency in the zebrafish embryo (Danio rerio). Environ. Sci. Technol. 53, 3898–3907. [DOI] [PubMed] [Google Scholar]
- Wang J. X., Shi G. H., Yao J. Z., Sheng N., Cui R. N., Su Z. B., Guo Y., Dai J. Y. (2020). Perfluoropolyether carboxylic acids (novel alternatives to PFOA) impair zebrafish posterior swim bladder development via thyroid hormone disruption. Environ. Int. 134, 105317. [DOI] [PubMed] [Google Scholar]
- Wang Q., Huang J., Liu S., Wang C., Jin Y., Lai H., Tu W. (2022). Aberrant hepatic lipid metabolism associated with gut microbiota dysbiosis triggers hepatotoxicity of novel PFOS alternatives in adult zebrafish. Environ. Int. 166, 107351. [DOI] [PubMed] [Google Scholar]
- Wang Z., Buser A. M., Cousins I. T., Demattio S., Drost W., Johansson O., Ohno K., Patlewicz G., Richard A. M., Walker G. W., et al. (2021). A new OECD definition for per- and polyfluoroalkyl substances. Environ. Sci. Technol. 55, 15575–15578. [DOI] [PubMed] [Google Scholar]
- Warner R. M., Sweeney L. M., Hayhurst B. A., Mayo M. L. (2022). Toxicokinetic modeling of per- and polyfluoroalkyl substance concentrations within developing zebrafish (Danio rerio) populations. Environ. Sci. Technol. 56, 13189–13199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasel O., Thompson K. M., Gao Y., Godfrey A. E., Gao J., Mahapatra C. T., Lee L. S., Sepúlveda M. S., Freeman J. L. (2021). Comparison of zebrafish in vitro and in vivo developmental toxicity assessments of perfluoroalkyl acids (PFAAs). J. Toxicol. Environ. Health A 84, 125–136. [DOI] [PubMed] [Google Scholar]
- Wen W., Xia X., Hu D., Zhou D., Wang H., Zhai Y., Lin H. (2017). Long-chain perfluoroalkyl acids (PFAAs) affect the bioconcentration and tissue distribution of short-chain PFAAs in zebrafish (Danio rerio). Environ. Sci. Technol. 51, 12358–12368. [DOI] [PubMed] [Google Scholar]
- Wen W., Xia X. H., Zhou D., Wang H. T., Zhai Y. W., Lin H., Chen J., Hu D. X. (2019). Bioconcentration and tissue distribution of shorter and longer chain perfluoroalkyl acids (PFAAs) in zebrafish (Danio rerio): Effects of perfluorinated carbon chain length and zebrafish protein content. Environ. Pollut. 249, 277–285. [DOI] [PubMed] [Google Scholar]
- Worley R. R., Moore S. M., Tierney B. C., Ye X., Calafat A. M., Campbell S., Woudneh M. B., Fisher J. (2017). Per- and polyfluoroalkyl substances in human serum and urine samples from a residentially exposed community. Environ. Int. 106, 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Dang Y., Liang L. X., Gong Y. C., Zeeshan M., Qian Z., Geiger S. D., Vaughn M. G., Zhou Y., Li Q. Q., et al. (2022). Perfluorooctane sulfonates induces neurobehavioral changes and increases dopamine neurotransmitter levels in zebrafish larvae. Chemosphere 297, 134234. [DOI] [PubMed] [Google Scholar]
- Wu Y., Huang J., Deng M., Jin Y., Yang H., Liu Y., Cao Q., Mennigen J. A., Tu W. (2019). Acute exposure to environmentally relevant concentrations of Chinese PFOS alternative f-53b induces oxidative stress in early developing zebrafish. Chemosphere 235, 945–951. [DOI] [PubMed] [Google Scholar]
- Xu Y. Y., Fletcher T., Pineda D., Lindh C. H., Nilsson C., Glynn A., Vogs C., Norstrom K., Lilja K., Jakobsson K., et al. (2020). Serum half-lives for short- and long-chain perfluoroalkyl acids after ceasing exposure from drinking water contaminated by firefighting foam. Environ. Health Persp. 128, 77004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S. J., Chen P. Y., Yang L. P., Zhu L. Y. (2019). Probing the hepatotoxicity mechanisms of novel chlorinated polyfluoroalkyl sulfonates to zebrafish larvae: Implication of structural specificity. Environ. Int. 133, 105262. [DOI] [PubMed] [Google Scholar]
- Yu J., Cheng W., Jia M., Chen L., Gu C., Ren H-q., Wu B. (2022). Toxicity of perfluorooctanoic acid on zebrafish early embryonic development determined by single-cell RNA sequencing. J. Hazard. Mater. 427, 127888. [DOI] [PubMed] [Google Scholar]
- Yu T., Zhou G., Cai Z., Liang W., Du Y., Wang W. (2021). Behavioral effects of early-life exposure to perfluorooctanoic acid might synthetically link to multiple aspects of dopaminergic neuron development and dopamine functions in zebrafish larvae. Aquat. Toxicol. 238, 105926. [DOI] [PubMed] [Google Scholar]
- Zhong Y., Shen L., Ye X., Zhou D., He Y., Zhang H. (2020). Mechanism of immunosuppression in zebrafish (Danio rerio) spleen induced by environmentally relevant concentrations of perfluorooctanoic acid. Chemosphere 249, 126200. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Yang D., Duan X., Zhang Y., Chen D., Gong Z., Liu C. (2021). Perfluorooctane sulfonate promotes doxycycline-induced liver tumor progression in male KRAS(v12) transgenic zebrafish. Environ. Res. 196, 110962. [DOI] [PubMed] [Google Scholar]
- Zou Y., Wu Y., Wang Q., Wan J., Deng M., Tu W. (2021). Comparison of toxicokinetics and toxic effects of PFOS and its novel alternative OBS in zebrafish larvae. Chemosphere 265, 129116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.