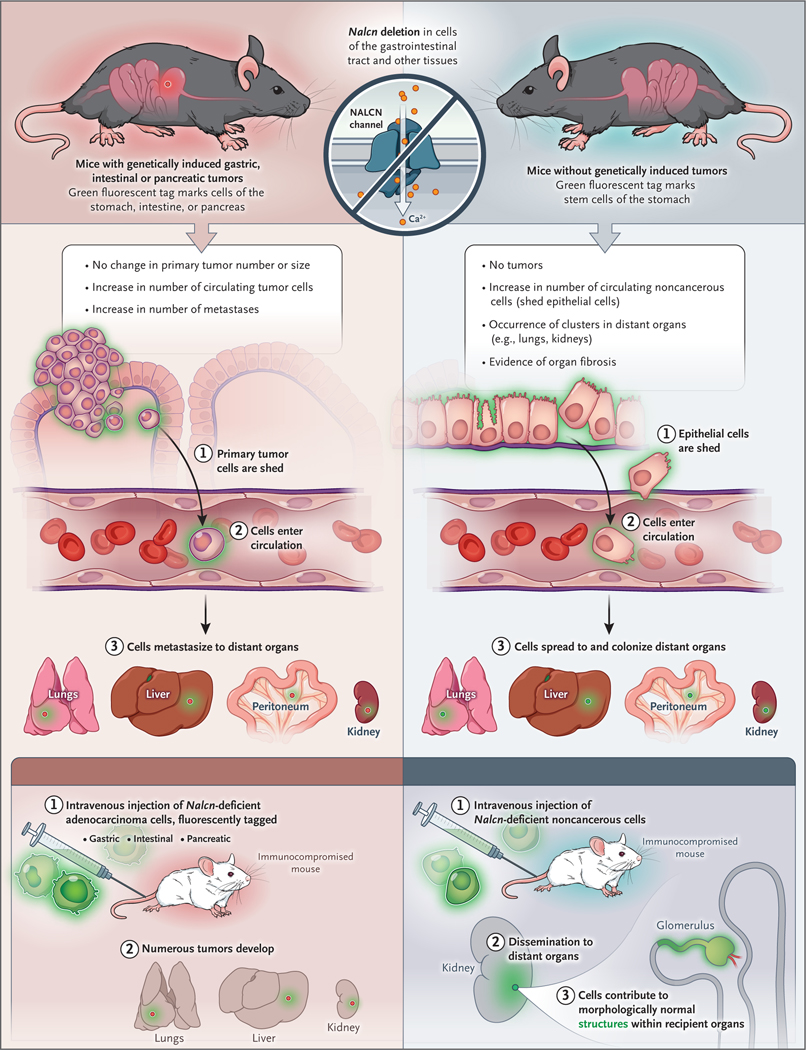
Cancer is thought to progress through a strict sequence of events: oncogenic mutations in normal cells drive hyperproliferation and tumor initiation, followed by invasion through the basement membrane, dissemination through the lymphovasculature, and finally, the metastatic colonization of distant organs.1 An assumption related to this invasion-to-metastasis cascade is that metastasis is a property exclusive to cells that have acquired oncogenic mutations. A recent study by Rahrmann et al. challenges this dogma, providing evidence that nontransformed epithelial cells that lack oncogenic mutations can be shed from organs, disseminate through the circulation, and colonize distant organs (Fig. 1).2
Figure 1. Dissemination of Tumor and Nontransformed Epithelial Cells.
In a recent study, Rahrmann et al.2 investigated the effects of deleting or inhibiting the function of the sodium leak channel nonselective protein (NALCN) in tumors and nontransformed epithelia. Using mouse models of gastric, intestinal, and pancreatic cancer harboring oncogenic Kras and Tp53 alleles, they found that organspecific NALCN deletion did not affect tumor initiation or primary tumor growth but increased tumor cell shedding into the circulation, circulating tumor-cell numbers, and metastases in multiple organs. When NALCN was deleted in the same organs in mice without any oncogenic mutations, no tumors formed, but increased numbers of NALCN-deficient cells were detected both in the circulation and in newly established cell colonies in distant organs of older mice, which showed evidence of fibrosis. NALCN-deleted fluorescent circulating nontumor cells that were injected into immunosuppressed recipient mice trafficked into organ parenchyma and generated morphologically normal structures, including renal glomeruli and tubules.
First, the authors used a mouse model of gastric cancer to show that the sodium leak channel nonselective protein (NALCN) is downregulated in gastric adenocarcinoma relative to gastric antral basal stem cells. Analysis of more than 10,000 human cancer genomes revealed that nonsynonymous mutations in NALCN were enriched in gastric and colorectal cancers, and structural prediction suggested that 75% of these mutations would block sodium-channel function. Deletion of Nalcn (the mouse counterpart of NALCN) in mouse models of gastric, intestinal, and pancreatic adenoma increased the number of circulating tumor cells and metastases without altering the number or size of primary tumors, and gene expression profiling of Nalcn-deficient circulating tumor cells from these mice revealed signatures similar to those of circulating tumor cells obtained from humans. These results support a role for NALCN in the suppression of metastatic dissemination without influence on tumor initiation.
Surprisingly, the deletion of NALCN in cells that expressed a fluorescent protein also increased circulating noncancerous cells, and fluorescent cells and clusters deficient in Nalcn could be detected in the lungs and kidneys of older mice. Circulating nontumor cells deficient in Nalcn that were intravenously injected into recipient mice could not only traffic into and form colonies in the parenchyma of multiple organs but, remarkably, also formed morphologically normal structures within recipient organs — for example, glomeruli and renal tubules in the kidney.
These provocative data show that epithelial cells can disseminate through the circulation, seed, and differentiate in response to local microenvironmental cues to colonize distant organs in the absence of oncogenic driver mutations, thus separating the process of metastasis from tumorigenesis. Nalcn-deficient circulating tumor and nontumor cells in mice have similar transcriptional profiles to circulating tumor cells in humans; however, with regard to driving metastasis, research has not shown the function of nonsynonymous NALCN mutations that have been observed in clinical tumor sequencing. The extent to which nontransformed epithelial cells can disseminate in patients is not known. The mechanisms by which NALCN deletion enables epithelial dissemination and the extent to which epithelial cells with wild-type (nonmutant) NALCN can disseminate (perhaps by blocking NALCN function) also remain unclear. Previous studies have shown that metastasis can occur early in tumorigenesis, but Rahrmann et al. provide direct in vivo evidence of epithelial dissemination even in the absence of oncogenic mutations. Uncoupling tumor initiation from metastasis has important implications for the understanding of tumor progression and potentially for the development of future cancer therapeutics.
Many cancer therapeutics target the driver mutations that are thought to initiate and sustain tumors, but such drugs would not eliminate the dissemination of nontransformed cells. If indeed the dissemination of nontransformed cells turns out to be a source of secondary cancers, new therapeutic approaches would be needed. Initial dissemination and seeding of distant organs by nontransformed epithelial cells, followed by later acquisition of oncogenic mutations, may underlie the clinical phenomenon of metastatic cancer of unknown primary site, which constitutes approximately 5% of cancers worldwide.3 These cancers typically have an unfavorable course that may potentially be explained by a distinct, highly plastic and migratory cell-state origin. The molecular profiling of cancers of unknown primary tumor site has been mostly limited to targeted exon sequencing of certain genes that are known to be associated with cancer; this approach has yielded few clues to their distinct clinical behavior. Informed by the work of Rahrmann et al., future studies that seek to identify NALCN mutations or other alterations specific to a dissemination-first, tumor-second phenotype may yield much-needed novel therapeutic approaches for these aggressive cancers.
Normal epithelial cells have been shown to have migratory capacity, notably in the context of wound healing. Such epithelial migration and transdifferentiation have previously been shown to occur within a contiguous epithelial bed, but the current study suggests that more widespread epithelial dissemination through circulation is possible and may serve as a physiological mechanism for reseeding and repairing tissues. Indeed, regeneration and metastasis have been shown to share programs that are distinct from the initiation of the primary tumor.4 Many cancers arise in the context of chronic tissue injury and repair, including colitis-associated colorectal cancer, hepatocellular cancer, head and neck squamous-cell cancer, and others. Acquisition of oncogenic mutations in epithelial cells in a regenerative state may lead to more rapid metastasis than the same mutation in a nonregenerative epithelium. Indeed, colitis-associated cancers are associated with a worse prognosis than sporadic colon cancers.5 In turn, these data may suggest the need for distinct treatments for tumors that arise in a regenerative milieu, and for preventive therapies specific to metastasis in patients with chronic inflammatory conditions to eliminate disseminated cells before they acquire oncogenic mutations. Conversely, increasing epithelial dissemination may enhance the efficacy of stem-cell therapeutics that are being developed to promote tissue regeneration. However, renal and skin fibrosis developed in older mice that were deficient in NALCN, findings that hint at complex interactions among epithelial electrical activity, regenerative programs, aging, and tumor progression; these issues merit further study. More generally, the study by Rahrmann et al. provides a conceptual and molecular framework for planning studies that further probe how cell dissemination relates to oncogenesis and tissue regeneration.
Footnotes
Disclosure forms provided by the author are available with the full text of this article at NEJM.org.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–74. [DOI] [PubMed] [Google Scholar]
- 2.Rahrmann EP, Shorthouse D, Jassim A, et al. The NALCN channel regulates metastasis and nonmalignant cell dissemination. Nat Genet 2022; 54: 1827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato S, Alsafar A, Walavalkar V, Hainsworth J, Kurzrock R. Cancer of unknown primary in the molecular era. Trends Cancer 2021;7 : 465–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganesh K, Basnet H, Kaygusuz Y, et al. L1CAM defines the regenerative origin of metastasis-initiating cells in colorectal cancer. Nat Cancer 2020; 1:2 8–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villarreal O, Zeineddine FA, Chacko R, et al. Outcomes of IBD-associated colorectal cancer and implications in early-onset colorectal cancer. In: Proceedings and Abstracts of the 2020 ASCO Gastrointestinal Cancers Symposium, January 23–25, 2020. San Francisco: American Society of Clinical Oncology, 2020. [Google Scholar]