Abstract
Mortality of candidemia in coronavirus disease 2019 (COVID-19) patients has not been deeply studied despite evidence suggesting an increased occurrence. We performed a systematic review and meta-analysis to summarize the available evidence about these patients’ mortality and length of stay. Data about the in-hospital, all-cause and 30-day mortality, and length of stay were pooled. Subgroup analyses were performed to assess sources of heterogeneity. Twenty-six articles out of the 1915 records retrieved during the search were included in this review. The pooled in-hospital mortality was 62.62% (95% CI, 54.77% to 69.86%), while the mortality in intensive care unit (ICU) was 66.77% (95% CI, 57.70% to 74.75%). The pooled median in-hospital length of stay was 30.41 (95% CI, 12.28 to 48.55) days, while the pooled median length of stay in the ICU was 28.28 (95% CI, 20.84 to 35.73) days. The subgroup analyses did not identify the sources of heterogeneity in any of the analyses. Our results showed high mortality in patients with candidemia and COVID-19, suggesting the need to consider screening measures to prevent this life-threatening condition.
Keywords: COVID-19, candidemia, meta-analysis, mortality, systematic review
Candidemia, the presence of Candida species in the blood, is a severe and life-threatening condition, especially in critically ill patients and those admitted to intensive care units (ICUs). Over the last few decades, the incidence of candidemia has increased [1]. Although its management has continuously evolved, the mortality of patients with this condition has also increased over time [2], ranging from at least 20% in non-ICU wards to >60% in the ICU [3–6], with variations that might be due to the underlying comorbidities of the considered sample [7]. The available data underline the severity of this life-threatening bloodstream infection.
Patients with coronavirus disease 2019 (COVID-19) have multiple reasons to be at higher risk for candidemia, such as iatrogenic immunosuppression [8], other bloodstream infections mainly due to bacteria and their associated high rates of antibiotic use [9], and indwelling vascular catheters [10]. Furthermore, severely ill COVID-19 patients are at even higher risk for developing candidemia due to the need for intensive care management [6, 11]. Therefore, patients with severe COVID-19 may present an exceptionally high incidence of candidemia and related mortality.
Although several studies have investigated candidemia's clinical and laboratory characteristics in COVID-19 patients [12–14], mortality has not yet been sufficiently explored. Most studies on this topic have employed small sample sizes and have been conducted at single centers, making it challenging to estimate mortality due to COVID-19 and candidemia with precision [10, 15, 16]. Knowledge of the impact of these conditions on mortality and length of stay of patients is important to inform the need for routine preventive screening measures for candidemia in patients with COVID-19 who are hospitalized or in intensive care units.
We designed this study to assess the mortality of hospitalized patients with candidemia and COVID-19. Secondly, we wanted to investigate this group of patients’ length of stay (LOS).
METHODS
The study protocol of this systematic review was registered on PROSPERO (CRD42021253891). An electronic search was performed on PubMed, Embase, Scopus, and Web of Science using keywords referring to candidemia, COVID-19 infection, mortality, and length of stay. The details of this search are reported in the Supplementary Materials (Supplementary Table 1). The electronic search was followed by manual searches of publications that might have been possibly considered for inclusion to identify any other study that might have been missed by the search strategy. Studies were included if (1) they assessed mortality in patients with both COVID-19 and candidemia and (2) they were cohort studies, retrospective observational studies, or case–control studies. Mortality was defined as in-hospital mortality, cause-specific mortality, all-cause mortality, 28-day or 30-day mortality, and 60-day or 90-day mortality. Having COVID-19 was described as having a positive polymerase chain reaction or antigenic test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); having candidemia was defined as having positive blood cultures for any Candida species. Studies were excluded if (1) they were reported in congress abstracts, letters, or commentaries; (2) they were case series or case reports; (3) they computed mortality excluding early deaths; (4) they were case–control studies that employed death to select cases; (5) they included only patients age <18 years old. Mortality was considered the primary outcome, whereas length of stay in the hospital and length of stay in the ICU were considered the secondary outcomes. All the searches were restricted to publications from 2020 onward and to those written in English, French, and Spanish.
Screening of the Articles and Data Extraction
The records identified through the electronic search were exported to Rayyan online software. Two authors independently screened the titles and abstracts of these records based on the inclusion and exclusion criteria. Then, the full texts of the documents that passed the first screening were retrieved and evaluated according to the inclusion and exclusion criteria. At both stages, discrepancies were resolved through consensus discussion with a third reviewer.
Data extraction was performed independently by 2 reviewers. Discrepancies were resolved through consensus. When we encountered missing data in an article under consideration, we contacted its authors to obtain the unreported data or additional details. Extracted variables were:
characteristics of the studies, namely author and title, country, study design, setting in which the patients were enrolled (hospital, ICU, other wards or groups of wards), the semester in which the study was started (eg, January–June 2020, July–December 2020, January–June 2021, July–December 2021), the inclusion of patients with severe COVID-19 only or with COVID-19 of any severity;
sociodemographic characteristics of the patients included in the studies, namely age and gender;
clinical characteristics of the patients included in the studies, namely comorbidities (hypertension, diabetes, obesity, chronic kidney disease [CKD], chronic obstructive pulmonary disease [COPD], immunosuppressive condition, Charlson comorbidity index) and known risk factors for candidemia (Candida score, parenteral nutrition exposure, multifocal Candida colonization, previous surgery, central venous catheter presence, endo-oral tube presence, renal replacement therapy, and extracorporeal membrane oxygenation);
treatments administered to the patients included in the studies, namely antifungal treatment, antibiotic treatment exposure, treatment with steroids, an antiviral (remdesivir), or immunomodulating agents (baricitinib, tocilizumab, anakinra);
microbiological data, such as the species isolated, antifungal (azole and echinocandin) resistance data, bacterial and viral coinfections, and beta-D-glucan (BDG) assay data;
ICU admission and days from COVID-19 diagnosis to candidemia diagnosis;
data about mortality (all-cause, specific, 28- or 30-day mortality, 90-day mortality, and in-hospital mortality), LOS in the hospital, and LOS in the ICU.
Risk of Bias (Quality) Assessment
The methodological quality of cohort and retrospective observational studies was performed using the National Institutes of Health (NIH) quality assessment tool for observational cohort studies [17]. This instrument assesses study quality based on the following domains: (1) research question, (2) study population, (3) whether the groups are recruited from the same population and using uniform eligibility criteria, (4) sample size justification, (5) exposure assessed before outcome measurement, (6) sufficient time frame to see an effect, (7) different levels of the exposure of interest, (8) exposure measures and assessment, (9) repeated exposure assessment, (10) outcome measures, (11) blinding of outcome assessors, (12) follow-up rate, (13) statistical analyses. The methodological quality of case–control studies was assessed using the NIH quality assessment tool for case–control studies [17]. This tool assesses study quality in the following domains: (1) research question, (2) study population, (3) target population and case representation, (4) sample size justification, (5) whether the groups were recruited from the same population, (6) whether inclusion and exclusion criteria are prespecified and applied uniformly, (7) case and control definitions, (8) random selection of study participants, (9) use of concurrent controls, (10) exposure assessed before outcome measurement, (11) exposure measures and assessment, (12) blinding of exposure assessors, (13) statistical analysis. Two reviewers performed this assessment independently, and discrepancies were resolved through consensus.
Strategy for Data Synthesis
Study characteristics were described using counts and percentages, or medians and interquartile ranges (IQRs), as appropriate. Mortality rates with 95% Wilson CIs were computed for each study. Per protocol, we planned to perform separate random-effects meta-analyses for each combination of outcomes (all-cause, specific, 28- or 30-day mortality, 90-day mortality and in-hospital mortality, LOS in the hospital, LOS in the ICU) and setting (ICU, hospital, specific wards) when at least 2 studies for each combination were present. Regarding the analyses on mortality, based on the available data, it was possible to pool the results of in-hospital mortality, mortality in the ICU, and 30-day mortality in the ICU. In-hospital mortality was retrieved from studies in which patients acquired candidemia both in- and outside the ICU. These analyses used generalized linear mixed models with a logit link [18]. Regarding the analyses on LOS in the hospital and the ICU, as LOS was summarized using medians and interquartile ranges in the original studies, we performed separate meta-analyses using the quantile-matching estimation method to compute aggregated median LOS with corresponding 95% confidence intervals [19].
In all the models, heterogeneity was assessed using Cochran's Q with a likelihood-ratio test of significance and its amount using the I2 statistic. This latter index can be interpreted as the percentage of the variability in effect estimates due to heterogeneity rather than sampling error. Values of 25%, 50%, and 75% were used as cutoff points for low, medium, and high heterogeneity. Subgroup analyses were performed to investigate sources of heterogeneity considering study design (case–control vs retrospective observational studies), the semester in which the study started (eg, January–June 2020, July–December 2020, January–June 2021, July–December 2021), the continent, and exclusion of patients without severe COVID-19. Per protocol, a meta-regression was planned to assess if mean age, percentage of patients with immunosuppression, and percentage of patients with each extracted comorbidity were associated with mortality. In addition, we also considered for inclusion in the meta-regression percent male, Candida species prevalence, and percentage of patients treated with central venous catheters (CVCs), steroids, and antibiotics. However, due to the low number of included studies and to avoid missing data bias, we included only variables for which data were available in at least 75% of the included studies. In addition, to avoid redundancy, we included only C. albicans prevalence to assess the impact of Candida species on mortality.
To examine the robustness of the results, which could be impaired by the inclusion of studies with a very small sample size or with a single patient, we decided to perform a sensitivity analysis excluding the studies with a sample size <10. The significance threshold was set at .05. Analyses were performed using the R (version 4.2.1) packages meta and metamedian [20, 21].
RESULTS
Study Characteristics
The electronic search yielded 1915 articles. After duplicate removal, 912 papers were selected for title and abstract screening. Of these, 854 were excluded and 58 were included for full-text review. Finally, 26 articles were included in this review. A flow diagram of the study selection is shown in Figure 1.
Figure 1.
Study selection chart. Abbreviation: COVID-19, coronavirus disease 2019.
The main characteristics of the included studies are reported in Table 1.
Table 1.
Characteristics of the Included Studies
| Author | Country | Study Design | Mono-/Multicentric | Study Period | Population | Patients With COVID-19, No. | Patients With COVID-19 and Candidemia, No. | Study Setting | Severity of COVID-19 | ICU Admission, % | ICU Admission Before Candidemia Diagnosis, % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Allaw, 2022 | Lebanon | Case–control | Monocentric | 1/10/20–15/6/21 | Cases: patients with severe COVID-19 and in whom C. auris was isolated during their hospital stay until their discharge or death Controls: patients with severe COVID-19 requiring ICU admission because of an increase in oxygen requirements/hemodynamic instability |
162 | 3 | ICU | Severe | 100 | n/a |
| Altinkaya Çavu, 2022 | Turkey | Retrospective cohort | Monocentric | 30/3/21–30/9/21 | All patients with COVID-19 admitted to ICU | 5583 | 78 | ICU | n/a | 100 | n/a |
| Arastehfar, 2021 | Iran | Retrospective cohort | Multicentric | 1/11/20–31/1/21 | All critically ill patients with COVID-19 admitted to ICU | 1988 | 6 | ICU | Severe | 100 | n/a |
| Arastehfar, 2022 | Turkey | Retrospective cohort | Multicentric | 4/20–12/20 | All patients with COVID-19 admitted to ICU | 148 | 28 | ICU | Severe | 100 | n/a |
| Avkan-Oğuz, 2022 | Turkey | Retrospective cohort | Monocentric | 1/3/20–31/10/20 | All patients with COVID-19 admitted to ICU | 118 | 3 | ICU | Severe | 100 | n/a |
| Ayalon, 2022 | Israel | Case–control | Monocentric | 1/9/20–31/3/21 | Cases: patients with COVID-19 with either documented invasive candidiasis or worsening respiratory function/infection Controls: COVID-19 critically ill ICU patients with worsening respiratory function |
311 | 11 | ICU | Severe | 100 | n/a |
| Bardi, 2022 | Spain | Retrospective cohort | Monocentric | 1/3/20–30/5/20 | All patients with COVID-19 admitted to ICU | 140 | 5 | ICU | Severe | 100 | n/a |
| Beştepe Dursun 2022 | Turkey | Retrospective cohort | Monocentric | 7/20–1/21 | All patients with COVID-19 admitted to ICU | 126 | 44 | ICU | Severe | 100 | n/a |
| Bishburg, 2021 | Israel | Retrospective cohort | Monocentric | 10/3/20–10/4/20 | All patients with COVID-19 admitted to ICU | 89 | 8 | ICU | n/a | 100 | n/a |
| Boachie, 2022 | USA | Retrospective cohort | Multicentric | 1/3/20–31/1/21 | All hospitalized patients with COVID-19 | 1301 | 23 | Hospital | Any severity | 91 | 91 |
| Bretagne, 2021 | France | Retrospective cohort | Multicentric | 1/2/20–31/5/20 | All patients with COVID-19 and invasive fungal disease | 244 | 79 | ICU | Severe | 100 | n/a |
| Ceccarelli, 2022 | Italy | Retrospective cohort | Monocentric | 1/10/20–28/2/21 | All hospitalized patients with COVID-19 | 589 | 1 | Hospital | Any severity | 0 | n/a |
| Coskun, 2021 | Turkey | Retrospective cohort | Monocentric | 13/3/20–1/2/21 | All patients with COVID-19 admitted to ICU | 627 | 15 | ICU | Severe | 100 | n/a |
| Dixit, 2022 | USA | Case–control | Multicentric | 8/20–08/21 | Cases: patients with severe COVID-19 with confirmed candidemia Controls: patients with severe COVID-19 without candidemia |
275 | 91 | Hospital | Severe | 87.9 | n/a |
| Ioannou, 2022 | Greece | Retrospective cohort | Monocentric | 2/20–8/22 | All patients with COVID-19 with a positive culture for Candida spp. from any body part | 1536 | 6 | Hospital | Any severity | 0 | n/a |
| Kubin, 2021 | USA | Retrospective cohort | Multicentric | 2/3/20–31/5/20 | All hospitalized patients with COVID-19 | 3028 | 29 | Hospital | Any severity | n/a | n/a |
| Macauley, 2021 | USA | Retrospective cohort | Monocentric | 1/5/14–31/10/20 | All patients with blood cultures positive for Candida | 12 | 12 | ICU | Severe | 100 | n/a |
| Machado, 2022 | Spain | Retrospective cohort | Monocentric | 1/19–12/20 | All hospitalized patients with candidemia | 32 | 32 | Hospital | Any severity | 71.9 | 71.9 |
| Moin, 2021 | Pakistan | Retrospective cohort | Monocentric | 4/20–12/20 | All hospitalized patients with candidemia | 2438 | 26 | Hospital | Any severity | 80.7 | 65 |
| Nucci, 2021 | Brazil | Retrospective cohort | Monocentric | 1/19–9/20 | All hospitalized patients with candidemia | 9 | 9 | Hospital | Any severity | 100 | 100 |
| Omrani, 2021 | Qatar | Case–control | Monocentric | 1/3/20–30/4/21 | Cases: patients with COVID-19 with 1 or more episodes of candidemia Controls: patients without candidemia |
84 | 80 | ICU | Severe | 100 | n/a |
| Papadimitriou-Olivgeris, 2022 | Greece | Retrospective cohort | Monocentric | 4/20–8/21 | All patients admitted to the ICU with at least 1 positive blood culture for Candida spp. | 196 | 77 | ICU | Severe | 100 | n/a |
| Routsi, 2022 | Greece | Retrospective cohort | Monocentric | 3/20–10/20 | All patients with COVID-19 admitted to ICU | 600 | 62 | ICU | Severe | 100 | n/a |
| Sarvestani, 2021 | Iran | Retrospective cohort | Monocentric | 3/20–3/21 | All patients with COVID-19 and with at least 1 microbiologic and at least 1 clinical criterion for candidemia | 153 | 12 | Hospital | Any severity | 100 | 100 |
| Seagle, 2022 | USA | Retrospective cohort | Multicentric | 4/20–8/20 | All hospitalized patients with candidemia | 64 | 64 | Hospital | Any severity | 81 | 81 |
| Silva, 2021 | Brazil | Retrospective cohort | Monocentric | 5/20–11/20 | 212 hospitalized patients with COVID-19 | 212 | 3 | Hospital | Severe | n/a | n/a |
Of the included studies, the majority were conducted in the ICU (14 studies), and 7 were multicentric. In most of the studies, patients with candidemia were included, independently from the degree of severity. The sample size of patients with candidemia was overall small (range, 1–91; median [IQR], 19 [6.5–57.5]), and 9 of the studies (34.62%) had a sample size <10 patients.
Abbreviations: COVID-19, coronavirus disease 2019; ICU, intensive care unit; IQR, interquartile range.
The studies were all published between 2021 and 2022. Twenty of the 26 studies (76.92%) were started in the first semester of 2020, and 1 was conducted only in 2021. All studies were published in English. Twelve studies were conducted in Asia (46.15%), 7 in Europe (27.92%), 5 in North America (19.23%), and 2 in South America (7.69%). Five were case–control studies, while 21 were cohort studies. Most of the studies were monocentric (19/26, 73%). If only patients with both COVID-19 and candidemia were considered, all the studies had a small sample size.
Risk of Bias Assessment
The methodological quality assessment results of the included studies are reported in the Supplementary Materials (Supplementary Table 2 for retrospective cohort studies and Supplementary Table 3 for case–control studies). Overall, the quality of the included studies was good.
Candidemia Episodes Characteristics
The microbiological characteristics of candidemia episodes are shown in Table 2.
Table 2.
Isolated Candida Species (%) in Patients With COVID-19 and Candidemia
| Candida Species, % | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | C. albicans | C. auris | C. dubliniensis | C. glabrata | C. kefyr | C. krusei | C. lusitaniae | C. parapsilosis | C. tropicalis | Other |
| Allaw, 2022 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Altinkaya Çavus, 2022 | 55.1 | 0 | 0 | 9 | 0 | 0 | 0 | 17.9 | 11.5 | 6.4 |
| Arastehfar, 2021 | 62.5 | 0 | 0 | 37.5 | 0 | 0 | 0 | 0 | 0 | 0 |
| Arastehfar, 2022 | 43 | 0 | 0 | 4 | 0 | 4 | 4 | 25 | 21 | 0 |
| Avkan-Oğuz, 2022 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | 66 | 0 | 0 |
| Ayalon, 2022 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Bardi, 2022 | 83.3 | 0 | 0 | 16.7 | 0 | 0 | 0 | 0 | 0 | 0 |
| Beştepe, 2022 | 50 | 0 | 2 | 23 | 0 | 0 | 0 | 16 | 9 | 0 |
| Bishburg, 2021 | 25 | 0 | 0 | 25 | 0 | 0 | 0 | 25 | 25 | 0 |
| Boachie, 2022 | 65 | 0 | 4.3 | 22 | 0 | 0 | 4.3 | 4.3 | 0 | 0 |
| Bretagne, 2021 | 59.3 | 0 | 0 | 12.3 | 0 | 1.23 | 4.9 | 16 | 3.7 | 0 |
| Ceccarelli, 2022 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Coskun, 2021 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 43.75 | 31.25 | 0 |
| Dixit, 2022 | 52.7 | 3.3 | 2.2 | 18.7 | 0 | 0 | 0 | 11 | 15.4 | 7.7 |
| Ioannou, 2022 | 66.7 | 0 | 0 | 16.7 | 0 | 0 | 0 | 16.7 | 0 | 0 |
| Kubin, 2021 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Macauley, 2021 | 31 | 0 | 8 | 15 | 0 | 0 | 0 | 23 | 15 | 8 |
| Machado, 2022 | 68.8 | 0 | 0 | 9.4 | 3.1 | 0 | 0 | 6.2 | 12.5 | 0 |
| Moin, 2021 | 30.7 | 15 | 0 | 19.2 | 0 | 0 | 0 | 15 | 0 | 0 |
| Nucci, 2021 | 55.6 | 0 | 0 | 11.1 | 0 | 0 | 0 | 0 | 22.2 | 1 |
| Omrani, 2021 | 21.4 | 8.3 | 0 | 26.2 | 0 | 0 | 0 | 23.8 | 13.1 | 0 |
| Papadimitriou-Olivgeris, 2022 | 32.5 | 0 | 0 | 6.5 | 0 | 0 | 0 | 50.5 | 6.5 | 3.9 |
| Routsi, 2022 | 24.2 | 19 | 0 | 10.6 | 0 | 0 | 0 | 66 | 2 | 0 |
| Sarvestani, 2021 | 58.3 | 0 | 16.6 | 8.3 | 0 | 8.3 | 0 | 0 | 8.3 | 0 |
| Seagle, 2022 | 43.8 | 0 | 4.7 | 26.6 | 0 | 0 | 4.7 | 12.5 | 6.3 | 1.6 |
| Silva, 2021 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Percentage of Candida species isolated in each of the included studies. Only 2 studies did not report the percentage for each species. Overall C. albicans was the most popular pathogen, followed by C. glabrata, C. parapsilosis, and C. tropicalis.
Abbreviation: COVID-19, coronavirus disease 2019.
It was possible to retrieve microbiological data from all the included studies except for 2. The most represented was C. albicans. In 1 case, the only species described was C. auris, while in 4 studies the most common species was C. parapsilosis. Routine screening with BDG was performed in only 1 of the included studies. Resistance data were reported by 9 out of 26 studies (34.6%), of which only 6 reported both azole and echinocandin resistance data. Resistant strains ranged from 1% to 48.4% for azole resistance and from 0% to 33.3% for echinocandin resistance. Twenty articles reported data on antifungal treatment exposure in patients with COVID-19 and candidemia. Overall, a high percentage of patients were treated with antifungal therapy (range, 40.3%–100%). Concerning the studies conducted in-hospital, 7 studies reported the percentage of patients who acquired candidemia in the ICU (range, 65%–100%).
Patients’ Characteristics
Patients’ characteristics for each of the included studies are reported in Table 3. Most studies did not report specific data regarding the comorbidities of the recruited COVID-19 patients with candidemia. Of those that evaluated the comorbidities and risk factors of the recruited patients, the most reported conditions were steroid treatment exposure (15/26; range, 50%–100%), CVC positioning (17/26; range, 53%–100%), and antibiotic treatment exposure (15/26; range, 89.1%–100%). Patients’ immunosuppressive status was evaluated in 14 of the 26 studies, ranging from 3.8% to 100%.
Table 3.
Sex, Age, and Clinical Characteristics of Patients With COVID-19 and Candidemia in the Included Studies
| Author | Age, Mean (SD) or Median [IQR] | Male, % | Antifungal Treatment, % | Antibiotic Treatment, % | Steroid Treatment, % | HTX, % | DM, % | CKD, % | COPD, % | IS, % | NET, % | CVC, % | EOT, % | RRT, % | Surgery, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allaw, 2022 | 68.4 (14.2) | 59.4 | n/a | 100 | 100 | n/a | 40.6 | 18.8 | 21.9 | 18.7 | 3.1 | 93.8 | 84.4 | 25 | n/a |
| Altinkaya Çavus, 2022 | 71.33 (13.67) | 69.2 | 43.5 | 96.1 | 62.8 | 38.4 | 29.4 | 17.9 | 17.9 | 16.6 | 30.8 | 53 | 62.8 | 14.2 | n/a |
| Arastehfar, 2021 | 51.5 (22.3) | n/a | 100 | 100 | n/a | n/a | 16.7 | n/a | n/a | 16.7 | 100 | 100 | 83.3 | n/a | 16.7 |
| Arastehfar, 2022 | 65 [50–73] | 75 | 96 | 100 | 71 | 39 | 21 | 14 | 24 | 32 | n/a | 86 | 89 | n/a | n/a |
| Avkan-Oğuz, 2022 | 69 | 33.3 | 100 | 100 | 100 | n/a | 33.3 | n/a | n/a | 33.3 | n/a | 100 | 100 | n/a | n/a |
| Ayalon, 2022 | n/a | n/a | 81.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Bardi, 2022 | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Beştepe, 2022 | 74.8 (10) | 61 | 52 | 100 | 100 | 61 | 50 | 21 | 25 | 73 | 87 | 80 | 89 | n/a | n/a |
| Bishburg, 2021 | 65 [48–73] | 50 | 100 | 100 | 100 | n/a | n/a | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a |
| Boachie, 2022 | 66 (10) | 61 | 82 | 91 | 96 | 65 | 52 | 22 | 9 | n/a | n/a | 74 | 87 | 39 | n/a |
| Bretagne, 2021 | 65 (18) | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Ceccarelli, 2022 | n/a | n/a | 100 | 100 | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | no | n/a | n/a |
| Coskun, 2021 | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a |
| Dixit, 2022 | 62 [54.5–72] | 67 | 93.8 | 100 | 74.7 | 63.7 | 41.8 | 8.8 | 13.2 | 6.6 | 9.9 | 94.5 | 69.2 | n/a | n/a |
| Ioannou, 2022 | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Kubin, 2021 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Macauley, 2021 | 62 (16) | 75 | n/a | 100 | 50 | n/a | 8 | 0 | n/a | 0 | 16 | 100 | 92 | 83 | n/a |
| Machado, 2022 | 65.5 [58–73.8] | 71.9 | 93.7 | 96.9 | 84.4 | n/a | 21.9 | 15.6 | 12.5 | 38.4 | 100 | 93.8 | n/a | 18.8 | 9.4 |
| Moin, 2021 | 56.8 [0.8–82] | 61.5 | 88.4 | 100 | 84.6 | n/a | n/a | n/a | n/a | 3.8 | n/a | 73.1 | 80.7 | n/a | n/a |
| Nucci, 2021 | 62 [54–87] | 55.5 | 66.7 | n/a | n/a | n/a | 33.3 | 11.1 | 0 | 11,1 | 0 | 100 | 100 | 0 | 0 |
| Omrani, 2021 | 61 [51–70] | 85 | 88 | n/a | 88.7 | 56.3 | 48.8 | 18.8 | 6.3 | 3.8 | n/a | 96.3 | 95 | 45 | 1.3 |
| Papadimitriou-Olivgeris, 2022 | 68 [58–77] | 62.3 | 40.3 | 100 | 94.8 | n/a | 28.6 | 6.5 | 15.6 | 16.9 | n/a | n/a | n/a | n/a | 7.8 |
| Routsi, 2022 | 69 [15.8] | 72.4 | n/a | n/a | n/a | n/a | 25.8 | n/a | n/a | 8.1 | n/a | n/a | n/a | n/a | n/a |
| Sarvestani, 2021 | 57.5 [32–85] | 33.3 | 100 | 100 | n/a | n/a | 50 | n/a | n/a | 100 | n/a | 100 | 100 | n/a | n/a |
| Seagle, 2022 | n/a | 64.1 | 85.9 | 89.1 | 53.1 | n/a | 53.1 | 7.8 | 28.1 | 15.7 | 6.3 | 79.7 | 81 | 37.5 | 4.7 |
| Silva, 2021 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Age is expressed as mean (SD) or median [IQR], and other variables are expressed as %.
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease; CVC, central venous catheter; DM, diabetes mellitus; EOT, endo-oral tube; HTX, hypertension; IQR, interquartile range; IS, immunosuppressive status; NET, enteral nutrition therapy; RRT, renal replacement therapy.
Analysis of Mortality
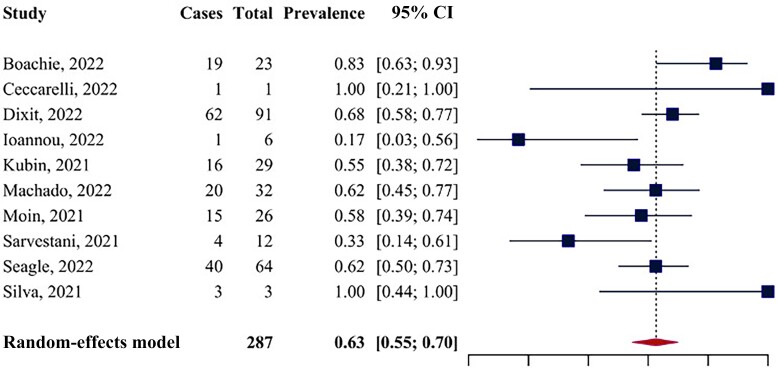
Ten studies assessed in-hospital mortality. The pooled mortality rate was 62.62% (95% CI, 54.77% to 69.86%) (Figure 2). Cochran's Q was significant (Q = 19.85; P = .019), and the I² index was equal to 32.7%, indicating low heterogeneity. The funnel plot did not reveal strong asymmetry, although the small number of included studies prevents reaching conclusions about the presence or absence of publication bias (Supplementary Figure 1). The sensitivity analysis performed excluding studies with a sample size <10 (n of studies = 7) showed similar results, with a mortality rate equal to 63.54% (95% CI, 57.71% to 69%) (Supplementary Figure 2). In this model, Cochran's Q was nonsignificant (Q = 10.66; P = .10), and the I² index was equal to 37.4%.
Figure 2.
Forest plot for in-hospital mortality.
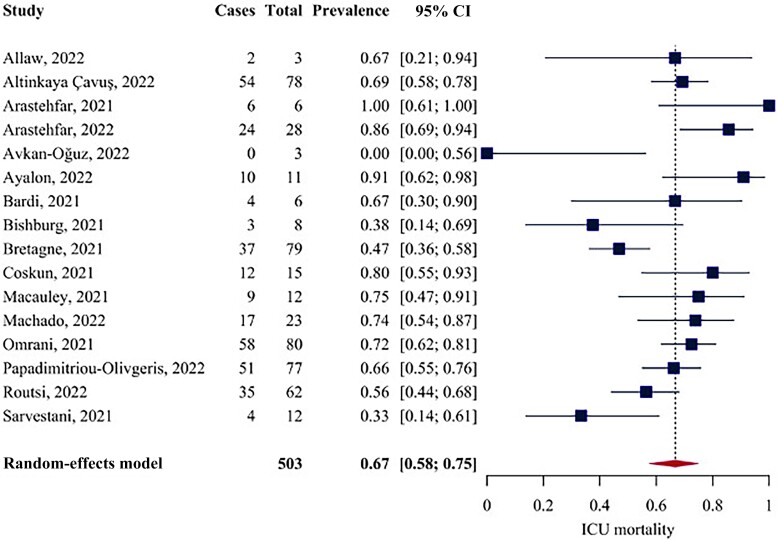
Analysis of in-ICU Mortality
Of the included studies, 16 assessed in-ICU mortality. The pooled mortality rate was 66.77% (95% CI, 57.70% to 74.75%) (Figure 3). Cochran's Q was significant (Q = 47.61; P < .001), and the I2 was equal to 53.5%, indicating medium heterogeneity. The funnel plot did not reveal strong asymmetry (Supplementary Figure 3). Excluding the 5 studies with a sample size <10, the sensitivity analysis (n of studies = 11) revealed a mortality rate of 67.98% (95% CI, 58.91% to 75.88%) (Supplementary Figure 4). This analysis detected higher heterogeneity (Q = 33.63; P < .001; I2 = 67.98%). Three studies reported the 30-day mortality rate in the ICU. The pooled 30-day mortality rate was 65.31% (95% CI, 57.27% to 75.55%). No heterogeneity was detected among these studies (Q = 2.27; P = .32; I2 = 10.2%).
Figure 3.
Forest plot for in-ICU mortality. Abbreviation: ICU, intensive care unit.
Length of Stay
Of the included studies, 4 assessed hospital LOS. The pooled median hospital LOS was 30.41 (95% CI, 12.28 to 48.55) days. However, high heterogeneity was detected (Q = 71.41; P < .001; I2 = 95.29%). The funnel plot of the study estimates was not drawn due to their low amount. Considering the studies carried only in ICU (n of studies = 7), the median ICU LOS was 28.29 (95% CI, 20.84 to 35.73) days. The heterogeneity was high (Q = 47.35; I2 = 86.87%). The funnel plot revealed a left asymmetry (Supplementary Figure 3, Supplementary Materials).
Subgroup Analyses and Meta-regression
Regarding in-hospital mortality, the subgroup analyses were performed using the following variables: study design, geographical area, the inclusion of only patients with severe COVID-19, and the semester in which the study was started (Supplementary Table 4). These analyses did not allow for identifying subgroups of studies with different mortality rates or explaining the heterogeneity found in the overall analysis. Regarding the geographical area, there were 2 studies conducted in Asia, 3 in Europe, 4 in North America, and only in 1 in South America. The test for differences was not significant, the minimum point estimate was 50, and the maximum was 100; however, this last estimate was retrieved from a single study with 9 patients. Because of the small number of included studies, it was impossible to perform a meta-regression to assess the impact of continuous variables on the estimated mortality rates.
Regarding in-ICU mortality, the subgroup analyses were performed using the following variables: study design, geographical area, and semester in which the study was started (Supplementary Table 5). Similar to the subgroup analyses on in-hospital mortality, none of these analyses identified subgroups with different mortality rates or helped explain the overall analysis's heterogeneity. In particular, concerning the geographical area, 10 studies were performed in Asia, 5 in Europe, and 1 in North America. The test for differences was not significant, with a minimum point estimate of 59.59 for Europe and a maximum of 75 for North America.
The variables that could be included in the meta-regression model were percent male, percentage of C. albicans isolates, and age. Overall, a higher percent male was associated with a significantly higher mortality rate (β = 0.42; 95% CI, 0.02 to 0.07; P = .0011). The analysis also showed that the percentage of C. albicans isolates was not associated with a higher mortality rate (β = 1.72; 95% CI, −0.02 to 3.46; P = .053), similar to age (β = −0.013%; 95% CI, −0.07 to 0.04; P = .65).
DISCUSSION
In this systematic review with meta-analysis, we found that patients with COVID-19 and candidemia have an in-hospital mortality rate of ∼63% and an in-ICU mortality rate of ∼67%. Moreover, the median LOS was 30 days.
Studies in the pre-COVID-19 era performed to assess the mortality of this condition found the rate to be as high as 50%–60% in ICU settings [22, 23], which is similar to the mortality rate in the ICU that we found in this study (20%–40% in non-ICU settings) [24, 25]. We acknowledge that none of the included papers excluded patients admitted to the ICU. In addition, in the studies conducted at the hospital level, we observed that a high percentage of patients were at some point admitted to the ICU. However, it was often impossible to obtain specific data on the timing of candidemia onset (eg, before or after the transfer to ICU). Hence, the lack of these data prevents us from attributing a specific mortality to COVID-19 patients with candidemia hospitalized in non-ICU settings.
Candidemia has been widely reported in patients hospitalized with severe SARS-CoV-2 infection [15]. These patients have greater exposure to known health care–associated candidemia risk factors, namely the presence of CVCs, underlying conditions such as malignancies, diabetes, or chronic kidney disease requiring hemodialysis, and receipt of corticosteroids, total parenteral nutrition, and antibiotics [26]. However, some authors have also suggested that COVID-19 is likely a risk factor for both candidemia and candidemia-associated mortality [27]. Despite providing information concerning the epidemiology of risk factors associated with candidemia development, the studies included in our systematic review only occasionally reported the putative source of infection. Therefore, we could not come to solid conclusions concerning the “primum movens” of candidemia in patients hospitalized with COVID-19. Additional data concerning the causes of candidemia would allow implementation of infection control procedures and diagnostic protocols aiming to reduce occurrence and mortality related to candidemia.
Concerning LOS, our results are in line with those of other studies, which showed a high LOS in patients with candidemia [28, 29]. However, it is to be noted that the estimates from the included studies had high heterogeneity and should therefore be assessed with caution. As we included studies from very different geographical areas and contexts, one could have expected important differences in mortality rates between different continents. However, the subgroup analyses based on continents showed that the range of in-hospital mortality across continents was between 59% and 75% for in-ICU mortality, and 50% to 69% (excluding the South American subgroup as it included only a single study with 9 patients and found a very imprecise 100% estimate) for in-hospital mortality. Therefore, even if there are differences across geographical areas, the mortality rate of Candida comorbid with COVID-19 remains high worldwide, and the variations in LOS likely could be due to unobserved hospital effects [30, 31].
All the analyses are influenced by the fact that the included studies had a small sample size, and more than one-third of them included <10 patients with both COVID-19 and candidemia. The sensitivity analyses performed excluding these latter studies found similar mortality rates compared with the overall calculations, suggesting that the sample size did not significantly impact the estimate’s magnitude. However, the small number of retrieved studies and their small sample sizes indicate that the precision of the pooled mortality estimates we found might be low. This was expected as comorbidity of COVID-19 and candidemia is not prevalent. Regarding heterogeneity, it was low in the analysis of in-hospital mortality, medium in the analysis of mortality in the ICU, nonsignificant in the analysis of 30-day mortality in the ICU, and high in the analysis of LOS. The preplanned subgroup analyses did not help to explain their sources in any of the analyses. As a result, the point values of the pooled mortality rates found in these analyses should be examined with caution. Nonetheless, as all the analyses and subgroup analyses found that both in-hospital and in-ICU mortality rates were consistently >50%, we believe it is safe to assume that patients with candidemia and COVID-19 have an increased risk of mortality.
In conclusion, our study emphasizes the high morbidity and mortality of candidemia in patients with COVID-19. This result requires us to be extremely cautious with all patients infected with SARS-CoV-2, especially those with already known risk factors for candidemia. More extensive clinical studies evaluating subjects suffering from this fungal infection and simultaneously infected with SARS-CoV-2 would be necessary to achieve more reliable findings on mortality and LOS.
Supplementary Material
Acknowledgments
Financial support. There was no specific funding source for this study.
Patient consent. Our study does not include factors necessitating patient consent.
Contributor Information
Marta Colaneri, Department of Clinical Sciences, Infectious Diseases and Immunopathology, Università di Milano, L. Sacco Hospital, Milan, Italy; Centre for Multidisciplinary Research in Health Science (MACH), University of Milano, Milano, Italy.
Emanuele Maria Giusti, EPIMED Research Center, Department of Medicine and Surgery, University of Insubria, Varese, Italy.
Camilla Genovese, Department of Clinical Sciences, Infectious Diseases and Immunopathology, Università di Milano, L. Sacco Hospital, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milano, Milano, Italy.
Lucia Galli, Department of Clinical Sciences, Infectious Diseases and Immunopathology, Università di Milano, L. Sacco Hospital, Milan, Italy; Department of Pathophysiology and Transplantation, University of Milano, Milano, Italy.
Andrea Lombardi, Department of Pathophysiology and Transplantation, University of Milano, Milano, Italy; Infectious Diseases Unit, Foundation IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milano, Italy.
Andrea Gori, Department of Clinical Sciences, Infectious Diseases and Immunopathology, Università di Milano, L. Sacco Hospital, Milan, Italy; Centre for Multidisciplinary Research in Health Science (MACH), University of Milano, Milano, Italy.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Bassetti M, Giacobbe DR, Vena A, et al. Incidence and outcome of invasive candidiasis in intensive care units (ICUs) in Europe: results of the EUCANDICU project. Crit Care 2019; 23:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Koehler P, Stecher M, Cornely OA, et al. Morbidity and mortality of candidaemia in Europe: an epidemiologic meta-analysis. Clin Microbiol Infect 2019; 25:1200–12. [DOI] [PubMed] [Google Scholar]
- 3. Kutlu M, Sayın-Kutlu S, Alp-Çavuş S, et al. Mortality-associated factors of candidemia: a multi-center prospective cohort in Turkey. Eur J Clin Microbiol Infect Dis 2022; 41:597–607. [DOI] [PubMed] [Google Scholar]
- 4. Leroy O, Gangneux J-P, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006). Crit Care Med 2009; 37:1612–8. [DOI] [PubMed] [Google Scholar]
- 5. Lausch KR, Søgaard M, Rosenvinge FS, et al. High incidence of candidaemia in a nationwide cohort: underlying diseases, risk factors and mortality. Int J Infect Dis 2018; 76:58–63. [DOI] [PubMed] [Google Scholar]
- 6. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med 2015; 373:1445–56. [DOI] [PubMed] [Google Scholar]
- 7. Falagas ME, Apostolou KE, Pappas VD. Attributable mortality of candidemia: a systematic review of matched cohort and case-control studies. Eur J Clin Microbiol Infect Dis 2006; 25:419–25. [DOI] [PubMed] [Google Scholar]
- 8. Antinori S, Bonazzetti C, Gubertini G, et al. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: an increased risk for candidemia? Autoimmun Rev 2020; 19:102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kokkoris S, Papachatzakis I, Gavrielatou E, et al. ICU-acquired bloodstream infections in critically ill patients with COVID-19. J Hosp Infect 2021; 107:95–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Machado M, Estévez A, Sánchez-Carrillo C, et al. Incidence of candidemia is higher in COVID-19 versus non-COVID-19 patients, but not driven by intrahospital transmission. J Fungi (Basel) 2022; 8:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paiva JA, Pereira JM, Tabah A, et al. Characteristics and risk factors for 28-day mortality of hospital acquired fungemias in ICUs: data from the EUROBACT study. Crit Care 2016; 20:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dursun ZB, Sipahioǧlu H, Yüksel RC, Sav H, Çelik I. Risk factors and lethality associated with candidemia in severe COVID-19 patients. Curr Med Mycol 2022; 8:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kayaaslan B, Eser F, Kaya Kalem A, et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021; 64:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCarty T. Candidemia and severe coronavirus disease 2019: which risk factors are modifiable? Clin Infect Dis 2022; 74:812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nucci M, Barreiros G, Guimarães LF, Deriquehem VAS, Castiñeiras AC, Nouér SA. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021; 64:152–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Macauley P, Epelbaum O. Epidemiology and mycology of candidaemia in non-oncological medical intensive care unit patients in a tertiary center in the United States: overall analysis and comparison between non-COVID-19 and COVID-19 cases. Mycoses 2021; 64:634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NHLBI, NIH . Study quality assessment tools. Available at: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed April 14, 2023.
- 18. Lin L, Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology 2020; 31:713–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McGrath S, Sohn H, Steele R, Benedetti A. Meta-analysis of the difference of medians. Biometrical J 2020; 62:69–98. [DOI] [PubMed] [Google Scholar]
- 20. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGrath S, Zhao X, Katzenschlager S, Ozturk O, Steele R, Benedetti A. metamedian: meta-analysis of medians. R package version 1.0.0. Available at: https://CRAN.R-project.org/package=metamedian. Accessed April 11, 2023.
- 22. Puig-Asensio M, Pemán J, Zaragoza R, et al. Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med 2014; 42:1423–32. [DOI] [PubMed] [Google Scholar]
- 23. Schroeder M, Weber T, Denker T, et al. Epidemiology, clinical characteristics, and outcome of candidemia in critically ill patients in Germany: a single-center retrospective 10-year analysis. Ann Intensive Care 2020; 10:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sbrana F, Sozio E, Bassetti M, et al. Independent risk factors for mortality in critically ill patients with candidemia on Italian internal medicine wards. Intern Emerg Med 2018; 13:199–204. [DOI] [PubMed] [Google Scholar]
- 25. Vena A, Bouza E, Valerio M, et al. Candidemia in non-ICU surgical wards: comparison with medical wards. PLoS One 2017; 12:e0185339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Poissy J, Damonti L, Bignon A, et al. Risk factors for candidemia: a prospective matched case-control study. Crit Care 2020; 24:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seagle EE, Jackson BR, Lockhart SR, et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin Infect Dis 2022; 74:802–11. [DOI] [PubMed] [Google Scholar]
- 28. Hassan I, Powell G, Sidhu M, Hart WM, Denning DW. Excess mortality, length of stay and cost attributable to candidaemia. J Infect 2009; 59:360–5. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Z, Zhu R, Luan Z, Ma X. Risk of invasive candidiasis with prolonged duration of ICU stay: a systematic review and meta-analysis. BMJ Open 2020; 10:e036452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Antinori S, Milazzo L, Sollima S, Galli M, Corbellino M. Candidemia and invasive candidiasis in adults: a narrative review. Eur J Intern Med 2016; 34:21–8. [DOI] [PubMed] [Google Scholar]
- 31. Muderris T, Kaya S, Ormen B, Aksoy Gokmen A, Varer Akpinar C, Yurtsever Gul S. Mortality and risk factor analysis for Candida blood stream infection: a three-year retrospective study. J Mycol Med 2020; 30:101008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.