Abstract
Background
Virological outcome data after programmatic transition from non-nucleoside reverse transcriptase inhibitor (NNRTI)-based to dolutegravir (DTG)-based antiretroviral therapy (ART) regimens in sub-Saharan Africa (SSA) outside of clinical trials are scarce. We compared viral suppression and associated factors in treatment-naïve people living with HIV (PLHIV) starting DTG- based versus NNRTI-based ART.
Methods
We compared virological suppression at 12 months, after treatment initiation in the two cohorts of participants aged ≥15 years, initiating DTG- and NNRTI-based ART. Drug resistance was assessed among participants with viremia ≥50 copies/mL on DTG.
Results
Viral suppression was achieved for 165/195 (85%) and 154/211 (73%) participants in the DTG- and NNRTI- cohorts, respectively (P = 0.003). DTG-based ART was associated with >2 times the odds of viral suppression versus NNRTI-based ART (adjusted odds ratio, 2.10 [95% confidence interval {CI}, 1.12–3.94]; adjusted risk ratio, 1.11 [95% CI, 1.00–1.24]). HIV-1 genotypic resistance testing (GRT) before ART initiation was done in 14 of 30 viremic participants on DTG, among whom nucleoside reverse transcriptase inhibitor (NRTI), NNRTI, and protease inhibitors resistance was detected in 0 (0%), 2 (14%) and 1 (7%), respectively. No resistance was found in the 2 of 30 participants with available GRT at the time of viremia ≥50 copies/mL.
Conclusions
Virological suppression at 1 year was higher in participants initiating DTG- versus NNRTI-based ART. In those with viremia ≥50 copies/mL on DTG-based ART, there was no pretreatment or acquired resistance to the DTG co-administered NRTIs, although the number of samples tested was small.
Keywords: HIV-1, ART-naive, dolutegravir, drug resistance, sub-Saharan Africa
Globally, almost 38 million people are living with human immunodeficiency virus (HIV) and two-thirds of these reside in sub-Saharan Africa (SSA) [1]. To end the HIV epidemic with the global 95-95-95 targets set by the Joint United Nations Programme on HIV/AIDS for 2025, 1 key element is access to antiretroviral therapy (ART) for all people with HIV (PWH) [2, 3]. Until recently, in most low- and middle-income countries (LMICs), first-line ART consisted of a nonnucleoside reverse transcriptase inhibitor (NNRTI)–based regimen containing either nevirapine or efavirenz. However, in the last years a substantial increase of pretreatment drug resistance (PDR) for NNRTIs has been observed in SSA [4], in some countries exceeding the World Health Organization (WHO)–recommended threshold of 10% [5], thus compromising the ambitious target to end the HIV epidemic by 2030 [6]. PDR resulting in virologic failure might go undetected for long timespans in LMICs due to limited resources for viral load (VL) and resistance testing, leading to increased morbidity, mortality, and onward transmission of virus [7]. Therefore, transition to integrase inhibitors (INSTIs), characterized by a faster viral suppression, a higher barrier to resistance, and fewer side effects compared to NNRTIs within randomized controlled trials (RCTs) [8], has been advocated by many national HIV programs.
By the end of 2017, dolutegravir (DTG)–based therapy became available to LMICs as the generic fixed-dose combination of tenofovir disoproxil fumarate, lamivudine, and dolutegravir at the favorable price of 75 US dollars per person per year [9]. Since then, many LMICs have rolled out DTG-based ART. Initially, the WHO advised women of childbearing age to avoid DTG-based ART when planning a pregnancy or not being on consistent contraception, due to a signal for an increased risk of neural tube defects in the offspring of women on DTG-based ART at conception in the Tsepamo study in Botswana [10]. After more data became available showing a considerably lowered risk of neural tube defects, the WHO recommended DTG-based ART for all adolescents and adults in July 2019.
So far, limited data outside of clinical trials are available on the clinical and virologic outcome after the transit from NNRTI-based to DTG-based regimens in SSA [11–13]. Evaluation of the rollout of DTG-based ART under programmatic conditions in resource-limited regions is important given the challenges outlined above. With this study, we aimed to compare viral suppression in treatment-naive patients at 12 months after initiating DGT-based versus NNRTI-based ART. Additionally, we assessed predictors associated with viral suppression and analyzed PDR mutations and acquired resistance, side effects, and pregnancy outcomes in patients initiated on a DTG-based regimen.
METHODS
Study Design and Setting
This is a retrospective, observational study nested within the Kilombero and Ulanga antiretroviral cohort (KIULARCO), a prospective cohort of PWH. The cohort includes patients seen at the Chronic Diseases Clinic of Ifakara, the care and treatment center for PWH of the Saint Francis Referral Hospital, located in rural southeastern Tanzania. The cohort was established in 2005 and captures comprehensive demographic and clinical data, including ART use and monitoring, comorbidities, and treatment outcomes, with details described previously [14, 15].
Study Population
Two groups of PWH aged ≥15 years attending KIULARCO were included in the analysis: (1) treatment-naive patients initiating NNRTI-based ART between 16 December 2016 and 15 December 2017 (referred to as the NNRTI cohort) and (2) treatment-naive patients initiating DTG-based ART between 16 March 2019 and 15 September 2020 (referred to as the DTG cohort). The separation of periods ensured that no patient in the NNRTI cohort would have been switched to a DTG-based regimen by the time of outcome assessment, which could have had an impact on VL results.
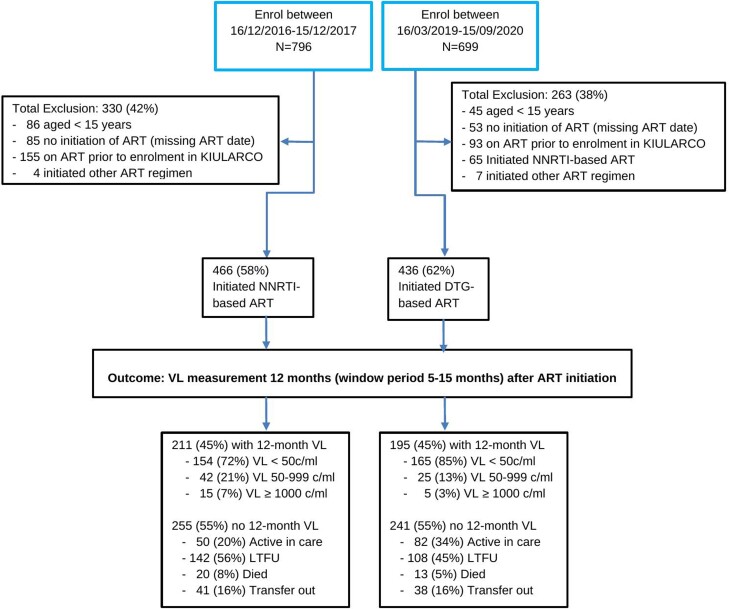
We excluded patients initiating protease inhibitor (PI)–based therapy in both periods, patients starting NNRTI-based ART between 16 March 2019 and 15 September 2020, treatment-experienced patients (assessed in the baseline questionnaire by asking if they had ever received ART before), those not starting ART during the given time periods, and those without written informed consent to KIULARCO (Figure 1). Reasons for excluding patients starting PI-based ART was that this is not the usual first-line therapy in Tanzania. Similarly, those initiating NNRTI-based ART during DTG rollout were excluded as they were only few and would have only started on this therapy due to specific criteria.
Figure 1.
Participant flowchart. Abbreviations: ART, antiretroviral therapy; c/mL, copies per milliliter; DTG, dolutegravir; KIULARCO, Kilombero and Ulanga Antiretroviral Cohort; LTFU, lost to follow-up; NNRTI, nonnucleoside reverse transcriptase inhibitor; VL, viral load.
Data Collection
Data for patient demographics, routine clinical information, and laboratory data as well as information on ART were extracted from OpenMRS, the KIULARCO electronic medical record system. As per routine care and according to the Tanzania National Guidelines for the Management of HIV and AIDS, patients receive a VL measurement at 6 and 12 months after treatment start, and once yearly thereafter for those with a VL <1000 copies/mL. At the same time point as VL measurements, blood samples are stored in a biobank at −80°C for research purposes.
Laboratory Measurements
VL measurement was done using the Abbott Real-time m2000 HIV-1 Assay (Abbott Laboratories, Chicago, Illinois), with a reportable range of 40–10 000 000 copies/mL for blood plasma. For this study, we performed HIV-1 genotypic resistance testing (GRT) from biobanked samples for those with an HIV VL ≥50 copies/mL. In addition, HIV-1 GRT was performed on samples available prior to treatment initiation for cases with a VL ≥50 copies/mL at 12 months using cryopreserved samples, to determine PDR before ART initiation in the DTG cohort.
GRT was performed using a validated in-house polymerase chain reaction (PCR) protocol to determine the HIV-1 drug resistance–associated mutations for reverse transcriptase, protease, and INSTI [16, 17]. In brief, RNA was extracted from 150 μL of plasma using the PureLink Viral RNA/DNA Mini Kit (Invitrogen, Thermo Fisher Scientific), according to the manufacturer's protocol. RNA extracts were retrotranscribed and amplified using the HIV-1 Genotyping Kit Amplification Module (Applied Biosystems, Thermo Fisher Scientific). A direct sequencing reaction was done using 6 overlapping primers, and assembly program (BioEdit 7.2) was used for sequence analysis. Mutations were interpreted according to the Stanford University's HIV Drug Resistance Database Program version 9.2 (http://hivdb.stanford.edu). Drug resistance mutations conferring low, intermediate, or high-level resistance were considered. The reported protease and reverse transcriptase sequences are available in GenBank (accession number OQ627458-OQ627474).
Definitions
Viral suppression was defined as <50 copies/mL and viremia as defined as ≥50 copies/mL, at 1 time-point measurement, and virological failure was defined as ≥1000 copies/mL after a minimum of 6 months on ART, based on 2 consecutive VL measurements in 3 months (with adherence support following the first VL test), in line with the most recent WHO guidelines [18]. Loss to follow-up (LTFU) was defined as being >60 days late for a scheduled visit.
Tuberculosis was recorded if, within 3 months from enrollment, acid-fast bacilli or a positive Xpert MTB/RIF assay (Cepheid, Sunnyvale, California) from sputum or an extrapulmonary site was documented, or if antituberculosis drugs with an International Classification of Diseases, Tenth Revision (ICD-10) code or clinical signs suggestive of tuberculosis were present. Unlikely tuberculosis was defined as no prescription of antituberculosis drugs and no diagnosis of tuberculosis by ICD-10. For other cases, an indeterminate tuberculosis status was stated and treated as missing data.
Study Outcomes
The primary outcome was viral suppression at 12 months, allowing for a time window of 5–15 months with a preference for the measurement closest to 12 months after treatment start. The secondary outcomes of this study were prevalence of PDR and acquired resistance among patients presenting with viremia on DTG-based ART (defined as the presence of resistance-associated mutations), plus, side effects and pregnancy outcomes in the DTG cohort.
Statistical Methods
Demographic characteristics were summarized using frequencies and percentages. We descriptively compared baseline characteristics between participants with and without a VL result at 12 months. The proportion of participants with viral suppression in the DTG and NNRTI cohorts was compared using a χ2 test. Factors associated with 12-month viral suppression were determined using logistic regression models, reporting odds ratios and 95% confidence intervals (CIs). We also calculated adjusted risk ratios (aRRs) after fitting the multivariable model with standard errors estimated using the delta method [19]. The multivariable model incorporated the following covariates, which were selected a priori: age, sex, marital status, disclosure of HIV status, education level, distance in kilometers of residence from the clinic, body mass index (BMI), HIV WHO stage, CD4 cell count, tuberculosis status, and cohort (DTG-based or NNRTI-based ART). No model selection was done. We performed a sensitivity analysis restricting VL measurement to a time window of 9–15 months from treatment start. The analysis was repeated under the assumption that those LTFU were not virally suppressed, as such participants are likely to be off treatment and therefore not suppressed.
The prevalence of HIV-1 drug resistance, the proportion of patients who discontinued DTG-based ART, and the proportion of women who conceived while on DTG-based ART and their pregnancy outcomes were described descriptively. All analyses were performed using Stata version 15 (StataCorp LP, College Station, Texas).
Ethical Considerations
Written informed consent of patients willing to participate in KIULARCO are obtained at registration. This study received ethical approval from the University of the Witwatersrand Human Research Ethics Committee (Medical) Clearance Certificate No. M210714. Yearly ethical approval for data and sample collection as well as analysis is sought from the Ifakara Health Institute institutional review board (IHI/IRB/No16-2006) and the Health Review Committee of the National Institute for Medical Research of Tanzania (NIMR/HQ/R.8c/Vol.I/378).
RESULTS
Study Population and Baseline Characteristics
There were 436 and 466 patients in the DTG and NNRTI cohorts, respectively (Figure 1). The combined median age in both cohorts was 38 years (interquartile range [IQR], 30–45 years). In the DTG and NNRTI cohorts, 280 (64%) and 300 (65%) patients were female, 292 (67%) and 263 (57%) were married/cohabiting, 290 (72%) and 318 (79%) had disclosed their HIV status, and 51 (12%) and 106 (25%) were resident ≥50 km from clinic, respectively. The clinical parameters were broadly comparable within the 2 groups, with a normal BMI (18.5–25 kg/m2) in 230 (57%) and 259 (62%), a WHO stage III/IV in 107 (25%) and 166 (39%), a CD4 count of ≥350 cells/mL in 142 (37%) and 134 (34%), and tuberculosis coinfection in 33 (8%) and 52 (11%) participants, respectively (Table 1).
Table 1.
Patient Characteristics at Initiation of Nonnucleoside Reverse Transcriptase Inhibitor– or Dolutegravir-Based Antiretroviral Therapy Regimens
| Patient Characteristics | Initiated NNRTI-Based ART 2016–2017 (n = 466) | Initiated DTG-Based ART 2019–2021 (n = 436) |
|---|---|---|
| Sociodemographics | ||
| Age, y | ||
| 15–24 | 52 (11) | 53 (12) |
| 25–34 | 136 (29) | 120 (28) |
| 35–44 | 164 (35) | 138 (32) |
| ≥45 | 111 (24) | 125 (29) |
| Sex, female | 300 (65) | 280 (64) |
| Marital status | ||
| Married/cohabiting | 263 (57) | 292 (67) |
| Never married | 63 (14) | 28 (6) |
| Separated/divorced/widowed | 137 (30) | 116 (27) |
| Disclosed HIV status | ||
| No | 85 (21) | 113 (28) |
| Yes | 318 (79) | 290 (72) |
| Missing | 60 (13) | 33 (8) |
| Education | ||
| None | 64 (14) | 28 (6) |
| Primary | 347 (75) | 368 (84) |
| Secondary and above | 52 (11) | 40 (9) |
| Distance of residence to clinic | ||
| <1 km | 182 (42) | 240 (56) |
| 2 to <50 km | 141 (33) | 135 (32) |
| ≥50 km | 106 (25) | 51 (12) |
| Missing | 34 (7) | 10 (2) |
| Clinical | ||
| ART regimen | ||
| DTG-based | 0 (0) | 436 (100) |
| EFV-based | 456 (98) | 0 (0) |
| NVP-based | 9 (2) | 0 (0) |
| Other | 1 (0) | 0 (0) |
| Tuberculosis statusa | ||
| Unlikely | 405 (89) | 395 (92) |
| Yes | 52 (11) | 33 (8) |
| Missing | 6 (1) | 8 (2) |
| BMIa, kg/m2 | ||
| Underweight (<18.5) | 66 (16) | 52 (13) |
| Normal (18.5–24.9) | 259 (62) | 230 (57) |
| Overweight (≥25) | 63 (15) | 123 (30) |
| Missing | 43 (9) | 31 (7) |
| HIV WHO stagea | ||
| I/II | 260 (61) | 316 (75) |
| III/IV | 166 (39) | 107 (25) |
| Missing | 37 (8) | 13 (3) |
| CD4 count, cells/µLa | ||
| <100 | 92 (24) | 62 (16) |
| 100–350 | 166 (42) | 184 (47) |
| ≥350 | 134 (34) | 142 (37) |
| Missing | 71 (15) | 48 (11) |
Data are presented as No. (column %) of those with nonmissing data; missing data rows are No. (column %).
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; DTG, dolutegravir; EFV, efavirenz; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; NVP, nevirapine; WHO, World Health Organization.
Tuberculosis, BMI, HIV WHO stage, and CD4 measurements closest to ART initiation within 6 months before and 3 months after.
We compared patients with and those without VL tests at 12 months and found them to be broadly comparable with the exception of those with 12-month VL more likely to have disclosed their HIV status (309 [81%] vs 301 [70%]) and to be resident <1 km from clinic (210 [54%] vs 213 [45%]), and less likely to be HIV WHO stage III/IV (115 [29%] vs 159 [35%]) and to have a CD4 count ≥350 cells/µL (118 [31%] vs 151 [38%]). Those without 12-month VL were more likely to have missed baseline characteristic measurements compared to those with VL (Supplementary Table 1).
Virological Outcome
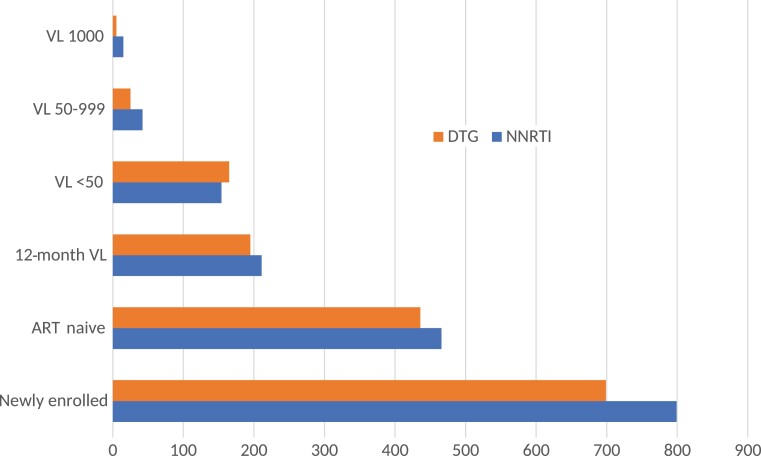
VL data at 12 months after ART initiation were available for 195 of 436 (45%) and 211 of 466 (45%) participants in the DTG and NNRTI cohorts, respectively (Figure 2). Among those with VL results available, viral suppression was achieved for 165 of 195 (85%) and 154 of 211 (73%) participants in the DTG and NNRTI cohorts, respectively (P = .003). For those with viremia in the DTG and NNRTI cohorts, 25 of 30 (83%) and 42 of 57 (74%) had a VL 50–999 copies/mL and 5 of 30 (17%) and 15 of 57 (26%) had VL ≥1000 copies/mL, respectively. At 1 year following ART initiation, 108 of 436 (25%) patients in the DTG cohort and 143 of 466 (31%) in the NNRTI cohort were LTFU (Figure 1). Assuming those LTFU were not virally suppressed, 165 of 303 (55%) and 154 of 353 (44%) achieved viral suppression in the DTG and NNRTI cohorts, respectively (P < .001).
Figure 2.
Virologic outcome at 12 mo after initiation of reverse transcriptase inhibitor– and dolutegravir-based antiretroviral therapy in treatment-naive participants. Abbreviations: ART, antiretroviral therapy; DTG, dolutegravir; NNRTI, nonnucleoside reverse transcriptase inhibitor; VL, viral load (copies/mL).
Factors Associated With Virological Suppression
Results of the univariable and multivariable analyses are shown in Table 2. DTG-based versus NNRTI-based ART was independently associated with improved viral suppression (adjusted odds ratio, 2.10 [95% CI, 1.12–3.94]; aRR, 1.11 [95% CI, 1.00–1.24]). In addition, the following factors were independently associated with viral suppression (Table 2): being separated/divorced/widowed (and to some extent married/cohabiting) versus never married, higher education level, and higher (worse) HIV WHO stage.
Table 2.
Factors Associated With Viral Suppression (HIV-1 RNA <50 Copies/mL) at 12 Months (Window Period 5–15 Months) Among Treatment-Naive Patients Initiating Nonnucleoside Reverse Transcriptase Inhibitor– or Dolutegravir-Based Antiretroviral Therapy
| Characteristics | Unadjusted | Adjusted | Adjusted |
|---|---|---|---|
| OR (95% CI)a | OR (95% CI)a,b | RR (95% CI)c | |
| ART regimen | |||
| NNRTI-based | Reference | Reference | Reference |
| DTG-based | 2.16 (1.29–3.62) | 2.10 (1.12–3.94) | 1.11 (1.00–1.24) |
| Age, y | |||
| 15–24 | Reference | Reference | Reference |
| 25–34 | 1.56 (.62–3.89) | 1.81 (.57–5.69) | 1.09 (.87–1.37) |
| 35–44 | 1.05 (.45–2.45) | 1.55 (.51–4.73) | 1.03 (.82–1.29) |
| ≥45 | 1.23 (.51–3.00) | 1.36 (.43–4.31) | 0.99 (.78–1.26) |
| Sex | |||
| Male | Reference | Reference | Reference |
| Female | 0.65 (.38–1.12) | 0.62 (.32–1.21) | 0.96 (.85–1.07) |
| Marital status | |||
| Never | Reference | Reference | Reference |
| Married/cohabiting | 3.19 (1.51–6.74) | 2.03 (.70–5.86) | 0.80 (.58–1.10) |
| Separated/divorced/widowed | 2.94 (1.29–6.71) | 3.32 (1.03–10.7) | 1.04 (.93–1.17) |
| Disclosed HIV status | |||
| No | Reference | Reference | Reference |
| Yes | 1.49 (.81–2.71) | 1.58 (.74–3.36) | 1.12 (.95–1.32) |
| Education | |||
| None | Reference | Reference | Reference |
| Primary | 1.42 (.65–3.09) | 1.79 (.72–4.49) | 1.14 (.90–1.45) |
| Secondary and above | 1.65 (.57–4.79) | 4.72 (1.09–20.4) | 1.31 (1.02–1.70) |
| Distance of residence to clinic | |||
| ≤1 km | Reference | Reference | Reference |
| 2 to <50 km | 1.07 (.60–1.89) | 1.27 (.64–2.54) | 1.02 (.91–1.15) |
| ≥50 km | 0.66 (.33–1.31) | 0.66 (.30–1.46) | 0.93 (.78–1.11) |
| Tuberculosis status | |||
| Unlikely | Reference | Reference | Reference |
| Yes | 0.73 (.32–1.64) | 1.21 (.44–3.33) | .98 (.81–1.19) |
| BMI, kg/m2 | |||
| Underweight (<18.5) | 0.70 (.35–1.41) | 0.86 (.37–2.01) | 0.96 (.81–1.15) |
| Normal (18.5–24.9) | Reference | Reference | Reference |
| Overweight (≥25) | 1.10 (.62–1.95) | 1.46 (.68–3.10) | 1.06 (.93–1.20) |
| HIV WHO stage | |||
| I/II | Reference | Reference | Reference |
| III/IV | 2.06 (1.24–3.41) | 2.33 (1.17–4.65) | 1.14 (1.00–1.34) |
| CD4 count, cells/µL | |||
| <100 | Reference | Reference | Reference |
| 100–349 | 1.03 (.54–1.96) | 0.70 (.32–1.54) | 0.93 (.81–1.06) |
| 350 | 2.13 (.99–4.56) | 0.98 (.39–2.45) | 1.00 (.87–1.16) |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; CI, confidence interval; DTG, dolutegravir; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; OR, odds ratio.
ORs and 95% CIs obtained from logistic regression.
Adjusted for all variables shown in the table; patients with missing data excluded (n = 330).
aRRs estimated from the multivariable logistic regression model with standard errors estimated using the delta method, adjusted for all variables shown in the table; patients with missing data excluded (n = 330).
In sensitivity analysis restricting VL measurements to a time window of 9–15 months including 59 of 436 (14%) and 133 of 466 (29%) patients in the DTG and NNRTI cohorts, ART regimen was not associated with viral suppression (aRR, 2.12 [95% CI, .82–5.49]), compared to using the 5- to 15-month VL window above (Supplementary Table 2).
HIV-1 Drug Resistance–Associated Mutations
For patients with viremia at 12 months after starting DTG-based ART, blood plasma samples prior to treatment initiation were available for 25 of 30 (83%) patients. Of those, 14 of 25 (56%) samples were successfully PCR amplified and sequenced for PDR determination. In 2 of 14 (14%) samples we detected 1 mutation each, which were V108I and K103N mutations, known to be associated with NNRTI resistance. Additionally, 1 of 14 (7%) patients harbored the Q58E mutation, which is a PI-associated resistance mutation. No mutations associated with NRTI were detected (Table 3).
Table 3.
Patterns of Mutations Detected in the Reverse Transcriptase and Protease Regions of HIV-1 pol Sequences in Participants on Dolutegravir-Based Antiretroviral Therapy With Viral Load ≥50 at 12 Months
| Description | At Baseline (n = 14)a |
At 12 mo (n = 2)b |
|---|---|---|
| Major NRTI resistance mutations | 0 (0) | 0 (0) |
| Major NNRTI resistance mutations | V108I: 1 (7) K103N: 1 (7) |
0 (0) |
| Major PI resistance mutations | Q58E 1: (7) | 0 (0) |
Data are presented as No. (%).
Abbreviations: NNRTI, nonnucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
Fourteen of 25 (56%) samples were tested for pretreatment HIV-1 drug resistance–associated mutations.
Acquired HIV-1 drug resistance–associated mutations were tested for only 2 of 13 (15%) patients at viremia viral load ≥50 copies/mL.
Blood plasma samples at VL ≥50 copies/mL were available for 13 of 30 (43%) patients. PCR amplification and sequencing for determination of acquired and/or persisting HIV-1 drug resistance–associated mutations was successful for only 2 of 13 (15%) patients. No mutations associated with HIV-1 drug resistance were detected (Table 3).
Side Effects and Pregnancy Outcomes
Seven of 436 (2%) patients discontinued DTG-based ART due to side effects. Of the women initiating DTG-based ART, 33 (12%) women had a documented pregnancy either at treatment initiation or during the follow-up period; 25 (76%) of those delivered a live-born infant with no obvious birth defect, 3 (9%) had a reported abortion, 1 (3%) mother had a stillbirth, and 4 (12%) were LTFU before giving birth.
DISCUSSION
In this prospective real-world study from a rural setting in eastern Africa, we compared the virological outcome of DTG- and NNRTI-based ART in treatment-naive PWH. We found higher virological suppression rates on DTG-based ART compared to NNRTI-based ART at 12 months after initiation. The documented difference in viral suppression in our cohort was even higher than in the first trials evaluating DTG-containing first-line regimens in African settings [20, 21]. The cohorts are comparable as there were no major changes regarding the clinical practice guidelines during the time frames; specifically, ART initiation was recommended for all, regardless of CD4 count, since October 2016 in Tanzania, and VL testing was recommended to monitor treatment success.
Furthermore, we assessed for the presence of PDR among patients with viremia ≥50 copies/mL at months after ART initiation in the DTG cohort. No PDR to NRTI was detected; PDR to NNRTI and PIs was rare, albeit the subgroup for available sequencing data was small. Thus, the observed elevated VL might be due to delayed suppression after treatment start or poor adherence with treatment interruption [22]. Analyses from RCTs and real-life data have shown good virological suppression of DTG-based ART among patients with PDR to NNRTIs [23–25]. In our study we did not systematically screen for PDR to DTG, as resistances to INSTIs would not be expected during the beginning of DTG-based ART rollout, as reported in recent studies in similar settings [26–28]. Moreover, of 30 patients with detectable VL in the DTG-based cohort, 25 (83%) had low viremia (ie, 50–999 copies/mL), which may be a result of momentarily subtherapeutic drug levels due to suboptimal adherence [22].
The fact that we found no HIV-1–associated drug resistance mutations in PWH with a detectable VL on a DTG-based ART is reassuring. However, these results are mitigated by the fact that genotypic results were only available for a small subgroup of participants. While the high proportion of patients with low viremia at 12 months in the DTG-based cohort might be due to early measurement with an expected further decrease, it remains worrisome, as most patients are expected to suppress VL within 3 months and low viremia due to poor adherence may subsequently lead to viral failure and emergence of resistance [29]. Even though resistance to DTG is still uncommon in this setting, evidence from other studies now shows that resistance to DTG may emerge over time, particularly following accumulation of resistance to the NRTI backbone [30–33]. Hence, vigilance and monitoring of low viremia among PWH on DTG-based therapy remain important.
Of note, more than half of the patients in both cohorts had no VL results in the given time span. One reason is the high rate of LTFU, which accounted for 56% and 44% of those without VL results in the NNRTI and the DTG-based cohorts, respectively. This rate is comparable to a previous report from the period 2005–2016 from the same clinic with LTFU rates of 41% in the first year after enrollment [34]. Though some of these patients may have silently transferred and collected medication from another clinic, and therefore have a suppressed VL, others are likely to have suboptimal adherence or stopped medication altogether. Importantly, viral suppression rate in the sensitivity analysis was still higher in the DTG-based cohort compared to the NNRTI-based cohort. Regardless, the high rate of LTFU in this rural setting in SSA remains a major concern of the treatment cascade and urgently needs a better understanding of patients’ needs and adequate interventions [35].
Being on DTG-based ART was an important factor associated with improved viral suppression, which is in line with existing data from RCTs that showed excellent pharmacokinetic profile and tolerability of DTG, rapid viral suppression, and fewer side effects compared to NNRTIs in treatment-naive and -experienced patients [36–38]. Similarly, in this study, we observed very few patients who discontinued DTG due to side effects. However more data on an extended time span is required and is the aim of future studies. Other factors associated with viral suppression were a higher education level and being separated/divorced/widowed. While other studies have indicated high odds of viral failure in clients who did not disclose their HIV status [39], we found no evidence of such an association. The association of virological suppression with advanced WHO clinical stages in our study might be due to patients feeling worse and therefore more likely to adhere to medication and also receiving closer care and monitoring (and therefore better adherence) [11]. However, this outcome might also be affected by a reporting bias, as these patients are attending the clinic and are tested more frequently.
As in other settings, in the first year of DTG rollout in Tanzania, women of childbearing potential were given the choice to start either DTG-based or NNRTI-based ART, based on informed consent according to the WHO guidelines [40]. Nevertheless, a number of women became pregnant while on DTG. Reassuringly, we did not document obvious birth defects in the 33 women with live births during the study period. One stillbirth and 3 abortions of unknown reasons were reported. While the numbers of this study are too low to draw firm conclusions, it is important to observe uptake among women and pregnancy outcomes as a large observational study from 11 LMICs has shown substantial disparities in the uptake of DTG affecting females of childbearing age [41]. The benefit of rapid viral suppression in pregnant women is of utmost importance to avoid viral transmission to their newborns.
To our knowledge, this is the first study addressing virological outcomes among treatment-naive patients initiating DTG-based ART in Tanzania under programmatic conditions. Our study has several limitations. Most importantly, a large proportion of patients in both cohorts had no VL result in the given time span due to a high rate of LTFU. Another major limitation is unmeasured confounding as these are observational data. Furthermore, many patients who were in active care also had no VL result. Most likely reasons were stockout of reagents or procedural challenges during VL testing implementation. In both timespans, national guidelines recommended VL testing to monitor treatment success. The lack of available VL results might have led to overestimating the rates of viral suppression. Another limitation is that that many blood samples were not available for drug resistance testing. Furthermore, there was significant PCR amplification failure, especially in those with a low VL, which might be due to poor sample storage quality. Finally, all genotypic data were obtained through standard Sanger sequencing; rates of HIV-associated drug resistance mutations would possibly be higher if ultra-sensitive HIV-1 drug resistance testing by next-generation sequencing had been used.
CONCLUSIONS
Our results underline the benefit of programmatic uptake of DTG-based ART in LMICs. We did not find pretreatment resistance to the DTG co-administered NRTIs nor acquired resistance among viremic patients on DTG-based ART, although the number of samples tested was small. Continuous monitoring of pretreatment and acquired resistance under programmatic condition during the rollout of DTG-based first-line is of utmost importance. LTFU remains high and needs further attention as it jeopardizes control of the HIV epidemic.
Supplementary Material
Contributor Information
Alex J Ntamatungiro, Division of Epidemiology and Biostatistics, School of Public Health, University of the Witwatersrand, Johannesburg, South Africa; Department of Interventions and Clinical Trials, Ifakara Health Institute, Ifakara, Tanzania.
Anna Eichenberger, Department of Infectious Diseases, Bern University Hospital, Bern, Switzerland.
James Okuma, Department of Medicine, Swiss Tropical and Public Health Institute, Basel, Switzerland.
Fiona Vanobberghen, Department of Medicine, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Robert Ndege, Division of Epidemiology and Biostatistics, School of Public Health, University of the Witwatersrand, Johannesburg, South Africa; Department of Medicine, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland.
Namvua Kimera, Department of Interventions and Clinical Trials, Ifakara Health Institute, Ifakara, Tanzania.
Joel M Francis, Department of Family Medicine and Primary Care, University of the Witwatersrand, Johannesburg, South Africa.
Juliana Kagura, Division of Epidemiology and Biostatistics, School of Public Health, University of the Witwatersrand, Johannesburg, South Africa.
Maja Weisser, Department of Interventions and Clinical Trials, Ifakara Health Institute, Ifakara, Tanzania; Department of Medicine, Swiss Tropical and Public Health Institute, Basel, Switzerland; University of Basel, Basel, Switzerland; Division of Infectious Diseases and Hospital Epidemiology, University Hospital Basel, Basel, Switzerland.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. J. N., A. E., J. F., J. K., and M. W. conceptualized the study, designed the experiments; A. J. N. wrote the manuscript; A. N., N. K. performed the viral load and resistance testing; A. J. N., A. E., J. O., and F. V. analysed the data; A. J. N., A. E., J. O., F. V., R. N., N. K., J. F., J. K., and M. W. participated in the interpretation of the data, writing, reviewing, and approving the final manuscript. The corresponding author A. J. N. is the guarantor of the paper.
Acknowledgments. We are thankful to all the participants of the KIULARCO cohort. We thank the staff of the Chronic Diseases Clinic of St Francis Referral Hospital in Ifakara for making this study possible.
Data availability. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclaimer. The funders had no rule in the study design, data collection and analysis, preparation of the manuscript, or decision to submit the manuscript for publication.
Financial support. KIULARCO receives funds from the Ministry of Health and Social Welfare of Tanzania, the government of the Canton of Basel, the Swiss Tropical and Public Health Institute, the Ifakara Health Institute, and the US Agency for International Development through the local implementer. A. J. N. was supported by the Consortium for Advanced Research Training in Africa (CARTA). CARTA is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (grant number G-19-57145), Sida (grant number 54100113), Uppsala Monitoring Centre, and the DELTAS Africa Initiative (grant number 107768/Z/15/Z). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences' Alliance for Accelerating Excellence in Science in Africa and supported by the New Partnership for Africa's Development Planning and Coordinating Agency with funding from the Wellcome Trust and the United Kingdom government.
References
- 1. Joint United Nations Programme on HIV/AIDS (UNAIDS) . UNAIDS 2021 epidemiological estimates. Geneva, Switzerland: UNAIDS; 2021. [Google Scholar]
- 2. Joint United Nations Programme on HIV/AIDS (UNAIDS) . 2025 AIDS targets. Available at: https://aidstargets2025.unaids.org/. Accessed 27 October 2022.
- 3. Joint United Nations Programme on HIV/AIDS (UNAIDS) . Political declaration on HIV and AIDS: on the fast track to accelerating the fight against HIV and to ending the AIDS epidemic by 2030. Available at: https://www.unaids.org/en/resources/documents/2016/2016-political-declaration-HIV-AIDS. Accessed 27 October 2022.
- 4. Bertagnolio S, Hermans L, Jordan MR, et al. Clinical impact of pretreatment human immunodeficiency virus drug resistance in people initiating nonnucleoside reverse transcriptase inhibitor–containing antiretroviral therapy: a systematic review and meta-analysis. J Infect Dis 2021; 224:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ntamatungiro AJ, Kagura J, Weisser M, Francis JM. Pre-treatment HIV-1 drug resistance in antiretroviral therapy-naive adults in eastern Africa: a systematic review and meta-analysis. J Antimicrob Chemother 2022; 77:3231–41 [DOI] [PubMed] [Google Scholar]
- 6. United Nations Department of Economic and Social Affairs . Goal 3: ensure healthy lives and promote well-being for all at all ages. Available at: https://sdgs.un.org/goals/goal3. Accessed 27 October 2022.
- 7. Mesic A, Spina A, Mar HT, et al. Predictors of virological failure among people living with HIV receiving first line antiretroviral treatment in Myanmar: retrospective cohort analysis. AIDS Res Ther 2021; 18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mondi A, Cozzi-Lepri A, Tavelli A, et al. Effectiveness of dolutegravir-based regimens as either first line or switch antiretroviral therapy: data from the ICONA cohort. J Int AIDS Soc 2019; 22:e25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Unitaid . New high-quality antiretroviral therapy to be launched in South Africa, Kenya and over 90 low-and middle-income countries at reduced price [press release]. 2017. Available at: https://unitaid.org/news-blog/new-high-quality-antiretroviral-therapy-launched-south-africa-kenya-90-low-middle-income-countries-reduced-price/#en. Accessed 23 June 2023.
- 10. Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med 2019; 381:827–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mehari EA, Muche EA, Gonete KA. Virological suppression and its associated factors of dolutegravir based regimen in a resource-limited setting: an observational retrospective study in Ethiopia. HIV AIDS (Auckl) 2021; 13:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phillips AN, Venter F, Havlir D, et al. Risks and benefits of dolutegravir-based antiretroviral drug regimens in sub-Saharan Africa: a modelling study. Lancet HIV 2019; 6:e116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCluskey SM, Pepperrell T, Hill A, Venter WDF, Gupta RK, Siedner MJ. Adherence, resistance, and viral suppression on dolutegravir in sub-Saharan Africa: implications for the TLD era. AIDS 2021; 35(Suppl 2):S127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Letang E, Kalinjuma AV, Glass TR, et al. Cohort profile: the Kilombero and Ulanga Antiretroviral Cohort (KIULARCO)—a prospective HIV cohort in rural Tanzania. Swiss Med Wkly 2017; 147:w14485. [DOI] [PubMed] [Google Scholar]
- 15. Vanobberghen F, Letang E, Gamell A, et al. A decade of HIV care in rural Tanzania: trends in clinical outcomes and impact of clinic optimisation in an open, prospective cohort. PLoS One 2017; 12:e0180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ntamatungiro A, Muri L, Glass T, et al. Strengthening HIV therapy and care in rural Tanzania affects rates of viral suppression. J Antimicrob Chemother 2017; 72:2069–74.. [DOI] [PubMed] [Google Scholar]
- 17. Muri L, Gamell A, Ntamatungiro AJ, et al. Development of HIV drug resistance and therapeutic failure in children and adolescents in rural Tanzania: an emerging public health concern. AIDS 2017; 31:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization (WHO) . Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. Geneva, Switzerland: WHO; 2021. [PubMed] [Google Scholar]
- 19. Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J 2013; 13:492–509. [Google Scholar]
- 20. Zash R, Jacobson DL, Diseko M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health 2018; 6:e804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 22. Castillo-Mancilla JR, Morrow M, Coyle RP, et al. Low-level viremia is associated with cumulative adherence to antiretroviral therapy in persons with HIV. Open Forum Infect Dis 2021; 8:ofab463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schramm B, Temfack E, Descamps D, et al. Viral suppression and HIV-1 drug resistance 1 year after pragmatic transitioning to dolutegravir first-line therapy in Malawi: a prospective cohort study. Lancet HIV 2022; 9:e544–53. [DOI] [PubMed] [Google Scholar]
- 24. Paton NI, Musaazi J, Kityo C, et al. Dolutegravir or darunavir in combination with zidovudine or tenofovir to treat HIV. N Engl J Med 2021; 385:330–41. [DOI] [PubMed] [Google Scholar]
- 25. Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial. Lancet Infect Dis 2019; 19:253–64. [DOI] [PubMed] [Google Scholar]
- 26. Ndashimye E, Avino M, Kyeyune F, et al. Absence of HIV-1 drug resistance mutations supports the use of dolutegravir in Uganda. AIDS Res Hum Retroviruses 2018; 34:404–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCluskey SM, Kamelian K, Musinguzi N, et al. Pre-treatment integrase inhibitor resistance is uncommon in antiretroviral therapy–naive individuals with HIV-1 subtype A1 and D infections in Uganda. AIDS 2021; 35:1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Henerico S, Lyimo E, Makubi AN, et al. Primary resistance against integrase strand transfer inhibitors in integrase strand transfer inhibitor–naive patients failing first- and second-line ART in Tanzania. J Antimicrob Chemother 2022; 77:3138–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. An J, Lao Y, Tang S, Lou J, Li T, Dong X. The impact of low-level viraemia on virological failure—results from a multicenter HIV antiretroviral therapy cohort study in Yunnan, China. Front Med (Lausanne) 2022; 9:939261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hocqueloux L, Raffi F, Prazuck T, et al. Dolutegravir monotherapy versus dolutegravir/abacavir/lamivudine for virologically suppressed people living with chronic human immunodeficiency virus infection: the Randomized Noninferiority Monotherapy of Tivicay trial. Clin Infect Dis 2019; 69:1498–505. [DOI] [PubMed] [Google Scholar]
- 31. Blanco JL, Marcelin A-G, Katlama C, Martinez E. Dolutegravir resistance mutations: lessons from monotherapy studies. Curr Opin Infect Dis 2018; 31:237–45. [DOI] [PubMed] [Google Scholar]
- 32. Wijting IEA, Lungu C, Rijnders BJA, et al. HIV-1 resistance dynamics in patients with virologic failure to dolutegravir maintenance monotherapy. J Infect Dis 2018; 218:688–97. [DOI] [PubMed] [Google Scholar]
- 33. Braun DL, Scheier T, Ledermann U, et al. Emergence of resistance to integrase strand transfer inhibitors during dolutegravir containing triple-therapy in a treatment-experienced patient with pre-existing m184v/i mutation. Viruses 2020; 12:1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kalinjuma AV, Glass TR, Weisser M, et al. Prospective assessment of loss to follow-up: incidence and associated factors in a cohort of HIV-positive adults in rural Tanzania. J Int AIDS Soc 2020; 23:e25460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehrenkranz P, Rosen S, Boulle A, et al. The revolving door of HIV care: revising the service delivery cascade to achieve the UNAIDS 95-95-95 goals. PLoS Med 2021; 18:e1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Inzaule SC, Hamers RL, Doherty M, et al. Curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect Dis 2019; 19:e246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mondi A, Cozzi-Lepri A, Tavelli A, et al. Effectiveness of dolutegravir-based regimens as either first-line or switch antiretroviral therapy: data from the Icona cohort. J Int AIDS Soc 2019; 22:e25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393:143–55. [DOI] [PubMed] [Google Scholar]
- 39. Meshesha HM, Nigussie ZM, Asrat A, Mulatu K. Determinants of virological failure among adults on first-line highly active antiretroviral therapy at public health facilities in Kombolcha town, northeast, Ethiopia: a case–control study. BMJ Open 2020; 10:e036223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. National AIDS Control Programme . The United Republic of Tanzania. 2019. Available at: https://differentiatedservicedelivery.org/wp-content/uploads/national_guidelines_for_the_management_of_hiv_and_aids_2019.pdf. Accessed 7 November 2022.
- 41. Romo ML, Patel RC, Edwards JK, et al. Disparities in dolutegravir uptake affecting females of reproductive age with HIV in low- and middle-income countries after initial concerns about teratogenicity: an observational study. Ann Intern Med 2022; 175:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.