Abstract
Background
Cardiometabolic outcomes were investigated 3 years after switching to the 2-drug regimen dolutegravir/lamivudine (DTG/3TC) vs continuing 3-/4-drug tenofovir alafenamide (TAF)–based regimens in a multicenter phase 3 noninferiority study based on an open-label randomized design.
Method
Adults with virologically suppressed HIV-1 switched to once-daily DTG/3TC (n = 369) or continued TAF-based regimens (n = 372). Cardiometabolic health parameters were assessed through week 144 via mixed-model repeated measures or logistic regression analyses, adjusting for baseline variables.
Results
At week 144, 13% (42/316) of the DTG/3TC group and 12% (37/303) of the TAF-based regimen group had ≥10% weight gain from baseline (adjusted odds ratio, 1.11; 95% CI, .68–1.80). Adjusted change from baseline in serum leptin, a surrogate marker of adiposity, was similar between groups (treatment ratio, 1.00; 95% CI, .89–1.13). The lipid profile generally favored DTG/3TC in the baseline boosted subgroup. Adjusted odds revealed no clinically meaningful differences between groups: homeostatic model assessment of insulin resistance ≥2 (adjusted odds ratio, 0.79; 95% CI, .50–1.26), metabolic syndrome (International Diabetes Federation criteria, 0.99; .59–1.68), hepatic fibrosis (fibrosis-4 index score ≥1.45, 1.39; .63–3.06), and coronary artery disease risk (Framingham risk score ≥10%, 0.92; .56–1.49). Baseline variables and characteristics associated with odds of each cardiometabolic parameter outcome were consistent with known risk factors, including age, sex, race, and some disease characteristics.
Conclusions
Cardiometabolic health 3 years after switching to DTG/3TC was comparable to that for individuals continuing TAF-based regimens, further supporting DTG/3TC as a robust switch option with a stable metabolic profile.
Trial registration
ClinicalTrials.gov NCT03446573
Keywords: 2-drug regimen, cardiometabolic health, insulin resistance, leptin, weight
Cardiometabolic health outcomes 3 years after switching to the 2-drug regimen dolutegravir/lamivudine were comparable to those for individuals continuing 3-/4-drug tenofovir alafenamide–based regimens, further supporting dolutegravir/lamivudine as a robust switch option with a stable metabolic profile.
As people with HIV (PWH) age on lifelong antiretroviral therapy (ART), risk of comorbidities and polypharmacy increases [1, 2]. Regimen simplification via 2-drug regimens (2DRs) reduces the number of antiretroviral agents used and potentially decreases long-term toxicities, drug-drug interactions, and costs [3]. Older antiretrovirals have been associated with weight suppression and metabolic toxicity [4, 5]. Although progress has been made in mitigating metabolic toxicity, the holistic and long-term impact of newer antiretrovirals on metabolic health is unclear, particularly for tenofovir alafenamide (TAF) and the second-generation integrase strand transfer inhibitors (INSTIs) dolutegravir (DTG) and bictegravir.
International guidelines recommend that initial ART regimens include an INSTI with ≥1 nucleoside reverse transcriptase inhibitor [6–8]. However, ART regimens containing the INSTIs DTG or bictegravir or the nucleoside reverse transcriptase inhibitor TAF have been independently associated with weight gain [4, 9–12]. The first-generation INSTIs elvitegravir and raltegravir have generally been associated with less weight gain than DTG and bictegravir, underscoring potential differences in metabolic health impact between INSTI generations [13–15]. Understanding the causality and degree of association between any antiretroviral and weight gain is complex and requires consideration of confounders such as lifestyle, concomitant medications and comorbidities, the return-to-health phenomenon, and effect of prior regimens on weight (eg, suppressive effect of antiretrovirals such as tenofovir disoproxil fumarate [TDF] and efavirenz) [4].
Beyond change in weight, parameters related to body fat, insulin resistance, metabolic syndrome, metabolic-associated liver disease, and cardiovascular disease (CVD) provide a more complete understanding of cardiometabolic health. For example, plasma leptin assesses total fat mass [16], and homeostatic model assessment of insulin resistance (HOMA-IR) indicates insulin sensitivity, which may be predictive of increased CVD risk [17]. Assessing and preventing metabolic syndrome and CVD is important, given the occurrence of obesity in ART-treated individuals and heightened risk of CVD in PWH [18–21].
The 2DR dolutegravir/lamivudine (DTG/3TC) is recommended as initial therapy for most PWH who are ART naive and as a switch option for PWH who are virologically suppressed [6–8], as supported by phase 3 clinical trial evidence in ART-naive (GEMINI-1/-2) and virologically suppressed (TANGO and SALSA) populations for up to 3 years [22–24]. In TANGO, switching to a DTG/3TC fixed-dose combination tablet was noninferior to continuing 3-/4-drug TAF-based regimens for maintenance of virologic suppression, demonstrating durable efficacy, good safety and tolerability, and a high barrier to resistance for 3 years [22]. Change in weight from baseline to week 144 was similar between participants who switched to DTG/3TC (2.2 kg) and those continuing TAF-based regimens (1.7 kg) and between treatment groups across baseline body mass index (BMI) categories [22]. Participants who switched to DTG/3TC had favorable changes in lipid profile, with small and similar changes observed between treatment groups from baseline to week 144 in terms of HOMA-IR and inflammatory biomarkers associated with cardiometabolic disorders [22, 25, 26]. We present in-depth post hoc analyses of cardiometabolic outcomes and baseline variables associated with cardiometabolic outcomes through week 144 of TANGO.
METHODS
Study Design
TANGO (ClinicalTrials.gov NCT03446573) is a phase 3 open-label study based on a randomized noninferiority design that evaluated the efficacy and safety of switching to the 2DR DTG/3TC vs continuing 3-/4-drug TAF-based regimens in adults who are virologically suppressed. Methods have previously been published [22, 27]. Briefly, adults with HIV-1 who were virologically suppressed for >6 months while taking TAF-based regimens were randomized 1:1 (stratified by baseline third agent class) to switch to once-daily DTG (50 mg)/3TC (300 mg) or continue their TAF-based regimen. Switch from TDF to TAF ≥3 months before screening was permitted; during the study, no regimen modifications were allowed in the TAF-based regimen group, except for switching between ritonavir and cobicistat [22, 27]. The primary endpoint was the proportion of participants with HIV-1 RNA ≥50 copies/mL at week 48.
Patient Consent Statement
TANGO was conducted in accordance with the International Conference on Harmonization’s good clinical practice and the Declaration of Helsinki, with protocol approvals obtained before participant screening. All participants provided written informed consent before study initiation.
Metabolic Health Assessments
Metabolic outcomes included change from baseline in weight and BMI and percentage change from baseline in fasting lipids (total cholesterol, low- and high-density lipoprotein cholesterol [LDL-C and HDL-C], total cholesterol/HDL-C ratio, and triglycerides), fasting glucose and insulin, and hemoglobin A1c (HbA1c). Insulin resistance was assessed with HOMA-IR and defined as a score ≥2 [28–30]. Metabolic syndrome was defined by criteria of the International Diabetes Federation [31]. Hepatic fibrosis was assessed through fibrosis-4 index (FIB-4) ≥1.45 [32–35]. Framingham risk score ≥10% was used in the present analysis [36, 37]. Data collection timelines and formulas used for analysis are provided in the Supplementary Methods.
Statistics
Adjusted mean change and adjusted geometric mean ratio for loge-transformed endpoints from baseline to week 144 were calculated for weight, leptin, fasting lipids, HOMA-IR, fasting glucose and insulin, HbA1c, and FIB-4. Data were compared between treatment groups per mixed-model repeated measures analyses adjusting for relevant baseline variables in the safety population. Multivariable logistic regression models were used for determining adjusted odds ratios (aORs) of relevant baseline variables’ association with the following binary endpoints defined at week 144: ≥10% weight gain, metabolic syndrome, HOMA-IR ≥2, FIB-4 ≥1.45, and Framingham risk score ≥10%.
Exploratory subgroup analyses for prior TAF duration (<1 vs ≥1 year), boosting status (presence or absence of pharmacokinetic boosting agent at baseline), and baseline BMI category (kg/m2; underweight/normal, <25; overweight, 25 to <30; obesity, ≥30) were performed with mixed-model repeated measures or logistic regression as appropriate. Adjustment of baseline variables per analysis is detailed in the figures.
RESULTS
Demographics and Baseline Characteristics
Overall, 743 participants were randomized to switch to DTG/3TC (n = 371) or continue their TAF-based regimen (n = 372), and 369 and 372 participants, respectively, received study treatment (intention-to-treat–exposed population). The median age was 40 and 39 years for the DTG/3TC and TAF-based regimen groups, respectively (Table 1). Most participants were male and White. The most common baseline third agent class was INSTI, with elvitegravir/cobicistat being the most common INSTI used (66% and 67% for the DTG/3TC and TAF-based regimen groups). Most participants used boosted regimens at baseline (74% in each group). Baseline weight (kg, mean [SD]: DTG/3TC, 81.2 [15.4]; TAF-based regimen, 81.7 [15.9]) and cardiometabolic characteristics were similar between treatment groups.
Table 1.
Demographics and Baseline Characteristics
| Parameter, No. (%)a | ||
|---|---|---|
| DTG/3TC (n = 369) | TAF-Based Regimen (n = 372) | |
| Age, median (range), y | 40 (20–74) | 39 (18–73) |
| Female | 25 (7) | 33 (9) |
| Race | ||
| White | 297 (80) | 289 (78) |
| African American/African heritage | 50 (14) | 58 (16) |
| Asian | 13 (4) | 13 (3) |
| Other racesb | 9 (2) | 12 (3) |
| Ethnicity | ||
| Hispanic/Latinx | 69 (19) | 66 (18) |
| Not Hispanic/Latinx | 300 (81) | 306 (82) |
| CD4+, median (range), cells/mm3 | 682 (133–1904) | 720 (119–1810) |
| Baseline third agent class | ||
| INSTI | 289 (78) | 296 (80) |
| EVG/c | 243 (66) | 249 (67) |
| NNRTI | 51 (14) | 48 (13) |
| RPV | 43 (12) | 45 (12) |
| PI | 29 (8) | 28 (8) |
| bDRV | 25 (7) | 27 (7) |
| Baseline boosted regimen | 272 (74) | 277 (74) |
| Duration before day 1, median (range), mo | ||
| ART | 33.8 (7.1–201.2) | 35.1 (7.0–160.8) |
| TAF-based regimen | 17.7 (3.6–73.7) | 18.2 (3.9–71.2) |
| Cardiometabolic characteristics | ||
| Mean (SD) | ||
| Weight, kg | 81.2 (15.4) | 81.7 (15.9)c |
| BMI, kg/m2 | 26.3 (4.8) | 26.7 (5.1)c |
| BMI categoryd | ||
| Underweight | 3 (<1) | 4 (1) |
| Normal | 172 (47) | 153 (41) |
| Overweight | 131 (36) | 137 (37) |
| Obesity | 63 (17) | 77 (21) |
| Diabetes | ||
| Type 2 | 12 (3) | 18 (5) |
| Type 1 | 1 (<1) | 1 (<1) |
| Prevalence of metabolic syndromee,f | 36 (10) | 41 (11)g |
| Fasting insulin, pmol/L | ||
| Median (range)f | 72.0 (11–582) | 72.0 (11–690) |
| Geometric mean (95% CI) | 73.8 (69.2–78.8) | 71.7 (67.4–76.4) |
| Geometric mean (95% CI) | ||
| Fasting glucose, | 5.07 (5.00–5.13) | 5.09 (5.02–5.16) |
| HbA1c, % | 5.07 (5.02–5.12) | 5.13 (5.06–5.20) |
| HOMA-IRh | 2.81 (2.62–3.02) | 2.74 (2.55–2.93) |
| HOMA-IR ≥2i | 193 (72) | 186 (72) |
| Leptin, geometric mean (95% CI)f | 4.98 (4.47–5.54) | 4.97 (4.43–5.58) |
| BMI: underweight/normal | 2.79 (2.43–3.21) | 2.49 (2.15–2.89) |
| BMI: overweight | 6.82 (5.98–7.77) | 5.67 (4.97–6.48) |
| BMI: obesity | 13.12 (10.61–16.22) | 16.18 (13.45–19.47) |
| FIB-4 | ||
| Median (range)f | 0.869 (0.20–3.08) | 0.867 (0.26–3.51) |
| Geometric mean (95% CI) | 0.843 (.804–.884) | 0.862 (.824–.903) |
| Proportion of participants | ||
| Past or current hypertension | 59 (16) | 59 (16) |
| Framingham risk score ≥10%j | 90 (24) | 80 (22) |
Abbreviations: ART, antiretroviral therapy; bDRV, boosted darunavir; BMI, body mass index; DTG, dolutegravir; EVG/c, elvitegravir/cobicistat; FIB-4, fibrosis-4 index; HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; RPV, rilpivirine; TAF, tenofovir alafenamide; 3TC, lamivudine.
Data are presented as No. (%) unless otherwise indicated.
Other races included American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, mixed White race, and multiple races.
n = 371.
BMI categories based on World Health Organization classification.
Participants who have BMI ≥30 kg/m2 and satisfy any 2 of the raised/reduced factors within the baseline visit window: raised triglycerides, ≥150 mg/dL or treatment; reduced HDL cholesterol, <40 mg/dL (men), <50 mg/dL (women), or treatment; raised blood pressure, systolic ≥130 mm Hg or diastolic ≥85 mm Hg, or treatment for hypertension; fasting glucose ≥100 mg/dL or previous diagnosis of type 2 diabetes.
Post hoc analysis.
n = 370.
HOMA-IR = fasting plasma insulin (mU/L) × fasting plasma glucose (mmol/L) / 22.5.
Percentages based on participants with HOMA-IR data at baseline and week 144: DTG/3TC, n = 268; TAF-based regimen, n = 257.
DTG/3TC, n = 368; TAF-based regimen, n = 370.
Changes in Weight, BMI, and Leptin
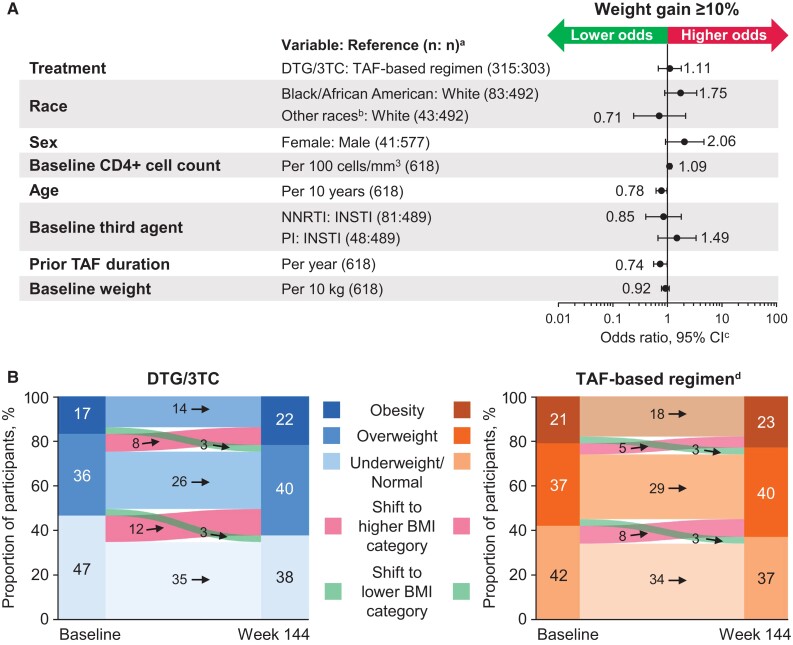
At week 144, weight gain was generally similar between treatment groups, with ≥5% gain from baseline in 39% (123/316) switching to DTG/3TC and 31% (94/303) continuing a TAF-based regimen and ≥10% gain in 13% (42/316) switching to DTG/3TC and 12% (37/303) continuing a TAF-based regimen (aOR, 1.11; 95% CI, .68–1.80; Figure 1A). Older age (per 10-year increase) was associated with lower odds of ≥10% weight gain (aOR, 0.78; 95% CI, .62–.98). At week 144, 12% (69/577) of male participants and 24% (10/41) of female participants had ≥10% weight gain (aOR, 2.06; 95% CI, .92–4.66). In subgroup analyses based on baseline BMI, adjusted odds of ≥10% weight gain were highest among participants with obesity at baseline, although the 95% CI overlapped with 1 for all BMI categories (aOR [95% CI]: underweight/normal, 0.97 [.48–1.95]; overweight, 1.04 [.45–2.40]; obesity, 1.76 [.56–5.58]). In subgroup analyses evaluating prior TAF duration as a continuous variable, adjusted odds of ≥10% weight gain were lower with longer prior TAF duration (aOR, 0.74; 95% CI, .56–.99). When prior TAF duration was evaluated categorically, adjusted odds of ≥10% weight gain were slightly higher between treatment groups in the <1-year category (1.35; .53–3.44) vs ≥1 year (1.02; .58–1.80), although the 95% CI overlapped with 1 for both categories. Proportions of participants with an adverse event of increased weight were similar between treatment groups: DTG/3TC, 5% (17/369); TAF-based regimen, 5% (19/371; Supplementary Table 1).
Figure 1.
(A) Adjusted odds (95% CI) of ≥10% weight gain by relevant parameters and (B) change in BMI category in the DTG/3TC and TAF-based regimen groups (post hoc analyses). BMI, body mass index; DTG, dolutegravir; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide; 3TC, lamivudine. aOdds ratios are presented in relation to the reference variable; the number of participants included in final model with covariate data at baseline and weight data at baseline and week 144. bOther races included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, mixed White race, and multiple races. cOdds ratios and 95% CIs were calculated with a logistic regression model adjusting for treatment, race, sex, baseline CD4+ cell count (continuous), age (continuous), baseline weight (continuous), baseline third agent class, and prior TAF duration (continuous). dIn the TAF-based regimen group, 1 (<1%) participant with obesity at baseline had a normal BMI at week 144, and 1 (<1%) participant with a normal BMI had obesity at week 144.
Changes in BMI categories from baseline to week 144 were similar between treatment groups, with 8% (24/316) and 6% (17/303) shifting from baseline BMI <30 (underweight, normal, and overweight) to postbaseline ≥30 (obesity) in the DTG/3TC and TAF-based regimen groups, respectively (Figure 1B).
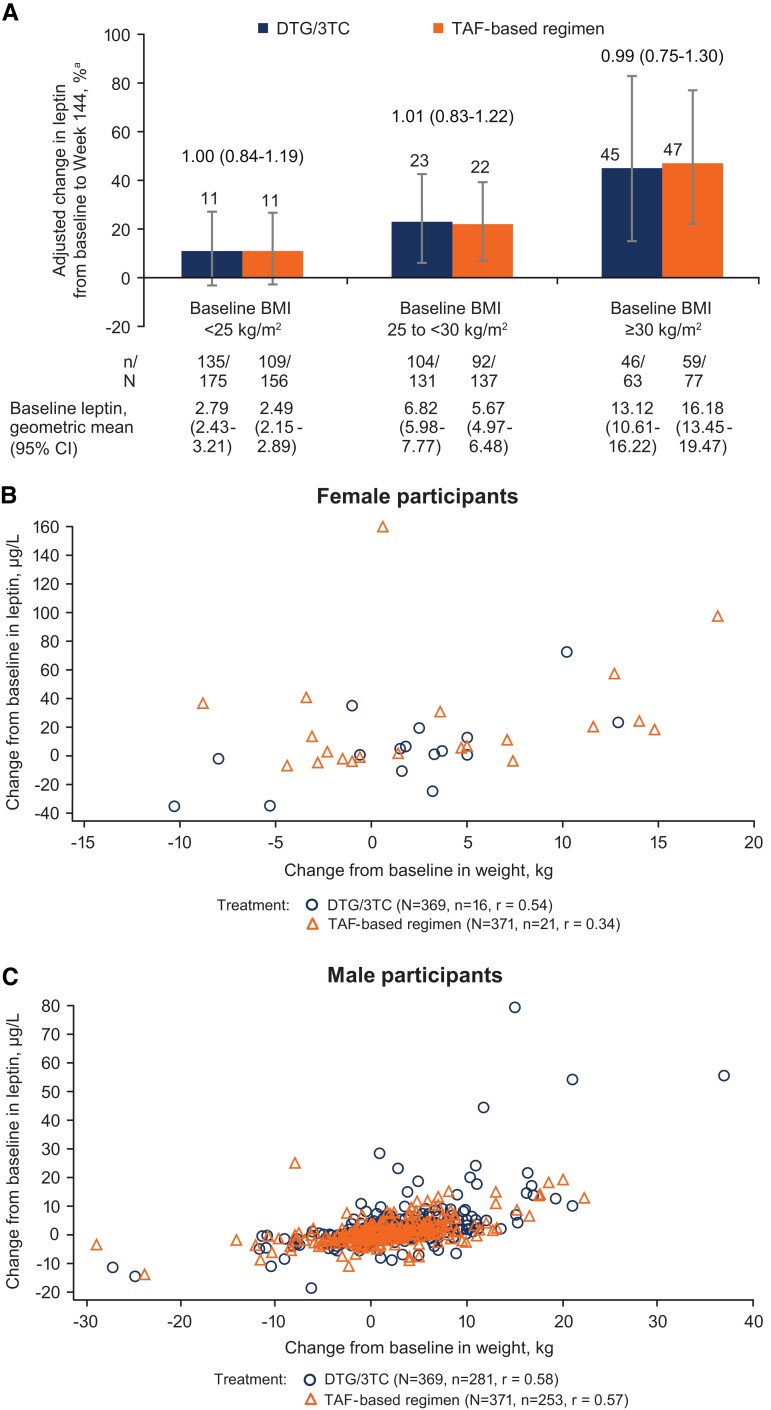
Adjusted percentage change in leptin from baseline through week 144 was comparable between the DTG/3TC (21.4%; 95% CI, 11.2%–32.5%; n = 285) and TAF-based regimen (21.2%; 12.1%–31.0%; n = 260) groups (treatment ratio, 1.00; 95% CI, .89–1.13). Change from baseline in leptin was comparable between treatment groups within each BMI category (Figure 2A), with a higher percentage change from baseline in leptin observed with a higher baseline BMI. Change in weight was positively correlated with change in leptin levels in both treatment groups among women (DTG/3TC, r = 0.54; TAF, r = 0.34; Figure 2B) and men (DTG/3TC, r = 0.58; TAF, r = 0.57; Figure 2C).
Figure 2.
(A) Adjusted percentage change from baseline in leptin and correlation between change from baseline in leptin and body weight in (B) female and (C) male participants (post hoc analysis). BMI, body mass index; DTG, dolutegravir; TAF, tenofovir alafenamide; 3TC, lamivudine. aPercentage change from baseline and 95% CI based on adjusted ratio (week 144 to baseline) in each group were calculated via a mixed-model repeated measures analysis applied to change from baseline in loge-transformed data, adjusting for the following: treatment, baseline BMI category, baseline third agent class, prior TAF duration (continuous), loge-transformed baseline leptin (continuous), baseline insulin (continuous), baseline CD4+ cell count (continuous), age (continuous), sex, treatment × visit, loge-transformed baseline leptin × visit, treatment × baseline BMI category, baseline BMI category × visit, and baseline BMI category × treatment × visit—with visit as the repeated factor. Treatment ratio (95% CI) is shown above the bars in panel A.
Change in Lipid Profile
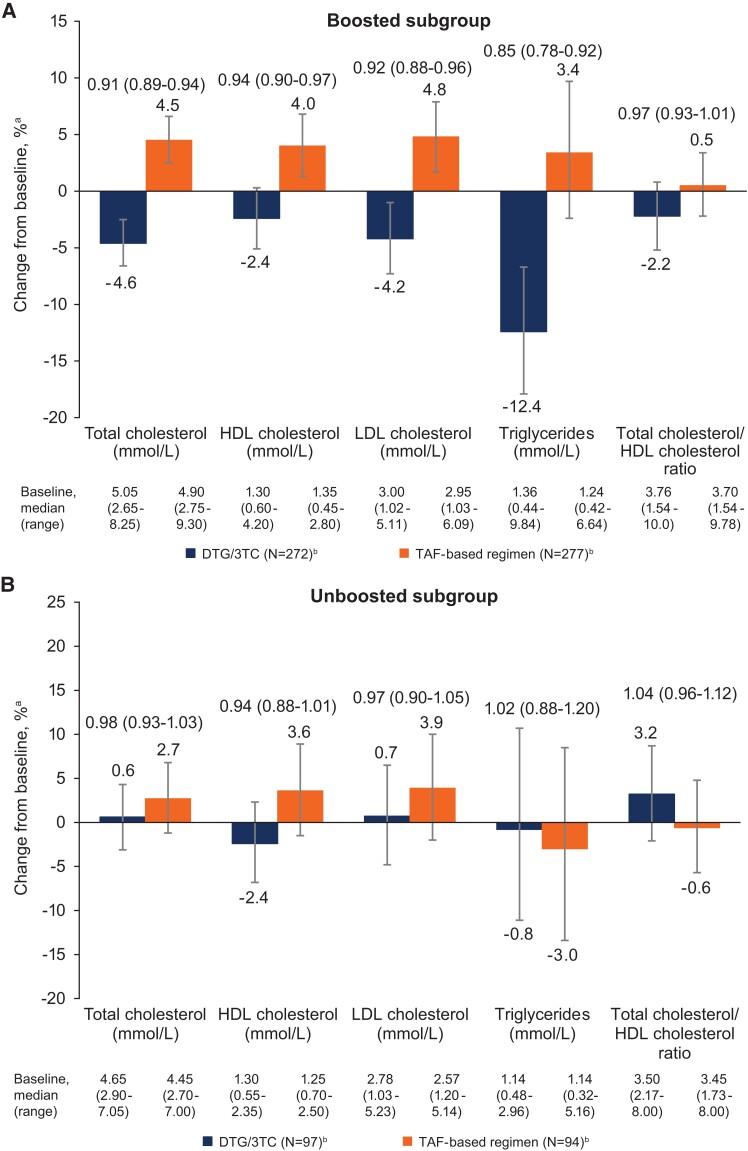
At week 144, the proportion of participants with desirable or optimal lipid levels was numerically higher in the DTG/3TC group for total cholesterol, LDL-C, and triglycerides (Supplementary Table 2). In the baseline boosted subgroup, changes in lipid profile favored DTG/3TC for total cholesterol, LDL-C, and triglycerides and favored TAF-based regimens for HDL-C, with no difference between groups for total cholesterol/HDL-C ratio (Figure 3). No significant differences in lipid changes were observed between treatment groups in the baseline unboosted subgroup.
Figure 3.
Adjusted percentage change from baseline (95% CI) in lipids in the baseline (A) boosted and (B) unboosted subgroups (post hoc analysis). BMI, body mass index; DTG, dolutegravir; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TAF, tenofovir alafenamide; 3TC, lamivudine. aPercentage change from baseline and 95% CI based on adjusted ratio (week 144 to baseline) in each group were calculated via mixed-model repeated measures analysis applied to change from baseline in loge-transformed data, adjusting for the following: treatment, baseline boosting status, CD4+ cell count (continuous), age (continuous), loge-transformed baseline value (continuous), race, baseline BMI (continuous), treatment × visit, baseline value × visit, treatment × baseline boosting status, baseline boosting status × visit, and baseline boosting status × treatment × visit—with visit as the repeated factor. bNumber of participants with nonmissing fasting lipid data at baseline and week 144, removing those with lipid-modifying agent administered at baseline. Treatment ratio (95% CI) is shown above the bars.
Insulin Resistance
Adjusted percentage change from baseline through week 144 in fasting serum glucose was −0.4% (95% CI, −1.6% to .9%) and 0.5% (−.9% to 1.9%) in the DTG/3TC and TAF-based regimen groups, respectively (treatment ratio, 0.99; 95% CI, .97–1.01). Percentage change from baseline in fasting serum insulin was 14.2% (95% CI, 8.0%–20.7%) and 12.1% (5.9%–18.7%) in the DTG/3TC (n = 272) and TAF-based regimen (n = 258) groups (treatment ratio, 1.02; 95% CI, .94–1.10). Percentage change from baseline in serum HbA1c was 3.6% (95% CI, 2.8%–4.3%) and 2.9% (2.1%–3.8%) in the DTG/3TC and TAF-based regimen groups (treatment ratio, 1.01; 95% CI, 1.00–1.02). The proportions of participants with an adverse event of type 2 diabetes were low and similar between treatment groups: DTG/3TC, <1% (2/369); TAF-based regimen, <1% (1/371; Supplementary Table 1).
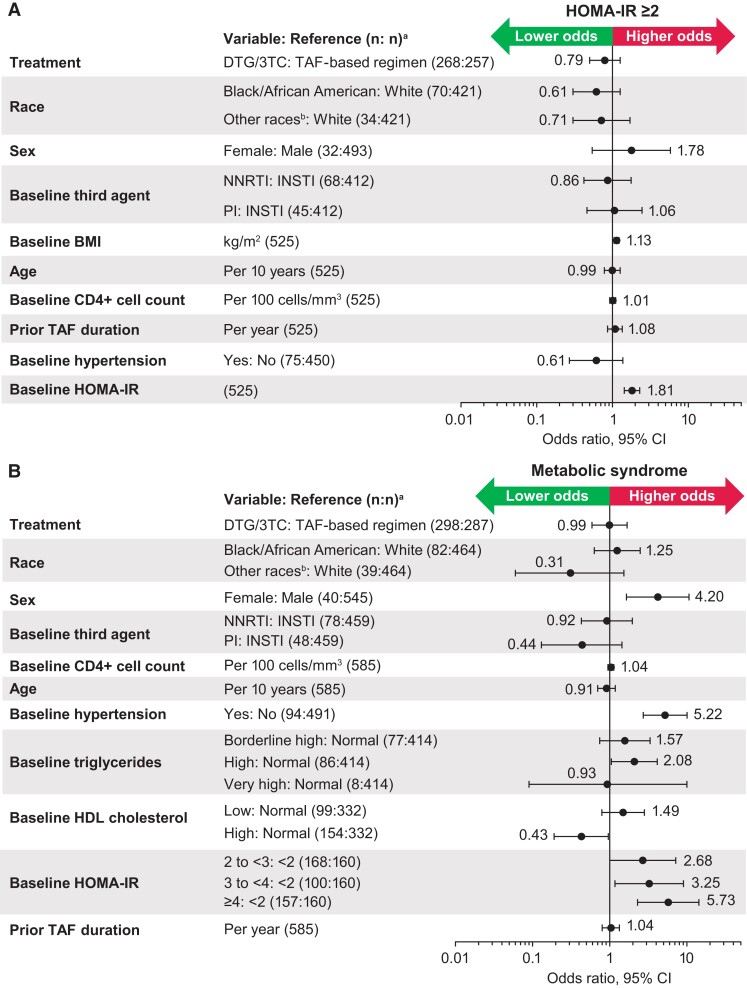
Adjusted percentage change from baseline in HOMA-IR was similar in the DTG/3TC (11.5%; 95% CI, 4.7%–18.7%) and TAF-based regimen (10.2%; 3.7%–17.2%) groups (treatment ratio, 1.01; 95% CI, .93–1.10). The proportions of participants with HOMA-IR ≥2 at baseline were 72% (193/268) and 72% (186/257) in the DTG/3TC and TAF-based regimen groups, respectively, and 78% (208/268) and 82% (211/257) at week 144 (aOR, 0.79; 95% CI, .50–1.26). Higher baseline HOMA-IR (1.81; 1.43–2.29) and BMI (1.13; 1.05–1.22) were associated with higher odds of HOMA-IR ≥2 at week 144 (Figure 4A). The odds of HOMA-IR ≥2—when analyzed by baseline boosting status (aOR [95% CI]: boosted, 0.81 [.47–1.40]; unboosted, 0.74 [.29–1.90]) and baseline BMI (underweight/normal, 0.74 [.42–1.31]; overweight, 0.79 [.28–2.20]; obesity, 1.05 [.21–5.28])—were generally similar between the DTG/3TC and TAF-based regimen groups.
Figure 4.
Adjusted odds (95% CI) of (A) HOMA-IR ≥2 and (B) metabolic syndrome (post hoc analysis). Each logistic regression adjusted for all variables assessed within the analysis. BMI, body mass index; DTG, dolutegravir; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; INSTI, integrase strand transfer inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TAF, tenofovir alafenamide; 3TC, lamivudine. aOdds ratios are presented in relation to the reference variable; the number of participants included in final model with covariate data at baseline and outcome data at baseline and week 144. Participants with evidence of prestudy diabetes were excluded. bOther races included American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander, mixed White race, and multiple races.
Metabolic Syndrome Assessed by International Diabetes Federation Criteria
Proportions of participants with metabolic syndrome were similar between treatment groups at baseline (DTG/3TC, 10% [36/369]; TAF-based regimen, 11% [41/370]) and week 144 (DTG/3TC, 14% [43/298]; TAF-based regimen, 16% [46/287]; aOR, 0.99 [95% CI, .59–1.68]; Figure 4B). Female sex (aOR, 4.20; 95% CI, 1.65–10.68), the presence of baseline hypertension (5.22; 2.72–10.04), and baseline HOMA-IR (2 to <3, 2.68 [1.00–7.17]; 3 to <4, 3.25 [1.17–9.02]; ≥4, 5.73 [2.29–14.32]) were associated with higher odds of metabolic syndrome at week 144, whereas high baseline HDL-C (aOR, 0.43; 95% CI, .19–.96) was associated with lower odds. Odds of metabolic syndrome were generally similar in the DTG/3TC and TAF-based regimen groups when analyzed by baseline boosting status (aOR [95% CI]: boosted, 0.93 [.51–1.72]; unboosted, 1.14 [.42–3.05]).
Hepatic Fibrosis as Measured by FIB-4
Adjusted percentage change from baseline in FIB-4 showed a decrease in both treatment groups and was similar between the DTG/3TC (−11.9%; 95% CI, −14.5% to −9.2%; n = 299) and TAF-based regimen (−10.6%; −13.3% to −7.9%; n = 289) groups (treatment ratio, 0.99; 95% CI, .95–1.03). The proportions of participants with FIB-4 ≥1.45 were similar between treatment groups at week 144: DTG/3TC, 8% (25/299); TAF-based regimen, 7% (21/289; aOR, 1.39 [95% CI, .63–3.06]; Supplementary Figure 1A). Parameters associated with higher odds of FIB-4 ≥1.45 at week 144 were Black/African American vs White race (aOR, 4.52; 95% CI, 1.68–12.16), older age (1.91; 1.24–2.94), and higher baseline FIB-4 (28.68; 9.49–86.71). Adjusted odds of FIB-4 ≥1.45 between treatment groups were generally similar to the overall analysis when analyzed by baseline boosting status (aOR [95% CI]: boosted, 1.62 [.58–4.49]; unboosted, 1.16 [.32–4.17]).
Coronary Artery Disease Risk as Measured by Framingham Risk Score ≥10%
The proportions of participants with Framingham risk score ≥10% were similar between treatment groups at baseline (DTG/3TC, 24% [90/368]; TAF, 22% [80/370]) and week 144 (DTG/3TC, 24% [58/238]; TAF, 25% [57/231]; aOR, 0.92 [95% CI, .56–1.49]; Supplementary Figure 1B). At week 144, the presence of baseline hypertension (aOR, 11.25; 95% CI, 5.22–24.26) and higher baseline total cholesterol (1.02; 1.01–1.03) were associated with higher odds of Framingham risk score ≥10%, whereas higher baseline HDL-C (0.98; 0.96–0.99) was associated with lower odds. The proportions of participants with hypertension and other cardiac adverse events were generally similar between treatment groups (Supplementary Table 3).
DISCUSSION
At week 144 in the TANGO study, the adjusted mean change in weight from baseline was similar between participants who switched to DTG/3TC and continued TAF-based regimens through 3 years and was consistent with the annual rate expected in the general population (0.5–1.0 kg/y) [22, 38]. Shift in BMI category was similar between treatment groups, with similar odds of ≥10% weight gain in the DTG/3TC and TAF-based regimen groups. Increases in leptin from baseline to week 144 were similar between treatment groups (∼21%). Leptin is predominantly secreted by white adipose tissue; circulating levels positively correlate with amount of body fat [16, 39–41]. Plasma leptin measurement allows for evaluation of whether weight gain is fat or lean muscle mass [16, 41]. Across groups, magnitude of leptin increase was aligned with baseline BMI, and there was a positive correlation between change from baseline in serum leptin and weight in male and female participants. Together, these data suggest a similar change in total fat mass over 3 years between treatment groups.
More female than male participants gained ≥10% weight, which is consistent with other studies reporting female sex as a risk factor for greater weight gain on INSTI-based regimens, including after switch to INSTIs [12, 13, 42]. In the ADVANCE trial of PWH initiating ART (N = 1053), greater weight gain was observed in female participants, particularly among those taking DTG + TAF/emtricitabine [43]. Older age was associated with lower odds of ≥10% weight gain, similar to findings from a retrospective observational study of PWH who were virologically suppressed who switched to INSTIs (N = 2272), reporting that younger age (<50 years) was associated with greater risk of weight gain [44]. Shorter prior TAF duration, when evaluated as a continuous variable, was associated with higher odds of weight gain. Shorter prior TAF duration may indicate shorter overall ART duration, and return to health characterized by weight gain has been well described in PWH initiating ART [4]. Additionally, the inclusion criteria allowed participants a TDF-to-TAF switch ≥3 months before randomization; these participants would have shorter prior TAF durations and could have experienced weight gain associated with a TDF-to-TAF switch, which has been demonstrated to be greatest during the first 6 to 12 months, followed by a plateau [9].
In the overall TANGO population, DTG/3TC had a generally favorable lipid profile as compared with TAF-based regimens at week 144, particularly for total cholesterol, LDL-C, and triglycerides [22]. The present study showed favorable changes in lipids after switch to DTG/3TC in the baseline boosted subgroup, with the exception of HDL-C, whereas changes were comparable between groups in the baseline unboosted subgroup. The differences observed within the boosted subgroup may be explained by increased TAF exposure when given with cobicistat—even when dosed at 10 mg, as in elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (EVG/c/FTC/TAF), the most common baseline regimen in this study—and/or by a direct effect of the booster drug. In a study assessing the effect of switch from TDF to TAF on LDL-C (N = 221), use of cobicistat with TAF was associated with an increased risk of LDL-C above the cardiovascular target (aOR, 2.4; 95% CI, 1.0–5.1; P = .03) [45].
At week 144 in TANGO, similar proportions of participants in the DTG/3TC (78%) and TAF-based regimen (82%) groups had insulin resistance (HOMA-IR ≥2) [22]. The present analyses support this finding by showing that changes from baseline in markers of insulin resistance were comparable between the DTG/3TC and TAF-based regimen groups through week 144, including HOMA-IR, glucose, insulin, and HbA1c. Furthermore, switching to DTG/3TC vs continuing TAF-based regimens was associated with similar odds of HOMA-IR ≥2. Of note, at week 48 of TANGO, the odds of HOMA-IR ≥2 were lower with DTG/3TC (aOR, 0.59; 95% CI, .40–.87) [46]. However, between weeks 48 and 144, differences in HOMA-IR between groups were no longer apparent. Baseline BMI and HOMA-IR were associated with higher odds of HOMA-IR ≥2 at week 144. A post hoc analysis demonstrated that a greater proportion of participants continuing TAF-based regimens with obesity at baseline were referred for diet/weight management counseling, which may have influenced weight fluctuations and changes in HOMA-IR through week 144 [22]. Visceral adipose tissue, which is strongly associated with HOMA-IR [47], was not measured in TANGO, yet leptin data indicate that body fat increase was similar between groups.
We observed higher odds of metabolic syndrome in women, consistent with a prior study in 1861 PWH reporting that female sex increased odds of metabolic syndrome (aOR, 2.24; 95% CI, 1.69–2.97) [21]. Change in FIB-4 from baseline was similar between DTG/3TC (−11.9%) and TAF-based regimens (−10.6%), with older age associated with greater odds of hepatic fibrosis. Data from 1137 PWH in the ICONA Foundation Study support this finding, reporting that each 10-year increase in age was associated with a greater likelihood of FIB-4 progression (adjusted relative hazard, 1.40; 95% CI, 1.20–1.64; P < .001) [48]. Proportions of participants with Framingham risk score ≥10% were similar between treatment groups (DTG/3TC, 24%; TAF, 25%). The presence of baseline hypertension was associated with considerably higher odds of Framingham risk score ≥10% (aOR, 11.25), whereas higher baseline HDL-C was associated with odds just under 1 (aOR, 0.98). Overall, the baseline characteristics associated with lower or higher risk of various cardiometabolic parameters in the current analysis are consistent with the literature. These baseline characteristics may help inform cardiometabolic profiling to proactively identify PWH who would benefit from focused cardiometabolic health counseling.
There are several limitations to this analysis. Notably, the TANGO study population was primarily composed of White male participants. Although female sex and Black race were associated with higher odds of metabolic syndrome and FIB-4 ≥1.45, respectively, higher INSTI-related weight gain has been observed in Black PWH [12], and the relatively low number of participants in these groups may have affected the ability to detect associations with other cardiometabolic health outcomes as well as the precision of the odds ratio point estimates. The SALSA study recruited a more diverse population switching to DTG/3TC vs continuing 3-/4-drug current antiretroviral regimens [24]. Generally similar changes in insulin resistance and lipids were observed through 1 year in SALSA; however, comparing cardiometabolic endpoints between TANGO and SALSA is complicated by the significant heterogeneity of baseline regimens, as well as SALSA being limited to 1 year of follow-up. It remains important that clinical trials recruit diverse populations to help ensure appropriate efficacy and safety assessments of therapies in participants representing the global diversity of PWH. Given the low numbers of female participants and participants of Black race in this analysis, findings may not be as generalizable to the majority of PWH worldwide.
Another limitation is that many of the analyses were post hoc. While a 144-week follow-up is lengthy for a clinical trial, cardiometabolic parameters that fluctuate over time (eg, weight) are not completely captured in an analysis at a single time point, and longer follow-up is needed for a comprehensive understanding of the impact of ART on metabolic health. The majority of participants switched from EVG/c/FTC/TAF to DTG/3TC, limiting generalizations for switch from other INSTI-based regimens. Similar to many large analyses of this nature, several potential confounders were not adequately measured, including diet, lifestyle, and risk factors before ART initiation [10]. Finally, proportions of participants with ≥10% weight increase over 3 years were relatively small (12%–13%)—although in line with a 2022 study reporting that ∼10% of PWH who were virologically suppressed experienced ≥10% weight gain within 2 years of switching to TAF or INSTIs [49]—and analyses of the association between this postbaseline status and week 144 cardiometabolic health parameters were not performed due to statistical methodology challenges. Conversely, the strengths of the current analyses include the relatively large sample size, with high retention over 3 years, regular assessments every 12 weeks, and the randomized nature of the comparison between treatment groups. Additionally, the comprehensive nature of this metabolic health evaluation, which included assessment of diet/exercise counseling and leptin levels, is novel for a contemporary clinical trial of a second-generation INSTI.
Our findings support that cardiometabolic parameters in PWH who switched to DTG/3TC were similar to those who continued TAF-based regimens and were generally stable for both groups over 3 years of follow-up, except for lipids, which favored DTG/3TC in the boosted subgroup. Furthermore, demographic and cardiometabolic risk factors known to be associated with lower or higher odds of each cardiometabolic parameter examined were aligned in the TANGO population, underscoring their importance in clinical management and prevention of adverse outcomes in PWH receiving lifelong ART. As the 2DR DTG/3TC has been shown to be potent with durable efficacy, a high barrier to resistance, and good safety, including a comprehensive evaluation of laboratory parameters and inflammatory biomarkers associated with cardiometabolic health [22–24], the long-term use of an additional third antiretroviral agent for the treatment of HIV should be justified. Our findings, in combination with previously demonstrated 3-year durable efficacy and good safety and tolerability, support the 2DR DTG/3TC as a robust switch option [22].
Supplementary Material
Contributor Information
Rachel L Batterham, Department of Medicine, Centre for Obesity Research, University College London, London, UK; National Institute of Health Research, University College London Hospitals Biomedical Research Centre, London, UK.
Nuria Espinosa, Hospital Universitario Virgen del Rocío, Sevilla, Andalucía, Spain.
Christine Katlama, Service de Maladies Infectieuses et Tropicales, AP-HP, Hôpital Pitié-Salpêtrière, INSERM–Sorbonne Universités, Paris, France.
Mehri McKellar, Department of Medicine, School of Medicine, Duke University, Durham, North Carolina, USA.
Stefan Scholten, Praxis Hohenstaufenring, Cologne, Germany.
Don E Smith, Albion Centre, Sydney, Australia.
Mounir Ait-Khaled, ViiV Healthcare, Brentford, UK.
Nisha George, GSK, Bangalore, India.
Jonathan Wright, GSK, Brentford, UK.
Lori A Gordon, ViiV Healthcare, Durham, North Carolina, USA.
Riya Moodley, ViiV Healthcare, Brentford, UK.
Brian Wynne, ViiV Healthcare, Durham, North Carolina, USA.
Jean van Wyk, ViiV Healthcare, Brentford, UK.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. M. A.-K. and J. v. W. contributed to the conception of the study. M. A.-K., L. A. G., and J. v. W. contributed to the design of the study. N. E., C. K., M. M., S. S., D. E. S., and R. M. contributed to the acquisition of data. N. G., J. W., and R. M. contributed to the analysis of data. R. L. B., M. A.-K., N. G., J. W., L. A. G., B. W., and J. v. W. contributed to the interpretation of data. M. A.-K. and L. A. G. contributed to drafting the manuscript. All authors contributed to critically revising the manuscript for important intellectual content and approve the manuscript for publication.
Acknowledgments. We thank the study participants and their families and caregivers; the investigators and site staff who participated in the study; and the study team members of ViiV Healthcare, GSK, Pharmaceutical Product Development, and Phastar. Editorial assistance was provided under the direction of the authors by Seth Hurley, PhD, and Jennifer Rossi, MA, ELS, MedThink SciCom, and was funded by ViiV Healthcare.
Data availability. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Financial support. This work was supported by ViiV Healthcare.
References
- 1. Wing EJ. HIV and aging. Int J Infect Dis 2016; 53:61–8. [DOI] [PubMed] [Google Scholar]
- 2. Althoff KN, Smit M, Reiss P, Justice AC. HIV and ageing: improving quantity and quality of life. Curr Opin HIV AIDS 2016; 11:527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Back D. 2-Drug regimens in HIV treatment: pharmacological considerations. Germs 2017; 7:113–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wood BR, Huhn GD. Excess weight gain with integrase inhibitors and tenofovir alafenamide: what is the mechanism and does it matter? Open Forum Infect Dis 2021; 8:ofab542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Willig AL, Overton ET. Metabolic complications and glucose metabolism in HIV infection: a review of the evidence. Curr HIV/AIDS Rep 2016; 13:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Panel on Antiretroviral Guidelines for Adults and Adolescents, Department of Health and Human Services . Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. Available at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf. Accessed 4 February 2022.
- 7. European AIDS Clinical Society . Guidelines version 11.0. October 2021. Available at: https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf. Accessed 4 February 2022.
- 8. Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society–USA Panel. JAMA 2020; 324:1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a US cohort study. J Int AIDS Soc 2021; 24:e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71:1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schafer JJ, Sassa KN, O’Connor JR, Shimada A, Keith SW, DeSimone JA. Changes in body mass index and atherosclerotic disease risk score after switching from tenofovir disoproxil fumarate to tenofovir alafenamide. Open Forum Infect Dis 2019; 6:ofz414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hester EK, Greenlee S, Durham SH. Weight changes with integrase strand transfer inhibitor therapy in the management of HIV infection: a systematic review. Ann Pharmacother 2022; 56:1237–49. [DOI] [PubMed] [Google Scholar]
- 13. Erlandson KM, Carter CC, Melbourne K, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73:1440–51. [DOI] [PubMed] [Google Scholar]
- 14. Ando N, Nishijima T, Mizushima D, et al. Long-term weight gain after initiating combination antiretroviral therapy in treatment-naive Asian people living with human immunodeficiency virus. Int J Infect Dis 2021; 110:21–8. [DOI] [PubMed] [Google Scholar]
- 15. Rolle C-P, Castano J, Nguyen V, Patel K, Hinestrosa F, DeJesus E. Early discontinuations and adverse events among treatment-naive patients initiating integrase inhibitors in a real-world setting [poster 889]. Presented at: IDWeek 2021; 29 September–3 October 2021; virtual. [DOI] [PubMed]
- 16. Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med 1996; 334:292–5. [DOI] [PubMed] [Google Scholar]
- 17. Bonora E, Formentini G, Calcaterra F, et al. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care 2002; 25:1135–41. [DOI] [PubMed] [Google Scholar]
- 18. Koethe JR, Jenkins CA, Lau B, et al. Rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retroviruses 2016; 32:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drozd DR, Kitahata MM, Althoff KN, et al. Increased risk of myocardial infarction in HIV-infected individuals in North America compared with the general population. J Acquir Immune Defic Syndr 2017; 75:568–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freiberg MS, Chang CC, Kuller LH, et al. HIV Infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sears S, Buendia JR, Odem S, et al. Metabolic syndrome among people living with HIV receiving medical care in southern United States: prevalence and risk factors. AIDS Behav 2019; 23:2916–25. [DOI] [PubMed] [Google Scholar]
- 22. Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: results through week 144 from the phase 3, noninferiority TANGO randomized trial. Clin Infect Dis 2022; 75:975–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naive adults with HIV-1 infection. AIDS 2022; 36:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llibre JM, Brites C, Cheng C-Y, et al. Efficacy and safety of switching to the 2-drug regimen dolutegravir/lamivudine versus continuing a 3- or 4-drug regimen for maintaining virologic suppression in adults living with human immunodeficiency virus 1 (HIV-1): week 48 results from the phase 3, noninferiority SALSA randomized trial. Clin Infect Dis 2023; 76:720–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Béténé A Dooko C, De Wit S, Neuhaus J, et al. Interleukin-6, high sensitivity C-reactive protein, and the development of type 2 diabetes among HIV-positive patients taking antiretroviral therapy. J Acquir Immune Defic Syndr 2014; 67:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duprez DA, Neuhaus J, Kuller LH, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One 2012; 7:e44454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71:1920–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Galaviz KI, Schneider MF, Tien PC, et al. Expanding the Finnish Diabetes Risk Score for predicting diabetes incidence in people living with HIV. AIDS Res Hum Retroviruses 2021; 37:373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013; 13:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hedblad B, Nilsson P, Janzon L, Berglund G. Relation between insulin resistance and carotid intima-media thickness and stenosis in non-diabetic subjects: results from a cross-sectional study in Malmö, Sweden. Diabet Med 2000; 17:299–307. [DOI] [PubMed] [Google Scholar]
- 31. International Diabetes Federation . The IDF consensus worldwide definition of the metabolic syndrome. Brussels: International Diabetes Federation, 2006. [Google Scholar]
- 32. Iogna Prat L, Roccarina D, Lever R, et al. Etiology and severity of liver disease in HIV-positive patients with suspected NAFLD: lessons from a cohort with available liver biopsies. J Acquir Immune Defic Syndr 2019; 80:474–80. [DOI] [PubMed] [Google Scholar]
- 33. Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology 2019; 156:1264–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Perazzo H, Cardoso SW, Yanavich C, et al. Predictive factors associated with liver fibrosis and steatosis by transient elastography in patients with HIV mono-infection under long-term combined antiretroviral therapy. J Int AIDS Soc 2018; 21:e25201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection: comparison with liver biopsy and fibrotest. Hepatology 2007; 46:32–6. [DOI] [PubMed] [Google Scholar]
- 36. Brindle PM, McConnachie A, Upton MN, Hart CL, Davey Smith G, Watt GCM. The accuracy of the Framingham risk-score in different socioeconomic groups: a prospective study. Br J Gen Pract 2005; 55:838–45. [PMC free article] [PubMed] [Google Scholar]
- 37. Achhra AC, Lyass A, Borowsky L, et al. Assessing cardiovascular risk in people living with HIV: current tools and limitations. Curr HIV/AIDS Rep 2021; 18:271–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hutfless S, Maruthur NM, Wilson RF, et al. Strategies to prevent weight gain among adults. Comparative effectiveness review No. 97. AHRQ publication No. 13-EHC029-EF. Rockville: Agency for Healthcare Research and Quality, 2013. [PubMed] [Google Scholar]
- 39. Obradovic M, Sudar-Milovanovic E, Soskic S, et al. Leptin and obesity: role and clinical implication. Front Endocrinol (Lausanne) 2021; 12:585887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ahima RS, Flier JS. Leptin. Annu Rev Physiol 2000; 62:413–37. [DOI] [PubMed] [Google Scholar]
- 41. Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL. Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 1997; 82:3647–54. [DOI] [PubMed] [Google Scholar]
- 42. Lake JE, Wu K, Bares SH, et al. Risk factors for weight gain following switch to integrase inhibitor-based antiretroviral therapy. Clin Infect Dis 2020; 71:e471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. [DOI] [PubMed] [Google Scholar]
- 44. McComsey GA, Sax P, Althoff KN, et al. Weight gain after switching different integrase strand transfer inhibitors (InSTIs) [abstract 503]. Presented at: Conference on Retroviruses and Opportunistic Infections; 6–10 March 2021; virtual.
- 45. Gazzola L, Tagliaferri G, De Bona A, et al. Dyslipidaemia after switch to tenofovir alafenamide (TAF)–based cART regimens in a cohort of HIV-positive patients: what clinical relevance? HIV Med 2021; 22:140–5. [DOI] [PubMed] [Google Scholar]
- 46. van Wyk J, Ait-Khaled M, Santos J, et al. Brief report: improvement in metabolic health parameters at week 48 after switching from a tenofovir alafenamide-based 3- or 4-drug regimen to the 2-drug regimen of dolutegravir/lamivudine: the TANGO study. J Acquir Immune Defic Syndr 2021; 87:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham Heart Study. Obesity (Silver Spring) 2010; 18:2191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saracino A, Cozzi-Lepri A, Shanyinde M, et al. HIV-1 co-receptor tropism and liver fibrosis in HIV-infected patients. PLoS One 2018; 13:e0190302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Verburgh ML, Wit FWNM, Boyd A, Verboeket SO, Reiss P, van der Valk M. One in 10 virally suppressed persons with HIV in the Netherlands experiences ≥10% weight gain after switching to tenofovir alafenamide and/or integrase strand transfer inhibitor. Open Forum Infect Dis 2022; 9:ofac291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.