Abstract
Background
Iron is essential to brain function, and iron deficiency during youth may adversely impact neurodevelopment. Understanding the developmental time course of iron status and its association with neurocognitive functioning is important for identifying windows for intervention.
Objectives
This study aimed to characterize developmental change in iron status and understand its association with cognitive performance and brain structure during adolescence using data from a large pediatric health network.
Methods
This study included a cross-sectional sample of 4899 participants (2178 males; aged 8–22 y at the time of participation, M [SD] = 14.24 [3.7]) who were recruited from the Children’s Hospital of Philadelphia network. Prospectively collected research data were enriched with electronic medical record data that included hematological measures related to iron status, including serum hemoglobin, ferritin, and transferrin (33,015 total samples). At the time of participation, cognitive performance was assessed using the Penn Computerized Neurocognitive Battery, and brain white matter integrity was assessed using diffusion-weighted MRI in a subset of individuals.
Results
Developmental trajectories were characterized for all metrics and revealed that sex differences emerged after menarche such that females had reduced iron status relative to males [all R2partial > 0.008; all false discovery rates (FDRs) < 0.05]. Higher socioeconomic status was associated with higher hemoglobin concentrations throughout development (R2partial = 0.005; FDR < 0.001), and the association was greatest during adolescence. Higher hemoglobin concentrations were associated with better cognitive performance during adolescence (R2partial = 0.02; FDR < 0.001) and mediated the association between sex and cognition (mediation effect = −0.107; 95% CI: −0.191, −0.02). Higher hemoglobin concentration was also associated with greater brain white matter integrity in the neuroimaging subsample (R2partial = 0.06, FDR = 0.028).
Conclusions
Iron status evolves during youth and is lowest in females and individuals of low socioeconomic status during adolescence. Diminished iron status during adolescence has consequences for neurocognition, suggesting that this critical period of neurodevelopment may be an important window for intervention that has the potential to reduce health disparities in at-risk populations.
Keywords: iron, hemoglobin, ferritin, transferrin, adolescence, cognition, white matter, executive function, menarche, puberty
Introduction
Iron plays an essential role in many aspects of brain function, including metabolism, myelination of white matter pathways, and neurotransmitter synthesis [[1], [2], [3], [4]], and brain iron concentrations continue to mature throughout youth [40,[75], [76], [77], [78], [79]]. The body’s iron requirements fluctuate throughout development according to demand from ongoing maturational processes; insufficient iron during critical developmental windows can disrupt these processes with long-lasting consequences [2,5]. The specific impact of iron deficiency on brain maturation and cognition during these vulnerable developmental windows may differ depending on the iron-dependent neurodevelopmental processes unfolding at the time they occur.
The impact of iron status has been most heavily studied during the first years of life, when iron deficiency impacts as many as 15% of children [6,7]. Iron deficiency during this vulnerable window can lead to short-term and long-term developmental delays, motor impairments, and cognitive deficits [5,[8], [9], [10], [11]]. Animal models of early life iron deficiency have shown that these deficits are accompanied by morphological abnormalities and reduced integrity of the white matter surrounding subcortical brain areas [2,[12], [13], [14], [15]].
Although sparsely investigated, adolescence may represent a late-occurring window of vulnerability to iron deficiency during development. Rapid growth at puberty onset increases iron demand [16,17], and at the same time, increasing autonomy over dietary decisions may reduce adolescent iron intake [18]. These factors are thought to underlie prior reports that the prevalence of iron deficiency during adolescence may rise to a level similar to that seen in early life [7]. Although earlier work using population survey data has coarsely documented mean-level increases in measures of iron status over 3-y age-bins during youth [19], the developmental trajectories of iron status metrics during the adolescent period have not been delineated in detail. Furthermore, the links between individual differences in adolescent iron status and neurocognitive development are not well characterized. Understanding the developmental window of vulnerability to iron deficiency is a necessary prerequisite for identifying windows for intervention.
Certain populations have been found to be at particularly elevated risk for adolescent iron deficiency, including females and individuals of low socioeconomic status (SES) [8,[19], [20], [21], [22]]. Following menarche, blood loss associated with menstruation can increase iron requirements by an average of 0.5 mg (25%) per day [17], placing female adolescents at heightened and prolonged risk relative to males [23]. Accordingly, population level data from the NHANES have shown that adolescent females have lower median hemoglobin (Hgb) and ferritin concentrations relative to adolescent males [19]. Socioenvironmental factors such as low SES can function as additional risk factors because of limited availability of access to high quality nutrition [24,25]. Globally, iron deficiency is a primary cause of disease burden in low- and middle-income populations [21]. Females and individuals of low SES may thus be particularly vulnerable to iron deficiency during adolescence and, as a result, may be at greater risk for adverse neurocognitive consequences. Understanding how and when these effects emerge over the course of development is critical for understanding and reducing health disparities in at-risk populations.
Low iron status during adolescence may have a significant impact on developmentally sensitive aspects of cognition and brain structure. Higher-order cognitive abilities, such as executive function, undergo protracted development during adolescence [[26], [27], [28], [29]]. This pattern of higher-order cognitive development is thought to be driven by a critical period of developmental plasticity in the brain’s executive system during adolescence and young adulthood [30,31]. One of the most pronounced aspects of this developmental plasticity during adolescence is the myelination of white matter pathways that link the spatially distributed brain regions that comprise the executive system [32,33]. Critically, the myelination process is metabolically demanding and requires substantial iron [1]. However, the effect of iron status on myelination and executive function during adolescence remains unknown.
Here, we leveraged a large community sample of preadolescent and adolescent aged youth from the Philadelphia area who participated in detailed cognitive assessment and neuroimaging. Using health system data from electronic medical records (EMRs), we assessed 3 hematological measures of iron status: Hgb, an indicator of functional iron level that, when reduced, indicates that iron deficiency has progressed to the point of compromising red blood cell production (anemia); ferritin, an indicator of iron storage that is reduced during iron deficiency even before developing anemia; and transferrin, an indicator of iron transport that is elevated during iron deficiency [34]. The use of EMR data allowed us to investigate the development during youth and then investigate associations with neurocognitive assessments performed during adolescence at the time of participation in this study. Using this unique dataset, we pursued 3 related objectives. First, we delineated the developmental trajectories of 3 iron status measures over the first 2 decades of life and assessed how they differed by sex, pubertal timing, and SES during youth. Second, we investigated how hematological measures of iron status were associated with overall cognitive performance as well as the subdomains of executive function, social cognition, and memory. We further assessed whether these effects differed by sex and whether they were present in individuals subthreshold for iron deficiency anemia. Finally, we investigated whether adolescent Hgb concentrations were associated with the integrity of brain’s major white matter pathways. We hypothesized that the iron status measures would evolve in youth, with sex differences emerging during puberty and lower SES being associated with lower iron status. We further predicted that better iron status during adolescence would be positively associated with executive performance and that greater Hgb concentrations would be positively associated with the white matter integrity of executive pathways.
Methods
Sample
This sample was drawn from the Philadelphia Neurodevelopmental Cohort (PNC). As previously detailed, the PNC sample consists of 9498 participants, aged 8–22 y, who underwent cognitive assessment and a subset of 1601 youths who also completed neuroimaging [35,36]. This study used a subset of the PNC sample for which EMR data included ≥1 indicator of peripheral iron status (Hgb, transferrin, or ferritin; EMR data available: n = 6926 and 92,849 observations). Study participants were excluded if their EMR data indicated a moderate or severe medical condition (n = 1954), which was previously determined in this sample [37] (Supplemental Methods). Laboratory results with serum ferritin concentrations above the normal reference range, suggesting that ferritin was either indicating iron overload or being influenced by inflammation in addition to iron storage, were excluded from all analyses (described below; 307 total observations excluded). For participants with repeated measures, all measures of iron status that met inclusion criteria were included. The final sample included 30,317 Hgb concentrations from 4874 participants, 1358 ferritin concentrations from 537 participants, and 1340 transferrin concentrations from 526 participants (see Supplemental Table 1 for demographic and hematology data summary statistics). Participants who had ≥1 iron status metric within 6 mo before neurocognitive testing as part of the PNC were also included in analysis of cognitive (n = 1429; male/female = 573/856; aged 8.08–21.92 y, M [SD] = 14.33 [3.26]) and neuroimaging data (n = 126; male/female = 51/75; aged 8.83–22.58 y, M [SD] = 15.22 [3.23]). A summary of all inclusions and exclusions can be found in Supplemental Figure 1. The institutional review boards of both the University of Pennsylvania and the Children’s Hospital of Philadelphia (CHOP) approved all study procedures.
Measures
Demographic variables
Participant sex and age of menarche (to the nearest year) were self-reported. Age at the time of hematological, cognitive, and neuroimaging measurements was determined from participant birth date and the date of measurement. A total of 1809 participants reported menarche at the time of enrollment in the PNC study. Neighborhood SES was determined on the basis of geocoded block-level data as previously detailed [38].
Iron status
Serum Hgb, transferrin, and ferritin were used as hematological indicators of peripheral iron status. Laboratory results reporting serum ferritin concentrations above the upper normal reference value of 358 ng/dL were excluded from all analyses (307 total observations). This was done for 2 reasons. First, elevated ferritin concentrations can indicate excess iron storage, which is not the focus of the current study. Second, ferritin is an acute phase reactant that is elevated during inflammation, and although it is a highly sensitive and specific indirect indicator of iron status at low levels, it is nonspecific to iron status at elevated levels [39].
Cognitive assessment
Cognitive performance was assessed using the Computerized Neurocognitive Battery (CNB). The CNB consists of 14 tests to evaluate a broad range of cognitive domains [26], including executive control, complex cognition, episodic memory, and social cognition (Supplemental Methods). As previously described, accuracy on each test was entered into a factor analysis, and factor scores were calculated separately for an overall factor and 3 correlated subfactors that correspond to executive function, social cognition, and memory [40,41].
Brain imaging
Neuroimaging was performed in 1601 participants from the total PNC sample. Participants were excluded from the current study on the basis of previously reported quality assurance assessment of diffusion-weighted imaging (DWI) data [42], or for lacking available EMR data (n = 693). Finally, participants were included in DWI analyses if a hematological measure was collected within 6 mo before neuroimaging (see Supplemental Figure 1 for an overview of sample inclusions and exclusions). A final sample of 126 participants was included in analysis of the association between DWI data and Hgb concentrations. As only 9 individuals met inclusion criteria for ferritin and transferrin data, associations with DWI were not conducted for these measures. Details of the neuroimaging acquisition and preprocessing have been previously reported [36] and can be found in the Supplemental Methods. Briefly, fractional anisotropy (FA), a commonly used measure of white matter microstructure, was calculated from DWI data and extracted for a set of major white matter pathways (JHU White Matter Tracts [43]).
Statistical analysis
Regression and mediation analyses
All developmental analyses included longitudinal data and were modeled using generalized additive mixed-effect models (GAMMs) that accounted for the nested data structure. Associations between iron status metrics and participant age were analyzed using GAMMs, which modeled chronological age using penalized smooth functions to flexibly capture both linear and nonlinear developmental effects while protecting against overfitting (see Supplemental Methods for further details). Moderating effects of sex and neighborhood SES were assessed by using interaction terms. Random slopes and intercepts were estimated for each individual to account for within-participant variance. Development in relation to the onset of menarche in female participants was assessed by centering age on the self-reported age of menarche and modeling it as a penalized smooth function in a GAMM. Windows of significant age-related change were identified using the derivative of the smooth function of age (see Supplemental Methods). All analyses of cognitive and white matter metrics were conducted on cross-sectional data using generalized additive models (GAMs). The GAMs included a penalized smooth function for iron status and included sex and a smooth function of age as covariates. Effect sizes for smooth terms were estimated as the partial R2 (R2partial) of the full model relative to a reduced model that omitted the smooth term of interest. To account for multiple comparisons, P values from related models were corrected using the false discovery rate (FDR) as implemented using p.adjust in R. To test whether Hgb mediated the association between SES and overall cognitive accuracy, we conducted a mediation analysis that included covariates for age and sex. To test whether sex differences in Hgb mediated sex differences in cognition over development, we conducted a moderated mediation analysis. The moderated mediation analysis tested whether the strength of Hgb as a mediator of the association between sex and overall cognitive accuracy differed with age (see Supplemental Methods for details).
Principal component analysis
Individual iron status measures can have limited interpretability when evaluated in isolation and are often assessed in combination when evaluating iron status. In addition to indexing iron status, ferritin and transferrin are also acute phase reactants that are sensitive to the inflammatory response. These metrics can become nonspecific to iron status in the context of acute or chronic inflammation or infection. Furthermore, Hgb concentrations can be reduced because of anemias that are not caused by iron deficiency. For example, iron deficiency is associated with reduced Hgb, reduced ferritin, and elevated transferrin; in contrast, anemia of chronic disease is associated with reduced Hgb, increased ferritin, and reduced transferrin. To address this issue and gain greater specificity in the interpretability of our findings, we conducted a data-driven decomposition using principal component analysis (PCA) to derive a composite metric that parsed effects of iron status from effects of inflammation or infection (Supplemental Methods). This approach yielded a composite iron status index (low scores on this index reflected reduced ferritin, reduced Hgb, and elevated transferrin) and an inflammatory index (elevated ferritin, reduced transferrin, and reduced Hgb). To confirm our interpretation of the inflammatory index, we repeated the PCA in a subset of the sample that also had the inflammatory marker CRP available in the EMR (n = 137) and compared the resulting PC scores with the original PCA model. We then assessed the association between the composite iron status index and development, sex, SES, and cognition using GAMs as described above. Because only 6participants included in this data-driven analysis completed neuroimaging within 6 mo of blood testing, associations with white matter integrity were not investigated.
Results
The development of iron status is moderated by sex and SES during adolescence
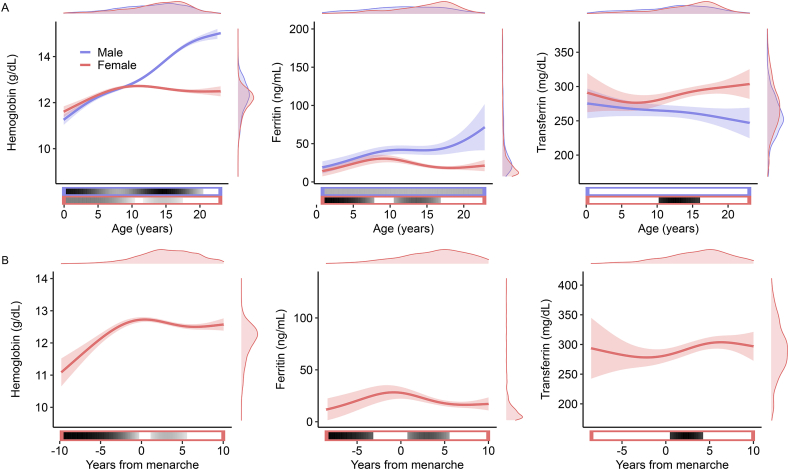
Developmental analyses were conducted on EMR measures of peripheral iron status that spanned the first 23 y of life and included longitudinal assessments (Supplemental Table 1). Over this period of development, each peripheral iron status measure followed a nonlinear developmental trajectory that differed by sex (Figure 1A, Supplemental Table 2). In each case, sex differences in the developmental trajectory were most prominent during the adolescent years. In males, developmental trajectories were largely consistent throughout youth: Hgb concentrations increased monotonically until age 20, ferritin concentrations increased throughout the age range, and transferrin concentrations did not significantly differ with age. In contrast, females displayed a different pattern: Hgb increased to a plateau at age 11 and subsequently decreased throughout adolescence (aged 12–18 y), ferritin concentrations increased in childhood and transiently decreased during adolescence (aged 11–16 y), whereas transferrin concentrations declined during early childhood (aged 0–5 y) before increasing over adolescence (aged 10–15 y).
FIGURE 1.
Development of peripheral indicators of iron status. Hemoglobin (left), ferritin (center), and transferrin (right) were significantly associated with age throughout the first 2 decades of life (A). Sex differences in developmental trajectories emerged during adolescence such that females tended to decline in iron status (red lines) relative to males (blue lines). Rescaling chronological age to reflect years from self-reported menarche revealed that developmental trajectories changed after menarche for females (B). Solid lines reflect spline fits from generalized additive models; shaded areas reflect 95% CIs of the fit. Density plots on right and top edges depict the distribution of the plotted variables. Horizontal bars below the x-axis depict the significance of age-related change (derivatives of the fitted spline function). The outline of the bar indicates age-related change for males (blue) or females (red). The filled portion of the bar indicates windows of significant change; the saturation of the fill represents the magnitude of change (derivative magnitude).
The emergence of sex differences in the developmental trajectories of iron status metrics near the onset of adolescence suggested a role of pubertal development. To assess this, we evaluated how each iron status metric developed relative to the self-reported age of menarche in female participants. This analysis revealed changes in the developmental trajectory of iron status that occurred within 1 y of menarche for all metrics. Specifically, Hgb significantly increased until 0.3 y before menarche before significantly decreasing between 1.1 and 5.5 y after menarche; ferritin began to significantly decrease 0.8 y after menarche; and transferrin began to significantly increase 0.5 y after menarche (Figure 1B, Supplemental Table 3).
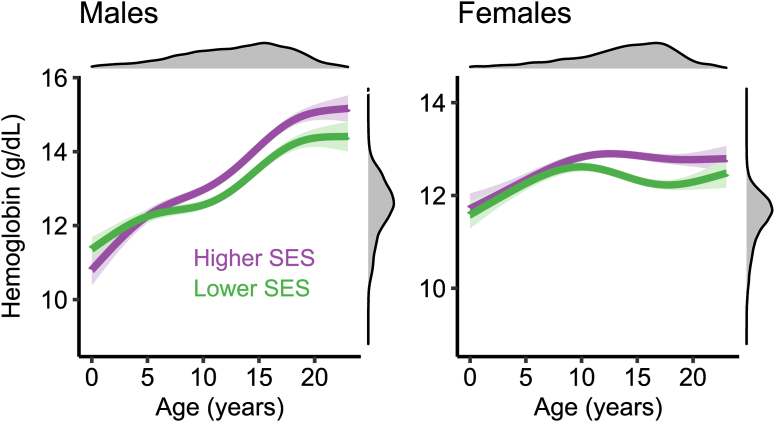
We next tested whether measures of iron status were associated with neighborhood SES. Higher SES was positively associated with higher Hgb concentrations (R2partial = 0.005, FDR < 0.001) but was not associated with ferritin (R2partial ≤ 0.001, FDR = 0.32) or transferrin concentrations (R2partial ≤ 0.001, FDR = 0.84). We then assessed whether the developmental trajectory of Hgb was moderated by neighborhood SES by testing a nonlinear age-by-SES interaction for males and females separately. We found that the development of Hgb was significantly moderated by SES for both males (R2partial = 0.002, FDR < 0.001) and females (R2partial = 0.002, FDR < 0.001). In both cases, higher SES was associated with higher Hgb during adolescence (Figure 2).
FIGURE 2.
Developmental trajectory of hemoglobin is moderated by neighborhood socioeconomic status. To visualize the significant interaction between age and neighborhood SES (both continuous variables) for males (left) and females (right), fitted lines are displayed that depict the relationship between age and hemoglobin at the top (higher SES, orange lines) and bottom (lower SES, purple lines) deciles of the range for neighborhood SES. Solid lines smooth fits from generalized additive models. Shaded areas reflect 95% CIs of the fit. Density plots represent the distributions of the plotted variables. SES, socioeconomic status.
Hgb is associated with cognitive performance during adolescence
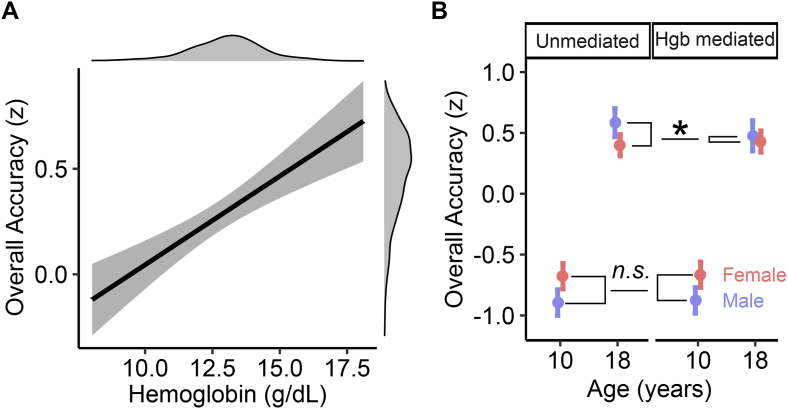
Cognitive testing was performed during adolescence at the time of participation in the PNC study (aged 8–22 y). Cognitive measures were compared with average iron status measures collected in the 6 mo before cognitive testing. As such, cognitive models were conducted on cross-sectional data. Hgb concentrations were positively associated with overall accuracy (R2partial = 0.02, FDR < 0.001; Figure 3A). This effect was significant for both males (R2partial = 0.01, P = 0.014, n = 529) and females (R2partial = 0.25, P < 0.001, n = 762) and did not vary by sex (that is, no significant Hgb-by-sex interaction; R2partial < 0.001, P = 0.82) or age (no significant Hgb-by-age interaction R2partial = 0.004, P = 0.27). The positive association between Hgb and overall accuracy remained significant when covarying for neighborhood SES (R2partial = 0.01, P = 0.004), and Hgb significantly mediated the association between neighborhood SES and overall cognition (average causal mediation effect = 0.007, 95% CI: 0.002, 0.02). To evaluate whether the association between Hgb and overall cognitive accuracy was driven by Hgb values that would meet the clinical threshold for iron deficiency, we repeated the analysis excluding observations that would meet the WHO’s threshold for iron deficiency anemia (Hgb < 12 g/dL, n = 266). Variation of Hgb values within this range remained significantly associated with overall cognitive accuracy (n = 1036; R2partial = 0.01, P = 0.006). Post-hoc analyses of cognitive subdomains revealed that the Hgb was significantly associated with all 3 cognitive domains surveyed, with the strongest impact on executive function (Supplemental Table 4).
FIGURE 3.
Hemoglobin concentrations are associated with cognitive performance. (A) Blood hemoglobin concentrations were positively associated with overall accuracy across the cognitive battery. Solid line represents the model fit for hemoglobin covarying for age and sex. Shaded portion reflects the CI of the fit. Density plots depict the distributions of the plotted variables. (B) Hemoglobin significantly mediated the effect of participant sex on overall accuracy, and the strength of the mediation effect was dependent on age (a moderated mediation effect). The moderated mediation effect was such that hemoglobin significantly mediated the association between overall accuracy and participant sex during adolescence—when sex differences in hemoglobin emerge—but not during childhood. ∗P = 0.020, n.s. not significant (P = 0.508).
Because sex differences in Hgb emerged during puberty, we next tested whether Hgb mediated sex differences in cognition and whether this effect was stronger after puberty. Females tended to perform better than males at cognitive tests before puberty but conversely tended to perform slightly worse after puberty. However, we found that these postpubertal sex differences in cognition largely disappeared when accounting for differences in Hgb concentrations. Specifically, our analysis revealed a significant moderated mediation effect such that Hgb significantly mediated sex differences in cognition during adolescence—but not childhood (age 18 compared with 10: Δaverage causal mediation effect = −0.08, 95% CI: −0.15, −0.02, P = 0.01; Figure 3B, Supplemental Figure 2).
Though the effect size for the association between ferritin and overall and executive cognitive performance was similar to Hgb, the association did not reach statistical significance (overall: R2partial = 0.014, P = 0.3; executive: R2partial = 0.024, P = 0.22; Supplemental Figure 3, Supplemental Table 5). Transferrin was not significantly associated with cognitive performance (all FDR > 0.5; Supplemental Figure 1) or any of the cognitive subdomains (Supplemental Table 6). Notably, these analyses had greatly reduced sample sizes (n = 60) and statistical power relative to analyses of Hgb data and sensitivity may have been influenced by the nonspecificity of ferritin and transferrin to iron status in the normative range or in the presence of inflammatory effects. To address this issue, we repeat these cognitive analyses using a composite iron status index below.
A data-driven approach dissociates iron status and inflammatory status and their links to cognition
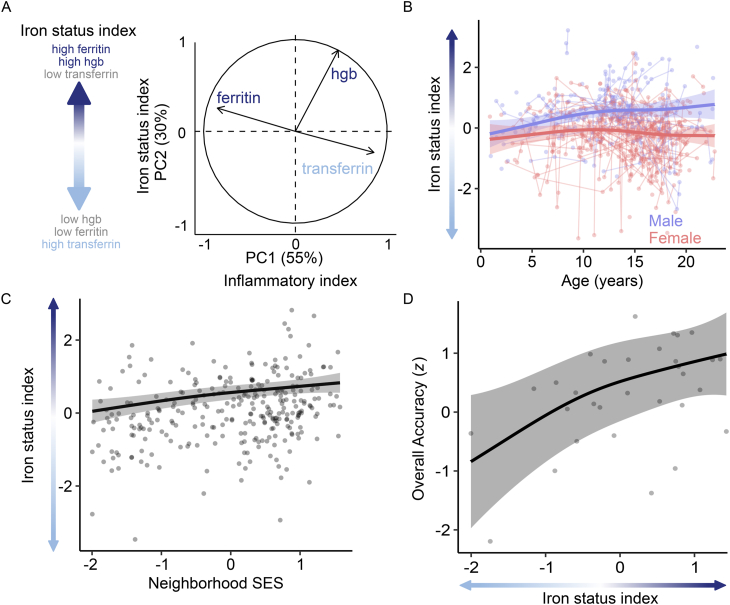
Ferritin and transferrin vary in response to iron status as well as acute inflammation or infection, potentially reducing our sensitivity and specificity to iron status effects in EMR data. Similarly, low Hgb concentrations can be caused by iron deficiency or other forms of anemia. As such, iron status measures are typically jointly evaluated to determine an individual’s iron status rather than relying on a single indicator. To disentangle iron status and inflammatory effects, we used PCA to derive a composite index of iron status (increased expression of the iron status index is related to increased ferritin, reduced transferrin, and increased Hgb) that was orthogonal an inflammatory index (that is, reduced expression of this component indicated elevated ferritin, reduced transferrin, and reduced Hgb; a pattern associated with anemia of chronic disease [44]; Figure 4A). To bolster our interpretation of these principal components, we conducted a confirmatory PCA in a subset of observations (n = 137) that also included the inflammatory biomarker CRP (Supplemental Figure 4A). We found a highly similar PCA solution such that the principal component scores of the initial model and the confirmatory model were correlated at r = 0.91. This result suggests that the simpler 3-variable model distinguished inflammatory effects from iron status effects as well as a model that also included an inflammatory biomarker while retaining a larger sample for further analysis (Supplemental Figure 4B).
FIGURE 4.
A data-driven iron status composite index is associated with age, sex, SES, and cognitive performance. (A) We generated a composite iron status index and a composite inflammatory index using PCA. The PCA (n = 690) separated patterns of covariation in Hgb, ferritin, and transferrin attributable to iron status effects from inflammatory effects. Greater values of the iron status index indicate greater Hgb, greater ferritin, and lower transferrin. Conversely, lower values indicate lower Hgb, lower ferritin, and higher transferrin (as would be expected in the case of iron deficiency). In contrast, lower scores on the inflammatory index reflect greater ferritin, lower Hgb, and higher transferrin (as would be expected in inflammation). These indices were highly similar to a confirmatory model that included a subset of 137 individuals for whom the inflammatory marker CRP was also available (r = 0.91; Supplemental Figure 4). The composite iron status index was significantly associated with age and sex [n = 690; panel (B)], neighborhood SES [n = 690; panel (C)], and overall accuracy on a battery of cognitive tests [n = 31; R2partial = 0.31; panel (D)]. Individual points represent observations with lines connecting repeated observations from the same individual; data across time are collapsed for each individual in panel C. Thick lines reflect model fits and shaded areas reflect 95% CIs of the fit (B–D). Hgb, hemoglobin; PC, principal component; PCA, principal component analysis; SES, socioeconomic status.
We next assessed whether this data-driven, composite indicator of iron status captured demographic and cognitive effects. As expected from prior analyses, the composite iron status index increased with age, with females having reduced iron status relative to males over adolescence (R2partial = 0.01, P = 0.04; Figure 4B). Furthermore, the iron status index was positively associated with neighborhood SES (R2partial = 0.05, P < 0.001; Figure 4C). Finally, the iron status index was significantly positively associated with overall cognitive accuracy (R2partial = 0.31, P = 0.011; Figure 4D) as well as the cognitive subdomains of executive function and memory (Supplemental Table 7). Notably, the effect size of the association between this data-driven iron status index and cognition was greater than any of the individual iron status measures alone (for example, R2partial = 0.31 compared with 0.02 for Hgb alone). In contrast, the inflammatory index was not significantly associated with cognition (R2partial = 0.004, P = 0.743; Supplemental Figure 4C). Together, these results suggest that our data-driven approach heightened our sensitivity to the association between cognition and iron status by disentangling inflammatory and iron status effects on our hematological measures.
Hgb is positively associated white matter integrity during adolescence
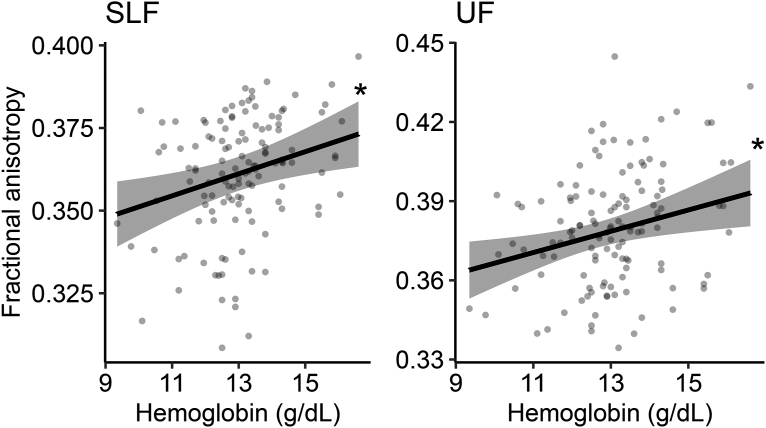
As a final step, we sought to investigate whether iron status measures were associated with the integrity of the brain’s white matter during adolescence. There were not sufficient data to test this association for ferritin and transferrin (n = 9 participants with ferritin or transferrin concentrations assessed within 6 mo before neuroimaging assessment), so this analysis was limited to Hgb data. We assessed the relationship between Hgb concentrations and the FA of the brain’s major white matter pathways in a subset of 126 participants who completed neuroimaging within 6 mo after Hgb measurement. All analyses used cross-sectional data. Hgb was significantly positively associated with white matter FA in 2 pathways critical for executive function: the superior longitudinal fasciculus (F = 8.6, edf = 1, R2partial = 0.06, FDR = 0.028) and the uncinate fasciculus (F = 7.5, edf = 1, R2partial = 0.05, FDR = 0.028) (Figure 5; Supplemental Table 8).
FIGURE 5.
Hemoglobin concentrations are significantly related to brain white matter integrity. Hemoglobin concentrations (n = 126) were positively associated with white matter fractional anisotropy in the superior longitudinal fasciculus and uncinate fasciculus. Solid lines reflect the model fit and shaded areas reflect 95% CIs of the fit. ∗FDR < 0.05. FDR, false discovery rate; SLF, superior longitudinal fasciculus; UF, uncinate fasciculus.
Discussion
Leveraging a large community-based sample, we found that the development of iron status measures varied by sex and SES during adolescence. Values indicative of lower iron status during this period were associated with poorer cognitive performance and reduced integrity of 2 of the brain’s major white matter pathways. Our findings suggest that adolescence may be a critical window of vulnerability to iron deficiency that has consequences for neurocognitive development and function.
Our findings suggest that females and individuals of low SES are at elevated risk for low iron status during adolescence. Aligning with results from population survey data in the United States and Europe [6,19,20,45,46], we show that postmenarchal females had lower Hgb, lower ferritin, and higher transferrin than males of similar age. Whereas prior population-based studies compared average iron status measures between males and females, the current study was able to characterize the developmental trajectories of iron status measures in males and females over the first 2 decades of life. Our nonlinear modeling approach revealed that the developmental trajectory of each iron status measure changed within ∼1 y of self-reported menarche such that female exhibited age-related decreases in iron status, whereas male values reflected age-related increases. We also found that individuals from lower SES neighborhoods have lower iron status than individuals from higher SES neighborhoods, which agrees with prior work indicating that lower SES is associated with lower iron status during youth [8] and global statistics linking the prevalence of iron deficiency to the sociodemographic index [21]. Our findings extend this work by revealing that the effect of neighborhood SES on measures of iron status is greatest over the adolescent period. Together, these findings highlight that postpubertal females from low SES neighborhoods are a particularly vulnerable population. Addressing iron status in these at-risk individuals may be crucial to reducing health disparities and improving neurocognitive outcomes during this stage of development.
We found that lower Hgb concentrations and lower scores on the composite iron status index during adolescence were broadly linked to lower cognitive performance. The significant association between Hgb concentrations and overall cognitive performance is consistent with earlier work linking frank iron deficiency with poor scholastic achievement in school-aged youth [47,48] as well as links between iron deficiency anemia and poorer academic performance in adolescent females [49]. Across the cognitive subdomains we examined, we found that Hgb concentrations and the iron status index were most strongly associated with the domain of executive function. Notably, in a Chinese cohort of early adolescents (age 12 y old), iron deficiency was also found to be most strongly associated with performance on an executive function task included in the Penn CNB [50,51]. Though findings have been mixed [52], other work has shown that frank iron deficiency is associated with poorer executive functioning in young-adult women compared with healthy controls [53] and that executive processes such as attention can be improved with dietary intervention [[54], [55], [56], [57]]. The association between iron status and executive function during this period is notable, because executive function undergoes protracted maturation into young adulthood [26,58]. Together, these findings may suggest that the development of executive function may be disrupted by poor iron status. An important direction of future work will be to understand how the impact of iron deficiency during adolescence interacts with iron status during different developmental epochs, because iron deficiency during infancy has also been found to predict long-term cognitive deficits that extend into the adolescent period [2,8,59]. Another critical extension of this work will be to understand the prospective relationship between iron status and cognitive performance over development. The present study provided evidence for a contemporaneous link between iron status and cognitive performance, and future work can begin to employ longitudinal study designs measure temporal dynamics of this association over longer time periods. Another critical extension of this work will be to understand the prospective relationship between iron status and cognitive performance over development. The present study provided evidence for a contemporaneous link between iron status and cognitive performance, and future work can begin to employ longitudinal study designs measure temporal dynamics of this association over longer time periods.
Our analyses revealed that the link between Hgb concentrations and cognitive performance was dimensional and not limited to individuals who would meet the laboratory threshold for iron deficiency anemia. Prior work on the cognitive consequences of iron deficiency in adolescence and young adulthood has investigated differences in cognitive performance in individuals with frank iron deficiency anemia relative to healthy controls [47,48], has focused specifically on female participants [53,[60], [61], [62]], or has assessed cognition in the context of iron supplementation intervention [[54], [55], [56],63]. Our results suggest that the association between Hgb concentrations and cognition is continuous rather than categorical; lower Hgb was dimensionally associated with lower overall cognitive performance even after excluding individuals that would meet the threshold for anemia. Notably, the association between Hgb and cognitive performance remained significant when covarying for neighborhood SES and even significantly mediated the association between neighborhood SES and overall cognitive ability. Hgb concentrations are nonspecific to iron status when examined in isolation. Providing support for the role of iron status in contributing to these results, we found that the dimensional association with cognitive performance was replicated and markedly strengthened when we combined Hgb, ferritin, and transferrin into a composite iron status index. Together, these results suggest that individual differences in iron status may impact cognitive ability even in individuals that would be considered in the normative range on laboratory tests, and that our composite iron status index may have utility in capturing individual variation in iron status even when Hgb is normal. It is important to note that these analyses did not include individuals with atypically high iron status. Investigating the association between excess iron and cognitive performance will be an important area for future investigation, particularly considering that iron overload has been linked to disorders characterized by impaired cognition such as Alzheimer’s disease and schizophrenia [[64], [65], [66]]. Furthermore, because the present study investigated the link between iron status and cognitive performance contemporaneously, future work using longitudinal study designs will be critical for understanding the temporal dynamics of this association using prospective analyses over longer time periods.
Critically, the strength of the association between Hgb concentrations and cognition was consistent across males and females. This finding has potentially important implications for diagnosis and treatment given that lower Hgb concentrations are considered “normal” in females because of sex-specific laboratory reference ranges. The impact of this effect is underscored by the finding that accounting for postpubertal sex differences in Hgb concentrations erased sex differences in cognitive performance. This pattern aligns with recent studies showing that increased dietary iron intake improves cognitive performance in young-adult women irrespective of baseline iron status [56,67]. Together, these findings suggest that the current sex-specific reference ranges for Hgb may need to be reconsidered and that improving iron status in individuals who do not meet current clinical criteria for anemia may still be beneficial to cognitive development. Gaining a broader understanding of how puberty-related sex differences in iron status interact with puberty-related aspects neurodevelopment will be an important aim of future work. For example, developmental changes in in synaptic density and inhibitory circuitry have been linked to pubertal hormones and are sensitive to sex differences in the age of puberty onset [[68], [69], [70], [71], [72], [73], [74]].
Finally, we found that low Hgb was associated with reduced white matter integrity during adolescence. Brain iron continues to develop during adolescence [40,[75], [76], [77], [78], [79]] and is critical to the myelination of white matter pathways [1,15]. This association between lower Hgb and reduced white matter integrity observed in this study may thus be a result of insufficient iron resources available to support ongoing myelination. The magnitude and effect size of this association is similar to deficits in white matter integrity observed in studies of patients with schizophrenia, a neuropsychiatric disease that emerges during this developmental stage [80,81]. Notably, these findings were specific to 2 major white matter pathways: the uncinate fasciculus and the superior longitudinal fasciculus. These pathways are notable because they have been consistently shown to be the white matter pathways that undergo the most protracted development, and are critical for executive function [32,33,82]. The protracted neurodevelopmental plasticity of the brain’s executive system is thought to support the maturation of executive function during this period while also being especially sensitive to environmental influences on development [30,31,83]. Our finding that low iron status is associated with both reduced integrity of the executive pathways and poorer executive function suggests that the most developmentally sensitive aspects of neurocognition may also be the most vulnerable to low iron availability during adolescence.
Limitations
The present study assessed iron status using EMR data from individuals seeking treatment in the CHOP pediatric network. Though screening for iron deficiency anemia is recommended by the United States Centers for Disease Control for women of childbearing age, there is a likely selection bias in EMR data for laboratory tests ordered for the evaluation of a medical indication. As such, these data should be considered as reflective of the CHOP health system rather than a strictly normative sample. In this study sample, medical conditions leading to acute or chronic inflammation may have a greater contribution to ferritin and transferrin concentrations than in a random sample that screened for healthy individuals, and Hgb concentrations may be impacted by other forms of anemia. We addressed these limitations in 2 ways. First, we excluded individuals with diagnoses of moderate or severe medical conditions that require standing medications or monitoring. Second, we used PCA to derive a composite iron status index that separated iron status effects from inflammatory effects. Analyses using our PCA-derived iron status index supported our assessment that the developmental and cognitive effects we observed were related to iron status rather than acute or chronic inflammation, and the effect size for the association between the iron status index and cognitive performance was greater than that of any individual iron status measure. This approach may be useful in future studies using available health record data. Despite these limitations, a significant strength of our approach is that it allowed us to detail the development of iron status in a large, existing dataset of over 30,000 laboratory tests from nearly 5000 individuals who also underwent rigorous cognitive assessment and neuroimaging.
The results of this study may have important translational implications. Iron status interventions in adolescence are actionable: iron supplementation is routinely used to address iron deficiency and its effects on cognitive development during the early window of vulnerability that occurs in the first years of life. Future work should address whether these treatments can be used to similarly facilitate cognitive development in adolescents, particularly in adolescent females of low SES.
Acknowledgments
The authors’ responsibilities were as follows—BL, MKG, and TDS: conceptualized the study; BL: developed methodology, wrote the original draft, and acquired funding; BL, TMM, and DRR: conducted formal analyses; EBB: validated the findings; MEC, DRR, TDS, RCG, REG, and TDS: conducted the original investigation; BL, NL, and KR: curated data; all authors: reviewed and edited the manuscript; and TDS: supervised the project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.05.005.
Conflicts of interest
The authors report no conflicts of interest.
Funding
This study was supported by NIH grants K99 MH127293 (to BL), T32 MH019112 (to EBB), R01 MH119185, R01 MH120174, and R56 AG066656 (to DRR), MH089983 and MH089924 (to REG), K08MH079364 (to MEC), R01 MH112847 (to TDS), R01 MH120482, R37 MH125829, and R01 MH113550 (to TDS). The source had no involvement in study or restrictions for publication.
Data availability
Demographic, cognitive, and neuroimaging data for the Philadelphia Neurodevelopmental Cohort are publicly available (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2). A detailed guide to these analyses and all data and codes used to generate the analyses for the current study are publicly available on GitHub (https://pennlinc.github.io/IronStatus_Development/).
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Todorich B., Pasquini J.M., Garcia C.I., Paez P.M., Connor J.R. Oligodendrocytes and myelination: the role of iron. Glia. 2009;57:467–478. doi: 10.1002/glia.20784. [DOI] [PubMed] [Google Scholar]
- 2.Lozoff B., Beard J., Connor J., Barbara F., Georgieff M., Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr. Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fitzpatrick P.F. The metal requirement of rat tyrosine hydroxylase. Biochem. Biophys. Res. Commun. 1989;161:211–215. doi: 10.1016/0006-291x(89)91582-9. [DOI] [PubMed] [Google Scholar]
- 4.Paul B.T., Manz D.H., Torti F.M., Torti S.V. Mitochondria and iron: current questions. Expert Rev. Hematol. 2017;10:65–79. doi: 10.1080/17474086.2016.1268047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgieff M.K. Long-term brain and behavioral consequences of early iron deficiency. Nutr. Rev. 2011;69(Suppl 1):S43–S48. doi: 10.1111/j.1753-4887.2011.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta P.M., Hamner H.C., Suchdev P.S., Flores-Ayala R., Mei Z. Iron status of toddlers, nonpregnant females, and pregnant females in the United States. Am. J. Clin. Nutr. 2017;106(Suppl 6):1640S–1646S. doi: 10.3945/ajcn.117.155978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Centers for Disease Control and Prevention . National Center for Environmental Health; Atlanta (GA): 2012. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population; p. 495. [Google Scholar]
- 8.Lozoff B., Jimenez E., Smith J.B. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch. Pediatr. Adolesc. Med. 2006;160:1108–1113. doi: 10.1001/archpedi.160.11.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deinard A.S., List A., Lindgren B., Hunt J.V., Chang P.-N. Cognitive deficits in iron-deficient and iron-deficient anemic children. J. Pediatr. 1986;108:681–689. doi: 10.1016/s0022-3476(86)81041-1. [DOI] [PubMed] [Google Scholar]
- 10.Shafir T., Angulo-Barroso R., Jing Y., Angelilli M.L., Jacobson S.W., Lozoff B. Iron deficiency and infant motor development. Early Hum. Dev. 2008;84:479–485. doi: 10.1016/j.earlhumdev.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gingoyon A., Borkhoff C.M., Koroshegyi C., Mamak E., Birken C.S., Maguire J.L., et al. Chronic iron deficiency and cognitive function in early childhood. Pediatrics. 2022;150 doi: 10.1542/peds.2021-055926. [DOI] [PubMed] [Google Scholar]
- 12.Beard J.L., Wiesinger J.A., Connor J.R. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Dev. Neurosci. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- 13.Jorgenson L.A., Wobken J.D., Georgieff M.K. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Dev. Neurosci. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- 14.Kwik-Uribe C.L., Gietzen D., German J.B., Golub M.S., Keen C.L. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J. Nutr. 2000;130:2821–2830. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- 15.Ortiz E., Pasquini J.M., Thompson K., Felt B., Butkus G., Beard J., et al. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. J. Neurosci. Res. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- 16.Dallman P.R. Nestec Ltd., Vevey/Raven Press, Ltd.; New York: 1992. Changing iron needs from birth through adolescence, Nutritional Anemias; p. 10. [Google Scholar]
- 17.DeMaeyer E.M., Dallman P., Gurney J.M., Hallberg L., Sood S.K., Srikantia S.G., et al. World Health Organization; 1989. Preventing and controlling iron deficiency anaemia through primary health care: a guide for health administrators and programme managers.https://apps.who.int/iris/handle/10665/39849 [Internet] [date updated; date cited]. Available from: [Google Scholar]
- 18.Mesías M., Seiquer I., Navarro M.P. Iron nutrition in adolescence. Crit. Rev. Food Sci. Nutr. 2013;53:1226–1237. doi: 10.1080/10408398.2011.564333. [DOI] [PubMed] [Google Scholar]
- 19.Yip R., Johnson C., Dallman P.R. Age-related changes in laboratory values used in the diagnosis of anemia and iron deficiency. Am. J. Clin. Nutr. 1984;39:427–436. doi: 10.1093/ajcn/39.3.427. [DOI] [PubMed] [Google Scholar]
- 20.Levi M., Simonetti M., Marconi E., Brignoli O., Cancian M., Masotti A., et al. Gender differences in determinants of iron-deficiency anemia: a population-based study conducted in four European countries. Ann. Hematol. 2019;98:1573–1582. doi: 10.1007/s00277-019-03707-w. [DOI] [PubMed] [Google Scholar]
- 21.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators, Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasricha S.-R., Tye-Din J., Muckenthaler M.U., Swinkels D.W. Iron deficiency. Lancet. 2021;397:233–248. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 23.Brabin L., Brabin B.J. The cost of successful adolescent growth and development in girls in relation to iron and vitamin A status. Am. J. Clin. Nutr. 1992;55:955–958. doi: 10.1093/ajcn/55.5.955. [DOI] [PubMed] [Google Scholar]
- 24.Oski F.A. Iron deficiency in infancy and childhood. N. Engl. J. Med. 1993;329:190–193. doi: 10.1056/NEJM199307153290308. [DOI] [PubMed] [Google Scholar]
- 25.Thomas M., Coneyworth L., Welham S. Influence of income on diet quality and daily iron and zinc intake: analysis of the National Diet and Nutrition Survey of British females aged 11–14 and 15–18 years. Eur. J. Nutr. 2023;62:499–510. doi: 10.1007/s00394-022-03000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gur R.C., Richard J., Calkins M.E., Chiavacci R., Hansen J.A., Bilker W.B., et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26:251–265. doi: 10.1037/a0026712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gur R.E., Gur R.C. Sex differences in brain and behavior in adolescence: findings from the Philadelphia Neurodevelopmental Cohort. Neurosci. Biobehav. Rev. 2016;70:159–170. doi: 10.1016/j.neubiorev.2016.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montez D.F., Calabro F.J., Luna B. Working memory improves developmentally as neural processes stabilize. PLOS ONE. 2019;14 doi: 10.1371/journal.pone.0213010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baum G.L., Ciric R., Roalf D.R., Betzel R.F., Moore T.M., Shinohara R.T., et al. Modular segregation of structural brain networks supports the development of executive function in youth. Curr. Biol. 2017;27:1561–1572.e8. doi: 10.1016/j.cub.2017.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larsen B., Luna B. Adolescence as a neurobiological critical period for the development of higher-order cognition. Neurosci. Biobehav. Rev. 2018;94:179–195. doi: 10.1016/j.neubiorev.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sydnor V.J., Larsen B., Bassett D.S., Alexander-Bloch A., Fair D.A., Liston C., et al. Neurodevelopment of the association cortices: patterns, mechanisms, and implications for psychopathology. Neuron. 2021;109:2820–2846. doi: 10.1016/j.neuron.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lebel C., Gee M., Camicioli R., Wieler M., Martin W., Beaulieu C. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2012;60:340–352. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 33.Urger S.E., De Bellis M.D., Hooper S.R., Woolley D.P., Chen S.D., Provenzale J. The superior longitudinal fasciculus in typically developing children and adolescents: diffusion tensor imaging and neuropsychological correlates. J. Child. Neurol. 2015;30:9–20. doi: 10.1177/0883073813520503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfeiffer C.M., Looker A.C. Laboratory methodologies for indicators of iron status: strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017;106(Suppl 6):1606S–1614S. doi: 10.3945/ajcn.117.155887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calkins M.E., Merikangas K.R., Moore T.M., Burstein M., Behr M.A., Satterthwaite T.D., et al. The Philadelphia Neurodevelopmental Cohort: constructing a deep phenotyping collaborative. J. Child. Psychol. Psychiatry. 2015;56:1356–1369. doi: 10.1111/jcpp.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Satterthwaite T.D., Elliott M.A., Ruparel K., Loughead J., Prabhakaran K., Calkins M.E., et al. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merikangas K.R., Calkins M.E., Burstein M., He J.-P., Chiavacci R., Lateef T., et al. Comorbidity of physical and mental disorders in the neurodevelopmental genomics cohort study. Pediatrics. 2015;135:e927–e938. doi: 10.1542/peds.2014-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore T.M., Martin I.K., Gur O.M., Jackson C.T., Scott J.C., Calkins M.E., et al. Characterizing social environment’s association with neurocognition using census and crime data linked to the Philadelphia Neurodevelopmental Cohort. Psychol. Med. 2016;46:599–610. doi: 10.1017/S0033291715002111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siimes M.A., Addiego J.E., Jr., Dallman P.R. Ferritin in serum: diagnosis of iron deficiency and iron overload in infants and children. Blood. 1974;43:581–590. [PubMed] [Google Scholar]
- 40.Larsen B., Bourque J., Moore T.M., Adebimpe A., Calkins M.E., Elliott M.A., et al. Longitudinal development of brain iron is linked to cognition in youth. J. Neurosci. 2020;40:1810–1818. doi: 10.1523/JNEUROSCI.2434-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore T.M., Reise S.P., Gur R.E., Hakonarson H., Gur R.C. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roalf D.R., Quarmley M., Elliott M.A., Satterthwaite T.D., Vandekar S.N., Ruparel K., et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage. 2016;125:903–919. doi: 10.1016/j.neuroimage.2015.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hua K., Zhang J., Wakana S., Jiang H., Li X., Reich D.S., et al. Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39:336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Punnonen K., Irjala K., Rajamäki A. Serum transferrin receptor and its ratio to serum ferritin in the diagnosis of iron deficiency. Blood. 1997;89:1052–1057. [PubMed] [Google Scholar]
- 45.Beard J.L. Iron requirements in adolescent females. J. Nutr. 2000;130:440S–442S. doi: 10.1093/jn/130.2.440S. [DOI] [PubMed] [Google Scholar]
- 46.Looker A.C., Dallman P.R., Carroll M.D., Gunter E.W., Johnson C.L. Prevalence of iron deficiency in the United States. JAMA. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- 47.Halterman J.S., Kaczorowski J.M., Aligne C.A., Auinger P., Szilagyi P.G. Iron deficiency and cognitive achievement among school-aged children and adolescents in the United States. Pediatrics. 2001;107:1381–1386. doi: 10.1542/peds.107.6.1381. [DOI] [PubMed] [Google Scholar]
- 48.Webb T.E., Oski F.A. Iron deficiency anemia and scholastic achievement in young adolescents. J. Pediatr. 1973;82:827–830. doi: 10.1016/s0022-3476(73)80074-5. [DOI] [PubMed] [Google Scholar]
- 49.Teni M., Shiferaw S., Asefa F. Anemia and its relationship with academic performance among adolescent school girls in Kebena District, Southwest Ethiopia. Biotech. Health Sci. 2017;4 [Google Scholar]
- 50.Ji X., Cui N., Liu J. Neurocognitive function is associated with serum iron status in early adolescents. Biol. Res. Nurs. 2017;19:269–277. doi: 10.1177/1099800417690828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ji X., Compher C.W., Irving S.Y., Kim J., Dinges D.F., Liu J. Serum micronutrient status, sleep quality and neurobehavioural function among early adolescents. Public Health Nutr. 2021;24:5815–5825. doi: 10.1017/S1368980021002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samson K.L.I., Fischer J.A.J., Roche M.L. Iron status, anemia, and iron interventions and their associations with cognitive and academic performance in adolescents: a systematic review. Nutrients. 2022;14:224. doi: 10.3390/nu14010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scott S.P., Murray-Kolb L.E. Iron status is associated with performance on executive functioning tasks in nonanemic young women. J. Nutr. 2016;146:30–37. doi: 10.3945/jn.115.223586. [DOI] [PubMed] [Google Scholar]
- 54.Wenger M.J., Murray Kolb L.E., Scott S.P., Boy E., Haas J.D. Modeling relationships between iron status, behavior, and brain electrophysiology: evidence from a randomized study involving a biofortified grain in Indian adolescents. BMC Public Health. 2022;22:1299. doi: 10.1186/s12889-022-13612-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scott S.P., Murray-Kolb L.E., Wenger M.J., Udipi S.A., Ghugre P.S., Boy E., et al. Cognitive performance in Indian school-going adolescents is positively affected by consumption of iron-biofortified pearl millet: a 6-month randomized controlled efficacy trial. J. Nutr. 2018;148:1462–1471. doi: 10.1093/jn/nxy113. [DOI] [PubMed] [Google Scholar]
- 56.Wenger M.J., Rhoten S.E., Murray-Kolb L.E., Scott S.P., Boy E., Gahutu J.-B., et al. Changes in iron status are related to changes in brain activity and behavior in Rwandan female university students: results from a randomized controlled efficacy trial involving iron-biofortified beans. J. Nutr. 2019;149:687–697. doi: 10.1093/jn/nxy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Finkelstein J.L., Fothergill A., Hackl L.S., Haas J.D., Mehta S. Iron biofortification interventions to improve iron status and functional outcomes. Proc. Nutr. Soc. 2019;78:197–207. doi: 10.1017/S0029665118002847. [DOI] [PubMed] [Google Scholar]
- 58.Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 59.DiGirolamo A.M., Ochaeta L., Flores R.M.M. Early childhood nutrition and cognitive functioning in childhood and adolescence. Food Nutr. Bull. 2020;41:S31–S40. doi: 10.1177/0379572120907763. [DOI] [PubMed] [Google Scholar]
- 60.Blanton C.A., Green M.W., Kretsch M.J. Body iron is associated with cognitive executive planning function in college women. Br. J. Nutr. 2013;109:906–913. doi: 10.1017/S0007114512002620. [DOI] [PubMed] [Google Scholar]
- 61.Bruner A.B., Joffe A., Duggan A.K., Casella J.F., Brandt J. Randomised study of cognitive effects of iron supplementation in non-anaemic iron-deficient adolescent girls. Lancet. 1996;348:992–996. doi: 10.1016/S0140-6736(96)02341-0. [DOI] [PubMed] [Google Scholar]
- 62.Bahrami A., Khorasanchi Z., Tayefi M., Avan A., Seifi N., Tavakoly Sany S.B., et al. Anemia is associated with cognitive impairment in adolescent girls: a cross-sectional survey. Appl. Neuropsychol. Child. 2020;9:165–171. doi: 10.1080/21622965.2018.1550405. [DOI] [PubMed] [Google Scholar]
- 63.Wenger M.J., DellaValle D.M., Murray-Kolb L.E., Haas J.D. Effect of iron deficiency on simultaneous measures of behavior, brain activity, and energy expenditure in the performance of a cognitive task. Nutr. Neurosci. 2019;22:196–206. doi: 10.1080/1028415X.2017.1360559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ayton S., Wang Y., Diouf I., Schneider J.A., Brockman J., Morris M.C., et al. Brain iron is associated with accelerated cognitive decline in people with Alzheimer pathology. Mol. Psychiatry. 2020;25:2932–2941. doi: 10.1038/s41380-019-0375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Becerril-Ortega J., Bordji K., Fréret T., Rush T., Buisson A. Iron overload accelerates neuronal amyloid-β production and cognitive impairment in transgenic mice model of Alzheimer’s disease. Neurobiol. Aging. 2014;35:2288–2301. doi: 10.1016/j.neurobiolaging.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 66.Lotan A., Luza S., Opazo C.M., Ayton S., Lane D.J.R., Mancuso S., et al. Perturbed iron biology in the prefrontal cortex of people with schizophrenia. Mol. Psychiatry. 2023:1–13. doi: 10.1038/s41380-023-01979-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blanton C. Improvements in iron status and cognitive function in young women consuming beef or non-beef lunches. Nutrients. 2013;6:90–110. doi: 10.3390/nu6010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delevich K., Klinger M., Okada N.J., Wilbrecht L. Coming of age in the frontal cortex: the role of puberty in cortical maturation. Semin. Cell. Dev. Biol. 2021;118:64–72. doi: 10.1016/j.semcdb.2021.04.021. [DOI] [PubMed] [Google Scholar]
- 69.Piekarski D.J., Boivin J.R., Wilbrecht L. Ovarian hormones organize the maturation of inhibitory neurotransmission in the frontal cortex at puberty onset in female mice. Curr. Biol. 2017;27:1735–1745.e3. doi: 10.1016/j.cub.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Segarra A.C., McEwen B.S. Estrogen increases spine density in ventromedial hypothalamic neurons of peripubertal rats. Neuroendocrinology. 1991;54:365–372. doi: 10.1159/000125915. [DOI] [PubMed] [Google Scholar]
- 71.Blakemore S.-J., Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 72.Yildirim M., Mapp O.M., Janssen W.G.M., Yin W., Morrison J.H., Gore A.C. Postpubertal decrease in hippocampal dendritic spines of female rats. Exp. Neurol. 2008;210:339–348. doi: 10.1016/j.expneurol.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Juraska J.M., Willing J. Pubertal onset as a critical transition for neural development and cognition. Brain Res. 2017;1654:87–94. doi: 10.1016/j.brainres.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drzewiecki C.M., Willing J., Juraska J.M. Synaptic number changes in the medial prefrontal cortex across adolescence in male and female rats: a role for pubertal onset. Synapse. 2016;70:361–368. doi: 10.1002/syn.21909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Larsen B., Olafsson V., Calabro F., Laymon C., Tervo-Clemmens B., Campbell E., et al. Maturation of the human striatal dopamine system revealed by PET and quantitative MRI. Nat. Commun. 2020;11:846. doi: 10.1038/s41467-020-14693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larsen B., Luna B. vivo evidence of neurophysiological maturation of the human adolescent striatum. Dev. Cogn. Neurosci. 2015;12:74–85. doi: 10.1016/j.dcn.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hect J.L., Daugherty A.M., Hermez K.M., Thomason M.E. Developmental variation in regional brain iron and its relation to cognitive functions in childhood. Dev. Cogn. Neurosci. 2018;34:18–26. doi: 10.1016/j.dcn.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Peterson E.T., Kwon D., Luna B., Larsen B., Prouty D., Bellis M.D.D., et al. Distribution of brain iron accrual in adolescence: evidence from cross-sectional and longitudinal analysis. Hum. Brain Mapp. 2019;40:1480–1495. doi: 10.1002/hbm.24461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parr A.C., Calabro F., Larsen B., Tervo-Clemmens B., Elliot S., Foran W., et al. Dopamine-related striatal neurophysiology is associated with specialization of frontostriatal reward circuitry through adolescence. Prog. Neurobiol. 2021;201 doi: 10.1016/j.pneurobio.2021.101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Karlsgodt K.H., Sun D., Jimenez A.M., Lutkenhoff E.S., Willhite R., van Erp T.G.M., et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev. Psychopathol. 2008;20:1297–1327. doi: 10.1017/S095457940800062X. [DOI] [PubMed] [Google Scholar]
- 81.Lee J., Oh J.-S., Park C.-I., Bang M., Sung G., Jung S., et al. White matter microstructure of superior longitudinal fasciculus II is associated with intelligence and treatment response of negative symptoms in patients with schizophrenia. Schizophrenia (Heidelb). 2022;8:43. doi: 10.1038/s41537-022-00253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31:10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tooley U.A., Bassett D.S., Mackey A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021;22:372–384. doi: 10.1038/s41583-021-00457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Demographic, cognitive, and neuroimaging data for the Philadelphia Neurodevelopmental Cohort are publicly available (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2). A detailed guide to these analyses and all data and codes used to generate the analyses for the current study are publicly available on GitHub (https://pennlinc.github.io/IronStatus_Development/).