Abstract
Ca2+-ATPases are membrane pumps that transport calcium ions across the cell membrane and are dependent on ATP. The mechanism of Listeria monocytogenes Ca2+-ATPase (LMCA1) in its native environment remains incompletely understood. LMCA1 has been investigated biochemically and biophysically with detergents in the past. This study characterizes LMCA1 using the detergent-free Native Cell Membrane Nanoparticles (NCMNP) system. As demonstrated by ATPase activity assays, the NCMNP7-25 polymer is compatible with a broad pH range and Ca2+ ions. This result suggests that NCMNP7-25 may have a wider array of applications in membrane protein research.
Keywords: Ca2+-ATPase, LMCA1, NCMN system, Single-partide cryo-EM
Homeostasis of the calcium ion (Ca2+) is essential to numerous physiological and pathological functions of living organisms. Ca2+ is involved in the process of muscle contraction, and the Sarco/Endoplasmic Reticulum Calcium ATPase (SERCA) acts as a pump to transport calcium ions from the cytosol back to the sarcoplasmic reticulum (SR) to maintain cellular homeostasis [1]. Ca2+ concentration in bacterial cells is kept between 100 and 300 nM by the SERCA homolog, Ca2+-ATPase, the primary ion transporters [2].
Based on the isoforms, Ca2+-ATPase of pathogenic Listeria monocytogenes (LMCA1) shares 34–39 % sequence identity with human Sarco/Endoplasmic Reticulum Ca2+-ATPase (SERCA1), the best understood P-type ATPases [2,3]. SERCA1 also participates in signal transduction, exocytosis, apoptosis, motility, and transcription [1]. Both LMCA1 and SERCA1 are members of the phosphorylation-type (P-type) ATPase subfamily II. Members of this protein family share a similar structural architecture and function in maintaining intracellular calcium homeostasis by actively transporting calcium ions across the cell membrane and consuming ATP as an energy source by forming and degrading a phosphoenzyme intermediate [2].
LMCA1 and other P-type ATPases share a similar structural architecture, but LMCA1 has unique features corresponding to calcium ATPases [4,5]. LMCA1 transports a single Ca2+ across the membrane, whereas SERCA transports two Ca2+ per ATP molecule [2]. LMCA1 is a critical determinant of calcium homeostasis in L. monocytogenes, a known pathogen of listeriosis, which makes it a potential therapeutic target [2]. Due to its location in the plasma membrane, bacteria are able to survive at high Ca2+ concentrations and an alkaline pH, and exposure to alkaline pH increases LMCA1 transcription [6].
The structural studies of LMCA1 in detergent micelles shed substantial light on its molecular mechanism [7,8]. Nevertheless, the structural information of a number of reaction intermediates, especially in their natural environment, is unknown. For the elucidation of the molecular mechanisms underlying the activity of membrane proteins and the subsequent structure-based drug discovery and development, it is necessary to comprehend the exact structural details. To understand the integrity of the protein-lipid interaction, it is also essential to examine its functionality in natural environments. It is well known that membrane proteins require a native lipid environment to maintain their native structures and functions. Therefore, it may be preferable to study the structure and function of LMCA1 in its native cell membrane environment.
Guo and colleagues have been developing a detergent-free, native cell membrane nanoparticles (NCMNP) system for membrane protein research [9]. Unlike the SMALP system, the NCMN system features a membrane-active polymer library including a diversity of polymers, each with its own set of characteristics that make it well-suited to the isolation of membrane proteins that need specialized circumstances for their native functionality and stability. In comparison to the SMALP approach, the NCMN system uses distinct protocols for nanoparticle preparation. One such instance is that NCMNPs employ a single-step nickel affinity column purification to determine a high-resolution structure; the effect of these NCMNPs protocols resulted in a 3.2 and 3.0 Å cryo-EM AcrB structures, whereas employing the SMALP method, resulted in a cryo-EM AcrB structure with a resolution of 8.8 Å [10,11]. Moreover, our earlier studies have demonstrated that the NCMN system is highly promising for various membrane proteins, and the protein samples prepared with the NCMN system are appropriate for various biophysical and biochemical studies [9].
Here, we demonstrate that the NCMNP7-25, a modified form of NCMNP7-1 with a grafting ratio of 2-aminoethanesulfonic acid at 25 % [9], from the NCMN polymer library can be utilized to characterize membrane proteins under a wide pH range and in the presence of divalent cations, such as calcium ions. This study lays the foundation for further structural investigation of LMCA1 in a detergent-free system.
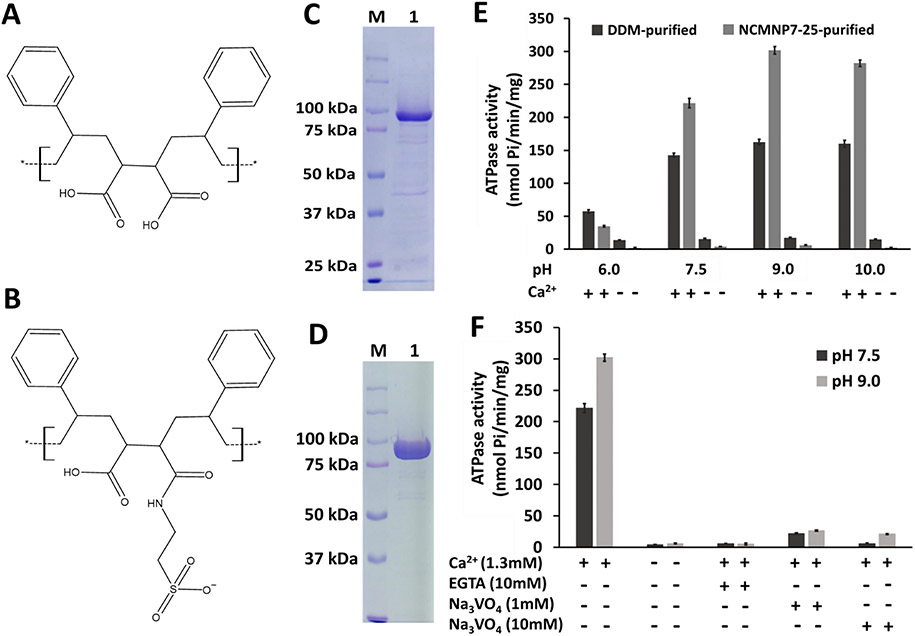
The LMCA1 gene-containing expressing plasmid (pET22b-LMCA1), a gift from UVA Professor Robert Nakamoto, was transformed into E. coli strain, BL21-C43, for overexpression. The overexpressed LMCA1 was solubilized with either NCMNP7-25 or n-Dodecyl-beta-Maltoside Detergent (DDM) detergent following a previously published protocol [10,12]. NCMNP7-25 is a modified polymer of NCMNP1-1, made in our lab, and the chemical structures of both polymers are shown in Fig. 1A and B. The Ni-NTA bound LMCA1 was eluted using an elution buffer comprising 250 mM imidazole and either NCMNP7-25 or DDM. Furthermore, the eluted fraction of LMCA1 with NCMNP7-25 was analyzed and shown in Fig. 1C, which depicted the purity of NCMNP7-25 purified LMCA1. In contrast, a two-step purification procedure was conducted with DDM, and Fig. 1D showed the LMCA1 purity and yield following the size exclusion column (Superdex 200, increase 10/300 GL) purification. A molecular weight of around 95 kDa was observed on SDS-PAGE gel for purified LMCA1, which corresponds to the estimated value.
Fig. 1.
Biochemical characterization of the purified LMCA1 with the NCMN system and DDM. (A) NCMNP1-1 (B) NCMNP7-25 (C) The purity of LMCA1 within NCMN particles was assessed on 10 % SDS-PAGE gel. Lane 1 shows the purified LMCA1. Lane M shows the prestained protein marker. (D) The purity of LMCA1 with DDM was assessed on 10 % SDS-PAGE gel. Lane 1 shows the purified LMCA1. Lane M shows the prestained protein marker. (E) The ATPase activity of DDM purified (black bars) and NCMNP7-25 purified (gray bars) LMCA1 at different pH in the presence and absence of Ca2+. The error bars indicated that the mean ± SEM was based on the three individual experiments. (F) ATPase activity and inhibition of the purified LMCA1 with NCMNP7-25. The error bars indicated that the mean ± SEM was based on the three individual experiments.
Using a malachite green phosphate assay kit (Sigma), the ATPase activity of LMCA1 was determined at four distinct pH conditions: pH 6.0, pH 7.5, pH 9.0, and pH 10.0. With calcium ions present, the ATPase activity of LMCA1 purified with DDM is approximately 162.67 nmol Pi/min/mg protein at pH 9.0 and is reduced to 160.14, 142.66, and 57.50 nmol Pi/min/mg protein at pH 10, 7.5, and 6.0, respectively (Fig. 1E). This observation is consistent with the previously reported ATPase activity of detergent micelles for LMCA1 [2]. Similar to DDM-purified LMCA1, NCMNP7-25 purified LMCA1 showed a maximum ATPase activity (301.80 nmol Pi/min/mg protein) at pH 9.0 (Fig. 1F). Calcium is required for LMCA1 ATPase activity (Fig. 1F). However, DDM and NCMNP7-25-purified LMCA1 nearly lost their activity without calcium ions in the reactions.
With the calcium chelating agent ethylene glycol tetraacetic acid (EGTA) in the buffer, ATPase activity was inhibited by approximately 97.26 % and 98.27 % at pH 7.5 and pH 9.0, respectively (Fig. 1F). These results indicate that calcium ions are required for LMCA1 ATP hydrolysis. In addition, 1 mM sodium vanadate inhibits the ATPase activity of NCMNP7-25 purified LMCA1 at pH 7.5 and pH 9.0 by approximately 90.18 % and 91.21 %, respectively (Fig. 1F). At pH 7.5, an increase in vanadate concentration inhibited ATPase activity by up to 97.44 %, and at pH 9.0, by up to 93.01 %. These findings suggest that sodium vanadate influences ATPase activity at both pH levels. According to the results, sodium vanadate is an efficient inhibitor of LMCA1 [13].
The native lipids may play a crucial role in maintaining the structure and function of the protein. In order to comprehend the structural mechanism of Ca2+ transport in a detergent-free system, a functional analysis of LMCA1 is required in the native environment. So far, L. monocytogenes LMCA1 was extracted and characterized using a detergent-based method and crystallized after re-lipidation to restore LMCA1 to its native environment [7,8]. According to our knowledge, this is the first study of LMCA1 conducted with a detergent-free system.
Traditional SMA2000 (NCMNP1-1) polymer does have limitations such as incompatibility with divalent ions and low pH, for structural and functional membrane protein studies [9,14]. According to a study by Yang et al., the NCMNP7-1 polymer effectively restored the functioning T74S KcsA channel using a dehydration/rehydration strategy, while the NCMNP1-1 polymer was effective at reconstituting MscS and MscL into proteoliposomes [9]. Since only KcsA is a pH-gated potassium channel, this reveals that NCMNP7-1 is more stable at a wide pH range and in the presence of divalent ions than the more regularly employed NCMNP1-1 polymer [9]. The NCMN system has some significant advantages for preserving the native environment of membrane proteins, but it also has some disadvantages. Each polymer possesses unique characteristics. We discovered that SMA polymers were not always able to extract membrane proteins effectively from native cell membranes. To identify the optimal polymer for the structural and functional investigation of a given membrane protein, a polymer screen will be required.
We demonstrate that the NCMNP7-25 polymer from the NCMN polymer library can preserve enzyme activity. The pH dependence of purified LMCA1 was investigated in this study. As reported by Kristina Faxe'n et al. with detergent-solubilized LMCA1, the ATPase activity at pH 9.0 and Ca2+-dependent ATP hydrolysis were more active [2]. The ATPase activity results demonstrated that NCMNP7-25 purified LMCA1 is more active than detergent-purified LMCA1, indicating that LMCA1 might be dependent on lipid molecules. It has been reported that NCMN polymers from our NCMN system, extract native lipid molecules more efficiently during the membrane solubilization stage, which may aid in retaining enzyme activity [15]. Furthermore, the presence of EGTA in the assay buffer suggests that Ca2+ is necessary for LMCA1 activity. Vanadate, a well-known inhibitor of the P-type ATPase family, inhibited the activity of LMCA1. Our research indicates that the LMCA1 in the form of NCMN particles retains transport activity, which implies that the NCMNP7-25 polymer is stable in the presence of divalent cations and a wide range of pH, the rational behind is that 2-aminoethanesulfonic acid has a much lower pKa, and more compatible comparing with a carboxyl group, therefore, grafting of 2-aminoethanesulfonic acid to the polymer makes NCMNP7-25 more compatible with divalent ions and lower pH conditions.
Our findings indicate that the NCMN system works well in characterizing the pH- and calcium-dependent ATPase activity of LMCA1. This study suggests the potential of wide application of the NCMN system for the large P-type ATPase family, including mammalian calcium pumps and possibly other membrane proteins whose activity or natural structure is dependent on low pH conditions or divalent cations.
Supplementary Material
Acknowledgments
YG was supported by the VCU School of Pharmacy and Department of Medicinal Chemistry through startup funds, the Institute for Structural Biology, Drug Discovery and Development through laboratory space and facilities, and NIH Grant R01-GM132329. The funders had no role in study design, data collection, analysis, publishing decisions, or manuscript preparation. The content is solely the authors' responsibility. It does not necessarily represent the National Institutes of Health or other funding organizations' official views. We thank Professor Robert Nakamoto for providing the LMCA1 plasmids and constructive discussion with us.
Footnotes
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Youzhong Guo reports financial support was provided by National Institutes of Health. Youzhong Guo has patent pending to VCU. Thi Kim Hoang Trinh has patent pending to VCU.
CRediT authorship contribution statement
WQ and YG conceived the project and designed the experiments. PD performed the purification and activity experiment and wrote the manuscript. HT produced the polymers. YG provided the lab space and facility for this project and the NCMN system. WQ and YG contributed to the revision of the manuscript.
Y.G. is the inventor of the NCMN system. Y.G and T.K.H.T are the inventors of the NCMNP7-25 polymer.
Appendix A. Supplementary data
The Supporting information containing the experimental procedure, additional figures, and results are free of charge at http://pubs.acs.org. Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbamem.2023.184143.
References
- [1].Primeau JO, Armanious GP, Fisher MLE, Young HS, The sarcoendoplasmic reticulum calcium, in: ATPaseMembrane protein complexes: structure and function, 2018, pp. 229–258. [DOI] [PubMed] [Google Scholar]
- [2].Faxén K, Andersen JL, Gourdon P, Fedosova N, Morth JP, Nissen P, Møller JV, Characterization of a listeria monocytogenes Ca2+ pump: a SERCA-type ATPase with only one Ca2+-binding site, J. Biol. Chem 286 (2) (2011) 1609–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dyla M, Andersen JL, Kjaergaard M, Birkedal V, Terry DS, Altman RB, Blanchard SC, Nissen P, Knudsen CR, Engineering a prototypic P-type ATPase listeria monocytogenes Ca2+-ATPase 1 for single-molecule FRET studies, Bioconjug. Chem 27 (9) (2016) 2176–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Desrosiers MG, Gately LJ, Gambel AM, Menick DR, Purification and characterization of the Ca2+-ATPase of Flavobacterium odoratum (*), J. Biol. Chem 271 (7) (1996) 3945–3951. [DOI] [PubMed] [Google Scholar]
- [5].Maya-Hoyos M, Rosales C, Novoa-Aponte L, Castillo E, Soto CY, The P-type ATPase CtpF is a plasma membrane transporter mediating calcium efflux in mycobacterium tuberculosis cells, Heliyon 5 (11) (2019), e02852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Giotis ES, Muthaiyan A, Blair IS, Wilkinson BJ, McDowell DA, Genomic and proteomic analysis of the alkali-tolerance response (AlTR) in listeria monocytogenes 10403S, BMC Microbiol. 8 (1) (2008) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Andersen JL, Gourdon P, Møller JV, Morth JP, Nissen P, Crystallization and preliminary structural analysis of the listeria monocytogenes Ca2+-ATPase LMCA1, Acta Crystallogr. Sect. F: Struct. Biol. Cryst. Commun 67 (6) (2011) 718–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hansen SB, Dyla M, Neumann C, Quistgaard EMH, Andersen JL, Kjaergaard M, Nissen P, The crystal structure of the Ca2+-ATPase 1 from listeria monocytogenes reveals a pump primed for dephosphorylation, J. Mol. Biol 433 (16) (2021), 167015. [DOI] [PubMed] [Google Scholar]
- [9].Yang L, Catalano C, Xu Y, Qiu W, Zhang D, McDermott A, Guo Y, Blount P, A native cell membrane nanopartides system allows for high-quality functional proteoliposome reconstitution, BBA advances 1 (2021), 100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qiu W, Fu Z, Xu GG, Grassucci RA, Zhang Y, Frank J, Hendrickson WA, Guo Y, Structure and activity of lipid bilayer within a membrane-protein transporter, Proceedings of the National Academy of Sciences 115 (51) (2018) 12985–12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parmar M, Rawson S, Scarff CA, Goldman A, Dafforn TR, Muench SP, Postis VL, Using a SMALP platform to determine a sub-nm single partied cryo-EM membrane protein structure, Biochim. Biophys. Acta, Biomembr 1860 (2) (2018) 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kroeck KG, Qiu W, Catalano C, Trinh TKH, Guo Y, Native cell membrane nanoparticles system for membrane protein-protein interaction analysis, J. Vis. Exp 161 (2020), e61298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pick U, The interaction of vanadate ions with the ca-ATPase from sarcoplasmic reticulum, J. Biol. Chem 257 (11) (1982) 6111–6119. [PubMed] [Google Scholar]
- [14].Catalano C, Ben-Hail D, Qiu W, Blount P, des Georges A, Guo Y, Cryo-EM structure of mechanosensitive channel Ynal using SMA2000: challenges and opportunities, Membranes 11 (11) (2021) 849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Trinh TKH, Catalano C, Guo Y, Membrane-active polymers: NCMNP13-X, NCMNP21-X and NCMNP21b-x for membrane protein structural biology, bioRxiv, 2022, 2022.01.10.475744. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.