Abstract
Obesity is increasing at an alarming rate. The effectiveness of currently available strategies for the treatment of obesity (including pharmacologic, surgical, and behavioral interventions) is limited. Understanding the neurobiology of appetite and the important drivers of energy intake (EI) can lead to the development of more effective strategies for the prevention and treatment of obesity. Appetite regulation is complex and is influenced by genetic, social, and environmental factors. It is intricately regulated by a complex interplay of endocrine, gastrointestinal, and neural systems. Hormonal and neural signals generated in response to the energy state of the organism and the quality of food eaten are communicated by paracrine, endocrine, and gastrointestinal signals to the nervous system. The central nervous system integrates homeostatic and hedonic signals to regulate appetite. Although there has been an enormous amount of research over many decades regarding the regulation of EI and body weight, research is only now yielding potentially effective treatment strategies for obesity. The purpose of this article is to summarize the key findings presented in June 2022 at the 23rd annual Harvard Nutrition Obesity Symposium entitled “The Neurobiology of Eating Behavior in Obesity: Mechanisms and Therapeutic Targets.” Findings presented at the symposium, sponsored by NIH P30 Nutrition Obesity Research Center at Harvard, enhance our current understanding of appetite biology, including innovative techniques used to assess and systematically manipulate critical hedonic processes, which will shape future research and the development of therapeutics for obesity prevention and treatment.
Keywords: appetite, neurobiology, endocrine, brain, genetics, obesity, GLP-1
Introduction
The prevalence of obesity is increasing tremendously in the last 3 decades with a 27.5% increase in adults and a 47.1% increase in children worldwide [1]. People with obesity are at an increased risk of type 2 diabetes mellitus, CVD, nonalcoholic fatty liver disease, neurodegenerative disease, and many cancers. The health problems associated with obesity are substantial and lead to increased healthcare costs. Indeed, annual healthcare costs have increased by 36% with a 77% increase in medication costs in individuals with obesity as compared with those with normal weight [1]. Studies have shown that weight loss of >5% can lead to beneficial effects on metabolic and cardiovascular complications [2]. Although some recently available pharmacological agents can result in effective and sustained weight loss in conjunction with lifestyle and behavioral treatments, their use can be limited by their availability, tolerability, contraindications, and cost. Therefore, a substantial unmet need exists for effective strategies to treat patients with obesity. Understanding the genetic, neural, and hormonal pathways that regulate weight and appetite is critical to finding therapeutic targets for the prevention and treatment of obesity [3]. Although the field of obesity research is large and extends over several decades with many major advances, this manuscript summarizes findings presented by the coauthors at the 23rd annual Harvard Nutrition Obesity Symposium entitled “The Neurobiology of Eating Behavior in Obesity: Mechanisms and Therapeutic Targets” in June 2022. This report is not a comprehensive review of the available research in the field but presents insights from experts speaking on major topics of relevance to our understanding of appetite physiology, covered at the symposium.
Lessons from Genetic Studies on Development of Obesity
The development of obesity is strongly influenced by social and environmental factors that affect the balance between energy intake (EI) and expenditure and further modulated by genetic factors. Twin studies show that identical twins have an extremely high concordance for the body weight and fat mass distribution compared with nonidentical twins, even if they were separated at birth, indicating a strong effect of “nature over nurture” with regard to obesity [4]. In the past few decades, many genetic variations have been identified, which may modulate the response to similar environmental triggers. Although there are rare monogenic causes of obesity that provide insights into the key homeostatic appetite pathways (like the leptin–melanocortin pathway), there are also many common genetic variants with small effect sizes that contribute to obesity in the general population. People with a high polygenic risk score derived from over 2 million common and rare variants were found to be on average 12 kg heavier than those with a low-risk score by 18 y of age [5]. Studies have also shed light on the genetics of thinness [6], with recent work from Study into Lean and Thin Subjects (STILTS) demonstrating genes that may protect against weight gain and provide novel drug targets for obesity prevention (https://www.stilts.org.uk/). The results from genetic studies can help understand the physiological and behavioral pathways involved in the development of obesity, which is essential in developing effective preventive and therapeutic interventions.
Neurobiology of Appetite and Weight
The neurobiology of food intake and appetite is complex. Critically, these pathways evolved and adapted to ensure survival in times of food scarcity. Within this system, peripheral hormones and neural signals act centrally to influence appetite and food intake. These hormones and neural impulses result in direct and indirect activation of the key systems throughout the brain—homeostatic, hedonic (reward-based), and cognitive pathways—that are involved in energy homeostasis, reward processing, and cognitive control of appetitive behaviors (Table 1) [7].
TABLE 1.
Brain regions involved in appetite and weight control1
| Homeostatic (hypothalamic) circuitry | Arcuate nucleus, paraventricular nucleus (PVN), dorsomedial hypothalamic nucleus (DMH), and lateral hypothalamus (LH) |
| Reward circuitry | Amygdala, striatum [caudate putamen and nucleus accumbens (NAcc)], ventral tegmental area (VTA), prefrontal cortex (PFC), anterior cingulate cortex, lateral habenula (LHb), and cerebellum |
| Cognitive control | Prefrontal cortex (PFC), anterior cingulate cortex, and insula |
Many brain areas are involved in >1 type of appetite control pathway.
Leptin, ghrelin, and the melanocortin pathway
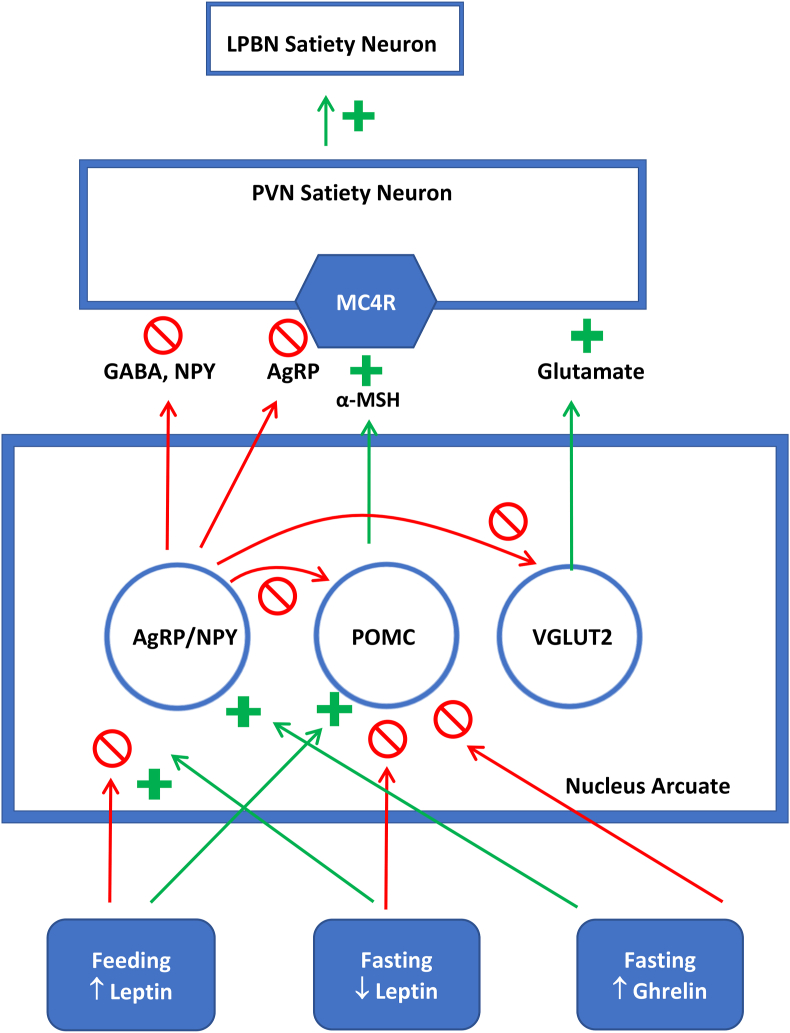
The melanocortin pathway is the key regulator in the homeostatic regulation of appetite but also influences the reward circuitry (Figure 1). Leptin is an adipokine that is released in proportion to the amount of fat mass. In the fed state, leptin binds to leptin receptors on the surface of proopiomelanocortin (POMC) neurons, resulting in release of α-melanocyte-stimulating hormone, which binds to melanocortin-4 receptors (MC4Rs) in the paraventricular nucleus (PVN) and leads to decreased food intake by stimulating satiety neurons in the lateral parabrachial nucleus (LPBN) [8,9]. Leptin also regulates food intake by modulating neuronal activation of the reward system in the striatum [caudate putamen and nucleus accumbens (NAcc)] [10]. Leptin deficiency or defects in its signaling result in hyperphagia and severe obesity in both human and rodent models [[11], [12], [13], [14], [15], [16], [17], [18], [19]]. The resulting obesity from leptin deficiency is reversed with the recombinant leptin therapy [20], further emphasizing the critical role of this hormone in body weight homeostasis. In acute caloric restriction, leptin concentrations decrease to defend against starvation [[21], [22], [23]] and are similarly decreased in the chronic weight reduced state [24,25]. Thus, leptin is a key adipokine involved in regulating appetite via homeostatic and hedonic regulation.
FIGURE 1.
Melanocortin pathway of homeostatic appetite regulation. Leptin binds to the leptin receptors present on AgRP and POMC neurons. In the fasting state, the fall in leptin concentrations strongly activates AgRP neurons while inhibiting POMC neurons. MC4R-expressing neurons in PVN receive strong and selective inhibitory synaptic input from AgRP neurons. The MC4R-experssing neurons of PVN exhibited their antiobesity effect by stimulating satiety neurons in the LPBN. AgRP neurons inhibit the POMC neurons as well as VGLUT2 neurons, all within the arcuate nucleus. VGLUT2 neurons release the excitatory neurotransmitter glutamate that competes with the inhibitory neurotransmitters NPY and GABA released by AgRP. In addition, postsynaptic activity of the glutamatergic inputs to MC4R-expressing neurons in the PVN is upregulated by the actions of α-MSH/MC4R signaling, thereby further stimulating the MC4R-expressing PVN satiety neurons and the satiety neurons of the LPBN. AgRP, agouti-related protein; α-MSH, α-melanocyte-stimulating hormone; LPBN, lateral parabrachial nucleus; MC4R, melanocortin-4 receptor; POMC, proopiomelanocortin; PVN, paraventricular nucleus of the hypothalamus; VGLUT2, vesicular-glutamate transporter 2.
In contrast, ghrelin is a gut peptide, released in the starved state to promote food intake. Ghrelin acts by binding to its endogenous receptor, the growth hormone secretagogue receptor, in the arcuate nucleus to stimulate orexigenic agouti-related neuropeptide (AgRP) and NPY-expressing neurons [26,27]. Growth hormone secretagogue receptor neurons are also coexpressed with dopamine neurons in the ventral tegmental area (VTA), which is involved in regulating hedonic appetite [28]. Ghrelin also modulates the dopaminergic activity in this area, thereby regulating hedonic appetite [28].
MC4R
MC4R is a key integrator of signals governing the regulation of appetite and body weight. Studies investigating defects in the melanocortin pathway have highlighted its role in regulating weight and appetite. Genetic deficiency of POMC results in severe obesity in mice [29] and humans [30]. Indeed, several studies have shown that loss-of-function mutations in the MC4R gene result in obesity, with a correlation between the degree of loss of function and hyperphagia [31,32]. Heterozygous mutations in the MC4R gene have been detected more commonly than previously suspected in association with obesity [33,34]. Setmelanotide is an MC4R agonist that results in 10% weight loss in states of POMC or leptin receptor deficiency [[35], [36], [37]]. In addition, human studies showed that gain-of-function MC4R variants are protective against obesity [38].
AgRP and POMC neurons
The AgRP and POMC neurons project to several sites in the brain including the PVN. Genetic defects or lesions of the PVN have been shown to cause hyperphagia and obesity [39,40]. Moreover, genetic disruption of the transcription factor Sim1, which is essential for the development of the PVN, leads to hyperphagia and obesity in mice [41,42] as well as humans [43]. In fact, signaling via the MC4R on PVN neurons is critical in preventing hyperphagia and obesity (illustrated in Figure 1) [44,45].
In contrast to the effects of ablation of AgRP neurons, which lead to reduced food intake and starvation [46,47], activation of AgRP neurons leads to hyperphagia [48,49]. Regulation of AgRP hunger neurons is partly mediated via feedback signals that report the current state of energy needs (for example, leptin) and by the nutrient-induced signals from the gut, which also reduce AgRP neuron activity [50,51]. Sensory cues, like the smell of food, that predict future eating will also rapidly inhibit AgRP neuron activity [[52], [53], [54]]. These effects are mediated via inhibitory signals from the dorsomedial hypothalamic nucleus (DMH) [55].
Stimulation of AgRP neurons results in an aversive (unpleasant) state [53]. Upon eating, the activity of AgRP neurons is decreased, which reduces the aversive state and is thereby rewarding, encouraging food-seeking behavior. In fact, AgRP neurons are inhibited by the inhibitory neurons of the DMH [55], which are activated by the glutamatergic neurons from the lateral hypothalamus (LH) [56]. Blocking this synaptic transmission results in impaired inhibition of AgRP neurons by food cues as well as impaired learning of food-seeking tasks [56]. This knowledge supplies a neurobiological basis for how food deprivation leads to food-seeking behavior to avoid aversive states, helping to explain why dieting is difficult.
As satiety signals are transmitted from the PVN to the LPBN, an important future direction is to identify the specific satiety neurons in the LPBN (Figure 1) and determine the sites in the brain where they project to regulate hunger/satiety. As the LPBN projects to “higher” brain sites that set emotional valence, this will provide a deeper understanding of what really happens in the brain to control hunger/satiety. Identification of LPBN neurons will be greatly enhanced by the latest techniques that are based on single-neuron transcriptomics to identify all the different transcriptionally defined subtypes of neurons that exist in the LPBN.
Alterations in Brain Reward Responses Contributing to Obesity
In the food-replete modern world, reward signaling in appetitive pathways can supersede the homeostatic regulation, and knowledge about its regulation is crucial to prevent and treat obesity. Functional MRI studies show that in response to food cues, individuals with obesity have greater activations of brain regions that regulate reward and motivational processes including the striatum, prefrontal complex (PFC), and amygdala, compared with lean individuals [57,58], with recent data identifying the cerebellum as a critical driver of reward-related hyperphagic behavior [59]. The magnitude of these responses to food cues predicts future weight gain and poor weight loss outcomes after weight loss interventions [[60], [61], [62]]. Thus, studies in humans suggest that the function of the reward circuits that influence food craving and food-seeking behaviors are enhanced in people who are susceptible to diet-induced weight gain.
Furthermore, obesity-prone rats show stronger food-seeking and basal enhancements in striatal function compared with obesity-resistant rats [63]. Also, eating a sugary, fatty “junk-food” diet enhances excitatory transmission within the striatum of obesity-prone, but not obesity-resistant rats [64]. Specifically, diet manipulation in an experimental setting, in the absence of obesity, enhances the expression and function of calcium-permeable-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors in the striatum of obesity-prone but not obesity-resistant male and female rats [64]. Calcium-permeable-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor activity is required for the expression of cue-triggered food-seeking [63], which is also enhanced after consumption of “junk-food” [65]. These data provide evidence that there are inherent enhancements in incentive motivational responses to food cues in obesity-prone rodent populations and that eating sugary fatty foods enhances striatal function to promote cue-triggered food-seeking in populations that are susceptible to diet-induced obesity [66]. The elaboration of these pathways in humans is ongoing and may help identify obesity prevention strategies in the high-risk population.
Effect of macronutrient content on homeostatic and reward regions
Multiple studies have investigated the effect of dietary interventions on appetite pathways using neuroimaging techniques. The Framingham State Food Study (FS2), a randomized controlled trial evaluating the effects of different macronutrients on weight regain, shows that 20-wk diets (in the weight loss maintenance phase) that vary in carbohydrate and fat content contribute to weight regain by decreasing energy expenditure (EE) and altering appetite [67]. As shown in the FS2 study, after 18–20 wk of adherence diets with different carbohydrate content, in the pre-prandial state, regional blood flow to the NAcc, which is a surrogate measure of the activity of this area, is higher in the high-carbohydrate group as compared with the low-carbohydrate group [68]. Furthermore, in the post-prandial state, individuals on a low- compared with high-carbohydrate diet demonstrate lower blood flow to the NAcc and thereby have a lower hedonic drive for food intake [68]. The blood flow in the NAcc is negatively associated with insulin concentrations in the high-carbohydrate diet group [68]. These findings augment findings from preclinical [69] and clinical [70] studies showing significant effects of short-term intake of carbohydrates on synchronous activity in homeostatic and hedonic circuits, contributing to the appetitive drive. Furthermore, the blood flow in the NAcc is negatively associated with insulin concentrations in the high-carbohydrate diet group [68]. Consistent with prior work demonstrating that intact NAcc insulin signaling is required for adequate control of food intake according to metabolic needs [71]. This suggests that individuals with high insulin secretion might be more susceptible to the effects of chronically high carbohydrate intake on brain reward activity, contributing to challenges in the maintenance of diet-induced weight loss. Further investigations in this field may elaborate individual characteristics that may predict weight regain and thus highlight modifiable targets for successful weight loss maintenance. These advancements will pave the way toward precision treatments in obesity.
Mechanisms of compulsive eating in obesity
Compulsive eating is an uncontrollable urge to eat even in the absence of hunger and is 1 of the appetite phenotypes that are associated with obesity. Investigators have been interested in the mechanisms of compulsive eating for several years. When unlimited daily access to a cafeteria-style diet is allowed, rats gain rapid amounts of weight quickly and develop obesity within a few weeks compared with rats that have restricted (1 h) or no access to food [72]. The food consumption in the rats with obesity is resistant to aversive conditioned stimuli, suggesting a pattern of compulsive-like feeding behavior [72]. Striatal dopamine D2 receptors are downregulated in rats with obesity similar to the downregulation observed in humans with addiction problems [73]. Similar data are seen in humans in whom lower dopamine signaling in the striatum is noted in individuals with obesity [74]. There are other models of compulsive eating like intermittent access to a high-energy diet, which has also been shown to induce compulsive eating in mouse models [75,76]. These data demonstrate that overconsumption of palatable food triggers maladaptive neurological responses in brain reward circuits, leading to compulsive eating.
To further evaluate these pathways, a recent study used whole-brain imaging technology to map the connectivity of LH glutamatergic neurons with brain regions that process food-relevant sensory, appetitive, and motivational information [77]. It demonstrates that the development of obesity modifies the connectivity of LH glutamatergic neurons with brain areas that regulate states of aversion, especially the lateral habenula, which plays a critical role in integration of reward and aversive stimuli [78]. Subsequent experiments show that LH-derived glutamatergic transmission in the lateral habenula regulates compulsive eating as well as other obesity-related abnormalities [77]. Future elucidation of these pathways in the context of development of obesity in humans will provide a novel therapeutic target to treat obesity.
Association of Obesity and Other Behavioral Traits
Identification of the associations of obesity and other behavioral traits, such as anxiety, aggression, autism, or depression has led to new mechanistic insights of obesity development. Disruption of the transcription factor orthopedia homeobox, which affects the development and function of hypothalamic circuits, is associated with obesity and anxiety [79]. Similarly, deletion of the Src homology 2B adaptor protein 1 gene, which modulates insulin and leptin signaling, is associated with severe obesity, insulin resistance, as well as behavioral abnormalities, including aggression and social isolation [[80], [81], [82]]. Src homology 2B adaptor protein 1 has also been shown to mediate the effects of the brain-derived neurotrophic factor on neuronal integrity and may contribute to the obesity and disordered behavior seen in patients with Prader-Willi syndrome (PWS) [83]. Studies have also suggested that ghrelin has psychological effects like coping with psychosocial stress. For example, mice with exogenously elevated ghrelin concentrations exhibit lower anxiety and depressive behaviors, which are not observed in ghrelin receptor knockout mice, whereas chronic stress leads to elevated ghrelin concentrations and subsequent increased appetite, which persist after cessation of the stressor [84]. Similarly, individuals with hyperphagic major depressive disorder have higher concentrations of ghrelin in response to a meal [85]. Taken together, these findings from preclinical and clinical models support a role for ghrelin in stress-induced hyperphagia. These studies can pave way for specific treatments when obesity is associated with significant psychosocial stress.
Inflammatory Modulators of Food Consumption and Endocrine Action
Our evolving understanding of inflammation in the CNS has demonstrated that cellular inflammatory responses engage in regular maintenance and remodeling of neuronal pathways [86,87]. This remodeling allows humans to learn and adapt in response to environmental or physiologic stimuli [86,87]. Microglia and astrocytes, specific types of glial cells that interact with neurons, undergo conformational responses to stimuli that induce a reactive state accompanied by changes in their morphology, known as gliosis [86,87]. Gliosis involves a release of cytokines that act locally on other glial cells and other neurons [86,87]. These cytokines can have positive effects on the surrounding tissues manifested as neuroprotection and synaptic pruning and negative effects resulting in glial scar formation that can inhibit neuronal function in the region [86,87]. Therefore, cellular inflammation in the CNS is known to affect the function of neurons and neural pathways, and study of these changes in CNS regions regulating energy homeostasis is relevant to the understanding of eating behavior.
Hypothalamic inflammation and gliosis in rodent models of diet-induced obesity
Overnutrition with a high-fat diet (HFD) in mice results in increased cytokine release in the hypothalamus consistent with hypothalamic inflammation [88]. The microglia within the arcuate nucleus of the hypothalamus are inflamed as early as 3 d after starting an HFD, and this precedes the onset of hyperphagia, weight gain, and obesity in mice [89]. These data suggest that hypothalamic inflammation may be involved in obesity pathogenesis in rodent models. The HFD also induces apoptosis and endoplasmic reticulum stress in POMC neurons with resulting inflammation contributing to insulin and leptin resistance [[90], [91], [92]]. These observations are further supported by the improvement of diet-induced obesity in a mouse model of an astrocyte-specific inhibitor of nuclear factor kappa-B kinase subunit beta knockout, which is an essential cofactor in inflammation [93]. Comparable results are also seen with inhibitor of nuclear factor kappa-B kinase subunit beta knockouts in microglia [94] or AgRP neurons [95]. These data confirm that hypothalamic inflammation plays a major role in obesity pathogenesis in rodent models.
Evidence for hypothalamic inflammation and gliosis in human obesity
A post-mortem study of brain histology in tissues from human adults shows increased reactive microglial staining in the hypothalamus as compared with the cortex in those with obesity and compared with the hypothalamic tissue of those with healthy weight [96]. In addition, studies demonstrate radiologic evidence of hypothalamic gliosis and inflammation in both adults and children with obesity [89,[97], [98], [99], [100], [101], [102]]. Radiologic measures of hypothalamic gliosis are positively and prospectively associated with changes in the BMI z-score in overweight children, suggesting that hypothalamic gliosis may predict a change of adiposity before the onset of obesity [103]. Furthermore, evidence of hypothalamic gliosis is positively correlated with visceral adiposity in adult men [104] and with glucose intolerance, insulin resistance, and diabetes status in adults with obesity [105], linking gliosis to the metabolic complications of obesity.
Impact of inflammation on eating behavior
In rodents, suppression of inflammatory signaling in astrocytes or microglia reduces hyperphagia [93,94], indicating that gliosis is driving excess consumption of HFD in rodent models. In humans, hypothalamic gliosis is associated with lesser reductions in connectivity within the salience network after meals. The salience network is a functional brain network that is important for coordinating behavioral responses to internal and external stimuli and includes regions such as the insula and anterior cingulate cortex [106].
Potential target for therapeutics development
Hypothalamic gliosis is implicated in the disruption of energy homeostasis with resultant hyperphagia and increased energy storage. Furthermore, hypothalamic gliosis, in mice, is reversible by switching mice from an HFD to a feed pellets diet [107]. Similarly, humans experiencing weight loss after bariatric surgery showed partial reversibility of hypothalamic gliosis [108]. Therefore, developing therapeutic agents targeting hypothalamic inflammation and gliosis may be helpful in the prevention and treatment of obesity.
Gut–Brain Axis in Control of Appetitive Behavior
The gut–brain axis refers to the communication between the brain and the gut, including the microbiome, enteral nervous system, and endocrine hormones.
GLP-1 and the enteric nervous system
Proglucagon-derived glucagon-like peptide-1 (GLP-1) is a peptide secreted by enteroendocrine L cells in the lower intestine after direct contact with nutrients [[109], [110], [111], [112], [113], [114], [115], [116], [117], [118]]. GLP-1 agonists result in a relaxation of the gastric fundus, leading to delayed gastric emptying and reduced food intake [113,119]. GLP-1 is an endocrine hormone [120,121], which is rapidly inactivated by a local enzyme, dipeptidyl peptidase-4, expressed at high concentrations in the intestine and liver [122,123]. GLP-1 also exerts its systemic effects via local neural circuits mediated by gut enteric neurons [120,124]. In fact, ileal GLP-1 activates local ileal myenteric neurons, known as intestinofugal neurons, which subsequently activate the abdominal sympathetic celiac ganglion [125]. The abdominal sympathetic celiac ganglion connects downstream to gastric nitric oxide (Nos1) neurons, the main inhibitory gut motor neurons [126], the activation of which results in gastroparesis and appetite suppression [125]. These signals are also transmitted via spinal sensory neurons to the hypothalamus, elaborating an intricate neural network linking gut peptides to brain centers associated with the perception of gastric distension and appetite suppression [125].
Microbiome and behavior
The influence of the microbiome on brain and behavior is important from birth. Indeed, mice born via cesarean section (CS) have a different microbiome signature early in life and demonstrate long-lasting behavioral differences as compared with mice born via vaginal birth [127]. These changes are reversed either by co-housing CS mice with vaginally born mice (in which case fecal matter is exchanged) or by giving probiotic or prebiotic supplements [127]. Administration of antibiotics to mothers leads to microbiome alteration during fetal development and results in long-standing changes in anxiety and cognitive behaviors in offspring mice [128]. Administration of microbial metabolite SCFAs can ameliorate increases in stress responsiveness induced by chronic psychosocial stress [129] as well as regulate metabolism and appetite by directly modulating satiety pathways and nutrient sensing [130]. Human studies demonstrate that adults born via CS have an elevated immune and psychological response to acute and chronic stressors compared with those born vaginally [131]. Numerous studies evaluating the effects of microbiome changes during various life stages on obesity development are underway and will provide essential insights into the pathophysiology of obesity.
Microbiome and brain function
Studies show that germ-free mice evidence changes in myelination, neurogenesis, neurotransmitters, synaptic plasticity, dendritic growth, microglia, and blood–brain barrier function [[132], [133], [134], [135], [136], [137], [138], [139], [140], [141], [142]]. Dietary manipulation of adolescent mice affects the gut microbiome composition and results in altered expression of genes related to neuroinflammation, neurotransmission, and myelination in the PFC [143]. Furthermore, it is known that the vagus nerve is essential for transmission of microbiota–gut–brain axis signaling [144,145]. The microbiota–gut–brain axis is also implicated in regulating brain reward function and affecting social, eating, sexual, and drug addiction behaviors in mice [146,147]. Data in humans with regard to the microbiome are also evolving but are beyond the scope of this review.
Microbiome and its relevance to obesity
Mice born via CS have increased weight gain, demonstrating an obesogenic phenotype [148]. In addition, early alteration of the gut microbiome in mice by administration of antibiotics to mothers during fetal development leads to lasting metabolic effects with an increase in the obesogenic phenotype [149].
Potential therapeutic targets
The term “psychobiotic” is used to describe agents that target the microbiome for health benefits [150]. Studies in animal models have used specific probiotic strains such as Lactobacillus rhamnosus JB1, prebiotics such as fructo-oligosaccharides and galacto-oligosaccharides, postbiotics such as SCFAs, or fermented food such as milk kefir, some of which have proven to be effective psychobiotics [129,144,[151], [152], [153]]. Other studies have used fecal microbiota transplantation, with studies showing evidence that microbiota isolated from HFD-fed mice result in significant neurobehavioral changes in exploratory and cognitive behavior in feed pellets diet–fed mice [154]. Similarly, microbiota from humans with depression lead to neurobehavioral changes in rats and result in increased anxiety, anhedonia, and inflammation [155].
Studies have shown effects of probiotics on obesity, reducing inflammation and metabolic changes [156,157], decreasing abdominal adiposity and body weight [[158], [159], [160], [161]], and decreasing appetite by increasing satiety hormone secretions [162]. Efforts have been focused on identifying probiotics that can influence gut–brain signaling [163]. This led to the identification of a novel Bifidobacterium strain that produces metabolites that modulate ghrelin signaling and have antiobesity effects [164]. Supplementation with this strain results in improved glucose tolerance, decreased corticosterone production, and decreased weight gain in mice [165]. Although supplementation of this Bifidobacterium strain does not result in decreased body weight in humans, it results in improvements in blood glucose concentrations, reduction in cortisol awakening response, and an increase in active ghrelin [165]. Therefore, although microbiome manipulation has not yet led to effective obesity treatment, it may serve as a useful adjunctive therapy [166].
Lessons from Targeted Interventions to Modulate Appetite Pathways
Studies using specific interventions, summarized in Table 2, provide a unique opportunity to evaluate the appetite pathways, providing further pathophysiologic insights.
TABLE 2.
Weight management treatments and interventions
| Approved treatments | Bariatric surgery | Effective and durable weight loss Several underlying mechanistic changes may contribute to the degree of weight loss |
| GLP-1 analogs | Successful weight loss in conjunction with lifestyle interventions Chronic administration required for sustained weight loss |
|
| Experimental interventions | Intranasal oxytocin | Potential weight loss via reduced caloric intake, increased EE, and increased lipolysis |
| Transcranial stimulation | Potential weight loss via reduced caloric intake and food cravings |
Bariatric surgery
Bariatric surgery, currently the most effective and durable means of weight loss in individuals with obesity, provides mechanistic insights into appetite physiology. Roux-en-Y gastric bypass (RYGB) leads to a 30% total body weight loss after 1 y, whereas sleeve gastrectomy (SG), the most commonly performed bariatric operation worldwide, leads to a 25% total body weight loss [167]. Although some weight gain is common after maximal weight loss, both surgeries lead to a durable weight loss of around 20%–25% at 5 y after surgery [167]. Bariatric surgery also leads to remission of many comorbidities associated with obesity such as hypertension, diabetes, and dyslipidemia [168].
Although the initial hypothesis was that RYGB decreases EI by restricting food intake and causing caloric malabsorption, these simple mechanistic proposals for the actions of bariatric surgery do not completely represent the underlying pathways [169,170]. Concentrations of ghrelin, an orexigenic hormone, decrease acutely after RYGB, unlike after diet-induced weight loss that is associated with increased ghrelin concentrations [171]. However, ghrelin concentrations increase about a year after RYGB, suggesting that lowering of ghrelin concentrations is not the only mechanism by which RYGB results in weight loss [172]. Data show an increase in post-prandial GLP-1 concentrations after RYGB, but the benefits from RYGB persist even after the use of GLP-1 antagonists in humans, implying that GLP-1 is not the only driver of RYGB outcomes [172].
These data suggest multiple mechanistic pathways that contribute to metabolic changes after bariatric surgery, with no single mechanism yet identified as the primary driver of the weight loss or metabolic benefit of surgery. Multiple pathways involved in appetite control are impacted by surgery including changes in microbiome and bile acids [173,174]. Changes in intestinal metabolism may also contribute to the metabolic benefits of RYGB. In rodent models in which nutrient exposure is removed from the proximal bowel to mimic RYGB, the sodium-glucose cotransporter 3 (SGLT3), a proximal bowel nutrient sensor that regulates glucose absorption, demonstrates reduced signaling capacity, resulting in a decreased intestinal glucose absorption [175]. Furthermore, the portal vein milieu, which modulates hepatic glucose handling and improves glucose tolerance, changes after RYGB, highlighting the role of portal circulation in mediating metabolic effects in this context [175,176].
In human studies, after SG, activation of hedonic circuitry activation during craving of highly palatable food decreases, whereas cognitive control region activation increases [177]. Furthermore, hedonic (NAcc) and homeostatic (hypothalamus) brain activation and connectivity significantly predict weight loss 1 y after SG [177,178]. These data identify important changes in neurocircuitry after SG, which are potentially modifiable and can impact outcomes after SG.
GLP-1 mechanisms for appetite effects
GLP-1 analogs such as liraglutide, administrated as daily subcutaneous injections, and semaglutide, a longer-acting GLP-1 analog administered once weekly, are now more frequently utilized in adults and children for weight management. GLP-1 analogs appear to be effective in achieving sustained weight loss in conjunction with current lifestyle and behavioral treatments. In fact, semaglutide administration for 68 wk in adults with overweight or obesity resulted in a decrease in mean weight by 15.8% [179]. The use of these interventions has shed further light into the effects of GLP-1 on weight and appetite homeostasis (Figure 2).
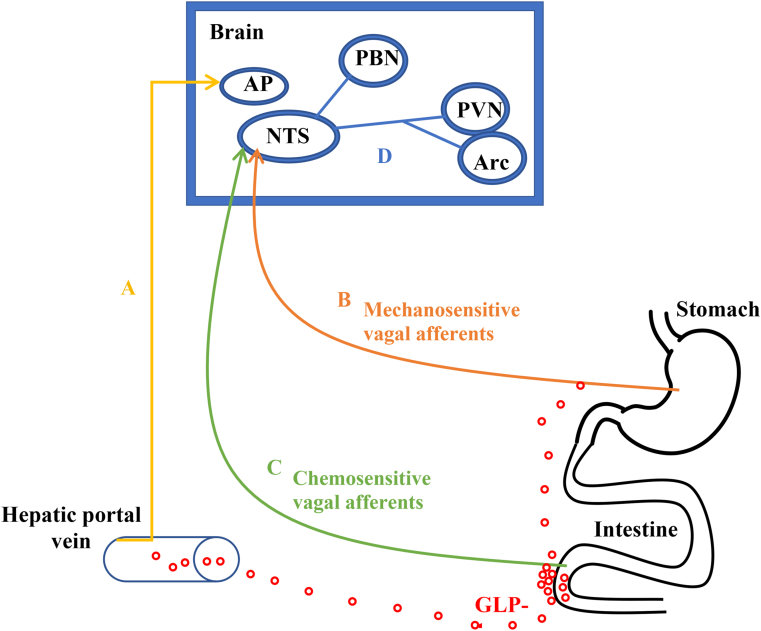
FIGURE 2.
GLP-1 mechanisms for appetite effects. (A) Local GLP-1 release causes an increase in concentration in the hepatic portal vein to reach area postrema. (B) GLP-1 receptors on vagus nerve afferent fibers modulate the sensation of gastric distension. (C) Chemosensitive vagal afferents stimulated by GLP-1 terminate in NTS. (D) GLP-1 receptors are found in key appetite regulating areas of the brain. Arc, arcuate nucleus; AP, area postrema; NTS, nucleus of the solitary tract; PBN, parabrachial nucleus; PVN, paraventricular nucleus of the hypothalamus.
Native GLP-1 decreases blood glucose concentrations by stimulating insulin secretion, suppressing glucagon secretion, and slowing gastric emptying. The effects of intestinal GLP-1 on eating behaviors are primarily mediated via the hepatic portal vein to allow sufficient GLP-1 concentrations to enter the systemic circulation and reach GLP-1 receptors in the area postrema [180]. GLP-1 may regulate gastrointestinal distention and its subsequent anorexigenic effects via mechanosensitive vagal afferents [180]. Other populations of chemosensitive vagal afferents end in the intestinal mucosa or reach the nucleus of the solitary tract in the brain, partly mediating the effects of GLP-1 on food intake [180]. GLP-1 receptors are found on key appetite-regulating neurons in the hypothalamus particularly in the PVN and on POMC neurons in the arcuate nucleus, allowing native GLP-1 to exert its effects on food intake [181].
Individuals with obesity have a lower GLP-1 response to oral glucose and food intake as compared with individuals with normal weight [182]. To achieve appropriate sensation of fullness after eating, individuals with obesity have to consume more food to achieve GLP-1 concentrations that will signal fullness [180]. Peripheral GLP-1 infusion in healthy, normal weight men increases fullness and concomitantly decreases hunger [183]. Comparable results are also seen with subcutaneous injections of GLP-1 analogs with increased satiety and fullness, as well as decreased hunger and prospective food consumption [184,185]. GLP-1 analogs have also been shown to regulate activity in brain areas such as the amygdala and insula in response to food cues [186], suggesting further modulation of appetite and food intake through reward-related mechanisms.
Although the administration of GLP-1 analogs has shown effective results, long-term chronic administration appears to be necessary to achieve and sustain weight loss [179,187]. Use of GLP-1 analogs carries additional considerations, including potential adverse effects as well as insurance coverage and cost [187]. Therefore, additional studies are needed to further investigate these therapeutics as well as other weight loss interventions. Newer studies have used combined GLP-1 and gastric inhibitory peptide analogs to produce even greater weight responses, and further research is ongoing into the optimal use of these agents [188].
Oxytocin effects on food intake
Oxytocin, a 9-amino acid peptide hormone produced by the hypothalamic PVN and supraoptic nucleus, acts at a G protein-coupled receptor to regulate important physiologic processes, including food intake and body weight [189]. Oxytocin is released into brain regions involved in homeostatic, reward-related, and cognitive control of food intake, as well as into the circulation through the posterior pituitary gland [189]. Oxytocin is also produced locally within the gastrointestinal tract where it binds to oxytocin receptors [189]. Although endogenous oxytocin does not readily cross the blood–brain barrier, peripheral oxytocin signals reach the caudal brainstem through the vagus nerve [189].
Studies in animal models have shown that exogenous oxytocin administration, given centrally or peripherally, induces weight loss by reducing food intake (particularly of more palatable carbohydrate and HFDs), increasing EE, and inducing lipolysis [190]. Independent of its effects on weight, oxytocin improves glucose homeostasis by increasing insulin release and sensitivity [190]. In a randomized double-blinded placebo-controlled crossover study in men across the weight spectrum, single-dose administration of 24 IU intranasal oxytocin reduced caloric intake (particularly of fat), increased fat utilization, and improved insulin sensitivity [191]. Decreased food consumption in response to intranasal oxytocin has also been demonstrated in women [192]. Effects of oxytocin in suppressing homeostatic food consumption appear to be greater in individuals with obesity compared with those with normal weight [193], whereas effects on reward-driven eating are consistently seen across the weight spectrum [193,194]. Mechanistic studies in humans using fMRI show that intranasal oxytocin modulates hedonic (for example, reduction of VTA activation, decreased functional connectivity between the VTA and other food motivation brain regions), homeostatic (for example, reduction of hypothalamic activation), and impulse control (for example, increase in anterior cingulate cortex activation) neurocircuitry [[195], [196], [197]]. Exogenous oxytocin also results in a reduction in impulsive behavior [198]. A small pilot study of intranasal oxytocin administration before meals and at bedtime for 8 wk in adults with obesity demonstrates significant (8.9 ± 5.4 kg) weight loss [199].
Effects of transcranial stimulation on eating behaviors and weight
Transcranial stimulation is a technique used to induce transient changes in cortical activity and is being investigated as a therapeutic method to alter behavior and help regulate food intake and food choice. There are 2 widely used types of noninvasive brain stimulation: repetitive transcranial magnetic stimulation and transcranial direct current stimulation (tDCS).
The PFC is a brain region involved in higher executive function, such as behavioral inhibition and flexibility [200], and in regulating control and reward-related aspects of eating behavior [201]. After a meal, regional cerebral blood flow to the left dorsolateral PFC (DLPFC), 1 of the areas involved in satiety, and meal termination, is reduced in individuals with obesity compared with lean individuals [202,203]. DLPFC is hence a potential target for intervention for regulating appetitive behavior.
Repetitive transcranial magnetic stimulation is administered via a large magnet over the scalp, which creates a magnetic field that activates neurons of the left DLPFC. When used in women with obesity, it results in stability in the number of food cravings compared with increased food cravings in women receiving the sham procedure [204]. Administration of tDCS to the PFC, which is performed by applying 2 surface electrodes with anodal and cathodal stimulation, results in weight loss and modification of eating behaviors by reducing food cravings, caloric intake, and ratings of hunger and urge to eat [[205], [206], [207], [208], [209]]. Variability in the magnitude of response is noted by the duration of sessions and the type of tDCS (anodal compared with cathodal) [210]. Furthermore, long-term (15 sessions) repeated anodal compared with sham tDCS to the left DLPFC decreases hunger ratings and urge to eat and reduces total EI during a food taste test but did not result in weight change [209]. These data suggest that activation of the left DLPFC promotes cognitive executive control and modifies nonhomeostatic eating behavior in individuals with obesity.
In conclusion, the 23rd Harvard Nutrition Obesity Symposium explored the neurobiological processes involved in appetite regulation from multiple perspectives. Biological, genetic, imaging, and therapeutic advances were reviewed. This article summarizes state-of-the-art data presented at the symposium on the latest techniques used in the evaluation of appetite regulation and outlines important opportunities for potential modulation of these systems through behavioral, pharmacological, surgical, and other techniques. Importantly, the symposium also emphasized those areas where more research is needed to more fully understand the critical mechanisms underlying appetite regulation. It is crucial to explore these pathways with the goal of developing therapeutic and preventive strategies for controlling obesity and its complications.
Acknowledgments
The authors’ responsibilities were as follows—IB and VS: drafted the manuscript based on talks presented by the coauthors during the conference and had primary responsibility for the final content; IB and ELB: designed the figures; SKG: supervised writing and revision of manuscript; and all authors: critically helped in the synthesis of data presented at the conference, revised the manuscript, provided relevant intellectual input, and read and approved the final manuscript.
JA receives research support from Nestle Healthcare Nutrition, Eli Lilly, Boehringer Ingelheim, Epitomee, UnitedHealth Group R&D, KVKTech, and WW. He is a consultant for Nestle Healthcare Nutrition, Eli Lilly, UnitedHealth Group R&D, Novo Nordisk, and Spokes Health. He also serves on the advisory board of Novo Nordisk, Nestle Healthcare Nutrition, Eli Lilly, and Level2. JFC is funded by the Science Foundation Ireland (SFI/12/RC/2273_P2), Saks Kavanaugh Foundation and Swiss National Science Foundation project CRSII5_186,346/NMS 2068, and has received research funding from Cremo, Dupont/IFF, Reckitt, Nutricia, and Pharmavite. ISF is an advisor for a number of companies including Eli Lilly, Rhythm Pharmaceuticals, and Novo Nordisk, all unrelated to this manuscript. PJK holds a financial interest in Eolas Therapeutics, which has a licensing agreement with AstraZeneca Inc and is on the advisory board of EpiVario Inc. EAL has served on the Scientific Advisory Board and has/had a financial interest in OXT Therapeutics, a company that developed oxytocin-based therapeutics for obesity and metabolic disease. EAL has an investigator-initiated grant from Tonix Pharmaceuticals to investigate intranasal oxytocin as a treatment of obesity and binge eating disorder. EAS receives research funding from NIDDK, NHLBI, American Diabetes Association, and University of Washington Nutrition and Obesity Research Center, all unrelated to this manuscript. TLS serves as a Scientific Advisor for RosVivo Therapeutics, unrelated to this manuscript, and receives research funding to her institution from Pfizer, Inc, also unrelated to this manuscript. AT is a cofounder of and consultant at AltrixBio, unrelated to this manuscript. SKG serves on the Scientific Advisory Board of Marathon Asset Management and consults for Theratechnologies, both unrelated to this manuscript. He receives research funding from Gilead, KOWA, and ViiV through his institution, also unrelated to this manuscript. IB, ELB, IEdA, CRF, MEG, LMH, BBL, VS, no conflicts of interest.
Conflict of interest
Each Author:
Dr. Imen Becetti has no conflicts of interest to disclose.
Esther L Bwenyi has no conflicts of interest to disclose.
Dr. Ivan E. de Araujo has no conflicts of interest to disclose.
Dr. Jamy Ard receives research support from Nestle Healthcare Nutrition, Eli Lilly, Boehringer Ingelheim, Epitomee, UnitedHealth Group R&D, KVKTech, and WW. He is a consultant for Nestle Healthcare Nutrition, Eli Lilly, UnitedHealth Group R&D, Novo Nordisk, and Spokes Health. He also serves on the advisory board of Novo Nordisk, Nestle Healthcare Nutrition, Eli Lilly, and Level2.
Dr. John F. Cryan is funded by the Science Foundation Ireland (SFI/12/RC/2273_P2), Saks Kavanaugh Foundation and Swiss National Science Foundation project CRSII5_186,346/NMS 2068, and has received research funding from Cremo, Dupont/IFF, Reckitt, Nutricia, and Pharmavite.
Dr. I. Sadaf Farooqi is an advisor for a number of companies including Eli Lilly, Rhythm Pharmaceuticals and Novo Nordisk, all unrelated to this manuscript.
Dr. Carrie R. Ferrario has no conflicts of interest to disclose.
Dr. Marci E.Gluck has no conflicts of interest to disclose.
Dr. Laura M. Holsen has no conflicts of interest to disclose.
Dr. Paul J. Kenny holds a financial interest in Eolas Therapeutics, which has a licensing agreement with AstraZeneca Inc, and is on the advisory board of EpiVario Inc.
Dr. Elizabeth A. Lawson has served on the Scientific Advisory Board and has/had a financial interest in OXT Therapeutics, a company that developed oxytocin-based therapeutics for obesity and metabolic disease. Dr. Lawson has an investigator-initiated grant from Tonix Pharmaceuticals to investigate intranasal oxytocin as a treatment for obesity and binge eating disorder.
Dr. Bradford B. Lowell has no conflicts of interest to disclose.
Dr. Ellen A. Schur receives research funding from NIDDK, NHLBI, American Diabetes Association, and UW Nutrition & Obesity Research Center, all unrelated to this manuscript.
Dr. Takara L. Stanley serves as a Scientific Advisor for RosVivo Therapeutics, unrelated to this manuscript, and receives research funding to her institution from Pfizer, Inc., also unrelated to this manuscript.
Dr. Ali Tavakkoli is a cofounder of and consultant at AltrixBio, unrelated to this manuscript.
Dr. Steven K. Grinspoon serves on the Scientific Advisory Board of Marathon Asset Management and consults for Theratechnologies, both unrelated to this manuscript. He receives research funding from Gilead, KOWA and ViiV through his institution, also unrelated to this manuscript.
Dr. Vibha Singhal has no conflicts of interest to disclose.
Funding
The funding for this article is: NIH P30 DK 040561; CRF supported by NIDDK 1R01DK130246 and 1R01DK115526.
References
- 1.Apovian C. Obesity: definition, comorbidities, causes, and burden. Am. J. Manag. Care. 2016;22(7):s176–s185. [PubMed] [Google Scholar]
- 2.Goldstein D.J. Beneficial health effects of modest weight loss. Int. J. Obes. Relat. Metab. Disord. 1992;16(6):397–415. [PubMed] [Google Scholar]
- 3.Styne D.M., Arslanian S.A., Connor E.L., Farooqi I.S., Murad M.H., Silverstein J.H., et al. Pediatric obesity-assessment, treatment, and prevention: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017;102(3):709–757. doi: 10.1210/jc.2016-2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stunkard A.J., Harris J.R., Pedersen N.L., McClearn G.E. The body-mass index of twins who have been reared apart. N. Engl. J. Med. 1990;322(21):1483–1487. doi: 10.1056/NEJM199005243222102. [DOI] [PubMed] [Google Scholar]
- 5.Khera A.V., Chaffin M., Wade K.H., Zahid S., Brancale J., Xia R., et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–596. doi: 10.1016/j.cell.2019.03.028. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riveros-McKay F., Mistry V., Bounds R., Hendricks A., Keogh J.M., Thomas H., et al. Genetic architecture of human thinness compared to severe obesity. PLOS Genet. 2019;15(1) doi: 10.1371/journal.pgen.1007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthoud H.R. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol. Behav. 2007;91(5):486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 8.Lowell B.B. New neuroscience of homeostasis and drives for food, water, and salt. N. Engl. J. Med. 2019;380(5):459–471. doi: 10.1056/NEJMRA1812053. [DOI] [PubMed] [Google Scholar]
- 9.Andermann M.L., Lowell B.B. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95(4):757–778. doi: 10.1016/J.NEURON.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farooqi I.S., Bullmore E., Keogh J., Gillard J., O’Rahilly S., Fletcher P.C. Leptin regulates striatal regions and human eating behavior. Science. 2007;317(5843):1355. doi: 10.1126/SCIENCE.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campfield L.A., Smith F.J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. doi: 10.1126/SCIENCE.7624778. [DOI] [PubMed] [Google Scholar]
- 12.Halaas J.L., Gajiwala K.S., Maffei M., Cohen S.L., Chait B.T., Rabinowitz D., et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269(5223):543–546. doi: 10.1126/SCIENCE.7624777. [DOI] [PubMed] [Google Scholar]
- 13.Pelleymounter M.A., Cullen M.J., Baker M.B., Hecht R., Winters D., Boone T., et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995;269(5223):540–543. doi: 10.1126/SCIENCE.7624776. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J.M., et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 15.Chua S.C., Chung W.K., Wu-Peng X.S., Zhang Y., Liu S.M., Tartaglia L., et al. Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science. 1996;271(5251):994–996. doi: 10.1126/SCIENCE.271.5251.994. [DOI] [PubMed] [Google Scholar]
- 16.Lee G.H., Proenca R., Montez J.M., Carroll K.M., Darvishzadeh J.G., Lee J.I., et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632A0. [DOI] [PubMed] [Google Scholar]
- 17.Chen H., Charlat O., Tartaglia L.A., Woolf E.A., Weng X., Ellis S.J., et al. Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell. 1996;84(3):491–495. doi: 10.1016/S0092-8674(00)81294-5. [DOI] [PubMed] [Google Scholar]
- 18.Montague C.T., Farooqi I.S., Whitehead J.P., Soos M.A., Rau H., Wareham N.J., et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 19.Farooqi I.S., Keogh J.M., Kamath S., Jones S., Gibson W.T., Trussell R., et al. Partial leptin deficiency and human adiposity. Nature. 2001;414(6859):34–35. doi: 10.1038/35102112. [DOI] [PubMed] [Google Scholar]
- 20.Farooqi I.S., Jebb S.A., Langmack G., Lawrence E., Cheetham C.H., Prentice A.M., et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 1999;341(12):879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 21.Ahima R.S., Prabakaran D., Mantzoros C., Qu D., Lowell B., Maratos-Flier E., et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382(6588):250–252. doi: 10.1038/382250A0. [DOI] [PubMed] [Google Scholar]
- 22.Chan J.L., Heist K., DePaoli A.M., Veldhuis J.D., Mantzoros C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Invest. 2003;111(9):1409–1421. doi: 10.1172/JCI17490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grinspoon S.K., Askari H., Landt M.L., Nathan D.M., Schoenfeld D.A., Hayden D.L., et al. Effects of fasting and glucose infusion on basal and overnight leptin concentrations in normal-weight women. Am. J. Clin. Nutr. 1997;66(6):1352–1356. doi: 10.1093/AJCN/66.6.1352. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbaum M., Murphy E.M., Heymsfield S.B., Matthews D.E., Leibel R.L. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J. Clin. Endocrinol. Metab. 2002;87(5):2391–2394. doi: 10.1210/JCEM.87.5.8628. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum M., Goldsmith R., Bloomfield D., Magnano A., Weimer L., Heymsfield S., et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 2005;115(12):3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baldini G., Phelan K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 2019;241(1):R1–R33. doi: 10.1530/JOE-18-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosoda H., Kojima M., Kangawa K. Biological, physiological, and pharmacological aspects of ghrelin. J. Pharmacol. Sci. 2006;100(5):398–410. doi: 10.1254/JPHS.CRJ06002X. [DOI] [PubMed] [Google Scholar]
- 28.Abizaid A., Liu Z.W., Andrews Z.B., Shanabrough M., Borok E., Elsworth J.D., et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J. Clin. Invest. 2006;116(12):3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yaswen L., Diehl N., Brennan M.B., Hochgeschwender U. Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat. Med. 1999;5(9):1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 30.Krude H., Biebermann H., Luck W., Horn R., Brabant G., Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat. Genet. 1998;19(2):155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 31.Yeo G.S.H., Farooqi I.S., Aminian S., Halsall D.J., Stanhope R.G., O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat. Genet. 1998;20(2):111–112. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 32.Farooqi I.S., Keogh J.M., Yeo G.S.H., Lank E.J., Cheetham T., O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 2003;348(12):1085–1095. doi: 10.1056/NEJMOA022050. [DOI] [PubMed] [Google Scholar]
- 33.Collet T.H., Dubern B., Mokrosinski J., Connors H., Keogh J.M., Mendes de Oliveira E., et al. Evaluation of a melanocortin-4 receptor (MC4R) agonist (setmelanotide) in MC4R deficiency. Mol. Metab. 2017;6(10):1321–1329. doi: 10.1016/J.MOLMET.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade K.H., Lam B.Y.H., Melvin A., Pan W., Corbin L.J., Hughes D.A., et al. Loss-of-function mutations in the melanocortin 4 receptor in a UK birth cohort. Nat. Med. 2021;27(6):1088–1096. doi: 10.1038/S41591-021-01349-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kühnen P., Clément K., Wiegand S., Blankenstein O., Gottesdiener K., Martini L.L., et al. Proopiomelanocortin deficiency treated with a melanocortin-4 receptor agonist. N. Engl. J. Med. 2016;375(3):240–246. doi: 10.1056/NEJMOA1512693. [DOI] [PubMed] [Google Scholar]
- 36.Clément K., Biebermann H., Farooqi I.S., van der Ploeg L., Wolters B., Poitou C., et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018;24(5):551–555. doi: 10.1038/S41591-018-0015-9. [DOI] [PubMed] [Google Scholar]
- 37.Clément K., van den Akker E., Argente J., Bahm A., Chung W.K., Connors H., et al. Efficacy and safety of setmelanotide, an MC4R agonist, in individuals with severe obesity due to LEPR or POMC deficiency: single-arm, open-label, multicentre, phase 3 trials. Lancet Diabetes Endocrinol. 2020;8(12):960–970. doi: 10.1016/S2213-8587(20)30364-8. [DOI] [PubMed] [Google Scholar]
- 38.Lotta L.A., Mokrosiński J., Mendes de Oliveira E., Li C., Sharp S.J., Luan J., et al. Human gain-of-function MC4R variants show signaling bias and protect against obesity. Cell. 2019;177(3):597–607.e9. doi: 10.1016/J.CELL.2019.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shor-Posner G., Azar A.P., Insinga S., Leibowitz S.F. Deficits in the control of food intake after hypothalamic paraventricular nucleus lesions. Physiol. Behav. 1985;35(6):883–890. doi: 10.1016/0031-9384(85)90255-0. [DOI] [PubMed] [Google Scholar]
- 40.Sims J.S., Lorden J.F. Effect of paraventricular nucleus lesions on body weight, food intake and insulin levels. Behav. Brain Res. 1986;22(3):265–281. doi: 10.1016/0166-4328(86)90071-9. [DOI] [PubMed] [Google Scholar]
- 41.Michaud J.L., Boucher F., Melnyk A., Gauthier F., Goshu E., Lévy E., et al. Sim1 haploinsufficiency causes hyperphagia, obesity and reduction of the paraventricular nucleus of the hypothalamus. Hum. Mol. Genet. 2001;10(14):1465–1473. doi: 10.1093/HMG/10.14.1465. [DOI] [PubMed] [Google Scholar]
- 42.Holder J.L., Zhang L., Kublaoui B.M., DiLeone R.J., Oz O.K., Bair C.H., et al. Sim1 gene dosage modulates the homeostatic feeding response to increased dietary fat in mice. Am. J. Physiol. Endocrinol. Metab. 2004;287(1):E105–E113. doi: 10.1152/AJPENDO.00446.2003/ASSET/IMAGES/LARGE/ZH10070417380007.JPEG. [DOI] [PubMed] [Google Scholar]
- 43.Holder J.L., Butte N.F., Zinn A.R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 2000;9(1):101–108. doi: 10.1093/HMG/9.1.101. [DOI] [PubMed] [Google Scholar]
- 44.Balthasar N., Dalgaard L.T., Lee C.E., Yu J., Funahashi H., Williams T., et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/J.CELL.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Shah B.P., Vong L., Olson D.P., Koda S., Krashes M.J., Ye C., et al. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. U S A. 2014;111(36):13193–13198. doi: 10.1073/PNAS.1407843111/SUPPL_FILE/PNAS.201407843SI.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luquet S., Perez F.A., Hnasko T.S., Palmiter R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/SCIENCE.1115524/SUPPL_FILE/LUQUET.SOM.PDF. [DOI] [PubMed] [Google Scholar]
- 47.Gropp E., Shanabrough M., Borok E., Xu A.W., Janoschek R., Buch T., et al. Agouti-related peptide–expressing neurons are mandatory for feeding. Nat. Neurosci. 2005;8(10):1289–1291. doi: 10.1038/NN1548. [DOI] [PubMed] [Google Scholar]
- 48.Aponte Y., Atasoy D., Sternson S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14(3):351–355. doi: 10.1038/NN.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krashes M.J., Koda S., Ye C.P., Rogan S.C., Adams A.C., Cusher D.S., et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121(4):1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beutler L.R., Chen Y., Ahn J.S., Lin Y.C., Essner R.A., Knight Z.A. Dynamics of gut-brain communication underlying hunger. Neuron. 2017;96(2):461–475.e5. doi: 10.1016/J.NEURON.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su Z., Alhadeff A.L., Betley J.N. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell. Rep. 2017;21(10):2724–2736. doi: 10.1016/J.CELREP.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Lin Y.C., Kuo T.W., Knight Z.A. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160(5):829–841. doi: 10.1016/J.CELL.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Betley J.N., Xu S., Cao Z.F.H., Gong R., Magnus C.J., Yu Y., et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521(7551):180–185. doi: 10.1038/NATURE14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandelblat-Cerf Y., Ramesh R.N., Burgess C.R., Patella P., Yang Z., Lowell B.B., et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife. 2015;4 doi: 10.7554/ELIFE.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garfield A.S., Shah B.P., Burgess C.R., Li M.M., Li C., Steger J.S., et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 2016;19(12):1628–1635. doi: 10.1038/NN.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berrios J., Li C., Madara J.C., Garfield A.S., Steger J.S., Krashes M.J., et al. Food cue regulation of AGRP hunger neurons guides learning. Nature. 2021;595(7869):695–700. doi: 10.1038/S41586-021-03729-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rothemund Y., Preuschhof C., Bohner G., Bauknecht H.C., Klingebiel R., Flor H., et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–421. doi: 10.1016/J.NEUROIMAGE.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Stice E., Spoor S., Bohon C., Veldhuizen M.G., Small D.M. Relation of reward from food intake and anticipated food intake to obesity: a functional magnetic resonance imaging study. J. Abnorm. Psychol. 2008;117(4):924–935. doi: 10.1037/A0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Low A.Y.T., Goldstein N., Gaunt J.R., Huang K.P., Zainolabidin N., Yip A.K.K., et al. Reverse-translational identification of a cerebellar satiation network. Nature. 2021;600(7888):269–273. doi: 10.1038/S41586-021-04143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Demos K.E., Heatherton T.F., Kelley W.M. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J. Neurosci. 2012;32(16):5549–5552. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yokum S., Ng J., Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity (Silver Spring) 2011;19(9):1775–1783. doi: 10.1038/OBY.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burger K.S., Stice E. Greater striatopallidal adaptive coding during cue-reward learning and food reward habituation predict future weight gain. Neuroimage. 2014;99:122–128. doi: 10.1016/J.NEUROIMAGE.2014.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Derman R.C., Ferrario C.R. Enhanced incentive motivation in obesity-prone rats is mediated by NAc core CP-AMPARs. Neuropharmacology. 2018;131:326–336. doi: 10.1016/J.NEUROPHARM.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oginsky M.F., Goforth P.B., Nobile C.W., Lopez-Santiago L.F., Ferrario C.R. Eating “junk-food” produces rapid and long-lasting increases in NAc CP-AMPA receptors: implications for enhanced cue-induced motivation and food addiction. Neuropsychopharmacology. 2016;41(13):2977–2986. doi: 10.1038/NPP.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Derman R.C., Ferrario C.R. Junk-food enhances conditioned food cup approach to a previously established food cue, but does not alter cue potentiated feeding; implications for the effects of palatable diets on incentive motivation. Physiol. Behav. 2018;192:145–157. doi: 10.1016/J.PHYSBEH.2018.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferrario C.R. Why did I eat that? Contributions of individual differences in incentive motivation and nucleus accumbens plasticity to obesity. Physiol. Behav. 2020;227:113114. doi: 10.1016/J.PHYSBEH.2020.113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ebbeling C.B., Feldman H.A., Klein G.L., Wong J.M.W., Bielak L., Steltz S.K., et al. Effects of a low carbohydrate diet on energy expenditure during weight loss maintenance: randomized trial. BMJ. 2018;363:k4583. doi: 10.1136/BMJ.K4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holsen L.M., Hoge W.S., Lennerz B.S., Cerit H., Hye T., Moondra P., et al. Diets varying in carbohydrate content differentially alter brain activity in homeostatic and reward regions in adults. J. Nutr. 2021;151(8):2465–2476. doi: 10.1093/JN/NXAB090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zeeni N., Nadkarni N., Bell J.D., Even P.C., Fromentin G., Tome D., et al. Peripherally injected cholecystokinin-induced neuronal activation is modified by dietary composition in mice. Neuroimage. 2010;50(4):1560–1565. doi: 10.1016/J.NEUROIMAGE.2010.01.065. [DOI] [PubMed] [Google Scholar]
- 70.Ulrich M., Endres F., Kölle M., Adolph O., Widenhorn-Müller K., Grön G. Glucose modulates food-related salience coding of midbrain neurons in humans. Hum. Brain Mapp. 2016;37(12):4376–4384. doi: 10.1002/HBM.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Woods C.A., Guttman Z.R., Huang D., Kolaric R.A., Rabinowitsch A.I., Jones K.T., et al. Insulin receptor activation in the nucleus accumbens reflects nutritive value of a recently ingested meal. Physiol. Behav. 2016;159:52–63. doi: 10.1016/J.PHYSBEH.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson P.M., Kenny P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010;13(5):635–641. doi: 10.1038/NN.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kenny P.J., Voren G., Johnson P.M. Dopamine D2 receptors and striatopallidal transmission in addiction and obesity. Curr. Opin. Neurobiol. 2013;23(4):535–538. doi: 10.1016/J.CONB.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stice E., Spoor S., Bohon C., Small D.M. Relation between obesity and blunted striatal response to food is moderated by TaqIA A1 allele. Science. 2008;322(5900):449–452. doi: 10.1126/SCIENCE.1161550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Di Segni M., Patrono E., Patella L., Puglisi-Allegra S., Ventura R. Animal models of compulsive eating behavior. Nutrients. 2014;6(10):4591–4609. doi: 10.3390/NU6104591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Czyzyk T.A., Sahr A.E., Statnick M.A. A model of binge-like eating behavior in mice that does not require food deprivation or stress. Obesity (Silver Spring) 2010;18(9):1710–1717. doi: 10.1038/OBY.2010.46. [DOI] [PubMed] [Google Scholar]
- 77.O’Connor R., Mathis V., Johnson P., Micioni Di Bonaventura M., Smith A., Caligiuri S., et al. 2022. Habenular foraging circuits that link environmental threats to food valuation drive obesity. [Google Scholar]
- 78.Mondoloni S., Mameli M., Congiu M. Reward and aversion encoding in the lateral habenula for innate and learned behaviours. Transl. Psychiatry. 2022;12(1):3. doi: 10.1038/s41398-021-01774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moir L., Bochukova E.G., Dumbell R., Banks G., Bains R.S., Nolan P.M., et al. Disruption of the homeodomain transcription factor orthopedia homeobox (Otp) is associated with obesity and anxiety. Mol. Metab. 2017;6(11):1419–1428. doi: 10.1016/J.MOLMET.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Doche M.E., Bochukova E.G., Su H.W., Pearce L.R., Keogh J.M., Henning E., et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. J. Clin. Invest. 2012;122(12):4732–4736. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bochukova E.G., Huang N., Keogh J., Henning E., Purmann C., Blaszczyk K., et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463(7281):666–670. doi: 10.1038/NATURE08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flores A., Argetsinger L.S., Stadler L.K.J., Malaga A.E., Vander P.B., DeSantis L.C., et al. Crucial role of the SH2B1 PH domain for the control of energy balance. Diabetes. 2019;68(11):2049–2062. doi: 10.2337/DB19-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bochukova E.G., Lawler K., Croizier S., Keogh J.M., Patel N., Strohbehn G., et al. A transcriptomic signature of the hypothalamic response to fasting and BDNF deficiency in Prader-Willi syndrome. Cell. Rep. 2018;22(13):3401–3408. doi: 10.1016/J.CELREP.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lutter M., Sakata I., Osborne-Lawrence S., Rovinsky S.A., Anderson J.G., Jung S., et al. The orexigenic hormone ghrelin defends against depressive symptoms of chronic stress. Nat. Neurosci. 2008;11(7):752–753. doi: 10.1038/NN.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cerit H., Christensen K., Moondra P., Klibanski A., Goldstein J.M., Holsen L.M. Divergent associations between ghrelin and neural responsivity to palatable food in hyperphagic and hypophagic depression. J. Affect. Disord. 2019;242:29–38. doi: 10.1016/J.JAD.2018.07.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.DiSabato D.J., Quan N., Godbout J.P. Neuroinflammation: the devil is in the details. J. Neurochem. 2016;139(Suppl 2):136–153. doi: 10.1111/JNC.13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giovannoni F., Quintana F.J. The role of astrocytes in CNS inflammation. Trends Immunol. 2020;41(9):805–819. doi: 10.1016/J.IT.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/EN.2004-1520. [DOI] [PubMed] [Google Scholar]
- 89.Thaler J.P., Yi C.X., Schur E.A., Guyenet S.J., Hwang B.H., Dietrich M.O., et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 2012;122(1):153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Valdearcos M., Robblee M.M., Benjamin D.I., Nomura D.K., Xu A.W., Koliwad S.K. Microglia dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep. 2014;9(6):2124–2138. doi: 10.1016/J.CELREP.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Moraes J.C., Coope A., Morari J., Cintra D.E., Roman E.A., Pauli J.R., et al. High-fat diet induces apoptosis of hypothalamic neurons. PLOS ONE. 2009;4(4):e5045. doi: 10.1371/JOURNAL.PONE.0005045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jais A., Brüning J.C. Hypothalamic inflammation in obesity and metabolic disease. J. Clin. Invest. 2017;127(1):24–32. doi: 10.1172/JCI88878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douglass J.D., Dorfman M.D., Fasnacht R., Shaffer L.D., Thaler J.P. Astrocyte IKKβ/NF-κB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol. Metab. 2017;6(4):366–373. doi: 10.1016/J.MOLMET.2017.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Valdearcos M., Douglass J.D., Robblee M.M., Dorfman M.D., Stifler D.R., Bennett M.L., et al. Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell, Metab. 2017;26(1):185–197.e3. doi: 10.1016/J.CMET.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/J.CELL.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baufeld C., Osterloh A., Prokop S., Miller K.R., Heppner F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132(3):361–375. doi: 10.1007/S00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schur E.A., Melhorn S.J., Oh S.K., Lacy J.M., Berkseth K.E., Guyenet S.J., et al. Radiologic evidence that hypothalamic gliosis is associated with obesity and insulin resistance in humans. Obesity (Silver Spring) 2015;23(11):2142–2148. doi: 10.1002/oby.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kreutzer C., Peters S., Schulte D.M., Fangmann D., Türk K., Wolff S., et al. Hypothalamic inflammation in human obesity is mediated by environmental and genetic factors. Diabetes. 2017;66(9):2407–2415. doi: 10.2337/DB17-0067. [DOI] [PubMed] [Google Scholar]
- 99.Sewaybricker L.E., Schur E.A., Melhorn S.J., Campos B.M., Askren M.K., Nogueira G.A.S., et al. Initial evidence for hypothalamic gliosis in children with obesity by quantitative T2 MRI and implications for blood oxygen-level dependent response to glucose ingestion. Pediatr. Obes. 2019;14(2) doi: 10.1111/IJPO.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Thomas K., Beyer F., Lewe G., Zhang R., Schindler S., Schönknecht P., et al. Higher body mass index is linked to altered hypothalamic microstructure. Sci. Rep. 2019;9(1):17373. doi: 10.1038/S41598-019-53578-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kullmann S., Abbas Z., Machann J., Shah N.J., Scheffler K., Birkenfeld A.L., et al. Investigating obesity-associated brain inflammation using quantitative water content mapping. J. Neuroendocrinol. 2020;32(12) doi: 10.1111/JNE.12907. [DOI] [PubMed] [Google Scholar]
- 102.Sewaybricker L.E., Huang A., Chandrasekaran S., Melhorn S.J., Schur E.A. The significance of hypothalamic inflammation and gliosis for the pathogenesis of obesity in humans. Endocr. Rev. 2023;44(2):281–296. doi: 10.1210/ENDREV/BNAC023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sewaybricker L.E., Kee S., Melhorn S.J., Schur E.A. Greater radiologic evidence of hypothalamic gliosis predicts adiposity gain in children at risk for obesity. Obesity (Silver, Spring) 2021;29(11):1770–1779. doi: 10.1002/OBY.23286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Berkseth K.E., Rubinow K.B., Melhorn S.J., Webb M.F., Rosalynn B.D.L.M., Marck B.T., et al. Hypothalamic gliosis by MRI and visceral fat mass negatively correlate with plasma testosterone concentrations in healthy men. Obesity (Silver Spring) 2018;26(12):1898–1904. doi: 10.1002/OBY.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenbaum J.L., Melhorn S.J., Schoen S., Webb M.F., de Leon M.R.B., Humphreys M., et al. Evidence that hypothalamic gliosis is related to impaired glucose homeostasis in adults with obesity. Diabetes Care. 2022;45(2):416–424. doi: 10.2337/DC21-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sewaybricker L.E., Melhorn S.J., Askren M.K., Webb M.F., Tyagi V., de Leon M.R.B., et al. Salience network connectivity is reduced by a meal and influenced by genetic background and hypothalamic gliosis. Int. J. Obes. (Lond). 2020;44(1):167–177. doi: 10.1038/S41366-019-0361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berkseth K.E., Guyenet S.J., Melhorn S.J., Lee D., Thaler J.P., Schur E.A., et al. Hypothalamic gliosis associated with high-fat diet feeding is reversible in mice: a combined immunohistochemical and magnetic resonance imaging study. Endocrinology. 2014;155(8):2858–2867. doi: 10.1210/EN.2014-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van de Sande-Lee S., Melhorn S.J., Rachid B., Rodovalho S., De-Lima-Junior J.C., Campos B.M., et al. Radiologic evidence that hypothalamic gliosis is improved after bariatric surgery in obese women with type 2 diabetes. Int. J. Obes. (Lond). 2020;44(1):178–185. doi: 10.1038/S41366-019-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Herrmann-Rinke C., Vöge A., Hess M., Göke B. Regulation of glucagon-like peptide-1 secretion from rat ileum by neurotransmitters and peptides. J. Endocrinol. 1995;147(1):25–31. doi: 10.1677/JOE.0.1470025. [DOI] [PubMed] [Google Scholar]
- 110.Layer P., Holst J.J., Grandt D., Goebell H. Ileal release of glucagon-like peptide-1 (GLP-1). Association with inhibition of gastric acid secretion in humans. Dig. Dis. Sci. 1995;40(5):1074–1082. doi: 10.1007/BF02064202. [DOI] [PubMed] [Google Scholar]
- 111.Ritzel U., Fromme A., Ottleben M., Leonhardt U., Ramadori G. Release of glucagon-like peptide-1 (GLP-1) by carbohydrates in the perfused rat ileum. Acta Diabetol. 1997;34(1):18–21. doi: 10.1007/S005920050059. [DOI] [PubMed] [Google Scholar]
- 112.Müller T.D., Finan B., Bloom S.R., D’Alessio D., Drucker D.J., Flatt P.R., et al. Glucagon-like peptide 1 (GLP-1) Mol. Metab. 2019;30:72–130. doi: 10.1016/J.MOLMET.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schirra J., Göke B. The physiological role of GLP-1 in human: incretin, ileal brake or more? Regul. Pept. 2005;128(2):109–115. doi: 10.1016/J.REGPEP.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 114.Holst J.J. The physiology of glucagon-like peptide 1. Physiol. Rev. 2007;87(4):1409–1439. doi: 10.1152/PHYSREV.00034.2006. [DOI] [PubMed] [Google Scholar]
- 115.Dailey M.J., Moran T.H. Glucagon-like peptide 1 and appetite. Trends Endocrinol, Metab. 2013;24(2):85–91. doi: 10.1016/J.TEM.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paternoster S., Falasca M. Dissecting the physiology and pathophysiology of glucagon-like peptide-1. Front. Endocrinol. (Lausanne) 2018;9:584. doi: 10.3389/FENDO.2018.00584/BIBTEX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eissele R., Göke R., Willemer S., Harthus H.-P., Vermeer H., Arnold R., et al. Glucagon-like peptide-1 cells in the gastrointestinal tract and pancreas of rat, pig and man. Eur. J. Clin. Invest. 1992;22(4):283–291. doi: 10.1111/J.1365-2362.1992.TB01464.X. [DOI] [PubMed] [Google Scholar]
- 118.Panaro B.L., Yusta B., Matthews D., Koehler J.A., Song Y., Sandoval D.A., et al. Intestine-selective reduction of Gcg expression reveals the importance of the distal gut for GLP-1 secretion. Mol. Metab. 2020;37:100990. doi: 10.1016/J.MOLMET.2020.100990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Drucker D.J. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27(4):740–756. doi: 10.1016/J.CMET.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 120.Burcelin R., Serino M., Cabou C. A role for the gut-to-brain GLP-1-dependent axis in the control of metabolism. Curr. Opin. Pharmacol. 2009;9(6):744–752. doi: 10.1016/J.COPH.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 121.D’Alessio D. Is GLP-1 a hormone: whether and when? J. Diabetes. Investig. 2016;7(Suppl 1):50–55. doi: 10.1111/JDI.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deacon C.F. What do we know about the secretion and degradation of incretin hormones? Regul. Pept. 2005;128(2):117–124. doi: 10.1016/J.REGPEP.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 123.Hansen L., Deacon C.F., Ørskov C., Holst J.J. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase IV in the capillaries supplying the L cells of the porcine intestine. Endocrinology. 1999;140(11):5356–5363. doi: 10.1210/ENDO.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 124.Holst J.J., Andersen D.B., Grunddal K.V. Actions of glucagon-like peptide-1 receptor ligands in the gut. Br. J. Pharmacol. 2022;179(4):727–742. doi: 10.1111/BPH.15611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhang T., Perkins M.H., Chang H., Han W., de Araujo I.E. An inter-organ neural circuit for appetite suppression. Cell. 2022;185(14):2478–2494.e28. doi: 10.1016/J.CELL.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Furness J.B., Callaghan B.P., Rivera L.R., Cho H.J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3/FIGURES/7. [DOI] [PubMed] [Google Scholar]
- 127.Morais L.H., Golubeva A.V., Moloney G.M., Moya-Pérez A., Ventura-Silva A.P., Arboleya S., et al. Enduring behavioral effects induced by birth by caesarean section in the mouse. Curr. Biol. 2020;30(19):3761–3774.e6. doi: 10.1016/J.CUB.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 128.O’Connor R., Moloney G.M., Fulling C., O’Riordan K.J., Fitzgerald P., Bastiaanssen T.F.S., et al. Maternal antibiotic administration during a critical developmental window has enduring neurobehavioural effects in offspring mice. Behav. Brain Res. 2021;404:113156. doi: 10.1016/J.BBR.2021.113156. [DOI] [PubMed] [Google Scholar]
- 129.van de Wouw M., Boehme M., Lyte J.M., Wiley N., Strain C., O’Sullivan O., et al. Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 2018;596:4923–4944. doi: 10.1113/JP276431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.van de Wouw M., Schellekens H., Dinan T.G., Cryan J.F. Microbiota-gut-brain axis: modulator of host metabolism and appetite. J. Nutr. 2017;147(5):727–745. doi: 10.3945/JN.116.240481. [DOI] [PubMed] [Google Scholar]
- 131.Dinan T.G., Kennedy P.J., Morais L.H., Murphy A., Long-Smith C.M., Moloney G.M., et al. Altered stress responses in adults born by Caesarean section. Neurobiol. Stress. 2022;16:100425. doi: 10.1016/J.YNSTR.2021.100425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6(263):263ra158. doi: 10.1126/SCITRANSLMED.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chu C., Murdock M.H., Jing D., Won T.H., Chung H., Kressel A.M., et al. The microbiota regulate neuronal function and fear extinction learning. Nature. 2019;574(7779):543–548. doi: 10.1038/S41586-019-1644-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., et al. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18(6):666–673. doi: 10.1038/MP.2012.77. [DOI] [PubMed] [Google Scholar]
- 135.Erny D., de Angelis A.L.H., Jaitin D., Wieghofer P., Staszewski O., David E., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18(7):965–977. doi: 10.1038/NN.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gacias M., Gaspari S., Santos P.M.G., Tamburini S., Andrade M., Zhang F., et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. eLife. 2016;5 doi: 10.7554/ELIFE.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Heijtz R.D., Wang S., Anuar F., Qian Y., Björkholm B., Samuelsson A., et al. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. U S A. 2011;108(7):3047–3052. doi: 10.1073/PNAS.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hoban A.E., Stilling R.M., Ryan F.J., Shanahan F., Dinan T.G., Claesson M.J., et al. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 2016;6(4):e774. doi: 10.1038/TP.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hoban A.E., Stilling R.M., Moloney G., Shanahan F., Dinan T.G., Clarke G., et al. The microbiome regulates amygdala-dependent fear recall. Mol. Psychiatry. 2018;23(5):1134–1144. doi: 10.1038/MP.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Luczynski P., Whelan S.O., O’Sullivan C., Clarke G., Shanahan F., Dinan T.G., et al. Adult microbiota-deficient mice have distinct dendritic morphological changes: differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016;44(9):2654–2666. doi: 10.1111/EJN.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23(3):255–264. doi: 10.1111/J.1365-2982.2010.01620.X. [DOI] [PubMed] [Google Scholar]
- 142.Ogbonnaya E.S., Clarke G., Shanahan F., Dinan T.G., Cryan J.F., O’Leary O.F. Adult hippocampal neurogenesis is regulated by the microbiome. Biol. Psychiatry. 2015;78(4):e7–e9. doi: 10.1016/J.BIOPSYCH.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 143.Fülling C., Lach G., Bastiaanssen T.F.S., Fouhy F., O’Donovan A.N., Ventura-Silva A.P., et al. Adolescent dietary manipulations differentially affect gut microbiota composition and amygdala neuroimmune gene expression in male mice in adulthood. Brain Behav. Immun. 2020;87:666–678. doi: 10.1016/J.BBI.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 144.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. U S A. 2011;108(38):16050–16055. doi: 10.1073/PNAS.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fülling C., Dinan T.G., Cryan J.F. Gut microbe to brain signaling: what happens in vagus…. Neuron. 2019;101(6):998–1002. doi: 10.1016/J.NEURON.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 146.García-Cabrerizo R., Carbia C., O´Riordan K.J., Schellekens H., Cryan J.F. Microbiota-gut-brain axis as a regulator of reward processes. J. Neurochem. 2021;157(5):1495–1524. doi: 10.1111/JNC.15284. [DOI] [PubMed] [Google Scholar]
- 147.Peterson V.L., Richards J.B., Meyer P.J., Cabrera-Rubio R., Tripi J.A., King C.P., et al. Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. eBioMedicine. 2020;55:102769. doi: 10.1016/J.EBIOM.2020.102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Martinez K.A., Devlin J.C., Lacher C.R., Yin Y., Cai Y., Wang J., et al. Increased weight gain by C-section: functional significance of the primordial microbiome. Sci. Adv. 2017;3(10) doi: 10.1126/SCIADV.AAO1874/SUPPL_FILE/AAO1874_SM.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cox L.M., Yamanishi S., Sohn J., Alekseyenko A.V., Leung J.M., Cho I., et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. 2014;158(4):705–721. doi: 10.1016/J.CELL.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Long-Smith C., O’Riordan K.J., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Microbiota-gut-brain axis: new therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 2020;60:477–502. doi: 10.1146/ANNUREV-PHARMTOX-010919-023628. [DOI] [PubMed] [Google Scholar]
- 151.Savignac H.M., Hyland N.P., Dinan T.G., Cryan J.F. The effects of repeated social interaction stress on behavioural and physiological parameters in a stress-sensitive mouse strain. Behav. Brain Res. 2011;216(2):576–584. doi: 10.1016/J.BBR.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 152.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., et al. Targeting the microbiota-gut-brain axis: prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biol. Psychiatry. 2017;82(7):472–487. doi: 10.1016/J.BIOPSYCH.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 153.van de Wouw M., Walsh A.M., Crispie F., van Leuven L., Lyte J.M., Boehme M., et al. Distinct actions of the fermented beverage kefir on host behaviour, immunity and microbiome gut-brain modules in the mouse. Microbiome. 2020;8(1):67. doi: 10.1186/S40168-020-00846-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bruce-Keller A.J., Salbaum J.M., Luo M., Blanchard E., Taylor C.M., Welsh D.A., et al. Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol. Psychiatry. 2015;77(7):607–615. doi: 10.1016/J.BIOPSYCH.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kelly J.R., Borre Y., O’Brien C., Patterson E., El Aidy S., Deane J., et al. Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016;82:109–118. doi: 10.1016/J.JPSYCHIRES.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 156.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U S A. 2013;110(22):9066–9071. doi: 10.1073/PNAS.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Amar J., Chabo C., Waget A., Klopp P., Vachoux C., Bermúdez-Humarán L.G., et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol. Med. 2011;3(9):559–572. doi: 10.1002/EMMM.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kadooka Y., Sato M., Imaizumi K., Ogawa A., Ikuyama K., Akai Y., et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur. J. Clin. Nutr. 2010;64(6):636–643. doi: 10.1038/EJCN.2010.19. [DOI] [PubMed] [Google Scholar]
- 159.Kang J.H., Yun S.I., Park H.O. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J. Microbiol. 2010;48(5):712–714. doi: 10.1007/S12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 160.Kondo S., Xiao J.Z., Satoh T., Odamaki T., Takahashi S., Sugahara H., et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci. Biotechnol. Biochem. 2010;74(8):1656–1661. doi: 10.1271/BBB.100267. [DOI] [PubMed] [Google Scholar]
- 161.Lee H.Y., Park J.H., Seok S.H., Baek M.W., Kim D.J., Lee K.E., et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim. Biophys. Acta. 2006;1761(7):736–744. doi: 10.1016/J.BBALIP.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 162.Yadav H., Lee J.H., Lloyd J., Walter P., Rane S.G. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J. Biol. Chem. 2013;288(35):25088–25097. doi: 10.1074/JBC.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bambury A., Sandhu K., Cryan J.F., Dinan T.G. Finding the needle in the haystack: systematic identification of psychobiotics. Br. J. Pharmacol. 2018;175(24):4430–4438. doi: 10.1111/BPH.14127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Torres-Fuentes C., Golubeva A.V., Zhdanov A.V., Wallace S., Arboleya S., Papkovsky D.B., et al. Short-chain fatty acids and microbiota metabolites attenuate ghrelin receptor signaling. FASEB J. 2019;33(12):13546–13559. doi: 10.1096/FJ.201901433R. [DOI] [PubMed] [Google Scholar]
- 165.Schellekens H., Torres-Fuentes C., van de Wouw M., Long-Smith C.M., Mitchell A., Strain C., et al. Bifidobacterium longum counters the effects of obesity: partial successful translation from rodent to human. eBioMedicine. 2021;63:103176. doi: 10.1016/J.EBIOM.2020.103176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Boscaini S., Leigh S.J., Lavelle A., García-Cabrerizo R., Lipuma T., Clarke G., et al. Microbiota and body weight control: weight watchers within? Mol. Metab. 2022;57:101427. doi: 10.1016/J.MOLMET.2021.101427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Arterburn D., Wellman R., Emiliano A., Smith S.R., Odegaard A.O., Murali S., et al. Comparative effectiveness and safety of bariatric procedures for weight loss: a PCORrnet cohort study. Ann. Intern. Med. 2018;169(11):741–750. doi: 10.7326/M17-2786/ASSET/IMAGES/LARGE/M172786FF3_FIGURE_3_PROPORTIONS_OF_AGB_RYGB_AND_SG_PATIENTS_WITH_TWL_5_10_20_AND_30_AT_1_3_AND_5.JPEG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Courcoulas A.P., King W.C., Belle S.H., Berk P., Flum D.R., Garcia L., et al. Seven-year weight trajectories and health outcomes in the Longitudinal Assessment of Bariatric Surgery (LABS) Study. JAMA Surg. 2018;153(5):427–434. doi: 10.1001/JAMASURG.2017.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hao Z., Mumphrey M.B., Townsend R.L., Morrison C.D., Münzberg H., Ye J., et al. Reprogramming of defended body weight after Roux-en-Y gastric bypass surgery in diet-induced obese mice. Obesity (Silver Spring) 2016;24(3):654–660. doi: 10.1002/OBY.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stylopoulos N., Hoppin A.G., Kaplan L.M. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 2009;17(10):1839–1847. doi: 10.1038/OBY.2009.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Cummings D.E., Weigle D.S., Frayo R.S., Breen P.A., Ma M.K., Dellinger E.P., et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N. Engl. J. Med. 2002;346(21):1623–1630. doi: 10.1056/NEJMOA012908. [DOI] [PubMed] [Google Scholar]
- 172.Luo J.N., Tavakkoli A. Physiologic mechanisms of weight loss following metabolic/bariatric surgery. Surg. Clin. North Am. 2021;101(2):223–237. doi: 10.1016/J.SUC.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 173.Aron-Wisnewsky J., Prifti E., Belda E., Ichou F., Kayser B.D., Dao M.C., et al. Major microbiota dysbiosis in severe obesity: fate after bariatric surgery. Gut. 2019;68(1):70–82. doi: 10.1136/GUTJNL-2018-316103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Albaugh V.L., Banan B., Antoun J., Xiong Y., Guo Y., Ping J., et al. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2019;156(4):1041–1051.e4. doi: 10.1053/J.GASTRO.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pal A., Rhoads D.B., Tavakkoli A. Foregut exclusion disrupts intestinal glucose sensing and alters portal nutrient and hormonal milieu. Diabetes. 2015;64(6):1941–1950. doi: 10.2337/DB14-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pal A., Rhoads D.B., Tavakkoli A. Effect of portal glucose sensing on systemic glucose levels in SD and ZDF rats. PLOS ONE. 2016;11(11) doi: 10.1371/JOURNAL.PONE.0165592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Holsen L.M., Davidson P., Cerit H., Hye T., Moondra P., Haimovici F., et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int. J. Obes. (Lond). 2018;42(4):785–793. doi: 10.1038/IJO.2017.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cerit H., Davidson P., Hye T., Moondra P., Haimovici F., Sogg S., et al. Resting-state brain connectivity predicts weight loss and cognitive control of eating behavior after vertical sleeve gastrectomy. Obesity (Silver, Spring) 2019;27(11):1846–1855. doi: 10.1002/OBY.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Rubino D.M., Greenway F.L., Khalid U., O’Neil P.M., Rosenstock J., Sørrig R., et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–150. doi: 10.1001/JAMA.2021.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Krieger J.P. Intestinal glucagon-like peptide-1 effects on food intake: physiological relevance and emerging mechanisms. Peptides. 2020;131:170342. doi: 10.1016/J.PEPTIDES.2020.170342. [DOI] [PubMed] [Google Scholar]
- 181.Muscogiuri G., DeFronzo R.A., Gastaldelli A., Holst J.J. Glucagon-like Peptide-1 and the central/peripheral nervous system: crosstalk in diabetes. Trends Endocrinol. Metab. 2017;28(2):88–103. doi: 10.1016/J.TEM.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 182.Færch K., Torekov S.S., Vistisen D., Johansen N.B., Witte D.R., Jonsson A., et al. GLP-1 response to oral glucose is reduced in prediabetes, screen-detected type 2 diabetes, and obesity and influenced by sex: The Addition-Pro Study. Diabetes. 2015;64(7):2513–2525. doi: 10.2337/DB14-1751. [DOI] [PubMed] [Google Scholar]
- 183.Flint A., Raben A., Astrup A., Holst J.J. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J. Clin. Invest. 1998;101(3):515–520. doi: 10.1172/JCI990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.van Can J., Sloth B., Jensen C.B., Flint A., Blaak E.E., Saris W.H.M. Effects of the once-daily GLP-1 analog liraglutide on gastric emptying, glycemic parameters, appetite and energy metabolism in obese, non-diabetic adults. Int. J. Obes. (Lond). 2014;38(6):784–793. doi: 10.1038/IJO.2013.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Blundell J., Finlayson G., Axelsen M., Flint A., Gibbons C., Kvist T., et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes. Obes. Metab. 2017;19(9):1242–1251. doi: 10.1111/DOM.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.van Bloemendaal L., IJzerman R.G., ten Kulve J.S., Barkhof F., Konrad R.J., Drent M.L., et al. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes. 2014;63(12):4186–4196. doi: 10.2337/DB14-0849. [DOI] [PubMed] [Google Scholar]
- 187.Chao A.M., Tronieri J.S., Amaro A., Wadden T.A. Clinical insight on semaglutide for chronic weight management in adults: patient selection and special considerations. Drug Des. Devel. Ther. 2022;16:4449–4461. doi: 10.2147/DDDT.S365416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N. Engl. J. Med. 2022;387(3):205–216. doi: 10.1056/NEJMOA2206038. [DOI] [PubMed] [Google Scholar]
- 189.Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat. Rev. Neurosci. 2011;12(9):524–538. doi: 10.1038/NRN3044. [DOI] [PubMed] [Google Scholar]
- 190.Blevins J.E., Baskin D.G. Translational and therapeutic potential of oxytocin as an anti-obesity strategy: insights from rodents, nonhuman primates and humans. Physiol. Behav. 2015;152(B):438–449. doi: 10.1016/J.PHYSBEH.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lawson E.A., Marengi D.A., Desanti R.L., Holmes T.M., Schoenfeld D.A., Tolley C.J. Oxytocin reduces caloric intake in men. Obesity (Silver Spring) 2015;23(5):950–956. doi: 10.1002/OBY.21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Burmester V., Gibson E.L., Butler G., Bailey A., Terry P. Oxytocin reduces post-stress sweet snack intake in women without attenuating salivary cortisol. Physiol. Behav. 2019;212:112704. doi: 10.1016/J.PHYSBEH.2019.112704. [DOI] [PubMed] [Google Scholar]
- 193.Fritsche M. Thienel A., Heinrichs M., Peter A., Ewers M., Lehnert H., et al. Oxytocin’s inhibitory effect on food intake is stronger in obese than normal-weight men. Int. J. Obes. (Lond). 2016;40(11):1707–1714. doi: 10.1038/IJO.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Spetter M.S., Feld G.B., Thienel M., Preissl H., Hege M.A., Hallschmid M. Oxytocin curbs calorie intake via food-specific increases in the activity of brain areas that process reward and establish cognitive control. Sci. Rep. 2018;8(1):2736. doi: 10.1038/S41598-018-20963-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kerem L., Hadjikhani N., Holsen L., Lawson E.A., Plessow F. Oxytocin reduces the functional connectivity between brain regions involved in eating behavior in men with overweight and obesity. Int. J. Obes. (Lond). 2020;44(5):980–989. doi: 10.1038/S41366-019-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.van der Klaauw A.A., Ziauddeen H., Keogh J.M., Henning E., Dachi S., Fletcher P.C., et al. Oxytocin administration suppresses hypothalamic activation in response to visual food cues. Sci. Rep. 2017;7(1):4266. doi: 10.1038/S41598-017-04600-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Plessow F., Marengi D.A., Perry S.K., Felicione J.M., Franklin R., Holmes T.M., et al. Effects of intranasal oxytocin on the blood oxygenation level-dependent signal in food motivation and cognitive control pathways in overweight and obese men. Neuropsychopharmacology. 2018;43(3):638–645. doi: 10.1038/NPP.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Plessow F., Marengi D.A., Perry S.K., Lawson E.A. Oxytocin administration increases proactive control in men with overweight or obesity: a randomized, double-blind, placebo-controlled crossover study. Obesity (Silver Spring) 2021;29(1):56–61. doi: 10.1002/OBY.23010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang H., Wu C., Chen Q., Chen X., Xu Z., Wu J., et al. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLOS ONE. 2013;8(5) doi: 10.1371/JOURNAL.PONE.0061477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Bickel W.K., Jarmolowicz D.P., Mueller E.T., Gatchalian K.M., McClure S.M. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacol. (Berl). 2012;221(3):361–387. doi: 10.1007/S00213-012-2689-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Carnell S., Gibson C., Benson L., Ochner C.N., Geliebter A. Neuroimaging and obesity: current knowledge and future directions. Obes. Rev. 2012;13(1):43–56. doi: 10.1111/J.1467-789X.2011.00927.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Le D.S.N.T., Pannacciulli N., Chen K., del Parigi A., Salbe A.D., Reiman E.M., et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am. J. Clin. Nutr. 2006;84(4):725–731. doi: 10.1093/AJCN/84.4.725. [DOI] [PubMed] [Google Scholar]
- 203.Le D.S., Pannacciulli N., Chen K., Salbe A.D., Del Parigi A., Hill J.O., et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am. J. Clin. Nutr. 2007;86(3):573–579. doi: 10.1093/AJCN/86.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Uher R., Yoganathan D., Mogg A., Eranti S.v., Treasure J., Campbell I.C., et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biol. Psychiatry. 2005;58(10):840–842. doi: 10.1016/J.BIOPSYCH.2005.05.043. [DOI] [PubMed] [Google Scholar]
- 205.Kekic M., McClelland J., Campbell I., Nestler S., Rubia K., David A.S., et al. The effects of prefrontal cortex transcranial direct current stimulation (tDCS) on food craving and temporal discounting in women with frequent food cravings. Appetite. 2014;78:55–62. doi: 10.1016/J.APPET.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 206.Fregni F., Orsati F., Pedrosa W., Fecteau S., Tome F.A.M., Nitsche M.A., et al. Transcranial direct current stimulation of the prefrontal cortex modulates the desire for specific foods. Appetite. 2008;51(1):34–41. doi: 10.1016/J.APPET.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lapenta O.M., di Sierve K., de Macedo E.C., Fregni F., Boggio P.S. Transcranial direct current stimulation modulates ERP-indexed inhibitory control and reduces food consumption. Appetite. 2014;83:42–48. doi: 10.1016/J.APPET.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 208.Jauch-Chara K., Kistenmacher A., Herzog N., Schwarz M., Schweiger U., Oltmanns K.M. Repetitive electric brain stimulation reduces food intake in humans. Am. J. Clin. Nutr. 2014;100(4):1003–1009. doi: 10.3945/AJCN.113.075481. [DOI] [PubMed] [Google Scholar]
- 209.Heinitz S., Reinhardt M., Piaggi P., Weise C.M., Diaz E., Stinson E.J., et al. Neuromodulation directed at the prefrontal cortex of subjects with obesity reduces snack food intake and hunger in a randomized trial. Am. J. Clin. Nutr. 2017;106(6):1347–1357. doi: 10.3945/AJCN.117.158089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gluck M.E., Alonso-Alonso M., Piaggi P., Weise C.M., Jumpertz-Von Schwartzenberg R., Reinhardt M., et al. Neuromodulation targeted to the prefrontal cortex induces changes in energy intake and weight loss in obesity. Obesity (Silver Spring) 2015;23(11):2149–2156. doi: 10.1002/OBY.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]