Abstract
Background
The iron regulatory hormones erythroferrone (ERFE), erythropoietin (EPO), and hepcidin, and the cargo receptor nuclear receptor coactivator 4 (NCOA4) are expressed in the placenta. However, determinants of placental expression of these proteins and their associations with maternal or neonatal iron status are unknown.
Objectives
To characterize expression of placental ERFE, EPO, and NCOA4 mRNA in placentae from newborns at increased risk of iron deficiency and to evaluate these in relation to maternal and neonatal iron status and regulatory hormones.
Methods
Placentae were collected from 114 neonates born to adolescents carrying singletons (14–18 y) and 110 neonates born to 54 adults (20–46 y) carrying multiples. Placental EPO, ERFE, and NCOA4 mRNA expression were measured by RT-qPCR and compared with maternal and neonatal iron status indicators (SF, sTfR, total body iron, serum iron) and hormones.
Results
Placental ERFE, EPO, and NCOA4 mRNA were detected in all placentae delivered between 25 and 42 wk of gestation. Relationships between placental ERFE and EPO differed by cohort. In the multiples cohort, placental EPO and ERFE were positively correlated (P = 0.004), but only a positive trend (P = 0.08) was evident in the adolescents. Placental EPO and ERFE were not associated with maternal or neonatal iron status markers or hormones in either cohort. Placental NCOA4 was not associated with placental EPO or ERFE in either cohort but was negatively associated with maternal SF (P = 0.03) in the multiples cohort and positively associated with neonatal sTfR (P = 0.009) in the adolescents.
Conclusions
The human placenta expresses ERFE, EPO, and NCOA4 mRNA as early as 25 wk of gestation. Placental expression of ERFE and EPO transcripts was not associated with maternal or neonatal iron status. Greater placental NCOA4 transcript expression was evident in women and newborns with poor iron status (lower SF and higher sTfR, respectively). Further research is needed to characterize the roles of these proteins in the human placenta.
Trial registration number
These clinical trials were registered at clinicaltrials.gov as NCT01019902 (https://clinicaltrials.gov/ct2/show/NCT01019902) and NCT01582802 (https://clinicaltrials.gov/ct2/show/NCT01582802).
Keywords: placenta, anemia, iron deficiency, placental mRNA, nuclear receptor coactivator 4
Introduction
The placenta is a dynamic, fetally derived organ that serves as the sole interface between the mother and fetus. The placenta must respond to competing demands because it supports its own high metabolic requirements while simultaneously transferring nutrients to the fetus in response to maternal and fetal signals [1]. Once imported into the placenta, iron can be used by the placenta, stored as ferritin, or exported to the fetus. Although iron trafficking through the placenta is not fully understood, efficient placental iron transport is essential to support fetal growth and prevent adverse neonatal outcomes [2,3]. Three regulatory hormones (hepcidin, erythropoietin [EPO], and erythroferrone [ERFE]) and the protein nuclear receptor coactivator 4 (NCOA4) are involved in iron homeostasis, but their role in the placenta is not fully understood.
Hepcidin is produced by the liver and considered the master regulator of iron status. Hepcidin decreases circulating iron by inhibiting ferroportin-mediated export of iron from cells [4,5]. Placental hepcidin mRNA has been detected within our adolescent cohort [6]. Previous analysis demonstrated that placental hepcidin mRNA expression was unrelated to the expression of several placental iron transporters and was not significantly associated with maternal or neonatal iron status [6]. In pregnant women with malarial infection, placental hepcidin expression was increased but was not associated with maternal hematological status, suggesting that hepcidin’s antimicrobial activity predominates in the placenta [7]. Nonetheless, more data are needed to understand how placental hepcidin upregulation in the setting of infection or inflammation may impact placental iron dynamics.
Erythropoietin is produced in the kidney and liver in response to hypoxia and increased erythropoietic drive, which then leads to an increase in iron utilization to support erythropoiesis [8,9]. Placental EPO mRNA and protein have been detected in both animal and human placental tissue [[10], [11], [12], [13]], but its role in pregnancy is poorly understood and data on possible relationships between placental EPO and placental iron content or maternal and neonatal iron status are limited. A study in sheep found that placental EPO expression increased significantly under hypoxic conditions [13], and one study in human twin pregnancies (n = 26 placentae) found that placental EPO expression was induced by neonatal hypoxia (diagnosed using umbilical artery Doppler) and severe growth restriction [12].
A third iron-related regulatory hormone, ERFE, is produced by erythroid precursors during stress erythropoiesis and functions to suppress hepcidin production [14]. In mice, placental ERFE mRNA expression was shown to be appreciably lower than that observed in maternal bone marrow or embryonic liver and was not affected by maternal iron status [15]. To our knowledge, only one study evaluated ERFE by immunofluorescence staining in human placentas (n = 26) and found ERFE to be expressed in trophoblast and extravillous cytotrophoblast cells [16]. In addition, ERFE signal intensity was found to be significantly lower in placentas delivered by pregnant individuals with preeclampsia, although the mechanisms of this response are unknown [16]. Expression of ERFE mRNA in human placental tissue and possible associations between this hormone and maternal or neonatal hematological traits and iron status has not yet been evaluated.
The NCOA4 is a cargo receptor that plays an important role in cellular iron homeostasis by mediating the autophagic degradation of ferritin in response to increased intracellular iron demands [17]. Although NCOA4 is a key regulator of cellular iron availability, expression of this cargo receptor in human or mouse placental tissue has not been evaluated, and little is known regarding factors that impact expression of this cargo receptor in the placenta.
The objective of this study was to characterize placental ERFE, EPO, hepcidin, and NCOA4 mRNA expression in a cohort of newborns delivered to women and teens at higher risk of gestational iron deficiency and anemia. Possible relationships between expression of these proteins and maternal and neonatal iron status indicators and iron regulatory hormones were evaluated.
Methods
Participants
Placental tissue was obtained from 2 study cohorts. The first cohort was comprised of placental tissue (n = 114) obtained from placentas delivered by adolescents (aged 14–18 y) carrying singletons (adolescent cohort) [18,19]. The second cohort was comprised of placental tissue (n = 110) obtained from placentas delivered from adult women (aged 20–46 y; n = 54) carrying twins (n = 73) or triplets (n = 37) (multiples cohort) [20,21]. Pregnant adolescents were recruited from the Rochester Adolescent Maternity Program in Rochester, NY, between 2006 and 2012 and women carrying multiples were recruited from Strong Memorial Hospital and Highland Hospital in Rochester, NY from 2011 to 2014. Women carrying multiples were eligible if they were healthy and ≥20 y of age and pregnant adolescents were eligible if they were healthy and carrying a singleton fetus. In all studies, individuals were excluded if they had HIV, eating disorders, pre-existing diabetes, malabsorption diseases, hemoglobinopathies, or other pre-existing medical conditions at the time of enrollment or taking any medications known to potentially impact iron homeostasis. Informed written consent was obtained at baseline from all participants ≥15 y of age, and parental consent and teen assent were obtained from adolescents ≤14 y of age. All studies were approved by the Institutional Review Boards at Cornell University and the University of Rochester.
Demographic information was self-reported upon entry to the study. Gestational age (GA) was determined based on self-reported menstrual history and sonogram data, or by date of in vitro fertilization when applicable. If self-reported menstrual history and sonogram data differed by more than 10 d, ultrasound estimates were used to determine GA. Maternal anthropometric information was recorded at each study visit. At delivery, infant weight and length were recorded and cord blood and placental tissue were obtained. Gestational weight gain was calculated as the difference between final weight at delivery and self-reported prepregnancy weight. As part of their prenatal care, all pregnant individuals were prescribed prenatal supplements containing on average 27 mg of iron and those with anemia received additional iron supplements providing 60–120 mg of iron per day. Information on self-reported prenatal supplement use has been published [19,21,22]. Descriptive data on iron status indicators, regulatory hormones, and inflammatory markers from mothers and neonates within the adolescent cohort and multiples cohort have previously been published [18,19,[21], [22], [23], [24], [25], [26], [27]].
Placental collection
At delivery in the adolescent cohort, placental untrimmed weight and dimensions (length, width, thickness, and volume) were recorded by study staff and in the multiples cohort, placental trimmed weight and dimensions were recorded by pathologists in the University of Rochester Pathology Department. In the multiples cohort, the majority (70%) of placentae were fused; therefore, individual placental weights for fused placentae were estimated by dividing the entire placental mass by the number of placentas. Placental efficiency was calculated as the ratio of placental weight (g):birth weight (g) for each individual placenta and infant [28,29]. Placental samples were taken from the center of the interior of the placental disk, after removing fetal membranes and superficial tissue on the maternal side. Placental tissue samples were excised from multiple cotyledons of each placenta and pooled to obtain a representative sample. The mixture was placed into RNAlater (Ambion) and kept at −80°C until analysis. Placental iron concentration [p[Fe] reported as μg of iron per g of wet placental tissue weight (μg/g wet weight)] of each placenta was analyzed by inductively coupled plasma-mass spectrometry [30,31]. Data on mRNA and/or protein expression of placental non-heme iron-related proteins (transferrin receptor 1 [32]) and heme iron-related proteins (feline leukemia virus subgroup C receptor [33], LDL receptor-related protein 1 [34], the proton-coupled folate transporter [6]), in addition to placental elemental content [30], vitamin D hydroxylase enzymes, and vitamin D receptor [35,36] have been previously measured in these samples.
RT-qPCR
For all placentae, total RNA was extracted using the RNeasy Microarray Tissue Mini Kit (Qiagen) and RNA purity was checked by the ratio of absorbance at 260 and 280 nm on a Nanodrop spectrophotometer (Thermo Scientific). A total of 1 μg RNA was reverse transcribed into cDNA with the transcriptor cDNA synthesis kit (Roche Applied Sciences). All RT-qPCR reactions used a total volume of 10 μL per reaction, which contained 2 μL of the cDNA template, 5 μL of SYBR Green I Master reaction mix, and 0.7 μM of primers. All samples were run in triplicate in 384-well plates on a LightCycler 480 instrument (Roche Applied Sciences). The cycling conditions for ERFE included an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 10 s, annealing at 63°C for 20 s, and extension at 72°C for 15 s. The cycling conditions for EPO involved an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 10 s, annealing at 62°C for 15 s, and extension at 72°C for 20 s. NCOA4 mRNA expression was measured in a subset of samples (n = 98, adolescent cohort; n = 90, multiples cohort). The cycling conditions for NCOA4 consisted of an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 10 s, annealing at 60°C for 10 s, and extension at 72°C for 10 s. The cycling conditions and primer sequences for hepcidin were previously published [6]. Specificity of amplifications was verified by melting curve analysis and only a single peak was observed in all samples. Relative ERFE, EPO, hepcidin, and NCOA4 mRNA expression was normalized to β-actin and compared with that obtained from a control placenta sample using the 2−ΔΔCt method. This method was calculated using the following equations: ΔCt = Ct (EPO, ERFE or hepcidin) – Ct (β-actin); ΔΔCt = ΔCt (sample) – ΔCt (control placenta); and Fold Change = 2−ΔΔCt. The primer sequences for ERFE, hepcidin and β-actin have been previously published [6,14,34]. The primer sequences for EPO and NCOA4 were as follows: EPO forward 5′-AGAATATCACGACGGGCTGT-3′ and EPO reverse 5′-AGGCCCTGCCAGACTTCTAC-3′, NCOA4 forward 5′-GCAGACCTTGGAGAACAGT-3′ and NCOA4 reverse 5′-TCACTCTTGAGGAGCCAGT-3′.
Serum collection and biochemical analysis
Nonfasted maternal blood (15 mL) was collected from women at midgestation [multiples: 21.8 wk (95% CI: 19.7, 24.1); adolescents: 25.9 wk (95% CI: 25.2, 26.7)]. At delivery [multiples: 35.2 wk (95% CI: 34.5, 35.6); adolescents: 40.2 wk (95% CI: 39.7, 40.2)], additional samples of maternal blood and umbilical cord blood (∼15 mL) were collected. Whole blood was sent to the University of Rochester core laboratory for assessment of hemoglobin (Hb) concentrations using a Cell-Dyn 4000 hematology analyzer (Abbott Diagnostics). The remaining blood samples were centrifuged, and serum was stored at −80° C until analysis. Anemia across pregnancy was defined as Hb concentration <11.0 g/dL in the first and third trimesters and <10.5 g/dL in the second trimester [37]. Race adjustments for anemia cutoffs were assessed (Hb <10.2 g/dL in the first and third trimesters and Hb <9.7 g/dL in the second trimester for Black women [37]) but significance of key findings did not change; therefore, nonadjusted values were utilized. Neonatal anemia was defined as umbilical cord Hb concentration <13.0 g/dL [37].
Serum ERFE was measured using a validated ELISA with a stated lower limit of detection (LOD) of 1.5 ng/mL (Intrinsic LifeSciences). The ERFE assay provides quantitative measures of ERFE down to 0.001 ng/mL so absolute values of this hormone were utilized for statistical analyses. Serum EPO was measured by immunoassay (Siemens Immulite 2000). SF and sTfR were measured by ELISA as previously described [18,21]. Iron deficiency was defined if either SF <12 μg/L [38] or sTfR >8.5 mg/L [39]. Total body iron (TBI) was calculated using SF and sTfR [40]. Serum iron was measured by atomic absorption spectrophotometry (Perkin Elmer Aanalyst 800). Folate and vitamin B12 were measured by an Immulite 2000 immunoassay system (Siemens Healthcare). Folate insufficiency was defined as folate concentrations <6.8 nmol/L and vitamin B12 insufficiency was defined as vitamin B12 concentrations <148 pmol/L [41,42]. Hepcidin, CRP, and IL-6 were measured using different assays between cohorts. For the adolescent cohort, hepcidin was measured with an ELISA and IL-6 and CRP were measured using a commercial immunoassay [18]. The LOD for hepcidin was 5 μg/L, and values below were assigned a value of 2.5 μg/L for analysis purposes. For the multiples cohort, hepcidin, CRP, and IL-6 were measured by ELISA as previously described [21]. The hepcidin assay had an LOD of 0.39 ng/mL, and a value of 0.195 was assigned for analysis purposes.
Statistical analysis
Placental characteristics are presented as the median or geometric mean [95% CI] or percent for continuous and categorical outcomes, respectively. The Shapiro-Wilk test was used to assess normality of data, and non-normal variables were log transformed to achieve normality. Student’s t-test and ANOVA were conducted to test whether normally distributed variables differed by maternal cohorts and Wilcoxon’s rank-sum test was used to test statistical differences between nonparametric variables. Chi-square test of independence was used for analysis of differences between categorical variables between cohorts. Linear regression models were constructed to explore associations of placental mRNA expression with placental, neonatal, and maternal variables in each cohort separately. In the multiples cohort, a maternal identification variable was included in the model as a random effect to account for the lack of independence between siblings. Statistical analyses were performed using JMP 14.0 (SAS Institute Inc.). Results of analyses were considered significant if P < 0.05.
Results
Descriptive characteristics and iron status of the study population
Placental, maternal, and neonatal descriptive characteristics are shown in Table 1. The majority (65%) of placentas and neonates from the multiples cohort were delivered preterm (<37 wk of gestation). p[Fe] (heme and nonheme, p[Fe]) (μg/g wet weight) was measured in a subset of placentae (multiples: n = 65, adolescent: n = 77), and p[Fe] (μg/g wet weight) per each placenta did not significantly differ between cohorts (P = 0.3) (Table 1). In the multiples cohort, there was a significantly higher percentage of placentas delivered by women with parity >1 and with a higher prepregnancy BMI compared with the adolescent cohort.
TABLE 1.
Placental, maternal, and neonatal characteristics in adult women carrying multiple fetuses and their neonates and pregnant adolescents and their neonates1
| Multiples cohort | Adolescents cohort | P value | |
|---|---|---|---|
| Placental characteristics | (n = 110) | (n = 114) | |
| Gestational age (wk) | 35.2 [34.5, 35.6] | 40.2 [39.7, 40.2] | <0.001 |
| Placenta weight per fetus2 (g) | 297 [278.2, 316.0] | 586 [577.9, 622.6] | — |
| Sum placental weight2 (g) | 667 [606.9, 738.7] | 586 [577.9, 622.6] | — |
| p[Fe] (μg/g wet weight) | 94.3 [59.3,150.0] | 67.8 [51.8, 88.7] | 0.31 |
| Maternal characteristics | (n = 54) | (n = 114) | |
| Age (y) | 30 [29.4, 32.1] | 17.4 [17.2, 18.1] | <0.001 |
| Race3 (% Black women) | 22 | 64 | <0.001 |
| Ethnicity3 (% Hispanic women) | 7 | 26 | 0.002 |
| ppBMI (kg/m2) | 26.1 [26.4, 31.0] | 23.0 [23.7, 25.8] | 0.001 |
| GWG4 (kg) | 18.6 [17.1, 22.2] | 18.2 [16.7, 19.4] | 0.22 |
| Parity <1 (%) | 37 | 79 | <0.001 |
| Prenatal supplement use5, >2 times/wk (%) | 90 (44/49) | 77 (82/106) | 0.07 |
| Current cigarette use (%) | 6 (3/52) | 7 (7/107) | 0.80 |
| Delivery anemia6 (%) | 44 | 33 | 0.20 |
| Neonatal characteristics | (n = 110) | (n = 114) | |
| Birth weight (kg) | 2.2 [2.1, 2.3] | 3.3 [3.2, 3.4] | <0.001 |
| Birth length (cm) | 47.0 [44.7, 46.4] | 51.5 [50.9, 51.8] | <0.001 |
| Sex (% male) | 43 | 51 | 0.19 |
| C-section (%) | 73 | 11 | <0.001 |
| Placenta weight/birth weight2 | 0.13 [0.13, 0.15] | 0.18 [0.18, 0.19] | — |
| Anemic7 (%) | 14 | 25 | 0.09 |
Data presented as median [95% CI] or %. GWG, gestational weight gain; ppBMI, prepregnancy body mass index; p[Fe], placental iron concentration.
Differences between placental weight or placental efficiency were not compared between cohorts because placental weight measurements in the adolescent cohort were taken before trimming and in the multiples cohort after trimming took place.
Self-reported maternal race (Black individual or White individual) and ethnicity (Hispanic individual or non-Hispanic individual).
Gestational weight gain categories were determined using the Institute of Medicine categories with adjustment for gestational age at delivery. For the adolescent cohort, recommended gestational weight gain was 12.7–18.1 kg for underweight women, 11.3–15.9 kg for normal-weight women, 6.8–11.3 kg for women with overweight, and 5.0–9.1 kg (11–20 lb) for women with obesity. For the multiples cohort, recommended gestational weight gain was 22.7–28.1 kg for underweight women, 16.8–24.5 kg for normal-weight women, 14.1–22.7 kg for women with overweight, and 11.3–19.1 kg for women with obesity.
Self-reported use of prenatal supplements that typically contained 27 mg of iron per day or intake of 2 pediatric chewable supplements that provided 20 mg of iron per day.
Prevalence of anemia at delivery. Maternal anemia was defined as hemoglobin concentration <11.0 g/dL in the first and third trimesters and <10.5 g/dL in the second trimester [37].
Neonatal anemia was defined as umbilical cord Hb concentration <13.0 g/dL [37].
Data on maternal iron status at midgestation and delivery are presented in Supplemental Table 1 and data on neonatal iron status are presented in Supplemental Table 2. The prevalence of maternal anemia and iron deficiency (defined as SF <12 μg/L or sTfR >8.5 mg/L) at midgestation and delivery did not differ between the multiples and adolescent cohorts (Supplemental Table 1). Of note, the prevalence of maternal anemia in both cohorts was 5–8 times higher than the reported United States national average [43] as expected given that multiple pregnancies and teen pregnancies are 2 higher-risk obstetric populations. The prevalence of neonatal anemia did not differ between cohorts, but mean neonatal Hb concentration was higher in multiple birth neonates compared with the singletons (Supplemental Table 2).
Placental ERFE and EPO mRNA expression
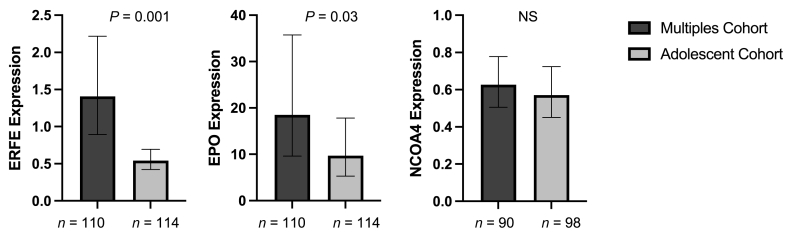
Placental ERFE and EPO mRNA were detected in all placental samples analyzed from deliveries that occurred across 25–42 wk of gestation. Placental ERFE mRNA expression was significantly higher in the multiples cohort compared with the adolescent cohort (P = 0.001) (Figure 1), even after controlling for GA at delivery (P = 0.004). Placental EPO mRNA expression was not significantly different between cohorts (P = 0.2) (Figure 1).
FIGURE 1.
Fold change expression of placental hormones and NCOA4 (ddCT) in placental tissue obtained from neonates born to women carrying multiples or adolescents carrying singletons. Data presented as geometric mean [95% CI]. EPO, erythropoietin; ERFE, erythroferrone; NCOA4, nuclear receptor coactivator 4; NS, not significantly different (P > 0.05).
ERFE expression is known to be regulated by EPO [14]. In the multiples cohort, there was a significant positive relationship between placental EPO and ERFE (β = 0.2, P = 0.004, n = 110) which was not evident in the adolescent cohort (β = −0.07, P = 0.08, n = 114). In both cohorts, placental ERFE and EPO did not significantly differ as a function of placental weight per fetus, neonatal birth weight, placental efficiency, or neonatal sex (all P > 0.05). In the multiples cohort, placental ERFE and EPO mRNA was not impacted by chorionicity (ERFE: P = 0.9, EPO: P = 0.5) or amnionicity (ERFE: P = 0.4, EPO: P = 0.3) and did not differ between fused and discrete placentae (ERFE: P = 0.1, EPO: P = 0.3).
In the adolescent cohort, placental ERFE mRNA expression increased over the observed GA range at delivery in this cohort (34–42 wk, β = 0.1, P = 0.002), but this relationship was not evident in the multiples cohort across the observed GA at delivery (25–38 wk, P = 0.9). This relationship was not evident even when the gestational window was restricted to 34–38 wk to more closely match that of the adolescent cohort (P = 0.5). Placental ERFE did not significantly differ between Black and White mothers (multiples: P = 0.2, adolescents: P = 0.1). Placental ERFE mRNA expression was higher in multiparous compared with primiparous individuals (P = 0.04) in the multiples cohort, but this difference was not observed in the adolescent cohort (P = 0.3).
Placental EPO expression did not significantly change as a function of GA in either cohort (multiples: P = 0.8, adolescents: P = 0.1). However, placental EPO expression significantly differed between Black and White mothers in both cohorts (multiples: P = 0.02, adolescents: P = 0.03). Placental EPO did not differ as a function of parity in either group (both P = 0.4).
Placental hormone relationships in women carrying multiples
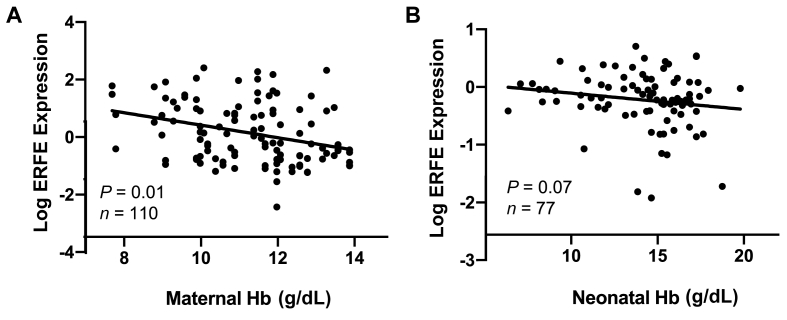
Within the multiples cohort, higher placental ERFE expression was significantly associated with lower maternal Hb concentration at delivery (β = −0.2, P = 0.01, n = 110) (Figure 2). Placental EPO expression showed a nonsignificant inverse trend with Hb concentration at delivery (β = −0.2, P = 0.06, n = 110). This trend, however, appeared to be driven by differences between Black mothers and White mothers (P = 0.4). Placental EPO and ERFE mRNA expression was not significantly associated with maternal or neonatal iron status indicators (SF, serum sTfR, TBI, and serum iron), regulatory hormones (serum EPO, ERFE, and hepcidin), or inflammatory markers (CRP and IL-6) evaluated (all P > 0.05). Placental ERFE expression increased as p[Fe] (μg/g wet weight) increased (β = 0.5, P = 0.004, n = 65), whereas placental EPO was not significantly associated with p[Fe] (P = 0.8).
FIGURE 2.
Placental ERFE fold change expression as a function of maternal and neonatal hemoglobin in placental tissue obtained from neonates born to women carrying multiples (A) or adolescents carrying singletons (B). ERFE, erythroferrone; Hb, hemoglobin.
Placental hormone relationships in adolescents carrying singletons
Relationships between EPO and ERFE mRNA expression in placentae from adolescents carrying single fetuses differed from associations observed in the multiples cohort. In the adolescent cohort, placental EPO was significantly positively associated with maternal serum ERFE concentrations at midgestation (β = 0.3, P = 0.005, n = 92) and delivery (β = 0.3, P = 0.02, n = 70) and with Hb concentration at delivery (β = 0.3, P = 0.03, n = 110). After adjustment for maternal race, the association of placental EPO mRNA and maternal Hb was no longer significant (P = 0.1). Placental EPO mRNA was not associated with maternal serum EPO or hepcidin measured at midgestation or delivery, nor was it associated with any of the neonatal iron regulatory hormones measured (serum EPO, ERFE, or hepcidin) (all P > 0.05). Placental EPO mRNA was not associated with maternal or neonatal iron status indicators (SF, serum sTfR, TBI, and serum iron) or inflammatory markers (CRP and IL-6) evaluated, or with neonatal Hb (all P > 0.05).
Placental ERFE expression demonstrated a nonsignificant inverse association with neonatal Hb (β = −0.04, P = 0.07, n = 77) (Figure 2). In contrast to the findings in the multiples cohort, placental ERFE expression was not significantly associated with maternal Hb in the adolescents (P = 0.4). In addition, in the adolescent cohort placental ERFE was significantly positively associated with neonatal serum EPO (β = 0.2, P = 0.002), although this relationship was no longer significant after controlling for delivery GA (β = 0.1, P = 0.06). No significant associations were identified when placental ERFE mRNA was evaluated in relation to other maternal or neonatal iron status indicators (SF, serum sTfR, TBI, and serum iron) or inflammatory markers (CRP and IL-6) evaluated (all P > 0.05).
In the subset of adolescents with data on placental hepcidin mRNA expression (n = 103), hepcidin mRNA expression was not significantly associated with placental ERFE expression (β = 0.2, P = 0.3) or placental EPO expression (β = 0.02, P = 0.9). Furthermore, maternal and neonatal serum ERFE levels were not associated with placental hepcidin mRNA expression. Finally, neither placental EPO nor placental ERFE were associated with p[Fe] in the adolescent cohort (EPO: P = 0.4, ERFE: P = 0.8; n = 77).
Placental NCOA4 mRNA expression and relationships
Placental NCOA4 was detected in all placentae delivered between 25 and 42 wk of gestation. Placental NCOA4 expression did not significantly differ between the multiples cohort and adolescent cohort (P = 0.6). In both cohorts, placental NCOA4 was not associated with GA at delivery, placental weight per fetus, neonatal birth weight, placental efficiency, or neonatal sex, nor did it differ as a function of maternal age, race, parity, and prepregnancy BMI (all P > 0.05). In the multiples cohort, placental NCOA4 mRNA was not impacted by chorionicity or amnionicity (both P = 0.8) and did not differ between fused and discrete placentae (P = 0.9).
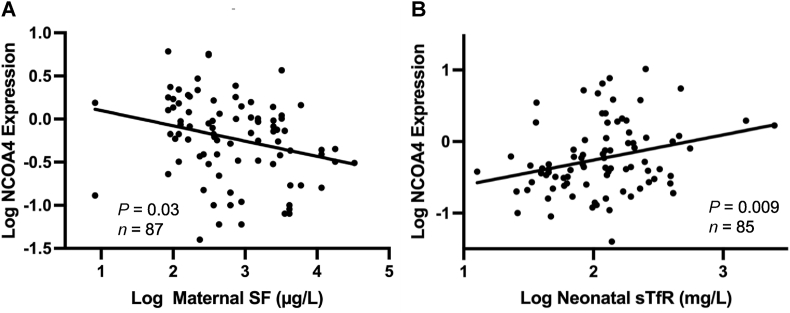
In the multiples cohort, higher placental NCOA4 expression was associated with lower maternal Hb concentration at midgestation (β = −0.09, P = 0.04, n = 87) and impaired iron status as evidenced by lower SF at midgestation (β = −0.2, P = 0.03, n = 87) (Figure 3). A nonsignificant inverse trend was observed between placental NCOA4 mRNA and cord Hb levels in neonates born to adult women carrying multiples (β = −0.03, P = 0.09, n = 82). Of the 3 iron regulatory hormones evaluated, placental NCOA4 was positively associated with maternal ERFE (β = 0.3, P = 0.01, n = 65) and EPO at delivery (β = 0.2, P = 0.008, n = 76), but no association was observed with maternal hepcidin at midgestation (P = 0.4, n = 87) or delivery (P = 0.7, n = 78). In the multiples cohort also, placental NCOA4 mRNA expression was not associated with placental ERFE mRNA (P = 0.4, n = 98), EPO mRNA (P = 0.2, n = 98), or p[Fe] (P = 0.9, n = 61).
FIGURE 3.
Placental NCOA4 fold change expression as a function of maternal and neonatal iron status in placental tissue obtained from neonates born to women carrying multiples (A) or adolescents carrying singletons (B). NCOA4, nuclear receptor coactivator 4.
In the adolescent cohort, placental NCOA4 was more strongly associated with neonatal rather than maternal iron status indicators and hormones. Higher placental NCOA4 expression was associated with higher cord sTfR (β = 0.3, P = 0.009, n = 85) (Figure 3) and higher cord ERFE (β = 0.3, P = 0.03, n = 73), and these associations remained after controlling for GA at delivery (P = 0.006 and P = 0.03, respectively). Of the maternal iron status indicators and regulatory hormones measured, only a weak positive association between placental NCOA4 and maternal ERFE at delivery was observed (β = 0.2, P = 0.05, n = 71). In contrast to the lack of associations observed in the multiples cohort, in the adolescents, placental NCOA4 showed a strong inverse association with p[Fe] (β = −0.4, P = 0.002, n = 72). However, there was no association between placental NCOA4 expression and placental EPO (P = 0.7, n = 90) or ERFE (P = 0.2, n = 90) mRNA expression.
Discussion
To our knowledge, this is the first study to measure placental ERFE, EPO, and NCOA4 mRNA expression in humans and to assess relationships between these measures and placental iron content, maternal, and neonatal iron status and hematological parameters in 2 groups of mothers and newborns at higher risk of anemia and iron deficiency. We demonstrated that all 3 iron regulatory hormones (EPO, ERFE, and hepcidin) are expressed in placental tissue as early as 25 wk of gestation. Although the biological role of the placental expression of ERFE, hepcidin, EPO, and NCOA4 or the placental cell type that produces these remains unclear, understanding their expression and regulation in the placenta sets the stage for future mechanistic investigations and explorations of their potential usefulness as biomarkers of iron homeostasis in pregnancy.
In placental tissue collected from newborns born to women carrying multiples, placental expressions of EPO and ERFE were increased when maternal Hb concentrations were low, as would be expected based on the known regulatory mechanism linking anemia to EPO, and EPO to ERFE. Likewise, in the placentae obtained from newborns born to adolescents carrying single fetuses, placental ERFE expression showed a trend for an inverse association with neonatal Hb, and placental EPO showed a significant positive association with maternal serum ERFE. These findings suggest that placental expression of these hormones is partly driven by erythropoietic activity in both cohorts.
Consistent with murine data [15], placental ERFE mRNA was not significantly associated with maternal or neonatal iron status biomarkers. In systemic circulation these hormones are associated with iron status and erythropoietic activity, but in placental tissue, the mRNA expression of these hormones was not significantly related to maternal or neonatal iron status. The lack of any significant associations between transcript expression of these known iron regulatory hormones and the iron status indicators measured may indicate that other known functions of these hormones, such as erythropoietic [44] and antimicrobial activity [45], predominate in the placenta.
The placenta is the sole interface for iron transfer to the developing fetus, but the amount of iron devoted to fetal needs must be balanced against the iron requirements needed to support placental functions and maternal needs. Consistent with animal data [46] and recent data in adult women [31], the placenta maintains a relatively constant placental iron content even in adult pregnant individuals with lower iron and hematologic status. The current study also found a nonsignificant trend for higher placental expression of EPO in adult women with lower Hb levels, suggesting low oxygen levels may increase the placental expression of this hypoxia-driven hormone. In contrast, placental sequestration of iron in the face of maternal anemia was not evident in placental tissue obtained from adolescents. In the adolescent cohort, placental EPO expression and p[Fe] [31] were positively associated with maternal Hb concentrations and iron status, respectively. Of note, the mean p[Fe] in both the multiples and adolescent cohorts was similar to the mean p[Fe] reported in other pregnant populations with varying risk factors [31]. These findings of altered iron partitioning in biologically immature gravida are consistent with findings from adolescent sheep models that have found that nutrients are prioritized in support of maternal growth over the developing fetus [47,48].
Hypoxia increases EPO production, and EPO in turn stimulates erythroblasts to produce ERFE [14]. Associations between placental ERFE and maternal Hb differed between cohorts. In adult women carrying multiples, maternal Hb concentration was significantly negatively associated with placental ERFE expression, whereas this association was not evident in the adolescents. In the adolescent cohort, placental ERFE exhibited a positive association with neonatal EPO and a suggestive negative trend with neonatal Hb. These results further suggest that there are likely other unidentified factors that differ between these 2 obstetric cohorts that impact placental ERFE expression.
Both cellular hypoxia and iron deficiency stimulate cellular NCOA4 expression, a known cargo receptor that mediates iron release from ferritin via ferritinophagy [49]. Consistent with known regulation of NCOA4 expression in other tissues, placental NCOA4 expression was inversely associated with maternal or neonatal iron status. In the multiples cohort, placental NCOA4 expression was higher in women with poor iron status (lower Hb and SF and higher ERFE and EPO), and in the adolescent cohort, higher placental NCOA4 expression was observed in neonates with poor iron status (higher sTfR and ERFE) and lower p[Fe]. Although NCOA4 was not associated with maternal iron status in the adolescents, previous findings in pregnant adolescents have shown a significantly lower p[Fe] in adolescents with poor iron status [31]. Increased placental NCOA4 expression was consistently associated with poor iron status across cohorts, but its expression was more strongly associated with maternal iron status indicators and hormones in the multiples cohort and with neonatal iron status indicators and hormones in the adolescents. These findings further suggest differing regulatory mechanisms or factors between these 2 populations that are yet to be identified.
There are limitations to our investigation that need to be considered. Measurement of mRNA concentrations evaluates gene expression, but regulation of gene expression can occur post-transcriptionally and translationally. EPO [50,51] and NCOA4 [17] are known to be regulated at both the transcriptional and post-transcriptional levels and little is known about whether ERFE is also regulated at both the transcriptional or post-transcriptional levels [44]. We did not measure placental protein concentrations and do not know if placental mRNA transcript expression and protein abundance are related in these cohort. In addition, placental tissue is composed of syncytiotrophoblasts and other cell types including macrophages and dendritic cells, and it is yet unknown which cells are responsible for the production of these regulatory proteins or how potentially variable cell-type composition between samples may affect study findings. Both cohorts studied are at greater risk for maternal anemia, and the racial and ethnic composition differed between cohorts with more minorities among the adolescents, both of which may limit the generalizability of findings. Lastly, although the data from the adolescent cohort support existing evidence of a competition for nutrients in biologically immature gravida, possible effects of sociodemographic characteristics and diet composition that likely differ in pregnant adolescents cannot be excluded.
In conclusion, these data demonstrate that the human placenta expresses EPO, ERFE, hepcidin, and NCOA4 as early as 25 wk of gestation. No strong consistent relationships were evident between placental ERFE or EPO transcripts and any of the measured markers of iron status in the mother, placenta, or neonate. Consistent with known regulation of NCOA4 expression, placental NCOA4 mRNA was inversely associated with maternal, neonatal, and placental iron status but determinants differed between the 2 cohorts studied. More work is needed to characterize the role of these proteins in placental tissue and evaluate these in relation to maternal characteristics given the different associations noted between the pregnant adolescents and adult women carrying multiples.
Author contributions
The authors’ responsibilities were as follows—KMD, AB: conducted research, analyzed and interpreted the data, and wrote the manuscript; KOO: designed and conducted research, analyzed and interpreted the data, and wrote the manuscript; RG, EKP: were responsible for the clinical implementation of the studies, and assisted with the design of the research and analysis and interpretation of the data and with the preparation of the manuscript; EN, TG: assisted with the design of the research and analysis and interpretation of the data and with the preparation of the manuscript; LFC, CMH, PJK: conducted research and acquired the data; and all authors: read and approved the final manuscript.
Data availability
Data described in the article, code book, and analytic code will not be made available because of the composition of the patient population and the confidential nature of the data collected.
Funding
The United States NIH NICHD Grant 1R21HD098864, The NIH NIDDK Grant T32-DK007158, and The Gerber Foundation and USDA grants 2005-35200-15218 and 2009-35200-05171.
Author disclosures
KMD, AB, LFC, CMH, RG, EKP, PJK, and KOO, no conflicts of interest. TG and EN are scientific founders of Intrinsic LifeSciences and Silarus Pharma, companies that have interests related to ERFE.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tjnut.2023.05.023.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sangkhae V., Nemeth E. Placental iron transport: the mechanism and regulatory circuits. Free Radic. Biol. Med. 2018;133:254–261. doi: 10.1016/j.freeradbiomed.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Georgieff M.K. Iron deficiency in pregnancy. Am. J. Obstet. Gynecol. 2020;223(4):516–524. doi: 10.1016/j.ajog.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgieff M.K. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem. Soc. Trans. 2008;36(Pt 6):1267–1271. doi: 10.1042/BST0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camaschella C., Nai A., Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105(2):260–272. doi: 10.3324/haematol.2019.232124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeth E., Ganz T. The role of hepcidin in iron metabolism. Acta. Haematol. 2009;122(2–3):78–86. doi: 10.1159/000243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Best C.M., Pressman E.K., Cao C., Cooper E., Guillet R., Yost O.L., et al. Maternal iron status during pregnancy compared with neonatal iron status better predicts placental iron transporter expression in humans. FASEB J. 2016;30(10):3541–3550. doi: 10.1096/fj.201600069R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muehlenbachs A., Fried M., Lachowitzer J., Mutabingwa T.K., Duffy P.E. Genome-wide expression analysis of placental malaria reveals features of lymphoid neogenesis during chronic infection. J. Immunol. 2007;179(1):557–565. doi: 10.4049/jimmunol.179.1.557. [DOI] [PubMed] [Google Scholar]
- 8.Gammella E., Diaz V., Recalcati S., Buratti P., Samaja M., Dey S., et al. Erythropoietin's inhibiting impact on hepcidin expression occurs indirectly. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015;308(4):R330–R335. doi: 10.1152/ajpregu.00410.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chateauvieux S., Grigorakaki C., Morceau F., Dicato M., Diederich M. Erythropoietin, erythropoiesis and beyond. Biochem. Pharmacol. 2011;82(10):1291–1303. doi: 10.1016/j.bcp.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 10.Conrad K.P., Benyo D.F., Westerhausen-Larsen A., Miles T.M. Expression of erythropoietin by the human placenta. FASEB J. 1996;10(7):760–768. doi: 10.1096/fasebj.10.7.8635693. [DOI] [PubMed] [Google Scholar]
- 11.Fairchild Benyo D., Conrad K.P. Expression of the erythropoietin receptor by trophoblast cellsin the human placenta. Biol. Reprod. 1999;60(4):861–870. doi: 10.1095/biolreprod60.4.861. [DOI] [PubMed] [Google Scholar]
- 12.Chang Y.L., Chao A.S., Peng H.H., Chang S.D., Chen K.J., Cheng P.J., et al. Placental erythropoietin expression is upregulated in growth-restricted fetuses with abnormal umbilical artery Doppler findings: a case-control study of monochorionic twins. BMC Pregnancy Childbirth. 2018;18(1):321. doi: 10.1186/s12884-018-1963-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis L.E., Widness J.A., Brace R.A. Renal and placental secretion of erythropoietin during anemia or hypoxia in the ovine fetus. Am. J. Obstet. Gynecol. 2003;189(6):1764–1770. doi: 10.1016/s0002-9378(03)00874-3. [DOI] [PubMed] [Google Scholar]
- 14.Kautz L., Jung G., Valore E.V., Rivella S., Nemeth E., Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat. Genet. 2014;46(7):678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangkhae V., Yu V., Coffey R., O'Brien K.O., Ganz T., Nemeth E. Erythroferrone contributes to iron mobilization for embryo erythropoiesis in iron-deficient mouse pregnancies. Am. J. Hematol. 2022;97(10):1348–1358. doi: 10.1002/ajh.26680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoumi Z., Hansson L.R., Hansson E., Ahlm E., Mezey E., Erlandsson L., et al. Assessing erythroferrone and iron homeostasis in preeclamptic and normotensive pregnancies: a retrospective study. Placenta. 2023;133:10–18. doi: 10.1016/j.placenta.2023.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santana-Codina N., Mancias J.D. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals (Basel). 2018;11(4):114. doi: 10.3390/ph11040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S., Guillet R., Cooper E.M., Westerman M., Orlando M., Kent T., et al. Prevalence of anemia and associations between neonatal iron status, hepcidin, and maternal iron status among neonates born to pregnant adolescents. Pediatr. Res. 2016;79(1–1):42–48. doi: 10.1038/pr.2015.183. [DOI] [PubMed] [Google Scholar]
- 19.Lee S., Guillet R., Cooper E.M., Westerman M., Orlando M., Pressman E., et al. Maternal inflammation at delivery affects assessment of maternal iron status. J. Nutr. 2014;144(10):1524–1532. doi: 10.3945/jn.114.191445. [DOI] [PubMed] [Google Scholar]
- 20.Ru Y., Pressman E.K., Guillet R., Katzman P.J., Bacak S.J., O'Brien K.O. Predictors of anemia and iron status at birth in neonates born to women carrying multiple fetuses. Pediatr. Res. 2018;84(2):199–204. doi: 10.1038/s41390-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 21.Ru Y., Pressman E.K., Cooper E.M., Guillet R., Katzman P.J., Kent T.R., et al. Iron deficiency and anemia are prevalent in women with multiple gestations. Am. J. Clin. Nutr. 2016;104(4):1052–1060. doi: 10.3945/ajcn.115.126284. [DOI] [PubMed] [Google Scholar]
- 22.Lee S., Young B.E., Cooper E.M., Pressman E., Queenan R.A., Olson C.M., et al. Nutrient inadequacy is prevalent in pregnant adolescents, and prenatal supplement use may not fully compensate for dietary deficiencies, Infant Child Adolesc. Nutr. 2014;6(3):152–159. [Google Scholar]
- 23.Cao C., Pressman E.K., Cooper E.M., Guillet R., Westerman M., O'Brien K.O. Prepregnancy body mass index and gestational weight gain have no negative impact on maternal or neonatal iron status. Reprod. Sci. 2016;23(5):613–622. doi: 10.1177/1933719115607976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinl G.K., Gandelman J.S., Katzman P.J., Ru Y., Guillet R., Pressman E., et al. Umbilical cord coiling in high -risk pregnancies: associations with determinants of adverse birth outcomes and iron status. Pediatr. Dev. Pathol. 2018;21(6):537–547. doi: 10.1177/1093526618770318. [DOI] [PubMed] [Google Scholar]
- 25.Ru Y., Pressman E.K., Guillet R., Katzman P.J., Vermeylen F., O'Brien K.O. Umbilical cord hepcidin concentrations are positively associated with the variance in iron status among multiple birth neonates. J. Nutr. 2018;148(11):1716–1722. doi: 10.1093/jn/nxy151. [DOI] [PubMed] [Google Scholar]
- 26.Ru Y.P., Pressman E.K., Guillet R., Katzman P.J., Bacak S.J., O'Brien K.O. Predictors of anemia at birth in neonates born to women carrying multiple fetuses. Pediatrics. 2018;84(2):199–204. doi: 10.1038/s41390-018-0044-6. [DOI] [PubMed] [Google Scholar]
- 27.Delaney K.M., Guillet R., Fleming R.E., Ru Y., Pressman E.K., Vermeylen F., et al. Umbilical cord serum ferritin concentration is inversely associated with umbilical cord hemoglobin in neonates born to adolescents carrying singletons and women carrying multiples. J. Nutr. 2019;149(3):406–415. doi: 10.1093/jn/nxy286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornburg K.L., Kolahi K., Pierce M., Valent A., Drake R., Louey S. Biological features of placental programming. Placenta. 2016;48(Suppl 1):S47–S53. doi: 10.1016/j.placenta.2016.10.012. Suppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson M.E., Ford S.P. Comparative aspects of placental efficiency. Reprod. Suppl. 2001;58:223–232. [PubMed] [Google Scholar]
- 30.de Angelis P., Miller R.K., Darrah T.H., Katzman P.J., Pressman E.K., Kent T.R., et al. Elemental content of the placenta: a comparison between two high-risk obstetrical populations, adult women carrying multiples and adolescents carrying singletons. Environ. Res. 2017;158:553–565. doi: 10.1016/j.envres.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Barad A., Guillet R., Pressman E.K., Katzman P.J., Miller R.K., Darrah T.H., et al. Placental iron content is lower than previously estimated and is associated with maternal iron status in women at greater risk of gestational iron deficiency and anemia. J. Nutr. 2022;152(3):737–746. doi: 10.1093/jn/nxab416. [DOI] [PubMed] [Google Scholar]
- 32.Young M.F., Pressman E., Foehr M.L., McNanley T., Cooper E., Guillet R., et al. Impact of maternal and neonatal iron status on placental transferrin receptor expression in pregnant adolescents. Placenta. 2010;31(11):1010–1014. doi: 10.1016/j.placenta.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 33.Jaacks L.M., Young M.F., Essley B.V., McNanley T.J., Cooper E.M., Pressman E.K., et al. Placental expression of the heme transporter, feline leukemia virus subgroup C receptor, is related to maternal iron status in pregnant adolescents. J. Nutr. 2011;141(7):1267–1272. doi: 10.3945/jn.110.135798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao C., Pressman E.K., Cooper E.M., Guillet R., Westerman M., O'Brien K.O. Placental heme receptor LRP1 correlates with the heme exporter FLVCR1 and neonatal iron status. Reproduction. 2014;148(3):295–302. doi: 10.1530/REP-14-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Brien K.O., Li S., Cao C., Kent T., Young B.V., Queenan R.A., et al. Placental CYP27B1 and CYP24A1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. J. Clin. Endocrinol. Metab. 2014;99(4):1348–1356. doi: 10.1210/jc.2013-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Young B.E., Cooper E.M., McIntyre A.W., Kent T., Witter F., Harris Z.L., et al. Placental vitamin D receptor (VDR) expression is related to neonatal vitamin D status, placental calcium transfer, and fetal bone length in pregnant adolescents. FASEB J. 2014;28(5):2029–2037. doi: 10.1096/fj.13-246736. [DOI] [PubMed] [Google Scholar]
- 37.CDC Recommendations to prevent and control iron deficiency in the United States. MMWR Recomm. Rep. 1998;47(Rr-3):1–29. [PubMed] [Google Scholar]
- 38.WHO guideline on use of ferritin concentrations to assess iron status in individuals and populations. World Health Organization; Geneva: 2020. [PubMed] [Google Scholar]
- 39.Akesson A., Bjellerup P., Berglund M., Bremme K., Vahter M. Serum transferrin receptor: a specific marker of iron deficiency in pregnancy. Am. J. Clin. Nutr. 1998;68(6):1241–1246. doi: 10.1093/ajcn/68.6.1241. [DOI] [PubMed] [Google Scholar]
- 40.Cook J.D., Flowers C.H., Skikne B.S. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 41.Yetley E.A., Pfeiffer C.M., Phinney K.W., Bailey R.L., Blackmore S., Bock J.L., et al. Biomarkers of vitamin B-12 status in NHANES: a roundtable summary. Am. J. Clin. Nutr. 2011;94(1):313s–321s. doi: 10.3945/ajcn.111.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yetley E.A., Pfeiffer C.M., Phinney K.W., Fazili Z., Lacher D.A., Bailey R.L., et al. Biomarkers of folate status in NHANES: a roundtable summary. Am. J. Clin. Nutr. 2011;94(1):303s–312s. doi: 10.3945/ajcn.111.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mei Z., Cogswell M.E., Looker A.C., Pfeiffer C.M., Cusick S.E., Lacher D.A., et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am. J. Clin. Nutr. 2011;93(6):1312–1320. doi: 10.3945/ajcn.110.007195. [DOI] [PubMed] [Google Scholar]
- 44.Srole D.N., Ganz T. Erythroferrone structure, function, and physiology: iron homeostasis and beyond. J. Cell Physiol. 2021;236(7):4888–4901. doi: 10.1002/jcp.30247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park C.H., Valore E.V., Waring A.J., Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 46.Parrow N.L., Fleming R.E. The selfishly selfless placenta. J. Clin. Invest. 2020;130(2):590–592. doi: 10.1172/JCI134272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace J., Bourke D., Da Silva P., Aitken R. Nutrient partitioning during adolescent pregnancy. Reproduction. 2001;122(3):347–357. doi: 10.1530/rep.0.1220347. [DOI] [PubMed] [Google Scholar]
- 48.Wallace J.M. Competition for nutrients in pregnant adolescents: consequences for maternal, conceptus and offspring endocrine systems. J. Endocrinol. 2019;242(1):T1–T19. doi: 10.1530/JOE-18-0670. [DOI] [PubMed] [Google Scholar]
- 49.Mancias J.D., Wang X., Gygi S.P., Harper J.W., Kimmelman A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGary E.C., Rondon I.J., Beckman B.S. Post-transcriptional regulation of erythropoietin mRNA stability by erythropoietin mRNA-binding protein. J. Biol. Chem. 1997;272(13):8628–8634. doi: 10.1074/jbc.272.13.8628. [DOI] [PubMed] [Google Scholar]
- 51.Ebert B.L., Bunn H.F. Regulation of the erythropoietin gene. Blood. 1999;94(6):1864–1877. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will not be made available because of the composition of the patient population and the confidential nature of the data collected.