Abstract
The cytoplasmic accumulation of exogenous betaine stimulates the growth of Lactococcus lactis cultivated under hyperosmotic conditions. We report that L. lactis possesses a single betaine transport system that belongs to the ATP-binding cassette (ABC) superfamily of transporters. Through transposon mutagenesis, a mutant deficient in betaine transport was isolated. We identified two genes, busAA and busAB, grouped in an operon, busA (betaine uptake system). The transcription of busA is strongly regulated by the external osmolality of the medium. The busAA gene codes for the ATP-binding protein. busAB encodes a 573-residue polypeptide which presents two striking features: (i) a fusion between the regions encoding the transmembrane domain (TMD) and the substrate-binding domain (SBD) and (ii) a swapping of the SBD subdomains when compared to the Bacillus subtilis betaine-binding protein, OpuAC. BusA of L. lactis displays a high affinity towards betaine (Km = 1.7 μM) and is an osmosensor whose activity is tightly regulated by external osmolality, leading the betaine uptake capacity of L. lactis to be under dual control at the biochemical and genetic levels. A protein presenting the characteristics predicted for BusAB was detected in the membrane fraction of L. lactis. The fusion between the TMD and the SBD is the first example of a new organization within prokaryotic ABC transporters.
As a consequence of the permeability of biological membranes to water, the active adaptation to osmotic changes of the external medium is essential for the maintenance of homeostasis which characterizes living systems. In nonhalophilic bacteria, the most efficient strategy to cope with hyperosmotic conditions consists of the cytoplasmic accumulation of compatible solutes. According to a recent definition, a compatible solute is a “cytoplasmic cosolvent (solute) whose levels can be modulated over a broad range without disrupting cellular function” (51). A large set of organic molecules have been found to possess such a property: they include sugars, amino acids and derivatives, tertiary sulfonium compounds, tetrahydropyrimidines (ectoine), and quaternary ammonium compounds (9). Betaine (N,N,N-trimethylglycine) belongs to the last chemical class. The accumulation of betaine upon an osmotic upshift restores the cellular volume and increases the hydration of the cytoplasm of Escherichia coli cells (6). Furthermore, this compound is excluded from the hydration shell of proteins and stabilizes macromolecules against denaturation through its ability to increase water structure (kosmotrope) (3, 9, 51). These properties make betaine one of the most potent and universally compatible solutes that are used by eukaryotic organisms, archea, and many bacterial species (10, 35). The de novo synthesis of betaine occurs rarely among microorganisms, which have evolved highly efficient osmodependent transport systems dedicated to its accumulation.
The mechanisms of adaptation to hyperosmolality through compatible solute uptake has been extensively studied in E. coli and Salmonella typhimurium, and the data accumulated on these model organisms furnish the basic concepts of osmoadaptation in bacteria (7). Two uptake systems have been described as being responsible for the accumulation of organic compatible solutes: a secondary transporter, ProP, of relatively broad specificity (proline and betaine) and a high-affinity betaine uptake system, ProU, which belongs to the superfamily of ATP-binding cassette (ABC) transporters. The activity of these two transporters is modulated by the external osmolality (8, 38) and the expression of proU increased upon osmotic upshift (26).
Recent studies extended our knowledge of osmoadaptation to gram-positive organisms, and the description of osmodependent uptake capacities for compatible solutes has been accomplished at the molecular level in Bacillus subtilis and Corynebacterium glutamicum. B. subtilis was found to possess four compatible solute uptake systems. OpuA (betaine and related compounds) and OpuC (betaine and ectoine) are ABC-type transport systems (15, 18), while OpuD (betaine) and OpuE (proline) are secondary transporters (17, 49). In C. glutamicum, three secondary transporters have been identified and characterized: BetP (betaine) and EctP (ectoine, betaine, and proline) are sodium-solute symporters, and ProP (proline and ectoine), a member of the major facilitator superfamily, is an H+-compatible solute cotransporter (31, 32). These transport systems provide these soil organisms with a large osmoadaptation capacity.
We aimed to characterize the osmoadaptive capacity of Lactococcus lactis, a low-GC-content gram-positive organism originating from a different ecological habitat. Dairy products are the main origin of the presently known biodiversity of the genus Lactococcus, while plant materials could represent its natural source (41). In both industrial and natural environments, L. lactis encounters osmotic challenges. A previous study reported that the ML3 strain of L. lactis accumulated betaine under hyperosmotic conditions but, intriguingly, the transport system was not found to be induced or activated by the medium osmolality (29).
In the present work, we focused our attention on the betaine uptake capacity of L. lactis NCDO763. We report the characterization of busA, the operon coding for the single high-affinity betaine transport system of L. lactis. The betaine transport capacity of L. lactis was found to be regulated at both the biochemical and genetic levels. Sequence analysis revealed that BusA belongs to the ABC transporter superfamily, but a striking genetic organization was observed: the regions encoding transmembrane- and betaine-binding domains are fused in a single gene. The biochemical consequences of such a feature are discussed.
MATERIALS AND METHODS
Bacterial strains, media, culture conditions, and chemicals.
E. coli TG1 supE hsdΔ5 thi Δ(lac-proAB) F′ (traD36 proAB+ lacIq lacZΔM15) and TG1rep (containing a chromosomal copy of the repA gene) were grown on Luria-Bertani medium (LB) with shaking at 37°C (28). Erythromycin (Em) (150 μg/ml) was added when required. B. subtilis JH642 was grown on LB or LB plus 0.5 M NaCl at 37°C with shaking. L. lactis subsp. cremoris NCDO763 was grown at 30°C in M17 (44) with 0.5% (wt/vol) glucose or in chemically defined medium (CDM) containing vitamins, salts, nucleotides, sodium phosphate buffer (pH 6.5), amino acids, and 0.5% glucose (wt/vol) as described previously (29), except for the presence of cysteine (0.17 g/liter) and the absence of proline unless indicated. When required, CDM was supplemented with 1 mM betaine (0.117 g/liter) or 5.9 mM proline (0.68 g/liter), and the osmolality of the medium was raised by the addition of NaCl or sorbitol. All solutions were sterilized by filtration. Growth rate experiments were performed with a Microbiology Reader Bioscreen C (Labsystems, Helsinki, Finland) in 100-well sterile microplates, each well containing 300 μl of culture medium. The optical density was monitored at 600 nm (OD600). The osmolality levels of the solutions and media were measured by freezing-point depression with a digital micro-osmometer (Hermann Roebling, Berlin, Germany). Betaine and sorbitol were from Sigma (St. Louis, Mo.). Ectoine was from Bitop (Witten, Germany). [1-14C]betaine (specific activity, 55 mCi/mmol) was purchased from Isotopchim (Ganagobie-Peyruis, France).
DNA manipulations.
Plasmid DNA manipulation and transformation of E. coli TG1 and TG1rep were performed as previously described (40). L. lactis NCDO763 was transformed by electroporation (22). PCR amplifications were performed on 0.1 μg of chromosomal DNA of NCDO763 by using Taq polymerase (Appligene Oncor, Illkirch, France) and a GeneAmp PCR system 2400 (Perkin Elmer Corp., Norwalk, Conn.). The DyeTerminator kit and a 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.) were used for DNA sequencing.
Insertional mutagenesis and selection of an osmosensitive mutant.
Insertional mutagenesis with pGh9:ISS1 (Emr) in L. lactis NCDO763 was performed as described earlier (27). pGh9:ISS1 transposants were selected at 37°C on M17 plates containing 0.5% glucose and 1 μg of Em per ml. The transposition frequency was found to be 3 · 10−3. Southern hybridization analysis of 12 Emr clones indicated that transposition occurred randomly and that 9 of them (75%) were monocopy transposition events. We identified osmosensitive mutants by their inability to grow on replica CDM plates (1.5% [wt/vol] agar) containing 0.6 M NaCl and 1 mM betaine. The screening of 5,000 transposants led to the isolation of 42 osmosensitive mutants, including the betaine uptake-deficient mutant OSM35. To isolate the stable mutant OSM35 (Ems), the integrated vector was excised as described earlier (27). A single transposition event and plasmid excision were confirmed by Southern hybridization analysis.
Cloning and sequencing of busA.
Chromosomal sequences upstream and downstream of the inserted plasmid pGh9:ISS1 were recovered by plasmid rescue. OSM35 genomic DNA was digested with PstI or HindIII, and ligated DNA was transformed in E. coli TG1rep. This procedure permitted the isolation of chromosomal L. lactis DNA fragments of 3 and 0.3 kb upstream and downstream of the pGh9:ISS1 insertion, respectively. L. lactis NCDO763 DNA fragments were sequenced with oligonucleotide primers designed in the transposon. Data of the diagnostic sequence of the genome of L. lactis subsp. lactis IL1403 were used to design a primer in the 3′ part of the operon (4). After the sequencing of PCR products, a pair of oligonucleotide primers, OSM-17 (GTT GTT GCA ATT TTA CAG AAT GAA G) and OSM-3446 (TCA CTG AGA TTT TCT TAG TTA ACT C), were designed for PCR amplification of the whole region. PCR amplification gave rise to a unique band of 3.4 kb (cycling conditions: 94°C for 1 min, 62°C for 1 min, and 72°C for 3 min 30 s for 30 cycles). The purified PCR product was cloned into pGEM-T Easy vector (Promega Corp., Madison, Wis.) and amplified in E. coli TG1. Sequence data were confirmed on two independent clones sequenced on both strands.
Site-directed inactivation of busA.
busA disruption mutation was independently reconstructed through plasmid integration. A 0.98-kb fragment (positions 586 to 1564) of busAA was cloned into the KpnI site of the integrative vector pORInewlux (Emr) (39), a derivative of pORI5 (23). The resulting construction, pTIL451, was used to transform L. lactis NCDO763. Emr transformants, which harbored a chromosomal copy of pTIL451, were obtained. The disruption of the busA operon, located after codon 81 of busAA, was verified by PCR amplification and Southern hybridization, and a single strain designated TIL451 was selected. TIL451 and OSM35 displayed the same phenotype, and TIL451 was deficient in betaine transport capacity.
Northern blot analysis.
L. lactis NCDO763 was grown in M17 containing 0.5% glucose at 30°C up to an OD600 of 0.5. The culture was split into two aliquots of 35 ml, and 5 ml of either water or 2.4 M NaCl (0.3 M, final concentration) was added. After a further incubation of 40 min at 30°C, total RNA was isolated (2). Then, 10 μg of RNA denatured by the addition of glyoxal was separated on a 1% agarose gel and transferred to a Hybond-N+ membrane (Amersham, Uppsala, Sweden). The membrane was probed with the oligonucleotide EXT1 (GTT CAA TTT TGA CTT TTA CTG GCA) labeled with [γ-32P]ATP by using T4 kinase (Gibco/BRL, Eggenstein, Germany) (40). Washing and autoradiography were performed under standard conditions (40).
Preparation of membrane fractions.
L. lactis cells were grown in M17 without or with 0.3 M NaCl up to an OD600 of 1. Cells were harvested by centrifugation at 5,000 × g for 10 min and washed twice with 25 mM Tris-HCl (pH 8.0). The cell pellet was resuspended in 25 mM Tris-HCl (pH 8.0) at a final OD600 of 10. The cells were disrupted with a cell disrupter at a pressure of 2,700 bars (Constant Systems Ltd., Kenilworth, United Kingdom). The homogenate was centrifuged at 5,000 × g for 15 min to remove unbroken cells. The supernatant was subjected to ultracentrifugation at 100,000 × g for 1 h. The pellet corresponding to the membrane fraction was resuspended at a protein concentration of 2 to 4 mg/ml in 25 mM Tris-HCl (pH 8.0) and stored at −20°C until use. Total cell extracts of B. subtilis JH642 were prepared as described earlier (18).
Electrophoresis and Western blotting.
To allow the solubilization of the membrane proteins before electrophoresis, 30 μg of protein was resuspended in 20 μl of 25 mM Tris-HCl (pH 8.0)–1% Triton X-100 containing 1 mM phenylmethylsulfonyl fluoride and incubated for 3 h at room temperature. Then, 20 μl of twice-concentrated sample buffer (4 M urea, 2% sodium dodecyl sulfate [SDS], 0.02% Coomassie blue, and 10% glycerol) was added. Samples were applied onto a SDS-polyacrylamide gel (3.5%-12.5%) (21), and electrophoresis was performed by using a Bio-Rad Miniprotean II cell (Bio-Rad Laboratories, Ltd., Hercules, Calif.). Proteins were then transferred onto a 0.45-μm-pore-size nitrocellulose membrane (BA85; Schleicher & Schuell) in ice-cold transfer buffer (25 mM Tris; 192 mM glycine; 20% [vol/vol] methanol; 0.02% [wt/vol] SDS, pH 8.3) at 80 mA of constant current for 2 h. The membrane was blocked for 1 h at room temperature in 10 ml of PLT (10 mM sodium phosphate, pH 7.4; 145 mM NaCl; 0.2% Tween 20; 3% low-fat milk) and incubated for 1 h at room temperature with a 1:5,000 dilution of a rabbit antiserum raised against the purified OpuAC protein from B. subtilis (19). The membrane was washed twice for 10 min with 10 ml of PLT and incubated with 2 μl of horseradish peroxidase-coupled second goat anti-rabbit antibody (1:5,000, final dilution) (Bio-Rad) in 10 ml of phosphate-buffered saline (PBS; 10 mM sodium phosphate, pH 7.4; 145 mM NaCl) for 1 h. The membrane was washed twice in PBS for 5 min. Detection of the formed complexes was accomplished with the Bio-Rad Opti-4CN substrate kit. A Bio-Rad prestained SDS-polyacrylamide gel electrophoresis (PAGE) standard was used as a molecular weight marker.
Analysis and quantification of accumulated amino acids and betaine.
Cells were grown to exponential phase in CDM with the indicated additives. Samples (2 to 4 ml) corresponding to 1 mg of protein were filtered through 0.45-μm-pore-size prewetted cellulose acetate filters (MillexHA; Millipore, Bedford, Mass.) by using a vacuum manifold 1225 connected to a vacuum pump (−600 mm Hg). Immediately after filtration, filters were washed with 5 ml of 200 mM sodium phosphate buffer with 0.5% glucose (pH 6.5), isoosmotic to CDM and complemented with NaCl at a final osmolality corresponding to that of the growth medium. Filters were extensively dried and resuspended in 0.4 ml of HCl at 4 mM. Volumes of 0.3 ml were withdrawn and briefly centrifuged to remove unbound cells. Supernatants (0.25 ml) were used for either betaine or amino acid analysis. For the analysis and quantification of amino acids, samples were diluted 1/1 in 0.16 N lithium acetate buffer (pH 2.2), and 50 μl was injected onto an amino acid analyzer, the Biotronix LC 3000 (Biotronik, Hamburg, Germany). Betaine was quantified by high-pressure liquid chromatography (HPLC) analysis on a Waters system (Milford). Then, 50 μl of the supernatant was injected into a strong cation-exchanger column (Protein-Pak SP 8HR, 100 by 5 mm; Waters). The sample elution was isocratic in HCl at 4 mM at a flow rate of 0.8 ml/min at 40°C. UV detection was performed at 195 nm, and betaine was found to elute at a retention time of 11 min (capacity factor, k′ = 4.8). Quantification was obtained from a standard curve. This method was also used to assess the absence of betaine in sorbitol solutions. Alternatively, intracellular accumulation of betaine was measured by filtration experiments on cells grown in the presence of [1-14C]betaine (1 mM; specific activity, 0.1 mCi/mmol). Cells (3 OD600 U, equivalent to 0.6 mg of protein) were harvested during the culture and immediately filtered as described above. Filters were immediately washed with 5 ml of isoosmotic buffer and then dried, and the radioactivity was counted by liquid scintillation. Both methods were found to give the same results.
Betaine transport assays.
Cells were grown in CDM with additives (NaCl, sorbitol, betaine, and proline) as indicated in the legends to the figures. Cells were harvested in exponential phase (OD600 = 0.8 to 1) by centrifugation and washed twice in 0.12 osM hypotonic buffer, 50 mM MES [2-(N-morpholino)ethanesulfonic acid]–NaOH (pH 6.5), at 4°C and containing 0.5% (wt/vol) glucose, which was present in all further steps. The presence of a metabolizable carbon source (glucose or lactose) was found to be essential for the betaine transport activity. Cells were resuspended at a protein concentration of 0.6 to 1 mg/ml in the same buffer and kept on ice. Prior to transport experiments, the cells were diluted (0.04 to 0.1 mg of protein per ml) in the same buffer containing chloramphenicol (50 μg/ml). Cells were preincubated at 30°C for 5 min. Transport was initiated by the addition of [1-14C]betaine simultaneously with NaCl or sorbitol. In uptake experiments to equilibrium, [1-14C]betaine was added at 0.5 mM (0.25 mCi/mmol), and 150-μl portions were withdrawn at the indicated time intervals. For the determinations of initial rates of uptake, [1-14C]betaine was added at a final concentration of 20 μM (2.5 mCi/mmol), and aliquots of 500 μl were filtered at time intervals of 1 min. The rate of betaine uptake was linear for 4 min. For Km determination experiments, the final [1-14C]betaine concentration ranged from 0.2 to 50 μM (2.5 to 55 mCi/mmol). In all experiments, filters were immediately washed after filtration with 5 ml of the corresponding assay buffer and dried, and the radioactivity associated was counted by liquid scintillation. Initial rates of betaine uptake were nonlinearly fitted to the Michaelis-Menten equation with the computer package SigmaPlot 3.0 (Jandel Scientific).
Miscellaneous.
Protein concentrations were determined by the method of Lowry et al. (25) with bovine serum albumin as a standard.
Sequence analysis was performed by using the Wisconsin Package, version 9.1 (Genetics Computer Group, Madison, Wis.). For sequence similarity searches in unfinished microbial genomes, the NCBI BLAST program with microbial genomes page was used (1, 29a). Sequence alignments were performed with CLUSTAL W (1.5) (45) and drawn with GeneDoc, version 2.5.0 (30).
Nucleotide sequence accession number.
The nucleotide sequence data reported here have been submitted to GenBank under accession number AF139575.
RESULTS
Osmoprotective properties of betaine in L. lactis.
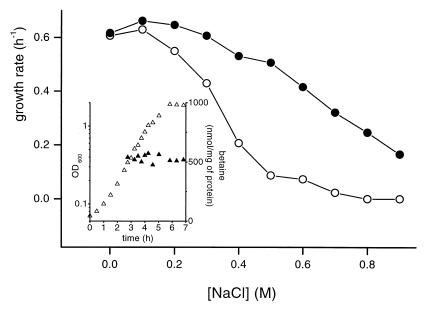
The growth of the proline prototroph strain L. lactis NCDO763 under hyperosmotic constraint was studied in CDM in the absence of proline. Figure 1 reports the maximal growth rates in a range of osmolality from 0.32 to 2.1 osM obtained by the addition of NaCl. In the absence of betaine, the growth rate declined sharply at concentrations greater than 0.1 M NaCl and growth was totally inhibited in the presence of 0.8 M NaCl. When the medium was supplemented with 1 mM betaine, the growth rate was restored to a normal level at up to 0.3 M NaCl (1.1 osM), and the compatible solute extended the osmotic range of growth to at least 0.9 M NaCl (2.1 osM). The same beneficial effect was also observed with 10 μM betaine (data not shown).
FIG. 1.
Effect of increased osmolality on the growth rates of L. lactis NCDO763. Cells were grown in CDM without (○) or with (●) 1 mM betaine. The osmolality was increased by the addition of NaCl. The inset shows a growth curve (▵) in CDM containing 0.3 M NaCl and 1 mM betaine, and the intracellular betaine content (▴) was measured by HPLC analysis at different times.
HPLC analysis of the cytoplasmic content indicated that the amount of betaine accumulated remained constant from exponential phase to stationary phase (OD600 = 0.3 to 2) (inset, Fig. 1). The amount of cytoplasmic betaine was found to be dependent upon the osmolality of the growth medium, rising from 520 to 2,000 nmol/mg of protein at between 0.3 and 0.6 M NaCl (data not shown). It has been previously reported that betaine does not confer osmotic tolerance on Lactobacillus plantarum or L. lactis when the bacteria are challenged with nonionic solutes (13, 29). This observation was confirmed for L. lactis NCDO763 grown in the presence of sorbitol. Betaine was found to be accumulated in these conditions but at levels that were consistently lower than those measured in the presence of NaCl at the same osmolality (data not shown). Ectoine, carnitine, taurine, and the betaine precursor choline were tested for their possible protective properties. None of them conferred any osmotolerance on L. lactis cultivated in the presence of salts or nonionic solutes (data not shown). These data suggest the existence of an osmodependent betaine transport system, which we undertook to characterize at the genetic level.
Identification of a mutant deficient in betaine transport.
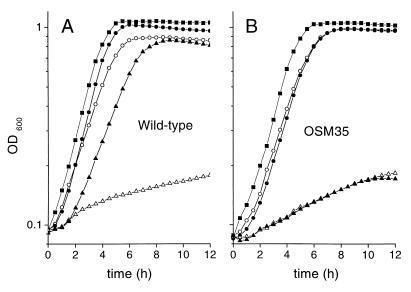
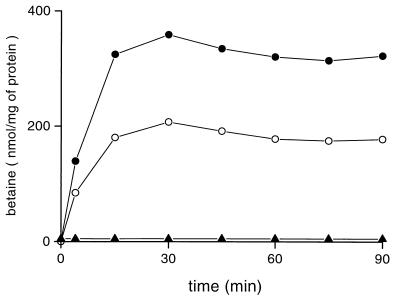
To characterize the betaine transport system(s) of L. lactis, we constructed a library of transposon mutants, which were screened for their inability to grow on CDM-NaCl agar plates containing betaine (see Materials and Methods). An osmosensitive mutant obtained by insertion of the ISS1 element was isolated and called OSM35. The growth capacity of OSM35 was examined in liquid CDM under the range of osmolalities tested for the wild-type strain. Figure 2 displays the growth curves of wild-type and OSM35 cells in three osmotic conditions. In the absence of osmotic challenge, OSM35 was barely distinguishable from the wild type. At 0.3 M NaCl in the absence of betaine, OSM35 was found to possess a slightly better growth capacity than the wild type (μ = 0.51 versus 0.43 h−1). The most noticeable property of OSM35 was its inability to take advantage of the presence of betaine. A deficiency of the betaine uptake system(s) was the most probable explanation for such a phenotype. We tested this hypothesis by measuring the betaine uptake activity of whole cells cultivated under osmotic constraint (Figure 3). A strong energy-dependent betaine uptake activity, measurable in the presence of NaCl or sorbitol was detected for wild-type cells. No betaine uptake activity was detectable for OSM35 cells. This result confirmed that the lack of growth stimulation by betaine in the mutant was due to the abolition of its transport capacities.
FIG. 2.
Comparison of the growth curves of L. lactis NCDO763 wild-type and OSM35 cells. Wild-type (A) and OSM35 (B) cells were grown at 30°C in CDM (■), CDM plus 0.3 M NaCl (○), CDM plus 0.3 M NaCl and 1 mM betaine (●), CDM plus 0.6 M NaCl (▵), or CDM plus 0.6 M NaCl and 1 mM betaine (▴).
FIG. 3.
Betaine uptake activity in L. lactis NCDO763. Wild-type (circles) and OSM35 (triangles) cells were grown in CDM containing 0.3 M NaCl (solid) or 0.45 M sorbitol (open) supplemented with 1 mM betaine. Cells were washed and resuspended in 50 mM MES-NaOH (pH 6.5) containing 0.5% glucose and 50 μg of chloramphenicol per ml. After 5 min of preincubation at 30°C, uptake was initiated by the addition of [1-14C]betaine (final concentration, 0.5 mM) and 0.3 M NaCl (solid) or 0.45 M sorbitol (open) (final concentrations).
Sequence analysis of busA coding for the betaine uptake system of L. lactis.
The complete nucleotide sequence of a 3.4-kb DNA fragment from L. lactis NCDO763 corresponding to the chromosome regions surrounding the transposon integration site in OSM35 was determined after plasmid rescue and with the help of the diagnostic sequence of L. lactis IL1403 (4). Sequence analysis revealed two open reading frames (ORFs), named busAA and busAB for betaine uptake system. The genetic organization of the busA genes was identical in L. lactis NCDO763 and IL1403 strains, which share 86.7% identity at the nucleotide level. The first ORF, busAA, codes for a putative protein 407 residues in length and was likely to start with a TTG initiation codon. The ISS1 insertion site was found in this ORF. The second ORF, busAB, is oriented in the same direction. It potentially encodes a polypeptide of 573 residues. The first codons of busAB overlap the last two codons of busAA. Twenty nucleotides downstream of the stop codon of busAB, a 15-nucleotide inverted repeat (ΔG = −15.6 kCal/mol) could be used as a rho-independent terminator. Altogether, these data suggest that busAA and busAB constitute an operon. To verify that the sole busA inactivation was responsible of the OSM35 phenotype, directed disruption was performed. The resulting mutant, TIL451, exhibited the same phenotype as OSM35 (data not shown; see Materials and Methods). BusAA (407 amino acids; Mr, 45,679) shared strong similarities with the ATP-binding proteins OpuAA (417 amino acids) and ProV (400 amino acids) of the high-affinity betaine ABC transporters B. subtilis OpuA and E. coli ProU, respectively. The sequence alignment of the three proteins was straightforward, with the L. lactis and B. subtilis proteins showing 56% identical amino acids.
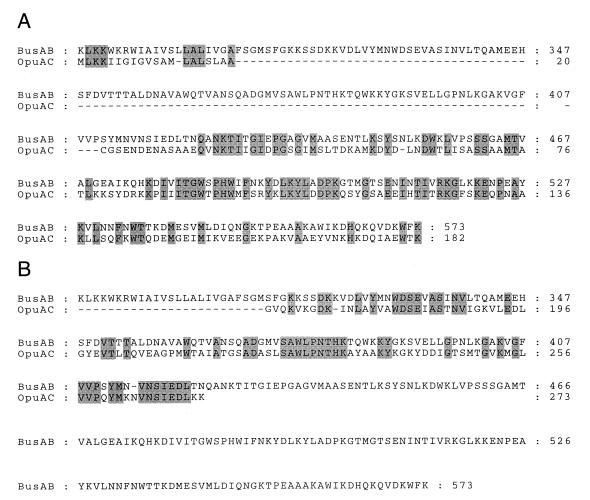
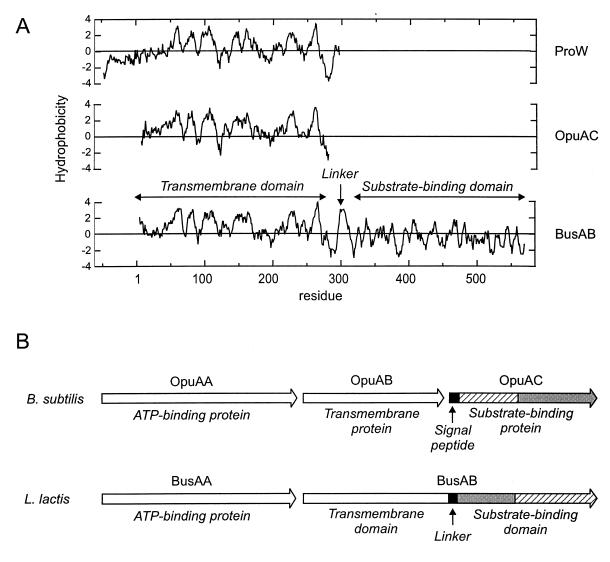
BusAB (573 amino acids; Mr, 61,996), the protein encoded by the second ORF of busA, presents a surprising organization. It is composed of a hydrophobic N-terminal part and a hydrophilic C-terminal part, which correspond to two functional domains, expressed as distinct polypeptides in the prokaryotic ABC transporters characterized so far. The C-terminal part of BusAB, probably corresponding to the substrate-binding domain (SBD), showed significant similarity to the betaine-binding protein of B. subtilis, OpuAC (Fig. 4). However, the subdomain organization was inverted: residues 313 to 423 of BusAB aligned with residues 163 to 273 of OpuAC (43% identity; Fig. 4B), and residues 424 to 573 of BusAB aligned with residues 1 to 162 of OpuAC (45% identity; Fig. 4A). The 282 N-terminal residues of BusAB can be aligned with OpuAB and ProW, the transmembrane components of the high-affinity betaine ABC transporters of B. subtilis and E. coli, respectively. The hydrophobic profile of the N-terminal domain of BusAB displayed striking similarity to OpuAB and ProW, which is a seven-membrane-spanning segment permease (14) (Fig. 5A). Interestingly, in the region encompassing residues 295 to 315 of BusAB, an additional potential membrane-spanning segment was detected, which linked the transmembrane N-terminal domain (TMD) to its hydrophilic C-terminal part (residues 316 to 573) (Fig. 5A). The protein organization of BusA is summarized Fig. 5B and is compared with that of OpuA, the high-affinity betaine ABC transporter of B. subtilis.
FIG. 4.
(A) Alignment of the substrate-binding domain of BusAB (residues 288 to 573) with the N-terminal part of pro-OpuAC (residues 1 to 182). (B) Alignment of the substrate-binding domain of BusAB (residues 288 to 573) with the C-terminal part of mature OpuAC (residues 163 to 273) of B. subtilis.
FIG. 5.
(A) Hydropathy plot of the transmembrane components of ProW of E. coli, OpuAB of B. subtilis, and BusAB of L. lactis NCDO763. The hydrophobicity scale is that of Kyte and Doolittle (20). The profile was obtained with a window of seven residues. The hydrophobicity profiles of ProW, OpuAB, and BusAB were superimposed on the basis of the aligned proteins. The numbering is that of BusAB. (B) Schematic organization of the polypeptides encoded by busA and comparison with those of opuA of B. subtilis. Regions presenting high similarity scores are filled identically (see the text).
The biochemical and genetic data indicated that the osmosensitive phenotype of OSM35 was the consequence of the inactivation of a gene coding for a betaine ABC transporter presenting a new modular arrangement. The complete abolition of betaine transport in OSM35 strongly suggested that L. lactis NCDO763 possesses only one osmoregulated betaine transport system.
Expression of busA is under osmotic control.
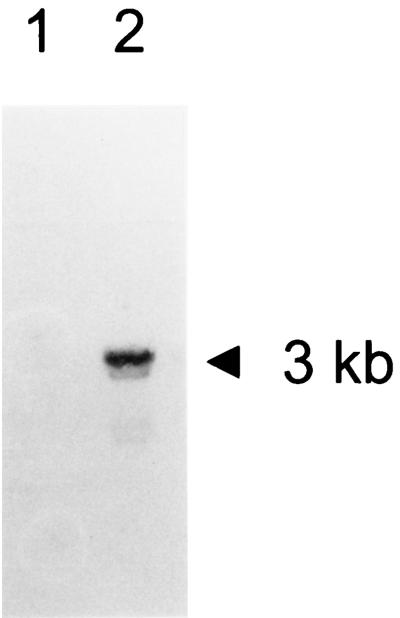
The expression of busA and its induction in response to an osmotic upshock was examined by Northern analysis. RNA extracted from L. lactis NCDO763 cells grown without or after a 40-min osmotic upshift were probed with an oligonucleotide corresponding to a 5′ coding segment of busA. A band corresponding to a 3-kb transcript was detected only on RNA issued from osmotically shocked cells (Fig. 6, lane 2). An overexposition of the membrane revealed a band of similar size in lane 1, indicating that a weak expression of busA exists in cells growing at low osmolality (0.32 osM) (data not shown). This result confirmed that the busAA and busAB genes are organized in an operon whose expression is under osmotic control.
FIG. 6.
Induction of the expression of busA in response to osmotic upshock. RNA was isolated from wild-type cells grown on M17 glucose (lane 1) or M17 glucose containing 0.3 M NaCl (lane 2). After electrophoresis, the RNA was transferred to a membrane and probed with a busA-specific oligonucleotide probe. The size of the transcript is indicated.
Immunodetection of BusAB in the membrane fraction of L. lactis.
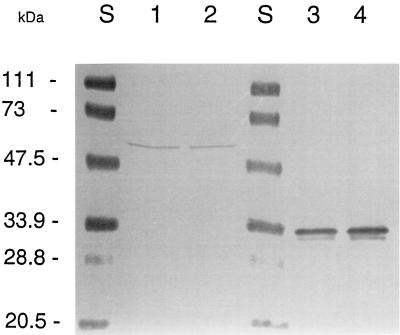
From the nucleotide sequence, BusAB is expected to be composed of an integral membrane domain fused to a hydrophilic domain homologous to the betaine-binding protein of B. subtilis, OpuAC. The presence of BusAB in the membrane fractions and whole cell extracts of L. lactis was verified by immunodetection by using polyclonal antibodies raised against the OpuAC protein of B. subtilis (19). Western blotting revealed the OpuAC protein with the expected size (ca. 30 kDa) in the control experiment performed on B. subtilis extracts, while a 55-kDa band was detected in the membrane fraction of L. lactis (Fig. 7). The observed molecular mass of 55 kDa was consistent with the predicted value of 62 kDa, as it has been widely observed that membrane proteins display an anomalous rapid migration during SDS-PAGE (36, 38). Since we did not find any evidence for an isolated SBD, these experiments show that BusAB is present in the membrane as a fusion between the TMD and the SBD and that no cleavage of the protein occurred during or after membrane translocation. It should be noted that, under the conditions tested, the amount of protein detected was not significantly increased in cells cultivated at high osmolality.
FIG. 7.
Western blotting of BusAB and OpuAC proteins. Proteins were separated by SDS-PAGE (12.5% polyacrylamide) and transferred to a nitrocellulose membrane. Shown are proteins of the membrane fractions of L. lactis cells grown in M17 (lanes 1) or in M17 containing 0.3 M NaCl (lane 2) and proteins from whole-cell extracts of B. subtilis JH642 obtained after growth on LB (lane 4) or LB containing 0.5 M NaCl (lane 5). BusAB and OpuAC were detected with an antiserum against the purified OpuAC protein of B. subtilis JH642. The molecular mass marker was from Bio-Rad (lanes S).
BusA is an osmosensor.
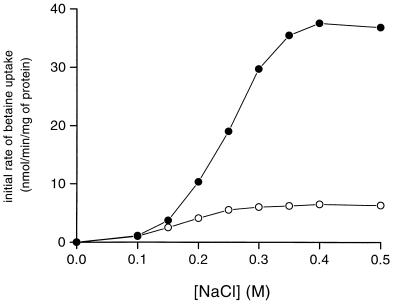
It has been widely observed that the activity of compatible solute transport systems can be modulated by the osmotic pressure independently of de novo protein synthesis (33). This phenomenon, called activation, is a property of high-affinity betaine ABC transporters characterized to date (5, 8, 11). The activation of BusA was investigated on cells grown in CDM (unadapted cells) or CDM plus 0.3 M NaCl and betaine (adapted cells). The initial rates of betaine uptake were measured in the presence of chloramphenicol, and the osmolality of the assay buffer was increased by the addition of NaCl (Fig. 8). No net uptake could be measured at 0.12 osM, which corresponded to the osmolality of the assay buffer. The addition of 0.1 M NaCl (0.3 osM) triggered the uptake. At this osmolality, the initial rate was identical for cells cultivated at low or high osmolality. The activity of BusA increased further with the osmolality of the assay buffer and reached a maximum in the presence of 0.4 M NaCl (0.84 osM). At between 0.1 and 0.4 M NaCl, the betaine uptake rate increased 6-fold for cells grown in CDM, while the activation reached 35-fold for cells adapted to the osmolality. The presence of a betaine transport capacity in cells grown in CDM confirmed that BusA was synthesized at a basal level in L. lactis, a result in agreement with Northern blot and immunodetection experiments. We also observed that the activation of BusA was obtained to a similar extent by raising the osmolality with a nonionic solute (data not shown). Altogether, these results indicated that the activity of BusA was regulated by the external osmolality.
FIG. 8.
Activation of the betaine uptake system in L. lactis NCDO763 as a function of NaCl concentration. Wild-type cells were grown in CDM (○) or CDM–0.3 M NaCl (●) containing 1 mM betaine. Cells were harvested (OD600 = 1 to 1.5), washed twice, and resuspended in 50 mM MES-NaOH (pH 6.5) containing 0.5% glucose and 50 μg of chloramphenicol per ml. After 5 min of preincubation at 30°C, uptake was initiated by the addition of 20 μM [1-14C]betaine (specific activity, 2.5 mCi/mmol), and NaCl final concentrations and initial rates of betaine uptake were determined as indicated.
Transport parameters of BusA.
The presence of BusA as the sole betaine transport system in L. lactis facilitated the determination of its transport parameters. The initial rates of betaine transport have been measured over a range of substrate concentrations (0.2 to 50 μM) with cells grown in CDM or CDM–0.3 M NaCl (see Materials and Methods). Transport assays were performed in the presence of 0.3 M NaCl. For both kinds of experiments, the saturation curve was monophasic and was found to obey the Michaelis-Menten equation. The Kms were 1.7 ± 0.3 and 1.65 ± 0.4 μM for adapted and nonadapted cells, respectively. The maximal velocity measured in these conditions for adapted cells was approximately fivefold higher than for unadapted cells (29 versus 5 nmol/min/mg of protein). The similarity between the Kms was consistent with the idea that the osmodependent betaine uptake capacity of L. lactis NCDO763 is dependent upon the activity of a single, high-affinity transport system.
Osmodependent proline transport through BusA activity.
The role of proline as a compatible solute has been established in many microorganisms (7, 9). Proline can accumulate in the cytoplasm by de novo synthesis like in B. subtilis (50) or through the activity of uptake systems. In the latter case, several betaine transporters were found to also display an osmodependent proline transport activity (7, 9). However, it was not possible to measure a [14C]proline uptake activity by the filter-binding assay in L. lactis. To gain evidence concerning the potential role of BusA as a proline uptake system, we undertook a comparison of the growth properties of wild-type and OSM35 strains in various media with or without proline and then analyzed the cytoplasmic content of amino acids and betaine. For this analysis, we took advantage of the observation made on bacterial cells from various origins that hypoosmotic washes cause the release of solutes accumulated during growth (12, 16, 46). The growth-stimulatory property of proline on L. lactis NCDO763 (Table 1, compare lines 1 and 3), which was already reported for the ML3 strain (42), did not allow us to firmly conclude the osmoprotective role of the amino acid. However, a large proline accumulation was found in wild-type cells grown under osmotic constraint. This accumulation was only observed in the presence of the amino acid in the medium (Table 1, lines 2 and 4), suggesting that an osmodependent transporter was responsible. On the other hand, the addition of betaine to proline-containing media (line 6) abolished the proline accumulation and stimulated growth, confirming the prominent role of betaine as a compatible solute in L. lactis. The amount of betaine accumulated was 2.7-fold in excess over that measured for proline (lines 4 and 6), which could explain the poor beneficial effect of the amino acid. The betaine content measured by HPLC analysis on the same extracts was identical to that found by the radioactive procedure (Fig. 1), indicating that the quaternary amine compound was not further metabolized in the cell.
TABLE 1.
Growth rates and proline, betaine, and total amino acid contents of wild-type and OSM35 cells grown under various conditionsa
| Growth condition | μ (h−1)
|
Proline concn (nmol/mg of protein)
|
Betaine concn (nmol/mg of protein)
|
Amino acid concnb (nmol/mg of protein)
|
||||
|---|---|---|---|---|---|---|---|---|
| wt | OSM35 | wt | OSM35 | wt | OSM35 | wt | OSM35 | |
| CDM | 0.61 | 0.59 | 10 | 12 | –c | – | 460 | 490 |
| CDM + NaCl | 0.43 | 0.51 | 4 | 5 | – | – | 560 | 790 |
| CDM + Pro | 0.75 | 0.76 | 15 | 25 | – | – | 370 | 470 |
| CDM + Pro + NaCl | 0.62 | 0.59 | 201 | 17 | – | – | 750 | 680 |
| CDM + NaCl + Bet | 0.61 | 0.51 | 5 | 6 | 530 | – | 520 | 730 |
| CDM + Pro + NaCl + Bet | 0.70 | 0.54 | 16 | 15 | 560 | – | 510 | 660 |
The cells were cultivated in the indicated medium. The final concentrations of the various additives were as follows: proline, 5.9 mM; NaCl, 0.3 M; betaine, 1 mM. A volume of culture in exponential phase (A600 = 0.8 to 1) corresponding to 1 mg of protein was filtered, and cytoplasmic content was released by osmotic downshock. Growth rates were determined independently in microplate assays, and the values are the means of triplicate experiments. Amino acid analysis was performed on an amino acid analyzer. Glycine betaine was quantified by HPLC. wt, wild type; Pro, proline; Bet, betaine.
The data represent the sum of the 19 amino acids (with proline) detected by the analysis. Data are the means of duplicate experiments.
–, Value below the detection limit (<2).
Similar measurements were done for OSM35. As reported above, the mutant cultivated under osmotic constraint in the absence of proline or betaine (Table 1, line 2) displayed a higher growth rate than did the wild type. This behavior was found to be associated with an increase of the total amino acid pool. Under these conditions, it is possible that the expression of busA downregulates other osmoadaptive processes, such as the accumulation of a larger pool of amino acids. The most noticeable result, however, was the absence of an osmodependent proline accumulation in OSM35 (line 4). This observation demonstrated unambiguously that BusA was also responsible for the osmodependent proline transport observed in the wild-type cells. The absence of a detectable proline uptake activity by the filter-binding assay in wild-type cells was probably due to the low affinity of the transporter for proline: a 1,000-times excess of proline (50 mM) over betaine in a competition experiment did not inhibit betaine uptake (not shown).
DISCUSSION
In bacteria, the transport of compatible solute with a quaternary ammonium group (betaine, choline, and carnitine) occurs through the activity of either carrier-type transporters, which use energy of chemiosmotic origin, or through ATP-dependent transporters (7, 51). In the present study we report that the betaine transport capacity of L. lactis NCDO763 is linked to a single high-affinity ABC transporter, encoded by busA, an operon composed of only two genes. The betaine transport capacity of L. lactis was found to be under osmotic control at both the genetic and biochemical levels.
Betaine transport activity in L. lactis is triggered above an osmolality threshold and is further stimulated by its increase. This activation of betaine uptake is independent of de novo synthesis and was demonstrated for secondary transport systems such as ProP or BetP in E. coli and C. glutamicum, respectively (32, 38), or ABC transporter-like ProU in E. coli (8) or QacT in L. plantarum (11). The molecular mechanisms underlying this property are not yet understood. In theory, membrane-embedded osmodependent transport systems could be responsive to many osmotically driven changes of their environment such as altered membrane strain or osmotic gradient (33, 51). Intriguingly, the activity of the betaine transporter of L. lactis ML3 was reported not to be osmotically controlled (29). However, in that study cells were cultivated in complex media (containing an unknown amount of a putative compatible solute) or in CDM containing proline. trans inhibition or feedback regulation of the transport systems by internalized substrate could explain the apparent lack of activation (12, 34, 48).
Surprisingly, although the betaine transport activity was fivefold higher in cells cultivated at 0.3 M NaCl, Western blot experiments did not show a significant increase in the amount of BusAB in the membrane. This observation suggests that, besides the transcriptional control exerted by external osmolality on busA expression, additional mechanisms are involved in the regulation of the transporter.
The final level of betaine accumulation in the presence of sorbitol was lower than that measured with NaCl at the same osmolality (Fig. 3). In L. plantarum, diffusion-controlled transport of sugar into the cytoplasm counteracts the external osmotic pressure and prevents betaine accumulation at a high level (13). Our results (Fig. 3), together with the lack of a protective effect of betaine in a culture performed in the presence of sorbitol, suggest that a similar mechanism exists in L. lactis NCDO763.
The strong sequence similarities among BusA, OpuA, and ProU indicate that BusA belongs to the ABC-type superfamily of transporters. Bacterial ATP-driven uptake permeases are typically composed of three functional modules (24): (i) the ATP-binding-hydrolyzing protein, a homo- or heterodimer whose role is to fuel the transport reaction; (ii) the two integral TMDs, organized as a dimer; and (iii) the extracytoplasmic substrate-binding protein, which traps the substrate and delivers it to the transport machinery. The genetic organization of busA does not correspond to this archetype. The transporter deduced from the sequence of busA is unusual in two respects: first, there is a fusion between the TMD and SBD, and second, there is a swapping of the N- and C-terminal subdomains of the SBD compared to other betaine-binding proteins.
In gram-positive organisms, the substrate-binding proteins of ABC transporters are synthesized with a precursor signal peptide, which is cleaved by a specific signal peptidase. After this hydrolysis, the new N-terminal cysteine is acylated with a fatty acid, a process which is believed necessary for the membrane anchoring of the protein (19, 43). However, the lipid attachment could not be an absolute requirement in gram-positive organisms, since a potential substrate-binding protein of Lactobacillus fermentum was shown to be anchored to the cell surface through electrostatic interactions (47). At this time, we can speculate on two possibilities about the biochemical organization of functional BusA. After its translocation across the membrane, the SBD could be released outside the cell by an unknown proteolytic event. In that case, the absence of a lipoprotein consensus sequence in the region preceding the betaine-binding domain makes it unlikely that the N terminus is acylated. The second and more plausible fate of BusAB would be that both domains remain fused. This hypothesis is reinforced by the presence of a 55-kDa protein in the membrane fraction of L. lactis cross-reacting with an anti-OpuAC antiserum. These data are fully consistent with the idea that the functional betaine transporter of L. lactis involves a bifunctional protein composed of a TMD and an SBD. Interestingly, the linker region of BusAB connecting the TMD to the SBD displays sequence similarities to the signal peptide of OpuAC and its hydropathic properties indicate a transmembrane-spanning segment. Such an organization would be very similar to that of an unprocessed OpuAC mutant, for which a normal betaine uptake activity has been demonstrated in vivo (19). A search in the unfinished bacterial genome databank indicated that BusAB is not an isolated example of a fusion between the TMD and SBD coding regions. The putative betaine ABC transporters of Enterococcus faecalis and Streptococcus pyogenes, two organisms that are phylogenetically similar to L. lactis, display the same features. The polypeptides deduced from these sequences are similar in size and share more than 60% identity with BusAB (data not shown).
Another interesting characteristic of the polypeptide organization of BusAB concerns the interchange of the two halves of the SBD compared to OpuAC. The Km measured for BusA is in the micromolar range and is close to those reported for other betaine transporters, indicating that the betaine-binding capacity is not affected by the inversion. Although the three-dimensional structure of a betaine-binding protein has not yet been reported, we can hypothesize that the global fold conforms to the classical organization and consists of two hinge-connected lobes, which is the structural signature for the substrate-binding component of bacterial ABC transporters (37). On the basis of such a hypothesis, it is conceivable that the domain swapping observed in the SBD preserves this spatial arrangement. Furthermore, the sequence inversion is probably independent from the fusion with the TMD, since the putative betaine-binding protein of Borrelia burgdorferi AE001125, which is encoded by a distinct gene, displays the same permutation. It must also be noted that significant similarity (30% identical or highly similar residues) was detected between the two halves of the betaine-binding protein OpuAC of B. subtilis. This property, unusual among substrate-binding proteins, may indicate that a gene duplication event is at the origin of this set of proteins.
ABC transporters are encoded by the largest group of paralogous genes in prokaryotic genomes. Multidomain proteins arising from gene fusions are well recognized in this superfamily, but until now the only known cases involved the ATP-binding domains and the TMDs (24). The new organization observed for the high-affinity betaine uptake system of L. lactis might provide an important tool for the understanding of the control of transport by the substrate-binding component. Moreover, the association of the transmembrane component with a hydrophilic domain could help in the crystallization of BusAB, making BusA an attractive model for further structural study of an ABC transporter.
ACKNOWLEDGMENTS
This work was supported by the Programme de Recherche Fondamentale en Microbiologie, Maladies Infectieuses et Parasitaires from the Ministère de l’Education Nationale de la Recherche et de la Technologie (MENRT). D.O. is the recipient of a fellowship from the MENRT.
We thank S. D. Ehrlich and A. Sorokin for the release of diagnostic sequence data of L. lactis IL1403. We are indebted to E. Bremer for the gift of the antiserum raised against OpuAC. We are grateful to Jamila Anba for her help in RNA isolation and to Emmanuelle Maguin for her advice in the early steps of the mutagenesis experiments.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Anba J, Bidnenko E, Hillier A, Ehrlich S D, Chopin M C. Characterization of the lactococcal abiD1 gene coding for phage abortive infection. J Bacteriol. 1995;177:3818–3823. doi: 10.1128/jb.177.13.3818-3823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arakawa T, Timasheff S N. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolotin A, Mauger S, Malarme K, Sorokin A, Ehrlich S D. Abstracts of the Conference on Streptococcal Genetics of the American Society for Microbiology, Vichy 1998. Washington, D.C.: American Society for Microbiology; 1998. Lactococcus lactis IL1403 diagnostic genomics, abstr. O3; pp. 10–11. [Google Scholar]
- 5.Cairney J, Booth I R, Higgins C F. Osmoregulation of gene expression in Salmonella typhimurium: proU encodes an osmotically induced betaine transport system. J Bacteriol. 1985;164:1224–1232. doi: 10.1128/jb.164.3.1224-1232.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cayley S, Lewis B A, Record M T., Jr Origins of the osmoprotective properties of betaine and proline in Escherichia coli K-12. J Bacteriol. 1992;174:1586–1595. doi: 10.1128/jb.174.5.1586-1595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csonka L N, Epstein W. Osmoregulation. In: Neidhart F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbager H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1210–1224. [Google Scholar]
- 8.Faatz E, Middendorf A, Bremer E. Cloned structural genes for the osmotically regulated binding-protein-dependent glycine betaine transport system (ProU) of Escherichia coli K-12. Mol Microbiol. 1988;2:265–279. doi: 10.1111/j.1365-2958.1988.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 9.Galinski E A. Osmoadaptation in bacteria. Adv Microb Physiol. 1995;37:273–328. [PubMed] [Google Scholar]
- 10.Gilles R. “Compensatory” organic osmolytes in high osmolarity and dehydration stresses: history and perspectives. Comp Biochem Physiol. 1997;117A:279–290. doi: 10.1016/s0300-9629(96)00265-4. [DOI] [PubMed] [Google Scholar]
- 11.Glaasker E, Heuberger E H, Konings W N, Poolman B. Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactobacillus plantarum. J Bacteriol. 1998;180:5540–5546. doi: 10.1128/jb.180.21.5540-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaasker E, Konings W N, Poolman B. Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypo-osmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 13.Glaasker E, Tjan F S, Ter Steeg P F, Konings W N, Poolman B. Physiological response of Lactobacillus plantarum to salt and nonelectrolyte stress. J Bacteriol. 1998;180:4718–4723. doi: 10.1128/jb.180.17.4718-4723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haardt M, Bremer E. Use of phoA and lacZ fusions to study the membrane topology of ProW, a component of the osmoregulated ProU transport system of Escherichia coli. J Bacteriol. 1996;178:5370–5381. doi: 10.1128/jb.178.18.5370-5381.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jebbar M, von Blohn C, Bremer E. Ectoine functions as an osmoprotectant in Bacillus subtilis and is accumulated via the ABC-transport system OpuC. FEMS Microbiol Lett. 1997;154:325–330. [Google Scholar]
- 16.Jewell J B, Kashket E R. Osmotically regulated transport of proline by Lactobacillus acidophilus IFO 3532. Appl Environ Microbiol. 1991;57:2829–2833. doi: 10.1128/aem.57.10.2829-2833.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kappes R M, Kempf B, Bremer E. Three transport systems for the osmoprotectant glycine betaine operate in Bacillus subtilis: characterization of OpuD. J Bacteriol. 1996;178:5071–5079. doi: 10.1128/jb.178.17.5071-5079.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Biol Chem. 1995;270:16701–16713. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 19.Kempf B, Gade J, Bremer E. Lipoprotein from the osmoregulated ABC transport system OpuA of Bacillus subtilis: purification of the glycine betaine binding protein and characterization of a functional lipidless mutant. J Bacteriol. 1997;179:6213–6220. doi: 10.1128/jb.179.20.6213-6220.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyte J, Doolittle F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 22.Langella P, Chopin A. Effect of restriction-modification systems on transfer of foreign DNA into Lactococcus lactis. FEMS Microbiol Lett. 1989;59:301–306. [Google Scholar]
- 23.Leenhouts K J. Integration strategies and vectors. In: Ferretti J J, Brown F, Gilmore M S, Klaenhammer T R, editors. Genetics of streptococci, enterococci and lactococci. S. Basel, Switzerland: Karger AG; 1995. pp. 523–530. [Google Scholar]
- 24.Linton K J, Higgins C F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol Microbiol. 1998;28:5–13. doi: 10.1046/j.1365-2958.1998.00764.x. [DOI] [PubMed] [Google Scholar]
- 25.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Lucht J M, Bremer E. Adaptation of Escherichia coli to high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 27.Maguin E, Prevost H, Ehrlich S D, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller J H. Experiments in molecular microbiology. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 29.Molenaar D, Hagting A, Alkema H, Driessen A J, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29a.National Center for Biotechnology Information. copyright date. [Online.] BLAST program. http://www.ncbi.nlm.nih.gov/BLAST /unfinished genome.html. [26 August 1999, last date accessed.] 14 July 1999. [Google Scholar]
- 30.Nicholas K B, Nicholas H B J. copyright date. [Online.] GeneDoc: a tool for editing and annotating multiple sequence alignments, version 2.5.0. http://www.cris.com/∼Ketchup/genedoc.shtml. [23 July 1999, last date accessed.] 1999. [Google Scholar]
- 31.Peter H, Burkovski A, Kramer R. Isolation, characterization, and expression of the Corynebacterium glutamicum betP gene, encoding the transport system for the compatible solute glycine betaine. J Bacteriol. 1996;178:5229–5234. doi: 10.1128/jb.178.17.5229-5234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peter H, Weil B, Burkovski A, Kramer R, Morbach S. Corynebacterium glutamicum is equipped with four secondary carriers for compatible solutes: identification, sequencing, and characterization of the proline/ectoine uptake system, ProP, and the ectoine/proline/glycine betaine carrier, EctP. J Bacteriol. 1998;180:6005–6012. doi: 10.1128/jb.180.22.6005-6012.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poolman B, Glaasker E. Regulation of compatible solute accumulation in bacteria. Mol Microbiol. 1998;29:397–407. doi: 10.1046/j.1365-2958.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 34.Pourkomailian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of the two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 35.Proctor L M, Lai R, Gunsalus R P. The methanogenic archaeon Methanosarcina thermophila TM-1 possesses a high-affinity glycine betaine transporter involved in osmotic adaptation. Appl Environ Microbiol. 1997;63:2252–2257. doi: 10.1128/aem.63.6.2252-2257.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Putman M, van Veen H W, Poolman B, Konings W N. Restrictive use of detergents in the functional reconstitution of the secondary multidrug transporter LmrP. Biochemistry. 1999;38:1002–1008. doi: 10.1021/bi981863w. [DOI] [PubMed] [Google Scholar]
- 37.Quiocho F A, Ledvina P S. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variation of common themes. Mol Microbiol. 1996;20:17–25. doi: 10.1111/j.1365-2958.1996.tb02484.x. [DOI] [PubMed] [Google Scholar]
- 38.Racher K I, Voegele R T, Marshall E V, Culham D E, Wood J M, Jung H, Bacon M, Cairns M T, Ferguson S M, Liang W J, Henderson P J, White G, Hallett F R. Purification and reconstitution of an osmosensor: transporter ProP of Escherichia coli senses and responds to osmotic shifts. Biochemistry. 1999;38:1676–1684. doi: 10.1021/bi981279n. [DOI] [PubMed] [Google Scholar]
- 39.Renault, P. Personal communication.
- 40.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 41.Sandine W E, Radich P C, Elliker P R. Ecology of the lactic streptococci. A review. J Milk Food Technol. 1972;35:176–185. [Google Scholar]
- 42.Smid E J, Konings W N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1990;172:5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutcliffe I C, Russell R R. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Terzaghi B E, Sandine W E. Improved medium for lactic streptococci and their bacteriophages. Appl Environ Microbiol. 1975;29:807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsapis A, Kepes A. Transient breakdown of the permeability barrier of the membrane of Escherichia coli upon hypoosmotic shock. Biochim Biophys Acta. 1977;469:1–12. doi: 10.1016/0005-2736(77)90320-0. [DOI] [PubMed] [Google Scholar]
- 47.Turner M S, Timms P, Hafner L M, Giffard P M. Identification and characterization of a basic cell surface-located protein from Lactobacillus fermentum BR11. J Bacteriol. 1997;179:3310–3316. doi: 10.1128/jb.179.10.3310-3316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenes Scott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Blohn C, Kempf B, Kappes R M, Bremer E. Osmostress response in Bacillus subtilis: characterization of a proline uptake system (OpuE) regulated by high osmolarity and alternative transcription factor sigma B. Mol Microbiol. 1997;25:175–187. doi: 10.1046/j.1365-2958.1997.4441809.x. [DOI] [PubMed] [Google Scholar]
- 50.Whatmore A M, Chudek J A, Reed R H. The effects of osmotic upshock on the intracellular solute pools of Bacillus subtilis. J Gen Microbiol. 1990;136:2527–2535. doi: 10.1099/00221287-136-12-2527. [DOI] [PubMed] [Google Scholar]
- 51.Wood J M. Osmosensing by bacteria: signals and membrane-based sensors. Microbiol Mol Biol Rev. 1999;63:230–262. doi: 10.1128/mmbr.63.1.230-262.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]