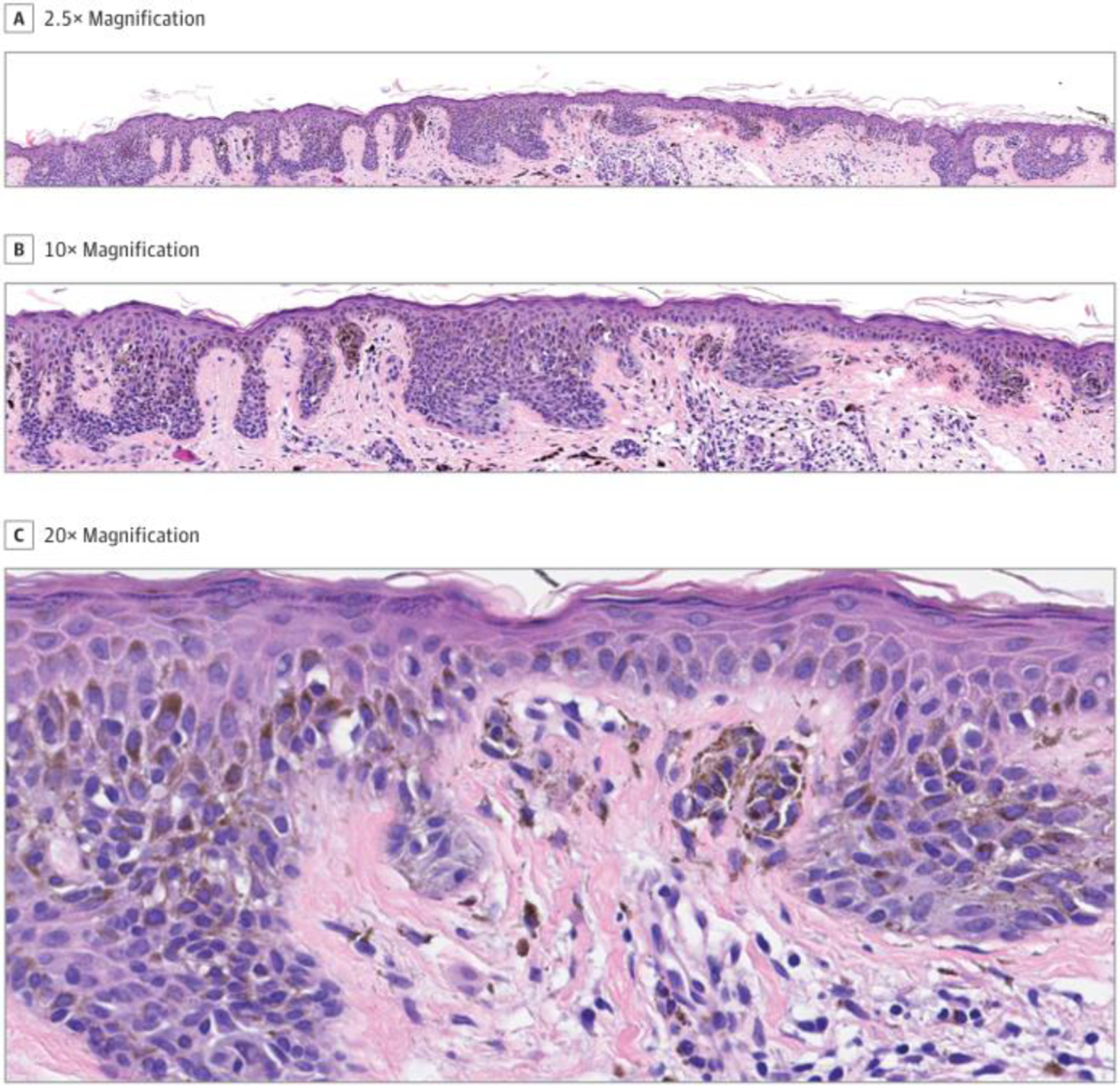
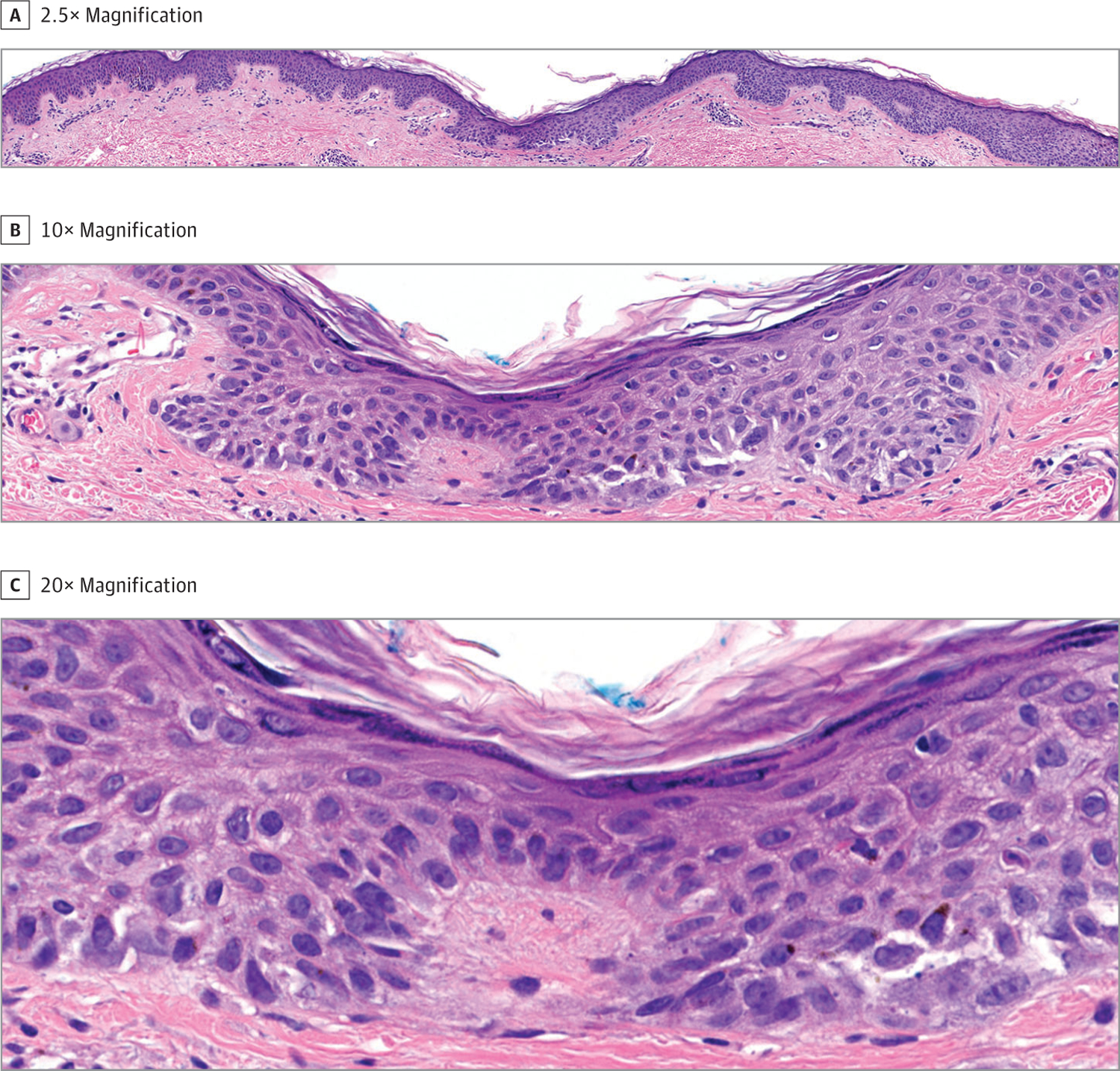
Raymond L Barnhill
Raymond L Barnhill, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
David E Elder
David E Elder, MBChB
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Michael W Piepkorn
Michael W Piepkorn, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Stevan R Knezevich
Stevan R Knezevich, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Lisa M Reisch
Lisa M Reisch, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Megan M Eguchi
Megan M Eguchi, MPH
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Boris C Bastian
Boris C Bastian, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Willeke Blokx
Willeke Blokx, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Marcus Bosenberg
Marcus Bosenberg, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Klaus J Busam
Klaus J Busam, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Richard Carr
Richard Carr, MBChB
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Alistair Cochran
Alistair Cochran, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Martin G Cook
Martin G Cook, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Lyn M Duncan
Lyn M Duncan, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Rosalie Elenitsas
Rosalie Elenitsas, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Arnaud de la Fouchardière
Arnaud de la Fouchardière, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Pedram Gerami
Pedram Gerami, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Iva Johansson
Iva Johansson, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Jennifer Ko
Jennifer Ko, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Gilles Landman
Gilles Landman, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Alexander J Lazar
Alexander J Lazar, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Lori Lowe
Lori Lowe, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Daniela Massi
Daniela Massi, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Jane Messina
Jane Messina, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Daniela Mihic-Probst
Daniela Mihic-Probst, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Douglas C Parker
Douglas C Parker, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Birgitta Schmidt
Birgitta Schmidt, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Christopher R Shea
Christopher R Shea, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Richard A Scolyer
Richard A Scolyer, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Michael Tetzlaff
Michael Tetzlaff, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Xiaowei Xu
Xiaowei Xu, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Iwei Yeh
Iwei Yeh, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Artur Zembowicz
Artur Zembowicz, MD, PhD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1,
Joann G Elmore
Joann G Elmore, MD
1Department of Translational Research, Institut Curie, Unit of Formation and Research of Medicine University of Paris, Paris, France (Barnhill); Department of Pathology and Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia (Elder, Xu); Division of Dermatology, Department of Medicine, University of Washington School of Medicine, Seattle (Piepkorn); Dermatopathology Northwest, Bellevue, Washington (Piepkorn); Pathology Associates, Clovis, California (Knezevich); Department of Biostatistics, University of Washington School of Medicine, Seattle (Reisch); Department of Medicine, David Geffen School of Medicine at University of California, Los Angeles (Eguchi, Elmore); Departments of Pathology and Dermatology, University of California, San Francisco (Bastian, Tetzlaff, Yeh); Department of Pathology, Division Laboratories, Pharmacy and Biomedical Genetics University Medical Center Utrecht, Utrecht, the Netherlands (Blokx); Departments of Dermatology, Pathology, and Immunobiology, Yale School of Medicine, New Haven, Connecticut (Bosenberg); Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, New York (Busam); Cellular Pathology, South Warwickshire NHS Trust, Warwick, United Kingdom (Carr); Department of Pathology and Laboratory Medicine, David Geffen School of Medicine at University of California, Los Angeles (Cochran); Department of Histopathology, Royal Surrey NHS Foundation Trust, Guildford, United Kingdom (Cook); Pathology Service, Massachusetts General Hospital, Harvard Medical School, Boston (Duncan); Department of Dermatology, Hospital of the University of Pennsylvania, Philadelphia (Elenitsas); Department of Biopathology, Centre Léon Bérard, Lyon, France (de la Fouchardière); University of Lyon, Université Claude Bernard Lyon 1, National Center for Scientific Research, Mixed Research Unit 5286, National Institute of Health and Medical Research U1052, Cancer Research Centre of Lyon, Lyon, France (de la Fouchardière); Department of Dermatology, Northwestern University Feinberg School of Medicine, Chicago, Illinois (Gerami); Department of Pathology, Sahlgrenska University Hospital, Gothenburg, Sweden (Johansson); Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio (Ko); Department of Pathology, Escola Paulista de Medicina, Universidade Federal de São Paulo, São Paulo, Brazil (Landman); Departments of Pathology, Dermatology, and Genomic Medicine, The University of Texas MD Anderson Cancer Center, Houston (Lazar); Departments of Pathology and Dermatology, University of Michigan, Ann Arbor (Lowe); Section of Pathology, Department of Health Sciences, University of Florence, Florence, Italy (Massi); Departments of Pathology and Cutaneous Oncology, Moffitt Cancer Center, Tampa, Florida (Messina); Department of Pathology and Molecular Pathology, University Hospital Zurich, Zurich, Switzerland (Mihic-Probst); Departments of Pathology and Dermatology, Emory University School of Medicine, Atlanta, Georgia (Parker); Department of Pathology, Boston Children’s Hospital, Harvard Medical School, Boston, Massachusetts (Schmidt); Department of Dermatology, University of Chicago Medicine, Chicago, Illinois (Shea); Charles Perkins Centre, The University of Sydney, Sydney, Australia (Scolyer); Melanoma Institute Australia, The University of Sydney, Sydney, Australia (Scolyer); Faculty of Medicine and Health, The University of Sydney, Sydney, Australia (Scolyer); Tissue Pathology and Diagnostic Oncology, Royal Prince Alfred Hospital and NSW Health Pathology, Sydney, Australia (Scolyer); Tufts University, Boston, Massachusetts (Zembowicz); Lahey Clinic, Burlington, Massachusetts (Zembowicz); Dermatopathology Consultations, Needham, Massachusetts (Zembowicz).
1