Abstract
This work introduces the use of 8-aminoquinoline subcomponents to generate complex three-dimensional structures. Together with a tris(formylpyridine), 8-aminoquinoline condensed around ZnII templates to produce a tris(tridentate) ligand. This ligand is incorporated into either a tricapped trigonal prismatic ZnII9L6 structure or a pair of pseudo-octahedral ZnII6L4 diastereomers, with S4 and D2 symmetries. Introduction of a methyl group onto the aminoquinoline modulated the coordination sphere of ZnII, which favored the ZnII9L6 structure and disfavored the ZnII6L4 assembly. The tricapped trigonal prismatic ZnII9L6 architecture converted into a single ZnII6L4 cage diastereomer following the addition of a dianionic 4,4′-dinitrostilbene-2,2′-disulfonate guest. Four of these guests clustered tightly at the four windows of the ZnII6L4 cage, held in place through electrostatic interactions and hydrogen bonding, stabilize a single diastereomeric configuration with S4 symmetry.
Introduction
Three-dimensional coordination cages with well-defined enclosed cavities have found applications in stabilizing reactive species,1,2 binding and sensing guests,3 chemical separations,4 and catalyzing reactions.5 The subcomponent self–assembly strategy has enabled the preparation of many metal–organic capsules from simple building blocks, as intricate products form from amine and aldehyde precursors together with metal ions via the concurrent formation of dynamic coordinative and reversible covalent imine bonds.6
Cages such as tetrahedra,7 cubes,8 and octahedra9−11 have thus been prepared. These high-symmetry cages contain pseudo-spherical cavities,12 which enable them to bind approximately spherical guests. Metal–organic cages with lower symmetry13 could enable the binding of non-spherical guests, such as biomolecules and pharmaceuticals. For example, a triangular prismatic architecture with an anisotropic cavity is capable of binding a series of asymmetric drugs and natural products.14 Clever and co-workers used a low-symmetry bowl-shaped metal–organic cage15 to act as a supramolecular mask of bound C60, to effect mono-functionalization of the fullerene on its unprotected face, rather than the bi-functionalization that occurs within a cubic coordination cage or a metal–organic framework.16 New methods of preparing low-symmetry cages are thus very much worth pursuing.
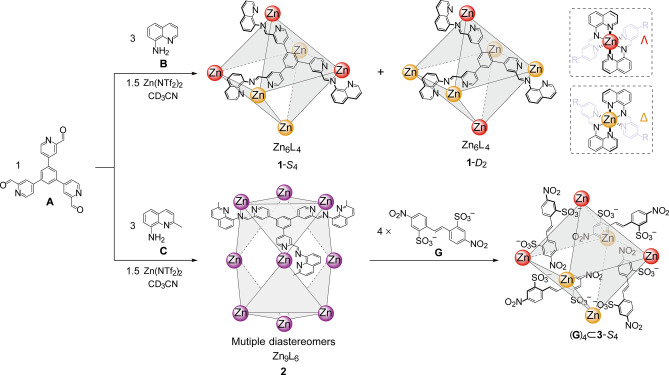
Methods that have been developed to construct low-symmetry cages13 include the use of low-symmetry or flexible ligands,17 solvent effects,18 the use of anions and other templates,9f,19 heteroleptic architectures,14 and multimetallic assemblies.20 However, creating low-symmetry cages by engineering the stereochemistry of metal vertices has proven challenging.21 M6L4 architectures with different C2-symmetric vertices (Table S1) usually display high symmetry, with rare exceptions.22 We hypothesized that the coordination-vector geometry of tris(tritopic) ligands shown in Figure 1 would lead to the generation of a new class of M6L4 cages, where steric hindrance within a ligand (Table S2) might lead to the formation of a lower-symmetry cage, and clashes between ligands may favor the formation of higher-nuclearity structures. The formation of structures 1 and 2 (Figure 1) supported these hypotheses, as detailed below.
Figure 1.
Subcomponent self–assembly of ZnII6L41 and ZnII9L62 and the guest-templated conversion of 2 to (G)4⊂3-S4. Δ and Λ metal stereoconfigurations are shown in yellow and red, respectively.
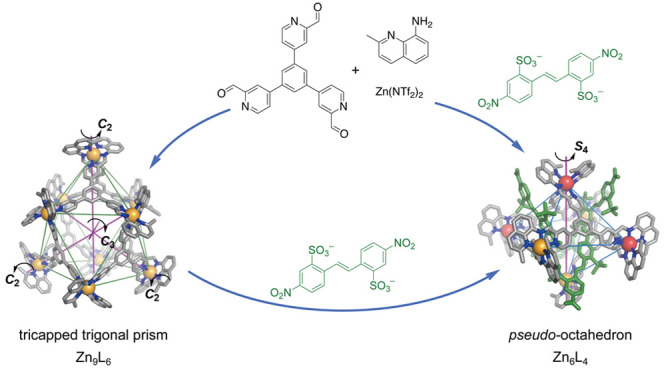
Here, we report the preparation of two novel types of low-symmetry metal–organic cage structures—a ZnII9L6 tricapped trigonal prism, and two ZnII6L4 architectures with octahedral metal-ion frameworks, but S4 and D2 point symmetries, assembled from the same tris(formylpyridine) subcomponent A (Figure 1) under different reaction conditions. The ZnII9L6 architecture, which is not among the more commonly observed Archimedean and Platonic solids, converts into a S4-symmetric ZnII6L4 cage through the action of disulfonate templates (Figure 1).
Results and Discussion
Self–Assembly of ZnII9L6 and ZnII6L4 Metal–Organic Cages
Subcomponent A was prepared through Suzuki–Miyaura cross-coupling of 1,3,5-tris(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl) benzene and 4-bromopicolinaldehyde (see Supporting Information Section 2). The reaction of A (4 equiv) and 8-aminoquinoline B (12 equiv) with zinc(II) bis(trifluoromethanesulfonyl)imide (triflimide or NTf2–, 6 equiv) in CD3CN at 70 °C resulted in the formation of ZnII6L4 cage 1. HR–ESI–MS showed a sharp set of peaks, corresponding to charge states from +8 to +4, all of which confirmed a ZnII6L4 composition (Figures S15 and S16).
The 1H NMR spectrum of 1 contained two sets of ligand signals in a 2:1 ratio (Figure 2b). Three magnetically distinct chemical environments for the ligand protons of 1 were observed, in a 1:1:1 integrated ratio. The 1H NMR diffusion-ordered spectrum (DOSY) of 1 showed that all of its signals had the same diffusion coefficient of 4.05 × 10–6 cm2 s–1 (Figure S10), consistent with the formation of multiple diastereomeric species with a common size of 3.0 nm modeled using the PM723 force field of Scigress24 (Figure S64). All of the protons of 1 were assigned using different two-dimensional NMR techniques (Figures S8, S11, and S12).
Figure 2.
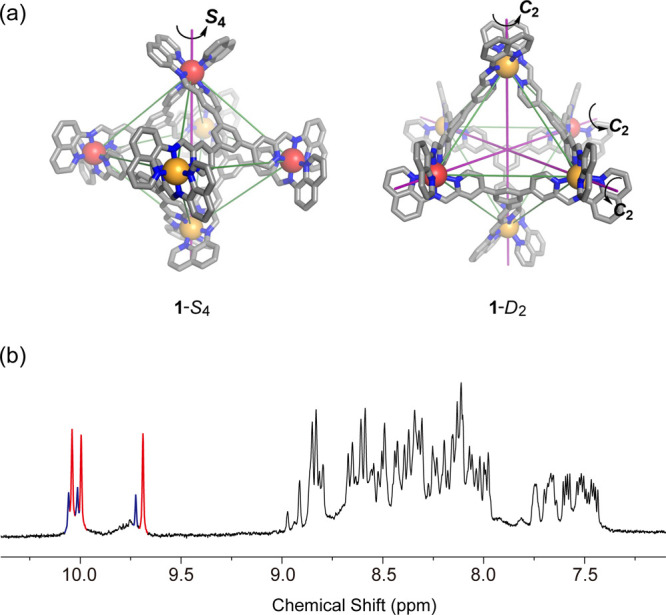
(a) PM7-optimized structure of the S4 and D2 diastereomers of cage 1. Color scheme: Δ-Zn, yellow; Λ-Zn red. (b) 1H NMR spectrum (400 MHz, 298 K, CD3CN) of cage 1. The two sets of imine peaks with a 1:1:1 integration ratio are highlighted.
We sought to elucidate the configurations adopted by cage 1 through NMR analysis. The ligands adopt configurations with different torsions between the central phenyl and peripheral pyridine rings to minimise ring eclipsing, similar to, but less regular than, the propeller-like configurations observed in higher-symmetry structures.25 Geometrical analysis of cage 1 reveals six enantiomeric pairs of diastereomers, with distinct point symmetries: Δ6/Λ6, T; Δ5Λ/ΔΛ5, C2; Δ4Λ2/Δ2Λ4, C1; Δ4Λ2/Δ2Λ4, D2; Δ3Λ3, C3; and Δ3Λ3, S4. These stereoisomers are shown in Figure S64.
M6L4 cages with T point symmetry11 afford 1H NMR spectra with only one set of ligand peaks, whereas such cages with reduced symmetry, e.g., with C2, C1, C3, S4, or D2 point symmetry, are predicted to give rise to spectra with 6, 12, 4, 3, or 3 imine singlet peaks, respectively. Because the 1H NMR spectrum of 1 exhibited two sets of three 1:1:1 imine singlet peaks (Figure 2b), we inferred that 1 existed in solution as a mixture of diastereomers with S4 and D2 symmetries.
To confirm this conclusion, energy minimization of the six stereoisomers was carried out at the PM723 level of theory (Figure S64 and Tables S8–S13) using Scigress.24 Although the calculated energies of these isomers were similar, the 1-S4 and 1-D2 diastereomers adopted larger dihedral angles between the ligand phenylene and pyridyl rings, which are closer to the values adopted by the free ligand, than for the other four isomers (Table S6). We infer that the larger dihedral angles may favor the 1-S4 and 1-D2 diastereomers, because in these diastereomers, the steric eclipsing of phenylene and pyridyl hydrogen atoms on the same ligand is reduced. These dihedral angles in the PM7-optimized model of 1-S4 are similar to those observed in the crystal structure of 3-S4 (Table S6, entries 6–7), and the Zn···Zn separations are similar between model and structure, further suggesting that the S4 diastereomer of 1, and its D2-symmetry analog, with larger dihedral angles according to the model, are energetically favored. We thus conclude that torsional steric hindrance of our new ligand, combined with the coordinate geometry of its vertices, led to the formation of M6L4 cages with D2 and S4 point symmetries, rather than the higher-symmetry pseudo-octahedra (Table S2).
As concentration dictates the formation of cages with different nuclearities according to Le Chatelier’s principle, we hypothesized that increasing the concentration might favor the conversion of a smaller cage into a larger one. Indeed, when the total concentration of A was increased from 0.5 to 2.0 mM during the synthesis of 1, a new species with a ZnII9L6 composition was detected by HR–ESI–MS alongside ZnII6L41. To promote the selective formation of ZnII9L62, we sought to modulate the coordination sphere of ZnII through methylation α to the quinoline nitrogen atom of subcomponent B. The analysis of models using the PM723 force field of Scigress24 (Table S7) suggests that the introduction of such a methyl group would favor the formation of ZnII9L6 cage 2 over ZnII6L4 cage 1. Thus, the assembly of subcomponent 2-methyl-8-aminoquinoline C (18 equiv) together with A (6 equiv) and ZnII(NTf2)2 (9 equiv) at [A] = 5.0 mM resulted in the formation of ZnII9L62 as the uniquely observed product.
The 1H NMR spectrum of 2 (Figure S17) was complex, with many signals, which we ascribe to the presence of multiple diastereoisomers. The 1H DOSY spectrum of 2 (Figure S19) indicated that all signals assigned to cage diastereomers had the same diffusion coefficient, consistent with the formation of isomers with similar sizes. The complex 1H NMR spectrum was assigned using different two-dimensional NMR techniques (Figures S20–S23). The construction of ZnII9L6 cage 2 is thus enabled through a detailed understanding of the subtle steric effects of the aminoquinoline methyl groups and the phenylene-pyridine torsion angles (Table S6).
Anionic Templates Drive Conversion of ZnII9L6 to ZnII6L4
The ZnII6L4 and ZnII9L6 frameworks of coordination cages 1 and 2 have distinct geometries, which imply different guest-binding preferences. We hypothesized that the conversion between these frameworks might be achieved following the addition of a suitable guest. Therefore, the use of anionic guests as templates (Figure S26) was investigated to effect the guest-induced conversion from ZnII9L62 to ZnII6L43, an analogue of cage 1 that incorporated methylated C instead of B.
Complete conversion of ZnII9L62 to the octahedral ZnII6L4 framework of 3 occurred after the addition of anionic metal cluster [PO4(WO3)12]3– in CD3CN. The NMR spectra of 3 were complex (Figure S27), suggesting the presence of multiple diastereomers. However, HR–ESI–MS showed only peaks corresponding to the [PO4(WO3)12]3– adduct of 3 (Figures S28 and S29). The addition of G (G = 4,4′-dinitrostilbene-2,2′disulfonate) to ZnII9L62 in acetonitrile, in contrast, led to complete conversion of 2 to (G)4⊂3 after 6 h,26 as confirmed by HR–ESI–MS (Figures S38 and S39). The conversion of 2 to (G)4⊂3 in dilute solution was accelerated due to the poor solubility of G and (G)4⊂3 (Figures S40–S42). NMR spectra (Figures 3b and S31–S37) indicated the formation of a single isomer with either S4 or D2 symmetry, as reflected in the presence of only three imine peaks in a 1:1:1 integral ratio. DOSY confirmed that all the ligand and guest peaks exhibited a single diffusion rate (Figure 3b).
Figure 3.
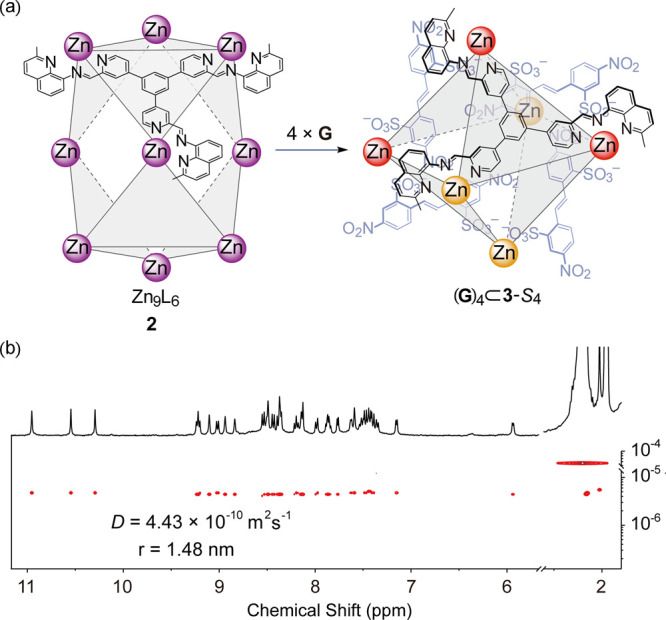
(a) Guest-templated transformation of 2 into (G)4⊂3-S4. (b) 1H DOSY NMR spectrum (400 MHz, 298 K, CD3CN) of (G)4⊂3-S4. G = 4,4′-dinitrostilbene-2,2′-disulfonate.
Vapor diffusion of diethyl ether into an acetonitrile solution of (G)4⊂3 containing KSbF6 resulted in the formation of cube-shaped yellow crystals.27 Single-crystal X-ray diffraction analysis revealed the solid state structure of (G)4⊂3 (Figure 4), having an S4 symmetry consistent with the NMR spectrum recorded in solution (Figure 3b). Inspection of the structure revealed three of the ZnII centers to have Δ handedness, with the other three adopting Λ handedness, lending the capsule achiral S4 point symmetry. Each metal center is thus related by the S4 symmetry operation (Figure 4) to a metal center of the opposite stereochemical configuration.
Figure 4.
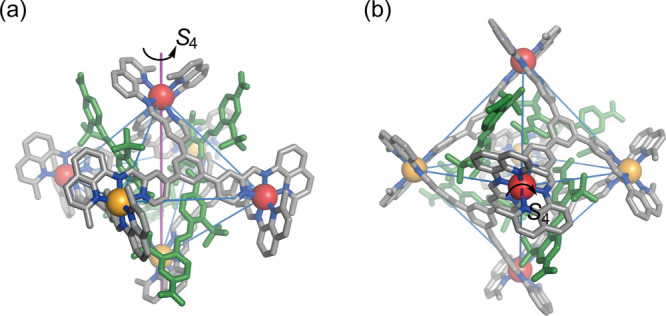
X-ray crystal structure of host–guest complex (G)4⊂3-S4. (a) View orthogonal to the S4 axis, showing the compression of the octahedral framework along this axis. (b) View down the S4 axis, showing the equatorial expansion of the framework. Color scheme: Δ-Zn, yellow; Λ-Zn, red; guest, green; S4 axis, purple. Ligand nitrogen atoms are blue, and the carbon atoms are gray.
We infer the structure of (G)4⊂3 to be stabilized by electrostatic attraction and hydrogen bonding (Figure S43) between the cationic cage framework and the anionic guests. Each guest occupies an open face of the octahedral host framework, with each sulfonate group oriented toward a ZnII center. This arrangement thus appears to stabilize the octahedral ZnII6L43 framework with respect to the larger tricapped trigonal prismatic ZnII9L62.
Structures having an octahedral metal framework but lower symmetry are rare;22 other M6L4 octahedra are observed to adopt Oh,9Td,10 or T(11) symmetry. The complex (G)4⊂3-S4 displayed antipodal metal···metal distances of 20.6 and 16.1 Å, with the ZnII centers on the S4 axis more closely spaced. We infer this axial compression and equatorial expansion to result from the reduced steric eclipsing of the phenylene and pyridyl hydrogen atoms within ligands, as compared to diastereomers with C1, C2, C3, and T configurations, as supported by PM7 calculations23 (Table S6). The dihedral angles between the chelate planes of two imino-quinoline moieties coordinated to each ZnII center range from 84–88°.
Host–Guest Chemistry of ZnII6L4
The complex NMR spectra of ZnII9L6 cage 2 hampered studies of its host–guest chemistry. We thus focused upon the host–guest chemistry of cage 1. To determine the binding stoichiometry and affinities for different guests, we performed titration experiments by 1H NMR spectroscopy. Job plots28 of the titration of B(p-C6H4Cl)4– into a solution of 1 were consistent with a 1:4 host–guest binding ratio (Table 1, and Figures S44 and S45). Given the size of the guest, we infer the guests to bind peripherally, at the four cage windows, in contrast to previously reported 1:1 peripheral binding of this guest to a different M6L4 cage with T symmetry.11c
Table 1. Summary of the Binding Constants of Anionic Guests to Cage 1.
The NMR titration data of guests B(p-C6H4Cl)4– and B(p-C6H4F)4– with 1 were plotted and fitted to the Hill function.29 These anions displayed Hill coefficients of ca. 1.3 and 1.4, respectively, indicating a weakly cooperative binding mode (Figures S50–S53). The association constants for the B(p-C6H4Cl)4– and B(p-C6H4F)4– were determined to be 2.16 × 102 and 1.36 × 102 M–1, respectively.
In contrast to the anionic guests B(C6H5)4–, B(p-C6H4Cl)4–, and B(p-C6H4F)4–, the more electron-deficient pentafluorophenyl and tetrakis[3,5-bis(trifluoromethyl)phenyl] borates were not bound by 1 (Figures S50–S53, S59, and S63). We infer that the increased electron-deficiency of the polyfluorinated tetraphenylborates could prevent binding due to the weaker electrostatic interaction between these anionic guests and cationic host 1.
The oxoanions IO4– and ReO4– bound with a 1:2 host–guest stoichiometry, with similar association constants of 5.73 × 102 and 5.98 × 102 M–1, respectively (Figures S46–S49 and S54–S57). Noting that four equivalents of the peripherally binding larger anions B(p-C6H4Cl)4– and B(p-C6H4F)4– bound to 1, we infer that the smaller ones ReO4– and IO4– bound internally. Two equivalents of these oxoanions fit easily within the cavity of 1 (Tables 1, S4, and S5),30,31 and internal binding of similar anions was observed in related M6L4 species.11c As the volume of [PO4(WO3)12]3– exceeds the cavity volume of 3 (Tables S4 and S5), we infer that this cluster is bound peripherally.
To further study the binding ability of 1 toward neutral guests, we treated the cage with polyaromatic hydrocarbons such as pyrene, corannulene, triphenylene, and dibenzo[g, p]chrysene, all of which were observed to bind to cage 1 in fast exchange on the NMR timescale (Figures S58 and S60–S62). However, the poor solubility of these guests precluded Ka determination.
Conclusions
Novel ZnII9L6 tricapped trigonal prism and ZnII6L4 structures with S4 and D2 symmetries thus establish the use of 8-aminoquinolines in subcomponent self–assembly of complex three-dimensional structures, beyond their use in copper(I) helicates.32 These zinc(II) architectures bind a diverse array of guests, and show the ability to reconfigure to optimize guest binding. Such dynamic reconfiguration might enable the preparation of new classes of heteroleptic structures with lower symmetries,33 capable of binding low-symmetry guests. Such species are potentially of interest in the purification of low-symmetry, complex molecules from mixtures.4b
Acknowledgments
This study was supported by the European Research Council (695009) and the UK Engineering and Physical Sciences Research Council (EPSRC, EP/T031603/1, and EP/P027067/1). H.K.L. thanks the Shanghai Institute of Organic Chemistry for a postdoctoral fellowship. We thank the Department of Chemistry NMR facility, University of Cambridge, for performing some NMR experiments and Diamond Light Source (UK) for the synchrotron beamtime on I19 (CY21497).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.3c03981.
Experimental procedures; NMR characterizations; mass spectrometry data; volume calculations; and X-ray crystallographic data (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- a Galan A.; Ballester P. Stabilization of reactive species by supramolecular encapsulation. Chem. Soc. Rev. 2016, 45, 1720–1737. 10.1039/c5cs00861a. [DOI] [PubMed] [Google Scholar]; b Wang K.; Jordan J. H.; Hu X.-Y.; Wang L. Supramolecular strategies for controlling reactivity within confined nanospaces. Angew. Chem., Int. Ed. 2020, 59, 13712–13721. 10.1002/anie.202000045. [DOI] [PubMed] [Google Scholar]
- a Mal P.; Breiner B.; Rissanen K.; Nitschke J. R. White phosphorus is air-stable within a self–assembled tetrahedral capsule. Science 2009, 324, 1697–1699. 10.1126/science.1175313. [DOI] [PubMed] [Google Scholar]; b Yamashina M.; Sei Y.; Akita M.; Yoshizawa M. Safe storage of radical initiators within a polyaromatic nanocapsule. Nat. Commun. 2014, 5, 4662. 10.1038/ncomms5662. [DOI] [PubMed] [Google Scholar]
- a Brzechwa-Chodzyńska A.; Drożdż W.; Harrowfield J.; Stefankiewicz A. R. Fluorescent sensors: a bright future for cages. Coord. Chem. Rev. 2021, 434, 213820. 10.1016/j.ccr.2021.213820. [DOI] [Google Scholar]; b Ahmad N.; Younus H. A.; Chughtai A. H.; Verpoort F. Metal–organic molecular cages: applications of biochemical implications. Chem. Soc. Rev. 2015, 44, 9–25. 10.1039/c4cs00222a. [DOI] [PubMed] [Google Scholar]; c Dhamija A.; Das C. K.; Ko Y. H.; Kim Y.; Mukhopadhyay R. D.; Gunnam A.; Yu X.; Hwang I.-C.; Schäfer L. V.; Kim K. Remotely controllable supramolecular rotor mounted inside a porphyrinic cage. Chem 2022, 8, 543–556. 10.1016/j.chempr.2021.12.008. [DOI] [Google Scholar]; d Chan A. K.; Lam W. H.; Tanaka Y.; Wong K. M.; Yam V. W. Multiaddressable molecular rectangles with reversible host–guest interactions: modulation of pH-controlled guest release and capture. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 690–695. 10.1073/pnas.1423709112. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Zhang Z.; Kim D. S.; Lin C. Y.; Zhang H.; Lammer A. D.; Lynch V. M.; Popov I.; Miljanic O. S.; Anslyn E. V.; Sessler J. L. Expanded porphyrin-anion supramolecular assemblies: environmentally responsive sensors for organic solvents and anions. J. Am. Chem. Soc. 2015, 137, 7769–7774. 10.1021/jacs.5b03131. [DOI] [PubMed] [Google Scholar]; f Custelcean R.; Bonnesen P. V.; Duncan N. C.; Zhang X.; Watson L. A.; Van Berkel G.; Parson W. B.; Hay B. P. Urea-functionalized M4L6 cage receptors: anion-templated self–assembly and selective guest exchange in aqueous solutions. J. Am. Chem. Soc. 2012, 134, 8525–8534. 10.1021/ja300677w. [DOI] [PubMed] [Google Scholar]; g Gemen J.; Białek M. J.; Kazes M.; Shimon L. J. W.; Feller M.; Semenov S. N.; Diskin-Posner Y.; Oron D.; Klajn R. Ternary host–guest complexes with rapid exchange kinetics and photoswitchable fluorescence. Chem 2022, 8, 2362–2379. 10.1016/j.chempr.2022.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wu K.; Li K.; Hou Y.-J.; Pan M.; Zhang L.-Y.; Chen L.; Su C.-Y. Homochiral D4-symmetric metal–organic cages from stereogenic Ru(II) metalloligands for effective enantioseparation of atropisomeric molecules. Nat. Commun. 2016, 7, 10487. 10.1038/ncomms10487. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Zhang D.; Ronson T. K.; Zou Y.-Q.; Nitschke J. R. Metal–organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. 10.1038/s41570-020-00246-1. [DOI] [PubMed] [Google Scholar]; c Fuertes-Espinosa C.; Pujals M.; Ribas X. Supramolecular purification and regioselective functionalization of fullerenes and endohedral metallofullerenes. Chem 2020, 6, 3219–3262. 10.1016/j.chempr.2020.11.003. [DOI] [Google Scholar]; d Cui P.-F.; Liu X.-R.; Lin Y.-J.; Li Z.-H.; Jin G.-X. Highly selective separation of benzene and cyclohexane in a spatially confined carborane metallacage. J. Am. Chem. Soc. 2022, 144, 6558–6565. 10.1021/jacs.2c01668. [DOI] [PubMed] [Google Scholar]; e Fuertes-Espinosa C.; Gómez-Torres A.; Morales-Martínez R.; Rodríguez-Fortea A.; García-Simón C.; Gándara F.; Imaz I.; Juanhuix J.; Maspoch D.; Poblet J. M.; Echegoyen L.; Ribas X. Purification of Uranium-based endohedral metallofullerenes (EMFs) by selective supramolecular encapsulation and release. Angew. Chem., Int. Ed. 2018, 57, 11294–11299. 10.1002/anie.201806140. [DOI] [PubMed] [Google Scholar]
- a Takezawa H.; Shitozawa K.; Fujita M. Enhanced reactivity of twisted amides inside a molecular cage. Nat. Chem. 2020, 12, 574–578. 10.1038/s41557-020-0455-y. [DOI] [PubMed] [Google Scholar]; b Kaphan D. M.; Levin M. D.; Bergman R. G.; Raymond K. N.; Toste F. D. A supramolecular microenvironment strategy for transition metal catalysis. Science 2015, 350, 1235–1238. 10.1126/science.aad3087. [DOI] [PubMed] [Google Scholar]; c Heard A. W.; Goldup S. M. Synthesis of a mechanically planar chiral rotaxane ligand for enantioselective catalysis. Chem 2020, 6, 994–1006. 10.1016/j.chempr.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Li T.-R.; Huck F.; Piccini G. M.; Tiefenbacher K. Mimicry of the proton wire mechanism of enzymes inside a supramolecular capsule enables β-selective O-glycosylations. Nat. Chem. 2022, 14, 985–994. 10.1038/s41557-022-00981-6. [DOI] [PubMed] [Google Scholar]; e Cullen W.; Misuraca M. C.; Hunter C. A.; Williams N. H.; Ward M. D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. 10.1038/nchem.2452. [DOI] [PubMed] [Google Scholar]; f Omagari T.; Suzuki A.; Akita M.; Yoshizawa M. Efficient catalytic epoxidation in water by axial N-ligand-free Mn-porphyrins within a micellar capsule. J. Am. Chem. Soc. 2016, 138, 499–502. 10.1021/jacs.5b11665. [DOI] [PubMed] [Google Scholar]; g Zhao L.; Jing X.; Li X.; Guo X.; Zeng L.; He C.; Duan C. Catalytic properties of chemical transformation within the confined pockets of Werner-type capsules. Coord. Chem. Rev. 2019, 378, 151–187. 10.1016/j.ccr.2017.11.005. [DOI] [Google Scholar]; h Fang Y.; Powell J. A.; Li E.; Wang Q.; Perry Z.; Kirchon A.; Yang X.; Xiao Z.; Zhu C.; Zhang L.; Huang F.; Zhou H.-C. Catalytic reactions within the cavity of coordination cages. Chem. Soc. Rev. 2019, 48, 4707–4730. 10.1039/c9cs00091g. [DOI] [PubMed] [Google Scholar]; i Dhamija A.; Gunnam A.; Yu X.; Lee H.; Hwang I.-C.; Ho Ko Y.; Kim K. Dramatically enhanced reactivity of fullerenes and tetrazine towards the inverse-electron-demand Diels–Alder reaction inside a porous porphyrinic cage. Angew. Chem., Int. Ed. 2022, 134, e202209326 10.1002/anie.202209326. [DOI] [PubMed] [Google Scholar]; j Chu D.; Gong W.; Jiang H.; Tang X.; Cui Y.; Liu Y. Boosting enantioselectivity of chiral molecular catalysts with supramolecular metal–organic cages. CCS Chem. 2022, 4, 1180–1189. 10.31635/ccschem.021.202100847. [DOI] [Google Scholar]; k Olivo G.; Capocasa G.; Del Giudice D.; Lanzalunga O.; Di Stefano S. New horizons for catalysis disclosed by supramolecular chemistry. Chem. Soc. Rev. 2021, 50, 7681–7724. 10.1039/d1cs00175b. [DOI] [PubMed] [Google Scholar]; l Gadzikwa T.; Bellini R.; Dekker H. L.; Reek J. N. H. Self–assembly of a confined rhodium catalyst for asymmetric hydroformylation of unfunctionalized internal alkenes. J. Am. Chem. Soc. 2012, 134, 2860–2863. 10.1021/ja211455j. [DOI] [PubMed] [Google Scholar]; m Yan D.-N.; Cai L.-X.; Cheng P.-M.; Hu S.-J.; Zhou L.-P.; Sun Q.-F. Photooxidase mimicking with adaptive coordination molecular capsules. J. Am. Chem. Soc. 2021, 143, 16087–16094. 10.1021/jacs.1c06390. [DOI] [PubMed] [Google Scholar]; n Wang J.; Young T. A.; Duarte F.; Lusby P. J. Synergistic noncovalent catalysis facilitates base-free Michael addition. J. Am. Chem. Soc. 2020, 142, 17743–17750. 10.1021/jacs.0c08639. [DOI] [PubMed] [Google Scholar]; o Samanta D.; Mukherjee S.; Patil Y. P.; Mukherjee P. S. Self–assembled Pd6 open cage with triimidazole walls and the use of its confined nanospace for catalytic Knoevenagel- and Diels-Alder reactions in aqueous medium. Chem.—Eur. J. 2012, 18, 12322–12329. 10.1002/chem.201201679. [DOI] [PubMed] [Google Scholar]
- a Zhang D.; Ronson T. K.; Nitschke J. R. Functional capsules via subcomponent self–assembly. Acc. Chem. Res. 2018, 51, 2423–2436. 10.1021/acs.accounts.8b00303. [DOI] [PubMed] [Google Scholar]; b Howson S. E.; Bolhuis A.; Brabec V.; Clarkson G. J.; Malina J.; Rodger A.; Scott P. Optically pure, water-stable metallo-helical ‘flexicate’ assemblies with antibiotic activity. Nat. Chem. 2012, 4, 31–36. 10.1038/nchem.1206. [DOI] [PubMed] [Google Scholar]; c Frischmann P. D.; Kunz V.; Wurthner F. Bright fluorescence and host–guest sensing with a nanoscale M4L6 tetrahedron accessed by self–assembly of zinc-imine chelate vertices and perylene bisimide edges. Angew. Chem., Int. Ed. 2015, 54, 7285–7289. 10.1002/anie.201501670. [DOI] [PubMed] [Google Scholar]; d Anhäuser J.; Puttreddy R.; Glanz L.; Schneider A.; Engeser M.; Rissanen K.; Lützen A. Subcomponent self–assembly of a cyclic tetranuclear FeII helicate in a highly diastereoselective self–sorting manner. Chem.—Eur. J. 2019, 25, 12294–12297. 10.1002/chem.201903164. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Sham K. C.; Yiu S. M.; Kwong H. L. Dodecanuclear hexagonal-prismatic M12L18 coordination cages by subcomponent self–assembly. Inorg. Chem. 2013, 52, 5648–5650. 10.1021/ic400665m. [DOI] [PubMed] [Google Scholar]; f Young M. C.; Holloway L. R.; Johnson A. M.; Hooley R. J. A supramolecular sorting hat: stereocontrol in metal–ligand self–assembly by complementary hydrogen bonding. Angew. Chem., Int. Ed. 2014, 53, 9832–9836. 10.1002/anie.201405242. [DOI] [PubMed] [Google Scholar]; g Ren D.-H.; Qiu D.; Pang C.-Y.; Li Z.; Gu Z.-G. Chiral tetrahedral iron(II) cages: diastereoselective subcomponent self–assembly, structure interconversion and spin-crossover properties. Chem. Commun. 2015, 51, 788–791. 10.1039/c4cc08041f. [DOI] [PubMed] [Google Scholar]; h Yi S.; Brega V.; Captain B.; Kaifer A. E. Sulfate-templated self–assembly of new M4L6 tetrahedral metal–organic cages. Chem. Commun. 2012, 48, 10295–10297. 10.1039/c2cc35095e. [DOI] [PubMed] [Google Scholar]; i Domer J.; Slootweg J. C.; Hupka F.; Lammertsma K.; Hahn F. E. Subcomponent assembly and transmetalation of dinuclear helicates. Angew. Chem., Int. Ed. 2010, 49, 6430–6433. 10.1002/anie.201002776. [DOI] [PubMed] [Google Scholar]; j Lavendomme R.; Ronson T. K.; Nitschke J. R. Metal and organic templates together control the size of covalent macrocycles and cages. J. Am. Chem. Soc. 2019, 141, 12147–12158. 10.1021/jacs.9b06182. [DOI] [PMC free article] [PubMed] [Google Scholar]; k Chen L.-J.; Yang H.-B.; Shionoya M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 2017, 46, 2555–2576. 10.1039/c7cs00173h. [DOI] [PubMed] [Google Scholar]; l Lewing D.; Koppetz H.; Hahn F. E. Reversible formation and transmetalation of Schiff-base complexes in subcomponent self–assembly reactions. Inorg. Chem. 2015, 54, 7653–7659. 10.1021/acs.inorgchem.5b01334. [DOI] [PubMed] [Google Scholar]
- a Bilbeisi R. A.; Clegg J. K.; Elgrishi N.; de Hatten X.; Devillard M.; Breiner B.; Mal P.; Nitschke J. R. Subcomponent self–assembly and guest-binding properties of face-capped Fe4L48+ capsules. J. Am. Chem. Soc. 2012, 134, 5110–5119. 10.1021/ja2092272. [DOI] [PubMed] [Google Scholar]; b Hu S.-J.; Guo X.-Q.; Zhou L.-P.; Yan D.-N.; Cheng P.-M.; Cai L.-X.; Li X.-Z.; Sun Q.-F. Guest-driven self–assembly and chiral induction of photofunctional lanthanide tetrahedral cages. J. Am. Chem. Soc. 2022, 144, 4244–4253. 10.1021/jacs.2c00760. [DOI] [PubMed] [Google Scholar]; c Fu J.; Zheng B.; Zhang H.; Zhao Y.; Zhang D.; Zhang W.; Yang X.-J.; Wu B. Chirality transcription in the anion-coordination-driven assembly of tetrahedral cages. Chem. Commun. 2020, 56, 2475–2478. 10.1039/c9cc09752j. [DOI] [PubMed] [Google Scholar]; d Yeh R. M.; Xu J.; Seeber G.; Raymond K. N. Large M4L4 ((M = Al(III), Ga(III), In(III), Ti(IV)) tetrahedral coordination cages: an extension of symmetry-based design. Inorg. Chem. 2005, 44, 6228–6239. 10.1021/ic0505145. [DOI] [PubMed] [Google Scholar]; e Li Y.; Dong J.; Gong W.; Tang X.; Liu Y.; Cui Y.; Liu Y. Artificial biomolecular channels: enantioselective transmembrane transport of amino acids mediated by homochiral zirconium metal–organic cages. J. Am. Chem. Soc. 2021, 143, 20939–20951. 10.1021/jacs.1c09992. [DOI] [PubMed] [Google Scholar]
- a Meng W.; Breiner B.; Rissanen K.; Thoburn J. D.; Clegg J. K.; Nitschke J. R. A self–assembled M8L6 cubic cage that selectively encapsulates large aromatic guests. Angew. Chem., Int. Ed. 2011, 50, 3479–3483. 10.1002/anie.201100193. [DOI] [PubMed] [Google Scholar]; b Otte M.; Kuijpers P. F.; Troeppner O.; Ivanović-Burmazović I.; Reek J. N. H.; de Bruin B. Encapsulated cobalt-porphyrin as a catalyst for size-selective radical-type cyclopropanation reactions. Chem.—Eur. J. 2014, 20, 4880–4884. 10.1002/chem.201400055. [DOI] [PubMed] [Google Scholar]; c Zhou X.-P.; Wu Y.; Li D. Polyhedral metal–imidazolate cages: control of self–assembly and cage to cage transformation. J. Am. Chem. Soc. 2013, 135, 16062–16065. 10.1021/ja4092984. [DOI] [PubMed] [Google Scholar]; d Browne C.; Brenet S.; Clegg J. K.; Nitschke J. R. Solvent-dependent host-guest chemistry of an Fe8L12 cubic capsule. Angew. Chem., Int. Ed. 2013, 52, 1944–1948. 10.1002/anie.201208740. [DOI] [PubMed] [Google Scholar]; e Zhou X.-C.; Wu L.-X.; Wang X.-Z.; Lai Y.-L.; Ge Y.-Y.; Su J.; Zhou X.-P.; Li D. Self–assembly of a Pd4Cu8L8 cage for epoxidation of styrene and its derivatives. Inorg. Chem. 2022, 61, 5196–5200. 10.1021/acs.inorgchem.2c00283. [DOI] [PubMed] [Google Scholar]; f Luo D.; Wu L.-X.; Zhang Y.; Huang Y.-L.; Chen X.-L.; Zhou X.-P.; Li D. Self–assembly of a photoluminescent metal–organic cage and its spontaneous aggregation in dilute solutions enabling time-dependent emission enhancement. Sci. China: Chem. 2022, 65, 1105–1111. 10.1007/s11426-022-1245-1. [DOI] [Google Scholar]; g Yang Y.; Jia J.-H.; Pei X.-L.; Zheng H.; Nan Z.-A.; Wang Q.-M. Diastereoselective synthesis of O-symmetric heterometallic cubic cages. Chem. Commun. 2015, 51, 3804–3807. 10.1039/c5cc00087d. [DOI] [PubMed] [Google Scholar]
- Examples of octahedral cages with Oh-symmetry. (b) and (d) are Oh-symmetry pseudo-octahedra.; a Saha R.; Ghosh A. K.; Samajdar R. N.; Mukherjee P. S. Self–assembled PdII6 molecular spheroids and their proton conduction property. Inorg. Chem. 2018, 57, 6540–6548. 10.1021/acs.inorgchem.8b00668. [DOI] [PubMed] [Google Scholar]; b Hiraoka S.; Harano K.; Shiro M.; Ozawa Y.; Yasuda N.; Toriumi K.; Shionoya M. Isostructural coordination capsules for a series of 10 different d5–d10 transition–metal ions. Angew. Chem., Int. Ed. 2006, 45, 6488–6491. 10.1002/anie.200601431. [DOI] [PubMed] [Google Scholar]; c Samanta D.; Mukherjee P. S. Component selection in the self–assembly of palladium(II) nanocages and cage-to-cage transformations. Chem.—Eur. J. 2014, 20, 12483–12492. 10.1002/chem.201402553. [DOI] [PubMed] [Google Scholar]; d Tessarolo J.; Lee H.; Sakuda E.; Umakoshi K.; Clever G. H. Integrative assembly of heteroleptic tetrahedra controlled by backbone steric bulk. J. Am. Chem. Soc. 2021, 143, 6339–6344. 10.1021/jacs.1c01931. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Jansze S. M.; Severin K. Palladium-based metal–ligand assemblies: the contrasting behavior upon addition of pyridine or acid. J. Am. Chem. Soc. 2019, 141, 815–819. 10.1021/jacs.8b12738. [DOI] [PubMed] [Google Scholar]; f Wang S.; Sawada T.; Ohara K.; Yamaguchi K.; Fujita M. Capsule–capsule conversion by guest encapsulation. Angew. Chem., Int. Ed. 2016, 55, 2063–2066. 10.1002/anie.201509278. [DOI] [PubMed] [Google Scholar]; g Chand D. K.; Biradha K.; Fujita M.; Sakamoto S.; Yamaguchi K. A molecular sphere of octahedral symmetry. Chem. Commun. 2002, 2486–2487. 10.1039/b206625b. [DOI] [Google Scholar]
- Example of octahedral cages with Td-symmetryFujita M.; Oguro D.; Miyazawa M.; Oka H.; Yamaguchi K.; Ogura K. Self–assembly of ten molecules into nanometer-sized organic host frameworks. Nature 1995, 378, 469–471. 10.1038/378469a0. [DOI] [Google Scholar]
- Examples of octahedral cages with T-symmetry.; a He C.; Lin Z.; He Z.; Duan C.; Xu C.; Wang Z.; Yan C. Metal–tunable nanocages as artificial chemosensors. Angew. Chem., Int. Ed. 2008, 47, 877–881. 10.1002/anie.200704206. [DOI] [PubMed] [Google Scholar]; b Chepelin O.; Ujma J.; Wu X.; Slawin A. M. Z.; Pitak M. B.; Coles S. J.; Michel J.; Jones A. C.; Barran P. E.; Lusby P. J. Luminescent, enantiopure, phenylatopyridine iridium-based coordination capsules. J. Am. Chem. Soc. 2012, 134, 19334–19337. 10.1021/ja309031h. [DOI] [PubMed] [Google Scholar]; c Rizzuto F. J.; Wu W. Y.; Ronson T. K.; Nitschke J. R. Peripheral templation generates an MII6L4 guest-binding capsule. Angew. Chem., Int. Ed. 2016, 55, 7958–7962. 10.1002/anie.201602135. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Carpenter J. P.; Ronson T. K.; Rizzuto F. J.; Héliot T.; Grice P.; Nitschke J. R. Incorporation of a phosphino(pyridine) subcomponent enables the formation of cages with homobimetallic and heterobimetallic vertices. J. Am. Chem. Soc. 2022, 144, 8467–8473. 10.1021/jacs.2c02261. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wei Z.; Jing X.; Yang Y.; Yuan J.; Liu M.; He C.; Duan C. A platinum(II)-based molecular cage with aggregation-induced emission for enzymatic photocyclization of alkynylaniline. Angew. Chem., Int. Ed. 2022, 62, e202214577 10.1002/anie.202214577. [DOI] [PubMed] [Google Scholar]; f Li K.; Zhang L.-Y.; Yan C.; Wei S.-C.; Pan M.; Zhang L.; Su C.-Y. Stepwise assembly of Pd6(RuL3)8 nanoscale rhombododecahedral metal–organic cages via metalloligand strategy for guest trapping and protection. J. Am. Chem. Soc. 2014, 136, 4456–4459. 10.1021/ja410044r. [DOI] [PubMed] [Google Scholar]
- a Yazaki K.; Akita M.; Prusty S.; Chand D. K.; Kikuchi T.; Sato H.; Yoshizawa M. Polyaromatic molecular peanuts. Nat. Commun. 2017, 8, 15914. 10.1038/ncomms15914. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Rizzuto F.; Nitschke J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 2017, 9, 903–908. 10.1038/nchem.2758. [DOI] [PubMed] [Google Scholar]
- McTernan C. T.; Davies J. A.; Nitschke J. R. Beyond platonic: how to build metal–organic polyhedra capable of binding low-symmetry, information-rich molecular cargoes. Chem. Rev. 2022, 122, 10393–10437. 10.1021/acs.chemrev.1c00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto F. J.; Carpenter J. P.; Nitschke J. R. Multisite binding of drugs and natural products in an entropically favorable, heteroleptic receptor. J. Am. Chem. Soc. 2019, 141, 9087–9095. 10.1021/jacs.9b03776. [DOI] [PubMed] [Google Scholar]
- Chen B.; Holstein J. J.; Horiuchi S.; Hiller W. G.; Clever G. H. Pd(II) coordination sphere engineering: pyridine cages, quinoline bowls, and heteroleptic pills binding one or two fullerenes. J. Am. Chem. Soc. 2019, 141, 8907–8913. 10.1021/jacs.9b02207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Brenner W.; Ronson T. K.; Nitschke J. R. Separation and selective formation of fullerene adducts within an MII8L6 cage. J. Am. Chem. Soc. 2017, 139, 75–78. 10.1021/jacs.6b11523. [DOI] [PubMed] [Google Scholar]; b Huang N.; Wang K.; Drake H.; Cai P.; Pang J.; Li J.; Che S.; Huang L.; Wang Q.; Zhou H.-C. Tailor-made pyrazolide-based metal–organic frameworks for selective catalysis. J. Am. Chem. Soc. 2018, 140, 6383–6390. 10.1021/jacs.8b02710. [DOI] [PubMed] [Google Scholar]
- a Lewis J. E. M.; Crowley J. D. Metallo–supramolecular self–assembly with reduced-symmetry ligands. ChemPlusChem 2020, 85, 815–827. 10.1002/cplu.202000153. [DOI] [PubMed] [Google Scholar]; b Stephenson A.; Argent S. P.; Riis-Johannessen T.; Tidmarsh I. S.; Ward M. D. Structures and dynamic behavior of large polyhedral coordination cages: An unusual cage-to-cage interconversion. J. Am. Chem. Soc. 2011, 133, 858–870. 10.1021/ja107403p. [DOI] [PubMed] [Google Scholar]
- Zarra S.; Clegg J. K.; Nitschke J. R. Selective assembly and disassembly of a water-soluble Fe10L15 prism. Angew. Chem., Int. Ed. 2013, 52, 4837–4840. 10.1002/anie.201209694. [DOI] [PubMed] [Google Scholar]
- a Zhang D.; Ronson T. K.; Mosquera J.; Martinez A.; Guy L.; Nitschke J. R. Anion binding in water drives structural adaptation in an azaphosphatrane-functionalized FeII4L4 tetrahedron. J. Am. Chem. Soc. 2017, 139, 6574–6577. 10.1021/jacs.7b02950. [DOI] [PubMed] [Google Scholar]; b Riddell I. A.; Smulders M. M. J.; Clegg J. K.; Hristova Y. R.; Breiner B.; Thoburn J. D.; Nitschke J. R. Anion-induced reconstitution of a self–assembling system to express a chloride-binding Co10L15 pentagonal prism. Nat. Chem. 2012, 4, 751–756. 10.1038/nchem.1407. [DOI] [PubMed] [Google Scholar]
- a Hardy M.; Struch N.; Holstein J. J.; Schnakenburg G.; Wagner N.; Engeser M.; Beck J.; Clever G. H.; Lützen A. Dynamic complex-to-complex transformations of heterobimetallic systems influence the cage structure or spin state of iron(II) ions. Angew. Chem., Int. Ed. 2020, 59, 3195–3200. 10.1002/anie.201914629. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Tranchemontagne D. J.; Ni Z.; O’Keeffe M.; Yaghi O. M. Reticular chemistry of metal–organic polyhedra. Angew. Chem., Int. Ed. 2008, 47, 5136–5147. 10.1002/anie.200705008. [DOI] [PubMed] [Google Scholar]
- a McTernan C. T.; Ronson T. K.; Nitschke J. R. Selective anion binding drives the formation of AgI8L6 and AgI12L6 six-stranded helicates. J. Am. Chem. Soc. 2021, 143, 664–670. 10.1021/jacs.0c11905. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Carpenter J. P.; McTernan C. T.; Ronson T. K.; Nitschke J. R. Anion pairs template a trigonal prism with disilver vertices. J. Am. Chem. Soc. 2019, 141, 11409–11413. 10.1021/jacs.9b05432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lang et. al. reportd low-symmetry vetices constructed octahedral metal organic cage which has a regular internal octahedral cavity.Bao S.-J.; Xu Z.-M.; Ju Y.; Song Y.-L.; Wang H.; Niu Z.; Li X.; Braunstein P.; Lang J.-P. The covalent and coordination Co-driven assembly of supramolecular octahedral cages with controllable degree of distortion. J. Am. Chem. Soc. 2020, 142, 13356–13361. 10.1021/jacs.0c07014. [DOI] [PubMed] [Google Scholar]; b Jin et. al. used two kind of vertices to build one C1-symmetry octahedral cage.Liu J.-J.; Lin Y.-J.; Li Z.-H.; Jin G.-X. Self–assembled half-sandwich polyhedral cages via flexible schiff-base ligands: an unusual macrocycle-to-cage conversion. Dalton Trans. 2016, 45, 13675–13679. 10.1039/c6dt02393b. [DOI] [PubMed] [Google Scholar]; c Howlader P.; Mondal S.; Ahmed S.; Mukherjee P. S. Guest-induced enantioselective self–assembly of a Pd6 homochiral octahedral cage with a C3-symmetric pyridyl donor. J. Am. Chem. Soc. 2020, 142, 20968–20972. 10.1021/jacs.0c11011. [DOI] [PubMed] [Google Scholar]
- Stewart J. J. P.MOPAC2016; Stewart Computational Chemistry: Colorado Springs, CO, USA, http://openmopac.net/MOPAC2016.html (accessed June 7, 2023).
- Fujitsu Ltd . SCIGRESS: Tokyo, Japan, 2013.; b Stewart J. J. P. Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. 10.1007/s00894-007-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D.; Ronson T. K.; Güryel S.; Thoburn J. D.; Wales D. J.; Nitschke J. R. Temperature controls guest uptake and release from Zn4L4 tetrahedra. J. Am. Chem. Soc. 2019, 141, 14534–14538. 10.1021/jacs.9b07307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Given the ability of the template G to convert 2 to a single diastereomer of octahedron 3 with an S4 symmetry, we investigated whether cage 1 with a mixture of S4 and D2 symmetry could also be converted into a single diastereomer of octahedron with an S4 symmetry. Experiments using G as a template resulted in precipitation under the same condition described above for the conversion between ZnII9L6 and ZnII6L4, presumbly due to the poor solubility of the host–guest complex without the methyl group.
- CCDC 2180301 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Center.
- Takezawa H.; Murase T.; Resnati G.; Metrangolo P.; Fujita M. Recognition of polyfluorinated compounds through self–aggregation in a cavity. J. Am. Chem. Soc. 2014, 136, 1786–1788. 10.1021/ja412893c. [DOI] [PubMed] [Google Scholar]
- Yang D.; Greenfield J. L.; Ronson T. K.; von Krbek L. K. S.; Yu L.; Nitschke J. R. LaIII and ZnII cooperatively template a metal–organic capsule. J. Am. Chem. Soc. 2020, 142, 19856–19861. 10.1021/jacs.0c09991. [DOI] [PubMed] [Google Scholar]
- We calculated the Van der Waals volumes using the MoloVol program, referring to the reported crystal structures of anions including B(p-C6H4Cl)4– (CCDC, 748991), B(p-C6H4F)4– (708376), IO4– (2168128), ReO4– (821877), PO4(WO3)123– (789283) based on the crystallographic data from The Cambridge Crystallographic Data Center. The caculated internal cavities of 1-S4, 1-D2, and crystal 3-S4 are 411.0, 452.7 and 558.9 Å3, respectively.
- Maglic J. B.; Lavendomme R. MoloVol: an easy-to-use program for analyzing cavities, volumes and surface areas of chemical structures. J. Appl. Crystallogr. 2022, 55, 1033–1044. 10.1107/s1600576722004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin M.; Bernardinelli G.; Nitschke J. R. Synthetic selectivity through avoidance of valence frustration. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 17655–17660. 10.1073/pnas.0607786103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson T. K.; Carpenter J. P.; Nitschke J. R. Dynamic optimization of guest binding in a library of diastereomeric heteroleptic coordination cages. Chem 2022, 8, 557–568. 10.1016/j.chempr.2021.12.017. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.