Abstract
Background:
Lower extremity amputations are common and post-operative neuropathic pain (phantom limb pain or symptomatic neuroma) is frequently reported. The use of active treatment of the nerve end has shown to reduce pain but requires additional resources and should therefore be performed primarily in high-risk patients. The aim of this study is to identify the factors associated with the development of neuropathic pain following above the knee amputation (AKA), knee disarticulation (KD) or below the knee amputation (BKA).
Methods:
Retrospectively, 1,565 patients with an average follow-up of 4.3 years who underwent a primary AKA, KD or BKA were identified. Amputation levels AKA and KD were combined as proximal amputation level, with BKAs being performed in 61% of patients. The primary outcome was neuropathic pain (phantom limb pain or symptomatic neuroma) based on medical chart review. Multivariable logistic regression was performed to identify independent factors associated with neuropathic pain.
Results:
Post-operative neuropathic pain was present in 584 (37%) of patients, with phantom limb pain occurring in 34% of patients and symptomatic neuromas occurring in 3.8% of patients. Proximal amputation level, normal creatinine levels and a history of psychiatric disease were associated with neuropathic pain. Diabetes, hypothyroidism and older age were associated with a lower odds of developing neuropathic pain.
Conclusions:
Neuropathic pain following lower extremity amputation is common. Factors influencing nerve regeneration, either increasing (proximal amputations and younger age) or decreasing (diabetes, hypothyroidism, chronic kidney disease) it, play a role in the development of post-amputation neuropathic pain.
Level of Evidence:
IV
Introduction
Lower extremity amputations are prevalent, with 623,000 Americans living with a major lower extremity amputation in 2005, and this number is expected to increase in the coming decades.1 The most commonly performed major amputations are above the knee amputations (AKA) and below the knee amputations (BKA).2
A recent meta-analysis reported that phantom limb pain occurs in 27–86% of patients following amputation, with this wide range being attributed to the heterogeneity in study design and outcome definition.3 Symptomatic neuromas often correlate with phantom limb pain as they can contribute to post-amputation neuropathic pain.4,5 As a consequence, rehabilitation and prosthesis use is negatively impacted which contributes to the residual limb patient experiencing an inferior overall quality of life and decreased survival.6,7
Since 2014, the application of “active” surgical treatments to address the nerve ends transected following amputation has gained popularity.8,9 Targeted muscle reinnervation (TMR) and regenerative peripheral nerve interfaces (RPNI) are surgical techniques that provide terminal nerve axons a path along which they can regrow as well as provide a functional task once the target muscle has been re-innervated. When applied at the time of amputation and/or after the development of neuropathic pain, both of these techniques have proven to reduce pain, decrease opioid use, and increase prosthesis use.9,10
Offering patients “active” management of the nerve endings with TMR or RPNI at the time of amputation involves a multidisciplinary team of orthopaedic, general, and peripheral nerve surgeons which requires additional resources during the initial amputation surgery. It is therefore important to identify patients most at risk for the development of post-amputation neuropathic pain so that they can receive optimal surgical care with appropriate allocation of required resources. The aim of this study is to identify those factors associated with the development of neuropathic pain following lower extremity amputation in an effort to aid in appropriate resource application and utilization.
Methods
Following Institutional Review Board approval, Current Procedural Terminology (CPT) codes, International Classification of Diseases (ICD), 9th and 10th Revision procedural and diagnosis codes were used to identify patients that underwent primary amputation of the lower extremity at one of five urban hospitals in the Northeastern United States, which included two level I trauma centers (n=4,253) (See Table, Supplemental Digital Content 1, which presents codes to identify patients with lower extremity amputation, INSERT HYPERLINK HERE).
All adult (>18 years) patients who underwent an AKA, knee disarticulation (KD) or BKA with a minimum post-operative survival of 12 months between January 1st, 2000 and August 1st, 2018 were included (n=2,266). A medical chart review was performed to confirm if a patient met inclusion criteria. Further exclusions included miscoding (i.e. the patient did not have an amputation n=219) or miscoded for an amputation level different than AKA, BKA or KD (n=83), TMR or RPNI at the time of amputation (n=16) or inaccessible operative notes (n=2). Amputations at the level of or distal to the tibiotalar joint were excluded due to the small nerve size at that level and the limitation identifying target neuromuscular junctions in this area. Additionally, patients with a follow-up of less than 6 months were excluded (n=381). After application of the exclusions noted previously, a total of 1,565 patients were included for analysis. Only initial amputations at one of the aforementioned levels were included. In cases where a BKA or KD was converted to an AKA, the final amputation level was included and conversion of amputation was included as an explanatory variable.
A combination of a medical chart review and a centralized clinical data registry for research (Research Patient Data Registry (RPDR)) was used to obtain patient characteristics, lab values, injury characteristics and outcome variables. Demographic characteristics such as age, sex, race, insurance status was extracted directly from RPDR. Median household income was calculated based on the median income by zip code from 2006–2010 reported by the United States Census Bureau.11 Using ICD-9 and ICD-10 codes, the Elixhauser comorbidity score was calculated, which takes into account 30 comorbidities.12,13 Additionally, several specific comorbidities including hypertension, diabetes mellitus, history of cardiac disease, chronic pulmonary disease, rheumatoid arthritis/collagen vascular disease, paralysis, neurodegenerative disorders, hypothyroidism, liver disease, alcohol abuse, drug abuse and a history of psychiatric disease were determined using the Elixhauser comorbidity score ICD9/10 codes and included as an explanatory variable. Tobacco use was determined using ICD-9 diagnosis codes (305.1, V15.82) and ICD-10 diagnosis codes (F17.20, F17.21, F17.22, F17.29, Z71.6, Z72.0, Z77.22, Z87.891, T65.2). In all instances where diagnosis codes were used, the comorbidity was considered to be present if it was coded for three months before or after the amputation.
The amputation level was determined based on CPT along with ICD9/10 codes, and patients who received a code for amputation twice were reviewed manually to determine the final amputation level or if conversion from BKA to AKA was performed. Bilateral amputations were defined as a bilateral amputation at the time of initial amputation surgery. The indication for amputation was based on medical chart review and grouped as peripheral vascular disease, infection, trauma, oncology and other indications (which included fracture nonunion, chronic pain, nerve palsy, deformity, dysfunctional limb, untreatable arthritis/Charcot deformity amongst others). Albumin level, calcium level, creatinine level, hemoglobin level and platelet count within three months prior to amputation were included as explanatory variables. Follow-up was defined as the time from surgery to the last visit at one of the hospitals within our health system.
Primary Outcome
Neuropathic pain was defined as the presence of phantom limb pain or a symptomatic neuroma, as based upon the patients’ reported history and clinical examination, that developed following amputation. This pain data was obtained based on the reports by treating physicians in the medical charts. Sub-categories included (1) phantom limb pain alone, (2) phantom limb pain in combination with a symptomatic neuroma, or (3) a symptomatic neuroma alone. To identify patients with neuropathic pain, CPT codes, ICD-9/10 diagnosis and procedure codes were used to flag patients (See Table, Supplemental Digital Content 2, which shows diagnosis and procedure codes used to flag patients with potential neuropathic stump pain, INSERT HYPERLINK HERE). Additionally, using data processing software (Stata, StataCorp, College Station, USA) the medical charts were reviewed and patients with “neurom” or “phantom” mentioned in their charts were flagged. A medical chart review was performed to confirm the presence of neuropathic pain in all flagged patients.
Statistical analysis
Continuous variables were reported as means and standard deviations or medians and interquartile ranges (IQR) depending on normality, and dichotomous and categorical variables were reported as frequencies and percentages. Normality was determined using the Shapiro-Wilk test. Bivariate analysis was performed using the Student’s t-test for age and label values, and the Mann-Whitney U test was used for median income and Elixhauser comorbidity score. For dichotomous and categorical variables the Fisher’s exact test was used to identify the factors associated with the development of neuropathic pain. The amputation levels AKA and KD were combined in analysis. Multivariable logistic regression was performed including all explanatory variables with a p<0.1 in bivariate analysis. Age, race, indication for amputation, amputation level, Elixhauser comorbidity score, diabetes, hypertension, history of cardiac disease, tobacco use, drug abuse, history of psychiatric disease, hypothyroidism, creatinine level were included in the multivariable analysis. Albumin level was not included in the multivariable analysis because this was missing in 27%. Additionally, a Locally Weighted Scatterplot Smoothing (LOWESS) curve was plotted for age using the probability of developing neuropathic based on the logistic regression coefficient for age. A p-value of <0.05 was deemed statistically significant. Analyses were performed using Stata 13.0 (Stata Corp. College Station, USA).
Study population
The patients in this cohort had an average age of 60.4±16.2 years with a median age of 61.4 years (interquartile range: 50.2–72.1), with 65% (n=1,021) being male (Table 1). BKA patients were more common (61%) than AKA patients (38%), and peripheral vascular disease (41%) was the most common indication for amputation followed by infection (40%). The median follow-up was 37 months (IQR: 19.0–67.9). According to the Elixhauser comorbidity score, 79% (n=1,233) of patients had three or more comorbidities.
Table 1:
Patient Characteristics (n=1565)
| Characteristic | Value |
|---|---|
| Mean age (SD), yr | 60.4 (16.2) |
| Male sex, n(%) | 1021 (65) |
| Race, n(%) | |
| White | 1229 (83) |
| NonWhite | 261 (18) |
| Bilateral, n(%) | 42 (2.7) |
| Conversion to above knee amputation | 75 (4.8) |
| Indication for amputation, n(%) | |
| Peripheral vascular disease | 643 (41) |
| Infection | 625 (40) |
| Trauma | 152 (9.7) |
| Oncology | 97 (6.2) |
| Other | 48 (3.1) |
| Amputation level, n(%) | |
| Above the knee | 590 (38) |
| Knee disarticulation | 19 (1.2) |
| Below the knee | 956 (61) |
Results
A total of 584 (37%) patients in the study group (n=1565) developed neuropathic pain following amputation, with the majority reporting phantom limb pain alone (34% of all patients) (Table 2). Additionally, 39 (2.5%) patients had phantom limb pain concurring with a symptomatic neuroma and 20 patients (1.3%) only had a symptomatic neuroma (Table 2).
Table 2:
Postamputation Symptoms
| Symptom | Value |
|---|---|
| Phantom limb pain, n(%) | 525 (34) |
| Phantom limb pain & symptomatic neuroma, n(%) | 39 (2.5) |
| Symptomatic neuroma, n(%) | 20 (1.3) |
| No symptoms, n(%) | 981 (63) |
After including explanatory variables with a p<0.1 in bivariate analysis (Tables 3–5), multivariable analysis showed that more proximal amputation level (AKA/KD) (OR: 1.5, 95% CI: 1.2–1.9, p=0.001); normal thyroid function (OR: 1.5, 95% CI: 1.1–2.0, p=0.019), normal creatine levels (OR: 1.3, 95% CI: 1.0–1.7, p=0.045) and a history psychiatric disease (OR: 1.6, 95% CI: 1.2–2.1, p<0.001) were independently associated with higher levels of neuropathic pain (Table 6). Additionally, older age (OR: 0.99, 95% CI: 0.98–0.99, p<0.001) and diabetes (OR: 0.70, 95% CI: 0.52, 0.93, p=0.015) were independently associated with a lower odds of neuropathic pain (Figure 1).
Table 3:
Patient and Injury Characteristics Associated with Neuropathic Pain
| Neuropathic pain |
p | ||
|---|---|---|---|
| Yes (n=584, 37%) | No (n=981, 63%) | ||
| Mean age (SD), yr | 57.0 (15.9) | 62.5 (16.0) | <0.001* |
| Male sex, n(%) | 376 (37) | 645 (63) | 0.31 |
| Median income, $ (IQR) | 67,118 (51,723–80,153) | 66,023 (51,723–80,153) | 0.62 |
| Insurance type, n(%) | 0.27 | ||
| Medicare + insurance | 349 (37) | 586 (63) | |
| Medicare alone | 9 (24) | 29 (76) | |
| Private insurance | 181 (38) | 298 (62) | |
| Medicaid | 27 (24) | 47 (64) | |
| Self-pay | 10 (42) | 14 (58) | |
| Worker’s compensation insurance | 3 (60) | 2 (40) | |
| Veterans insurance | 2 (100) | 0 | |
| Race | 0.008* | ||
| White | 487 (40) | 742 (61) | |
| NonWhite | 80 (31) | 181 (69) | |
| Bilateral, n(%) | 17 (41) | 25 (60) | 0.75 |
| Conversion to above-knee amputation | 33 (44) | 42 (56) | 0.22 |
| Indication for amputation, n(%) | <0.001* | ||
| Peripheral vascular disease | 227 (35) | 416 (65) | |
| Infection | 213 (34) | 412 (66) | |
| Trauma | 77 (51) | 75 (49) | |
| Oncology | 50 (52) | 47 (49) | |
| Other | 17 (35) | 31 (65) | |
| Amputation level, n(%) | <0.001* | ||
| Above the knee | 267 (44) | 342 (56) | |
| Below the knee | 317 (33) | 639 (67) | |
IQR, interquartile range.
Statistically significant.
Table 5:
Laboratory Values Associated with Neuropathic Pain
| Neuropathic pain |
p | ||
|---|---|---|---|
| Yes (n=584, 37%) | No (n=981, 63%) | ||
| Albumin, mean (SD), g/dl* | 3.3 (0.8) | 3.1 (0.8) | <0.001‡ |
| Calcium, mean (SD), mg/dl† | 8.8 (0.8) | 8.7 (0.8) | 0.20 |
| Creatinine, mean (SD), mg/dl† | <0.001‡ | ||
| >1.2 male, >1.1 female | 197 (30) | 451 (70) | |
| ≤ 1.2 male, ≤ 1.1 female | 367 (43) | 492 (57) | |
| Hemoglobin, mean (SD), g/dl† | 10.6 (2.1) | 10.5 (2.0) | 0.32 |
| Platelet count, mean (SD), count/μl† | 321 (163) | 323 (147) | 0.75 |
Twenty-seven percent missing data.
Less than 10 percent missing data.
Statistically significant.
Table 6:
Multivariable Logistic Regression Factors Associated with Neuropathic Pain (n=1435)*
| OR | SE | 95% CI | p | |
|---|---|---|---|---|
| Age | 0.99 | 0.004 | [0.98, 0.99] | <0.001† |
| Race (reference: nonwhite) | 1.3 | 0.21 | [0.97, 1.8] | 0.075 |
| Indication | ||||
| Peripheral vascular disease | Ref | Ref | Ref | Ref |
| Infection | 0.83 | 0.11 | [0.64, 1.1] | 0.15 |
| Trauma | 1.2 | 0.27 | [0.76, 1.8] | 0.46 |
| Oncology | 1.2 | 0.30 | [0.71, 1.9] | 0.53 |
| Other | 0.71 | 0.28 | [0.33, 1.5] | 0.39 |
| Amputation level (reference: above-the-knee amputation) | 1.5 | 0.18 | [1.2, 1.9] | 0.001† |
| Elixhauser comorbidity score | 1.0 | 0.03 | [0.99, 1.1] | 0.13 |
| Diabetes | 0.70 | 0.10 | [0.52, 0.93] | 0.015† |
| Cardiac disease | 0.77 | 0.12 | [0.58, 1.05] | 0.09 |
| Tobacco use | 1.2 | 0.15 | [0.90, 1.5] | 0.27 |
| Drug abuse | 1.3 | 0.24 | [0.87, 1.8] | 0.22 |
| History of psychiatric disease | 1.6 | 0.21 | [1.2, 2.1] | <0.001† |
| Hypothyroidism | 0.69 | 0.11 | [0.50, 0.94] | 0.019† |
| Creatinine, mean (SD), mg/dl | ||||
| ≤ 1.2 male, ≤ 1.1 female | Ref | Ref | Ref | Ref |
| >1.2 male, >1.1 female | 0.77 | 0.10 | [0.59, 0.98] | 0.045† |
Ref, reference
Area under the curve, 0.67.
Statistically significant.
Fig. 1.
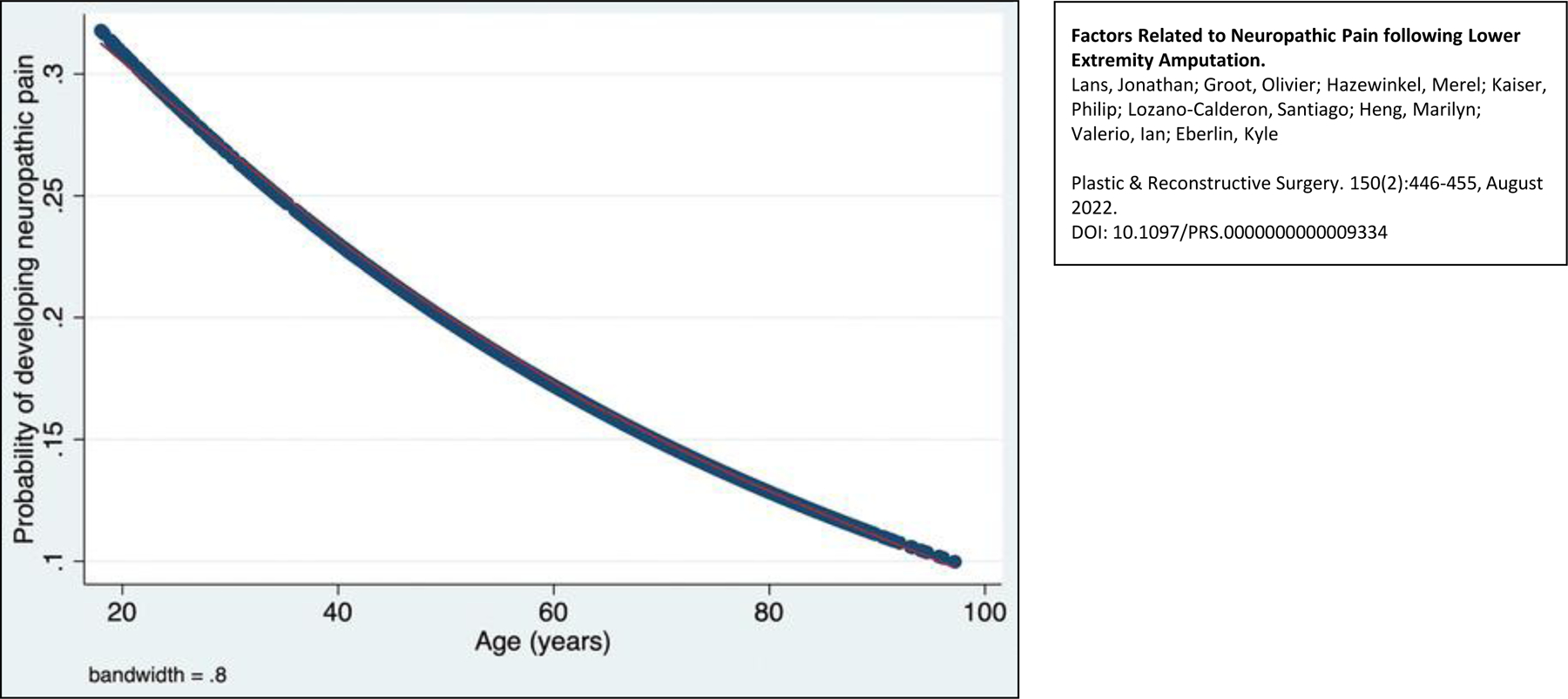
Locally weighted scatterplot smoothing curve for the probability of developing neuropathic pain depending on age.
Discussion
This study evaluated post-amputation neuropathic pain in 1,565 patients that underwent AKA, KD or BKA. In a mean follow-up period of 4.3 years, 584 of the patients (37%) developed neuropathic pain following their amputation, with phantom limb pain (36%) reported in the majority of patients. AKA, younger age and a history of psychiatric disease were associated with the development of neuropathic pain following amputation. Furthermore, patients with diabetes, elevated creatinine levels and hypothyroidism had lower odds of developing neuropathic stump pain.
There are several limitations to this study that need to be considered. First, the outcome of interest, “neuropathic pain”, was determined by retrospective medical chart review. This information was extracted from the descriptions and reports by the treating physicians. As a result, the rates of reported neuropathic pain likely may be an under-representation, but we suspect that the patients identified are those with clinically relevant neuropathic pain. Additionally, the size of the current cohort enables identification of clinically relevant associations. Second, we did not assess how neuropathic pain impacted the patient’s psychologic or overall well-being nor did we evaluate longitudinal changes in neuropathic pain over time nor the presence of pre-operative pain. It was noted that the patients who underwent amputation for chronic pain (n=14), eight (57%) developed neuropathic pain. Third, prosthesis use amongst the patients in this study was not assessed, but based on other studies it is known that neuropathic pain results in reduced prosthetic use.14 Fourth, gradual changes in phantom pain over time could not be accounted for as standardized follow-up was lacking, but phantom limb pain had to be present at an post-operative follow up visit. Although the nature of phantom pain may change over time, with our current knowledge regarding TMR/RPNI any neuropathic pain is clinically relevant. Lastly, some of the variables (i.e. Elixhauser comorbidity score, cardiac disease, diabetes, hypothyroidism, history of psychiatric disease) were based on ICD coding and therefore relies on adequate and accurate coding.
Over a third of the patients with an AKA, KD or BKA developed neuropathic pain in this study. Phantom limb pain is thought to result from maladaptive cortical, subcortical and spinal reorganization following nerve injury.15–17 Neuromas were specifically reported in only 3.8% of patients, which is likely an under-representation because not all healthcare providers typically document neuroma and/or are aware of their close relationship with neuropathic amputation pain. It is known that all transected nerves without a distal nerve target develop a neuroma as axonal regeneration takes place outside the epineurium in an unorganized manner.18–20 It remains unknown why some patients develop a symptomatic neuroma while others do not. However, an imbalance between the degenerative and regenerative processes and (over)expression of neurotrophic factors in the regenerative process may be causative.21–23
The incidence of phantom limb pain following lower extremity amputations ranges from 32% to 80% as reported in the literature.2,6,24–26 In a cross-sectional survey study Morgan et al. included 1,296 amputees, distal to the hip and proximal to the ankle, of which 48% developed phantom limb pain and 35% developed residual limb pain, which is similar to our findings.6 Ephraim et al. studied 914 patients who underwent upper or lower extremity amputation using surveys and reported phantom limb pain 80% of patients, which is almost twice the rate as in our series.25 Their reported findings of higher phantom limb pain may be explained by the higher percentage of proximal amputations and the survey nature of their study design. Interestingly, only 39% of patients reported severe phantom limb pain. Similarly, a higher rate of phantom limb pain (73%) and residual limb pain (70%) amongst 727 upper and lower extremity amputees was reported by Mioton et al., with 23% reporting moderate pain and 29% reporting severe pain.2 When interpreting studies using questionnaires one must be aware of responder bias, as it has been shown that responders report higher pain scores compared to non-responders.27 In the future it would be interesting to determine what degree of neuropathic pain and/or amputation pain is clinically relevant, as it seems that phantom limb pain only impacts physical or emotional function in roughly a quarter of those affected.26 Following lower extremity amputation the incidence of symptomatic neuroma, despite the association with phantom limb pain, has not been thoroughly studied and has been reported to range 4.2–14%.4,5,28
Patients with proximal amputations (44%) had a higher rate of neuropathic pain compared to more distal amputations (33%). Other studies have supported such findings of phantom limb pain being more prevalent in proximal amputations.2,29 This finding may be explained by the larger nerve caliber and concentration of affected axons proximally; however, experimental studies are needed to confirm the exact pathophysiology. Higher rates of neuropathic pain seen in younger age patients is likely due to the fact that the nerve regenerative potential decreases with age, and therefore younger patients’ nerves tend to regenerate more rapidly.30 This interesting finding is one that we have anecdotally noticed clinically in our residual limb patient population.31
Depression and anxiety have frequently been shown to be predictive for the development of neuropathic pain or phantom limb pain.25,32 Thirty-six percent of the patients in this study had a history of psychiatric disease and its presence was associated with higher odds of developing neuropathic pain (OR: 1.6 95% CI: 1.2–2.1). The interaction between pain and depression is known as the depression-pain syndrome, as both pain and depression have similar biological pathways and neurotransmitters, exacerbating each other.33–35 Clinically it is important to identify these patients prior to amputation because preventative measures such as pre-operative psychosocial counseling or pharmacologic treatment, including certain neuromodulating medications such as gabapentin, can decrease patient’s anxiety and pain-catastrophizing in an attempt to mitigate post-operative neuropathic pain.36
Diabetes and chronic kidney disease were associated with lower odds of developing neuropathic pain, and it seems likely that healing capacity – along with nerve regenerative potential – influences the development of neuropathic pain. Stenberg and Dahlin assessed axonal outgrowth along with activation transcription factor 3 (ATF-3) and cleaved caspase 3 staining in Schwann cells in diabetic and non-diabetic rats.37 Activation transcription factor 3 staining is a marker for Schwann cell activation and correlates with axonal outgrowth, whereas cleaved caspase 3 is a marker for Schwann cell apoptosis. Their findings showed decreased axonal outgrowth amongst diabetic rats and a larger number of cleaved caspase 3 Schwann cells in diabetic rats. These reported findings support the hypothesis that diabetic patients have decreased nerve regenerative potential and are thus less prone to develop neuropathic pain. Chronic kidney disease negatively impacts wound healing and the increased uremic toxics can impair nerve regeneration.38,39
Albumin is a biochemical indicator for chronic malnutrition, and nutrients play a role in many components of nerve regeneration.40,41 We did not include albumin level in the multivariable analysis, but in bivariate analysis higher albumin levels were associated with neuropathic pain. The average albumin level in patients who developed neuropathic pain was 3.3 g/dL compared to 3.1 g/dL in those that did not.
An interesting finding was that the rate of neuropathic pain was reduced in patients with hypothyroidism (OR: 0.69), which may be related to the regulatory role of thyroid hormone in nerve regeneration. Thyroid hormone regulates Schwann cell proliferation and stimulates axonal regeneration, as neurons express triiodothyronine (T3) receptors.42 In rats nerve regeneration has shown to be enhanced when nerve conduits are injected with T3.43 Many patients with hypothyroidism receive replacement therapy with levothyroxine but how this synthetic T4 impacts nerve regeneration remains unknown.
Targeted muscle reinnervation and RPNI have gained popularity to prevent and treat neuropathic pain and symptomatic neuroma formation following amputation.44,45 Several clinical studies have replicated significant reduction in phantom limb pain and residual limb pain, along with a decrease in opioid use.10,46–49 When TMR is performed, the terminal nerve branches of the amputation are redirected towards expendable motor nerve branches in close proximity.46 In some cases the neurorrhaphy is wrapped with a local vascularized muscle flap so that axonal escape can grow into the muscle’s motor end plate.50 In RPNI a non-vascularized muscle graft is wrapped around the terminal nerve ends so that the nerve can regenerate into and reinnervate the motor end plates.51 Both of these techniques rely on (1) giving the nerve a functional denervated target to reinnervate and (2) giving the nerve an endoneurial “runway” along which the regenerating axons can grow. These surgical techniques have been shown to limit the formation of symptomatic neuroma, and by recreating a functional neuromuscular unit, the central components of phantom limb pain may be more effectively addressed.52 Additionally, reducing nociceptive input towards the nerve end interferes with the chronic neuropathic pain cycle.53 While refractory neuropathic pain following TMR/RPNI surgery can occur, it is thought to be the result of persistent subcortical and peripheral changes that induce and maintain cortical changes, a situation that is often apparent in patients with long-standing neuropathic pain. Another explanation could be that central sensitization has taken place in patients with long-standing neuropathic pain.54
In conclusion, development of neuropathic pain following lower extremity amputation is common and seems to be related to those factors influencing nerve regeneration as well as those influencing pain experience. Younger age and proximal amputations were associated with post-amputation neuropathic pain, most likely due an increased nerve regenerative potential in these situations. On the contrary, diabetes, hypothyroidism and chronic kidney disease all decreased the odds of developing neuropathic pain, as such comorbidities impair nerve regeneration. Using the findings reported in this study, the authors suggest a clinical algorithm to aid decision making for TMR/RPNI in the setting of AKA, KD and BKA (Figure 2). Application of such an algorithm permits adequate resource allocation for patients at high risk of neuropathic pain and supports referral to a specialized center in certain instances. This concept of multi-disciplinary and centralized care for neuropathic pain needs to be further evaluated at the level of patient outcomes and cost-effectivity. Furthermore, additional research into the underlying pathophysiologic mechanisms of the factors identified in this study will allow for further advancement in treating post-amputation neuropathic pain.
Fig. 2.
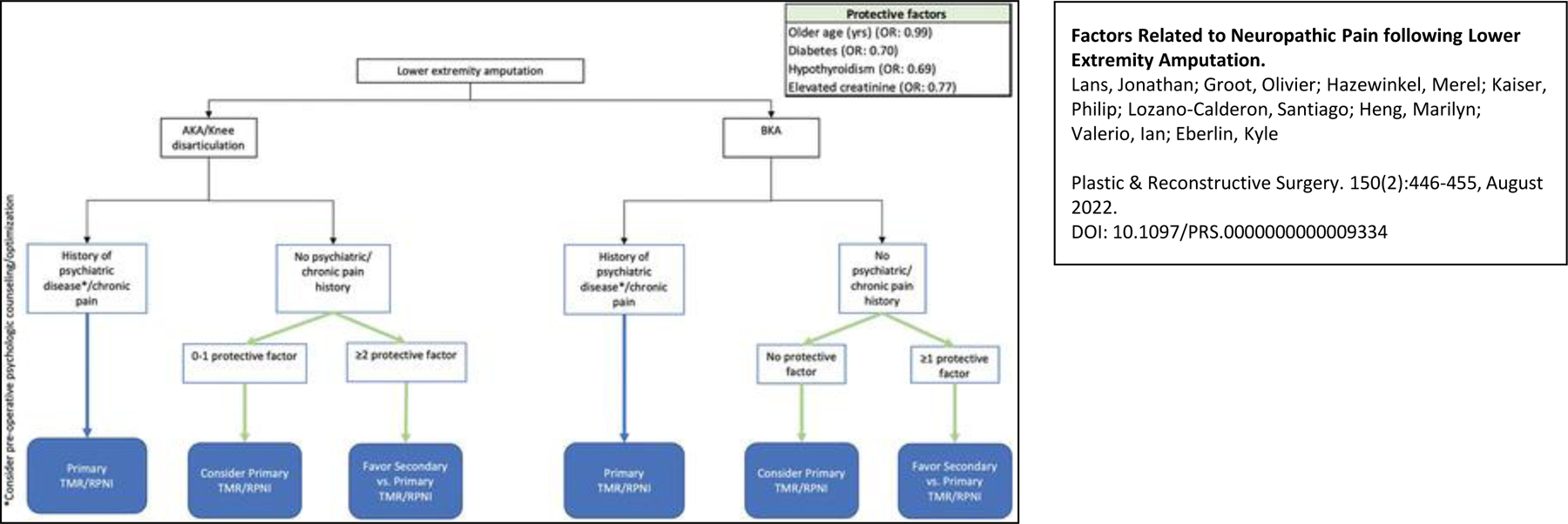
Algorithm for contemporary treatment of the nerve end following amputation. AKA, above-the-knee amputation; BKA, below-the-knee amputation, TMR, targeted muscle reinnervation; RPNI, regenerative peripheral nerve interfaces.
Supplementary Material
Table, Supplemental Digital Content 1: Codes to identify patients with lower extremity amputation
Table, Supplemental Digital Content 2: Diagnosis and procedure codes used to flag patients with potential neuropathic stump pain.
Table 4:
Patient Comorbidities Associated with Neuropathic Pain
| Neuropathic pain |
p | ||
|---|---|---|---|
| Yes (n=584, 37%) | No (n=981, 63%) | ||
| Elixhauser comorbidity score, median (IQR) | 6 (4–10) | 7 (4–10) | 0.022* |
| Diabetes mellitus, n(%) | 273 (31) | 615 (69) | <0.001* |
| Hypertension, n(%) | 397 (36) | 705 (64) | 0.11 |
| Cardiac disease, n(%) | 381 (35) | 710 (65) | 0.003* |
| Chronic pulmonary disease, n(%) | 145 (40) | 222 (61) | 0.32 |
| Obesity, n(%) | 95 (40) | 144 (60) | 0.42 |
| Tobacco use, n(%) | 187 (42) | 257 (58) | 0.015* |
| Alcohol abuse, n(%) | 17 (34) | 33 (66) | 0.66 |
| Drug abuse, n(%) | 83 (49) | 86 (51) | 0.001* |
| History of psychiatric disease, n(%) | 259 (46) | 302 (54) | <0.001* |
| Hypothyroidism, n(%) | 135 (33) | 276 (67) | 0.032* |
| Rheumatoid arthritis/collagen vascular diseases, n(%) | 82 (37) | 142 (63) | 0.88 |
| Paralysis, n(%) | 17 (30) | 39 (70) | 0.33 |
| Neurodegenerative disorders, n(%) | 45 (34) | 86 (66) | 0.51 |
| Liver disease, n(%) | 64 (40) | 98 (61) | 0.55 |
IQR, interquartile range.
Statistically significant.
Financial Disclosure Statement:
Dr. Lozano-Calderón, Dr. Heng, Dr. Kaiser, Mr. Groot and Ms. Hazewinkel have nothing to disclose. Dr. Lans is a consultant for Axogen. Dr. Valerio is a consultant for AxoGen, Integra Lifesciences and Stryker Corp. Dr. Eberlin is a consultant for AxoGen, Integra Lifesciences and Checkpoint. No funding was received for this article.
Footnotes
Conflict of Interest: None
Meeting presentation: none
References:
- 1.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008;89(3):422–429. doi: 10.1016/j.apmr.2007.11.005 [DOI] [PubMed] [Google Scholar]
- 2.Mioton LM, Dumanian GA, Fracol ME, et al. Benchmarking Residual Limb Pain and Phantom Limb Pain in Amputees through a Patient-reported Outcomes Survey. Plast Reconstr Surg - Glob Open 2020:1–7. doi: 10.1097/GOX.0000000000002977 [DOI] [PMC free article] [PubMed]
- 3.Limakatso K, Bedwell GJ, Madden VJ, Parker R. The prevalence and risk factors for phantom limb pain in people with amputations: A systematic review and meta-analysis. PLoS One 2020;15(10 October):1–21. doi: 10.1371/journal.pone.0240431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penna A, Konstantatos A, Cranwell W, Paul E, Bruscino-Raiola F. Incidence and associations of painful neuroma in a contemporary cohort of lower-limb amputees Anthony. ANZ J Surg 2018;88(5):491–496. [DOI] [PubMed] [Google Scholar]
- 5.Buch NS, Qerama E, Brix Finnerup N, Nikolajsen L. Neuromas and postamputation pain. Pain 2020;161(1):147–155. doi: 10.1097/j.pain.0000000000001705 [DOI] [PubMed] [Google Scholar]
- 6.Morgan SJ, Friedly JL, Amtmann D, Salem R, Hafner BJ. Cross-Sectional Assessment of Factors Related to Pain Intensity and Pain Interference in Lower Limb Prosthesis Users. Arch Phys Med Rehabil 2017;98(1):105–113. doi: 10.1016/j.apmr.2016.09.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh RK, Prasad G. Long-term mortality after lower-limb amputation. Prosthet Orthot Int 2016;40(5):545–551. doi: 10.1177/0309364615596067 [DOI] [PubMed] [Google Scholar]
- 8.Souza JM, Cheesborough JE, Ko JH, Cho MS, Kuiken TA, Dumanian GA. Targeted Muscle Reinnervation: A Novel Approach to Postamputation Neuroma Pain. Clin Orthop Relat Res 2014;472(10):2984–2990. doi: 10.1007/s11999-014-3528-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woo SL, Kung TA, Brown DL, Leonard JA, Kelly BM, Cederna PS. Regenerative Peripheral Nerve Interfaces for the Treatment of Postamputation Neuroma Pain: A Pilot Study. Plast Reconstr surgery Glob open 2016;4(12):e1038. doi: 10.1097/GOX.0000000000001038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumanian GA, Potter BK, Mioton LM, et al. Targeted Muscle Reinnervation Treats Neuroma and Phantom Pain in Major Limb Amputees. Ann Surg 2018;270(2):1. doi: 10.1097/sla.0000000000003088 [DOI] [PubMed] [Google Scholar]
- 11.Zip Code Characteristics: Mean and Median Household Income. Michigan Population Studies Center https://www.psc.isr.umich.edu/dis/census/Features/tract2zip/. Published 2010.
- 12.Van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009;47(6):626–633. doi: 10.1097/MLR.0b013e31819432e5 [DOI] [PubMed] [Google Scholar]
- 13.Quan H, Sundararajan V, Halfon P, et al. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med Care 2005;43(11):1130–1139. [DOI] [PubMed] [Google Scholar]
- 14.Raichle KA, Hanley MA, Molton I, et al. Prosthesis use in persons with lower- and upper-limb amputation Katherine. J Rehabil Res Dev 2008;45(7):961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finnerup NB, Nikolajsen L, Jensen TS. Are we neglecting spinal reorganization following nerve damage? Pain 2012;153(2):269–272. doi: 10.1016/j.pain.2011.10.030 [DOI] [PubMed] [Google Scholar]
- 16.Davis KD, Kiss ZHT, Luo L, Tasker RR, Lozano AM, Dostrovsky JO. Phantom sensations generated by thalamic microstimulation. Nature 1998;391(6665):385–387. doi: 10.1038/34905 [DOI] [PubMed] [Google Scholar]
- 17.Karl A, Birbaumer N, Lutzenberger W, Cohen LG, Flor H. Reorganization of motor and somatosensory cortex in upper extremity amputees with phantom limb pain. J Neurosci 2001;21(10):3609–3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oliveira KMC, Pindur L, Han Z, Bhavsar MB, Barker JH, Leppik L. Time course of traumatic neuroma development. PLoS One 2018;13(7):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg 1985;76(3):345–352. doi: 10.1097/00006534-198509000-00001 [DOI] [PubMed] [Google Scholar]
- 20.Watson J, Gonzalez M, Romero A, Kerns J. Neuromas of the Hand and Upper Extremity. J Hand Surg Am 2010;35(3):499–510. doi: 10.1016/j.jhsa.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 21.Davies AJ, Rinaldi S, Costigan M, Oh SB. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front Neurosci 2020;14(February):1–20. doi: 10.3389/fnins.2020.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanga FY, Raghavendra V, Nutile-McMenemy N, Marks A, DeLeo JA. Role of astrocytic S100β in behavioral hypersensitivity in rodent models of neuropathic pain. Neuroscience 2006;140(3):1003–1010. doi: 10.1016/j.neuroscience.2006.02.070 [DOI] [PubMed] [Google Scholar]
- 23.Anand P Nerve growth factor regulates nociception in human health and disease. Br J Anaesth 1995;75(2):201–208. doi: 10.1093/bja/75.2.201 [DOI] [PubMed] [Google Scholar]
- 24.Bosmans JC, Geertzen JHB, Post WJ, Van Der Schans CP, Dijkstra PU. Factors associated with phantom limb pain: A 3 1/2-year prospective study. Clin Rehabil 2010;24(5):444–453. doi: 10.1177/0269215509360645 [DOI] [PubMed] [Google Scholar]
- 25.Ephraim PL, Wegener ST, MacKenzie EJ, Dillingham TR, Pezzin LE. Phantom pain, residual limb pain, and back pain in amputees: Results of a national survey. Arch Phys Med Rehabil 2005;86(10):1910–1919. doi: 10.1016/j.apmr.2005.03.031 [DOI] [PubMed] [Google Scholar]
- 26.Richardson C, Crawford K, Milnes K, Bouch E, Kulkarni J. A Clinical Evaluation of Postamputation Phenomena Including Phantom Limb Pain after Lower Limb Amputation in Dysvascular Patients. Pain Manag Nurs 2015;16(4):561–569. doi: 10.1016/j.pmn.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Lonner JH, Nelson CL, Lotke PA. Response Bias: Effect on Outcomes Evaluation by Mail Surveys after Total Knee Arthroplasty. J Bone Jt Surg - Ser A 2004;86(1):15–21. doi: 10.2106/00004623-200401000-00004 [DOI] [PubMed] [Google Scholar]
- 28.Sehirlioglu A, Ozturk C, Yazicioglu K, Tugcu I, Yilmaz B, Goktepe AS. Painful neuroma requiring surgical excision after lower limb amputation caused by landmine explosions. Int Orthop 2009;33(2):533–536. doi: 10.1007/s00264-007-0466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lans J, Hoftiezer Y, Lozano-Calderón SA, Heng M, Valerio IL, Eberlin KR. Risk factors for neuropathic pain following major upper extremity amputation. J Reconstr Microsurg 2020. doi: 10.1055/s-0040-1718547 [DOI] [PMC free article] [PubMed]
- 30.Painter MW, Brosius Lutz A, Cheng YC, et al. Diminished Schwann cell repair responses underlie age-associated impaired axonal regeneration. Neuron 2014;83(2):331–343. doi: 10.1016/j.neuron.2014.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sobti N, Park A, Crandell D, et al. Interdisciplinary Care for Amputees Network: A Novel Approach to the Management of Amputee Patient Populations. Plast Reconstr Surg - Glob Open 2021. [DOI] [PMC free article] [PubMed]
- 32.Larbig W, Andoh J, Huse E, et al. Pre- and postoperative predictors of phantom limb pain. Neurosci Lett 2019;702:44–50. doi: 10.1016/j.neulet.2018.11.044 [DOI] [PubMed] [Google Scholar]
- 33.Blier P, Abbott FV. Putative mechanisms of action of antidepressant drugs in affective and anxiety disorders and pain. J Psychiatry Neurosci 2001;26(1):37–43. [PMC free article] [PubMed] [Google Scholar]
- 34.Gallagher R, Verma S. Managing pain and comorbid depression: A public health challenge. Semin Clin Neuropsychiatry 1999;4(3):203–220. [DOI] [PubMed] [Google Scholar]
- 35.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and Pain Comorbidity. Arch Intern Med 2003;163(20):2433. doi: 10.1001/archinte.163.20.2433 [DOI] [PubMed] [Google Scholar]
- 36.Clarke H, Kirkham KR, Orser BA, et al. Gabapentin reduces preoperative anxiety and pain catastrophizing in highly anxious patients prior to major surgery: A blinded randomized placebo-controlled trial. Can J Anesth 2013;60(5):432–443. doi: 10.1007/s12630-013-9890-1 [DOI] [PubMed] [Google Scholar]
- 37.Stenberg L, Dahlin LB. Gender differences in nerve regeneration after sciatic nerve injury and repair in healthy and in type 2 diabetic Goto-Kakizaki rats. BMC Neurosci 2014;15(1):1–10. doi: 10.1186/1471-2202-15-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maroz N, Simman R. Wound Healing in Patients With Impaired Kidney Function. J Am Coll Clin Wound Spec 2013;5(1):2–7. doi: 10.1016/j.jccw.2014.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moorthi RN, Doshi S, Fried LF, et al. Chronic kidney disease and peripheral nerve function in the Health, Aging and Body Composition Study. Nephrol Dial Transplant 2019;34(4):625–632. doi: 10.1093/ndt/gfy102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: A systematic review and meta-analysis. Nutrients 2017;9(8). doi: 10.3390/nu9080829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yildiran H, Macit MS, Özata Uyar G. New approach to peripheral nerve injury: nutritional therapy. Nutr Neurosci 2020;23(10):744–755. doi: 10.1080/1028415X.2018.1554322 [DOI] [PubMed] [Google Scholar]
- 42.Walter IB. Triiodothyronine exerts a trophic action on rat sensory neuron survival and neurite outgrowth through different pathways. Eur J Neurosci 1996;8(3):455–466. doi: 10.1111/j.1460-9568.1996.tb01229.x [DOI] [PubMed] [Google Scholar]
- 43.Barakat-Walter I, Krafsik R, Schenker M, Kuntzer T. Thyroid hormone in biodegradable nerve guides stimulates sciatic nerve regeneration: A potential therapeutic approach for human peripheral nerve injuries. J Neurotrauma 2007;24(3):567–577. doi: 10.1089/neu.2006.0104 [DOI] [PubMed] [Google Scholar]
- 44.Kuiken TA, Li G, Lock BA, et al. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA - J Am Med Assoc 2009;301(6):619–628. doi: 10.1001/jama.2009.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Langhals NB, Woo SL, Moon JD, et al. Electrically stimulated signals from a long-term Regenerative Peripheral Nerve Interface. Conf Proc IEEE Eng Med Biol Soc 2014:1989–1992. doi: 10.1109/EMBC.2014.6944004 [DOI] [PubMed]
- 46.Bowen JB, Ruter D, Wee C, West J, Valerio IL. Targeted muscle reinnervation technique in below-knee amputation. Plast Reconstr Surg 2019;143(1):309–312. doi: 10.1097/PRS.0000000000005133 [DOI] [PubMed] [Google Scholar]
- 47.Alexander JH, Jordan SW, West JM, et al. Targeted muscle reinnervation in oncologic amputees: Early experience of a novel institutional protocol. J Surg Oncol 2019;120(3):348–358. doi: 10.1002/jso.25586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kubiak CA, Kemp SWP, Cederna PS, Kung TA. Prophylactic Regenerative Peripheral Nerve Interfaces to Prevent Postamputation Pain. Plast Reconstr Surg 2019;144(3):421e–430e. doi: 10.1097/PRS.0000000000005922 [DOI] [PubMed] [Google Scholar]
- 49.Valerio IL, Dumanian GA, Jordan SW, et al. Preemptive Treatment of Phantom and Residual Limb Pain with Targeted Muscle Reinnervation at the Time of Major Limb Amputation. J Am Coll Surg 2019;228(3):217–226. doi: 10.1016/j.jamcollsurg.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 50.Valerio I, Schulz S, West J, Westenberg RF, Eberlin K. Targeted Muscle Reinnervation Combined with a Vascularized Pedicled Regenerative Peripheral Nerve Interface. Plast Reconstr Surg - Glob Open 2020. [DOI] [PMC free article] [PubMed]
- 51.Woo SL, Urbanchek MG, Cederna PS, Langhals NB. Revisiting nonvascularized partial muscle grafts: A novel use for prosthetic control. Plast Reconstr Surg 2014;134(2):344–346. doi: 10.1097/PRS.0000000000000317 [DOI] [PubMed] [Google Scholar]
- 52.Serino A, Akselrod M, Salomon R, et al. Upper limb cortical maps in amputees with targeted muscle and sensory reinnervation. Brain 2017;140(11):2993–3011. doi: 10.1093/brain/awx242 [DOI] [PubMed] [Google Scholar]
- 53.Birbaumer N, Lutzenberger W, Montoya P, et al. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci 1997;17(14):5503–5508. doi: 10.1523/jneurosci.17-14-05503.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011;152(SUPPL.3):S2–S15. doi: 10.1016/j.pain.2010.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table, Supplemental Digital Content 1: Codes to identify patients with lower extremity amputation
Table, Supplemental Digital Content 2: Diagnosis and procedure codes used to flag patients with potential neuropathic stump pain.


