Abstract
The aim of this study was to investigate whether the synthesis rates of some proteins change after the initiation of replication in Escherichia coli. An intR1 strain, in which chromosome replication is under the control of an R1 replicon integrated into an inactivated oriC, was used to synchronize chromosome replication, and the rates of protein synthesis were analyzed by two-dimensional polyacrylamide gel electrophoresis of pulse-labeled proteins. Computerized image analysis was used to search for proteins whose expression levels changed at least threefold after initiation of a single round of chromosome replication, which revealed 7 out of about 1,000 detected proteins. The various synthesis rates of three of these proteins turned out to be caused by unbalanced growth and the synthesis of one protein was suppressed in the intR1 strain. The rates of synthesis of the remaining three could be correlated only to the synchronous initiation of replication. These three proteins were analyzed by peptide mass mapping and appeared to be the products of the dps, gapA, and pyrI genes. Thus, the expression of the vast majority of proteins is not influenced by the state of chromosome replication, and a possible role of the replication-associated expression changes of the three identified proteins in the cell cycle is not clear.
A bacterial cell has to grow and duplicate its constituents before it can divide and give rise to two new daughter cells. Initiation of chromosome replication, partitioning of the sister chromosomes, septum formation, and cell division occur at specific times in the cell cycle (31), but the mechanisms controlling the timing of these events are poorly understood. It is possible that the occurrence of these cell cycle events partially requires, or induces, changes in synthesis rates of specific proteins at certain times in the cell cycle, as has been found for eukaryotic cells (3).
In the gram-negative bacterium Caulobacter crescentus, several proteins have been shown to be differentially synthesized during the cell cycle (8, 21). A recent two-dimensional polyacrylamide gel electrophoresis (2-D PAGE) analysis revealed that about 14% of the proteins detected in synchronized cells are expressed in a cell cycle-specific fashion (12a). In Escherichia coli, the synthesis rates of some outer membrane proteins (7), as well as the transcription rates of ftsZ (12, 33), gidA, mioC, dnaA (6, 23, 29), dam, mukB, seqA, iciA (32), and the nrd operon (28), have been shown to vary during the cell cycle. In contrast to the results obtained for C. crescentus, a 2-D PAGE analysis did not detect differential protein expression during the E. coli cell cycle (20). It is possible that the 2-D PAGE approach was not sensitive enough to identify cell cycle-specific protein synthesis. The 2-D PAGE technique has been improved, and the development of computerized image analysis has facilitated the analysis of complex protein spot patterns on 2-D PAGE gels. Another difficulty in studying cell cycle-related protein expression is to accurately synchronize large enough amounts of E. coli cells to allow detection of the proteins on a 2-D PAGE gel. This can be achieved with an E. coli intR1 strain, in which the initiation of replication is uncoupled from its cell cycle control, thereby enabling accurate synchronization of chromosome replication (but not cell size) of large populations by using relatively small temperature shifts (5). By using the intR1 strain MG::71CW(pOU420) and 2-D PAGE combined with computerized image analysis, we found that the expression of the vast majority of proteins does not change during the cell cycle. Out of about 1,000 proteins detected on the 2-D gels, 3 that had replication-associated expression changes were identified. These three proteins were analyzed by peptide mass mapping and appeared to be the products of the dps, gapA, and pyrI genes.
MATERIALS AND METHODS
Bacterial strains.
The intR1 strain MG::71CW(pOU420), derived from MG1655 (4), was used to synchronize initiation of replication (5). The intR1 strains are oriC mutants in which a part of the essential oriC sequence has been replaced by an R1 miniplasmid, pOU71 (Ampr). Thus, oriC is inactivated in these strains and chromosomal replication is governed by the plasmid R1 replicon (17). The strain MG::71CW(pOU420) also contains the nonintegrated plasmid pOU420 (Cmr), which results in temperature-dependent initiation of chromosome replication (5). At 40°C, initiation of replication is at a wild-type level, whereas at 36°C, initiation of replication is inhibited.
Media and growth conditions.
The bacteria were grown aerobically in M9 minimal medium (25) containing 0.2% (wt/vol) glucose in thermostatically controlled rotary water baths (Heto) with a maximum deviation of 0.2°C at 100 rpm. Chloramphenicol (50 μg/ml) and ampicillin (20 μg/ml) were added for the MG::71CW(pOU420) strain. Cell density was measured by spectrophotometry with an LKB Novaspec II spectrophotometer at 550 nm.
Synchronization of replication.
In order to initiate a synchronous single round of replication in an MG::71CW(pOU420) culture, basically the same procedure was used as described for EC::71CW(pOU420) (5). The cells were grown exponentially for at least 10 generations at 40°C. At an optical density at 550 nm of 0.040, the culture was shifted to 36°C for 150 min to inhibit initiation of chromosome replication and allow for completion of replication. The culture was then shifted to 40°C for 8 min to initiate one round of replication and thereafter returned to 36°C in order to block any further initiation (5).
Flow cytometry.
Synchronized cultures were monitored by flow cytometry (27). Cells of a growing culture (60 μl) were fixed directly in 1 ml of 99.5% ethanol plus 350 μl of 10 mM Tris (pH 7.5) and then stored at 4°C. The fixed cells were stained for flow cytometry as described previously (5) and analyzed with a Bryte HS flow cytometer (Bio-Rad).
Radioactive labeling.
At the appropriate time points, 6-ml aliquots of the culture were pulse-labeled for 7 min with 0.4 ml of 14C-amino acid mix (NEC445E; DuPont) and then chased for 2 min with 0.5 ml of nonradioactive amino acid mix, containing a 0.5-mg/ml concentration each of A, D, E, F, G, H, I, K, L, P, R, S, T, and Y (21). The labeled cells were immediately frozen in liquid nitrogen and stored at −20°C.
2-D PAGE.
Several 2-D PAGE gels were made, and four high-quality gels per time point were subjected to image analysis. Samples for pulse-labeling were taken from two independent experiments, and each sample was used for two independent gels. 2-D PAGE (11) was performed with Millipore Investigator equipment and chemicals according to the manual provided by Millipore. (The Investigator system and chemicals are now provided by Genomic Solutions, Inc., Chelmsford, Mass., but are referred to as Millipore products.) Protein extracts were prepared by boiling the cells in small amounts of sodium dodecyl sulfate (SDS) (Serva) before adding the high-molar-concentration urea solution according to the sample preparation method for bacteria in the Investigator manual. The first-dimension gels were focused to equilibrium for 18,000 V · h and contained ampholytes at pH 3 to 10 (3-10 2D; Millipore) and 10 mM ChapsO (Merck). The second-dimension slab gels contained 12% Duracryl (0.65% bis; Millipore) and analytic-grade C12 SDS. The amount of proteins loaded onto each gel was normalized by the optical density of the culture at the time of sampling. It was not necessary to load exactly the same amount of radioactivity on each 2-D PAGE gel, as the intensity differences between the gels were normalized by the computer software used for the analysis (see below). The gels were dried on Whatman paper and exposed to storage phosphor screens (Molecular Dynamics) for 7 days, and proteins containing radioactivity were detected with a storage phosphorimager (Molecular Dynamics) at a resolution of 176 μm per pixel.
Computerized image analysis.
2-D Analyzer 6.1 software (BioImage) was used for spot detection, quantification of the spot intensities, gel matching, and statistical analysis. The intensity of a spot reflects an arbitrary, relative unit for the rate of protein synthesis. To correct for intensity differences between the 2-D PAGE autoradiograms, each gel was normalized to the same reference gel by multiplying all its spot intensities with the ratio between the total gel intensities of the test gel and the reference gel. Intensity changes were considered to be significant when the mean values of a spot had an intensity ratio larger than three and a t test level of significance of 0.05 for two time points, or when a spot could not be detected at one time point. We decided that the intensity ratio must be larger than three because the mean relative standard error of the spot intensities was 15.8%, where the ratio of the two extreme values of the 95% confidence interval with 3 df is 3. All candidate spots were checked on the gels by eye and those with insufficient quality were deselected. The isoelectric points (pI) and molecular weights (MW) were estimated from the positions of comigrated 2-D PAGE marker proteins with known pI and MW (Bio-Rad).
Reverse staining of SDS-polyacrylamide gels.
The 2-D PAGE gels were stained essentially according to the imidazole-SDS-Zn reverse staining method developed by Fernandez-Patron et al. (10). After electrophoresis the gels were washed twice for 15 min each in distilled water and then soaked in a solution containing 0.2 M imidazole and 0.1% SDS for 15 min with gentle shaking. This solution was discarded and the gels were incubated in 0.2 M ZnSO4 solution until the gel background became white (after approximately 30 to 60 s). This reaction was stopped by washing the gels three times in distilled water. The protein spots of interest were cut out of the gels and were stored in distilled water at 4°C. Prior to protein identification, the gel pieces were soaked in 25 mM Tris-HCl–100 mM dithiothreitol (pH 8.3) solution (10) until they became transparent again.
Protein identification.
The procedures described by Shevchenko et al. (26) were used to identify the individual proteins. Briefly, the gel pieces were washed with 50 mM NH4HCO3-acetonitrile (1:1) followed by dehydration with acetonitrile and drying by vacuum centrifugation. The proteins were reduced with 200 μl of 10 mM dithiothreitol–50 mM NH4HCO3 for 1 h at 56°C and alkylated in 200 μl of 55 mM iodoacetamide–50 mM NH4HCO3 for 15 min. The gel pieces were washed several times in 50 mM NH4HCO3 followed by dehydration with acetonitrile and drying by vacuum centrifugation. The proteins were digested overnight with modified trypsin (Promega) at 37°C. A small aliquot of the generated peptide mixtures was analyzed by matrix-assisted laser desorption ionization–time of flight mass spectrometry on a Voyager-DE STR instrument (Perceptive Biosystems, Framingham, Mass.). The remaining peptides were extracted with 25 mM NH4HCO3-acetonitrile (1:1) followed by extraction with 25 mM NH4HCO3–5% formic acid. The combined extracts were dried by vacuum centrifugation. Peptide mixtures were purified on purification capillaries with POROS R2 perfusion chromatography material. The peptides were eluted in 50% MeOH–5% HCOOH directly into the nanospray capillary by centrifugation and analyzed on a prototype QqTOF mass spectrometer (Sciex, Toronto, Canada) equipped with a nanoelectrospray source (Protana A/S, Odense, Denmark). Proteins were identified by checking a nonredundant sequence database containing more than 320,000 entries by using the determined peptide masses and the partial amino acid sequences (peptide sequence tags) deduced from the tandem mass spectra. The search software used was PepSea version 1.1 (Protana A/S).
RESULTS
Synchronization of replication in MG::71CW(pOU420).
In the intR1 strain MG::71CW(pOU420), chromosome replication is under the control of an integrated R1 miniplasmid. Initiation of replication is temperature dependent and can be reversibly turned on and off by temperature shifts of 4°C, such that the entire population is synchronized with respect to initiation of chromosome replication without large secondary effects due to heat and cold shock responses (5).
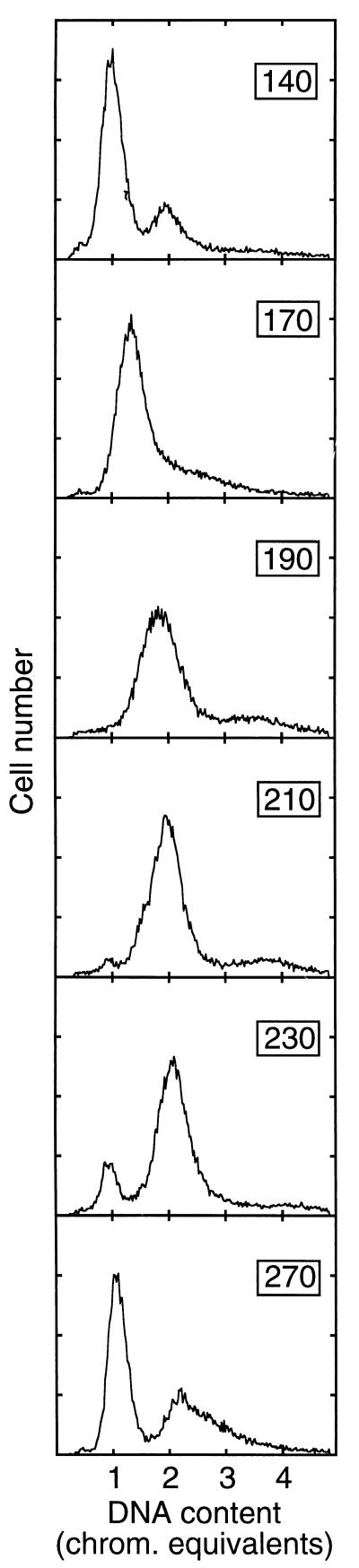
To synchronize the initiation of replication in MG::71CW(pOU420), the cells were essentially grown as described for strain EC::71CW(pOU420) (5). To block initiation of chromosome replication and allow subsequent completion of ongoing replications, an exponentially growing culture was shifted from 40 to 36°C (time zero) and incubated for 150 min (5). To initiate one round of chromosome replication, the culture was then shifted to 40°C for 8 min, and it was returned to 36°C for the rest of the experiment to inhibit further initiations. Flow cytometry data of a synchronized culture (Fig. 1) showed that 10 min before the transient temperature upshift (140 min after the downshift to 36°C), most cells (60 to 70% of the population) contained one chromosome. After the temperature upshift, the DNA content peak gradually increased to two chromosome equivalents for a little more than 40 min (until 190 min). It then remained stable at this position until 60 min after the temperature upshift (210 min), when a new peak at one chromosome equivalent appeared. This peak increased steadily until 120 min after the temperature upshift (270 min), when about half of the population contained one chromosome and the other half contained more DNA.
FIG. 1.
Flow cytometry data showing the DNA distribution of an MG::71CW(pOU420) culture with synchronized replication. A culture growing exponentially in M9 medium at 40°C was shifted to 36°C at time zero. After 150 min, the culture was shifted to 40°C for 8 min and then returned to 36°C. The samples were taken at the times (in minutes) indicated in the upper right corners of the panels. About 24,000 cells were counted in each sample. chrom., chromosome.
With the short temperature upshift after the completion of replication, replication was initiated and proceeded synchronously in most cells. The replication time was around 40 min, and the first cell divisions occurred around 60 min after initiation of replication (i.e., about 20 min after termination of replication). However, the cells continued to divide during the following 60 min, so the amount of cells containing one chromosome increased over time.
Replication-associated protein synthesis.
Several reports have shown that certain E. coli genes change their transcription rate during the cell cycle (see the introduction). To see whether such changes could be found at the protein expression level, we investigated the synthesis rates of individual proteins 10 min before (140 min after the downshift to 36°C) and 20 (170 min), 40 (190 min), 50 (200 min), and 60 (210 min) min after the transient temperature upshift to 40°C that initiated synchronous replication. These times were chosen since they represent specific events during the replication and cell division cycle: 140 min after the downshift to 36°C is just before initiation, 170 min is at about midreplication, 190 min is around replication termination, and 210 min is at the first cell divisions. At each point, the proteins were pulse-labeled with 14C-labeled amino acids for 7 min (see Materials and Methods).
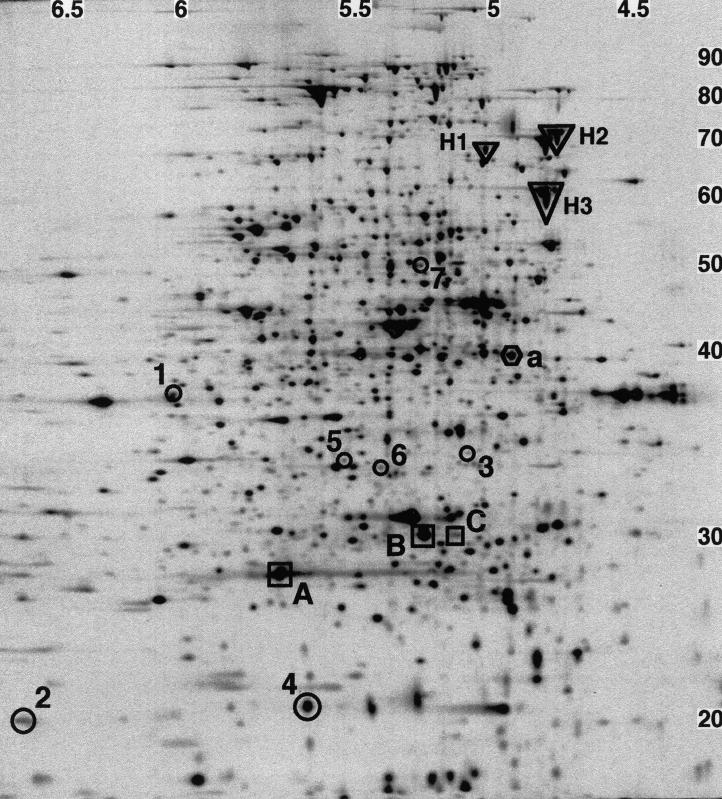
The pulse-labeled proteins were separated on 2-D PAGE gels, and nearly 1,000 spots were detected (Fig. 2). Because isoelectric focusing was run to equilibrium in the first dimension and a 12% polyacrylamide gel was used in the second dimension, only proteins with pI from 4 to 7 and MW from 15 × 103 to 100 × 103 were resolved. Most E. coli proteins are found within this pI and MW range (18, 22).
FIG. 2.
2-D PAGE autoradiogram of a 7-min pulse 50 min after initiation of a single round of replication in strain MG::71CW(pOU420). About 1,000 spots were detected on the gel. Spots 1 to 7 show significant changes in their intensities after synchronous initiation of replication in MG::71CW(pOU420). Proteins A, B, and C are intR1 specific. Protein a was chosen as an example of replication-independent protein synthesis. The heat shock proteins H1 (HtpG), H2 (DnaK), and H3 (GroEL) were identified by comparison with an E. coli 2-D PAGE database (30). The numbers at the top are pI, and the numbers on the right are MW, in thousands.
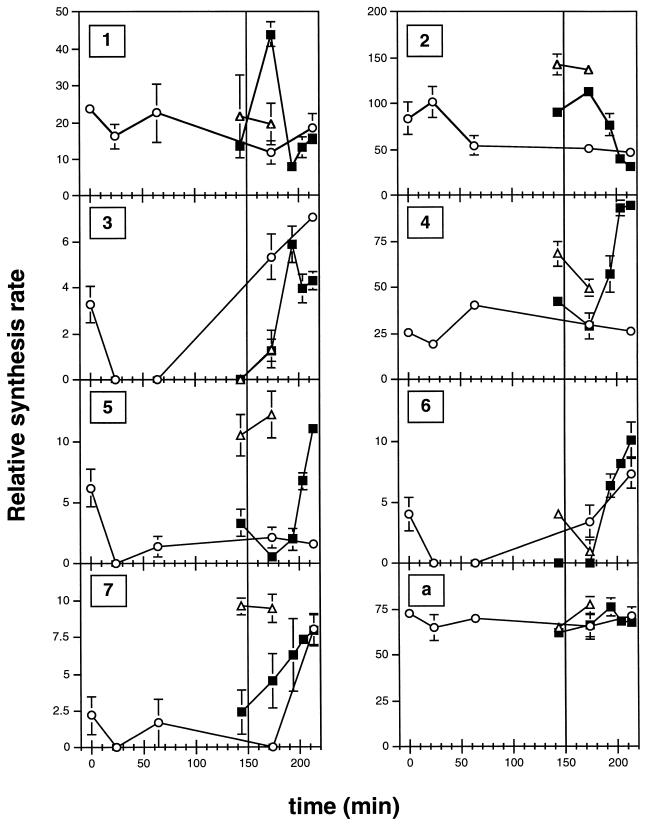
Four gels were made for each time point in order to enable a statistical analysis of the collected data (see Materials and Methods). The spots on the different 2-D gels were quantified and matched by computerized image analysis. Spots of good quality whose mean intensity increased or decreased at least threefold between two time points (see Materials and Methods) were sought. The intensity of a spot reflects an arbitrary, relative unit for the rate of protein synthesis. Seven candidate spots were found (proteins 1 to 7) (Fig. 2 to 4 and Tables 1 and 2) out of about 1,000 spots which could be detected by 2-D PAGE. Spots 1 and 2 had the highest intensity at 170 min (midreplication [Fig. 4]), spot 3 at 190 min (at replication termination [Fig. 4]), and spots 4 to 7 at 210 min (at first cell division [Fig. 4]).
FIG. 4.
Quantification (spot intensity; the scale is relative) of spots 1 to 7 and a during temperature shift experiments with MG::71CW(pOU420) and MG1655. In order to synchronize replication, exponentially growing MG::71CW(pOU420) cells were shifted from 40 to 36°C at time zero to block the initiation of replication. After 150 min the cells were shifted to 40°C for 8 min to synchronously initiate replication (filled squares). As a control, new MG::71CW(pOU420) cultures were grown as explained above without a shift to 40°C after 150 min (open circles). MG1655 (open triangles) was grown like MG::71CW(pOU420), with the 8-min temperature upshift to 40°C after 150 min at 36°C. The values for the relative synthesis rates were placed in the middle of the 7-min window during which the proteins were pulse-labeled. Each value is the mean for four gels. Vertical bars show standard errors. The start of the 8-min shift to 40°C for initiation of replication is indicated by a vertical line at 150 min.
TABLE 1.
Estimated pI and MW of proteins 1 to 7, whose expressions changed significantly after synchronous initiation of replication in MG::71CW(pOU420)
| Spot | pI | MW (103) |
|---|---|---|
| 1 | 6.0 | 38 |
| 2 | 6.9 | 20 |
| 3 | 5.1 | 34 |
| 4 | 5.7 | 21 |
| 5 | 5.5 | 34 |
| 6 | 5.4 | 33 |
| 7 | 5.3 | 49 |
TABLE 2.
Characteristics of proteins 1, 2, and 4
For spot 2, the two extreme values differed just threefold, but as the spot was located at the very basic end, a region which is difficult to reproduce, the interpretation of this result may be ambiguous.
Temperature effect.
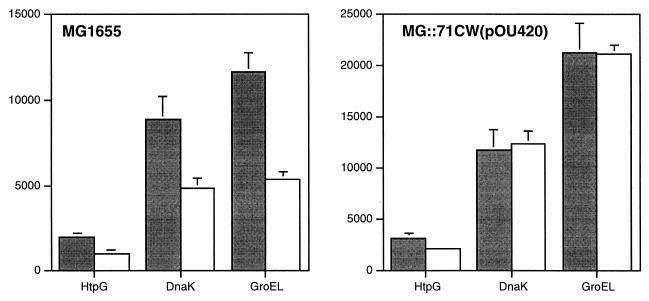
It is well documented that temperature shifts affect gene expression, and it is possible that the temperature shifts necessary to initiate synchronous replication in MG::71CW(pOU420) influenced the synthesis rates of some proteins. As a control for temperature response, the wild-type strain, MG1655, was grown as described for a synchronization-of-replication experiment, and the 2-D PAGE patterns 10 min before (140 min after the downshift to 36°C) and 20 min after (170 min) the transient temperature upshift to 40°C were investigated. No spots showed significant (at least threefold) changes in their intensities between the two time points (data not shown), indicating that no heat or cold shock genes were induced at significant levels. The known heat shock proteins HptG, DnaK, and GroEL could be identified on the 2-D gels by comparing them with an E. coli 2-D PAGE database (30), and their synthesis rates before and after the transient temperature upshift are shown in Fig. 5. For all three heat shock proteins, the transient 4°C temperature upshift did not induce any heat shock response after 20 min. The spot intensities of HptG and DnaK were similar in the intR1 and wild-type gels. However, the spot intensity of GroEL was twofold higher in the intR1 gels than in the wild-type gels, which, again, was not considered to be significant. Finally, none of the seven candidate spots showed a significant change in intensity between the two time points on the wild-type gels.
FIG. 5.
Quantification (spot intensity) of the heat shock proteins HtpG, DnaK, and GroEL 10 min before (140 min after the temperature shift to 36°C [grey bars]) and 20 min after (170 min [white bars]) the 8-min temperature upshift to 40°C in MG1655 and MG::71CW(pOU420). The heat shock proteins were identified by comparison with an E. coli 2-D PAGE database (30). Data are the means for four gels. Vertical bars show standard errors.
intR1-specific protein synthesis.
The intR1 strain contains the free plasmid pOU420 and, moreover, a deletion of the leftmost part of oriC, which is replaced by a derivative of the plasmid R1 replicon. These changes in the genotype might influence the interpretation of our results, and some of the observed variations in spot intensity may be effects of the intR1 construct. To find out which proteins were intR1 specific, the 2-D PAGE patterns of exponentially growing cultures of MG::71CW(pOU420) and the parental strain, MG1655, were compared. Three spots were found to be intR1 specific (spots A, B, and C in Fig. 2). These spots had pI and MW that are close to those predicted for the plasmid-encoded β-lactamase, chloramphenicol acetyltransferase, and λ cI857 repressor. One of the candidate spots (spot 5 in Fig. 2) was found to be repressed fourfold in the intR1 strain. Additionally, another two spots whose intensities differed around threefold between the two strains were found (not shown). Thus, although relatively few differences were found for the intR1 strain and the wild type during exponential growth conditions, one candidate spot was found to be repressed in the intR1 strain.
Influence of growth at the nonpermissive temperature.
In order to synchronize replication, initiation of replication was blocked in MG::71CW(pOU420) by a temperature shift from 40 to 36°C (nonpermissive temperature), and the culture was kept at the nonpermissive temperature for the rest of the experiment, except for the transient shift to 40°C to initiate a single round of replication. Thus, as the cells continue to grow in the absence of chromosome replication for a substantial period (data not shown), they may enter a state of unbalanced growth which could influence the expression level of certain proteins. To determine the effect of unbalanced growth, we grew new cultures of MG::71CW(pOU420) as described for the synchronization of replication without initiating a single round of replication. The 2-D PAGE patterns at 0 (exponentially growing culture), 20, 60, 170, and 210 min after the shift to the nonpermissive temperature were studied. Of the seven candidate spots, spots 3, 6, and 7 showed similar kinetics after 150 min in cultures both with and without a transient temperature upshift. Therefore, spots 3, 6, and 7 are unlikely to be regulated in a replication- or cell cycle-specific fashion. Hence, proteins 1, 2, and 4 were regarded to be cell cycle specific and were subjected to further analysis.
Protein identification.
After imidazole-SDS-Zn reverse staining of the gels, protein spots 1, 2, and 4 were cut out from the gels. The proteins were analyzed by peptide mass mapping and partial sequencing. The final identification was done by comparison with a sequence database by using the determined peptide masses and the partial amino acid sequences deduced from the tandem mass spectra. It turned out that spot 1 corresponds to glyceraldehyde-3-phosphate dehydrogenase (GAPDH, encoded by the gene gapA) and spot 2 corresponds to PyrI, the regulatory chain of aspartate transcarbamoylase (ATCase), which is involved in the synthesis of the pyrimidines from aspartate. Protein spot 4 was identified as Dps, also known as PexB, a stationary-phase-specific protein from E. coli (Table 2).
DISCUSSION
In this investigation, we studied replication-associated protein expression in E. coli using 2-D PAGE combined with computerized image analysis. Chromosome replication was synchronized in the intR1 strain MG::71CW(pOU420), in which initiation of replication is controlled by temperature (5). The observed cell cycle parameters were in agreement with previously published data (13); the replication time (the C period) was about 40 min, and the time from termination of replication until cell division (the D period) was around 20 min. However, although the cells replicated synchronously, cell division took place from 60 min until at least 120 min after initiation. This might be explained by the fact that cell division presumably occurs at certain critical lengths (5), and as the cell length was not synchronized in the population, individual cells reached the critical length at different times after termination of replication.
Using computer-aided analysis of the 2-D PAGE spot patterns of pulse-labeled proteins, we found that the synthesis rates of the vast majority of proteins remained constant after synchronous initiation of replication, which is in agreement with previously published data (20). A temperature effect on protein expression could not be detected; no significant changes were observed in the wild type 20 min after a transient temperature shift of 4°C. The expression of 7 proteins out of about 1,000 detected on the 2-D gels was found to vary at least threefold after synchronized initiation of replication. However, the expression of proteins 3, 6, and 7 showed similar changes in a nonshifted control culture, indicating that their expression changes were not replication specific but presumably an effect of unbalanced growth caused by the block of replication. Because protein 5 was repressed fourfold in the intR1 strain compared to the wild type, its observed expression changes are most likely an effect of the intR1 genotype. Therefore, the differential expression of finally only three proteins (i.e., 1, 2, and 4) could be correlated with the synchronous initiation of replication.
It should be pointed out that we did not consider spot intensity changes that differed less than threefold, and many cell cycle- or replication cycle-regulated proteins may thus have been missed, e.g., FtsZ, whose transcription rate has been shown to vary about twofold during the cell cycle (12). When the expression limit was lowered to twofold in the analysis procedure, the number of candidate spots increased to at least 20 (data not shown). Although these changes are below the mean 95% level of significance (see Materials and Methods), they may still represent proteins that are cell cycle regulated. In addition, as we measured only one time point during replication (20 min after synchronous initiation of replication), proteins expressed just after initiation (0 to 10 min) and just before termination (30 to 40 min) may have been missed. Also, all proteins which might be up- or down-regulated before the initiation of replication were missed because the initiation of replication was uncoupled from the normal cell cycle in the cells used for synchronization. Thus, our data presumably represents an underestimate of the number of proteins that are differentially expressed after initiation of replication.
The replication-dependently expressed proteins 1, 2, and 4 were identified by mass spectrometry analysis (Table 2). Both proteins 1 and 2 had their highest synthesis rates at midreplication, and they represent GAPDH (the product of gapA) and PyrI (ATCase), respectively. GAPDH is involved in one of the main metabolic pathways, glycolysis. It catalyzes the oxidative phosphorylation of d-glyceraldehyde-3-phosphate into 1,3-bisphosphoglycerate (14, 15). ATCase is an enzyme which catalyzes the first committed step in the synthesis of pyrimidines from aspartate (16, 24). It consists of six identical catalytic subunits, the gene products of pyrB, and six identical regulatory chains coded for by pyrI. The enzyme catalyzes the reaction of carbamoyl phosphate with l-aspartate to yield N-carbamoyl-l-aspartate and phosphate. Protein 4 was found to be the stationary-phase-specific protein Dps (PexB). It binds DNA unspecifically and protects it against oxidative stress. It is found mainly in stationary-phase cells, where its expression is controlled by ςs and integration host factor (2), but it can also be induced by oxidative stress in exponentially growing cells. In this case, induction is goverened by OxyR and ς70 (2). In our synchronization experiment it had the highest rate of expression at the time cell division started. Beside its role as a DNA-protecting protein, Dps also seems to have an effect on the global pattern of gene expression, which has been shown by use of dps null mutants and 2-D gel electrophoresis (1).
In conclusion, this report shows that the vast majority of proteins do not change their rates of expression after initiation of replication in E. coli. However, the synthesis rates of three proteins were found to change significantly during synchronous replication. These expression changes could be correlated only with the synchronous initiation of replication. The role of these three proteins in the E. coli cell cycle is not clear and will be further studied by cell cycle characterization of mutant strains. It is still possible that the synthesis of these three proteins varies depending on the state of replication without being involved in the progression of the cell cycle.
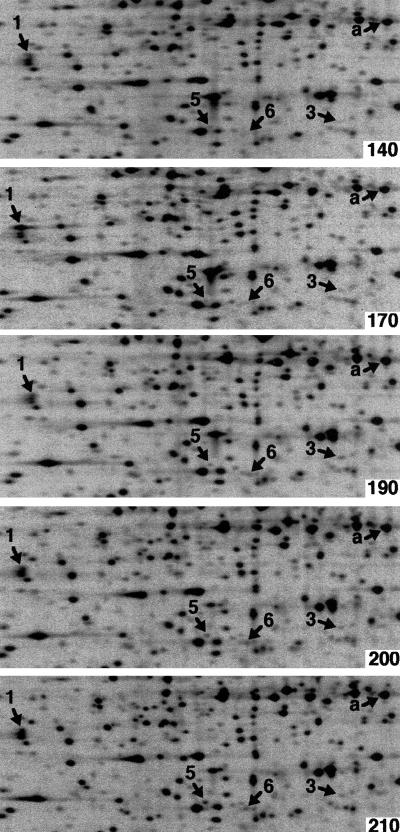
FIG. 3.
Identical sections of 2-D PAGE autoradiograms at each time point investigated after synchronous initiation of replication in MG::71CW(pOU420). Spots 1, 3, 5, and 6, whose intensities were found to change significantly after synchronous initiation of replication, and the example for continuous synthesis, protein a, are indicated. The time (in minutes) after the temperature downshift to 36°C is indicated in the upper right corner of each panel. At 150 min, replication was synchronously initiated by a transient (8-min) upshift to 40°C.
ACKNOWLEDGMENTS
We thank Santanu Dasgupta for critical reading of the manuscript and Rolf Bernander for much helpful advice during the work.
This work was supported by grants from the Swedish Natural Science Research Council and the Swedish Cancer Society. Björn Grünenfelder was supported by an Erasmus scholarship. The identification of the proteins was performed as contract work by Protana A/S.
Dorothée Bechtloff and Björn Grünenfelder contributed equally to this work.
REFERENCES
- 1.Almirón M, Link A J, Furlong D, Kolter R. A novel DNA binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 2.Altuvia S, Almirón M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςs in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Andrews B J, Herskowitz I. Regulation of cell cycle-dependent gene expression in yeast. J Biol Chem. 1990;265:14057–14060. [PubMed] [Google Scholar]
- 4.Bachmann B J. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2460–2488. [Google Scholar]
- 5.Bernander R, Åkerlund T, Nordström K. Inhibition and restart of initiation of chromosome replication: effects on exponentially growing Escherichia coli cells. J Bacteriol. 1995;177:1670–1682. doi: 10.1128/jb.177.7.1670-1682.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogan J A, Helmstetter C E. mioC transcription, initiation of replication, and the eclipse in Escherichia coli. J Bacteriol. 1996;178:3201–3206. doi: 10.1128/jb.178.11.3201-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd A, Holland I B. Regulation of the synthesis of surface protein in the cell cycle of E. coli B/r. Cell. 1979;18:287–296. doi: 10.1016/0092-8674(79)90048-5. [DOI] [PubMed] [Google Scholar]
- 8.Domian I J, Quon K C, Shapiro L. The control of temporal and spatial organization during the Caulobacter cell cycle. Curr Opin Genet Dev. 1996;6:538–544. doi: 10.1016/s0959-437x(96)80081-5. [DOI] [PubMed] [Google Scholar]
- 9.Feller A, Piérard A, Glansdorff N, Charlier D, Crabeel M. Mutation of gene encoding regulatory polypeptide of aspartate carbamoyltransferase. Nature. 1981;292:370–373. doi: 10.1038/292370a0. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Patron C, Calero M, Rodriguez Collazo P, Garcia J R, Madrazo J, Musacchio A, Soriano F, Estrada R, Frank R, Castellanos-Serra L R, Mendez E. Protein reverse staining: high-efficiency microanalysis of unmodified proteins detected on electrophoresis gels. Anal Biochem. 1995;224:203–211. doi: 10.1006/abio.1995.1031. [DOI] [PubMed] [Google Scholar]
- 11.Garrels J I. Two dimensional gel electrophoresis and computer analysis of proteins synthesized by clonal cell lines. J Biol Chem. 1979;254:7961–7977. [PubMed] [Google Scholar]
- 12.Garrido T, Sanchez M, Palacios P, Aldea M, Vicente M. Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J. 1993;12:3957–3965. doi: 10.1002/j.1460-2075.1993.tb06073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Grünenfelder, B. Unpublished data.
- 13.Helmstetter C E. Timing of synthetic activities in the cell cycle. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1627–1639. [Google Scholar]
- 14.Hillman J D, Fraenkel D G. Glyceraldehyde 3-phosphate dehydrogenase mutants of Escherichia coli. J Bacteriol. 1975;122:1175–1179. doi: 10.1128/jb.122.3.1175-1179.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irani M, Maitra P L. Isolation and characterization of Escherichia coli mutants defective in enzymes of glycolysis. Biochem Biophys Res Commun. 1974;56:127–133. doi: 10.1016/s0006-291x(74)80324-4. [DOI] [PubMed] [Google Scholar]
- 16.Jones M E, Spector L, Lipmann F. Carbamyl phosphate, the carbamyl donor in enzymatic citrulline synthesis. J Am Chem Soc. 1955;77:819–820. [Google Scholar]
- 17.Koppes L, Nordström K. Insertion of an R1 plasmid into the origin of replication of the E. coli chromosome: random timing of replication of the hybrid chromosome. Cell. 1986;44:117–124. doi: 10.1016/0092-8674(86)90490-3. [DOI] [PubMed] [Google Scholar]
- 18.Link A J, Robison K, Church G M. Comparing the predicted and observed properties of proteins encoded in the genome of Escherichia coli K-12. Electrophoresis. 1997;18:1259–1313. doi: 10.1002/elps.1150180807. [DOI] [PubMed] [Google Scholar]
- 19.Lomovskaya O L, Kidwell J P, Martin A. Characterization of the ς38-dependent expression of a core Escherichia coli starvation gene, pexB. J Bacteriol. 1994;176:3928–3935. doi: 10.1128/jb.176.13.3928-3935.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutkenhaus J F, Moore B A, Masters M, Donachie W D. Individual proteins are synthesized continuously throughout the Escherichia coli cell cycle. J Bacteriol. 1979;138:352–360. doi: 10.1128/jb.138.2.352-360.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milhausen M, Agabian N. Regulation of polypeptide synthesis during Caulobacter development: two-dimensional gel analysis. J Bacteriol. 1981;148:163–173. doi: 10.1128/jb.148.1.163-173.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Farrell P Z, Goodman H M, O’Farrell P H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977;12:1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Okazaki T. Cell cycle-dependent transcription from the gid and mioC promoters of Escherichia coli. J Bacteriol. 1994;176:1609–1615. doi: 10.1128/jb.176.6.1609-1615.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reichard P, Hanshoff G. Aspartate carbamyl transferase from Escherichia coli. Acta Chem Scand. 1956;10:548–566. [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Shevchenko A, Wilm M, Vorm O, Mann M. Mass spectrometric sequencing of proteins from silver-stained polyacrylamide gels. Anal Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 27.Skarstad K, Bernander R, Wold S, Steen H B, Boye E. Cell cycle analysis of microorganisms. In: Al-Rubeai M, Emery A N, editors. Flow cytometry applications in cell culture. New York, N.Y: Marcel Dekker; 1996. pp. 241–255. [Google Scholar]
- 28.Sun L, Jacobson B A, Dien B S, Srienc F, Fuchs J A. Cell cycle regulation of the Escherichia coli nrd operon: requirement for a cis-acting upstream AT-rich sequence. J Bacteriol. 1994;176:2415–2426. doi: 10.1128/jb.176.8.2415-2426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theisen P W, Grimwade J E, Leonard A C, Bogan J A, Helmstetter C E. Correlation of gene transcription with the time of initiation of chromosome replication in Escherichia coli. Mol Microbiol. 1993;10:575–584. doi: 10.1111/j.1365-2958.1993.tb00929.x. [DOI] [PubMed] [Google Scholar]
- 30.VanBogelen R A, Abshire K Z, Pertsemlidis A, Clark R L, Neidhardt F C. Gene-protein database of Escherichia coli K-12, edition 6. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2067–2117. [Google Scholar]
- 31.Vinella D, D’Ari R. Overview of controls in the Escherichia coli cell cycle. Bioessays. 1995;17:527–536. doi: 10.1002/bies.950170609. [DOI] [PubMed] [Google Scholar]
- 32.Zhou P, Bogan J A, Welch K, Pickett S R, Wang H J, Zaritsky A, Helmstetter C E. Gene transcription and chromosome replication in Escherichia coli. J Bacteriol. 1997;179:163–169. doi: 10.1128/jb.179.1.163-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou P, Helmstetter C E. Relationship between ftsZ gene expression and chromosome replication in Escherichia coli. J Bacteriol. 1994;176:6100–6106. doi: 10.1128/jb.176.19.6100-6106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]