Abstract
Objective
Glucocorticoid withdrawal syndrome (GWS) is a scarcely studied phenomenon that complicates the recovery following surgical remission of hypercortisolism. We aimed to characterize the presence and trajectory of glucocorticoid withdrawal symptoms in the postoperative period and to determine presurgical predictors of GWS severity.
Design
Longitudinal observational study.
Methods
Glucocorticoid withdrawal symptoms were prospectively evaluated weekly for the first 12 weeks following surgical remission of hypercortisolism. Quality of life (CushingQoL and Short-Form-36) and muscle function (hand grip strength and sit-to-stand test) were assessed at the baseline and at 12 weeks after surgery.
Results
Prevalent symptoms were myalgias and arthralgias (50%), fatigue (45%), weakness (34%), sleep disturbance (29%), and mood changes (19%). Most symptoms persisted, while myalgias, arthralgias, and weakness worsened during weeks 5-12 postoperatively. At 12 weeks after surgery, normative hand grip strength was weaker than at baseline (mean Z-score delta −0.37, P = .009), while normative sit-to-stand test performance improved (mean Z-score delta 0.50, P = .013). Short-Form-36 Physical Component Summary score worsened (mean delta −2.6, P = .015), but CushingQoL score improved (mean delta 7.8, P < .001) at 12 weeks compared to baseline. Cushing syndrome (CS) clinical severity was predictive of postoperative GWS symptomology.
Conclusion
Glucocorticoid withdrawal symptoms are prevalent and persistent following surgical remission of hypercortisolism with baseline CS clinical severity predictive of postoperative GWS symptom burden. Differential changes observed in muscle function and quality of life in the early postoperative period may reflect the competing influences of GWS and recovery from hypercortisolism.
Keywords: glucocorticoid withdrawal, Cushing syndrome, hypercortisolism, quality of life, myopathy
Significance.
Glucocorticoid withdrawal syndrome is a scarcely studied phenomenon that complicates the recovery following surgical remission of hypercortisolism and is challenging for patients to endure and for providers to manage. Limited data currently exist to guide patient education and management. In this longitudinal observational study, we characterized the presence, frequency, and trajectory of glucocorticoid withdrawal symptoms in the early postoperative period and identified clinical severity during active hypercortisolism to be a predictor of glucocorticoid withdrawal symptomology. These findings have direct implications on patient care and can be used to inform preoperative patient counseling and management as well as future interventions to treat glucocorticoid withdrawal syndrome.
Introduction
Endogenous neoplastic hypercortisolism or Cushing syndrome (CS) is most commonly due to a corticotropin (ACTH)-secreting pituitary lesion but may also be caused by an ectopic ACTH-producing tumor or ACTH-independent adrenocortical disease. Regardless of the etiology, the mainstay of treatment for CS is surgical resection of the disease-causing lesion.1,2 While surgery achieves biochemical remission rates ranging from 60%-80% in pituitary CS to virtually 100% in adrenal CS, recovery from the effects of hypercortisolism can be prolonged and challenging.3–5 Postoperatively, patients may develop adrenal insufficiency from long-standing suppression of the hypothalamic–pituitary–adrenal (HPA) axis and require glucocorticoid (GC) replacement. Many patients, however, continue to feel unwell despite GC therapy with symptoms that mimic cortisol deficiency (ie, fatigue, nausea, anorexia, myalgias, and arthralgias)—symptoms that may be ascribed to GC withdrawal instead.6
Glucocorticoid withdrawal syndrome (GWS) is a withdrawal reaction that occurs when physical dependence develops to supraphysiologic GC exposure and is precipitated by rapid reductions in serum GC concentrations—such as following successful surgery for CS.7 Because many of the symptoms of GWS overlap with those of adrenal insufficiency, GWS is often underrecognized as a separate entity in the postoperative period with limited data to guide patient counseling and management. In a retrospective study examining GWS in patients who underwent unilateral adrenalectomy for ACTH-independent hypercortisolism, GC withdrawal events were common with a prevalence of 67% in severe CS, 46% in moderate CS, and 40% in mild autonomous cortisol secretion (MACS)—defined as failure to suppress serum cortisol adequately following the 1 mg overnight dexamethasone suppression test, in the absence of physical manifestations of overt CS.8,9 Glucocorticoid withdrawal syndrome events were more likely to occur in patients with severe CS and when the 8:00 Am serum cortisol drawn 24 h off GC therapy was under 5 µg/dL.8 However, GWS events may not have been properly captured during the study due to the retrospective study design and reliance on patient-initiated communication to their provider. In addition, data on GWS following treatment for pituitary or ectopic CS are lacking.
Improved characterization of GWS and its clinical course may help inform preoperative counseling and provide anticipatory guidance for patients and their care teams, particularly as patients with CS report feeling unprepared for the postoperative experience.10 Here, we conducted an observational longitudinal study to (1) prospectively assess the presence, frequency, and trajectory of GC withdrawal symptoms in the first 12 weeks following successful surgery for endogenous hypercortisolism and to (2) identify presurgical variables that predict postoperative GWS symptom burden.
Methods
Participant selection
Since August 2019, we have prospectively enrolled patients with endogenous hypercortisolism (adrenal, pituitary, or ectopic origin) who are undergoing surgery for cortisol excess at Mayo Clinic in Rochester, MN, into an ongoing longitudinal observational study. Patients are enrolled before surgical treatment and then followed for up to 2 years postoperatively. The study protocol was approved by the Mayo Clinic Institutional Review Board and carried out in compliance with the Declaration of Helsinki. Written informed consent was obtained from all study participants at time of study entry.
For this study, we included patients with sustained biochemical remission after surgery who had 12 weeks of follow-up data available by December 31, 2021. Patients were considered to have achieved biochemical remission if they (1) underwent bilateral adrenalectomy, (2) underwent unilateral adrenalectomy for unilateral adrenal disease, and/or (3) developed postoperative adrenal insufficiency, defined as (i) 8:00 Am serum cortisol <10 µg/dL on postoperative day 1 or (ii) perioperative hemodynamic instability requiring empiric intravenous GC administration. Patients who did not achieve biochemical remission after surgery or who achieved biochemical remission but had recurrence of hypercortisolism within 12 weeks were excluded from this analysis.
Baseline assessment and longitudinal follow-up
We conducted a presurgical visit with each patient to confirm clinical findings and to obtain baseline measurements. Biochemical test results were extracted from the medical record. In line with current practice guidelines,9 MACS was diagnosed if the postdexamethasone serum cortisol concentration was >1.8 µg/dL in patients without obvious constellation of features of overt CS, based on the discretion of the treating endocrinologist.
Cushing syndrome disease severity classification
To allow for comparison across the spectrum of patients presenting with endogenous hypercortisolism, we utilized scoring systems to classify clinical and biochemical disease severity. The scoring systems were developed based on practice guidelines for the diagnosis of CS, extensive clinical experience (W.F.Y. and I.B.), and previous publications.2,8,11 For further description of the scoring systems, please see Tables S1 and S2.
Longitudinal follow-up
An electronic questionnaire was sent to patients on a weekly basis for the first 12 weeks after surgery to assess GC withdrawal symptoms and to confirm the type and dose of GC replacement. For those who underwent pituitary surgery for Cushing disease, routine evaluation of pituitary-dependent hormones was performed at weeks 6 and 12 postoperatively with initiation of hormone replacement therapies, if indicated.
All patients with postoperative adrenal insufficiency remained on GC replacement until documented recovery of the HPA axis, defined as 8:00 Am serum cortisol ≥10 µg/dL at least 24 h after the last GC dose. While the choice of GC therapy and rapidity of the taper was at the discretion of the treating endocrinologist, most patients (88%) followed the same standardized hydrocortisone taper schedule (Table S3).
Measurements
Glucocorticoid withdrawal syndrome
There are currently no validated assessments for GWS in the literature. However, because many of the symptoms of GWS overlap with those of adrenal insufficiency, we used the adrenal insufficiency disease-specific questionnaire (AddiQoL) to evaluate for possible GC withdrawal symptoms in the postoperative period. We grouped each of the 30 AddiQoL items according to the symptom being assessed (Table S4). For symptoms with multiple corresponding AddiQoL items (eg, fatigue), the symptom had to be reported on at least 50% of the corresponding items to be considered present, and the frequency of the symptom throughout the week (eg, “a good bit of the time,” “most of the time,” and “all the time”) was used as a measure of symptom severity. The total AddiQoL score was used as a surrogate for overall GWS symptomology with lower AddiQoL scores corresponding to higher GWS symptom burden.
Quality of life
The Short-Form-36 (SF-36), a general health-related questionnaire, and the CushingQoL, a CS disease-specific questionnaire, were used to assess quality of life at baseline and at 12 weeks postoperatively. Physical and Mental Component Summary scores were generated for the SF-36 and adjusted for age and sex using normative data for the US population.12–14 Physical, psychosocial, and overall Cushing score was generated for the CushingQoL.15,16 All results were standardized to range from 0 to 100 with higher SF-36 and CushingQoL scores corresponding to higher self-perceived quality of life.
Muscle function
Muscle function was assessed with hand grip strength and sit-to-stand test performance. Hand grip strength was measured in a seated position using the Jamar Plus+ digital hand dynamometer (Sammons Preston, Bolingbrook, IL, USA), and the maximum peak strength out of three attempts was recorded from the self-identified nondominant hand.17 Lower extremity muscle strength was assessed with the sit-to-stand test, and the maximum number of sit to stands completed by the patient in 30 s was recorded. Performance was reported using age- and sex-adjusted Z-scores based on normative data from the reference US adult population.18
Statistical analysis
Categorical variables were expressed as counts and proportions, and continuous variables as median and interquartile range (IQR). The normative data for the US adult population for hand grip strength and sit-to-stand test were used to convert the respective results into age- and sex- adjusted Z-scores.18 Paired t-tests were used to compare measurements at baseline and at 12 weeks after successful surgery for hypercortisolism. The Kruskal–Wallis test was used to compare the change in measurements (12 weeks-baseline) across the different CS clinical disease severity score groups. Univariate and multivariable linear regression models were used to predict the 12-week AddiQoL score (GWS symptom burden). A two-tailed probability value of P < .05 was considered statistically significant for all tests. Statistical analysis was conducted using R, version 4.1.2.
Results
Baseline characteristics
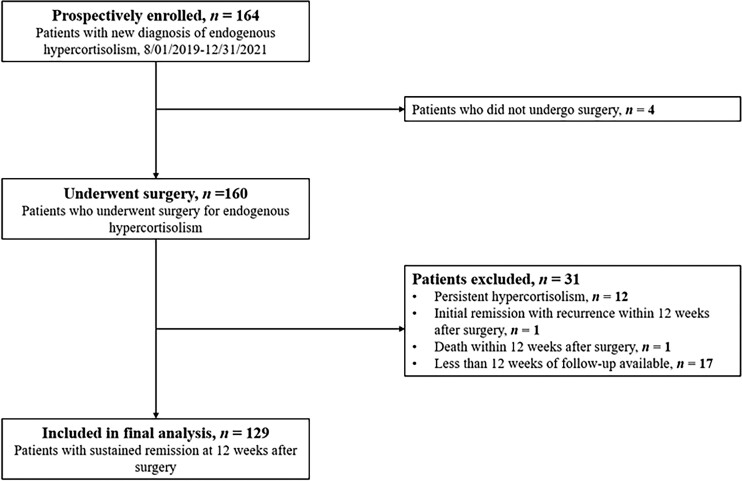
Between August 1, 2019, and December 31, 2021, a total of 160 patients were prospectively enrolled and underwent surgical treatment for endogenous neoplastic hypercortisolism (Figure 1). Thirteen patients had either persistent hypercortisolism after surgery (n = 12) or recurrence of hypercortisolism within 12 weeks (n = 1) and were excluded. One patient died during follow-up, and 17 patients did not have 12 weeks of follow-up available at the time of analysis. The final cohort consisted of 129 patients (MACS, n = 59 [46%]; adrenal CS, n = 12 [9%]; pituitary CS, n = 51 [40%]; and ectopic CS, n = 7 [5%]). The cohort was predominately female (n = 101 [78.3%]) with a median age at diagnosis of 51 years (IQR, 41-62 years).
Figure 1.
Flowchart of study participant inclusion.
Clinical and biochemical disease severity scores correlated positively with each other (R = 0.49, P < .001). As expected, patients with ectopic CS presented with the highest clinical (median, 17.0, IQR 15.5-17.0) and biochemical (median, 7.0, IQR 7.0-10.0) disease severity scores, while patients with MACS had the lowest disease severity scores (clinical score: median 6.0, IQR 3.0-9.5; biochemical score: median 3.0, IQR 2.0-4.0). Table 1 summarizes the demographics, clinical presentation, physical exam findings, and biochemical workup of the study participants.
Table 1.
Baseline characteristics of study participants.
| MACS (n = 59) | Adrenal CS (n = 12) | Pituitary CS (n = 51) | Ectopic CS (n = 7) | All participants (n = 129) | |
|---|---|---|---|---|---|
| Women, n (%) | 43 (72.9) | 9 (75.0) | 44 (86.3) | 5 (71.4) | 101 (78.3) |
| Caucasian, n (%) | 56 (94.9) | 12 (100.0) | 45 (88.2) | 7 (100.0) | 120 (93.0) |
| Age at diagnosis, median (IQR), years | 54 (48-65) | 56 (35-61) | 46 (37-59) | 42 (40-62) | 51 (41-62) |
| Body mass index, median (IQR), kg/m2 | 31.2 (27.4-38.0) | 31.6 (29.7-35.3) | 35.6 (30.5-43.7) | 31.5 (27.1-39.3) | 32.9 (28.5-40.6) |
| Duration of symptoms before diagnosis, median (IQR), months | - | 12.0 (12.0-29.2) | 36.0 (12.0-82.0) | 2.0 (2.0-9.0) | 12.0 (5.0-36.0) |
| Clinical characteristics | |||||
| Hypertension, n (%) | 47 (79.7) | 10 (83.3) | 41 (80.4) | 7 (100.0) | 105 (81.4) |
| Therapy with ≥3 antihypertensive medications, n (%) | 18 (30.5) | 4 (33.3) | 13 (25.5) | 2 (28.6) | 37 (28.7) |
| Hyperglycemia | |||||
| Prediabetes, n (%) | 19 (32.2) | 2 (16.7) | 16 (31.4) | 1 (14.3) | 38 (29.5) |
| Type 2 diabetes mellitus, n (%) | 18 (30.5) | 2 (16.7) | 21 (41.2) | 5 (71.4) | 46 (35.7) |
| Insulin therapy, n (%) | 10 (16.9) | 1 (8.3) | 10 (19.6) | 3 (42.9) | 24 (18.6) |
| Bone disease | |||||
| Osteopenia, n (%) | 15 (25.4) | 7 (58.3) | 17 (33.3) | 2 (28.6) | 41 (31.8) |
| Osteoporosis, n (%) | 10 (16.9) | 0 (0.0) | 9 (17.6) | 1 (14.3) | 20 (15.5) |
| Fragility fracture within 12 months, n (%) | 1 (1.7) | 0 (0.0) | 5 (9.8) | 2 (28.6) | 8 (6.2) |
| Dyslipidemia, n (%) | 38 (64.4) | 7 (58.3) | 33 (71.7) | 3 (60.0) | 81 (66.4) |
| Atherosclerotic cardiovascular disease, n (%) | 9 (15.3) | 0 (0.0) | 1 (2.0) | 2 (28.6) | 12 (9.3) |
| Venous thromboembolism within 12 months, n (%) | 0 (0.0) | 1 (8.3) | 4 (7.8) | 0 (0.0) | 5 (3.9) |
| Weight gain, n (%) | 28 (47.5) | 12 (100.0) | 48 (94.1) | 5 (71.4) | 93 (72.1) |
| Physical exam findings | |||||
| Central obesity, n (%) | 23 (39.0) | 10 (83.3) | 45 (88.2) | 7 (100.0) | 85 (65.9) |
| Supraclavicular and/or dorsocervical fat accumulation, n (%) | 15 (25.4) | 9 (75.0) | 42 (82.4) | 7 (100.0) | 73 (56.6) |
| Rounding of the face ± plethora, n (%) | 11 (18.6) | 9 (75.0) | 44 (86.3) | 7 (100.0) | 71 (55.0) |
| Skin changes (ie, violaceous striae, skin thinning, and/or bruising), n (%) | 17 (28.8) | 10 (83.3) | 39 (76.5) | 5 (71.4) | 71 (55.0) |
| Proximal muscle weaknessa, n (%) | 28 (47.5) | 9 (75.0) | 30 (58.8) | 6 (85.7) | 73 (56.6) |
| Biochemical assessment | |||||
| 1 mg DST, median (IQR), µg/dL | |||||
| Missing, n = 26 | 3.4 | 14.0 | 7.6 | - | 5.4 |
| Reference range: <1.8 µg/dL | (2.6-5.7) | (12.3-20.7) | (5.0-14.5) | (2.9-11.6) | |
| 8 mg DST, median (IQR), µg/dL | |||||
| Missing, n = 108 | 2.5 | 16.0 | 2.7 | 4.0 | 2.7 |
| Reference range: <1.0 µg/dL | (2.2-6.6) | (9.1-18.8) | (2.5-3.0) | (4.0-4.0) | (2.2-6.2) |
| 24-h urine cortisol, median (IQR), µg/24 h | |||||
| Missing, n = 34 | 23.0 | 197.0 | 103.5 | 803.0 | 70.0 |
| Reference range: <45 µg/24h | (15.5-38.0) | (113.0-273.1) | (49.8-216.5) | (440.0-1880.5) | (33.0-215.0) |
| Late night salivary cortisol, median (IQR), ng/dL | |||||
| Missing, n = 68 | 85.5 | 254.0 | 230.0 | 1540.0 | 230 |
| Reference range: < 100 ng/dL | (54.8-145.5) | (213.0-348.5) | (147.0-391.0) | (1185.5-1550.0) | (147.0-428.0) |
| ACTH, median (IQR), pg/mL | |||||
| Missing, n = 3 | 7.2 | <5.0 | 74.0 | 164.0 | 21.0 |
| Reference range: 5-63 pg/mL | (5.1-12.0) | (<5.0-<5.0) | (47.0-97.0) | (141.0-242.0) | (6.3-74.5) |
| DHEA-S, median (IQR), µg/dL | |||||
| Missing, n = 7 | 36.5 | 40.0 | 145.5 | 214.0 | 58.0 |
| Reference range age- and sex-dependent | (20.0-51.5) | (15.0-66.5) | (85.8-231.0) | (203.0-327.0) | (33.2-141.8) |
| CS disease severity score | |||||
| Clinical severity score, median (IQR) | 6.0 (3.0-9.5) | 15.5 (11.0-16.2) | 14.0 (11.0-17.5) | 17.0 (15.5-17.0) | 11.0 (6.0-16.0) |
| Clinical severity category, n (%) | |||||
| Mild, sum score 1-8 points | 39 (66.1) | 2 (16.7) | 6 (11.8) | 0 (0.0) | 47 (36.4) |
| Moderate, sum score 9-14 points | 14 (23.7) | 3 (25.0) | 20 (39.2) | 2 (28.6) | 39 (30.2) |
| Severe, sum score 15-22 points | 6 (10.2) | 7 (58.3) | 25 (49.0) | 5 (71.4) | 43 (33.3) |
| Biochemical severity score, median (IQR) | 3.0 (2.0-4.0) | 7.0 (6.0-8.0) | 6.0 (5.0-8.0) | 7.0 (7.0-10.0) | 5.0 (3.0-7.0) |
| Biochemical severity category, n (%) | |||||
| Mild, sum score 0-3 points | 37 (62.7) | 0 (0.0) | 4 (7.8) | 0 (0.0) | 41 (31.8) |
| Moderate, sum score 4-6 points | 21 (35.6) | 5 (41.7) | 22 (43.1) | 1 (14.3) | 49 (38.0) |
| Severe, sum score 7-11 points | 1 (1.7) | 7 (58.3) | 25 (49.0) | 6 (85.7) | 39 (30.2) |
Abbreviations: ACTH, corticotropin; CS, Cushing syndrome; DHEA-S, dehydroepiandrosterone sulfate; DST, dexamethasone suppression test; IQR, interquartile range; MACS, mild autonomous cortisol secretion.
Based on patient report and/or documented clinical physical exam.
Postoperative adrenal insufficiency developed in 112 patients, of whom 98 (88%) received hydrocortisone and 14 (12%) received prednisone. While the choice of GC therapy and the rapidity of the taper depended on the preference of the treating physician, most patients followed a standardized hydrocortisone taper schedule (Table S3). The median hydrocortisone dose equivalent was 50 mg/day (IQR 40-50) at the start of the taper and 25 mg/day (IQR 20-30) at 6 weeks after surgery. GC therapy was continued until documented recovery of the HPA axis. Of the 112 patients who developed postoperative adrenal insufficiency, 29 (26%) patients were no longer on GC therapy at 12 weeks after surgery.
Glucocorticoid withdrawal syndrome
Patient-reported symptoms
The most common patient-reported symptoms in the first 12 weeks after surgical remission of hypercortisolism were myalgias and arthralgias (50%), fatigue (45%), weakness (34%), sleep disturbance (29%), mood changes (19%), and headaches (17.0%) (Table 2). The median number of symptoms experienced by each patient was 7 (IQR 2-13), with 86% of patients reporting at least one symptom during the early postoperative period.
Table 2.
Prevalence of glucocorticoid withdrawal symptoms in the first 12 weeks following surgical remission of endogenous hypercortisolism.
| Symptom | Percentage of patients experiencing symptoma (%) | P-valueb | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Weeks 1-4 | Weeks 5-8 | Weeks 9-12 | Weeks 1-12 | Baseline vs weeks 1-4 | Weeks 1-4 vs weeks 9-12 | Baseline vs weeks 9-12 | |
| Fatigue | 56.8 | 43.2 | 44.2 | 46.8 | 44.7 | .007 | .811 | .076 |
| Myalgias and arthralgias | 64.0 | 38.1 | 53.1 | 60.4 | 50.3 | <.001 | <.001 | .586 |
| Weakness | 44.4 | 22.0 | 37.2 | 42.3 | 33.6 | <.001 | <.001 | .838 |
| Sleep disturbance | 50.4 | 29.7 | 31.9 | 26.1 | 29.2 | <.001 | .785 | <.001 |
| Mood changes | 40.8 | 16.9 | 19.5 | 21.6 | 19.3 | <.001 | .536 | <.001 |
| Headaches | 40.0 | 23.7 | 14.2 | 12.6 | 17.0 | .001 | .015 | <.001 |
| Sweating | 41.5 | 11.9 | 13.3 | 14.4 | 13.2 | <.001 | .889 | <.001 |
| Inability to concentrate | 34.4 | 13.6 | 12.4 | 12.6 | 12.9 | <.001 | .785 | <.001 |
| Nausea | 16.8 | 7.6 | 11.5 | 9.9 | 9.6 | .009 | .703 | .092 |
| Light-headedness | 13.6 | 7.6 | 8.0 | 3.6 | 6.4 | .203 | .334 | .020 |
| Limited ability to work | 34.4 | 37.3 | 29.2 | 32.4 | 33.0 | .887 | .555 | .920 |
| Predisposition to illness | 35.2 | 28.0 | 35.4 | 37.8 | 33.6 | .159 | .061 | .945 |
A particular symptom was considered present if reported on at least 50% of the weekly surveys during the specified time interval.
Statistical comparisons were made using logistic mixed effects models.
Trajectory of symptoms over time
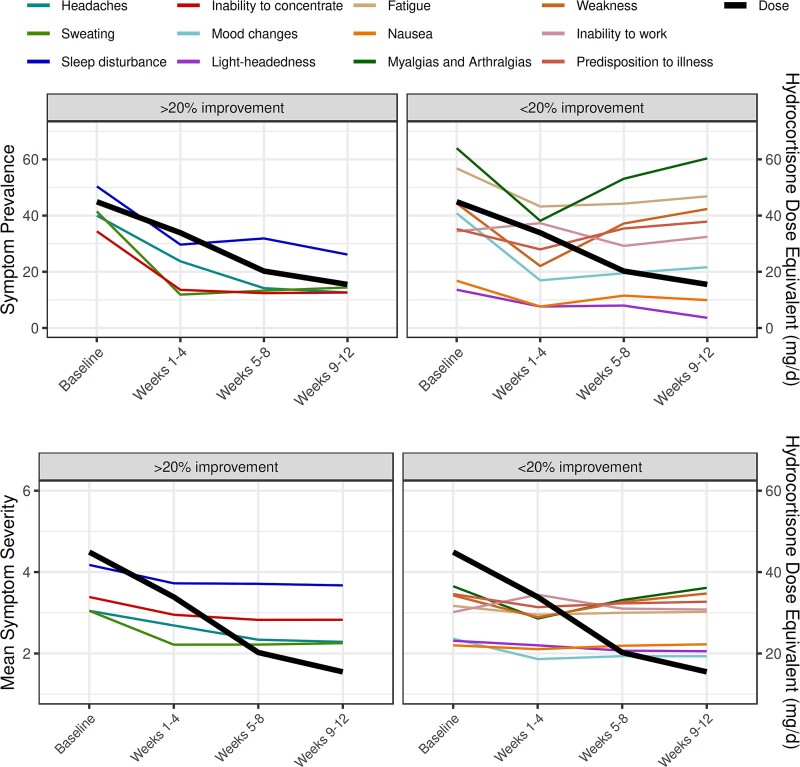
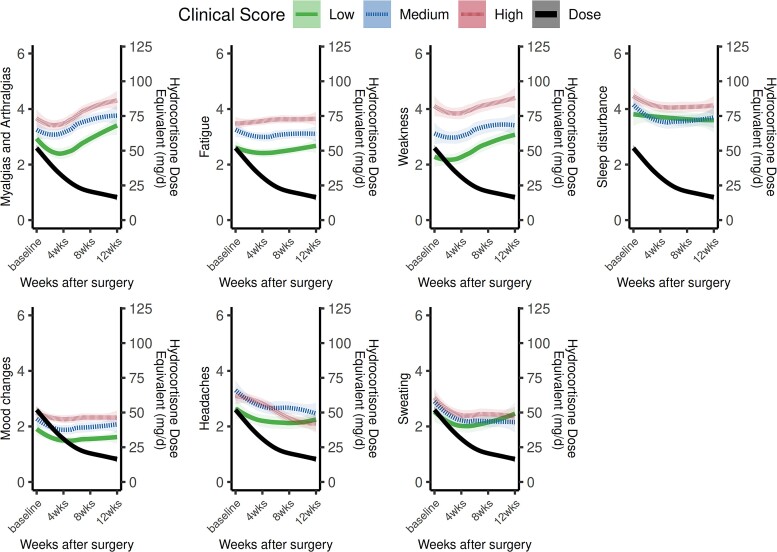
Most symptoms persisted throughout the first 12 weeks after surgery except for four—sleep disturbance, headaches, light-headedness, and sweating—which decreased in both prevalence and severity compared to baseline (Table 2 and Figure 2). In comparison to before surgery, fewer patients experienced myalgias/arthralgias (64% vs 38%, P < .001) and weakness (44% vs 22%, P < .001) in the first 4 weeks after surgery. However, the prevalence of these symptoms increased back to baseline during weeks 5-8 and weeks 9-12 postoperatively (Table 2 and Figure 2). In the subgroup of patients who were initiated on GC replacement for postoperative adrenal insufficiency, the prevalence and severity of myalgias/arthralgias and weakness symptoms increased when the median hydrocortisone equivalent dose tapered below 30 mg/day (IQR 20-30) (Figure 2). The severity and trajectory of individual symptoms also varied based on CS disease severity. Patients with the lowest clinical disease severity scores at baseline during active CS had decreased severity of GC withdrawal symptoms during recovery compared to those with higher clinical severity scores at baseline (Figure 3).
Figure 2.
Trajectory of glucocorticoid withdrawal symptoms following surgical remission of hypercortisolism. Symptoms are grouped into those that improved by >20% and those that improved by <20% within the first 12 weeks after surgery. The mean glucocorticoid dose (expressed as hydrocortisone dose equivalent/day) on the Y-axis on the right is superimposed. Mean symptom severity on the Y-axis on the left is based on frequency of symptom with 1 corresponding to the symptom being present “none of the time,” 2 corresponding to “a little of the time,” 3 corresponding to “some of the time,” 4 corresponding to “a good bit of the time,” 5 corresponding to “most of the time,” and 6 corresponding to “all of the time.”
Figure 3.
Trajectory of individual symptoms following surgical remission of hypercortisolism, stratified by baseline clinical Cushing syndrome disease severity. The trajectory of the most prevalent symptoms in the postoperative period are graphed individually and stratified by baseline clinical Cushing syndrome disease severity (low, medium, or high). Mean symptom severity on the Y-axis on the left is based on frequency of symptom with 1 corresponding to the symptom being present “none of the time,” 2 corresponding to “a little of the time,” 3 corresponding to “some of the time,” 4 corresponding to “a good bit of the time,” 5 corresponding to “most of the time,” and 6 corresponding to “all of the time.” The mean glucocorticoid dose (expressed as hydrocortisone dose equivalent/day) on the Y-axis on the right is superimposed.
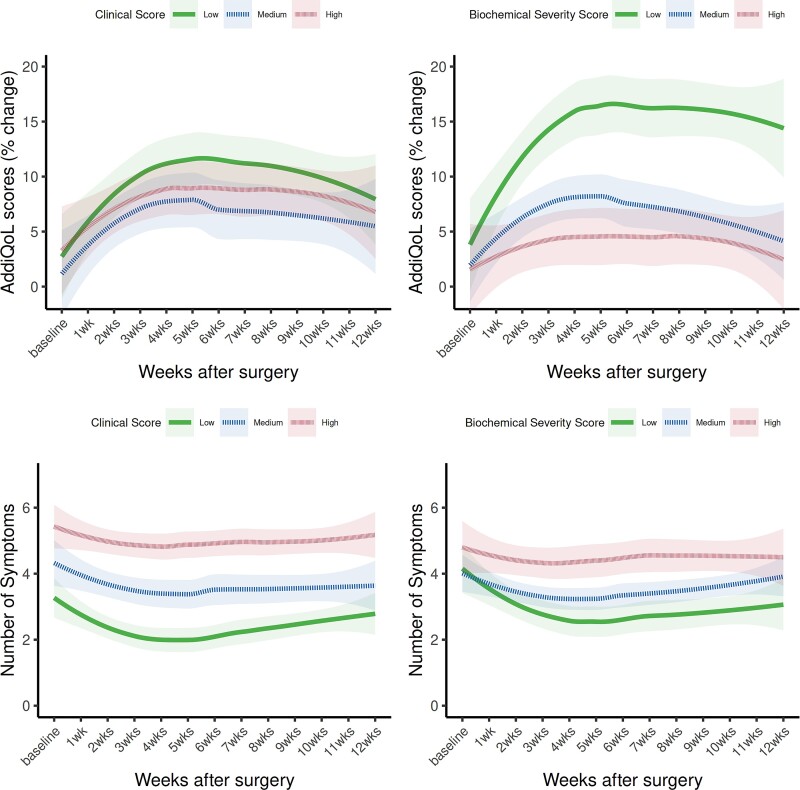
Glucocorticoid withdrawal syndrome symptom burden
The AddiQoL score was used as marker of overall GWS symptomology with lower scores corresponding to higher symptom burden. Glucocorticoid withdrawal syndrome symptom burden initially improved after surgery for hypercortisolism but then stagnated before worsening during weeks 5-12 (Figure 4). When stratified by baseline CS severity scores, patients with the lowest CS biochemical scores at baseline had decreased symptom burden throughout the first 12 weeks after surgery compared to patients with higher CS biochemical scores (low vs medium CS biochemical scores, P = .02; low vs high CS biochemical scores, P = .006). The number of symptoms reported per week was also used to assess overall symptom burden (Figure 4), with a greater number of symptoms in patients with higher baseline CS clinical severity scores (low vs medium CS clinical score, P = .02; low vs high CS clinical score, P < .001; and medium vs high CS clinical score, P = .04).
Figure 4.
Glucocorticoid withdrawal syndrome (GWS) symptom burden in the first 12 weeks following surgical remission of hypercortisolism, stratified by baseline clinical and biochemical Cushing syndrome disease severity. The AddiQoL score as well as the total number of symptoms was used as a marker of GWS symptom burden. A lower AddiQoL score corresponds to higher GWS symptom burden.
Quality of life
On the SF-36 questionnaire, the Physical Component Summary score worsened at 12 weeks compared to the presurgical baseline (mean difference −2.6, P = .015), while the Mental Component Summary score improved but did not reach statistical significance (mean difference 1.5, P = .225) (Table 3). On the CushingQoL questionnaire, the overall CushingQoL score (mean difference 7.8, P < .001) as well as both its physical (mean difference 5.4, P = .009) and psychosocial components scores (mean difference 14.2, P < .001) improved postoperatively at 12 weeks compared to baseline (Table 3).
Table 3.
Quality of life and muscle function measurements before and 12 weeks after surgical remission of hypercortisolism.
| Presurgical baseline | 12 weeks after surgery | Difference | P-valuea | |
|---|---|---|---|---|
| SF-36 | ||||
| Physical functioning | 36.9 (14.6) | 37.3 (13.3) | −0.4 (10.3) | .725 |
| Role–physical limitation | 35.3 (12.5) | 33.4 (10.4) | −2.2 (11.3) | .062 |
| Body pain | 43.4 (11.9) | 39.3 (10.6) | −4.3 (10.7) | <.001 |
| General health | 37.0 (11.9) | 38.4 (10.9) | 0.7 (8.0) | .392 |
| Social functioning | 37.1 (13.0) | 38.5 (12.0) | 0.5 (12.9) | .720 |
| Role–emotional limitation | 39.0 (12.7) | 39.1 (13.1) | −1.0 (13.7) | .406 |
| Vitality | 35.1 (10.5) | 35.1 (9.9) | −0.3 (11.4) | .791 |
| Mental health | 40.6 (11.8) | 43.8 (11.9) | 2.6 (10.7) | .018 |
| Physical Component Summary score | 37.2 (13.1) | 35.0 (11.9) | −2.6 (10.1) | .015 |
| Mental Component Summary score | 38.6 (12.5) | 41.1 (12.2) | 1.5 (12.3) | .225 |
| CushingQoL | ||||
| Physical score | 40.8 (21.8) | 45.9 (22.8) | 5.4 (19.4) | .009 |
| Psychosocial score | 36.1 (24.2) | 50.8 (22.4) | 14.2 (22.8) | <.001 |
| Overall Cushing score | 39.8 (20.6) | 47.1 (21.1) | 7.8 (17.8) | <.001 |
| Muscle function | ||||
| Hand grip (nondominant hand), Z-scoreb | −0.10 (0.97) | −0.52 (1.14) | −0.37 (0.88) | .009 |
| Sit-to-stand test performance, Z-scoreb | −0.67 (1.41) | −0.23 (1.85) | 0.50 (1.23) | .013 |
Abbreviation: SF-36, Short-Form-36.
Paired t-tests were used to compare measurements at presurgical baseline and 12 weeks after successful surgery for hypercortisolism. Values are expressed as means (standard deviation). Short-Form-36 and CushingQoL scores were standardized to range from 0 to 100, with higher scores corresponding with higher self-reported quality of life. Muscle function was reported using age- and sex-adjusted Z-scores based on normative data from a reference US adult population.
Pre- and postsurgical measurements were available for n = 43 (handgrip strength) and n = 40 (sit-to-stand test).
Muscle function
On assessment of muscle function, normalized hand grip strength from the nondominant side worsened at 12 weeks after surgery compared to the presurgical baseline (mean Z-score difference −0.37, P = .009), while normalized sit-to-stand test performance improved (mean Z-score difference 0.50, P = .013) (Table 3). Changes in muscle function varied based on the presurgical CS clinical disease severity score. Patients with the highest CS clinical scores had more significant worsening of hand grip strength but greater improvements in sit-to-stand test performance after surgery compared to those with lower CS clinical scores (Table 4).
Table 4.
Change in quality of life and muscle function measurements before and 12 weeks after surgical remission of hypercortisolism, stratified by baseline Cushing syndrome clinical disease severity.
| Mild Clinical disease severitya |
Moderate Clinical disease severitya |
Severe Clinical disease severitya |
P-valueb | |
|---|---|---|---|---|
| n = 47 | n = 39 | n = 43 | ||
| SF-36, delta (12 weeks-baseline) | ||||
| Physical functioning | −0.78 (10.70) | −2.26 (11.15) | 1.78 (8.79) | .091 |
| Role–physical limitation | −2.51 (13.56) | −5.22 (11.85) | 0.98 (6.10) | .097 |
| Body pain | −2.46 (10.48) | −8.51 (12.31) | −2.88 (8.65) | .074 |
| General health | −0.66 (7.17) | 0.42 (8.20) | 2.56 (8.70) | .292 |
| Social functioning | −2.24 (12.67) | −1.80 (10.28) | 5.66 (13.89) | .072 |
| Role–emotional limitation | −5.34 (13.79) | 1.81 (13.41) | 1.70 (12.73) | .061 |
| Vitality | −0.97 (12.36) | 0.16 (11.16) | 0.08 (10.56) | .994 |
| Mental health | 2.35 (9.20) | 1.72 (10.16) | 3.65 (12.82) | .812 |
| Physical Component Summary | −1.77 (10.13) | −6.13 (11.85) | −0.49 (7.88) | .132 |
| Mental Component Summary | −1.52 (11.78) | 3.16 (10.12) | 3.76 (14.06) | .199 |
| CushingQoL, delta (12 weeks-baseline) | ||||
| Physical score | 5.48 (19.80) | 14.66 (20.85) | 22.98 (24.48) | .009 |
| Psychosocial score | 6.72 (20.05) | 2.87 (18.27) | 6.31 (20.01) | .928 |
| Overall CushingQoL | 6.38 (17.36) | 5.82 (16.86) | 10.87 (19.09) | .555 |
| Muscle function (12 weeks-baseline) | ||||
| Hand grip (nondominant hand), Z-scorec | 0.17 (0.26) | −0.19 (1.06) | −0.82 (0.69) | .002 |
| Sit to stand test performance, Z-scorec | 1.01 (1.62) | 0.38 (1.20) | 0.41 (1.10) | .830 |
Abbreviation: SF-36, Short-Form-36.
Clinical disease severity scores were calculated for each patient prior to surgical treatment for hypercortisolism. A total score of 1-8 points was considered mild, 9-14 points moderate, and 15-22 points severe clinical disease.
Kruskal–Wallis test was used to compare the change in quality of life and muscle function measurements (12 weeks-baseline) across the different Cushing syndrome clinical disease severity groups. Values are expressed as means (standard deviation).
Pre- and postsurgical measurements were available for n = 43 (handgrip strength) and n = 40 (sit-to-stand test).
Predictors of glucocorticoid withdrawal syndrome
On univariable analysis, younger age at the time of diagnosis, female sex, higher CS clinical disease severity score, and decreased proximal muscle strength (as measured by the sit-to-stand test) at baseline were predictive of higher GWS symptom burden at 12 weeks postoperatively (Table 5). A higher body mass index and longer duration of symptoms prior to the diagnosis of hypercortisolism correlated positively with symptom burden but did not reach statistical significance. On multivariable analysis, baseline CS clinical disease severity was the only independent predictor of GWS at 12 weeks postoperatively, with higher presurgical CS clinical disease severity correlating with greater GWS symptom burden (Table 5).
Table 5.
Predictors of AddiQoL score (GWS symptom burden) 12 weeks after surgical remission of hypercortisolism.
| Presurgical parameter | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| Estimate | Standard error | P-value | Estimate | Standard error | P-value | |
| Age at diagnosis (per 10-year increase) | 0.266 | 0.088 | .003 | 0.159 | 0.090 | .081 |
| Sex, female | −7.935 | 3.036 | .010 | −3.633 | 2.987 | .227 |
| Body mass index (per 1 kg/m2 increase) | −0.275 | 0.146 | .063 | 0.006 | 0.148 | .968 |
| Duration of symptoms prior to diagnosisa (per 1-month increase) | −0.028 | 0.030 | .342 | - | - | - |
| Clinical disease severity score (per 1-point increase) | −0.977 | 0.220 | <.001 | −0.689 | 0.250 | .007 |
| Sit-to-stand test performance, Z-score | 1.865 | 0.794 | .021 | 1.249 | 0.769 | .107 |
The AddiQoL score was used as a marker of GWS symptom burden. A lower AddiQoL score corresponds to higher symptom burden.
Abbreviation: GWS, glucocorticoid withdrawal syndrome.
Patients with mild autonomous cortisol secretion were excluded from the specific analysis.
Discussion
This is the first longitudinal study to prospectively characterize GWS following surgical remission of endogenous hypercortisolism. We described the most prevalent GC withdrawal symptoms and their trajectory in the first 12 weeks after surgery. Baseline CS disease severity was found to be the only independent presurgical predictor of postoperative GWS symptom burden.
Glucocorticoid withdrawal syndrome is a scarcely studied phenomenon that complicates the recovery following successful surgery for cortisol excess and is both a challenge for patients to endure and for providers to manage. The symptoms are precipitated by the rapid decline in cortisol levels following surgery and can be difficult to distinguish from those of postoperative adrenal insufficiency or recurrence of cortisol excess. In this study, we examined patient-reported symptoms following documented surgical remission of hypercortisolism and after initiation of GC replacement for postoperative adrenal insufficiency—symptoms that may be ascribed to GWS.
We found that the most prevalent GC withdrawal symptoms in the postoperative period were myalgias/arthralgias, fatigue, sleep disturbance, weakness, and mood changes. While most symptoms persisted without significant improvement, four symptoms did improve by at least 20% during the first 12 weeks after successful surgery: headaches, sleep disturbance, inability to concentrate, and sweating. Myalgias, arthralgias, and weakness initially improved after surgery, but notably started to worsen at week 5 and were prominent symptoms during weeks 5-12 postoperatively.
The pattern of GC withdrawal symptoms observed in this study may be due to the natural trajectory of GWS itself or from the GC doses utilized postoperatively. In patients who are initiated on GC replacement for postoperative adrenal insufficiency, supraphysiologic GC doses are often used to try to prevent GWS symptomology immediately after surgery. If symptoms flare as the GC dose is being tapered, a commonly employed strategy is to increase the GC dose to the most recent prior dose.6 In this study, we found that exacerbation of myalgias and arthralgias and weakness symptoms occurred when the GC tapered below a hydrocortisone equivalent of 30 mg/day, which may serve as a clinically relevant threshold during GWS. Our data suggests that GC replacement at higher doses in the early postoperative remission phase appears to attenuate GC withdrawal symptoms and possibly the associated underlying inflammatory process. However, further analyses on the type and rapidity of GC taper and correlation with GWS symptoms were not possible with our sample size and the observational nature of the study.
The exacerbation of patient-reported myalgias/arthralgias and weakness symptoms during weeks 5-12 after surgery may explain the discordant changes in muscle strength observed in the early postoperative period. We found that while hand grip strength worsened at 12 weeks compared to baseline, proximal muscle strength (as measured by sit-to-stand test performance) improved. Similarly, in a longitudinal study from the German CS Registry that assessed muscle function following surgical remission of hypercortisolism (of pituitary, adrenal, or ectopic origin), hand grip strength temporarily worsened at 6 months after surgery compared to baseline, while chair rise test performance improved.19 A possible explanation is that hand grip strength may be disproportionally affected by the patient-reported myalgias/arthralgias that are a prominent part of GWS. Meanwhile, proximal muscle strength (as measured by the chair rise test) may be a more specific indicator of favorable change in underlying muscle function following resolution of hypercortisolism, although studies show that muscle function (both hand grip strength and sit-to-stand test performance) remains impaired in patients with treated CS compared to persons without CS.19,20
Similarly, discordant changes were noted in quality of life measurements at 12 weeks after surgical remission of hypercortisolism. While the SF-36 Physical Component Score worsened, the CushingQoL and both its physical and psychosocial components improved at 12 weeks after surgery compared to baseline. The decline in the SF-36 Physical Component score was driven by the body pain and role–physical limitations subdomains, which may be exacerbated by GWS symptomology. Meanwhile, the CushingQoL physical score focused on questions specific to hypercortisolism, such as wound healing and easy bruising16—symptoms that improve following surgical remission of cortisol excess. A previous longitudinal study from the German CS Registry also demonstrated an improvement in the overall CushingQoL summary score (subscales not reported) with no significant change in SF-36 Physical Component score at 6 months compared to baseline. Both quality-of-life measures subsequently improved with longer follow-up (1, 2, and 3 years).19 These findings suggest that the discrepant changes in SF-36 and CushingQoL in the early postoperative period may reflect the competing influences of GWS and recovery from hypercortisolism.
Overall GWS symptom burden (as measured by the AddiQoL score) persisted throughout the first 12 weeks after surgery, and patients with higher CS disease severity had greater symptom burden. Furthermore, baseline CS clinical disease severity was found to be the only independent presurgical predictor of postoperative GWS symptom burden. Patients with higher baseline CS clinical disease severity scores had greater GWS symptom burden at 12 weeks as measured by the AddiQoL score. Body mass index, age at the time of diagnosis, and baseline muscle strength were not predictive of GWS symptom burden on multivariable analysis. This finding is consistent with our previous retrospective study that showed higher CS disease severity correlated with a greater number of GWS events following unilateral adrenalectomy for ACTH-independent hypercortisolism.8
The strengths of this study include the longitudinal study design and the prospective assessment of GC withdrawal symptoms, which minimizes recall bias. Detailed clinical and biochemical data were available for each patient, and the sample size was relatively large for a rare disease. There are also several limitations. The study population was heterogenous and, in part, represented the spectrum of patients presenting with endogenous hypercortisolism. Clinical and biochemical severity scores were thus generated to allow for comparison across the different subtypes of hypercortisolism. The GC dosing was at the discretion of the treating endocrinologist, but most patients (88%) followed a similar hydrocortisone taper, and the GC dose was prospectively assessed on a weekly basis. At institutions that utilize different GC tapering regimens, the trajectory of GWS may differ than what was observed in this study. Finally, this study does not specifically address management of GWS, and the data presented were limited to the early postoperative period, where patients may struggle the most with GWS symptomology. Future studies with longer follow-up data and larger sample sizes are needed to delineate the full trajectory of GWS and to evaluate strategies to mitigate GWS symptomology.
In summary, our findings suggest that GC withdrawal symptoms are prevalent and persistent in the first 12 weeks following surgical remission of endogenous hypercortisolism with notable worsening of myalgias/arthralgias and patient-reported weakness during weeks 5-12. Baseline CS clinical disease severity was identified to be the only presurgical variable predictive of GWS symptom burden. Finally, discrepant changes in muscle function and quality-of-life measurements were observed at 12 weeks after surgery compared to baseline and may reflect the competing influences of GWS and recovery from hypercortisolism in the early postoperative period. The findings from this study may help inform patient education and expectations following surgical remission of hypercortisolism as well as future interventions to improve GWS.
Supplementary Material
Acknowledgments
The authors are grateful to the patients who participated in the study. We would also like to acknowledge the support from our neurosurgery, endocrine surgery, and endocrinology colleagues in the Pituitary-Gonadal-Adrenal Core group at Mayo Clinic in the care of patients with endogenous hypercortisolism.
Contributor Information
Catherine D Zhang, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Division of Endocrinology and Molecular Medicine, Medical College of Wisconsin, Milwaukee, WI 53226, USA.
Dingfeng Li, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Endocrine and Metabolism Institute, Cleveland Clinic, Cleveland, OH 44195, USA.
Sumitabh Singh, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Malavika Suresh, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Department of Internal Medicine, Medstar Health, Baltimore, MD 21237, USA.
Karthik Thangamuthu, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA.
Rohit Nathani, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA; Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX 75390, USA.
Sara J Achenbach, Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN 55905, USA.
Elizabeth J Atkinson, Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN 55905, USA.
Jamie J Van Gompel, Department of Neurosurgery, Mayo Clinic, Rochester, MN 55905, USA.
William F Young, Jr., Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA
Irina Bancos, Division of Endocrinology, Diabetes, Metabolism and Nutrition, Mayo Clinic, Rochester, MN 55905, USA.
Supplementary material
Supplementary material is available at European Journal of Endocrinology online.
Funding
This research was partially supported by a philanthropic gift from Lili and Keith Olin supporting patients with Cushing syndrome and by a grant from Recordati Rare Diseases in support of investigator-initiated research on glucocorticoid withdrawal syndrome. I.B. was partially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) USA under awards K23DK121888 and R03DK132121. The content is solely the responsibility of the authors and does not necessarily represent the views of the NIH.
Data availability
Datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.
References
- 1. Fleseriu M, Auchus R, Bancos I, et al. Consensus on diagnosis and management of Cushing’s disease: a guideline update. Lancet Diabetes Endocrinol. 2021;9(12):847–875. 10.1016/S2213-8587(21)00235-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nieman LK, Biller BMK, Findling JW, et al. Treatment of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100(8):2807–2831. 10.1210/jc.2015-1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stroud A, Dhaliwal P, Alvarado R, et al. Outcomes of pituitary surgery for Cushing’s disease: a systematic review and meta-analysis. Pituitary. 2020;23(5):595–609. 10.1007/s11102-020-01066-8 [DOI] [PubMed] [Google Scholar]
- 4. Alexandraki KI, Kaltsas GA, Isidori AM, et al. Long-term remission and recurrence rates in Cushing’s disease: predictive factors in a single-centre study. Eur J Endocrinol. 2013;168(4):639–648. 10.1530/EJE-12-0921 [DOI] [PubMed] [Google Scholar]
- 5. Ciric I, Zhao JC, Du H, et al. Transsphenoidal surgery for Cushing disease: experience with 136 patients. Neurosurgery. 2012;70(1):70–80; discussion 80-1. 10.1227/NEU.0b013e31822dda2c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He X, Findling JW, Auchus RJ. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary. 2022;25(3):393–403. 10.1007/s11102-022-01218-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24(4):523–538. 10.1210/er.2001-0014 [DOI] [PubMed] [Google Scholar]
- 8. Hurtado MD, Cortes T, Natt N, et al. Extensive clinical experience: hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin Endocrinol (Oxf). 2018;89(6):721–733. 10.1111/cen.13803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fassnacht M, Arlt W, Bancos I, et al. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2016;175(2):G1–G34. 10.1530/EJE-16-0467 [DOI] [PubMed] [Google Scholar]
- 10. Acree R, Miller CM, Abel BS, et al. Patient and provider perspectives on postsurgical recovery of Cushing syndrome. J Endocr Soc. 2021;5(8):bvab109. 10.1210/jendso/bvab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herndon J, Kaur RJ, Romportl M, et al. The effect of curative treatment on hyperglycemia in patients with Cushing syndrome. J Endocr Soc. 2021;6(1):bvab169. 10.1210/jendso/bvab169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ware JE, Kosinski M. SF-36 Physical & Mental Health Summary Scales: a Manual for Users of Version 1. 2 ed. Lincoln, RI: QualityMetric; 2001. [Google Scholar]
- 13. Ware JE Jr, Coutinho G, Smith AB, Tselenti E, Kulasekaran A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995;33(4 Suppl):As264-79. https://www.researchgate.net/publication/15476643_Comparison_of_methods_for_the_scoring_and_statistical_analysis_of_SF-36_health_profile_and_summary_measures_Summary_of_results_from_the_Medical_Outcomes_Study [PubMed] [Google Scholar]
- 14. Taft C, Karlsson J, Sullivan M. Do SF-36 summary component scores accurately summarize subscale scores? Qual Life Res. 2001;10(5):395–404. 10.1023/A:1012552211996 [DOI] [PubMed] [Google Scholar]
- 15. Webb SM, Badia X, Barahona MJ, et al. Evaluation of health-related quality of life in patients with Cushing’s syndrome with a new questionnaire. Eur J Endocrinol. 2008;158(5):623–630. 10.1530/EJE-07-0762 [DOI] [PubMed] [Google Scholar]
- 16. Tiemensma J, Depaoli S, Felt JM. Using subscales when scoring the Cushing's quality of life questionnaire. Eur J Endocrinol. 2016;174(1):33–40. 10.1530/EJE-15-0640 [DOI] [PubMed] [Google Scholar]
- 17. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing. 2011;40(4):423–429. 10.1093/ageing/afr051 [DOI] [PubMed] [Google Scholar]
- 18. Warden SJ, Liu Z, Moe SM. Sex- and age-specific centile curves and downloadable calculator for clinical muscle strength tests to identify probable sarcopenia. Phys Ther. 2022;102(3):pzab299. 10.1093/ptj/pzab299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vogel F, Braun LT, Rubinstein G, et al. Persisting muscle dysfunction in Cushing’s syndrome despite biochemical remission. J Clin Endocrinol Metab. 2020;105(12):e4490–e4498. 10.1210/clinem/dgaa625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berr CM, Stieg MR, Deutschbein T, et al. Persistence of myopathy in Cushing’s syndrome: evaluation of the German Cushing’s Registry. Eur J Endocrinol. 2017;176(6):737–746. 10.1530/EJE-16-0689 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets generated and/or analyzed during the study are available from the corresponding author on reasonable request.