Abstract
Myxococcus xanthus is a gram-negative bacterium that develops in response to starvation on a solid surface. The cells assemble into multicellular aggregates in which they differentiate from rod-shaped cells into spherical, environmentally resistant spores. Previously, we have shown that the induction of β-lactamase is associated with starvation-independent sporulation in liquid culture (K. A. O’Connor and D. R. Zusman, Mol. Microbiol. 24:839–850, 1997). In this paper, we show that the chromosomally encoded β-lactamase of M. xanthus is autogenously induced during development. The specific activity of the enzyme begins to increase during aggregation, before spores are detectable. The addition of inducers of β-lactamase in M. xanthus, such as ampicillin, d-cycloserine, and phosphomycin, accelerates the onset of aggregation and sporulation in developing populations of cells. In addition, the exogenous induction of β-lactamase allows M. xanthus to fruit on media containing concentrations of nutrients that are normally too high to support development. We propose that the induction of β-lactamase is an integral step in the development of M. xanthus and that this induction is likely to play a role in aggregation and in the restructuring of peptidoglycan which occurs during the differentiation of spores. In support of this hypothesis, we show that exogenous induction of β-lactamase can rescue aggregation and sporulation of certain mutants. Fruiting body spores from a rescued mutant are indistinguishable from wild-type fruiting body spores when examined by transmission electron microscopy. These results show that the signal transduction pathway leading to the induction of β-lactamase plays an important role in aggregation and sporulation in M. xanthus.
Myxococcus xanthus is a gram-negative bacterium with a complex life cycle in which multicellular associations are common. During growth, the cells feed upon organisms and macromolecules in their environment. Predation is aided by the secretion of antibiotics and catabolic enzymes. Upon starvation for nutrients, the cells cease growth and begin to aggregate into mounds within which the cells differentiate into spores. It was from these distinctive fruiting structures that the organism was first described (54). Sporulation involves the restructuring of the entire cell wall as the cell undergoes differentiation from a rod-shaped, vegetatively growing cell into a spherical spore possessing a thick spore coat (52).
In addition to the developmental pathway that leads to sporulation, the cells can be induced to make glycerol spores by the addition of 0.5 M glycerol to nutrient-rich medium (9). Previously, we have shown that the chromosomally encoded β-lactamase of M. xanthus (56) is induced concomitantly with the induction of glycerol sporulation and that the disruption of the synthesis or assembly of the peptidoglycan layer of the cell wall of growing M. xanthus is sufficient to induce both β-lactamase and glycerol sporulation (45). In the first description of β-lactamase, the activity was shown to purify as a single peak by gel chromatography, which leads to the assumption that there is a single enzyme responsible for β-lactamase activity in M. xanthus (56). However, it is formally possible that the β-lactamase activity measured in the experiments described in the previous paper and this paper is due to the combined activities of more than one β-lactamase enzyme.
The observation that β-lactamase activity is induced during glycerol sporulation suggests that it might also be induced during starvation-induced sporulation. In this paper we report that β-lactamase activity is indeed autogenously induced during fruiting body development. In addition, the exogenous induction of β-lactamase can have significant effects on the timing of aggregation and sporulation. Furthermore, mutants blocked in development can be phenotypically rescued for development by the inclusion of inducers of β-lactamase in the developmental medium.
MATERIALS AND METHODS
Strains and growth conditions.
M. xanthus was grown in CYE liquid culture (10 mM MOPS [3-(N-morpholino)-propanesulfonic acid], pH 7.6, 1% Difco Bacto Casitone, 0.5% Difco Bacto Yeast Extract, 0.1% MgSO4 · 7H2O) (3). Gel Gro was added at 0.5% for plates. Strains are described in Table 1.
TABLE 1.
Strains
| Strain | Genotype or description | Reference |
|---|---|---|
| DK1253 | tgl-1 | 17 |
| DK1622 | Fully motile | 22 |
| DK2657 | DK1622::Tn5-132 Ω1519 (csgA) | 53 |
| DK4290 | DK1622::Tn5lac Ω4273 (tps) | 29, 30 |
| DK4293 | DK1622::Tn5lac Ω4401 | 30 |
| DK4300 | DK1622::Tn5lac Ω4408 (sde) | 13, 30 |
| DK4368 | DK1622::Tn5lac Ω4403a | 30 |
| DK4469 | DK1622::Tn5lac Ω4469 | 30 |
| DK4514 | DK1622::Tn5lac Ω4514 | 30 |
| DK4521 | DK1622::Tn5lac Ω4521 | 30 |
| DK5204 | DK1622::Tn5lac Ω4435 | 30 |
| DK5206 | DK1622::Tn5lac Ω4455 | 30 |
| DK5274 | DK1622::Tn5lac Ω4414 | 30 |
| DK5285 | DK1622::Tn5lac Ω4491 | 30 |
| DZ2 | Wild type | 8 |
| DZ4148 | DZ2::Tn5 Ω226 (frzE) | 51 |
| DZ4169 | DZ2::Tn5tac1 Ω4017 (frzCD) | 51 |
| DZF1 | Leaky pilQ (sglA); wild-type development | 8, 57 |
The Tn5lac is inserted in a gene encoding an apparent serine protease (12).
Materials.
Difco Bacto Casitone, Bacto Yeast Extract, Bacto Agar, and salts were purchased through Fisher Scientific (Pittsburgh, Pa.). Ampicillin, cephaloridine, MOPS, Trizma Base, alcohol dehydrogenase (A7011), o-nitrophenyl-β-d-galactopyranoside, and lysozyme-agarose beads (L1129) were purchased from Sigma Chemical Company (St. Louis, Mo.). GelGro was purchased from ICN Biomedicals, Inc. (Irvine, Calif.). n-Dodecyl-β-d-maltoside and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) were purchased from Calbiochem-Novabiochem Corporation (San Diego, Calif.).
Development on plates.
M. xanthus was grown in CYE medium at 32°C with shaking at 275 rpm to a cell density of 2 × 108 to 6 × 108/ml. The cells were harvested by centrifugation for 10 min at 4°C at 7,700 × g in a Dupont Sorvall refrigerated centrifuge (unless stated otherwise, this was the standard centrifugation protocol used for M. xanthus). The culture medium was poured off. The cell pellet was washed one time in 10 mM MOPS, pH 7.6, 4 mM MgCl2, 2 mM CaCl2 (MMC) (50). The cells were resuspended in MMC at the cell density described for each experiment. The cells were allowed to develop on CF solid medium containing 0.5% GelGro or 1.5% Bacto Agar. [CF contains 10 mM MOPS (pH 7.6), 8 mM MgSO4, 1 mM KH2PO4 (pH 7.6), 0.2% C6H5Na3O7 (sodium citrate), 0.02% (NH4)2SO4, and 0.015% Bacto Casitone. Sodium pyruvate is added to a final concentration of 0.1% after autoclaving (16).] CF salts medium lacks Casitone and pyruvate.
Spores were harvested for counting by using a 7-mm-diameter glass tube to take a core of agar with developing cells on it. The agar was placed into 1 ml of water and sonicated for 30 s at 15 W to disrupt the agar, fruiting bodies, and cells that were sonication sensitive. Sonication-resistant spores were counted in a hemacytometer. Dense suspensions of spores were diluted for counting. We note that spores resuspended in water do not clump; thus, they can easily be counted as individuals. Those rare spores which occurred as doublets in the field were counted as single spores. The numbers of spores reported are the average of four independent samples from plates.
Development in submerged culture.
Development of M. xanthus in submerged culture was set up essentially as described by Kuner and Kaiser (33) with the buffer indicated for each experiment. Briefly, cells were inoculated in petri plates or tissue culture wells in CYE medium at a density of 0.5 × 108 to 1.0 × 108/ml. After overnight growth, the culture medium was removed by aspiration. The cells were washed with an equal volume of MMC for 5 min at room temperature with shaking at 50 rpm. The wash was aspirated. The cells were then overlaid with the buffer to be used for development. The volume of medium used for each size of culture dish was 40 ml for 150-mm-diameter petri plates, 25 ml for 100-mm-diameter petri plates, 2 ml for 35-mm-diameter petri plates, and 1 ml for 12-well tissue culture plates.
Spores were harvested for counting by releasing cells from the bottom of the dish with a rubber policeman. The culture medium and cells were transferred to a centrifuge tube. The cells were pelleted by centrifugation for 10 min at 4°C at 7,700 × g in a Dupont Sorvall refrigerated centrifuge or at 14,000 × g for 5 min at room temperature in a microcentrifuge, depending upon the volume of the culture. The supernatant was drawn off. The cells were resuspended in 0.1 ml of water and then sonicated for 15 s at 10 W to disrupt fruiting bodies and cells that were sonication sensitive. Sonication-resistant spores were counted in a hemacytometer. Dense suspensions of spores were diluted for counting.
Induction of sporulation in suspension.
Strain DZ2 was grown to a cell density of 2 × 108/ml in CYE. Glycerol was added to the culture to a final concentration of 0.5 M, or ampicillin was added to a final concentration of 1 mM, with continued incubation at 32°C for 24 h. For induction in MMC, cells were harvested from CYE medium by centrifugation. The cells were resuspended at 2 × 108/ml in MMC. In either CYE or MMC, refractile spores were present within 3 h of induction.
Expression of β-lactamase during development.
Cells were set up to develop as described above; 2.8 × 109 cells were spread evenly on each CF plate containing GelGro. The plates were incubated at 28 and 34°C. At the intervals indicated in Fig. 1, the plates were flooded with 1.5 ml of ice-cold MMC. The cells were scraped from the surface of the plate with a razor blade. The scraped cells suspended in MMC were transferred to a 1.5-ml microcentrifuge tube, in which the cells were pelleted at 14,000 × g for 5 min at room temperature. The supernatant was drawn off the cells and discarded, and the cell pellets were stored at −70°C until needed for assays. The cells were prepared for β-lactamase assays by thawing the pellet and then preparing the extract and assaying for β-lactamase as described below.
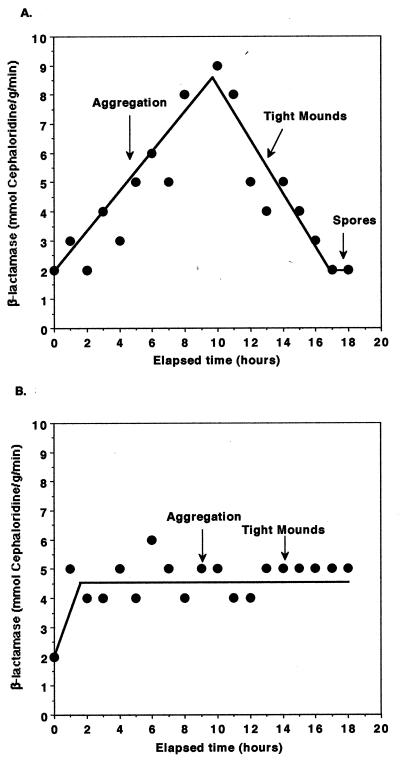
FIG. 1.
Expression of β-lactamase during development. DZF1 cells were plated for development on CF GelGro plates as described in Materials and Methods. Cells were harvested from the plates every hour, lysed, and assayed for β-lactamase activity with cephaloridine as a substrate for the enzyme. Specific activity of the enzyme based upon protein concentrations determined by the bicinchoninic acid assay is reported. The experiment was performed several times, and the results of a typical experiment are shown. (A) Specific activity of β-lactamase during development at 34°C. (B) Specific activity of β-lactamase during development at 28°C.
Tn5lac expression during development.
Tn5lac strains were grown on CYE containing 100 μg of kanamycin/ml. The cells were scraped from the growth plates and resuspended in MMC at a concentration of approximately 4 × 109/ml. Five-microliter aliquots were spotted on CF GelGro plates containing 40 μg of X-Gal/ml dissolved in dimethyl sulfoxide (CF X-Gal plates) or CF X-Gal plates containing 15 μg of d-cycloserine/ml. The plates were incubated at 28°C and examined daily for blue color associated with the developing colonies. This is not a sensitive assay for expression, but the sensitivity is sufficient to detect significant differences in the onset or level of expression.
Protein extracts and β-lactamase assays.
Cells were harvested from plates or culture and concentrated by centrifugation as described above. Protein extracts were made by the addition of 1 ml of 50 mM Tris-HCl (pH 8.0)–50 mM n-dodecyl-β-d-maltoside to 4 × 109 to 8 × 109 pelleted cells. The cells were lysed by vortexing. The extract was treated by sonic oscillation for 10 pulses at a 10% duty cycle at a power setting of 1 on a model 450 Sonifier equipped with a tapered microtip (Branson Ultrasonics Corporation, Danbury, Conn.) to complete lysis and to reduce viscosity. The protein concentrations in the extracts were determined by the bicinchoninic acid assay (Pierce, Rockford, Ill.). Twenty microliters of extract was assayed for β-lactamase activity in 980 μl of 100 μM cephaloridine in 0.1 M sodium phosphate buffer, pH 8.0 (42). Activity was monitored by measuring the decrease in absorbance at 260 nm in a Genesys 5 spectrophotometer (Milton Roy, Rochester, N.Y.) with the Simple Kinetics software programmed with a 30-s delay followed by readings taken every 15 s for 5 min. The specific activity was calculated based upon the molar extinction coefficient of cephaloridine (14,000 M−1 cm−1 under our assay conditions), the amount of total protein in the assay, and an elapsed time of 5 min.
β-Galactosidase assays.
Cell extracts were obtained as described for β-lactamase assays. Twenty microliters of extract was assayed in 1 ml of Z buffer with 0.8 mg of o-nitrophenyl-β-d-galactoside as a substrate. The assays were read at 420 nm (40).
Transmission electron microscopy of spores.
Transmission electron microscopy was performed by Kent McDonald at the Electron Microscope Laboratory at the University of California, Berkeley. Spores formed in suspension were collected by centrifugation after 24 h of incubation. Fruiting body spores from plates were collected after 1 week of incubation. Pelleted spores were transferred to 100-μm-deep specimen carriers (Ted Pella, Inc., Redding, Calif.) for high-pressure cryofixation in a Bal-Tec HPM 010 high-pressure freezer (Technotrade International, Manchester, N.H.) (39). Samples were freeze substituted in 0.2% osmium tetroxide plus 0.1% uranyl acetate in acetone for 2 days at −78°C and then warmed to −20°C over a 12-h period. The samples were then warmed to room temperature over a 6-h period. The samples were rinsed five times for 5 min each time in pure acetone, then infiltrated with Epon-Araldite resin without accelerator in the following steps: 1 h in 1 part resin–3 parts acetone, 2 h in 1 part resin–1 part acetone, 4 h in 3 parts resin–1 part acetone, and 1 h in resin without acetone, followed by overnight infiltration in pure resin. The samples were then transferred to resin plus accelerator for 4 h. Changes of solution were accomplished by pelleting the samples at 12,000 × g and then resuspending them in the next solution. After 4 h in resin plus accelerator, the pelleted samples were transferred to BEEM capsules and cured for 48 h at 60°C. Fifty- to 60-nm sections, cut on a microtome (Reichert Ultracut E), were transferred to Formvar-coated slot grids, poststained with uranyl acetate and lead citrate (48), and examined on a JEOL 100 CX transmission electron microscopy at 80 kV.
Photography.
Images of developing populations were taken at the College of Natural Resources Biological Imaging Facility at the University of California at Berkeley or in our laboratory with a CCD72 camera from MTI attached to a Zeiss microscope. Further formatting of images was accomplished with Adobe PhotoShop and ClarisWorks.
Germination of spores.
Glycerol sporulation of DZ2 in one liter of CYE nutrient broth was induced as described above. After 24 h of incubation, ≥95% of the cells had formed spores as judged by microscopy. The spores were harvested by centrifugation at 7,700 × g for 10 min at 4°C. The pelleted spores were resuspended in 15 ml of MMC and sonicated for 60 pulses at a duty cycle of 90% at a setting of 4 (approximately 15 W of power) to disrupt unsporulated cells. The sonicated suspension of spores was diluted to 100 ml in fresh CYE broth in a 500-ml Nephelometer flask with shaking at 275 rpm at 32°C. Five-milliliter aliquots were harvested by centrifugation at various times. The pellets were stored at −70°C until needed for assays.
To obtain fruiting body spores, DZ2 cells were plated for development in submerged culture in 150-mm-diameter petri dishes at 28°C. After 1 week of development, the supernatant was aspirated. Ten milliliters of fresh MMC was added to the plates, and fruiting bodies were released from the bottom of the plate by gentle scraping with a flame-sterilized rubber policeman. The cells were pelleted by centrifugation, resuspended in 10 ml of MMC, and then sonicated as described above to disrupt rods and to disperse spores from fruiting bodies. The sonicated spores were diluted into 50 ml of fresh CYE and incubated at 32°C with shaking at 275 rpm. Aliquots of germinating spores were taken at various times. The cells were pelleted by centrifugation; the pellets were stored at −70°C until they were processed for β-lactamase assays.
Protein extracts of germinating-spore samples were prepared by resuspending thawed pellets in 50 mM Tris-HCl (pH 8.0)–50 mM n-dodecyl-β-d-maltoside. Samples were sonicated (60 s at a 90% duty cycle at an output control yielding 30 W of power) and then centrifuged at 14,000 × g for 5 min at room temperature to pellet sonication-resistant spores. After centrifugation, the soluble supernatant was stored on ice until assay. The pellet, consisting of sonication-resistant spores and cell debris, was resuspended in 50 mM Tris-HCl, pH 8.0, and disrupted with 0.1-μm-diameter zirconium beads in the Mini-Beadbeater (BioSpec Products, Bartlesville, Okla.) according to the manufacturer’s instructions. Multiple 30-s treatments, followed by cooling on ice, were used to break all sonication-resistant spores (samples were screened for breakage by microscopy). n-Dodecyl-β-d-maltoside was added to a final concentration of 50 mM. The extract was vortexed to mix it well and then centrifuged at 14,000 × g at room temperature for 5 min to remove the beads and cellular debris. The supernatant was drawn off and stored on ice until assay. This constitutes the “spore” sample. The β-lactamase and protein contents were assayed as described above.
Rescue of development of Csg mutant by crude C-factor.
Crude C-factor was obtained by allowing DZ2 cells to develop in submerged culture in 100-mm-diameter petri plates for 24 h at 30°C. The 24-h culture supernatant was used as the developmental buffer for DK2657 cells (csgA), which were also inoculated for development in submerged culture in 100-mm-diameter petri dishes. After 1 week of development, the cells were scraped from the bottom of the plate and sonicated to disrupt unsporulated cells and fruiting bodies. Spores were counted in a hemacytometer.
RESULTS
β-Lactamase activity is increased during development of M. xanthus.
Because the induction of β-lactamase was found to be correlated with the induction of sporulation in rich medium (glycerol sporulation) (45), we were interested in examining autogenous expression of β-lactamase under conditions of fruiting body formation (Fig. 1). The specific activity of β-lactamase increased within 1 h of plating cells on CF. It should be noted that, although β-lactamase activity showed a significant increase at both 34 and 28°C, the pattern of expression of β-lactamase in developing cells was not the same at the two temperatures (Fig. 1A and B). A strong temperature effect on gene expression during development is a phenomenon previously reported for several other developmentally regulated proteins (7, 44). At 34°C, the activity of β-lactamase increased linearly during aggregation, reaching a peak during the early stages of mound formation; the peak activity was approximately 4.5-fold higher than that in vegetatively growing cells. Activity declined as aggregates formed tight mounds. At the time that refractile spores were observed and morphogenesis was completed, the activity of β-lactamase in nonsporulated cells was the same as that of vegetatively growing cells. At 28°C, β-lactamase activity increased by 2.5-fold over the activity in vegetatively growing cells and remained at nearly this level throughout aggregation and mound formation. It should be noted that the method chosen for making extracts of cells for assaying β-lactamase did not lyse spores. Therefore, the cells being assayed are peripheral rods and prespores (44).
Because there is a difference in the pattern of expression of β-lactamase at 28 and 34°C, subsequent experiments were performed at both temperatures. Both aggregation and sporulation initiate earlier at 34 than at 28°C. However, the outcomes of most of the experiments did not differ at the two temperatures. Both temperatures are within the range at which the mesophilic M. xanthus grows (21).
Cells in aggregates express β-lactamase at higher levels than peripheral rods.
Is the expression of β-lactamase induced equally in all developing cells? After scraping developing cells from a plate, it is possible to separate multicellular aggregates from nonaggregated cells (peripheral rods [44]). Samples were taken at two developmental stages at 28°C: (i) early aggregation, before discrete mounds were present and (ii) early mound formation, when discrete mounds were present but not all cells had completed aggregation. In both cases it was found that the activity of β-lactamase in nonaggregated cells was 2 to 2.5 mmol/g/min, similar to that in vegetatively growing cells. The activity of β-lactamase in aggregates was 5 to 6 mmol/g/min. This observation could account, at least in part, for the lower expression of β-lactamase observed at 28 than at 34°C at early times during development because the slower pace of development at 28°C results in there being fewer aggregated cells than at 34°C.
β-Lactamase expression is increased in cells shaken in buffer.
β-Lactamase is also induced when cells are starved while being shaken in suspension. Cells were grown in rich medium (CYE) and then washed and resuspended in starvation medium (MMC) as described in Materials and Methods. The resuspended cells were shaken at 34°C overnight. The specific activity of β-lactamase in cells at a density of 3 × 108/ml or 1 × 109/ml was 15 ± 2 mmol/g/min. The specific activity of β-lactamase in cells grown in CYE was 2.5 ± 0.5 mmol/g/min.
β-Lactamase is expressed early during spore germination.
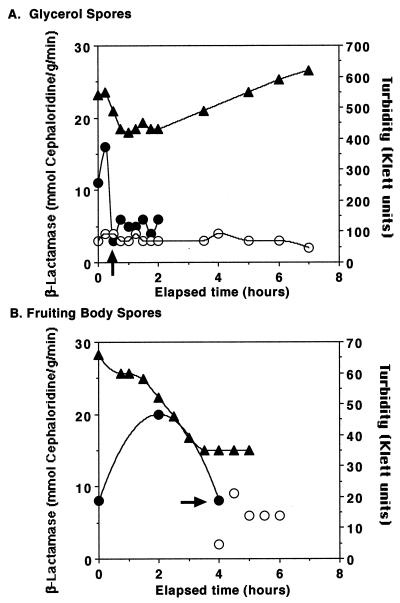
Glycerol spores and fruiting body spores were purified and resuspended in CYE medium to induce germination. At intervals, the spores were examined by microscopy to determine the extent of germination and aliquots were taken for assay of β-lactamase (Fig. 2). Glycerol spores completed germination more quickly than fruiting body spores, but both types of spores followed similar patterns of physical changes as seen by light microscopy, by measurement of turbidity, and by the expression of β-lactamase during germination. The patterns of changes in cell shape and refractility and culture turbidity were similar to those reported previously (47). There was a peak of expression of β-lactamase during the earliest stage of germination examined, when ≥98% of the spores were still refractile. β-Lactamase activity declined as the spores lost their refractility. The specific activity of β-lactamase in newly-germinated, sonication-sensitive cells from either glycerol spores or fruiting body spores was the same as the specific activity in vegetatively growing cells.
FIG. 2.
Expression of β-lactamase during germination of spores. Spores of DZ2 were obtained and allowed to germinate in CYE nutrient broth at 32°C. Aliquots of cells were harvested at intervals, examined by microscopy, extracted, and assayed for protein and β-lactamase, as described in Materials and Methods. Triangles, turbidity as measured with a 660-nm filter in a Klett-Summerson colorimeter; solid circles, β-lactamase-specific activity (mmol/g/min) from the sonication-resistant portion of the sample; open circles, β-lactamase-specific activity from the sonication-sensitive portion of the sample. The arrows point to the times at which spores were observed to lose refractility. The experiment was performed several times, and the results of a typical experiment are reported.
Exogenous induction of β-lactamase in developing cells accelerates aggregation and sporulation.
When cells were plated on CF plates containing 100 μg of ampicillin/ml, β-lactamase activity was increased 5- to 10-fold above normal developmental levels. At this level of induction, cells are not triggered to form glycerol spores (45). Maximum levels of β-lactamase activity were reached approximately 4 h after the cells were plated, which is similar to the time required for maximum induction in nutrient-rich media (45). Under these conditions, DK1622 developing in submerged culture in CF medium formed aggregates of cells detectable to the naked eye after shorter periods of incubation when d-cycloserine, phosphomycin, vancomycin, or ampicillin (data not shown), inducers of β-lactamase in M. xanthus (45), was added to the medium (Fig. 3). Similar results were obtained for strains DZF1 and DZ2 (data not shown). Development at 34°C was also accelerated by addition of inducers of β-lactamase, but the time differential was smaller between induced and uninduced cultures (data not shown). The onset and completion of sporulation were accelerated concomitantly with the acceleration of aggregation. Data are shown for development in d-cycloserine (Table 2). The number of spores produced in mature fruiting bodies was the same whether or not β-lactamase inducers were present.
FIG. 3.
Development of DK1622 on inducers of β-lactamase. DK1622 was allowed to develop in submerged culture in CF medium at 28°C for 1 day as described in Materials and Methods. Antibiotics were added at 5 μg/ml. All images are at the same magnification. Bar = 0.1 mm.
TABLE 2.
Sporulation of DK1622a
| Elapsed time (days) | CF
|
CF + d-cycloserine
|
||
|---|---|---|---|---|
| Stage of development | No. of spores | Stage of development | No. of spores | |
| 1 | No mounds | <102 | Tight mounds | 1 × 103 |
| 2 | Tight mounds | 2 × 106 | Fruiting bodies | 3 × 106 |
| 3 | Fruiting bodies | 3 × 106 | Fruiting bodies | 3 × 106 |
DK1622 cells were prepared for development in submerged culture in CF medium lacking pyruvate in 12-well tissue culture dishes as described in Materials and Methods, with 1 ml of medium/well. The plates were incubated at 28°C. The cells were scraped from the plates, pelleted by centrifugation, resuspended in 0.1 ml of water, sonicated to disrupt fruiting bodies for 15 cycles at 90% duty cycle at a setting of 1 with a microtip on a Branson model 450 Sonifier (approximately 10 W of power). Spores were counted in a hemacytometer after dilution in water, if necessary.
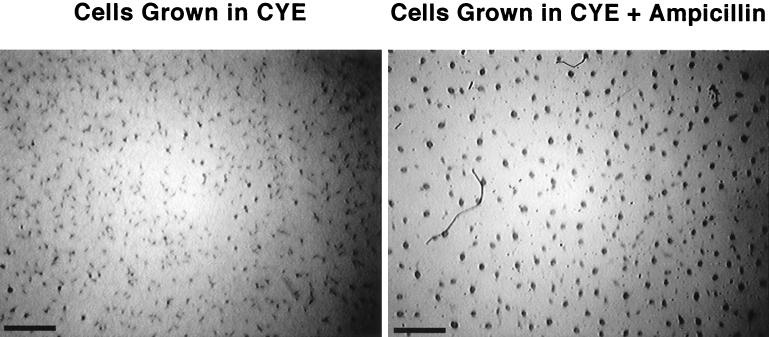
Cells pregrown in ampicillin aggregate and sporulate at an accelerated rate.
When strain DZ2 was grown in CYE containing 100 μg of ampicillin/ml in preparation for development in submerged culture at 28°C, the cells aggregated sooner than cells grown in CYE without ampicillin. For example, after 22 h of development at 28°C, cells pregrown in CYE had only begun to aggregate while cells pregrown in CYE plus ampicillin had already formed tight mounds (Fig. 4). Cells pregrown in CYE did not reach this stage until they had undergone approximately 20 additional hours of incubation. Cells pregrown in CYE plus ampicillin developing at 34°C also aggregated sooner than cells grown in CYE. However, the difference in timing was not as pronounced (data not shown).
FIG. 4.
Aggregation of cells grown in CYE plus ampicillin. DZF1 was prepared for development in MMC in submerged culture at 28°C in 35-mm-diameter petri dishes as described in Materials and Methods. The images were taken after 22 h of development. Bar = 1 mm.
Pregrowth of cells in CYE plus ampicillin also accelerated sporulation (Fig. 5). Cells developing at 34°C sporulated sooner than cells developing at 28°C. At 34°C, cells pregrown in CYE plus ampicillin had produced an order of magnitude more spores than cells grown in CYE after 1 day of development. Within 2 days of development at 34°C, the numbers of spores in cultures pregrown in CYE and CYE plus ampicillin were nearly identical. At 28°C, the slower pace of aggregation is reflected in the lower rate of accumulation of spores. After 2 days of development the culture started from cells pregrown in CYE containing 100 μg of ampicillin/ml had 100-fold more spores than the culture pregrown in CYE. After 3 days, the culture pregrown in CYE plus ampicillin had formed the maximum number of spores and was just 10-fold higher than the number of spores in the culture pregrown in CYE alone. Within 8 days of incubation at 28°C, the same number of spores had formed in both cultures. Thus, the addition of ampicillin to the pregrowth medium increased the rate at which sporulation occurred but did not affect the total number of spores formed at the completion of development.
FIG. 5.
Sporulation of cells pregrown in ampicillin. DZ2 was prepared for development in MMC in submerged culture in 35-mm-diameter petri dishes as described in Materials and Methods. The number of spores reported reflects the total number of spores for each sample. (A) Sporulation at 34°C. Open squares, cells grown in CYE lacking ampicillin; solid triangles, cells grown in CYE containing 100 μg of ampicillin/ml. (B) Sporulation at 28°C. Open circles, cells grown in CYE lacking ampicillin; solid circles, cells grown in CYE containing 100 μg of ampicillin/ml.
The β-lactamase activity of cells pregrown in CYE and in CYE plus ampicillin during development in submerged culture at 28°C was measured (Table 3). Under these culture conditions, the β-lactamase activity of cells pregrown in CYE increased from 1.6 to 7 mmol/g/min over 2 to 3 days as the cells aggregated and formed fruiting bodies. At the initiation of development, the activity of β-lactamase in cells pregrown in 100 μg of ampicillin/ml was 10-fold higher than that in cells pregrown in CYE. However, the activity of β-lactamase in the induced culture declined during the first 24 h until it reached a level similar to that in cells pregrown in CYE lacking ampicillin.
TABLE 3.
Expression of β-lactamase in developing cells pregrown in CYE plus ampicillina
| Elapsed time (days) | CYE-grown cells
|
CYE + ampicillin-grown cells
|
||
|---|---|---|---|---|
| Aggregate morphology | β-Lactamase activity | Aggregate morphology | β-Lactamase activity | |
| 0 | 1.6 | 15 | ||
| 1 | Mounds | 1.3 | Tight mounds | 7.0 |
| 2 | Tight mounds | 4.0 | Late tight mounds | 7.4 |
| 3 | Fruiting bodies | 7.0 | Fruiting bodies | 8.0 |
DZF1 cells were grown in CYE or CYE containing 100 μg of ampicillin/ml in 35-mm-diameter tissue culture plates for development in submerged culture. After 24 h of growth, the culture supernatant was aspirated. After being washed with 1.0 ml of MMC, the cells were overlaid with 2.0 ml of MMC and incubated at 28°C. At the intervals given in the table, the cells were scraped from the plates with a rubber policeman and harvested by centrifugation. Extracts were prepared, and β-lactamase was assayed as described in Materials and Methods. Activity is expressed in mmol/g/min.
Expression of some developmentally regulated Tn5lac insertions was changed by exogenous induction of β-lactamase.
Does accelerating development by the exogenous induction of β-lactamase bypass steps in the developmental pathway? We examined this question by investigating the expression of Tn5lac insertions creating transcriptional fusions between developmentally regulated promoters and the β-galactosidase reporter gene (30) (Table 1). Cells were grown and spotted for development on CF agar and CF agar containing an inducer of β-lactamase, d-cycloserine, as described in Materials and Methods. Both media contained X-Gal as an indicator for β-galactosidase activity. Although the precipitation of X-Gal is not a sensitive method for detecting expression, it is sufficiently sensitive to detect significant differences in the onset or level of expression.
Two developmentally regulated Tn5lac insertions gave blue precipitate hours earlier on CF X-Gal plates containing d-cycloserine than on CF plates: Ω4455, which is partially bsg dependent, and Ω4514, which is partially csg dependent. No difference in timing of expression was seen for Ω4491, Ω4408, Ω4521, Ω5206, Ω4469, Ω4273, Ω4414, Ω4403, Ω5204, or Ω4293 (Table 4). All Tn5lac insertions were expressed under conditions of exogenous induction of β-lactamase. A more careful study of the expression of Ω4521, an insertion in a well-characterized gene which is expressed early in development (15, 25, 59), showed that it turned on at the same time whether or not β-lactamase was exogenously induced. However, the peak of expression was reached 12 h earlier on d-cycloserine and was 25% of the peak activity seen under normal conditions of development (200 U) (data not shown).
TABLE 4.
Expression of Tn5lac insertions during development on d-cycloserinea
| Strain | Tn5lac insertionb | Timing (h)c | Dependenced | CF + d-cycloserinee |
|---|---|---|---|---|
| DK5285 | Ω4491 | 0 | asg | Same |
| DK4300 | Ω4408 | 1 | Partial bsg | Same |
| DK4521 | Ω4521 | 2 | asg | Same |
| DK5206 | Ω4455 | 3 | Partial bsg | Early |
| DK4469 | Ω4469 | 5 | Partial bsg | Same |
| DK4290 | Ω4273 (tps) | 5 | asg | Same |
| DK4514 | Ω4514 | 9 | Partial csg | Early |
| DK5274 | Ω4414 | 10 | bsg | Same |
| DK4368 | Ω4403 | 15 | csg | Same |
| DK5204 | Ω4435 | 25 | csg | Same |
| DK4293 | Ω4401 | 30 | csg | Same |
All strains are in a DK1622 background. The strains were incubated at 28°C for development as described in Materials and Methods. Plates were monitored for appearance of a blue precipitate. d-Cycloserine was added at 15 μg/ml.
Ω4408 is a mutation that causes delayed aggregation. Ω4414 is a mutation that causes poor aggregation. The rest of the Tn5lac insertions have no developmental defect (30).
The time at which Tn5lac insertions are expressed is taken from Kroos et al. (30) and was determined by spectrophotometric assays of β-galactosidase in extracts of developing cells.
The expression of Tn5lac insertions in Asg, Bsg, and Csg mutant backgrounds was monitored to determine the effects of these mutations on the expression of the Tn5lac insertions (31, 34).
Time at which blue precipitate was detected in comparison to CF plates (no d-cycloserine). As described in Materials and Methods, the plates were examined daily for the appearance of blue precipitate. This method is not as sensitive as that used by Kroos, et al. (30) to determine the time at which the genes are first expressed (see note b), but the sensitivity is sufficient to detect significant differences in the onset or level of expression.
Addition of ampicillin allows cells to develop at higher concentrations of nutrients.
The addition of nutrients to developmental medium can delay or abolish aggregation and sporulation (53). In submerged culture, development is usually studied in the total absence of added nutrients. After 4 days of incubation in MMC medium, cells had formed fruiting bodies containing spores. In MMC containing 0.2% Casitone, there were few aggregates and no spores at this time. However, the addition of inducers of β-lactamase to the MMC containing 0.2% Casitone promoted aggregation into tight mounds, although the course of development was still delayed by 2 days compared to development in MMC (data not shown). After 1 week of incubation, sonication-resistant spores were harvested from the plates and counted. Cells developing in 0.2% Casitone formed 1,000-fold-fewer spores than cells developing in MMC. Addition of an inducer of β-lactamase increased sporulation up to 10-fold. The number of spores formed was proportional to the amount of inducer added within a twofold range for d-cycloserine and phosphomycin (Table 5).
TABLE 5.
Sporulation in the presence of Casitonea
| Medium | Spores/plate |
|---|---|
| MMC (no Casitone) | 4 × 107 |
| MMC, 0.2% Casitone | 4 × 104 |
| MMC, 0.2% Casitone + d-cycloserine (5 μg/ml) | 2 × 105 |
| MMC, 0.2% Casitone + d-cycloserine (10 μg/ml) | 4 × 105 |
| MMC, 0.2% Casitone + phosphomycin (5 μg/ml) | 1 × 105 |
| MMC, 0.2% Casitone + phosphomycin (15 μg/ml) | 2 × 105 |
DK1622 cells were grown and plated for development in submerged culture in 100-mm-diameter petri plates as described in Materials and Methods. The plates were incubated at 28°C for 1 week before the cultures were scraped from the plate and sonicated to release spores from fruiting bodies. The spores were counted in a hemacytometer.
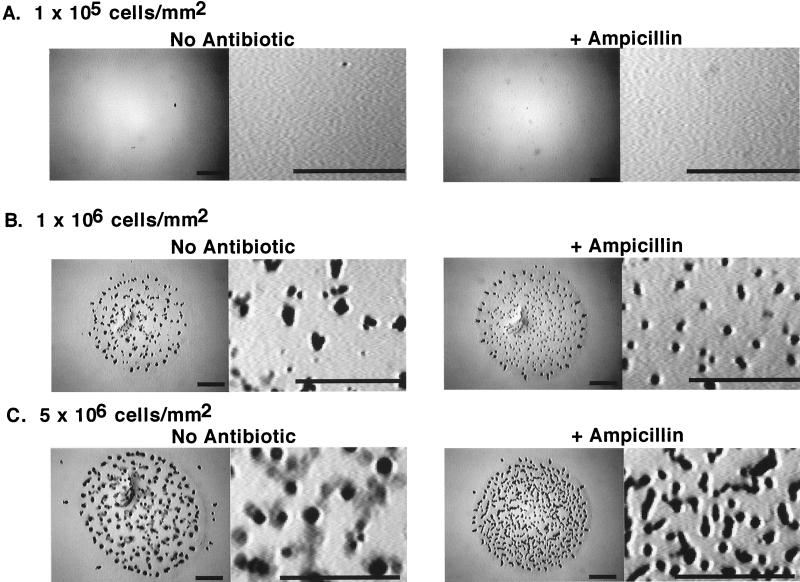
Addition of ampicillin influences development at high cell densities but not at low cell densities.
We examined the development of M. xanthus in the presence and absence of ampicillin at 1 × 105, 1 × 106, and 5 × 106 cells/mm2 (Fig. 6A, B, and C, respectively). The cells aggregated into fruiting bodies with well-defined boundaries with few spores outside the aggregates when they were plated at an average density of 1 × 106/mm2 (this is the standard cell density at which we spot cells for development). In contrast, at 5 × 106 cells/mm2, fruiting bodies on MMC agar had hazy boundaries which were made up of cells that had sporulated without entering the aggregate. When β-lactamase was exogenously induced, the aggregates resembled those formed at a moderate cell density (Fig. 6C). At the low cell density, 1 × 105/mm2, the cells had not aggregated within 6 days and no spores were observed by microscopy. The addition of ampicillin to the medium did not rescue development. Similar results were obtained on CF agar (data not shown).
FIG. 6.
Cell density and development. DZ2 was grown and prepared for development as described in Materials and Methods. Cells were resuspended in MMC buffer at 4 × 109 or at 4 × 108/ml. Five-microliter aliquots were spotted onto MMC agar plates or MMC agar plates containing ampicillin at 100 μg/ml. The cell density/mm2 was calculated based upon an average surface area of 19.6 mm2 for each spot of cells. The plates were incubated at 28°C for 6 days prior to photography. Bars = 1 mm.
Exogenous induction of β-lactamase does not alter the ultrastructure of spores.
Spores formed in suspension (glycerol spores [9]) cannot be distinguished by light microscopy from spores formed in fruiting bodies. However, transmission electron microscopy reveals that the ultrastructure of glycerol spore walls is different from the ultrastructure of walls of spores formed in fruiting bodies (18, 53). We examined spores formed in suspension in CYE (a nutrient-rich broth) and in MMC buffer by transmission electron microscopy to determine if the level of nutrients present during sporulation was responsible for the differences between the ultrastructures of fruiting body spores and glycerol spores (Fig. 7A and B). The ultrastructure of the walls of spores induced in CYE or MMC by the addition of glycerol or ampicillin showed no significant differences, even at higher magnification (Fig. 7C and D). The walls of spores formed in response to glycerol or ampicillin were identical in the two media (data not shown). Thus, the structure of the walls of mature spores formed in suspension in response to induction of β-lactamase is due to the process of sporulation in suspension and is not influenced by nutrition. However, spores formed under starvation, whether in suspension or in fruiting bodies, lacked the large number of vesicles found in spores induced in CYE.
FIG. 7.
Transmission electron microscopy of DZ2 spores. Spores were harvested and prepared for transmission electron microscopy as described in Materials and Methods. (A) Spore formed in suspension in CYE by the addition of 0.5 M glycerol; incubation was for 24 h. (B) Spore formed in suspension in MMC by the addition of 1 mM ampicillin (372 μg/ml); incubation was for 24 h. (C) Enlargement of wall region of the cell in panel A. (D) Enlargement of wall region of the cell in panel B. (E) Fruiting body spore formed in submerged culture in MMC lacking ampicillin. (F) Fruiting body spore formed on MMC containing 100 μg of ampicillin/ml. (G) Enlargement of wall of the cell in panel E. (H) Enlargement of wall of the cell in panel F. Bars = 1 μm.
Spores formed in fruiting bodies have a morphology different from that of glycerol spores and are characterized by thick cell walls (18, 53, 55). Mature spores from fruiting bodies developing in the presence or absence of 100 μg of ampicillin/ml were indistinguishable, even at high magnification (Fig. 7E to H). Both types of spores have the characteristic thickened cell wall of fruiting body spores. Thus, the accelerated formation of spores in response to exogenous induction of β-lactamase during development does not alter the morphology of the mature spores.
Inducers of β-lactamase rescue the development of a CsgA mutant.
CsgA mutants are unable to aggregate or sporulate on developmental medium but can aggregate and sporulate when mixed with wild-type cells (extracellular complementation [16, 35]). Extracellular complementation can also be achieved by the addition of peptidoglycan components (53) or by the addition of purified CsgA protein to the CsgA mutant (26, 27, 36).
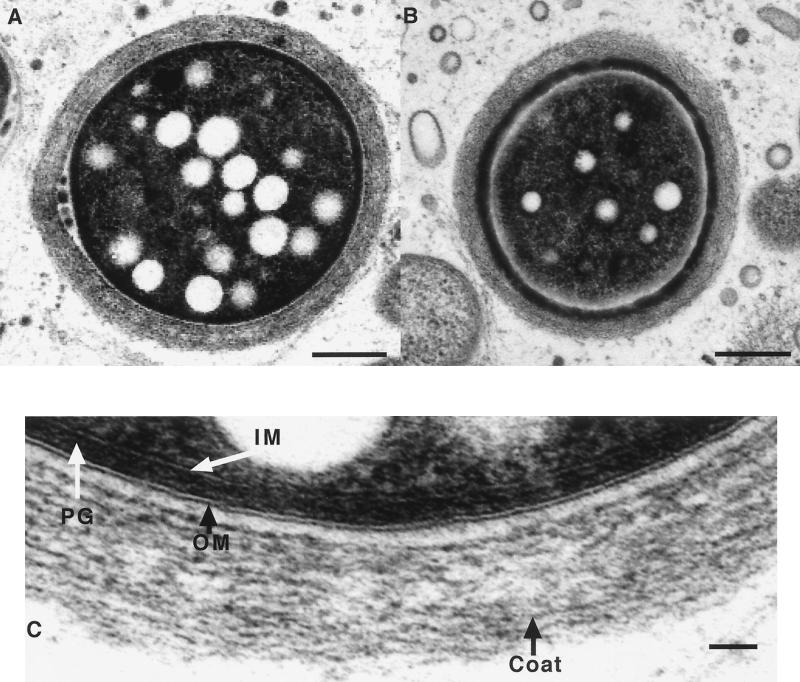
Figure 8 shows that ampicillin, d-cycloserine, and phosphomycin, three inducers of β-lactamase, could also rescue fruiting body formation of the CsgA mutant. The CsgA mutant failed to fruit in the absence of β-lactamase inducers, even after 1 week of incubation. Sporulation of CsgA was also rescued by the addition of inducers of β-lactamase (Table 6). Transmission electron microscopy showed that the ultrastructure of CsgA fruiting body spores formed on CF agar in the presence of an inducer of β-lactamase resembled wild-type fruiting body spores (compare Fig. 9 to Fig. 7). The outer and inner membranes, peptidoglycan layer, and spore coat are clearly visible (Fig. 9C).
FIG. 8.
Development of DK2657 (csgA) on inducers of β-lactamase. DK2657 was grown and plated for development in 5-μl aliquots at a cell density of 4 × 109/ml (106 cells/mm2) on CF agar. The plates were incubated at 28°C, and the images were taken after 48 h. d-Cycloserine was added to a final concentration of 15 μg/ml, phosphomycin was added to a final concentration of 15 μg/ml, and ampicillin was added to a final concentration of 100 μg/ml. All images are at the same magnification; bar = 1 mm.
TABLE 6.
Rescue of sporulation of CsgA
| Addition | Strain | Medium | Spores (%) |
|---|---|---|---|
| Crude C-factor from developing cellsa | DZ2 | MMC | 100 |
| DZ2 | Crude C-factor | 100 | |
| DK2657 (csgA) | MMC | 0.1 | |
| DK2657 (csgA) | Crude C-factor | 100 | |
| Ampicillin to CF saltsb | DK1622 | CF salts + ampicillin | 100 |
| DK2657 (csgA) | CF salts | ≤1 | |
| DK2657 (csgA) | CF salts + ampicillin | 33 | |
| Glycerol to CFc | DK1622 | CF | 100 |
| DK1622 | CF + glycerol | 100 | |
| DK2657 (csgA) | CF | ≤0.1 | |
| DK2657 (csgA) | CF + glycerol | 160 |
Rescue of sporulation of DK2657 (csgA) by crude C-factor. Crude C-factor was obtained from DZ2 developing in submerged culture as described in Materials and Methods. Freshly grown cells were then overlaid with the crude C-factor or MMC and allowed to develop for 1 week in submerged culture as described in Materials and Methods. Sonication-resistant spores were counted. The number of spores counted from the wild type developing in MMC was set to 100%.
Rescue of sporulation of DK2657 (csgA) by ampicillin. Strains were grown and prepared for development as described in Materials and Methods. Five-microliter aliquots of cells were spotted onto CF salts agar plates or CF salts agar plates containing ampicillin at 100 μg/ml. The plates were incubated at 28°C for 1 week. The spots of developing cells were harvested in 0.5 ml of water with a length of 7-mm-diameter glass tubing with a rubber bulb attached. The fruiting bodies were disrupted, and spores were released from the agar surface by sonication for 15 s at 30 W of power. The spores were counted in a hemacytometer and are reported as total spores per sample. The number of spores counted from the wild type developing on CF salts containing ampicillin was set to 100%.
Rescue of sporulation of DK2657 (csgA) by glycerol. Strains were grown and prepared for development as described in Materials and Methods. Five-microliter aliquots of cells were spotted onto CF agar plates or CF agar plates containing ampicillin at 100 μg/ml. The plates were incubated at 28°C for 1 week. The spots of developing cells were harvested in 0.5 ml of water with a length of 7-mm-diameter glass tubing with a rubber bulb attached. The fruiting bodies were disrupted, and the spores were released from the agar surface by sonication for 15 s at 30 W of power. The spores were counted in a hemacytometer and are reported as total spores per sample. The number of wild-type spores counted from CF plates was set to 100%.
FIG. 9.
Transmission electron microscopy of DK2657 (csgA) spores. One-week-old fruiting body spores from CF plates containing an inducer of β-lactamase were harvested and prepared for transmission electron microscopy as described in Materials and Methods. (A and B) Low-magnification images of two different spores; sections are not necessarily medial, and the apparent difference in size of the spores is related to the position of the section in the spore. Bar = 1 μm. (C) Enlargement of the wall of the cell in panel A. IM, inner membrane; PG, peptidoglycan; OM, outer membrane. Bar = 0.1 μm.
Inducers of β-lactamase rescue sporulation but not aggregation of chemotaxis and motility mutants.
We have proposed that the induction of β-lactamase plays a role in sporulation and aggregation. The data presented to this point support the hypothesis that the signal transduction pathway which leads to induction of β-lactamase is activated during aggregation and that the exogenous induction of β-lactamase accelerates aggregation. Is the concomitant influence on sporulation an incidental response due solely to the upstream effects on aggregation, or is there an effect on sporulation independent of aggregation? We studied this by examining the development of two classes of mutants incapable of aggregation: swarming mutants and motility mutants.
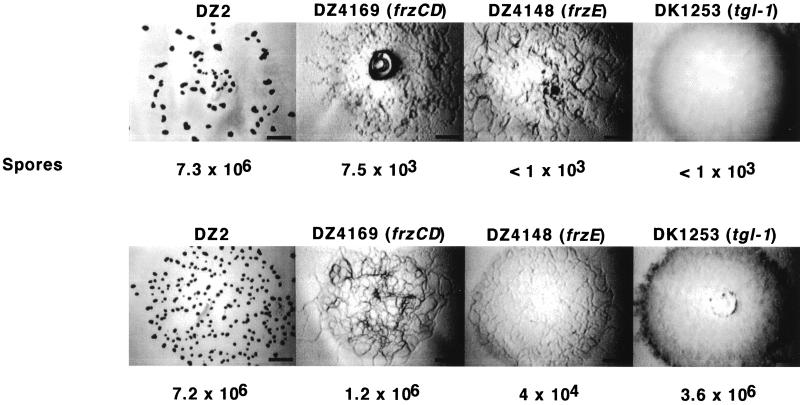
frzCD and frzE encode proteins which are similar to proteins essential for chemotaxis in bacteria. These genes are required for vegetative swarming and aggregation in M. xanthus (2). These mutants sporulate poorly in a fully motile background, such as DZ2 (23). When the mutants were plated for development on CF agar containing 100 μg of ampicillin/ml, aggregation was not restored but sporulation was improved by as much as 3,000-fold (Fig. 10). A mutation in tgl-1 renders the cells unable to engage in S motility, which involves the movement of groups of cells and is essential for aggregation. Like the csgA allele, tgl mutants can be extracellularly complemented for motility, aggregation, and sporulation by tgl-1+ cells (17). Although the Tgl mutant was rescued for sporulation by the exogenous induction of β-lactamase by ampicillin, motility and, consequently, aggregation were not rescued. Thus, induction of β-lactamase can independently influence aggregation and sporulation.
FIG. 10.
Rescue of developmental sporulation of mutants unable to aggregate. DZ2, DZ4169 (frzCD), DZ4148 (frzE), and DK1253 (tgl-1) cells were grown and plated for development in 5-μl aliquots at a density of 4 × 109/ml (106/mm2) on CF agar without (top row) or with (bottom row) ampicillin. The plates were incubated at 28°C, and the images were taken after 1 week. Ampicillin was added to a final concentration of 100 μg/ml. The spores were harvested and counted as described in Materials and Methods. Bar = 1 mm. Numbers of spores are shown below each image.
DISCUSSION
We were led to investigate the expression of β-lactamase of M. xanthus during development after observing that glycerol sporulation is associated with agents that induce β-lactamase (45). The β-lactamase activity of M. xanthus was first described by von Krüger and Parish (56), who showed that the monomeric form of the β-lactamase activity migrated as a single peak by gel chromatography. They also showed that the enzyme is chromosomally encoded. Despite the evidence from gel chromatography, it is still formally possible that there is more than one gene encoding β-lactamase activity. Whether the activity reported in this paper is due to a single enzyme or more than one enzyme, it is nevertheless clear that the chromosomal β-lactamase activity of M. xanthus is autogenously induced during development and during germination of spores. Furthermore, exogenous addition of inducers of β-lactamase to developing cells causes aggregation and sporulation to occur more rapidly without bypassing the pathways marked by Tn5lac insertions. Exogenous addition of inducers of β-lactamase also allows cells to develop at slightly increased levels of nutrients which would otherwise inhibit both aggregation and sporulation. A CsgA mutant which is unable to aggregate or sporulate is rescued by exogenous addition of inducers of β-lactamase. Mutants which are unable to move or participate in aggregation can be rescued for sporulation in the absence of aggregation. These data suggest roles for the β-lactamase signal transduction pathway in the developmental process of M. xanthus.
What is happening during development that could trigger the β-lactamase signal transduction pathway? We know that starvation for nutrients initiates the process of development in M. xanthus (52). Studies of starvation and stringent response in Escherichia coli have shown that peptidoglycan biosynthesis and cross-linking are altered upon starvation and that the expression of some peptidoglycan-altering enzymes is likely to be under the control of the starvation-specific sigma factor RpoS (10, 38, 49). It is also known that the β-lactamase signal transduction pathway in E. coli is integrally linked to peptidoglycan recycling (46). It is possible that upon starvation M. xanthus begins to alter its peptidoglycan, which leads to accumulation of the autogenous inducer(s) of β-lactamase. We have shown that starvation alone is indeed sufficient to induce β-lactamase in M. xanthus. We have not identified the specific inducer(s) of β-lactamase in M. xanthus, nor do we know if the inducer of the β-lactamase signal transduction pathway is exchanged between cells or if it is solely intracellular.
It is not clear why peptidoglycan components and β-lactamase induction rescue development of a CsgA mutant. csgA encodes a protein with strong similarity to short-chain alcohol dehydrogenases, but its precise role in development is not known. It has been proposed that the enzymatic activity of CsgA is required for the processing of the C-signal (37). Indeed, short-chain alcohol dehydrogenases are known to convert signaling molecules between active and inactive forms in both prokaryotes and eukaryotes (32). It was initially proposed that the rescue of CsgA by peptidoglycan might bypass the pathway that is dependent upon the activity of CsgA (53). This could also explain the rescue by inducers of β-lactamase. Data presented in this paper, that Tn5lac insertions dependent upon the csg pathway are expressed during exogenous induction of β-lactamase, show that if there is a bypass of the csg pathway it is partial; that is, not all genes dependent upon the expression of csgA are bypassed.
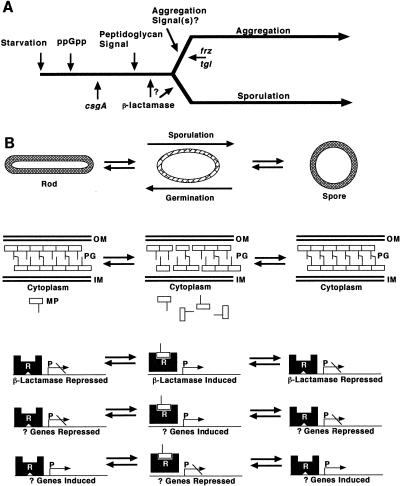
The exogenous induction of β-lactamase does not uncouple aggregation and sporulation in the wild type. Under normal conditions of development, sporulation is tightly regulated such that mature spores are observed only in aggregates (4, 43). However, it has long been known that sporulation and aggregation could be uncoupled by mutation, leading to a model of development in which the two processes of aggregation and sporulation are not mutually dependent (41). The rescue of sporulation but not of aggregation of Frz (unable to participate in mound formation) and Tgl (defective in S motility) mutants by β-lactamase induction is consistent with this model. The rescue of the aggregation of the CsgA mutant suggests that the induction of β-lactamase could be upstream of the induction of aggregation; however, we know that β-lactamase activity is higher in aggregated cells than in nonaggregated cells. One hypothesis that explains both of these results is that the same molecule which autogenously induces β-lactamase might be the aggregation signal itself or induce other genes which ultimately lead to the production and release of, and response to, aggregation signal(s). If β-lactamase of M. xanthus is regulated similarly to class C β-lactamases, then the inducer is likely to be a peptidoglycan component (19). We do know that addition of peptidoglycan at >2 mg/ml can inhibit aggregation of wild-type M. xanthus (unpublished results). This is the response that cells would be expected to show to an excess of an extracellular signal for aggregation because it would be expected to swamp the gradient of the signal necessary for aggregation. In Fig. 11A we present a model for how csgA, frz, tgl, and β-lactamase might fit into the aggregation and sporulation pathways. A null mutant in β-lactamase would elucidate whether β-lactamase is upstream or downstream of aggregation signals.
FIG. 11.
(A) Model for the induction of β-lactamase during development of M. xanthus. (B) Model for the expression of β-lactamase as a function of morphogenesis. We propose that the induction of β-lactamase is an integral step in the development of M. xanthus and that this induction is likely to play a role in the restructuring of peptidoglycan which occurs during the differentiation of spores. See the text for more details. OM, outer membrane; PG, peptidoglycan; IM, inner membrane; MP, muropeptides; R, repressor protein; P, promoter; ppGpp, guanosine 3′-diphosphate 5′-diphosphate.
In the wild type, the acceleration of sporulation in response to exogenously added inducers of β-lactamase could be due to their effects upon aggregation; the same could be said of the rescue of sporulation in the CsgA mutant. However, the rescue of sporulation, but not aggregation, of Frz and Tgl mutants by exogenously added inducers of β-lactamase suggests that the β-lactamase signal transduction pathway is involved in fruiting body sporulation as well as glycerol sporulation, as previously shown (45). Sporulation in M. xanthus involves conversion of the whole rod-shaped vegetative form of the cell to a spherical spore. Because the shape of a cell is constrained by peptidoglycan, peptidoglycan must be modified by de novo synthesis and/or breaking and reforming of cross-links between muropeptide polymers to permit changes in shape. Indeed, there is increased turnover of peptidoglycan during sporulation but there is no net change in the amount of peptidoglycan, and cross-linking between diaminopimelic acid and alanine increases by 11% (for a review, see reference 58). In addition, Kimura et al. (28) have shown that the activities of dd-carboxypeptidase and d-alanyl-d-alanine ligase increase during development and are correlated with sporulation. Thus, the peptidoglycan of sporulating cells is being significantly altered during sporulation.
The observation that the expression of β-lactamase is induced during sporulation led us to study the expression of β-lactamase in germinating spores. The timing of the expression of β-lactamase during germination correlates with the time reported for loss of heat and sodium dodecyl sulfate resistance, i.e., before loss of refractility (11).
β-Lactamase is autogenously induced in M. xanthus under conditions in which the cell undergoes a change in shape: during sporulation and germination. In Fig. 11B, we present a speculative model for future testing of the regulation of the chromosomal β-lactamase activity and other genes involved in development of M. xanthus. In this model, the chromosomal β-lactamase of M. xanthus is induced concomitantly with the changes in shape that accompany sporulation and germination. During starvation-induced development, it might be changes in peptidoglycan metabolism caused by starvation which induce the β-lactamase signal transduction pathway. We believe that this model is consistent with the model for induction of β-lactamase in gram-negative bacteria by components of peptidoglycan (20). The work of Kimura et al. (28) demonstrating that addition of d-alanyl-d-alanine to slowly sporulating subcultured cells accelerates sporulation is also consistent with this model. A similar model has been suggested to regulate processing and secretion of a β-lactamase in Streptomyces griseus (6). We have no evidence that the activity of β-lactamase itself is important in the reshaping of the peptidoglycan that happens during sporulation and germination, and current understanding of β-lactamases suggests that it is unlikely that the chromosomal β-lactamase of M. xanthus acts directly on peptidoglycan or its components (14). β-Lactamases are believed to have evolved from the small penicillin-binding proteins which have dd-carboxypeptidase activity (24). Although, no dd-carboxypeptidases specific for d-alanyl-d-alanine have been found to have β-lactamase activity (5), an alkaline d-endopeptidase specific for aromatic d-amino acids has been found to have β-lactamase activity (1). It is intriguing to hypothesize that the repressor(s) and inducer(s) which regulate the expression of β-lactamase also regulate penicillin-binding proteins which do shape and reshape the peptidoglycan.
ACKNOWLEDGMENTS
We thank Dave Astling, Kyungyun Cho, John Kirby, Helen Lew, Anke Treuner-Lange, Hera Vlamakis, and Mandy Ward for helpful discussions of the work presented in this paper. We also wish to thank Steve Ruzin and Denise Schichnes for instruction at the CNB Center for Biological Imaging and Kent McDonald of the Electron Microscope facility for doing the transmission electron microscopy.
This work was supported by grant GM20509 from the National Institutes of Health.
REFERENCES
- 1.Asano Y, Ito H, Dairi T, Kato Y. An alkaline D-stereospecific endopeptidase with β-lactamase activity from Bacillus cereus. J Biol Chem. 1996;271:30256–30262. doi: 10.1074/jbc.271.47.30256. [DOI] [PubMed] [Google Scholar]
- 2.Blackhart B D, Zusman D R. Cloning and complementation analysis of the “Frizzy” genes of Myxococcus xanthus. Mol Gen Genet. 1985;198:243–254. doi: 10.1007/BF00383002. [DOI] [PubMed] [Google Scholar]
- 3.Campos J M, Geisselsoder J, Zusman D R. Isolation of bacteriophage MX4, a generalized transducing phage for Myxococcus xanthus. J Mol Biol. 1978;119:167–178. doi: 10.1016/0022-2836(78)90431-x. [DOI] [PubMed] [Google Scholar]
- 4.Cho, K., and D. R. Zusman. Sporulation timing in Myxococcus xanthus is controlled by the espAB locus. Mol. Microbiol., in press. [DOI] [PubMed]
- 5.Damblon C, Zhao G H, Jamin M, Ledent P, Dubus A, Vanhove M, Raquet X, Christiaens L, Frere J M. Breakdown of stereospecificity of DD-peptidases and β-lactamases with thiolester substrates. Biochem J. 1995;309:431–436. doi: 10.1042/bj3090431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deák E, Szabó I, Kálmáczhelyi A, Gál Z, Barabás G, Penyige A. Membrane-bound and extracellular β-lactamase production with developmental regulation in Streptomyces griseus NRRL B-2682. Microbiology. 1998;144:2169–2177. doi: 10.1099/00221287-144-8-2169. [DOI] [PubMed] [Google Scholar]
- 7.Downard J S, Zusman D R. Differential expression of the protein S genes during Myxococcus xanthus development. J Bacteriol. 1985;161:1146–1155. doi: 10.1128/jb.161.3.1146-1155.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dworkin M. Nutritional requirements for vegetative growth of Myxococcus xanthus. J Bacteriol. 1962;84:250–257. doi: 10.1128/jb.84.2.250-257.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin M, Gibson S. A system for studying microbial morphogenesis: rapid formation of microcysts in Myxococcus xanthus. Science. 1964;146:243–244. doi: 10.1126/science.146.3641.243. [DOI] [PubMed] [Google Scholar]
- 10.Ehlert K, Holtje J V. Role of precursor translocation in coordination of murein and phospholipid synthesis in Escherichia coli. J Bacteriol. 1996;178:6766–6771. doi: 10.1128/jb.178.23.6766-6771.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elías M, Murillo F. Induction of germination in Myxococcus xanthus fruiting body spores. J Gen Microbiol. 1991;137:381–388. [Google Scholar]
- 12.Fisseha M, Gloudemans M, Gill R E, Kroos L. Characterization of the regulatory region of a cell interaction-dependent gene in Myxococcus xanthus. J Bacteriol. 1996;178:2539–2550. doi: 10.1128/jb.178.9.2539-2550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garza A G, Pollack J S, Harris B Z, Lee A, Keseler I M, Licking E F, Singer M. SdeK is required for early fruiting body development in Myxococcus xanthus. J Bacteriol. 1998;180:4628–4637. doi: 10.1128/jb.180.17.4628-4637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghuysen J-M. Serine β-lactamases and penicillin binding proteins. Annu Rev Microbiol. 1991;45:37–67. doi: 10.1146/annurev.mi.45.100191.000345. [DOI] [PubMed] [Google Scholar]
- 15.Gulati P, Xu D, Kaplan H B. Identification of the minimum regulatory region of Myxococcus xanthus A-signal-dependent developmental gene. J Bacteriol. 1995;177:4645–4651. doi: 10.1128/jb.177.16.4645-4651.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hagen D C, Bretscher A P, Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978;64:284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- 17.Hodgkin J, Kaiser D. Genetics of gliding motility in Myxococcus xanthus (Myxobacterales): two gene systems control movement. Mol Gen Genet. 1979;171:177–191. [Google Scholar]
- 18.Inouye M, Inouye S, Zusman D R. Biosynthesis and self-assembly of protein S, a development-specific protein of Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:209–213. doi: 10.1073/pnas.76.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs C, Joris B, Jamin M, Klarsov K, Van Beeumen J, Mengin-Lecreulx D, van Heijenoort J, Park J T, Normark S, Frere J M. AmpD, essential for both β-lactamase regulation and cell wall recycling, is a novel cytosolic N-acetylmuramyl-L-alanine amidase. Mol Microbiol. 1995;15:553–559. doi: 10.1111/j.1365-2958.1995.tb02268.x. [DOI] [PubMed] [Google Scholar]
- 20.Jacobs C, Frere J M, Normark S. Cytosolic intermediates for cell wall biosynthesis and degradation control inducible β-lactam resistance in Gram-negative bacteria. Cell. 1997;88:823–832. doi: 10.1016/s0092-8674(00)81928-5. [DOI] [PubMed] [Google Scholar]
- 21.Janssen G R, Wireman J W, Dworkin M. Effect of temperature on the growth of Myxococcus xanthus. J Bacteriol. 1977;130:561–562. doi: 10.1128/jb.130.1.561-562.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci USA. 1979;76:5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashefi K, Hartzell P L. Genetic suppression and phenotypic masking of a Myxococcus xanthus frzF− defect. Mol Microbiol. 1995;15:483–494. doi: 10.1111/j.1365-2958.1995.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 24.Kelley J A, Kujin A P, Charlier P, Fongé E. X-ray studies of enzymes that interact with penicillins. Cell Mol Life Sci. 1998;54:353–358. doi: 10.1007/s000180050163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keseler I M, Kaiser D. An early A-signal-dependent gene in Myxococcus xanthus has a sigma 54-like promoter. J Bacteriol. 1995;177:4638–4644. doi: 10.1128/jb.177.16.4638-4644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim S K, Kaiser D. C-factor: a cell-cell signaling protein required for fruiting body morphogenesis of M. xanthus. Cell. 1990;61:19–26. doi: 10.1016/0092-8674(90)90211-v. [DOI] [PubMed] [Google Scholar]
- 27.Kim S K, Kaiser D. Purification and properties of Myxococcus xanthus C-factor, an intercellular signaling protein. Proc Natl Acad Sci USA. 1990;87:3635–3639. doi: 10.1073/pnas.87.10.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimura Y, Ishida T, Ujibe A, Sato M. Penicillin and D-Alanyl-D-Alanine accelerate spore formation of Myxococcus xanthus subcultured cells. Biosci Biotechnol Biochem. 1998;62:2115–2119. doi: 10.1271/bbb.62.2115. [DOI] [PubMed] [Google Scholar]
- 29.Komano T, Furuichi T, Teintze M, Inouye M, Inouye S. Effects of deletion of the gene for the development-specific protein S on differentiation of Myxococcus xanthus. J Bacteriol. 1984;158:1195–1197. doi: 10.1128/jb.158.3.1195-1197.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroos L, Kuspa A, Kaiser D. A global analysis of developmentally regulated genes in Myxococcus xanthus. Dev Biol. 1986;117:252–266. doi: 10.1016/0012-1606(86)90368-4. [DOI] [PubMed] [Google Scholar]
- 31.Kroos L, Kaiser D. Expression of many developmentally regulated genes in Myxococcus depends on a sequence of cell interactions. Genes Dev. 1987;1:840–854. doi: 10.1101/gad.1.8.840. [DOI] [PubMed] [Google Scholar]
- 32.Krozowski Z. The short-chain alcohol dehydrogenase superfamily: variations on a common theme. J Steroid Biochem Mol Biol. 1994;51:125–130. doi: 10.1016/0960-0760(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 33.Kuner J M, Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982;151:458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuspa A, Kroos L, Kaiser D. Intercellular signaling is required for developmental gene expression in Myxococcus xanthus. Dev Biol. 1986;117:267–276. doi: 10.1016/0012-1606(86)90369-6. [DOI] [PubMed] [Google Scholar]
- 35.LaRossa R, Kuner J, Hagen D, Manoil C, Kaiser D. Developmental cell interactions of Myxococcus xanthus: analysis of mutants. J Bacteriol. 1983;153:1394–1404. doi: 10.1128/jb.153.3.1394-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee B-U, Lee K, Mendea J, Shimkets L J. A tactile sensory system of Myxococcus xanthus involves an extracellular NAD(P)+-containing protein. Genes Dev. 1995;9:2964–2973. doi: 10.1101/gad.9.23.2964. [DOI] [PubMed] [Google Scholar]
- 37.Lee K, Shimkets L J. Suppression of a signaling defect during Myxococcus xanthus development. J Bacteriol. 1996;178:977–984. doi: 10.1128/jb.178.4.977-984.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lessard I A, Pratt S D, McCafferty D G, Bussiere D E, Hutchins C, Wanner B L, Katz L, Walsh C T. Homologs of the vancomycin resistance D-Ala-D-Ala dipeptidase VanX in Streptomyces toyocaensis, Escherichia coli, and Synechocystis: attributes of catalytic efficiency, stereoselectivity and regulation with implications for function. Chem Biol. 1998;5:489–504. doi: 10.1016/s1074-5521(98)90005-9. [DOI] [PubMed] [Google Scholar]
- 39.McDonald K L. High pressure freezing for preservation of high resolution fine structure and antigenicity for immunolabeling. Methods Mol Biol. 1999;117:77–97. doi: 10.1385/1-59259-201-5:77. [DOI] [PubMed] [Google Scholar]
- 40.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 41.Morrison C E, Zusman D R. Myxococcus xanthus mutants with temperature-sensitive, stage-specific defects: evidence for independent pathways in development. J Bacteriol. 1979;140:1036–1042. doi: 10.1128/jb.140.3.1036-1042.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Callaghan C H, Muggleton P W, Ross G W. Effects of β-lactamase from gram-negative organisms on cephalosporins and penicillins. Antimicrob Agents Chemother. 1968;8:57–63. [PubMed] [Google Scholar]
- 43.O’Connor K A, Zusman D R. Patterns of cellular interactions during fruiting-body formation in Myxococcus xanthus. J Bacteriol. 1989;171:6013–6024. doi: 10.1128/jb.171.11.6013-6024.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connor K A, Zusman D R. Development in Myxococcus xanthus involves differentiation into two cell types, peripheral rods and spores. J Bacteriol. 1991;173:3318–3333. doi: 10.1128/jb.173.11.3318-3333.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor K A, Zusman D R. Starvation-independent sporulation in Myxococcus xanthus involves the pathway of β-lactamase induction and provides a mechanism for competitive cell survival. Mol Microbiol. 1997;24:839–850. doi: 10.1046/j.1365-2958.1997.3931757.x. [DOI] [PubMed] [Google Scholar]
- 46.Park J T. Why does Escherichia coli recycle its cell wall peptides? Mol Microbiol. 1995;17:421–426. doi: 10.1111/j.1365-2958.1995.mmi_17030421.x. [DOI] [PubMed] [Google Scholar]
- 47.Ramsey W S, Dworkin M. Microcyst germination in Myxococcus xanthus. J Bacteriol. 1968;95:2249–2257. doi: 10.1128/jb.95.6.2249-2257.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reynolds E S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodionov D G, Ishiguro E E. Dependence of peptidoglycan metabolism on phospholipid synthesis during growth of Escherichia coli. Microbiology. 1996;142:2871–2877. doi: 10.1099/13500872-142-10-2871. [DOI] [PubMed] [Google Scholar]
- 50.Rosenbluh A, Rosenberg E. Sporulation of Myxococcus xanthus in liquid shake culture. J Bacteriol. 1989;171:4521–4524. doi: 10.1128/jb.171.8.4521-4524.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shi W, Köhler T, Zusman D R. Chemotaxis plays a role in the social behavior of Myxococcus xanthus. Mol Microbiol. 1993;9:601–611. doi: 10.1111/j.1365-2958.1993.tb01720.x. [DOI] [PubMed] [Google Scholar]
- 52.Shimkets L J. Social and developmental biology of the myxobacteria. Microbiol Rev. 1990;54:473–501. doi: 10.1128/mr.54.4.473-501.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shimkets L J, Kaiser D. Murein components rescue developmental sporulation of Myxococcus xanthus. J Bacteriol. 1982;152:462–470. doi: 10.1128/jb.152.1.462-470.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thaxter R. On the Myxobacteriaceae, a new order of Schizomycetes. Bot Gaz. 1892;17:389–406. [Google Scholar]
- 55.Voelz H, Dworkin M. Fine Structure of Myxococcus xanthus during morphogenesis. J Bacteriol. 1962;84:943–952. doi: 10.1128/jb.84.5.943-952.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Von Krüger W M A, Parish J H. β-Lactamase activity and resistance to penicillin’s in Myxococcus xanthus. Arch Microbiol. 1981;130:150–154. doi: 10.1007/BF00411069. [DOI] [PubMed] [Google Scholar]
- 57.Wall D, Kolenbrander P E, Kaiser D. The Myxococcus xanthus pilQ (sglA) gene encodes a secretin homolog required for type IV pilus biogenesis, social motility, and development. J Bacteriol. 1999;181:24–33. doi: 10.1128/jb.181.1.24-33.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.White D. Structure and function of Myxobacteria cells and fruiting bodies. In: Rosenberg E, editor. Myxobacteria development and cell interactions. New York, N.Y: Springer-Verlag; 1984. pp. 51–67. [Google Scholar]
- 59.Xu D, Yang C, Kaplan H B. Myxococcus xanthus sasN encodes a regulator that prevents developmental gene expression during growth. J Bacteriol. 1998;180:6215–6223. doi: 10.1128/jb.180.23.6215-6223.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]