Abstract
Platinum-based chemotherapy combined with anti-PD-1 or PD-L1 monoclonal antibodies (mAbs) is now standard first-line therapy for mNSCLC patients without sensitizing driver mutations. Anti-PD-1 and anti-PD-L1 mAbs are considered to be equivalent in efficacy. In the absence of head-to-head randomized control trials (RCTs), we utilized network meta-analysis (NWM) to provide an indirect comparison of their efficacy. A systematic literature review and NWM were performed using RCTs that investigated anti-PD-1 or PD-L1 mAbs ± chemotherapy in patients with mNSCLC in the first-line setting. The primary outcome was comparative overall survival (OS), while secondary outcomes were comparative progression-free survival (PFS), objective response rate (ORR), and rate of grade 3 and higher toxicities. We identified 24 RCTs. Patients treated with anti-PD-1 mAb + chemotherapy compared with anti-PD-L1 mAb + chemotherapy showed superior mOS, mPFS, and ORR with a similar rate of grade 3 and higher toxicities. This difference in mOS was most pronounced in the PD-L1 TPS 1–49% population. The two mAbs were equivalent as single agents. Anti-PD-1 mAb + chemotherapy improved mOS when compared to anti-PD-1 mAb monotherapy, whereas anti-PD-L1 mAbs + chemotherapy did not when compared to anti-PD-L1 mAb monotherapy. Head-to-head RCTs are warranted in the future.
Keywords: immunotherapy, chemoimmunotherapy, non-small cell lung cancer, anti-PD-1, anti-PD-L1
1. Introduction
Lung cancer is the leading cause of cancer death worldwide [1]. Approximately 85% of occurrences are non-small cell lung cancers (NSCLC), of which 70% are diagnosed at an advanced or metastatic stage [2], defining a need for effective systemic therapy. NSCLC can be classified as squamous or non-squamous cell carcinoma. Platinum-based doublet chemotherapy has been the backbone treatment for metastatic NSCLC (mNSCLC) for decades.
Immune checkpoint inhibitors (ICIs) have revolutionized cancer therapies. Monoclonal antibodies (mAbs) that inhibit programmed death protein 1 (PD-1) or its ligand (PD-L1) are now widely used in various cancers [3,4,5,6]. These mAbs work because they can block the interaction between PD-1 on activated T cells and PD-L1 on tumor cells, thereby reprogramming and reactivating otherwise exhausted anti-tumor T cells [7]. Multiple anti-PD-1 and anti-PD-L1 mAbs have been developed to take advantage of this mechanism. Several large, randomized control trials (RCTs) have shown that to addition of anti-PD-1 or anti-PD-L1 mAb to standard chemotherapy can significantly improve the survival of patients with mNSCLC without sensitizing driver mutations compared with platinum-doublet chemotherapy, and these combinations are now a standard of care [8,9,10].
Because anti-PD-1 and anti-PD-L1 mAbs can both block the PD-1-PD-L1 interaction, their efficacy is thought to be comparable. However, numerous factors could cause differences in efficacy between the two mAbs, including: the greater ability of anti-PD-L1 mAbs (IgG1) to induce antibody-dependent complement-mediated cytotoxicity [11]; the presence of a second ligand for PD-1, PD-L2, which cannot be blocked by anti-PD-L1 mAb [12]; and the potential for chemotherapy to modulate PD-L1 expression within the tumor microenvironment (TME) [13,14]. To date, no head-to-head RCTs comparing PD-1 inhibitor therapy versus PD-L1 as monotherapy or in combination with chemotherapy have been performed to define differences in efficacy between these two classes of mAbs.
In 2021, several network meta-analyses (NWM)—a statistical method used to compare interventions indirectly [15]—have shown that pembrolizumab, an anti-PD-1 mAb, is more effective than atezolizumab, an anti-PD-L1 mAb, when combined with chemotherapy [16,17,18]. Because of the limited number of RCTs available at that time, the authors were unable to conclude if the differences represented an individual drug effect or a drug class effect.
More recently, multiple noval anti-PD-1 and anti-PD-L1 mAbs have been investigated in phase II/III RCTs in the first-line mNSCLC setting [19,20,21,22,23,24,25,26,27,28,29]. In light of these additional studies, we have performed NWM between anti-PD-1 and anti-PD-L1 mAbs with or without platinum-based chemotherapy doublets in an attempt to detect any differences in efficacy between these two mAbs as drug classes.
2. Materials and Methods
2.1. The Search Strategy and Selection Criteria
This systematic review and NWM were performed according to the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) Statement. The inclusion criteria and analytical plan were prespecified in a protocol submitted to PROSPERO (registration number CRD42022361215). Eligible studies were retrieved from MEDLINE, PubMed, and EMBASE published up to 1st December 2022. Two authors (JW and AY) developed the search strategy and performed manual searches independently. The following search terms were used to identify studies involving humans: “randomized” AND (“non-small cell lung cancer” OR “lung adenocarcinoma” OR “lung squamous cell carcinoma” OR “lung cancer”) AND (“immunotherapy” OR “PD-L1” OR “PD-1”).
To determine eligibility, titles and abstracts were manually screened by JW and AY independently. The full texts of studies were assessed if eligibility could not be determined by the titles or abstracts. Disagreements regarding eligibility were discussed verbally to reach a consensus. If consensus was not reached, a third author (SC) was involved for the tie-break. Inclusion criteria: phase II or III RCTs involving unresectable, stage IV NSCLC without sensitizing EGFR mutations or ALK rearrangements; an intervention arm involving anti-PD-1 or anti-PD-L1 monoclonal antibodies (mAb) with or without platinum-based chemotherapy; a control arm involving platinum-based chemotherapy; and studies with data on overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and rate of grade 3 and higher toxicities. Exclusion criteria: studies not performed in the first-line setting; studies involving only large cell lung carcinoma or bronchopulmonary neuroendocrine tumors; studies with tyrosine kinase inhibitors, anti-vascular endothelial growth factor (VEGF) inhibitors, or immunotherapies other than anti-PD-1 or anti-PD-L1 mAbs.
2.2. Data Collection and Risk of Bias within Individual Studies
The data were extracted by JW and AY separately in accordance with the PRISMA statement. Discrepancies were verbally resolved with SC. Patient characteristics extracted from the eligible studies included sample size, histologic type, and PD-L1 tumor proportion score (TPS). Clinical outcomes extracted included hazard ratios (HR) with 95% confidence intervals (95% CIs) for median OS (mOS) and median PFS (mPFS), the incidence over the total number evaluated (n/N) for ORR, and the rate of grade 3 and higher toxicities.
The risk of bias was assessed using the Cochrane Risk of Bias Tool 2.0 (RoB 2.0) for RCTs [30]. Five domains were assessed for bias: risk of bias arising from the randomization process (D1), risk of bias owing to deviations from the intended interventions (D2), risk of bias from missing outcome data (D3), risk of bias in the measurement of the outcome (D4), and risk of bias in the selection of the reported result (D5). A judgment of risk for each domain was assigned by the selection of one of the three levels of risk of bias for each domain—low risk of bias, some concerns, or high risk of bias. An overall bias assessment was given to the study by considering the results of the above assessments.
2.3. Statistical Analysis
The primary outcome was OS; secondary outcomes were PFS and ORR. The effective sizes were HRs with 95% CI for mOS and mPFS, and ORs with 95% CI for ORR and a rate of grade 3 and higher toxicities. Data were collected from studies and entered into REVMAN version 5.4. Using a random effects model, pairwise meta-analyses were performed for the following groups: group 1: anti-PD-1 mAbs + chemotherapy versus chemotherapy; group 2: anti-PD-L1 mAbs + chemotherapy versus chemotherapy; group 3: anti-PD-1 mAbs versus chemotherapy; group 4: anti-PD-L1 mAbs versus chemotherapy (see Figure 1). Subgroup meta-analyses for squamous and non-squamous histology types and PD-L1 TPS < 1%, 1–49%, and >50% were also performed. Studies without data on these subgroups were excluded from subgroup meta-analyses. Heterogeneity was evaluated using the Q test and I2 previously described [31]. A Q test p-value of >0.1 or I2 < 50% was considered to have a low-to-moderate level of heterogeneity; a Q test p-value of <0.05 or I2 > 50% was considered to have a substantial level of heterogeneity. A sensitivity analysis was performed to investigate the impact of excluding the likely study causing heterogeneity and to see whether the reported result in the pooled analysis was changed. Indirect comparisons of the outcomes described above and subgroup analyses according to PD-L1 TPS were performed between group 1 versus group 2, group 3 versus group 4, group 1 versus group 3, and group 2 versus group 4 (see Figure 1) using the Bayesian NWM described previously [15]. Group 1 versus group 4 and group 2 versus group 3 were not performed as the results could not be interpreted. A p-value of <0.05 was considered statistically significant with NMW.
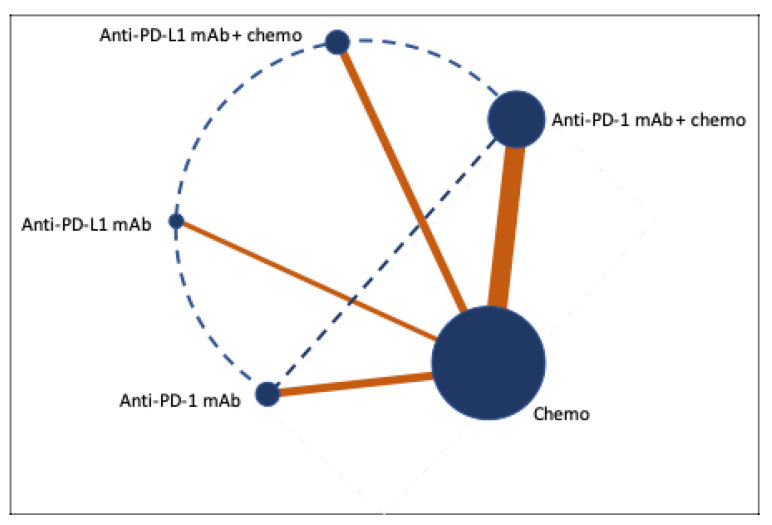
Figure 1.
Network meta-analysis plot. A Bayesian framework was used to generate comparisons between the median overall survival, the median progression-free survival, and the objective response rate. The size of the blue circle and thickness of the orange lines are proportional to the number of studies included. Solid lines represent meta-analyses, and dotted lines represent network meta-analyses performed.
3. Results
3.1. This Systematic Review and Study Characteristics
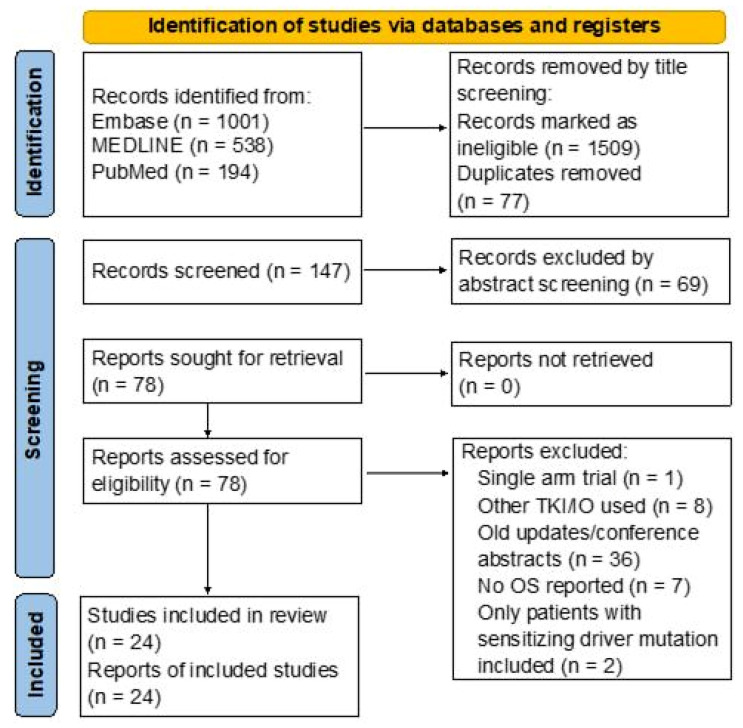
We identified 1686 records in the databases. After removing duplicates and ineligible studies by title and abstract screening, 78 full-text articles were retrieved and assessed for eligibility. Finally, 24 RCTs [8,21,22,23,24,26,27,28,29,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46] involving 14,123 patients met the eligibility criteria, and there were no studies with a high risk of bias (see Figure 2 and Figure A1). Sixteen RCTs used anti-PD-1 mAbs and eight used anti-PD-L1 mAbs. Seven different anti-PD-1 mAbs (nivolumab, pembrolizumab, cemiplimab, sintilimab, toripalimab, tislelizumab, and camrelizumab) and four different anti-PD-L1 mAbs (atezolizumab, durvalumab, avelumab, and sugemalimab) were investigated in the first-line setting. We did not identify RCTs that compared anti-PD-1 with anti-PD-L1 mAbs directly.
Figure 2.
PRISMA flow diagram: Literature search and selection following PRISMA guidelines. TKI, tyrosine kinase inhibitor; IO, immunotherapy; OS, overall survival; EGFR, epithelial growth factor receptor; ALK, anaplastic lymphoma kinase; ROS-1, ROS proto-oncogene 1 receptor kinase.
All 24 RCTs used 4–6 cycles of platinum-based doublet chemotherapy as the control arm. Carboplatin or cisplatin were used as platinum agents in all studies; pemetrexed was used in patients with non-squamous histopathology; and paclitaxel was used in patients with squamous histopathology in all studies. We excluded the following in our analyses to reduce the chance of heterogeneity: an arm involving ipilimumab in CheckMate 227; an arm involving tremelimumab in MYSTIC, and data involving EGFR sensitizing mutations or ALK rearrangements in IMpower 130 (see Table 1).
Table 1.
Study characteristics of studies included in the network meta-analyses.
| Study No. | Study Name | Number of Patients | Study Arms | Lung Cancer Histology Type | PD-L1 Status | Antibody Used to Determine PD-L1 Status |
|---|---|---|---|---|---|---|
| 1 | KEYNOTE 021 cohort G | ChemoIO: 60 Chemo: 63 |
Arm A: Pembrolizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 2 | KEYNOTE 189 | ChemoIO: 410 Chemo: 206 |
Arm A: Pembrolizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 3 | KEYNOTE 407 | ChemoIO: 278 Chemo: 281 |
Arm A: Pembrolizumab + Platinum doublet Arm B: Platinum doublet |
Squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 4 | CheckMate 227 | ChemoIO: 177 IO alone: 396 Chemo: 397 |
Arm A: Nivolumab + ipilimumab + Platinum doublet Arm B (PD-L1 < 1%): nivolumab + Platinum doublet Arm C (PD-L1 > 1%): nivolumab alone Arm D: Platinum doublet |
Non-small cell | PD-L1 < 1%, 1–49%, >50% | 28-8 pharmDX |
| 5 | CHOICE 01 | ChemoIO: 309 Chemo: 156 |
Arm A: Toripalimab + Platinum doublet Arm B: Platinum doublet |
Non-small cell | PD-L1 < 1%, 1–49%, >50% | JS311 MEDx |
| 6 | CameL | ChemoIO: 205 Chemo: 207 |
Arm A: Camrelizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 7 | CameL-Sq | ChemoIO: 193 Chemo: 196 |
Arm A: Camrelizumab + Platinum doublet Arm B: Platinum doublet |
Squamous | PD-L1 < 1%, 1–49%, >50% | E1L3N AmoyDx |
| 8 | EMPOWER-Lung 3 | ChemoIO: 312 Chemo: 154 |
Arm A: Cemiplimab + Platinum doublet Arm B: Platinum doublet |
Non-small cell | PD-L1 < 1%, 1–49%, >50% | SP263 |
| 9 | ORIENT 11 | ChemoIO: 266 Chemo: 131 |
Arm A: Sintilimab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 10 | ORIENT 12 | ChemoIO: 179 Chemo: 178 |
Arm A: Sintilimab + Platinum doublet Arm B: Platinum doublet |
Squamous | PD-L1 < 1%, 1–49%, >50% | 22C3 pharmDX |
| 11 | RATIONALE 304 | ChemoIO: 223 Chemo: 111 |
Arm A: Tislelizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% | SP263 |
| 12 | RATIONALE 307 | ChemoIO Arm A: 120 ChemoIO Arm B: 119 Chemo: 121 |
Arm A: Tislelizumab + Platinum + Paclitaxel Arm B: Tislelizumab + Platinum + Nab-paclitaxel Arm C: Platinum + Paclitaxel |
Squamous | PD-L1 < 1%, 1–49%, >50% | SP263 |
| 13 | IMpower 130 | ChemoIO: 451 Chemo: 228 |
Arm A: Atezolizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% (TC) PD-L1 < 1%, 1–9%, >10% (IC) |
SP142 |
| 14 | IMpower 131 | ChemoIO Arm A: 338 ChemoIO Arm B: 343 Chemo: 340 |
Arm A: Atezolizumab + Platinum + Paclitaxel Arm B: Atezolizumab + Platinum + Nab-paclitaxel Arm C: Platinum + Nab-paclitxel |
Squamous | PD-L1 < 1%, 1–49%, >50% (TC) PD-L1 < 1%, 1–9%, >10% (IC) |
SP142 |
| 15 | IMpower 132 | ChemoIO: 292 Chemo: 286 |
Arm A: Atezolizumab + Platinum doublet Arm B: Platinum doublet |
Non-squamous | PD-L1 < 1%, 1–49%, >50% (TC) PD-L1 < 1%, 1–9%, >10% (IC) |
SP142 |
| 16 | GEMSTONE 302 | ChemoIO: 320 Chemo: 159 |
Arm A: Sugemalimab + Platinum doublet Arm B: Platinum doublet |
Non-small cell | PD-L1 < 1%, 1–49%, >50% | SP263 |
| 17 | POSEIDON | ChemoIO: 338 Chemo: 337 |
Arm A: Durvalumab + Platinum doublet Arm B: Platinum doublet |
Non-small cell | PD-L1 < 1%, 1–49%, >50% | SP263 |
| 18 | Checkmate 026 | ChemoIO: 271 Chemo: 270 |
Arm A: Nivolumab Arm B: Platinum doublet |
Non-small cell | PD-L1 > 5%, >50% | 28-8 pharmDX |
| 19 | Keynote 024 | ChemoIO: 154 Chemo: 151 |
Arm A: Pembrolizumab Arm B: Platinum doublet |
Non-small cell | PD-L1 > 50% | 22C3 pharmDX |
| 20 | Keynote 042 | ChemoIO: 637 Chemo: 636 |
Arm A: Pembrolizumab Arm B: Platinum doublet |
Non-small cell | PD-L1 1–49%, >50% | 22C3 pharmDX |
| 21 | EMPOWER-Lung 1 | ChemoIO: 356 Chemo: 354 |
Arm A: Cemiplimab Arm B: Platinum doublet |
Non-small cell | PD-L1 >50% | 22C3 pharmDX |
| 22 | IMpower 110 | ChemoIO: 227 Chemo: 227 |
Arm A: Atezolizumab Arm B: Platinum doublet |
Non-small cell | PD-L1 > 1%, >5%, >50% | SP142 |
| 23 | JAVELIN Lung 100 | ChemoIO Arm A: 366 ChemoIO Arm B: 322 Chemo: 526 |
Arm A: Avelumab Q2W Arm B: Avelumab QW Arm C: Platinum doublet |
Non-small cell | PD-L1 > 1%, >50%, >80% | 73-10 pharmDx |
| 24 | MYSTIC | ChemoIO Arm A: 374 ChemoIO Arm B: 372 Chemo: 372 |
Arm A: Durvalumab Arm B: Durvalumab + Tremelimumab Arm C: Platinum doublet |
Non-small cell | PD-L1 > 25% | SP263 |
We identified some differences between the included studies (Table 1). Anti-PD-L1 mAbs used in immunohistochemistry staining (IHC) to classify TPS were different in various studies; IMpower 131 and 132 had a different way of classifying PD-L1 TPS (PD-L1 moderate population was defined as >1% and <50% tumor cell or >1% and <10% immune cell PD-L1 staining, and PD-L1 high population was defined as >50% tumor cell or >10% immune cell PD-L1 staining); only the PD-L1 TPS <1% population in CheckMate 227 received anti-PD-1 mAb + chemotherapy combination (see Table 1). These differences could contribute to heterogeneity in the subgroup analyses (see below).
3.2. Pairwise Meta-Analyses for Survival and Tumor Response
3.2.1. Overall Survival
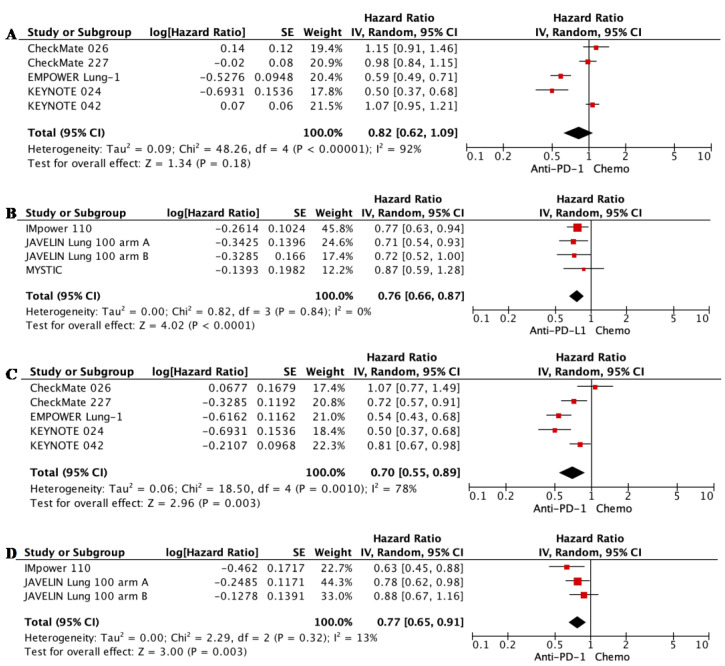
The addition of anti-PD-1 mAb to chemotherapy in patients with mNSCLC improved mOS significantly in patients with mNSCLC compared with chemotherapy alone (HR 0.67, 95% CI 0.62–0.73, p < 0.00001; see Figure 3A). Similarly, the addition of anti-PD-L1 mAb to chemotherapy also improved mOS significantly compared with chemotherapy alone (HR 0.83, 95% CI 0.76–0.91, p < 0.0001; see Figure 3B). As monotherapies, both anti-PD-1 or anti-PD-L1 mAbs achieved better mOS than chemotherapy in patients with tumors positive for PD-L1 (TPS >1%, see Figure A2; and TPS > 50%, see Figure 3C,D). The results are summarized in Table 2.
Figure 3.
Forest plot for meta-analyses of median overall survival (mOS). (A) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for all patients; (B) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for all patients; (C) anti-PD-1 mAb monotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%; (D) anti-PD-L1 mAb monotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%. Squares represent HRs of individual studies; rhombi represent HRs of meta-analyses.
Table 2.
Summary of overall survival results from meta-analyses.
| Treatment | Hazard Ratio (95% CI) | |
|---|---|---|
| Anti-PD-1 mAbs + chemotherapy > chemotherapy | All patients | 0.67 (0.62–0.73) |
| PD-L1 TPS < 1% | 0.76 (0.66–0.87) | |
| PD-L1 TPS 1–49% | 0.61 (0.52–0.71) | |
| PD-L1 TPS > 50% | 0.66 (0.54–0.81) | |
| Squamous | 0.69 (0.58–0.83) | |
| Non-squamous | 0.67 (0.58–0.76) | |
| Anti-PD-L1 + chemotherapy > chemotherapy | All patients | 0.83 (0.76–0.91) |
| PD-L1 TPS < 1% | 0.85 (0.74–0.99) | |
| PD-L1 TPS > 50% | 0.64 (0.51–0.81) | |
| Non-Squamous | 0.83 (0.73–0.93) | |
| Anti-PD-L1 + chemotherapy = chemotherapy | PD-L1 TPS 1–49% | 0.97 (0.82–1.15) |
| Squamous | 0.87 (0.74–1.01) | |
| Anti-PD-1 > chemotherapy | PD-L1 TPS > 1% | 0.83 (0.70–0.97) |
| PD-L1 TPS > 50% | 0.71 (0.61–0.84) | |
| Anti-PD-L1 > chemotherapy | PD-L1 TPS > 1% | 0.88 (0.79–0.99) |
| PD-L1 TPS > 50% | 0.78 (0.68–0.90) |
3.2.2. Progression-Free Survival
Consistent with mOS, adding anti-PD-1 or anti-PD-L1 mAb to chemotherapy also improved mPFS significantly in patients with mNSCLC compared with chemotherapy alone (anti-PD-1: HR 0.52, 95% CI 0.48–0.57, p < 0.00001; anti-PD-L1: HR 0.63, 95% CI 0.55–0.73, p < 0.00001; see Figure 4A,B; NB, please see below regarding addressing high levels of heterogeneity). As monotherapies, anti-PD-L1 mAbs were superior to chemotherapy alone (HR 0.76, 95% CI 0.66–0.87, p < 0.0001; see Figure A3B), but anti-PD-1 mAbs were not (HR 0.82, 95% CI 0.62–1.09, p < 0.18; see Figure A3A). In patients with tumors expressing a high level of PD-L1 (TPS > 50%), both anti-PD-1 and anti-PD-L1 mAb monotherapy improved mPFS when compared with chemotherapy (Figure A3C,D).
Figure 4.
Forest plot for meta-analyses of median progression-free survival (mPFS), objective response rate (ORR), and grade 3 and higher toxicities for chemoimmunotherapies. (A) mPFS, anti-PD-1 mAb + chemotherapy versus chemotherapy alone; (B) mPFS, anti-PD-L1 mAb + chemotherapy versus chemotherapy alone; (C) ORR, anti-PD-1 mAb + chemotherapy versus chemotherapy alone; (D) ORR, anti-PD-L1 mAb + chemotherapy versus chemotherapy alone; grade 3 and higher toxicities for anti-PD-1 mAb + chemotherapy (E) and anti-PD-L1 mAb + chemotherapy (F) versus chemotherapy alone. Squares represent HRs or ORs of individual studies; rhombi represent meta-analyses.
3.2.3. Objective Tumor Radiological Response Rate and Grade 3 and Higher Toxcities
Pairwise meta-analyses were also performed for ORR and grade 3 and higher toxicities between anti-PD-1 or anti-PD-L1 mAb ± chemotherapy compared with chemotherapy alone. Both anti-PD-1 and anti-PD-L1 mAbs achieved better ORR when combined with chemotherapy (anti-PD-1: OR 2.54, 95% CI 2.23–2.89, p = 0.00001; anti-PD-L1: OR 1.96, 95% CI 1.61–2.39, p = 0.00001; see Figure 4C,D), but were equivalent to chemotherapy when used alone (anti-PD-1: OR 1.27, 95% CI 0.81–1.97, p = 0.29; anti-PD-L1: OR 1.00, 95% CI 0.82–1.21, p = 0.96; see Figure A4). The grade 3 and higher toxicities were similar between ant-PD-1 and anti-PD-L1 mAbs (Figure 4E,F).
3.3. Pairwise Meta-Analyses for Overall Survival According to Histology Type and PD-L1 TPS
We predicted that histological subtype and PD-L1 TPS would be sources of possible interstudy heterogeneity; hence, we performed pairwise meta-analyses for these subgroups, and the results are summarized in Table 2. Anti-PD-1 mAb combined with chemotherapy achieved superior mOS regardless of the histological subtype (squamous: HR 0.69, 95% CI 0.59–0.83, p = 0.0001; non-squamous: HR 0.67, 95% CI 0.58–0.76, p = 0.00001; see Figure A5A,C) and PD-L1 TPS (PD-L1 TPS ≤ 1%: HR 0.76, 95% CI 0.66–0.87, p = 0.0001; PD-L1 TPS 1–49%: HR 0.61, 95% CI 0.52–0.71, p = 0.00001; PD-L1 TPS > 50%: HR 0.66, 95% CI 0.54–0.81, p = 0.0001; see Figure A6A,C,E). In contrast, anti-PD-L1 mAbs combined with chemotherapy achieved superior mOS for patients with non-squamous mNSCLC (HR 0.83, 95% CI 0.73–0.93, p = 0.002; see Figure A5D), PD-L1 TPS < 1% (HR 0.85, 95% CI 0.74–0.99, p = 0.03; see Figure A6B), and PD-L1 TPS > 50% (HR 0.64, 95% CI 0.51–0.81, p = 0.0002; see Figure A6F), but not in patients with squamous mNSCLC (HR 0.87, 95% CI 0.74–1.01, p = 0.08; see Figure A5B) or PD-L1 TPS 1–49% (HR 0.97, 95% CI 0.82–1.15, p = 0.76; see Figure A6D).
3.4. Heterogeneity and Transitivity Assessments
Network meta-analysis is only valid if the interstudy heterogeneity is low (I2 < 50%) with acceptable transitivity. The majority of the meta-analyses performed had low levels of heterogeneity, and transitivity was largely acceptable as only selected RCTs were utilized. There were a few studies with I2 > 50%. The meta-analyses with substantial heterogeneity are described below.
There was substantial heterogeneity in the mOS (I2 = 67%, p = 0.02), mPFS (I2 = 92%, p = 0.00001), and ORR (I2 = 87%, p = 0.00001) meta-analysis for anti-PD-1 mAb monotherapy (see Figure A2, Figure A3 and Figure A4). EMPOWER Lung-1 and KEYNOTE 024 were identified as the outliers. Unlike the other studies, these two studies only included patients with PD-L1 TPS >50%. Pairwise meta-analyses were then performed for patients with PD-L1 TPS >50%, and this reduced the heterogeneity level to low-moderate (I2 = 36%, p = 0.18, Figure 3) for mOS, but remained substantial for mPFS (I2 = 78%) and ORR (I2 = 81%).
We detected a moderate and substantial level of heterogeneity in the mPFS meta-analyses for anti-PD-1 mAb + chemotherapy (I2 = 43%, p = 0.05; see Figure 4A) and anti-PD-L1 mAb + chemotherapy versus chemotherapy (I2 = 66%, p = 0.02; see Figure 4B), respectively. Sensitivity analysis revealed CheckMate 227 and GEMSTONE 302 contributed to the heterogeneity and were largely within the PD-L1 TPS < 1% population (I2 = 71%, p = 0.008, see Figure A7). Removing these trials significantly reduced the heterogeneity (anti-PD-1 mAb + chemotherapy: I2 = 24%, p = 0.2; anti-PD-L1 mAb + chemotherapy: I2 = 2%, p = 0.38).
3.5. Network Meta-Analyses for Survival and Tumor Response
Using the pairwise meta-analyses results from above, indirect comparisons were made between anti-PD-1 and anti-PD-L1 mAbs ± chemotherapy (Figure 5). To ensure transitivity and consistency, we only used meta-analyses with a low to moderate level of heterogeneity (i.e., I2 < 50%) for our NWM.
Figure 5.
Forest plot for network meta-analyses of median overall survival (mOS), median progression-free survival (mPFS), objective response rate (ORR), and grade 3 or higher toxicities.
In the presence of chemotherapy, anti-PD-1 mAbs produced significantly better mOS compared with anti-PD-L1 mAbs (HR 0.81, 95% CI 0.71–0.91, p = 0.0006), but not as monotherapies (HR 0.91, 95% CI 0.74–1.13, p = 0.39). Subgroup NWM analyses were performed, and we demonstrated the superiority of anti-PD-1 mAb in the PD-L1 TPS 1–49% population (HR 0.63, 95% CI 0.50–0.79, p = 0.0001) and non-squamous histology (HR 0.81, 95% CI 0.67–0.97, p = 0.02 but not in PD-L1 TPS < 1% (HR 0.89, 95% CI 0.73–1.09, p = 0.27), PD-L1 TPS >50% HR 1.03, 95% CI 0.76–1.40, p = 0.84) and not significant for squamous histology (HR 0.79, 95% CI 0.63–1.01, p = 0.056; result not shown). We also demonstrated that anti-PD-1 mAb + chemotherapy had better mOS than anti-PD-1 mAb alone (HR 0.81, 95% CI 0.67–0.97, p = 0.02), but the mOS for anti-PD-L1 mAb + chemotherapy and anti-PD-L1 mAb alone were equivalent (HR 0.94, 95% CI 0.83–1.08, p = 0.39).
In the presence of chemotherapy, anti-PD-1 mAbs also had significantly better mPFS than anti-PD-L1 mAbs (even with GEMSTONE 302 included in the NWM, there was a significant difference between anti-PD-1 and anti-PD-L1 mAbs in mPFS (HR 0.84, 95% CI 0.71–1.00, p = 0.045) (HR 0.63, 95% CI 0.50–0.79, p = 0.0001) and ORR (OR 1.30, 95% CI 1.02–1.64, p = 0.03)). Consistent with mOS, mPFS was also significantly better in anti-PD-1 mAb + chemotherapy (HR 0.79, 95% CI 0.63–0.99, p = 0.04) in the PD-L1 TPS 1–49% population. Grade 3 and higher toxicities were not significantly different between anti-PD-1 mAbs and anti-PD-L1 mAbs with chemotherapy (OR 0.77, 95% CI 0.48–1.24, p = 0.29).
4. Discussion
This is the largest NWM comparing the efficacy of anti-PD-1 and anti-PD-L1 mAbs in mNSCLC. Our NWM has three main advantages over the previously published NWMs: We included a larger number of RCTs, giving more statistical power; we included 7 different anti-PD-1 mAbs and 4 different anti-PD-L1 mAbs, allowing comparison of anti-PD-1 to anti-PD-L1 mAbs as drug classes; we excluded studies with bevacizumab (IMpower 150 [9] and TASUKI 52 [47]), allowing a similar control arm for a more precise NWM.
In our meta-analyses, we confirmed the previous observation that anti-PD-1 with or without chemotherapy improves outcome and efficacy compared with chemotherapy alone for patients with mNSCLC without sensitizing driver mutations in the first-line setting. Interestingly, anti-PD-L1 with chemotherapy was equivalent in outcome and efficacy compared with chemotherapy in patients with PD-L1 TPS 1–49% and squamous histology (Table 2). This indicates that the tumor microenvironment and/or PD-L1 expression in these populations are heterogeneous and more prone to alteration, thus reducing anti-PD-L1 + chemotherapy’s efficacy. Numerically, the OS outcomes for anti-PD-1 or anti-PD-L1 mAb monotherapies were better in patients expressing high levels of PD-L1 in their tumors (TPS > 50%). In these patients, whether to add chemotherapy to the anti-PD-1 or anti-PD-L1 mAb remains debatable. A NWM published in 2021 has shown that in the PD-L1 TPS > 50% population, chemoimmunotherapy had a similar mOS compared with immunotherapy alone [48], suggesting that adding chemotherapy in this population may be unnecessary and preferable to minimizing toxicity. However, this study did not distinguish anti-PD-1 from anti-PD-L1 mAbs. Intriguingly, we showed that in order to achieve superior OS, chemotherapy is necessary in the setting of anti-PD-1 mAbs. Median OS was improved by 19% with anti-PD-1 mAb when combined with chemotherapy versus anti-PD-1 mAb monotherapy in patients with PD-L1 TPS > 50%. Of importance, this was not observed when anti-PD-L1 mAbs were used. Furthermore, in the presence of chemotherapy, anti-PD-1 mAbs were more effective than anti-PD-L1 mAbs. Compared with the anti-PD-L1 mAb + chemotherapy combination, anti-PD-1 mAb + chemotherapy improved mOS by 33%, mPFS by 22%, and response rate by 30%, with similar grade 3 or higher toxicities.
Together, these findings suggest chemotherapy may create a condition more favorable for anti-PD-1 mAbs as a plausible explanation. Chemotherapy has been recognized to favorably alter the immune TME in several ways: it increases immunogenic cell death [49], it improves dendritic cell maturation [50], it reduces the presence of immunosuppressive cells [51], it alters the expression of PD-L1 [52], and it increases the number of tumor-infiltrating lymphocytes [53]. However, these mechanisms should impact anti-PD-1 and anti-PD-L1 mAbs equally and do not explain the differences we have observed between the two mAbs in our NWM. A mechanistic difference between the two classes of mAbs will explain our observations.
Although both anti-PD-1 and anti-PD-L1 block the PD-1-PD-L1 interaction, anti-PD-1 and anti-PD-L1 mAbs are mechanistically unique. One of the key differences is that anti-PD-1 mAbs can block the interaction between PD-1 and both PD-L1 and PD-L2, while anti-PD-L1 mAbs cannot. PD-L2 is another ligand for PD-1, and its significance within the TME remains unclear. Several differences exist between PD-L1 and PD-L2. Firstly, PD-L1 is thought to be the main driver of T cell inhibition, as most tumors express higher levels of PD-L1 than PD-L2 [54,55]. This may explain why, when used as monotherapies, anti-PD-1 mAbs do not result in superior efficacy over anti-PD-L1 mAbs. Secondly, PD-L1 and PD-L2 expression do not correlate with one another. Poorly-differentiated tumors have been suggested to express more PD-L1 than PD-L2, while well-differentiated tumors express more PD-L2 than PD-L1 [56]. This differential expression of PD-L1 would be expected to impact the efficacy of anti-PD-L1 mAbs but not the efficacy of anti-PD-1 mAbs. Finally, PD-L2 binds to PD-1 with a higher affinity. If PD-L2 expression increases, it could outcompete PD-L1, making PD-L2 the predominant driver of immunosuppression over PD-L1. Chemotherapy is already known to upregulate PD-L2 expression on tumor cells [57] and is often more active in poorly differentiated tumors than well-differentiated tumors [58,59]. In the presence of chemotherapy, the TME might develop a higher PD-L2 to PD-L1 ratio. Out of the PD-L1 low (TPS < 1%), moderate (TPS 1–49%), and high (TPS > 50%) populations, only the PD-L1 moderate population is impacted by chemotherapy, suggesting this group of patients might have a more malleable TME for PD-L2 expression. Interestingly, a pooled analysis of two RCTs (PEMBRO-RT [60] and MDACC [61]) also showed that in the presence of radiotherapy, pembrolizumab is more effective, especially in the PD-L1 moderate population [62]. These observations warrant more investigations into the mechanisms that influence the tumor microenvironment of the PD-L1 TPS 1–49% population.
4.1. Implications
In our NWM, we have shown anti-PD-1 mAbs perform better than anti-PD-L1 mAbs in the presence of chemotherapy in patients with mNSCLC without sensitizing driver mutations in the first-line setting, especially for tumors expressing PD-L1 TPS 1–49%. In patients with mNSCLC who are suitable for chemotherapy, chemotherapy should be included in the regimen with anti-PD-1 mAb as it improves outcomes even in the PD-L1-high population. This has implications not just for mNSCLC but also for resectable NSCLC. In recent years, the appropriate neoadjuvant chemoimmunotherapy combination for resectable NSCLC has been investigated. Presented at the European Society of Medical Oncology (ESMO) in 2022, a phase II study showed that chemotherapy + nivolumab produced significantly better response rates than nivolumab alone, even in the PD-L1 high population (complete and major pathological response rates were 50% and 80% versus 18.2%, respectively) [63]. Consistent with our observation, chemotherapy was indeed required for a better response when combined with an anti-PD-1 mAb.
Furthermore, if PD-L2 indeed plays a role in the TME when chemotherapy is involved, then studies combining ICIs in conjunction with chemoradiotherapy may need to be performed using an anti-PD-1 mAb to achieve optimal disease-recurrence-free survival. Indeed, KEYNOTE 799 [64] already showed a numerically better ORR than the PACIFIC trial [65] (70.5% versus 28.4%, respectively). A RCT involving anti-PD-1 mAbs will be needed to confirm KEYNOTE 799’s findings.
In most trials, PD-L2 status is rarely reported, and it is difficult to determine if PD-L2 expression changes with chemotherapy administration. We recommend future clinical trials include PD-L2 analysis to give us a better understanding of the PD-1-PD-L2 interaction and confidence in the mAb being chosen. Furthermore, basic research is needed to better understand the complex interplay within the TME.
4.2. Limitations and Future Directions
There are several limitations to our study. Firstly, our indirect comparisons between anti-PD-1 and anti-PD-L1 mAbs do not replace a comparative prospective randomized study. Unfortunately, a RCT using all of the available anti-PD-1 and anti-PD-L1 mAbs would not be feasible or favorable to the industry. Thus, this NWM may offer the best evidence for the superiority of anti-PD-1 mAbs. Secondly, one of the important factors that could cause heterogeneity in our study was the inconsistent use of anti-PD-L1 antibodies used in IHC for PD-L1 TPS. Therefore, the difference in efficacy observed between the two mAbs by subgroup analyses using PD-L1 TPS needs to be interpreted with caution. Thirdly, we are still waiting for the OS results from RATIONALE 304 and 307. These results will add more power to our analyses once the data are more mature.
There are several questions left unanswered by our NWM:
Is chemotherapy still necessary in the PD-L1 TPS > 90% population? According to 3-year update from correlative analysis presented at the American Society for Clinical Oncology (ASCO) in 2022, the ORR for pembrolizumab in PD-L1 TPS > 90% is 47.9% [66]. Interestingly, KEYNOTE 189 showed chemoimmunotherapy could achieve an ORR of 61.4% in the PD-L1 TPS > 50% population [8]. These results indicate that chemotherapy may still be beneficial regardless of PD-L1 status;
Are anti-PD-1 mAbs better than anti-PD-L1 mAbs in mNSCLC with sensitizing driver mutations or in subsequent line therapies?
Are anti-PD-1 mAbs superior to anti-PD-L1 in combination with bevacizumab, TKIs, or other ICIs?
5. Conclusions
In conclusion, anti-PD-1 mAbs appear to be superior to anti-PD-L1 mAbs in combination with chemotherapy for mNSCLC in the first-line setting. The TME is a complex entity that can be influenced by multiple factors, including chemotherapy. A head-to-head comparison between anti-PD-1 and anti-PD-L1mAbs in a clinical trial is needed to confirm our findings. Importantly, basic science research to further understand the mechanisms that influence the TME is needed to optimize informed trial design with biomarker selection and deliver reliable therapies in the future to improve survival in this insidious disease.
Appendix A
Figure A1.
Risk of bias analysis for studies included in the network meta-analyses. D1, risk of bias arising from the randomization process; D2, risk of bias owing to deviations from the intended interventions; D3, risk of bias from missing outcome data; D4, risk of bias in the measurement of the outcome; D5, risk of bias in the selection of the reported result.
Figure A2.
Forest plot for meta-analyses of median overall survival (mOS). (A) anti-PD-1 mAb monotherapy versus chemotherapy alone for patients with PD-L1 TPS >1%; (B) anti-PD-L1 mAb monotherapy versus chemotherapy alone for patients with PD-L1 TPS >1%.
Figure A3.
Forest plot for meta-analyses of median progression-free survival (mPFS) for immunotherapy alone. (A) anti-PD-1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS >1%; (B) anti-PD-L1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS >1%; (C) anti-PD-1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS > 50%; (D) anti-PD-L1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS > 50%.
Figure A4.
Forest plot for meta-analyses of objective response rate (ORR) for immunotherapy alone. (A) anti-PD-1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS >1%; (B) anti-PD-L1 mAb monotherapy versus chemotherapy alone in patients with PD-L1 TPS >1%.
Figure A5.
Forest plot for meta-analyses of median overall survival (mOS) according to histology type. (A) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with squamous NSCLC; (B) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with squamous NSCLC; (C) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with non-squamous NSCLC; (D) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with non-squamous NSCLC.
Figure A6.
Forest plot for meta-analyses of median overall survival (mOS) according to PD-L1 TPS. (A) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS < 1%; (B) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS < 1%; (C) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS 1–49%; (D) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS 1-49%; (E) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%; (F) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%.
Figure A7.
Forest plot for meta-analyses of progression-free survival (PFS) according to PD-L1 TPS. (A) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS < 1%; (B) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS < 1%; (C) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS 1–49%; (D) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS 1–49%; (E) anti-PD-1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%; (F) anti-PD-L1 mAb + chemotherapy versus chemotherapy alone for patients with PD-L1 TPS > 50%.
Author Contributions
Conceptualization, J.Q.W.; Data curation, A.Y.; Formal analysis, J.Q.W. and D.L.C.; Methodology, J.Q.W. and A.Y.; Supervision, D.L.C. and S.J.C.; Writing—original draft, J.Q.W.; Writing—review and editing, J.Q.W., A.Y., M.I., B.Y.K., B.T.L., N.P., D.L.C. and S.J.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data used in this network meta-analysis are collected from published clinical trials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2018. CA Cancer J. Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.-J., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 4.Motzer R.J., Tannir N.M., McDermott D.F., Arén Frontera O., Melichar B., Choueiri T.K., Plimack E.R., Barthélémy P., Porta C., George S., et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018;378:1277–1290. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., Kudo M., Breder V., Merle P., Kaseb A.O., et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020;382:1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 6.Burtness B., Harrington K.J., Greil R., Soulières D., Tahara M., de Castro G., Jr., Psyrri A., Basté N., Neupane P., Bratland Å., et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet. 2019;394:1915–1928. doi: 10.1016/S0140-6736(19)32591-7. [DOI] [PubMed] [Google Scholar]
- 7.Belk J.A., Daniel B., Satpathy A.T. Epigenetic regulation of T cell exhaustion. Nat. Immunol. 2022;23:848–860. doi: 10.1038/s41590-022-01224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadgeel S., Rodríguez-Abreu D., Speranza G., Esteban E., Felip E., Dómine M., Hui R., Hochmair M.J., Clingan P., Powell S.F., et al. Updated Analysis from KEYNOTE-189: Pembrolizumab or Placebo Plus Pemetrexed and Platinum for Previously Untreated Metastatic Nonsquamous Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:1505–1517. doi: 10.1200/JCO.19.03136. [DOI] [PubMed] [Google Scholar]
- 9.Socinski M.A., Nishio M., Jotte R.M., Cappuzzo F., Orlandi F., Stroyakovskiy D., Nogami N., Rodríguez-Abreu D., Moro-Sibilot D., Thomas C.A., et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021;16:1909–1924. doi: 10.1016/j.jtho.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Paz-Ares L.G., Ciuleanu T.-E., Cobo-Dols M., Bennouna J., Cheng Y., Mizutani H., Lingua A., Reyes F., Reinmuth N., De Menezes J.J., et al. First-line (1L) nivolumab (NIVO) + ipilimumab (IPI) + 2 cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients (pts) with metastatic non–small cell lung cancer (NSCLC): 3-year update from CheckMate 9LA. J. Clin. Oncol. 2022;40((Suppl. S17)):LBA9026. doi: 10.1200/JCO.2022.40.17_suppl.LBA9026. [DOI] [Google Scholar]
- 11.Yu J., Song Y., Tian W. How to select IgG subclasses in developing anti-tumor therapeutic antibodies. J. Hematol. Oncol. 2020;13:45. doi: 10.1186/s13045-020-00876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin J., Chung J.-H., Kim S.H., Lee K.S., Suh K.J., Lee J.Y., Kim J.-W., Lee J.-O., Kim Y.-J., Lee K.-W., et al. Effect of Platinum-Based Chemotherapy on PD-L1 Expression on Tumor Cells in Non-small Cell Lung Cancer. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2019;51:1086–1097. doi: 10.4143/crt.2018.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo L., Song P., Xue X., Guo C., Han L., Fang Q., Ying J., Gao S., Li W. Variation of Programmed Death Ligand 1 Expression after Platinum-based Neoadjuvant Chemotherapy in Lung Cancer. J. Immunother. 2019;42:215–220. doi: 10.1097/CJI.0000000000000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Catalá-López F., Tobías A., Cameron C., Moher D., Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: An introduction. Rheumatol. Int. 2014;34:1489–1496. doi: 10.1007/s00296-014-2994-2. [DOI] [PubMed] [Google Scholar]
- 16.Addeo A., Banna G.L., Metro G., Di Maio M. Chemotherapy in Combination with Immune Checkpoint Inhibitors for the First-Line Treatment of Patients with Advanced Non-small Cell Lung Cancer: A Systematic Review and Literature-Based Meta-Analysis. Front. Oncol. 2019;9:264. doi: 10.3389/fonc.2019.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu L., Bai H., Wang C., Seery S., Wang Z., Duan J., Li S., Xue P., Wang G., Sun Y., et al. Efficacy and Safety of First-Line Immunotherapy Combinations for Advanced NSCLC: A Systematic Review and Network Meta-Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2021;16:1099–1117. doi: 10.1016/j.jtho.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Brito A.B.C., Camandaroba M.P.G., de Lima V.C.C. Anti-PD1 versus anti-PD-L1 immunotherapy in first-line therapy for advanced non-small cell lung cancer: A systematic review and meta-analysis. Thorac. Cancer. 2021;12:1058–1066. doi: 10.1111/1759-7714.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sezer A., Kilickap S., Gümüş M., Bondarenko I., Özgüroğlu M., Gogishvili M., Turk H., Çiçin I., Bentsion D., Gladkov O., et al. LBA52 EMPOWER-Lung 1: Phase III first-line (1L) cemiplimab monotherapy vs platinum-doublet chemotherapy (chemo) in advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥50% Ann. Oncol. 2020;31:S1182–S1183. doi: 10.1016/j.annonc.2020.08.2285. [DOI] [Google Scholar]
- 20.Gogishvili M., Mobashery N., Makharadze T., Navarro M., Snodgrass P., Chen H., Lowy I., Rietschel P., Lee S. P2.01-26 EMPOWER-Lung 3: Phase 3 Study of Combinations of Cemiplimab and Chemotherapy in First-Line Treatment of Advanced NSCLC. J. Thorac. Oncol. 2019;14:S649. doi: 10.1016/j.jtho.2019.08.1370. [DOI] [Google Scholar]
- 21.Lu S., Wang J., Yu Y., Yu X., Hu Y., Ai X., Ma Z., Li X., Zhuang W., Liu Y., et al. Tislelizumab Plus Chemotherapy as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC (RATIONALE 304): A Randomized Phase 3 Trial. J. Thorac. Oncol. 2021;16:1512–1522. doi: 10.1016/j.jtho.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Wang J., Yu X., Lu S., Hu Y., Sun Y., Wang Z., Zhao J., Yu Y., Hu C., Yang K., et al. Phase III study of tislelizumab plus chemotherapy vs chemotherapy alone as first-line (1L) treatment for advanced squamous non-small cell lung cancer (sq NSCLC) J. Clin. Oncol. 2020;38((Suppl. S15)):9554. doi: 10.1200/JCO.2020.38.15_suppl.9554. [DOI] [Google Scholar]
- 23.Rizvi N.A., Cho B.C., Reinmuth N., Lee K.H., Luft A., Ahn M.-J., Van Den Heuvel M.M., Cobo M., Vicente D., Smolin A., et al. Durvalumab with or without Tremelimumab vs Standard Chemotherapy in First-line Treatment of Metastatic Non-Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:661–674. doi: 10.1001/jamaoncol.2020.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson M.L., Cho B.C., Luft A., Alatorre-Alexander J., Geater S.L., Laktionov K., Kim S.-W., Ursol G., Hussein M., Lim F.L., et al. Durvalumab with or without Tremelimumab in Combination with Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J. Clin. Oncol. 2022;41:1213–1227. doi: 10.1200/JCO.22.00975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu S., Wu L., Jian H., Chen Y., Wang Q., Fang J., Wang Z., Hu Y., Sun M., Han L., et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): First interim results from a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1167–1179. doi: 10.1016/S1470-2045(22)00382-5. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L., Wang Z., Fang J., Yu Q., Han B., Cang S., Chen G., Mei X., Yang Z., Stefaniak V., et al. Final overall survival data of sintilimab plus pemetrexed and platinum as First-Line treatment for locally advanced or metastatic nonsquamous NSCLC in the Phase 3 ORIENT-11 study. Lung Cancer. 2022;171:56–60. doi: 10.1016/j.lungcan.2022.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhou C., Wu L., Fan Y., Wang Z., Liu L., Chen G., Zhang L., Huang D., Cang S., Yang Z., et al. Sintilimab Plus Platinum and Gemcitabine as First-Line Treatment for Advanced or Metastatic Squamous NSCLC: Results From a Randomized, Double-Blind, Phase 3 Trial (ORIENT-12) J. Thorac. Oncol. 2021;16:1501–1511. doi: 10.1016/j.jtho.2021.04.011. [DOI] [PubMed] [Google Scholar]
- 28.Wang J., Wang Z., Wu L., Li B., Cheng Y., Li X., Wang X., Han L., Wu X., Fan Y., et al. Final progression-free survival, interim overall survival, and biomarker analyses of CHOICE-01: A phase 3 study of toripalimab versus placebo in combination with first-line chemotherapy for advanced NSCLC without EGFR/ALK mutations. J. Clin. Oncol. 2022;40((Suppl. S16)):9028. doi: 10.1200/JCO.2022.40.16_suppl.9028. [DOI] [Google Scholar]
- 29.Zhou C., Wang Z., Sun Y., Cao L., Ma Z., Wu R., Yu Y., Yao W., Chang J., Chen J., et al. Sugemalimab versus placebo, in combination with platinum-based chemotherapy, as first-line treatment of metastatic non-small-cell lung cancer (GEMSTONE-302): Interim and final analyses of a double-blind, randomised, phase 3 clinical trial. Lancet Oncol. 2022;23:220–233. doi: 10.1016/S1470-2045(21)00650-1. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Awad M.M., Gadgeel S.M., Borghaei H., Patnaik A., Yang J.C.-H., Powell S.F., Gentzler R.D., Martins R.G., Stevenson J.P., Altan M., et al. Long-Term Overall Survival From KEYNOTE-021 Cohort G: Pemetrexed and Carboplatin with or without Pembrolizumab as First-Line Therapy for Advanced Nonsquamous NSCLC. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2021;16:162–168. doi: 10.1016/j.jtho.2020.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A., Gottfried M., Peled N., Tafreshi A., Cuffe S., et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019;37:537–546. doi: 10.1200/JCO.18.00149. [DOI] [PubMed] [Google Scholar]
- 34.Paz-Ares L., Vicente D., Tafreshi A., Robinson A., Parra H.S., Mazières J., Hermes B., Cicin I., Medgyasszay B., Rodríguez-Cid J., et al. A Randomized, Placebo-Controlled Trial of Pembrolizumab Plus Chemotherapy in Patients with Metastatic Squamous NSCLC: Protocol-Specified Final Analysis of KEYNOTE-407. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2020;15:1657–1669. doi: 10.1016/j.jtho.2020.06.015. [DOI] [PubMed] [Google Scholar]
- 35.Ren S., Chen J., Xu X., Jiang T., Cheng Y., Chen G., Pan Y., Fang Y., Wang Q., Huang Y., et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022;17:544–557. doi: 10.1016/j.jtho.2021.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Zhou C., Chen G., Huang Y., Zhou J., Lin L., Feng J., Wang Z., Shu Y., Shi J., Hu Y., et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir. Med. 2021;9:305–314. doi: 10.1016/S2213-2600(20)30365-9. [DOI] [PubMed] [Google Scholar]
- 37.Borghaei H., Hellmann M.D., Paz-Ares L.G., Ramalingam S.S., Reck M., O’Byrne K.J., Bhagavatheeswaran P., Nathan F.E., Brahmer J.R. Nivolumab (Nivo) + platinum-doublet chemotherapy (Chemo) vs chemo as first-line (1L) treatment (Tx) for advanced non-small cell lung cancer (NSCLC) with <1% tumor PD-L1 expression: Results from CheckMate 227. J. Clin. Oncol. 2018;36((Suppl. S15)) doi: 10.1200/JCO.2018.36.15_suppl.9001. [DOI] [Google Scholar]
- 38.Sezer A., Kilickap S., Gümüş M., Bondarenko I., Özgüroğlu M., Gogishvili M., Turk H.M., Cicin I., Bentsion D., Gladkov O., et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet. 2021;397:592–604. doi: 10.1016/S0140-6736(21)00228-2. [DOI] [PubMed] [Google Scholar]
- 39.Gogishvili M., Melkadze T., Makharadze T., Giorgadze D., Dvorkin M., Penkov K., Laktionov K., Nemsadze G., Nechaeva M., Rozhkova I., et al. Cemiplimab plus chemotherapy versus chemotherapy alone in non-small cell lung cancer: A randomized, controlled, double-blind phase 3 trial. Nat. Med. 2022;28:2374–2380. doi: 10.1038/s41591-022-01977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 41.West H., McCleod M., Hussein M., Morabito A., Rittmeyer A., Conter H.J., Kopp H.G., Daniel D., McCune S., Mekhail T., et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 42.Jotte R., Cappuzzo F., Vynnychenko I., Stroyakovskiy D., Rodríguez-Abreu D., Hussein M., Soo R., Conter H.J., Kozuki T., Huang K.-C., et al. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2020;15:1351–1360. doi: 10.1016/j.jtho.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 43.Nishio M., Barlesi F., West H., Ball S., Bordoni R., Cobo M., Longeras P.D., Goldschmidt J., Novello S., Orlandi F., et al. Atezolizumab Plus Chemotherapy for First-Line Treatment of Nonsquamous NSCLC: Results from the Randomized Phase 3 IMpower132 Trial. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2021;16:653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 44.Carbone D.P., Reck M., Paz-Ares L., Creelan B., Horn L., Steins M., Felip E., van den Heuvel M.M., Ciuleanu T.E., Badin F., et al. First-Line Nivolumab in Stage IV or Recurrent Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;376:2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herbst R.S., Giaccone G., de Marinis F., Reinmuth N., Vergnenegre A., Barrios C.H., Morise M., Felip E., Andric Z., Geater S., et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020;383:1328–1339. doi: 10.1056/NEJMoa1917346. [DOI] [PubMed] [Google Scholar]
- 46.Reck M., Barlesi F., Yang J.-H., Westeel V., Felip E., Özgüroğlu M., Dols M.C., Sullivan R., Kowalski D., Andric Z., et al. OA15.03 Avelumab vs Chemotherapy for First-line Treatment of Advanced PD-L1+ NSCLC: Primary Analysis from JAVELIN Lung 100. J. Thorac. Oncol. 2022;17:S39. doi: 10.1016/j.jtho.2022.07.072. [DOI] [PubMed] [Google Scholar]
- 47.Sugawara S., Lee J.-S., Kang J.-H., Kim H., Inui N., Hida T., Lee K., Yoshida T., Tanaka H., Yang C.-T., et al. Nivolumab with carboplatin, paclitaxel, and bevacizumab for first-line treatment of advanced nonsquamous non-small-cell lung cancer. Ann. Oncol. 2021;32:1137–1147. doi: 10.1016/j.annonc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 48.Pathak R., Lopes G.D.L., Yu H., Aryal M.R., Ji W., Frumento K.S., Wallis C.J.D., Klaassen Z., Park H.S., Goldberg S.B. Comparative efficacy of chemoimmunotherapy versus immunotherapy for advanced non-small cell lung cancer: A network meta-analysis of randomized trials. Cancer. 2021;127:709–719. doi: 10.1002/cncr.33269. [DOI] [PubMed] [Google Scholar]
- 49.Flieswasser T., Van Loenhout J., Boullosa L.F., Eynde A.V.D., De Waele J., Van Audenaerde J., Lardon F., Smits E., Pauwels P., Jacobs J. Clinically Relevant Chemotherapeutics Have the Ability to Induce Immunogenic Cell Death in Non-Small Cell Lung Cancer. Cells. 2020;9:1474. doi: 10.3390/cells9061474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma Y., Adjemian S., Mattarollo S.R., Yamazaki T., Aymeric L., Yang H., Portela Catani J.P., Hannani D., Duret H., Steegh K., et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity. 2013;38:729–741. doi: 10.1016/j.immuni.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Alizadeh D., Larmonier N. Chemotherapeutic targeting of cancer-induced immunosuppressive cells. Cancer Res. 2014;74:2663–2668. doi: 10.1158/0008-5472.CAN-14-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rojkó L., Reiniger L., Téglási V., Fábián K., Pipek O., Vágvölgyi A., Agócs L., Fillinger J., Kajdácsi Z., Tímár J., et al. Chemotherapy treatment is associated with altered PD-L1 expression in lung cancer patients. J. Cancer Res. Clin. Oncol. 2018;144:1219–1226. doi: 10.1007/s00432-018-2642-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zens P., Bello C., Scherz A., von Gunten M., Ochsenbein A., Schmid R.A., Berezowska S. The effect of neoadjuvant therapy on PD-L1 expression and CD8+lymphocyte density in non-small cell lung cancer. Mod. Pathol. 2022;35:1848–1859. doi: 10.1038/s41379-022-01139-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takamochi K., Hara K., Hayashi T., Kohsaka S., Takahashi F., Suehara Y., Shimokawa M., Suzuki K. Clinical relevance of PD-L2 expression in surgically resected lung adenocarcinoma. Lung Cancer. 2022;168:50–58. doi: 10.1016/j.lungcan.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Inoue Y., Yoshimura K., Mori K., Kurabe N., Kahyo T., Mori H., Kawase A., Tanahashi M., Ogawa H., Inui N., et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget. 2016;7:32113–32128. doi: 10.18632/oncotarget.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumitomo R., Huang C.L., Fujita M., Cho H., Date H. Differential expression of PD-L1 and PD-L2 is associated with the tumor microenvironment of TILs and M2 TAMs and tumor differentiation in non-small cell lung cancer. Oncol. Rep. 2022;47:73. doi: 10.3892/or.2022.8284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Okadome K., Baba Y., Yasuda-Yoshihara N., Nomoto D., Yagi T., Toihata T., Ogawa K., Sawayama H., Ishimoto T., Iwatsuki M., et al. PD-L1 and PD-L2 expression status in relation to chemotherapy in primary and metastatic esophageal squamous cell carcinoma. Cancer Sci. 2022;113:399–410. doi: 10.1111/cas.15198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saghatchian M., Fizazi K., Borel C., Ducreux M., Ruffié P., Le Chevalier T., Théodore C. Carcinoma of an unknown primary site: A chemotherapy strategy based on histological differentiation—Results of a prospective study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2001;12:535–540. doi: 10.1023/A:1011129429499. [DOI] [PubMed] [Google Scholar]
- 59.Saijo N., Niitani H., Tominaga K., Eguchi K., Koketsu H., Fujino T., Ishikawa S. Comparison of survival in nonresected well differentiated and poorly differentiated adenocarcinoma of the lung. J. Cancer Res. Clin. Oncol. 1980;97:71–79. doi: 10.1007/BF00411280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Theelen W.S.M.E., Peulen H.M.U., Lalezari F., van der Noort V., de Vries J.F., Aerts J.G.J.V., Dumoulin D.W., Bahce I., Niemeijer A.N., de Langen A.J., et al. Effect of Pembrolizumab after Stereotactic Body Radiotherapy vs Pembrolizumab Alone on Tumor Response in Patients with Advanced Non-Small Cell Lung Cancer: Results of the PEMBRO-RT Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:1276–1282. doi: 10.1001/jamaoncol.2019.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Welsh J., Menon H., Chen D., Verma V., Tang C., Altan M., Hess K., de Groot P., Nguyen Q.-N., Varghese R., et al. Pembrolizumab with or without radiation therapy for metastatic non-small cell lung cancer: A randomized phase I/II trial. J. Immunother. Cancer. 2020;8:e001001. doi: 10.1136/jitc-2020-001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Theelen W.S.M.E., Chen D., Verma V., Hobbs B.P., Peulen H.M.U., Aerts J.G.J.V., Bahce I., Niemeijer A.L.N., Chang J.Y., de Groot P.M., et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir. Med. 2021;9:467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 63.Liu S.-Y., Dong S., Liao R.-Q., Jiang B., Zhang J.-T., Lin J.-T., Zhang S., Yang J., Nie Q., Yang X., et al. LBA2 Phase II study of PD-L1 expression guidance on neoadjuvant (NA) nivolumab (Nivo) monotherapy with or without platinum-doublet chemotherapy in resectable NSCLC. Immuno-Oncol. Technol. 2022;16:1. doi: 10.1016/j.iotech.2022.100363. [DOI] [Google Scholar]
- 64.Jabbour S.K., Lee K.H., Frost N., Breder V., Kowalski D.M., Pollock T., Levchenko E., Reguart N., Martinez-Marti A., Houghton B., et al. Pembrolizumab Plus Concurrent Chemoradiation Therapy in Patients with Unresectable, Locally Advanced, Stage III Non-Small Cell Lung Cancer: The Phase 2 KEYNOTE-799 Nonrandomized Trial. JAMA Oncol. 2021;7:1351–1359. doi: 10.1001/jamaoncol.2021.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Antonia S.J., Villegas A., Daniel D., Vicente D., Murakami S., Hui R., Yokoi T., Chiappori A., Lee K.H., de Wit M., et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 66.Ricciuti B., Elkrief A., Alessi J.V.M., Wang X., Barrichello A.P.D.C., Pecci F., Lamberti G., Lindsay J., Sharma B., Felt K., et al. Three-year outcomes and correlative analyses in patients with non-small cell lung cancer (NSCLC) and a very high PD-L1 tumor proportion score (TPS) ≥ 90% treated with first-line pembrolizumab. J. Clin. Oncol. 2022;40((Suppl. S16)):9043. doi: 10.1200/JCO.2022.40.16_suppl.9043. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used in this network meta-analysis are collected from published clinical trials.