Abstract
Background:
Delineation of the cortical anomalies underpinning Attention-Deficit Hyperactivity Disorder (ADHD) can powerfully inform pathophysiological models. We previously found that ADHD is characterized by a delayed maturation of prefrontal cortical thickness. We now ask if this extends to the maturation of cortical surface area and gyrification.
Methods.
234 children with attention-deficit hyperactivity disorder and 231 typically developing children with 837 neuroanatomic magnetic resonance images, acquired longitudinally. We defined the developmental trajectories of cortical surfaces and gyrification and the sequence of cortical maturation, as indexed by the age at which each cortical vertex attained its peak surface area.
Results:
In both groups, the maturation of cortical surface area progressed in centripetal waves, both lateral (starting at the central sulcus and fronto-polar regions, sweeping towards the mid and superior frontal gyrus) and medial (descending down the medial prefrontal cortex, towards the cingulate gyrus). However, the surface area developmental trajectory was delayed in ADHD. For the right prefrontal cortex, the median age by which 50% of cortical vertices attained peak area was 14.6 years (SE 0.03) in ADHD, significantly later than in typically developing group at 12.7 yrs (SE 0.03) (log-rank test χ(1)2= 1300, p<0.00001). Similar, but less pronounced delay was found in the left hemispheric lobes. There were no such diagnostic differences in the developmental trajectories of cortical gyrification.
Conclusions.
The congruent delay in cortical thickness and surface area directs attention away from processes that selectively affect one cortical component towards mechanisms controlling the maturation of multiple cortical dimensions.
Keywords: Attention Deficit Hyperactivity Disorder, cerebral cortex, cortical gyrification, magnetic resonance imaging, typical development, structural neuroimaging
Introduction
Recent advances in neuroimaging acquisition and analytic techniques allow an increasingly detailed delineation of the cortical anomalies that underpin neuropsychiatric disorders. We previously reported that ADHD is partly characterized by a delay in the age at which the phase of childhood increase in cortical thickness gives way to an adolescent phase of cortical thinning (1). The mean age at which this peak cortical thickness was attained in the cerebrum was around seven years in typically developing children whereas in children with ADHD peak cortical thickness was reached around ten years, with the delay most prominent in lateral prefrontal cortex. We now ask whether a similar developmental delay is found in the other principal determinants of cortical structure, namely cortical surface area and cortical curvature, or gyrification.
It is by no means certain that a delay in the maturation of one component of cortical morphology entails a delay in all others. Dissociations between morphometric cortical properties have been reported in the few studies of neuropsychiatric disorders that have simultaneously considered the composite dimensions of the cortex. Thus reduced cortical surface area but intact cortical thickness have been reported in dyslexia, ADHD and autism (2–4) and the reverse in Alzheimer’s disease (5). Additionally, there is accumulating evidence that these morphometric properties may be partly biologically distinct as they can be manipulated independently in non-human animals (6). Human twin studies also find largely unique genetic factors to influence cortical surface area and thickness (7–9) and in vivo neuroimaging studies of healthy young adults suggest that while both cortical thickness and area are organized as networks, these networks have quite distinct organizational properties (10).
It is also equally true that cortical thickness, surface area and gyrification are unlikely to be completely independent. The demonstration of some unique underlying cellular processes does not preclude many common ones. Likewise twin studies report some common genetic factors controlling surface area and thickness, particularly in the orbitofrontal and pre and post-central prefrontal gyri (7). Finally, it should be noted that if a pathological process affects more than one dimension of cortical structure, then it would be more readily detected by poly-dimensional metrics that amplify the magnitude of anomalies. Thus findings of anomalies in surface area (a two dimensional construct) but intact cortical thickness (a one-dimensional entity) may reflect lack of power to detect disorder related change occurring in the unidimensional cortical thickness, rather than truly intact thickness.
To date studies of the development of cortical surface area in typical and atypical development have been mainly cross-sectional and conducted largely at the level of entire lobes or regions of interest (5, 11). Here, we define trajectories of cortical surface area development at the level of over 80,000 vertices throughout the cerebral cortex using longitudinal data in children with and without ADHD. This level of spatiotemporal resolution allows us to determine if the regional delay in cortical thickness development in ADHD extends to the development of surface area. Answering this question may guide future searches for etiological processes.
Methods
Participants:
Two hundred and four three children with ADHD participated. Details of this cohort are elsewhere (1, 12, 13), but in brief, diagnosis was based on the Parent Diagnostic Interview for Children and Adolescents (14), Conner’s Teacher Rating Scales (15), and the Teacher Report Form. 217 had combined type ADHD at baseline, 12 had inattentive subtype and 5 had hyperactive/impulsive subtype. Numbers of subjects at each wave of scanning and their age, sex and IQ are given in Table 1. The institutional review board of the National Institutes of Health approved the research protocol, and written informed consent and assent to participate in the study were obtained from parents and children, respectively. At study entry, 68% of the 208 on whom medication details could be confirmed were taking psychostimulants. This proportion held relatively constant throughout the remaining waves of scanning: 67% were medicated at time 2, and 66% at time 3. At study entry, 85% were of the medicated subjects were taking methylphenidate preparations.
Table 1:
Demographic details and number of participants at each wave of scanning.
| ADHD | Typically developing | Test of significance | |
|---|---|---|---|
| Total number (Male %) | 234 (151– 65%) | 231-(148– 64%) | χ2=0.005, p=0.95 |
| Scan 1 | N=234 10.2 (3.3) | N=231 10.6 (3.6) | t(463) = 1.2, P=0.22 |
| Scan 2 | N=115 12.8 (3.6) | N=121 13.4 (4.2) | t(234) = 1.0, P=0.29 |
| Scan 3 | N=60 15.1 (3.9) | N=46 13.8 (4.2) | t(104) = 1.7, P=0.09 |
| Scan 4 | N=17 17.7 (4.0) | N=13 16.1 (3.2) | t(28) = 1.2, P=0.23 |
| IQ | 113 (15) | 113 (15) | t(463)=0.02, p=0.98 |
The typically developing subjects were part of the NIH Intramural project of typical brain development, which has been reported upon previously (16, 17). The group was matched with the ADHD group on sex and IQ and number of scans. Each subject completed the Childhood Behavior Checklist as a screening tool and then underwent a structured diagnostic interview by a child psychiatrist to rule out any psychiatric or neurological diagnoses (18). The numbers of subjects at each wave of scanning and their age is given in Table 1.
Neuroimaging.
All children had neuroanatomic magnetic resonance imaging on the same 1.5-T General Electric Signa scanner (Milwaukee, WI) throughout the study. T1-weighted images with contiguous 1.5-mm slices in the axial plane were obtained using 3-dimensional spoiled gradient recalled echo in the steady state. Imaging parameters were echo time of 5 ms, repetition time of 24 ms, flip angle of 45°, acquisition matrix of 256 × 192, number of excitations equals 1, and 24 cm field of view. The images were processed using the cortical surface extraction pipeline CIVET developed at the Montreal Neurological Institute (19) The native MRI scans were first masked using Brain Extraction Tool (20) then registered into standardized stereotaxic space (MNI-ICBM152 non-linear 6th generation symmetric target (21) using a 9-parameter linear transformation (22) and corrected for non-uniformity artifacts (23). The registered and corrected volumes were segmented into white matter, gray matter, cerebrospinal fluid and background using an advanced neural net classifier (24, 25). The cortical surfaces were extracted by hemispheres using the Constrained Laplacian Anatomic Segmentation Using Proximities to generate surface meshes representing the white matter and grey matter interfaces (26). A 30mm surface blurring algorithm was used to reduce noise in the thickness and area measurements (27). Cortical surface area was measured at the middle cortical surface, which lies at the geometric center between the inner and outer cortical surfaces and thus provides a relatively unbiased representation of sulcal versus gyral regions (28, 29). This gives the surface area of every vertex in the surface mesh (40962 in each hemisphere) which can be summed to give the lobar surface areas. The gyrification index was calculated as the ratio between the total surface area and exposed cortical surface or convex hull area (30)
Statistical modeling.
First we determined developmental trajectories for the metrics of surface area and the gyrification index using mixed model regression analysis. This technique was used as our unbalanced longitudinal data contains both multiple observations per participant measured at different and irregular time periods and single observations per participant. The classification of developmental trajectories was based upon a step-down model selection procedure: at each cortical vertex we modeled cortical thickness using a mixed-effects polynomial regression model, testing for cubic, quadratic and linear age effects. If the cubic age effect was not-significant at p<0.05, it was removed and we stepped down to the quadratic model and so on. In this way, we were able to classify the development of each cortical measure as being best explained by a cubic, quadratic or linear function of age. For metrics where a quadratic models was appropriate the jth metric of the ith individual was modeled as
where dj is a random effect modeling within-person dependence; the intercept and β terms are fixed effects, and eij represents the residual error. Where a quadratic fit was appropriate, the age at which each vertex attained its peak surface area was calculated groups from the derivatives of the developmental curves and illustrated through dynamic time-lapse sequences (“movies”). Kaplan–Meier curves were constructed showing the proportion of cortical vertices that had reached peak surface area throughout the age range covered. The significance of the group difference in the mean age by which half of cortical vertices had attained their peak surface area was calculated by using the log-rank (Mantel–Cox) test.
Results.
Surface area.
At the time of the first scan (mean age 10.4 years, SD 3.1 years) both total and lobar surface areas were significantly decreased in the ADHD group- see Table 2. Analyses at the level of each vertex showed that surface reduction was most pronounced bilaterally in the prefrontal cortex (especially the medial wall, the lateral superior, middle and polar frontal regions) the right lateral temporal cortex, and left medial temporal cortex, extending to posterior left medial wall (fusiform, lingual gryi and cuneus).
Table 2:
Baseline values of total and lobar surface areas and gyrification index
| ADHD | Typically developing | Test of significance t(df 463) p value | |
|---|---|---|---|
| Right hemispheric surfaces | |||
| Total surface area | 99456 (7579) | 102480 (7651) | t=4.3, p<0.0001 |
| Frontal | 36291 (3097) | 37472 (3302) | t=4.1, p<0.0001 |
| Temporal | 24835 (2119) | 25523 (2112) | t=3.5, p=0.001 |
| Parietal | 21549 (2148) | 22188 (2360) | t=3.1, p=0.002 |
| Occipital | 12137 (1399) | 12527 (1438) | t=2.9, p=0.003 |
| Left hemispheric surfaces | |||
| Total surface area | 99230 (7579) | 102103 (7594) | t=4.1, p<0.0001 |
| Frontal | 36556 (3302) | 37537 (3212) | t=3.2, p=0.001 |
| Temporal | 24747 (2180) | 25441 (2240) | t=3.4, p=0.001 |
| Parietal | 21679 (1973) | 22429 (2360) | t=3.7, p=0.0002 |
| Occipital | 11455 (1399) | 11800 (1330) | t=2.7, p=0.006 |
| Gryification | |||
| Right gyrification | 2.43 (0.08) | 2.44 (0.07) | t=1.5, p=0.13 |
| Left gyrification | 2.41 (0.08) | 2.42 (0.07) | t=1.5, p=0.13 |
We then mapped the developmental trajectories of surface area in both groups. At the lobar level in typically developing children, surface areas showed an initial childhood increase followed by an adolescent phase of decrease. The shape of the developmental curve for the ADHD group was similar but shifted along the age axis. Figure 1 shows results for the right hemispheric lobes and Figure S1 in Supplement 1 for the left hemisphere
Figure 1:
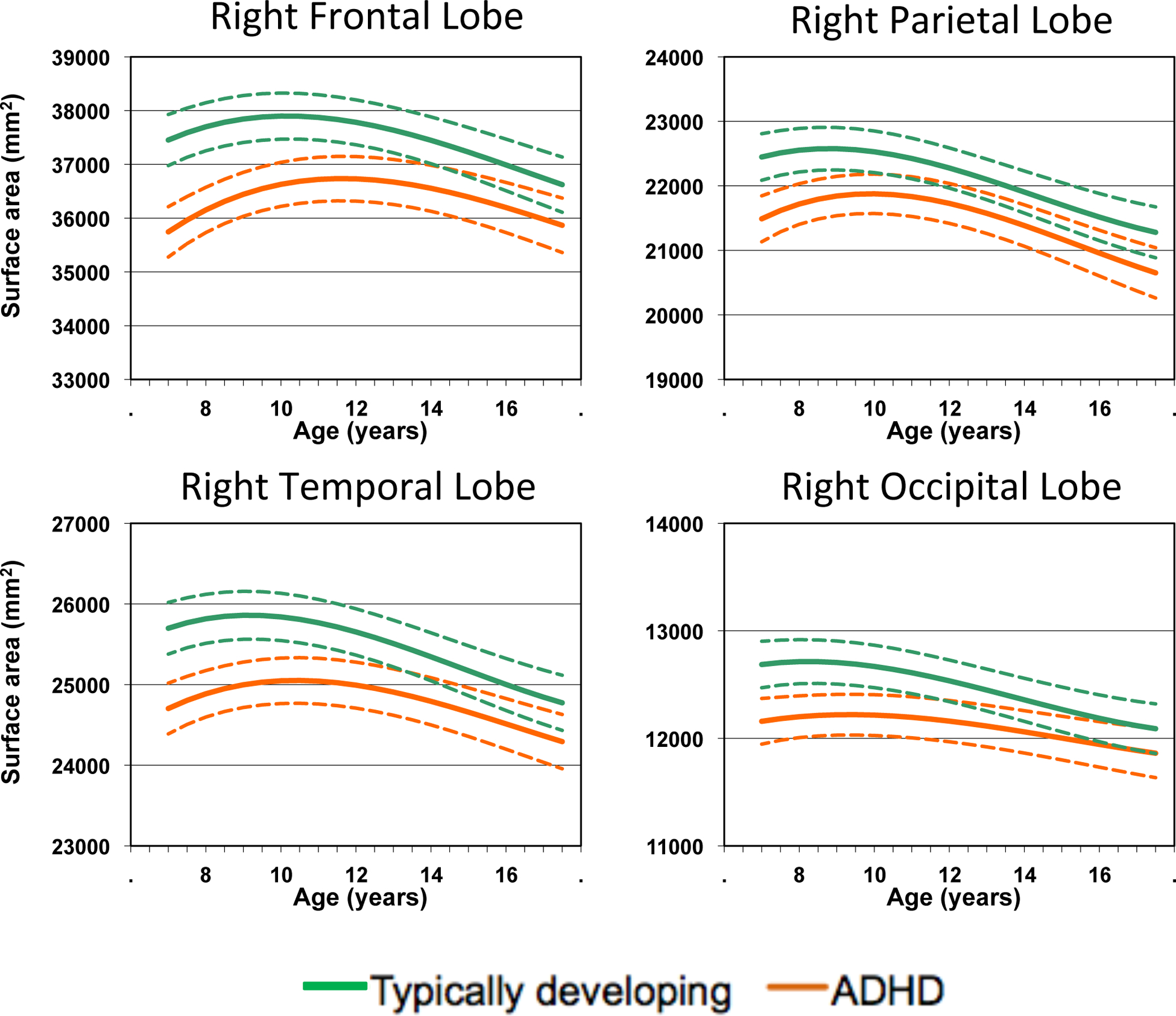
Developmental trajectories of right hemispheric lobar surface areas. 95% confidence intervals for the estimate are given in the dotted lines.
The trajectories were then mapped at the level of >80,000 vertices to define more precisely the sequence of maturation, shown as dynamic time-lapse sequences (Movies 1 and 2; see online supplements). In both typical development and ADHD there was a similar regional progression of maturation. The parietal and occipital cortex matured early, as indexed by surface area, with many regions attaining their peak surface area prior to the age of 6, and the remainder reaching this marker by around age 11. Maturation of the prefrontal cortex occurred earliest the pre and post central frontal gyri closely followed by fronto-polar regions. A wave of maturation then spread across the middle frontal gyrus with the last areas to attain peak surface area being the superior frontal gyrus and a smaller region of the inferior frontal gyrus. The temporal cortex matured in line with the prefrontal cortex, with some very early maturing areas (e.g. the angular gyrus) followed by the middle, polar and then superior temporal gyri. Medially, some regions matured early (such as the medial orbitofrontal cortex) followed by a centripetal wave of maturation down the medial prefrontal cortical wall, with the anterior regions of the cingulate gyrus attaining their peak surface area last.
While the order or sequences was similar there were marked diagnostic differences in the timing. At a lobar level, Kaplan- Meier analyses showed that the mean age by which 50% of the cortical vertices had attained peak surface area in the right prefrontal for the ADHD group was 14.6 years (SE 0.03), which was significantly later than the mean age of 12.7 years (SE 0.03) for the typically developing controls (log-rank test χ(1)2= 1300, p<0.00001). For the left prefrontal cortex, the corresponding values were 13.5 years (SE 0.03) for the ADHD group and 13.2 yrs (SE 0.03) for the typically developing group (log-rank test χ(1)2= 17, p<0.001). For the parietal cortex there was similar delay in both hemispheres - mean age of peak surface attained on right at 12.5 yrs (SE 0.03) for ADHD and 11.1 yrs (SE 0.03) for the typically developing group (log-rank test χ(1)2= 815, p<0.0001); for the left, 12.2 yrs (SE 0.06) for ADHD and 9.9 yrs (SE 0.05) for typical development (χ(1)2= 1075, p<0.0001). For the temporal lobe, results were similar for both hemispheres and the mean age of attaining peak surface area on the right was 13.9 yrs (SE 0.04) for the ADHD group and 13.2 rys (SE 0.04) for the typically developing group (χ(1)2= 32, p<0.0001); for the left, 12.6 yrs (SE0.04) for ADHD and 10.9 rys (SE 0.05) for typical development (χ(1)2= 249, p<0.0001). There was no delay for the occipital cortex, with the mean age of peak surface area in ADHD peak on the right of 12.7 yrs (SE 0.10) and 12.5 yrs (SE 0.04) for the typically developing group and on the left, 12.2 yrs (SE 0.07) for ADHD and 12.4 yrs (SE 0.06) for typical development (χ(1)2=2, p>0.05).
Figure 2 shows the magnitude of the delay in the ADHD group at a sublobar level. Delay was most prominent in the anterolateral prefrontal gyri bilaterally, especially on the right. Additionally, delay was prominent bilaterally in the medial temporal gryi, in the right postcentral and middle temporal gyri, and left supramarginal gyrus.
Figure 2:
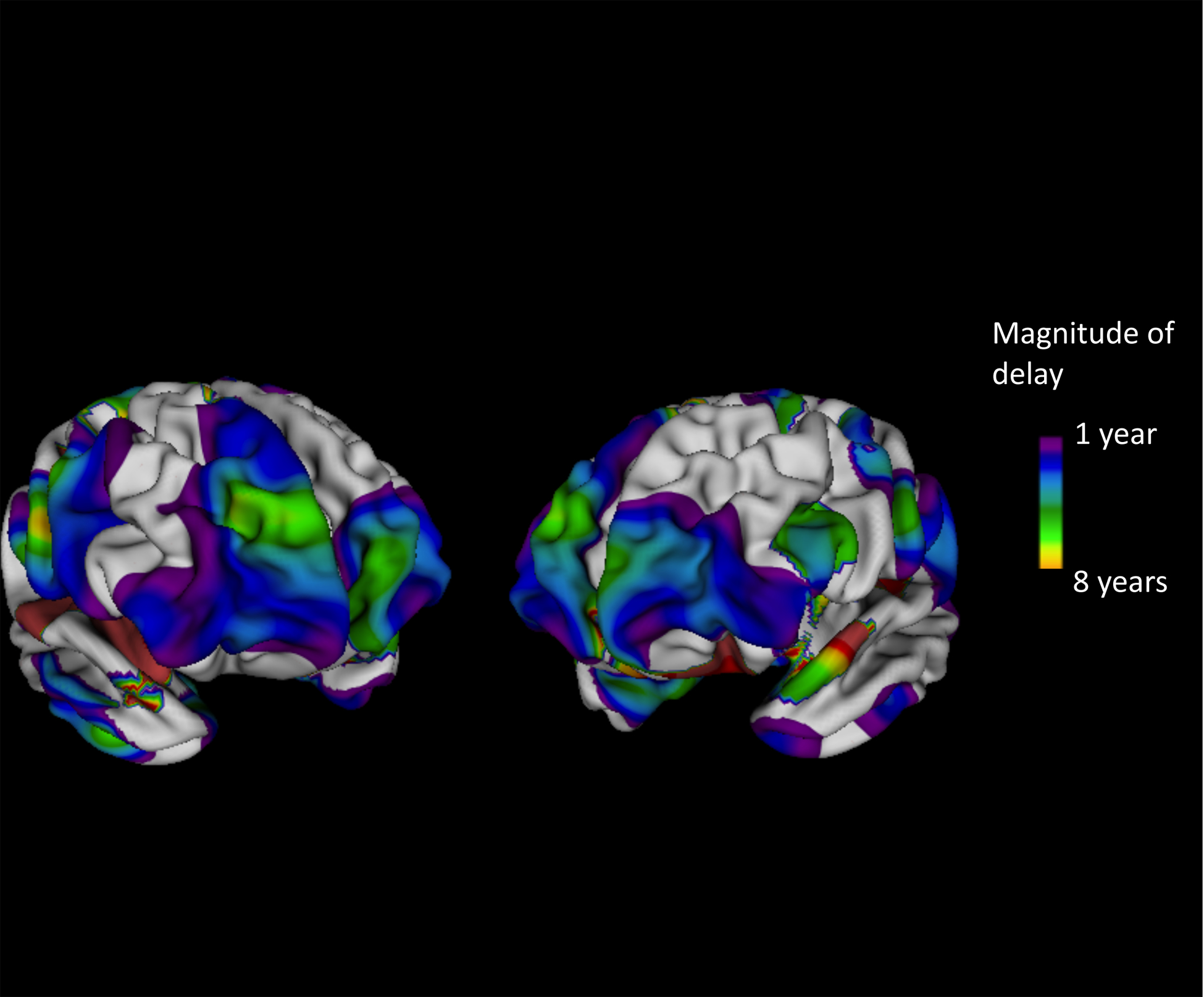
Regions where age of attaining peak surface area was delayed by more than one year in ADHD compared to typically developing participants.
The results for surface area are compared against data for cortical thickness maturation in the same cohort, taken from our earlier report in Figure 3 (Shaw et al 2007). Two features are clear. First, surface area tended to attain its peak dimensions later then cortical thickness for both groups. Second, the diagnostic delay is present for both prefrontal cortical thickness and cortical surface area.
Figure 3:
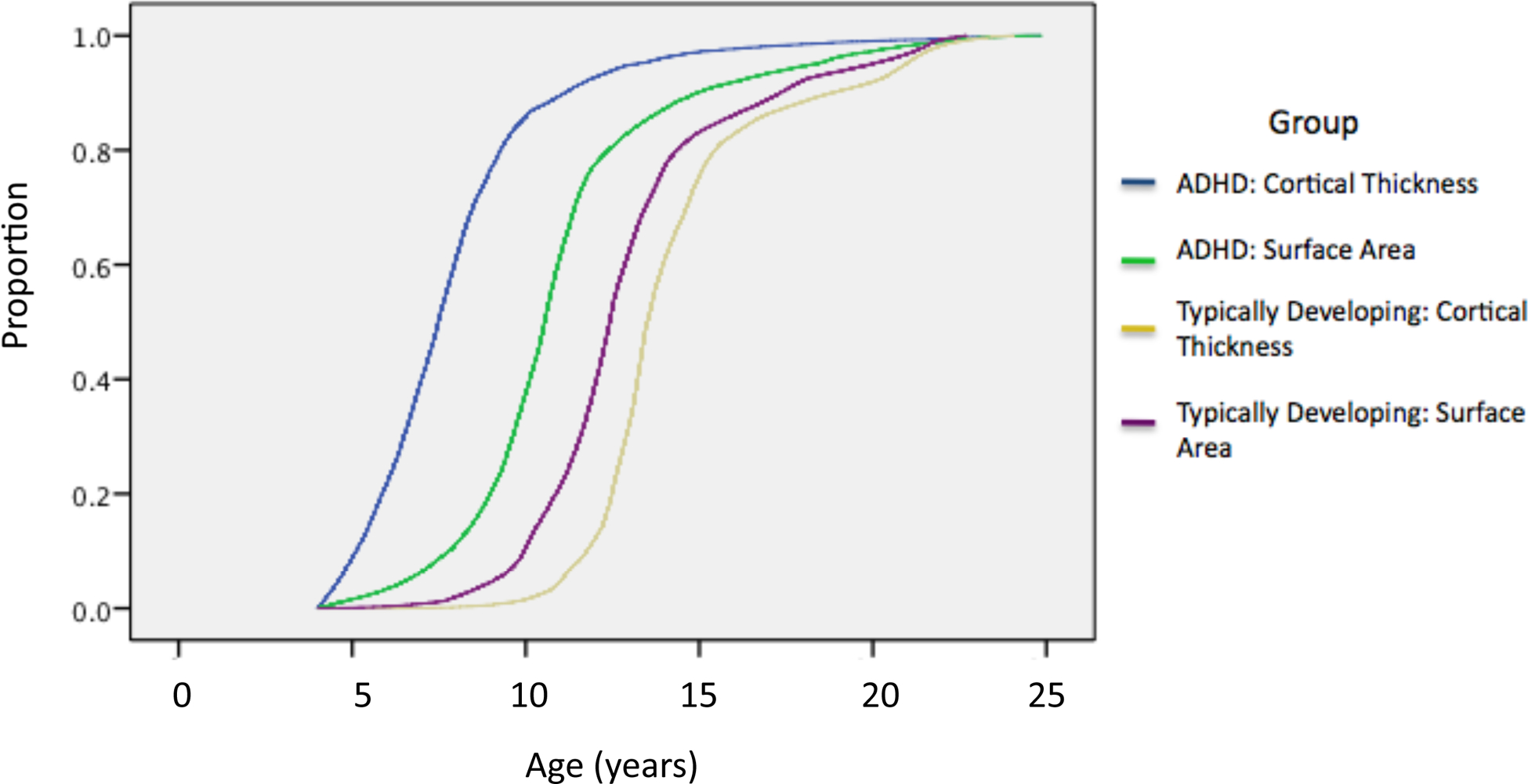
Kaplan–Meier curves showing the proportion of cortical vertices that attain peak thickness and peak surface area at each age for both the ADHD and typically developing groups. For both cortical thickness and surface area the age at which 50% of the cortical vertices attained peak dimensions was significantly later in ADHD (at P<1.0‒4).
The trajectory of the convex hull in ADHD showed a later peak and had a lower value than in typical development, similar to the trajectory for surface area – see Figure 4 for the right hemisphere and Figure S2 in Supplement 1 for the left hemisphere. As a result the trajectory of the gyrification index in ADHD, which is the ratio of convex hull to surface area did not differ from typical development (right F=0.39, p=0.76; left F=2.0, p=0.13). The developmental trajectories also showed that peak gyrification was attained for both groups before the start of the age period covered. Finally, at the time of study entry, the gyrification index did not differ significantly between the groups (see Table 2).
Figure 4:
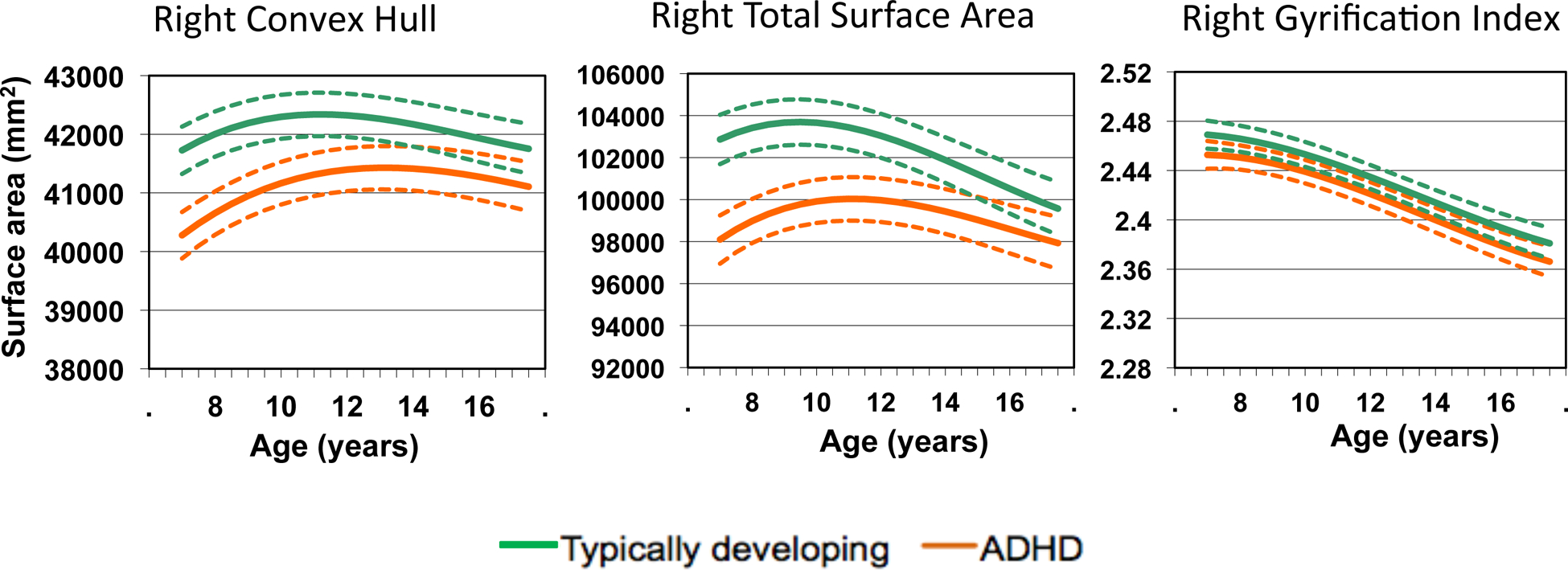
Developmental trajectories for the right hemispheric convex hull, total surface area and gyrification index. Both the convex hull and surface area curves show a similar delay in ADHD and thus the gyrification index –which is the ratio of the surface area to convex hull- does not differ diagnostically. Results for the left hemisphere are very similar.
Discussion.
There is a delay in the maturation of cortical surface area in ADHD which mirrors the delayed maturation of cortical thickness we previously reported (1). This suggests that ADHD is not on the list of neuropsychiatric disorders which have deficits of either just surface area or cortical thickness, such as dyslexia and autism (2, 3). This in turn has implications for the mechanisms that underpin ADHD. For example, deficits in cortical thickness but not surface area have been linked with mutations of Pax6, Ngn2, Id4 and other genes which impact on the abundance of intermediate progenitor cells which are critical for early neurogenesis (6). Our finding of a congruent delay in both cortical thickness and surface area in ADHD points instead to a more global perturbation in the mechanisms which guide cortical maturation. We can only speculate on the cellular mechanisms which might be responsible, although plausible candidates include neurotrophins, essential for the proliferation, differentiation, and survival of neuronal and nonneuronal cells. Indeed, polymorphisms within the brain-derived neurotrophic factor and nerve growth-factor 3 genes have already been linked with ADHD (31, 32).
Our finding runs counter to a previous report of reduced surface area and gyrification but intact cortical thickness in ADHD (4). Several factors may explain the discrepancy. The earlier study was cross-sectional, it had a smaller sample size and may thus have not been powered to detect change in a unidimensional metric such as cortical thickness. There are also some differences in the studies’ inclusion criteria, levels of medication use and the methods used to define surface area.
This study is the first to map the maturation of cortical surfaces at an exquisite level of spatiotemporal resolution in typically developing children. We find that posterior, parieto-occipital cortex matures earlier than more anterior regions. Prefrontal cortical surface area maturation comprises of centripetal waves, both lateral (starting at the central sulcus and fronto-polar regions, sweeping towards the mid and superior frontal gyrus) and medial (descending down the medial prefrontal cortex, towards the cingulate gyrus). This sequence of development resembles that found for cortical thickness (33, 34) highlighting the similarities in the maturation of cortical thickness and surface area.
At a lobar level, delay was found in the ADHD group in the frontal, temporal and parietal, but not occipital lobes. However, the pattern of delay was highly regional, being most marked in the anterior frontal gyri, particularly on the right. Structural cortical anomalies of the prefrontal regions have been frequently reported by others, particularly studies which have used the metric of cortical thickness. Four independent groups have reported thinning of the cortex in children with ADHD in prefrontal regions, particularly the anterior portions of the superior, middle and inferior frontal gyri (12, 35–37). This cortical thinning has been further linked with impairment in response inhibition, one of the most consistently implicated core cognitive deficits in ADHD (35). It will be interesting to see if a similar consensus emerges on reduction in cortical surface area for the anterolateral cortex. Additionally, in our study delay in attaining peak surface area in ADHD was prominent bilaterally in the medial temporal gryi, in the right postcentral and middle temporal gyri, and left supramarginal gyrus. These areas were less expected, although it is notable that cortical anomalies of the medial temporal cortex and underlying amygdala and hippocampus are being increasingly recognized, and may play a role in problems with the regulation of emotion found in many people who have ADHD (38–40). Task related functional imaging studies mostly find frontal hypoactivity in ADHD, affecting anterior cingulate, dorsolateral prefrontal, and inferior prefrontal cortices, but extending to portions of parietal cortex and temporal cortex, depending in the nature of the task being performed (41–48). Studies examining cortical activity during the resting state in ADHD likewise find a distributed pattern of anomalies, encompassing the cingulate, precuneus as well as lateral prefrontal cortex (49, 50). The distributed nature of task-related and resting state functional hypoactivation is congruent with the distributed nature of the structural delay we report.
Most data in this study (92% of scans) were from individuals under the age of 18. We were able to fit a quadratic growth curve to this data, which reflects a childhood phase of increase followed by an adolescent phase of decrease in cortical dimensions. Such modeling is helpful in capturing early milestones of cortical development, such as the age of attaining peak dimensions. However, it is not suited to characterization of later milestones, such as the age at which the cortex settles into relatively stable adult dimensions. More data from adults over 18 and perhaps a different analytic approach is needed to determine these adult milestones. Such adult data would allow is to determine whether by adulthood the degrees to which normalization occurs, or whether the delay in attaining peak dimensions is carried forward into persistent adult structural cortical deficits. Our developmental trajectories for surface area (Figure 1) suggest that some degree of normalization may occur, although extrapolation of the curves beyond the age range covered is not possible, and more data on adult ADHD is greatly needed.
It is interesting to speculate on the possible clinical significance that normalization as opposed to persistence of early cortical delay and deficits. One possibility is that normalization might accompany clinical remission, whereas persistent deficits might drive ADHD that lasts into adulthood. There is some evidence that this may be the case. In a study linking cerebellar growth with clinical course, we found that persistence of ADHD was associated with a progressive divergence away from the trajectory of typical development, whereas clinical improvement was associated with trajectories that resembled those of typical development (51). This study however dealt only with adolescence and there was little data on adult ADHD. In a recent cross-sectional study, Proal and colleagues found that adults with ADHD showed cortical thinning in the posterior cortical regions, whereas adults who had recovered from their childhood symptoms had no significant deficits relative to controls (52). These findings are compatible with the concept that persistence of ADHD into adulthood is underpinned by abnormal cortical development, which may manifest in childhood as delay in attaining an early milestone of development.
The typically developing and ADHD groups did not differ significantly in the degree of gyrification at study entry or in its developmental trajectory. This reflects the fact that both surface area and the exposed cortical surface or convex hull were similarly reduced in ADHD and had similarly delayed trajectories. Thus the gyrification index, which is the ratio of surface area and convex hull, did not differ from typical dimensions. It is also noteworthy that the gyrification index attained its peak value before the onset of the age period covered, in line with previous reports of early maturation of this measure (11, 53).
There are several limitations to this study. The majority of the subjects in the ADHD group were medicated at some stage and there was insufficient power to examine neurodevelopmental trajectories in medication naïve subjects only. However, several cross-sectional and one longitudinal observational study find that treatment with psychostimulants is associated with normalization of cortical structural deficits, making the finding of anomalies in surface area less likely to be attributable to medication effects (13, 54–56). This has been confirmed by a recent meta-analyses of voxel-based morphometric studies which also suggested that psychostimulant treatment was associated with more normative dimensions which would obscure any diagnostic effect (57). Our cohort was largely recruited from an affluent socioeconomic region, was free of major comorbidities beyond oppositional defiant disorder and had a high IQ, all factors which may limit the generalizability of the findings. We were unable to test for sex effects, as splitting the sample into separate sexes results in a loss of power to detect higher order effects of age on the development of most of the cortex, particularly in females, who constituted around 30% of the sample. There is evidence that sex effects may be important in ADHD, affecting patterns of neural activity (58, 59) and studies into sexual dimorphism in brain structure in ADHD is a priority when sufficient sample sizes are attained.
Our finding of congruent delays in the maturation of cortical thickness and surface directs attention towards mechanisms that may control the maturation of multiple dimensions of cortical structure as potentially pivotal in the pathogenesis of ADHD.
Supplementary Material
Disclosures and acknowledgements:
Dr. Shaw has received an unrestricted travel grant from Jansen-Cilag to attend the Nordic Psychiatry Assembly on attention-deficit/hyperactivity disorder in 2010 and from Jansen-Cilag to attend the Biennial Neuroscience Multidisciplinary Meeting in Madrid 2011. All other authors reported no biomedical financial interests or potential conflicts of interest. Supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References.
- 1.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. (2007): Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 104:19649–19654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frye R, Liederman J, Malmberg B, McLean J, Strickland D, Beauchamp M (2010): Surface Area Accounts for the Relation of Gray Matter Volume to Reading-Related Skills and History of Dyslexia Cereb Cortex. 20:2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raznahan A, Toro R, Daly E, Robertson D, Murphy C, Deeley Q, et al. (2010): Cortical Anatomy in Autism Spectrum Disorder: An In Vivo MRI Study on the Effect of Age. Cereb Cortex. 20:1332–1340. [DOI] [PubMed] [Google Scholar]
- 4.Wolosin SM, Richardson ME, Hennessey JG, Denckla MB, Mostofsky SH (2009): Abnormal cerebral cortex structure in children with ADHD. Hum Brain Mapp. 30:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickerson BC, Feczko E, Augustinack JC, Pacheco J, Morris JC, Fischl B, et al. (2009): Differential effects of aging and Alzheimer’s disease on medial temporal lobe cortical thickness and surface area. Neurobiology of Aging. 30:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pontious A, Kowalczyk T, Englund C, Hevner RF (2008): Role of intermediate progenitor cells in cerebral cortex development. Developmental Neuroscience. 30:24–32. [DOI] [PubMed] [Google Scholar]
- 7.Panizzon MS, Fennema-Notestine C, Eyler LT, Jernigan TL, Prom-Wormley E, Neale M, et al. (2009): Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, et al. (2009): Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. NeuroImage. 53:1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eyler LT, Prom-Wormley E, Panizzon MS, Kaup AR, Fennema-Notestine C, Neale MC, et al. (2011): Genetic and Environmental Contributions to Regional Cortical Surface Area in Humans: A Magnetic Resonance Imaging Twin Study. Cereb Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanabria-Diaz G, Melie-García L, Iturria-Medina Y, Alemán-Gómez Y, Hernández-González G, Valdés-Urrutia L, et al. (2010): Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. NeuroImage. 50:1497–1510. [DOI] [PubMed] [Google Scholar]
- 11.Raznahan A, Shaw P, Lalonde F, Stockman M, Wallace GL, Greenstein D, et al. (2011): How Does Your Cortex Grow? The Journal of Neuroscience. 31:7174–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, et al. (2006): Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 63:540–549. [DOI] [PubMed] [Google Scholar]
- 13.Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, et al. (2002): Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 288:1740–1748. [DOI] [PubMed] [Google Scholar]
- 14.Reich W (2000): Diagnostic interview for children and adolescents (DICA). J Am Acad Child Adolesc Psychiatry. 39:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Werry JS, Sprague RL, Cohen MN (1975): Conners’ Teacher Rating Scale for use in drug studies with children--an empirical study. J Abnorm Child Psychol. 3:217–229. [DOI] [PubMed] [Google Scholar]
- 16.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. (1999): Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 2:861–863. [DOI] [PubMed] [Google Scholar]
- 17.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, et al. (2006): Intellectual ability and cortical development in children and adolescents. Nature. 440:676–679. [DOI] [PubMed] [Google Scholar]
- 18.Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, et al. (1996): Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cereb Cortex. 6:551–560. [DOI] [PubMed] [Google Scholar]
- 19.AD-Dab’bagh Y (2006): The CIVET image-processing environment: A fully automated comprehensive pipeline for anatomical neuroimaging research. 12th Annual Meeting of the Organization for Human Brain Mapping. Florence, Italy. [Google Scholar]
- 20.Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp. 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL (2006): Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv. 9:58–66. [DOI] [PubMed] [Google Scholar]
- 22.Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 18:192–205. [PubMed] [Google Scholar]
- 23.Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 17:87–97. [DOI] [PubMed] [Google Scholar]
- 24.Zijdenbos AP, Forghani R, Evans AC (2002): Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Transactions on Medical Imaging. 21:1280–1291. [DOI] [PubMed] [Google Scholar]
- 25.Tohka J, Zijdenbos A, Evans A (2004): Fast and robust parameter estimation for statistical partial volume models in brain MRI. Neuroimage. 23:84–97. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Singh V, Lee JK, Lerch J, Ad-Dab’bagh Y, MacDonald D, et al. (2005): Automated 3-D extraction and evaluation of the inner and outer cortical surfaces using a Laplacian map and partial volume effect classification. NeuroImage. 27:210–221. [DOI] [PubMed] [Google Scholar]
- 27.Chung MK, Worsley KJ, Robbins S, Paus T, Taylor J, Giedd JN, et al. (2003): Deformation-based surface morphometry applied to gray matter deformation. NeuroImage. 18:198–213. [DOI] [PubMed] [Google Scholar]
- 28.Van Essen DC, Dierker D, Snyder AZ, Raichle ME, Reiss AL, Korenberg J (2006): Symmetry of Cortical Folding Abnormalities in Williams Syndrome Revealed by Surface-Based Analyses. J Neurosci. 26:5470–5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Im K, Lee JM, Lyttelton O, Kim SH, Evans AC, Kim SI (2008): Brain size and cortical structure in the adult human brain. Cereb Cortex. 18:2181–2191. [DOI] [PubMed] [Google Scholar]
- 30.Van Essen DC, Drury HA (1997): Structural and functional analyses of human cerebral cortex using a surface-based atlas. Journal of Neuroscience 17(18):7079–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ribases M, Hervas A, Ramos-Quiroga JA, Bosch R, Bielsa A, Gastaminza X, et al. (2008): Association study of 10 genes encoding neurotrophic factors and their receptors in adult and child attention-deficit/hyperactivity disorder. Biol Psychiatry. 63:935–945. [DOI] [PubMed] [Google Scholar]
- 32.Syed Z, Dudbridge F, Kent L (2007): An investigation of the neurotrophic factor genes GDNF, NGF, and NT3 in susceptibility to ADHD. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 144B:375–378. [DOI] [PubMed] [Google Scholar]
- 33.Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. (2008): Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 28:3586–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. (2004): Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 101:8174–8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Batty MJ, Liddle EB, Pitiot A, Toro R, Groom MJ, Scerif G, et al. (2010): Cortical Gray Matter in Attention-Deficit/Hyperactivity Disorder: A Structural Magnetic Resonance Imaging Study. Journal of the American Academy of Child & Adolescent Psychiatry. 49:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Almeida LG, Ricardo-Garcell J, Prado H, Barajas Lz, Fern√°ndez-Bouzas A, √Åvila D, et al. (2011): Reduced right frontal cortical thickness in children, adolescents and adults with ADHD and its correlation to clinical variables: A cross-sectional study. J Psychiatr Res. 44:1214–1223. [DOI] [PubMed] [Google Scholar]
- 37.Narr KL, Woods RP, Lin J, Kim J, Phillips OR, Del’Homme M, et al. (2009): Widespread Cortical Thinning Is a Robust Anatomical Marker for Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 48:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Posner J, Nagel BJ, Maia TV, Mechling A, Oh M, Wang Z, et al. (2011): Abnormal Amygdalar Activation and Connectivity in Adolescents With Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 50:828–837.e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plessen KJ, Bansal R, Zhu H, Whiteman R, Amat J, Quackenbush GA, et al. (2006): Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 63:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, et al. (2009): Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am J Psychiatry. 167:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickstein SG, Bannon K, Castellanos FX, Milham MP (2006): The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 47:1051–1062. [DOI] [PubMed] [Google Scholar]
- 42.Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H (2006): Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry. 60:1062–1070. [DOI] [PubMed] [Google Scholar]
- 43.Rubia K, Cubillo A, Smith AB, Woolley J, Heyman I, Brammer MJ (2010): Disorder-specific dysfunction in right inferior prefrontal cortex during two inhibition tasks in boys with attention-deficit hyperactivity disorder compared to boys with obsessive-compulsive disorder. Hum Brain Mapp. 31:287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cao Q, Zang Y, Zhu C, Cao X, Sun L, Zhou X, et al. (2008): Alerting deficits in children with attention deficit/hyperactivity disorder: event-related fMRI evidence. Brain Res. 1219:159–168. [DOI] [PubMed] [Google Scholar]
- 45.Suskauer SJ, Simmonds DJ, Fotedar S, Blankner JG, Pekar JJ, Denckla MB, et al. (2008): Functional magnetic resonance imaging evidence for abnormalities in response selection in attention deficit hyperactivity disorder: differences in activation associated with response inhibition but not habitual motor response. J Cogn Neurosci. 20:478–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubia K, Smith AB, Brammer MJ, Taylor E (2007): Temporal lobe dysfunction in medication-naive boys with attention-deficit/hyperactivity disorder during attention allocation and its relation to response variability. Biol Psychiatry. 62:999–1006. [DOI] [PubMed] [Google Scholar]
- 47.Vance A, Silk TJ, Casey M, Rinehart NJ, Bradshaw JL, Bellgrove MA, et al. (2007): Right parietal dysfunction in children with attention deficit hyperactivity disorder, combined type: a functional MRI study. Mol Psychiatry. 12:826–832. [DOI] [PubMed] [Google Scholar]
- 48.Valera EM, Faraone SV, Biederman J, Poldrack RA, Seidman LJ (2005): Functional neuroanatomy of working memory in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 57:439–447. [DOI] [PubMed] [Google Scholar]
- 49.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, et al. (2008): Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 63:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zang Y-F, He Y, Zhu C-Z, Cao Q-J, Sui M-Q, Liang M, et al. (2007): Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 29:83–91. [DOI] [PubMed] [Google Scholar]
- 51.Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF 3rd, et al. (2007): Cerebellar development and clinical outcome in attention deficit hyperactivity disorder.[see comment]. Am J Psychiatry. 164:647–655. [DOI] [PubMed] [Google Scholar]
- 52.Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, et al. (2011): Brain Gray Matter Deficits at 33-Year Follow-up in Adults With Attention-Deficit/Hyperactivity Disorder Established in Childhood. Arch Gen Psychiatry. 68:1122–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White T, Andreasen NC, Nopoulos P, Magnotta V (2003): Gyrification abnormalities in childhood- and adolescent-onset schizophrenia. pp 418–426. [DOI] [PubMed] [Google Scholar]
- 54.Bledsoe J, Semrud-Clikeman M, Pliszka SR (2009): A Magnetic Resonance Imaging Study of the Cerebellar Vermis in Chronically Treated and Treatment-Naïve Children with Attention-Deficit/Hyperactivity Disorder Combined Type. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Semrud-Clikeman M, Pliszka SR, Lancaster J, Liotti M (2006): Volumetric MRI differences in treatment-naive vs chronically treated children with ADHD.[erratum appears in Neurology. 2006 Dec 12;67(11):2091]. Neurology. 67:1023–1027. [DOI] [PubMed] [Google Scholar]
- 56.Shaw P, Sharp WS, Morrison M, Eckstrand K, Greenstein DK, Clasen LS, et al. (2009): Psychostimulant treatment and the developing cortex in attention deficit hyperactivity disorder. Am J Psychiatry. 166:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakao T, Radua J, Rubia K, Mataix-Cols D (2011): Gray Matter Volume Abnormalities in ADHD: Voxel-Based Meta-Analysis Exploring the Effects of Age and Stimulant Medication. Am J Psychiatry. 2011:24. [DOI] [PubMed] [Google Scholar]
- 58.Valera EM, Brown A, Biederman J, Faraone SV, Makris N, Monuteaux MC, et al. (2009): Sex differences in the functional neuroanatomy of working memory in adults with ADHD. Am J Psychiatry. 167:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermens DF, Kohn MR, Clarke SD, Gordon E, Williams LM (2005): Sex differences in adolescent ADHD: findings from concurrent EEG and EDA. Clinical Neurophysiology. 116:1455–1463. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


