Abstract
Fear learning allows us to identify and anticipate aversive events and adapt our behavior accordingly. This is often thought to rely on associative learning mechanisms where an initially neutral conditioned stimulus (CS) is repeatedly paired with an aversive unconditioned stimulus (US), eventually leading to the CS also being perceived as aversive and threatening. Importantly, however, humans also show verbal fear learning. Namely, they have the ability to change their responses to stimuli rapidly through verbal instructions about CS–US pairings. Past research on the link between experience-based and verbal fear learning indicated that verbal instructions about a reversal of CS–US pairings can fully override the effects of previously experienced CS–US pairings, as measured through fear ratings, skin conductance, and fear-potentiated startle. However, it remains an open question whether such instructions can also annul learned CS representations in the brain. Here, we used a fear reversal paradigm (female and male participants) in conjunction with representational similarity analysis of fMRI data to test whether verbal instructions fully override the effects of experienced CS–US pairings in fear-related brain regions or not. Previous research suggests that only the right amygdala should show lingering representations of previously experienced threat (“pavlovian trace”). Unexpectedly, we found evidence for the residual effect of prior CS–US experience to be much more widespread than anticipated, in the amygdala but also cortical regions like the dorsal anterior cingulate or dorsolateral prefrontal cortex. This finding shines a new light on the interaction of different fear learning mechanisms, at times with unexpected consequences.
SIGNIFICANCE STATEMENT Humans are able to learn about aversive stimuli both from experience (i.e., repeated pairings of conditioned stimulus (CS) and unconditioned stimulus (US; pavlovian conditioning), and from verbal instructions about stimulus pairings. Understanding how experience-based and verbal learning processes interact is key for understanding the cognitive and neural underpinnings of fear learning. We tested whether prior aversive experiences (CS–US pairings) affected subsequent verbal learning, searching for lingering threat signals after verbal instructions reversed a CS from being threatening to being safe. While past research suggested such threat signals can only be found in the amygdala, we found evidence to be much more widespread, including the medial and lateral PFC. This highlights how experience-based and verbal learning processes interact to support adaptive behavior.
Keywords: amygdala, fear instructions, learning, reversal learning
Introduction
Animals developed the adaptive ability to relate stimuli [conditioned stimulus (CS)] with harmful events [unconditioned stimulus (US)], which allows them to anticipate and avoid such events in the future (pavlovian conditioning; Maren, 2001; Öhman and Mineka, 2001). A fundamental advantage of human fear learning is the ability to also learn from verbal instructions (Olsson and Phelps, 2007). In most cases, a single instruction is enough to lead to the desired behavior, without the need for repeated trial and error learning (“Never leave electrical appliances near your bathtub”). Recently, the interaction between associative learning and verbal instructions has received increased attention (Koban et al., 2017; Mertens et al., 2018; Atlas, 2019). Recent studies have shown that behavioral conditioned responses can be fully reversed by merely verbally instructing a reversal of CS–US contingencies (Atlas et al., 2016; Mertens and De Houwer, 2016; Lonsdorf et al., 2017; Atlas and Phelps, 2018). Across these studies, merely instructing the discontinuation of an established CS–US pairing was shown to immediately result in a substantially reduced fear response.
Of all brain regions implicated in processing fear-relevant stimuli (Fullana et al., 2016), the amygdala (AMY) seems to have a specific role in experienced (vs merely instructed) CS–US pairings (Atlas et al., 2016; Braem et al., 2017). While instructed contingency reversal seems to fully override previously learned responses in most fear-relevant brain regions (Atlas, 2019), the right AMY (rAMY) shows lingering effects specifically of prior CS–US experience above and beyond the effects of verbal instructions (Braem et al., 2017). Whether prior CS–US pairings, either experienced or merely instructed, have lingering effects on behavior remains an open question. Here, we are interested only in the effect of previously experienced CS–US pairings, which we will call “pavlovian trace,” in line with previous research. Although previous findings demonstrated the limits of the effects of verbal instructions on prior CS–US experience, we know much less about the opposite effect of CS–US experience on verbal instruction implementation in fear reversal. One study has shown that instructed fear reversal effects on behavioral and psychophysiological measures can be largely explained through verbal instructions, with little additional effect attributable to CS–US experience (Mertens and De Houwer, 2016). Our goal was to test whether similar effects can be shown on the neural coding of fear-relevant stimuli as well, which remains unknown.
Previously, neural signals associated with experienced and instructed CS (CS+E) and merely instructed CS (CS+I) were dissociated to identify pavlovian traces in the right amygdala (Braem et al., 2017). This study used a static design with constant CS–US pairings, and it remains unclear whether such effects generalize to more dynamic reversal learning settings. Another fMRI study implemented a dynamic fear reversal learning paradigm to investigate experience–instruction interactions (Atlas et al., 2016). Here, the authors compared a condition in which participants relied on both experience and verbal instructions, with a condition in which participants relied on experience alone. Keeping experience constant across conditions while manipulating the presence of verbal instructions is a good way to demonstrate the effects of verbal instructions on experience-based learning. Yet it is not optimal to demonstrate specific effects of prior CS–US experience on verbal reversal instruction implementation, which requires keeping instructions constant across conditions and manipulating the presence of CS–US experience.
Here, we used a fear reversal paradigm, using representational similarity analysis (RSA) of fMRI data (Kriegeskorte et al., 2008) to measure neural coding of fear-relevant stimuli (Visser et al., 2013; Braem et al., 2017). Participants first performed a conditioning phase, where they received instructions about safe (CS–; never followed by US) and threatening (CS+; potentially followed by US) stimuli. Crucially, only some CS+s were actually followed by an aversive electrical stimulus (CS+E), while others were not (CS+I). After subsequent verbal reversal instructions, each CS was presented again in a second instructed reversal phase (which we will call “reversal phase” from now on, and in which no USs were presented). We then compared different CSs that became threatening just after the verbal reversal instructions, and which differed in their history during the conditioning phase (e.g., a safe CS that just became threatening vs a threatening CS which has been reinforced in the conditioning phase). In line with prior findings, we hypothesized that verbal instructions would be able to fully reverse both the behavioral and neural expressions of previously learned CS–US relations (Mertens and De Houwer, 2016). Only the (right) amygdala, we reasoned, could show the effects of prior experience after verbal reversal instructions.
Materials and Methods
Participants
Forty-two participants took part in the experiment (23 female, 19 male; mean age, 23.0 years; age range, 18–34 years; right handed; no history of neurologic or psychiatric disorders). Our sample size followed that of the study by Visser et al. (2013), who reported reliable multivariate fMRI results in the context of fear conditioning with n = 38 participants. All participants volunteered to participate and gave written informed consent, had normal or corrected-to-normal vision, and received 40€ for participation. The experiment was approved by the ethics committee of the Ghent University Hospital (registration #B670201421176). Two participants showed excessive head movements inside the MR scanner (>5 mm) and were excluded from all further analyses. The final sample consisted of 40 participants (21 female, 19 male; mean age, 22.9 years; age range, 18–34 years).
Procedure
Workup.
After entering the MR scanner, an experimenter attached the electrode to either the participant's right or left leg, and the workup procedure started. The electrotactile pain stimulus was delivered through a surface electrode (Speciality Developments) that was placed over the ankle (retromalleolar course of the sural nerve). The stimulated leg (left or right) was counterbalanced across participants. Stimulation was administered using an electrical stimulator (model DS5, Digitimer), and stimulation intensity was determined using an adaptive workup procedure. Individual pain thresholds were determined using an interleaved staircase procedure (Braem et al., 2017), consisting of a total of 20 trials. On each trial, an electrical stimulus was administered, and the participant was asked to verbally indicate pain intensity on a scale from 0 (no pain) to 10 (extreme pain). The 20 trials were randomly divided into two staircase sequences of 10 trials. Each staircase was initiated at a random intensity ranging between 0.2 and 0.7 mA, and between 0.7 and 1.2 mA, respectively. If the participant rated a stimulus >5, the intensity of the next step of the staircase would decrease by 0.1 mA. If the participant rated a stimulus <5, it would increase by 0.1 mA. If the participant rated a stimulus = 5, the intensity did not change. After both staircases were finished, the final values of both staircases were averaged, and that value was used as the stimulation intensity for the remainder of the experiment. Participants were instructed that the goal of this procedure was to ensure the electrical stimulation was unpleasant, but not extremely painful.
Conditioning phase.
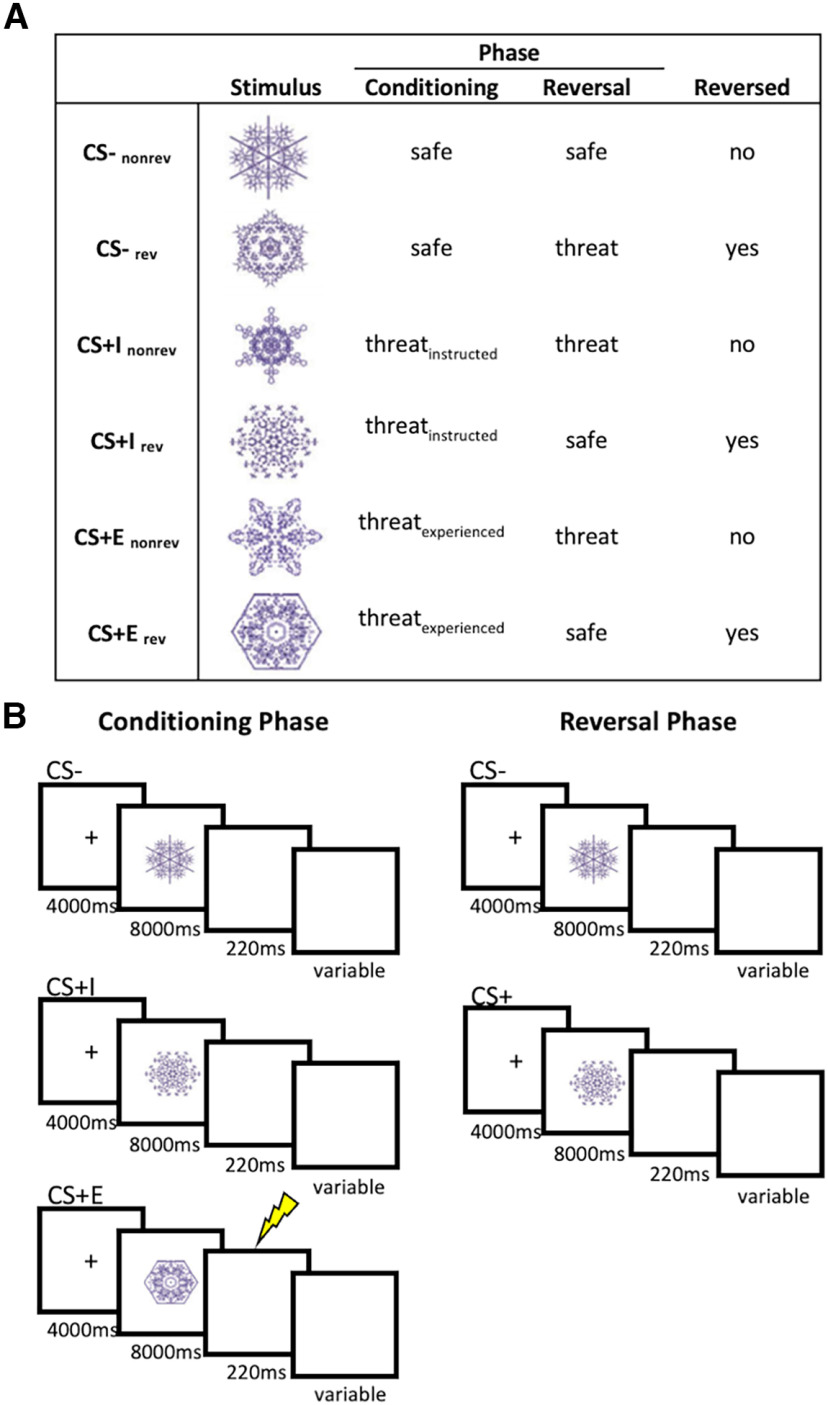
After the workup procedure, participants were exposed to a conditioning phase in which they observed six different fractal stimuli (CSs) on screen (Fig. 1A). CS conditions to which the stimuli were assigned were counterbalanced across participants. Two stimuli (CS–) were instructed to never be followed by an electrical stimulus (US). Two further stimuli were instructed to be followed by an electrical stimulus on some trials (CS+E), and the same instruction was given for the last two stimuli (CS+I). The key difference between CS+E and CS+I was that the former was indeed sometimes followed by an electrical stimulus (33% of the trials, chosen randomly), while the latter was not. Before the start of the conditioning phase, participants were asked to correctly identify each CS as either CS+ or CS–, and the experiment started only after they passed this test without errors.
Figure 1.
Stimuli and design. A, Conditions and stimuli used in this experiment. Stimulus to condition mappings were counterbalanced across participants. B, Trial structure in the conditioning and reversal phases, separately for each CS condition. The lightning bolt represents reinforcement, which was administered on 33% of the CS+E trials. In the reversal phase, stimuli were instructed to be either safe or threatening, but no stimuli were reinforced. Here, CS– included (CS+Ereversed, CS+Ireversed, CS–nonreversed), and CS+ included (CS+Enonreversed, CS+Inonreversed, CS–reversed) CS = conditions stimulus, CS– = safe stimulus, CS+I = merely instructed stimulus, CS+E = instructed and experienced stimulus.
The conditioning phase consisted of a total of 36 trials (6 repetitions of each CS; Fig. 1B). Each trial started with the presentation of a fixation cross (4000 ms) centrally on screen. This was followed by the presentation of a CS on screen (8000 ms). On reinforced trials (33% of CS+E trials), this was followed by an electrical stimulation; on all other trials, a blank screen was presented for 220 ms, which was the same duration as the electrical stimulation and was included to match all trials with respect to their duration. After a variable intertrial interval (ITI; 13, 15, or 17s), the next trial started. Conditioning trials were presented in a random order, and randomization was performed in mini-blocks of six trials that contained each CS once. No direct repetitions of the same CS were allowed. Additionally, we ensured that the CS condition was not correlated with either ITI duration or reversal condition, and that the CS condition on a particular trial could not be predicted on the basis of the previous trial.
After 12 trials, the experiment was paused and a rating block was presented. Participants were asked to rate their self-reported CS fear and US expectancy for each of the CSs presented in the last 12 trials. Each CS was presented twice, once with the question “How fearful were you while seeing this stimulus?” [fear rating: 9-point Likert scale anchored at 1 (“not at all”), 3 (“rather not”), 5 (“unsure”) 7 (“somewhat”), 9 (“strongly”)], and once with the question “To which degree did you expect an electric shock while seeing this stimulus?” [US expectancy rating: 9-point Likert scale anchored at 1 (“not at all”), 3 (“a little”), 5 (“average”), 7 (“somewhat”), 9 (“strongly”)]. Participants were instructed to base their ratings on the last time they saw each CS and responded in their own time. Once they answered each of the 12 rating items, the experiment continued. After the next 12 trials, another rating block was presented, and after the last 12 trials the third and last rating block was presented, splitting up the conditioning phase into three blocks separated by rating items. Overall, the condition phase lasted ∼18 min in total and consisted of a single fMRI run.
Reversal phase.
After the conditioning phase, participants were instructed that some of the CSs would change their meaning in the next phase (Fig. 1A). One CS– would remain a CS– (CS–norev), while the other could now be followed by an electrical stimulation (CS–rev), and two of four CS+s would now be safe. Specifically, one CS+I would remain a CS+ (CS+Inorev), while the other would no longer be followed by an electrical stimulus (CS+Irev), and one CS+E would remain a CS+ (CS+Enorev), while the other would no longer be followed by an electrical stimulation (CS+Erev). Please note that participants were not instructed on this difference between CS+Es and CS+Is; they merely received threat/safety instructions for each CS. Thus, the overall design of this study was a 3 (CS conditions: CS–, CS+E, CS+I) × reversal (reversed, nonreversed) × time (conditioning phase, reversal phase). Similar to the conditioning phase, the reversal phase started only after the participant correctly indicated the updated CS–US association for each CS. It was stressed that the second phase would be similar to the first phase, with some CSs to be followed by a US on a portion of the trials, and other CSs never followed by a US. However, similar to the study by Braem et al. (2017), no electrical stimulation was applied in the reversal phase (Fig. 1B).
The overall structure of the second run (reversal phase) was identical to the conditioning phase, with updated CS contingencies. Half of the CSs reversed, while the other retained their original association, and no electrical stimulation was administered in the reversal phase. After each block of 12 trials, participants were asked to rate their fear and US expectancy for each CS. The reversal phase lasted ∼18 min and consisted of a single fMRI run.
Debriefing.
After the reversal phase, participants left the MR scanner, were debriefed, and filled in the state-trait anxiety index [STAI (trait version); Spielberger et al., 1999]. The average STAI score was 36.53 (SD = 8.54). Within the context of this article, we did not further investigate STAI results. All participants were further asked to what degree they believed the instructions at the time they were given. Almost all participants believed the instructions; only two indicated the believability to be somewhat low.
Skin conductance response acquisition
For each subject, skin conductance responses (SCRs) were collected using Biopac Systems hardware (catalog #EDA100C-MRI, #PPG100C) and standard disposable Ag/AgCl electrodes attached to the thenar and hypothenar eminences of the nondominant (left) hand. The signal was measured using the ACQKnowledge software and digitized at 2000 Hz. For the SCR analysis, trials in which subjects were presented a US were excluded, as the electrical shocks might bias the SCRs. Data were further analyzed using MATLAB (version R2014b 8.4.0 150421; MathWorks; RRID:SCR_001622). They were first smoothed using a Gaussian kernel, and SCRs were calculated by subtracting the mean value of a baseline time period (2–0 s before CS onset) from the maximum amplitude within a 1–7 s interval after the CS onset. Any values <0.02, including negative values, were scored as zero. Then, SCRs were range corrected (Lykken and Venables, 1971) and square root transformed to normalize the data. This analysis procedure has been used successfully previously (Mertens and De Houwer, 2016). Unexpectedly, SCR signals were very weak, and we were unable to detect any SCR signal differences in the relevant response window (1–7 s after CS onset) for any condition (e.g., in contrast to the study by Costa et al., 2015), and thus did not analyze SCRs any further.
Behavioral data analysis
Manipulation check.
To test whether participants understood the difference between threatening and safe CSs (CS– vs CS+E), we tested whether US expectancy ratings differed between these conditions, and whether they reversed in line with the verbal instructions. For this purpose, we first averaged ratings across the three blocks within each phase, and then performed a three-factorial Bayesian ANOVA with the within-subject factors CS (CS–, CS+E), reversal (reversed, nonreversed), and phase (conditioning, reversal), and adding subjects as a random factor. All ANOVAs were computed in R (Rstudio, version 1.1.456; RRID:SCR_000432) using the BayesFactor package. Results are reported in terms of Bayes factors [BF10 (default inverse χ2 prior); scaling factor = 0.5]. Following previous research (Andraszewicz et al., 2015; Mertens and De Houwer, 2016), we considered BFs between 0.33 and 1 as anecdotal evidence, BFs between 0.1 and 0.33 as moderate evidence, and BFs <0.1 as strong evidence for the null hypothesis. BFs between 1 and 3 were considered as anecdotal evidence, BFs between 3 and 10 as moderate evidence, and BFs >10 as strong evidence for an alternative hypothesis. Effect sizes (ESs) were computed for each Bayesian test by computing the posterior distribution (posterior function, 10,000 iterations), and then computing the peak of that distribution (Schmalz et al., 2023).
We tested for a main effect of threat, expecting higher US expectancy ratings for threatening (CS+E) than for safe (CS–) stimuli. We also tested whether reversal instructions increased ratings for reversed CS–, and decreased ratings for reversed CS+E, which should be seen as a three-way interaction of CS, reversal, and phase (Mertens and De Houwer, 2016, for more information on this analysis logic in a highly similar design). The same tests were conducted on the fear ratings.
Experience effects.
In our main analysis, we tested whether experiencing a US affected fear reversal, as measured using US expectancy ratings. For this purpose, we computed a three-way Bayesian ANOVA again, using the factors CS (CS+I, CS+E), reversal (reversed, nonreversed), phase (conditioning, reversal), and modeling subjects as a random effect. By comparing CS+I and CS+E here, we were able to test whether reversals of CS–US contingencies across phases differed between CS+I and CS+E, in which case we should find evidence for a three-way interaction of CS, reversal, and phase. To test whether experience effects change over the course of the experiment, we first split each phase into three separate blocks (separated by rating items). We then estimated an additional Bayesian ANOVA, using the same three factors listed above, only adding block as an additional factor.
To investigate the effects of prior CS–US experience after verbal reversal instructions, we performed a number of tests on the reversal phase only. First, we tested whether expectancy ratings differed among CS–rev, CS+Inorev, and CS+Enorev using a one-factorial Bayesian ANOVA, modeling subjects as a random factor. All of these CSs were threatening during the reversal phase, and only differed in their history during the conditioning phase. While CS–rev only just became threatening, CS+Enorev was followed by USs and remained threatening, and CS+Inorev was not followed by USs and remained threatening. If learning history carried over from the conditioning to the reversal phase, we should see stronger responses to CS+Enorev than to either CS–rev and CS+Inorev. We used a similar approach to compare the three safe stimuli in the reversal phase, CS–norev, CS+Irev, and CS+Erev. These stimuli only differed in their learning history in the conditioning phase, and if this history carried over to the reversal phase, we would expect to see higher US expectancy for CS+Erev than for CS+Irev and CS–norev. Again, the same analyses were then performed on fear ratings as well. In line with the study by Mertens and De Houwer (2016), we expected no additional effects of having experienced CS–US pairings.
fMRI data acquisition
fMRI data were collected using a 3 T MRI scanner system (Magnetom Trio, Siemens Medical Systems), with a standard 32-channel radio frequency head coil. A 3D high-resolution anatomic image of the whole brain was acquired for coregistration and normalization of the functional images, using a T1-weighted MPRAGE sequence (TR = 2250 ms, TE = 4.18 ms, TI = 900 ms, acquisition matrix = 256 × 256, FOV = 256 mm, flip angle = 9°, voxel size = 1 × 1 × 1 mm). Furthermore, a field map was acquired for each participant to correct for magnetic field inhomogeneities (TR = 400 ms, TE1 = 5.19 ms, TE2 = 7.65 ms, image matrix = 64 × 64, FOV = 192 mm, flip angle = 60°, slice thickness = 3 mm, voxel size = 3 × 3 × 3 mm, distance factor = 20%, 33 slices). Whole-brain functional images were collected using a T2*-weighted EPI sequence (TR = 2000 ms, TE = 30 ms, image matrix = 64 × 64, FOV = 192 mm, flip angle = 78°, slice thickness = 3 mm, voxel size = 3 × 3 × 3 × mm, distance factor = 20%, 33 slices). Slices were oriented along the anterior commissure–posterior commissure line for each participant.
fMRI data analysis
Preprocessing.
fMRI data were analyzed using MATLAB (version R2014b 8.4.0 150241; MathWorks; RRID:SCR_001622) and the SPM12 toolbox (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/; version 6906; RRID:SCR_007037). Before the analysis, we discarded the first three acquired volumes of each run. Functional data were subsequently unwarped, realigned, and slice time corrected. The preprocessed data were then screened for possible scanner-related artifacts, using the Artifact Detection Tool (ART; version 2011–07; http://www.nitrc.org/projects/artifact_detect/; RRID:SCR_005994). ART automatically detects and marks outlier volumes based on the global mean brain activation, and movement parameters (z-threshold = 9, movement threshold = 2). We identified outlier volumes in 17 participants (mean number of identified volumes = 2.7, minimum number of identified volumes = 2, maximum number of identified volumes = 28). Variance attributable to these artifacts was removed by explicitly modeling the affected volumes in the respective first-level general linear models (GLMs).
First-level GLM estimation.
After preprocessing, a GLM (Friston et al., 1994) was estimated using unsmoothed, non-normalized data, separately for each participant (GLMmain). This allowed us to perform all representational similarity analyses in native space for each participant. Each combination of CS type (CS–, CS+I, CS+E), reversal (reversal, no reversal), and phase (conditioning, reversal) was modeled using a separate regressor. This resulted in 12 regressors of interest in this GLM. Each regressors was modeled as a boxcar locked to the onset of the CS presentation (duration = 8 s), and was convolved with a canonical HRF basis function, an approach used successfully before (Braem et al., 2017). Regressors of noninterest included the onset of each US, one regressor per identified outlier/artifact volume (as suggested in the ART documentation), and six movement regressors.
Next, we estimated a second GLM for each subject (GLMblock). This GLM was identical to GLMmain, only that we added block as an additional factor. As outlined above, each phase consisted of three blocks, separated by rating items. Adding block as a factor allowed us to investigate the temporal evolution of any observed effects, and allowed us to restrict analyses to, for example, only the first block of each phase, where instruction effects should be strongest. Overall, this GLM included 36 regressors of interest (CS × reversal × phase × block), and regressors of noninterest were identical to the previous GLM.
Region of interest definition.
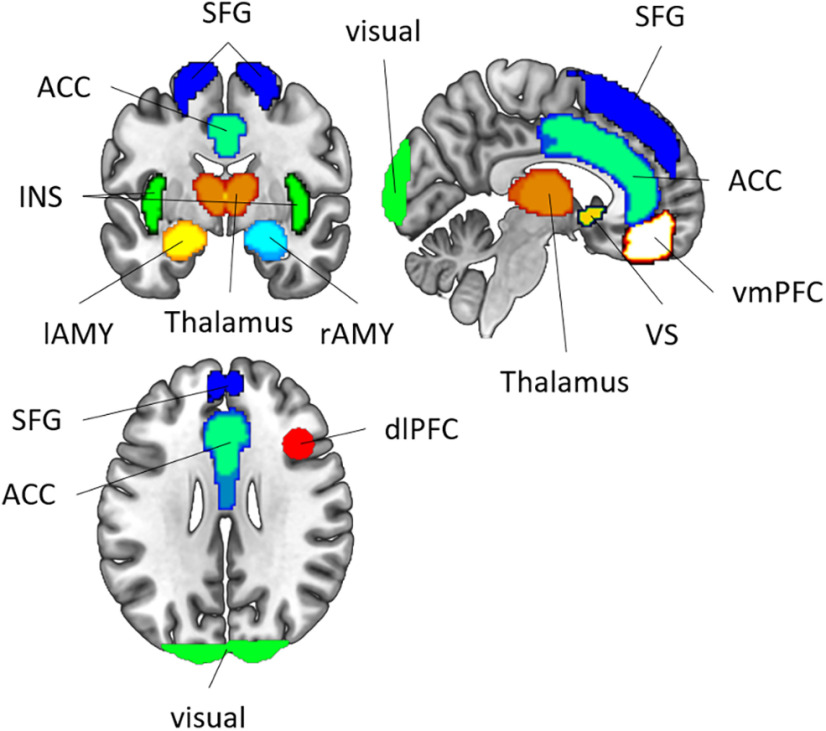
Our main analyses were performed within several a priori defined regions of interest (ROIs; Fig. 2) that have been found to be involved in fear conditioning (Visser et al., 2013; Fullana et al., 2016). Specifically, we included ROIs for the bilateral anterior cingulate cortex (ACC), bilateral insula (INS), bilateral ventral striatum (VS), bilateral thalamus (TH), bilateral ventromedial PFC (vmPFC), bilateral superior frontal gyrus (SFG), left AMY (lAMY), and rAMY. These ROIs were obtained from the Harvard-Oxford cortical and subcortical structural atlas (The Center for Morphometric Analysis, Harvard University, Boston, MA), using a probability threshold of 25%. Additionally, we constructed a spherical ROI (radius = 10 mm) in the right dorsolateral PFC (dlPFC), using coordinates ([39 × 20 × 31]) from a previous article (Demanet et al., 2016; as also used in Bourguignon et al., 2018) and the WFUpickatlas toolbox (version 3.0.5; RRID:SCR_007378; Maldjian et al., 2003). This region has been shown to be uniquely involved in instruction implementation of task rules and could, therefore, also be involved in implementing the reversal instructions in our dataset. All ROIs were then projected into native space, separately for each participant, using the inverse normalization field estimated during preprocessing.
Figure 2.
Regions of interest: ACC (anterior cingulate cortex), SFG (superior frontal gyrus), vmPFC (ventromedial prefrontal cortex), dlPFC (dorsolateral prefrontal cortex), VS (ventral striatum), TH (thalamus), lAMY (left amygdala), rAMY (right amygdala), INS (insula).
Representational similarity estimation.
We then used the β estimates from the GLMmain to perform RSAs (Kriegeskorte et al., 2008). This allowed us to measure the representational distances between individual CSs, and thus to test whether neural CS representations were more or less similar to each other depending on threat and/or prior CS–US experience. For each ROI, we first extracted β values for each of the 12 conditions in this experiment (i.e., each combination of CS × reversal × phase). Since we were unable to implement a leave-one-run-out cross-validation procedure with the current design, we instead z-scored the data across phases to avoid confounds related to global signal differences between the different runs of the experiment. Please note that all RSAs were performed in native space for each participant. Then, we performed multivariate noise normalization (Walther et al., 2016), after which we calculated Pearson correlation coefficients for each pairwise comparison of the 12 conditions. This resulted in a 12 × 12 correlation matrix [representational similarity matrix (RSM)]. Before running statistical tests on these correlations, they were first Fisher z-transformed. At the group level, correlations were assessed using either Bayesian t tests (Cauchy prior, scaling factor = 0.71) or Bayesian ANOVAs (inverse χ2 prior, scaling factor = 0.5).
Exploratory analyses: model-based RSA of expectancy and fear ratings.
There is an ongoing debate about the differences and similarities between expectancy and fear ratings (Mertens and De Houwer, 2016; Mertens et al., 2018), and to explore this issue in more detail, we also performed an additional exploratory analysis using model-based searchlight RSA (Nili et al., 2014). First, we computed two theoretical model RSMs from behavioral US expectancy ratings and fear ratings, respectively. For this purpose, we first extracted a vector of, for example, fear ratings for each CS across all participants, which yielded 12 rating vectors. Then, we calculated pairwise correlations between these rating vectors, and computed a 12 × 12 RSM (just like in the fMRI analysis). In a next step, we used a searchlight approach (radius = 3 voxels; Etzel et al., 2013) to determine the brain regions in which neural RSMs matched the theoretical predictions. In each searchlight, we computed the partial correlation between one model RSM and the data RSM, while controlling for the influence of the other model [e.g., r(US expectancy model, data)], while controlling for the fear model. This resulted in a whole-brain similarity map, showing which brain regions share unique variance with US expectancy ratings while controlling for fear ratings, and vice versa. Maps were then smoothed (FWHM, 6 mm) and normalized (MNI template, as implemented in SPM12). On the group level, we performed two separate GLMs, one for each model, testing where its unique shared variance with the data RSM was >0, and results were corrected for multiple comparisons using a voxel threshold of p < 0.05 (FWE corrected). This analysis allowed us to directly test which brain regions were specifically associated with either expectancy or fear ratings, potentially leading to new hypotheses that can be tested in future research.
Results
Behavioral results
Manipulation check
We first contrasted safe (CS–) and experienced (CS+E) stimuli to test for an effect of threat. For US expectancy ratings (Fig. 3A), we found strong evidence for a main effect of threat (BF10 > 150, ES = 3.82), as well as strong evidence for a three-way interaction of CS– type (CS– vs CS+E), reversal, and phase (BF10 > 150, ES = 3.82). For fear ratings (Fig. 3B), we found the following highly similar results: a main effect of threat (BF10 > 150, ES = 3.25), and a three-way interaction (BF10 > 150, ES = 3.28). These results demonstrated that participants clearly perceived threatening and safe stimuli differently, and that reversal instructions differentially affected CS– and CS+E stimuli, increasing ratings for reversed CS– and decreasing ratings for reversed CS+E.
Figure 3.
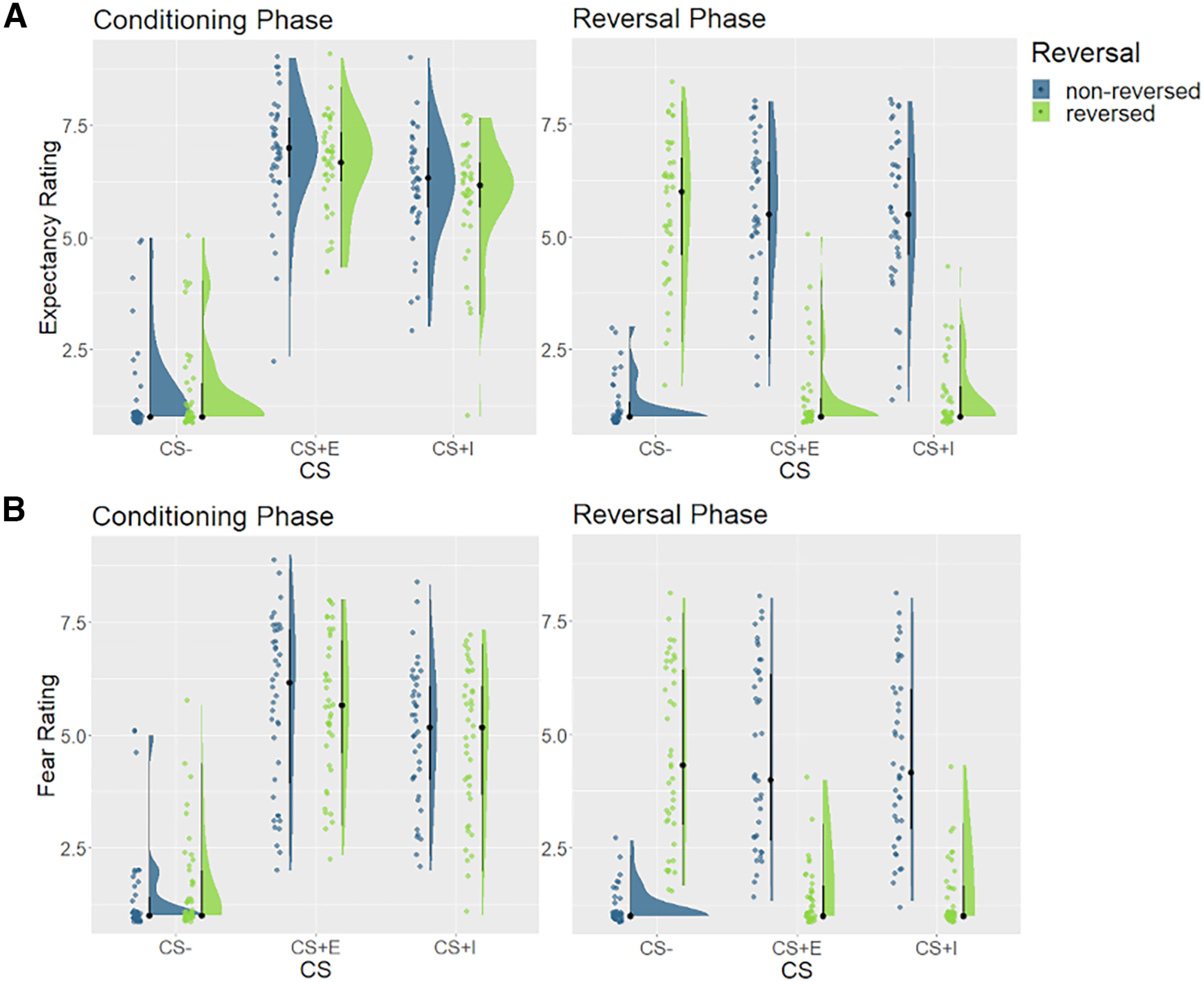
Expectancy and fear ratings. A, Retrospective US expectancy ratings for the conditioning phase (left), and the reversal phase (right), averaged across blocks within these phases. B, Retrospective fear ratings for the conditioning phase (left) and the reversal phase (right), averaged across blocks within these phases. Ratings were made on a 9-point Likert scale. CS = conditions stimulus, CS– = safe stimulus, CS+I = merely instructed stimulus, CS+E = instructed and experienced stimulus.
Experience effects
To test for the effect of CS–US experience, we then contrasted CS+I with CS+E. For US expectancy ratings, we found anecdotal evidence against a main effect of experience, suggesting that experienced stimuli showed similar ratings as merely instructed stimuli (BF10 = 0.38, ES = 4.97). Furthermore, we found moderate evidence against a three-way interaction of CS– type (CS+E vs CS+I), reversal, and phase (BF10 = 0.23, ES = 4.96). This suggests that reversal instructions affected experienced and merely instructed stimuli to an equal degree. We then assessed expectancy ratings in the reversal phase in more detail. If the learning history affected behavior, US expectancy ratings should be higher for safe stimuli that were threatening in the past (CS+Erev), compared with safe stimuli that were safe throughout the experiment (CS–norev). However, we found evidence against this hypothesis (BF10 = 0.28, ES = −0.20). Conversely, one would also expect threatening stimuli that were safe in the past (CS–rev) to show lower ratings, compared with threatening stimuli that were threatening throughout the experiment (CS+Enorev). We again found evidence against this hypothesis (BF10 = 0.11, ES = 0.01).
One potential reason why we found no differences between CS+E and CS+I stimuli in the reversal phase could be due the fact that US expectancies decayed over the course of the reversal phase. Indeed, we found strong evidence for block effects in the reversal phase (BF10 > 150, ES = 5.54), demonstrating that US expectancy ratings were higher in the beginning than in the end of the reversal phase. We repeated the analysis reported above, now only using data from the first block of each phase, where instructions should have the strongest impact. Even here, we found evidence against differences between CS+E and CS+I (BF10 values < 0.29, ES values < 0.04). Thus, in line with the study by Mertens and De Houwer (2016), we found evidence against an effect of CS–US experience on expectancy ratings in the reversal phase.
We found that fear ratings were overall higher for experienced stimuli (CS+E), compared with merely instructed stimuli (CS+I; BF10 = 3.43, ES = 4.16), which was mainly driven by differences in the conditioning phase. We again found moderate evidence against a three-way interaction, however (BF10 = 0.18, ES = 4.14), showing that reversal instructions had comparable effects on experienced and merely instructed stimuli. In the reversal phase, we found no evidence for an effect of learning history on ratings of threatening (BF10 = 0.09, ES = −0.11) and inconclusive evidence for a similar effect on safe stimuli (BF10 = 1.54, ES = −0.21). Additional exploratory analyses demonstrated that fear ratings also showed extinction effects in the reversal phase (BF10 > 150, ES = 4.57), yet restricting the analysis only to the first rating block still yielded evidence against differences between CS+E and CS+I (BF10 values < 0.31, ES values = 6.45). Overall, we replicated the study by Mertens and De Houwer (2016), finding evidence against an effect of CS–US experience on fear ratings in the reversal phase.
Note that we found evidence for a main effect of CS–US experience in the fear ratings, but not in the US expectancy ratings, driven by differences in the conditioning phase. To test whether this difference was robust, we repeated the ANOVA described above [factors: CS (CS+I, CS+E), reversal, phase], adding an additional factor item type (US expectancy, fear). We then assessed whether we found evidence for a CS × item type interaction, which would indicate that the main effect of CS (CS+E vs CS+I) was stronger for fear than for expectancy ratings. We found moderate evidence against this hypothesis (BF10 = 0.13, ES = 4.55). Thus, differences between US expectancy and fear ratings should be interpreted with caution.
fMRI results
Manipulation check
Conditioning phase.
As a first manipulation check, we assessed whether the experience of CS–US pairings affected voxel pattern responses to the different CSs in the conditioning phase (Fig. 4). If a region were responsive to instructed threat, we would expect the pattern similarity between threatening (CS+E, CS+I) and safe CSs (CS–) to be lower than between two threatening CSs (CS+E, CS+I). We thus estimated the representational similarities between each CS type in the conditioning phase, collapsing across reversal conditions here since this factor became relevant only after the reversal instructions. We then performed paired Bayesian t tests to test whether r(CS+E, CS+I) was higher than either r(CS+E, CS–) or r(CS+I, CS–). We found strong evidence for this effect in every ROI included here (all BF10 values > 150, all ES values > 0.049; Fig. 5), suggesting that each fear-related brain region encoded instructed threat.
Figure 4.
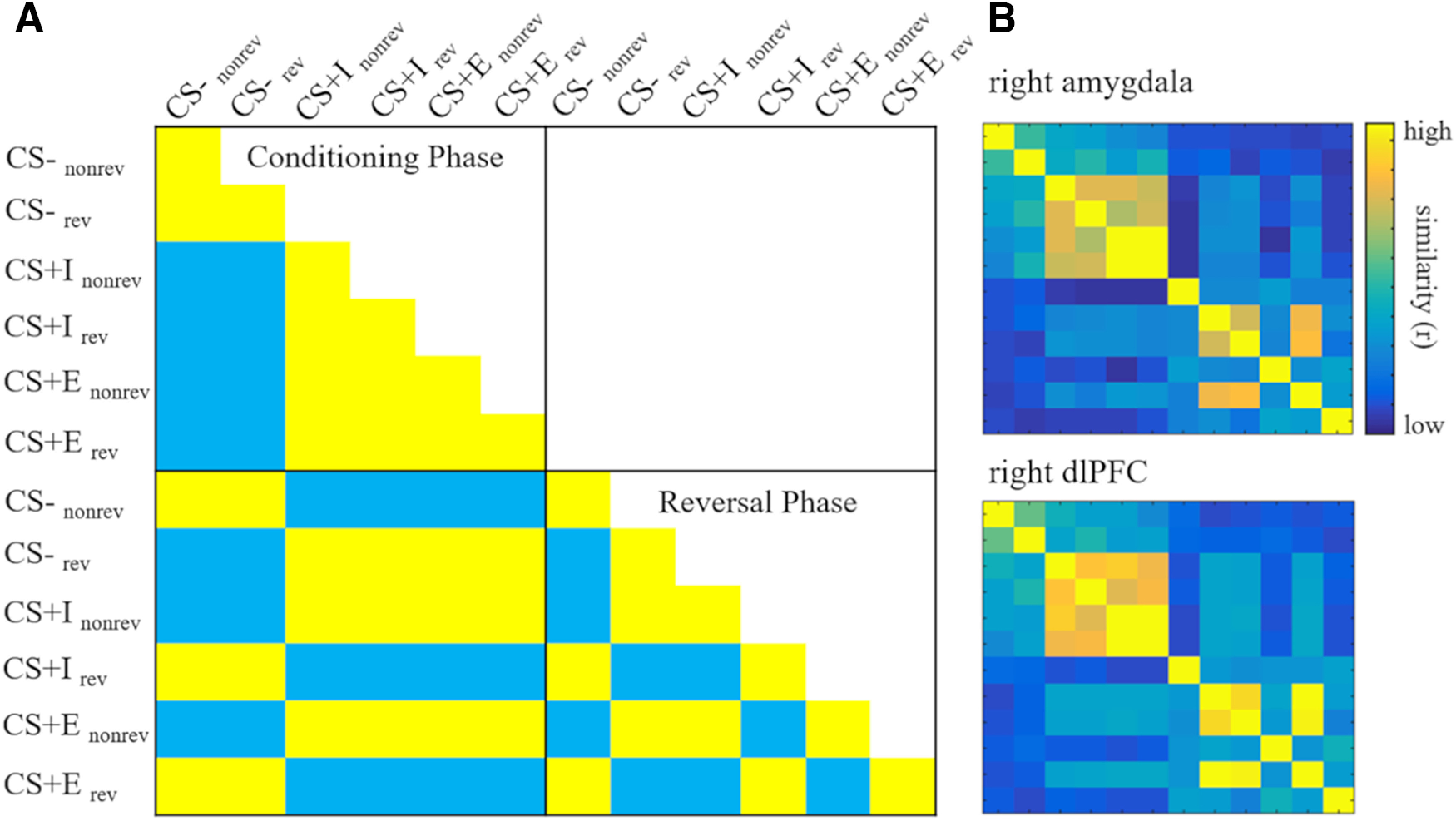
RSMs. A, Theoretical threat model RSM. Each cell depicts the expected pairwise correlations for each condition if a region coded for the presence of threat. Conditions: CS type (CS–, CS+I, CS+E), reversal (rev = reversed, nonrev = not reversed), phase (Conditioning Phase, Reversal Phase). B, RSMs extracted from two example regions of interest: the right amygdala and the right dlPFC. CS = conditions stimulus, CS– = safe stimulus, CS+I = merely instructed stimulus, CS+E = instructed and experienced stimulus.
Figure 5.
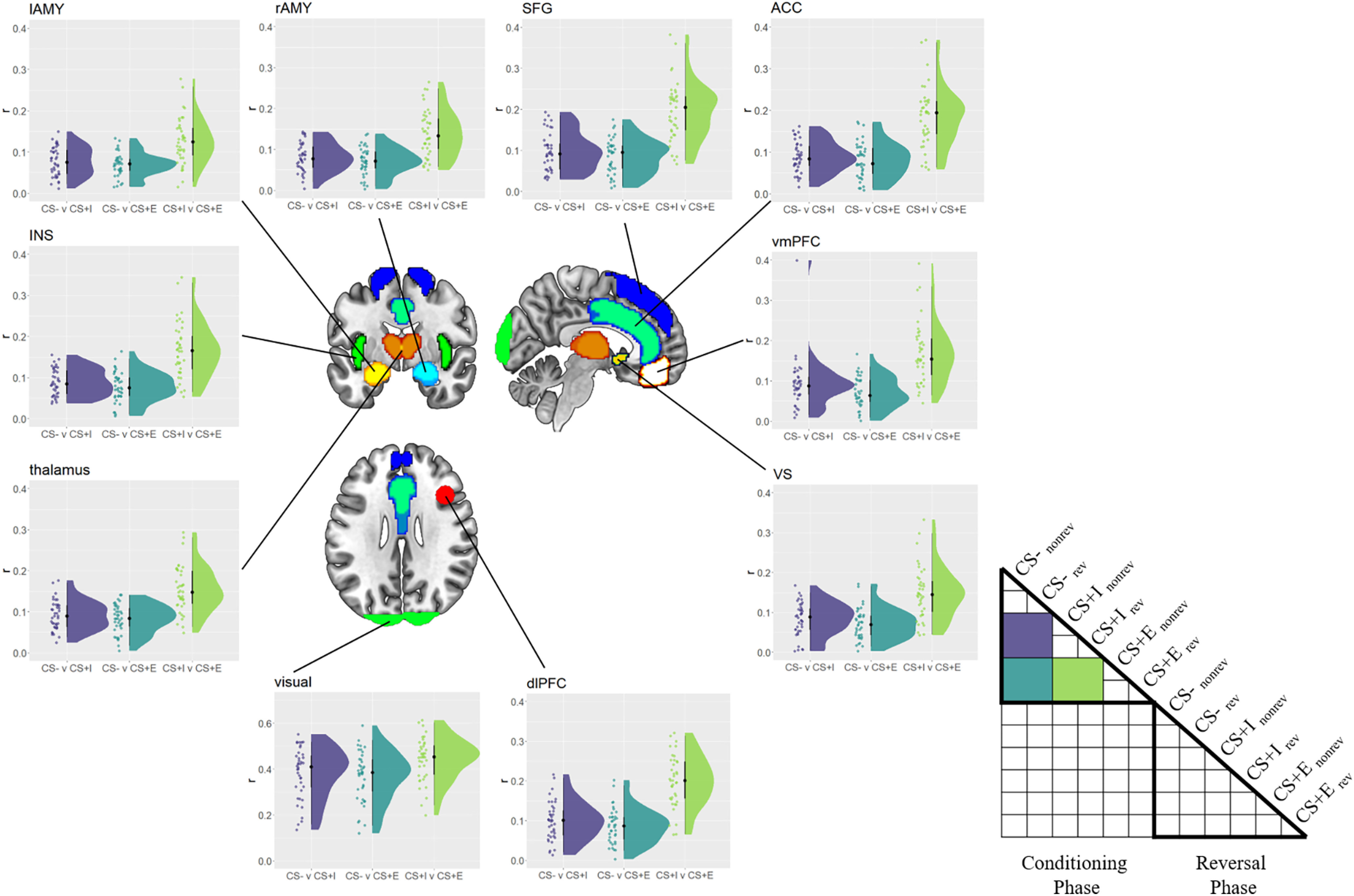
Between-CS similarity in the conditioning phase. Similarity (r) among safe (CS–), merely instructed (CS+I), and instructed + experienced (CS+E) CSs in the conditioning phase, separately for each ROI. Raincloud plots depict the raw data as dots on the left side and the data distribution on the right side. The schematic RSM denotes which pairwise representational similarity measures were used in this analysis. rev = reversed, norev = not reversed.
Next, we tested whether ROIs showed more consistent voxel pattern responses to threatening stimuli (CS+E, CS+I) than to safe stimuli (CS–) in the conditioning phase. There is some prior evidence that threat not only changes the overall activity level in fear-related brain regions, but also induces more consistent voxel pattern responses to threatening CSs (Visser et al., 2011; Braem et al., 2017), similar to emotional stimuli (Riberto et al., 2022). Moreover, and different than the study by Braem et al. (2017), here we could test whether the ROIs provide more consistent voxel patterns within CS type while controlling for low-level visual features. Namely, we tested this hypothesis by first computing the correlation between to be reversed and not to be reversed CS–s [r(CS–norev, CS–rev)]. Please note that at this time in the experiment, the meaning of these two stimuli is identical to all participants, they solely differ in, and therefore control for, their visual appearance. The same approach was used to estimate consistency of CS+E and CS+I. Following previous findings, we expected CS– values to show lower coding consistencies than CS+ values, which we tested using Bayesian paired t tests. We found strong evidence for more consistent coding of CS+E, compared with CS–, in every ROI (BF10 values > 150, ES values > 0.090; Fig. 6). Furthermore, we found evidence for more consistent coding of CS+I values, compared with CS– values (BF10 values > 21.4, ES values > 0.039) in almost all ROIs. The only exception here was the left amygdala, in which we found no evidence for (or against) differences between CS+I and CS– (BF10 = 0.98, ES = 0.026). To explore this finding further, we directly compared consistencies in the left and right amygdala, to see whether there is evidence for a hemispheric difference. Running an ANOVA using the factors CS (CS+I, CS–) and hemisphere (left, right), we found no evidence for (or against) either a main effect of hemisphere (BF10 = 1.00, ES = 0. 13) or an interaction of hemisphere and CS (BF10 = 0.71, ES = 0.13). Thus, differences between left and right amygdala should be interpreted with caution.
Figure 6.
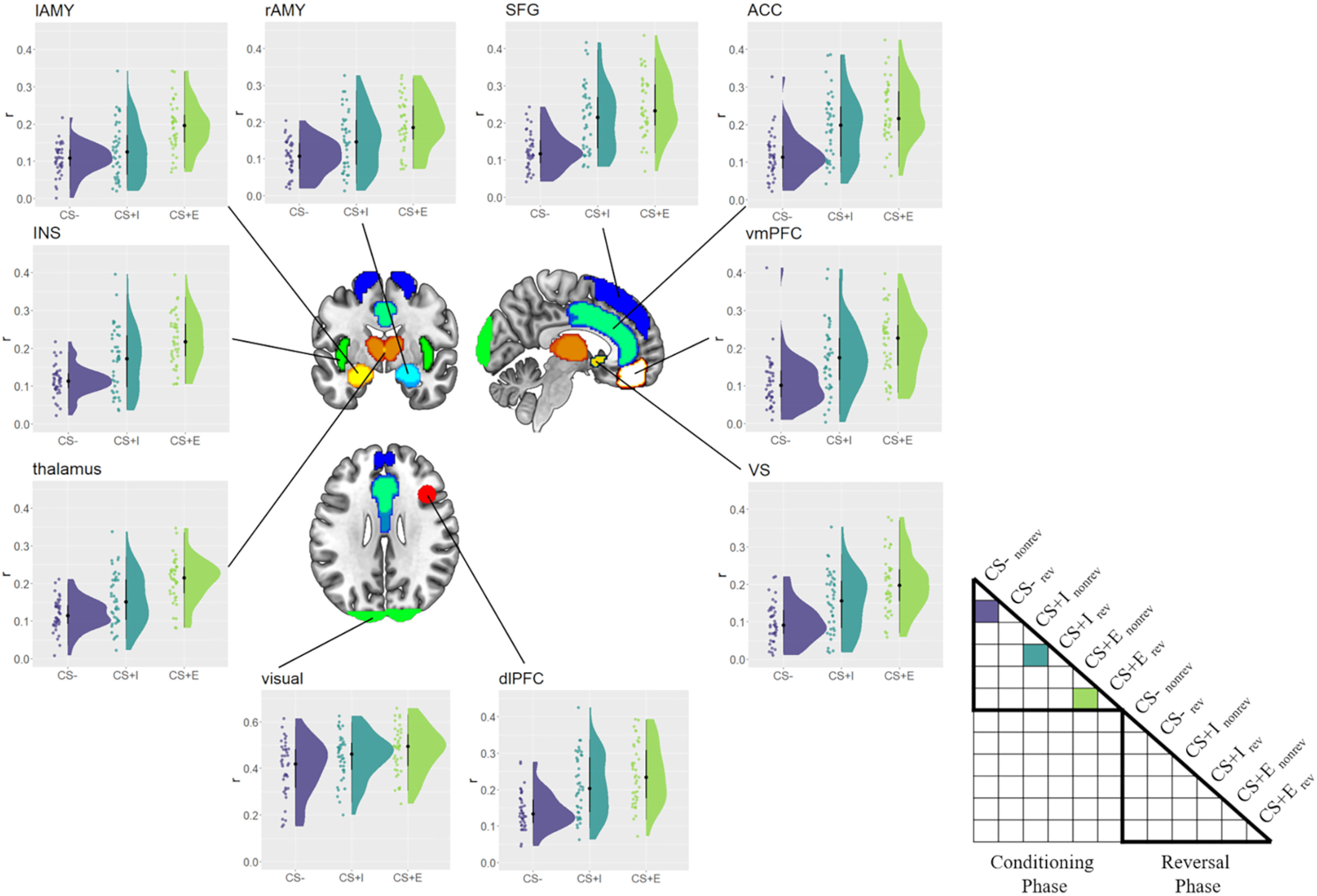
Within-CS consistency in the conditioning phase. Consistency (r) with which safe (CS–), merely instructed (CS+I), and instructed + experienced (CS+E) CSs were represented, separately for each ROI. Raincloud plots depict the raw data as dots on the left side and the data distribution on the right side. The schematic RSM denotes which pairwise representational similarity measures were used in this analysis. rev = reversed, norev = not reversed.
As an additional control analysis, we used this consistency measure to assess the effect of reversal instructions on neural coding of CSs. We reasoned that within-CS consistency should be relatively high in the conditioning phase when both CSs have an identical meaning (both safe or both threatening). Within-CS consistency should be lower in the reversal phase, however, since one CS was reversed while the other was not (one safe and one threatening). We compared consistencies between phases separately for each CS (CS–, CS+I, CS+E) and each ROI, using one-sided Bayesian paired t tests. We found evidence for the expected effect in each ROI, for each CS (CS–: all BF10 values > 4.1, all ES values > 0.033; CS+I: all BF10 values > 150, all ES values > 0.066; CS+E: all BF10 values > 150, ES values > 0.119). This demonstrated that reversal instructions effectively decreased similarity between originally identical CSs.
Reversal phase.
In the reversal phase, no reinforcements were given, making CS+I and CS+E identical with respect to the current, albeit not the past, threat experiences. Therefore, we were unable to repeat the manipulation check reported above, which relied on comparing r(CS+E, CS+I) with r(CS+E, CS–). We were, however, able to investigate the consistency of the voxel pattern response in a manner similar to that in the conditioning phase. Since no US was presented, we first collapsed across the CS+E and CS+I conditions, then separately computed coding consistency for safe CSs (CS–nonreversed, CS+Ireversed, CS+Ereversed) and threatening CSs (CS–reversed, CS+Inonreversed, CS+Enonreversed), respectively. We found coding consistency to be lower for safe CSs than for threatening CSs in the reversal phase in each ROI (all BF10 values > 150, all ES values > 0.077). This mirrors findings from the conditioning phase, although no reinforcements were given here.
Experience effects
Conditioning phase.
To test whether CS–US experience comes with a unique neural trace, we first investigated BOLD responses in the conditioning phase. We reasoned that if experience had an effect above and beyond verbal instructions, different CSs that were actually paired with a US might share more variance with each other than with threatening CSs that were merely instructed. To test this, we first extracted the correlation between CS+Ereversed and CS+Enonreversed during the conditioning phase, which captures any shared variance between two CSs with identical meaning during the conditioning phase but different visual features. To estimate the shared variance between different threatening CSs, we then computed and averaged the following correlations: r(CS+Ereversed, CS+Ireversed), r(CS+Ereversed, CS+Inonreversed), r(CS+Enonreversed, CS+Ireversed), and r(CS+Enonreversed, CS+Inonreversed). This measure served as our baseline and captured any shared variance between different threatening CSs, with different visual features and different CS–US experience, and therefore captures a general threat signal that is independent from CS–US experience. By computing the difference r(CS+Ereversed, CS+Enonreversed) – baseline, we can then test whether two experienced CSs share more variance with each other than they do with other threatening CSs, which were not experienced. We tested this hypothesis using Bayesian one-sided t tests against zero, and this served as one key measure of experience effects on neural coding in the conditioning phase. We found strong evidence for such an experience effect in the conditioning phase in each ROI (BF10 values > 150, ES values > 0.044; Fig. 7). Additionally, we explored whether CS+I values also showed a similar effect. We had no strong a priori hypotheses about this test, and we found an effect that was indistinguishable from 0 in each ROI (BF10 values < 0.37, ES values < 0.0095). These results indicate that during conditioning, different experienced CS+ values share more variance with each other than they do with other, merely instructed CS+ values, indicating experience effects that go beyond verbal threat instructions.
Figure 7.
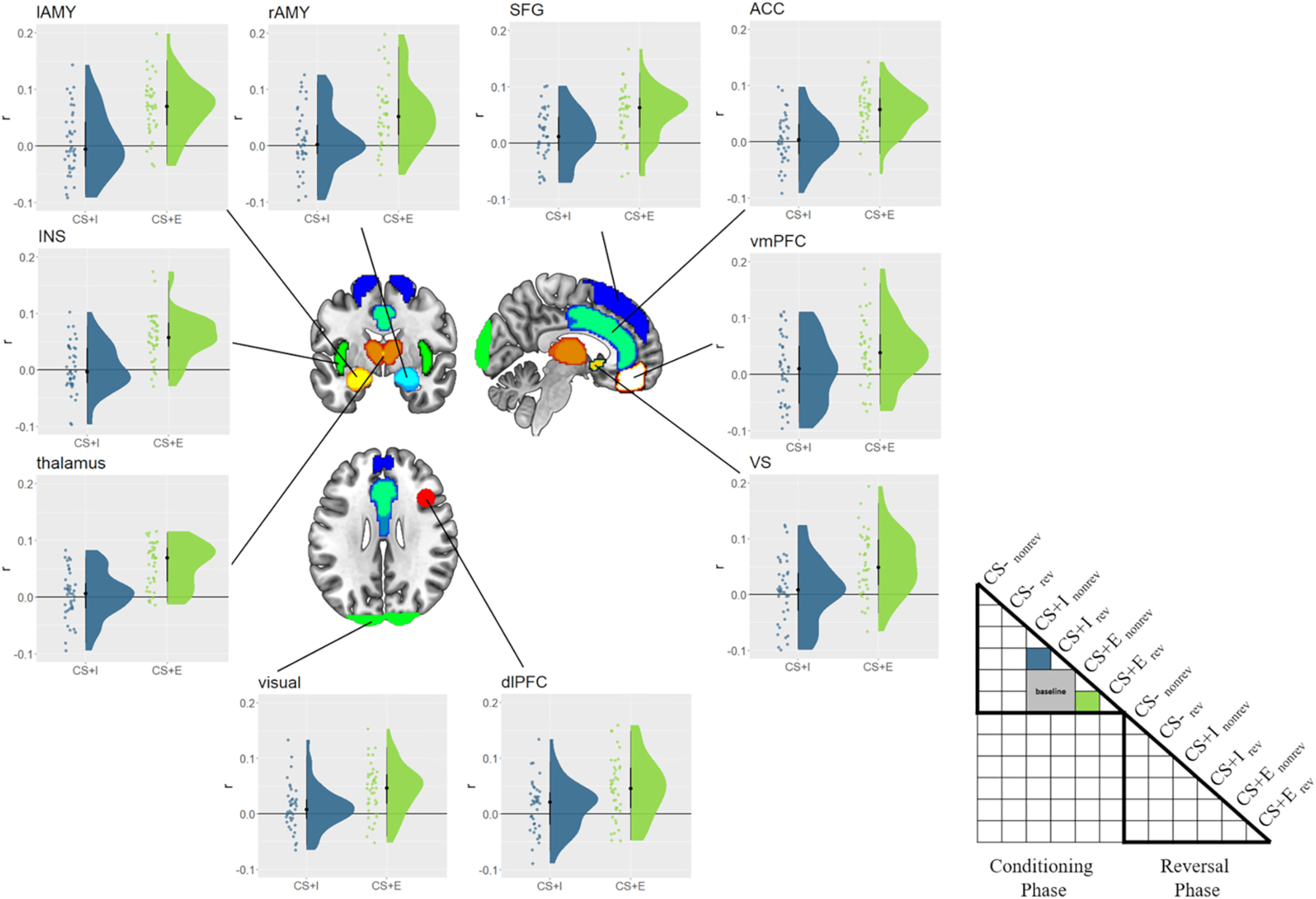
Experience effects in the conditioning phase. Similarity values for merely instructed (CS+I) and instructed + experienced (CS+E) CSs are depicted for each ROI, with the baseline subtracted. Raincloud plots depict the raw data as dots on the left side and the data distribution on the right side. The schematic RSM denotes which pairwise representational similarity measures were used in this analysis, including the baseline conditions (gray). rev = reversed, norev = not reversed.
Reversal phase.
Next, we evaluated our main research question: does the CS–US experience have an effect on neural responses above and beyond verbal instruction effects after reversal? Our behavioral results and prior research showed that verbal instructions alone can fully reverse and explain effects on US expectancy and psychophysiological measures (Mertens and De Houwer, 2016), with no unique effect attributable to CS–US experience. To test whether there was still a trace of CS–US experience in the neural voxel pattern responses, we compared the following three key CSs. (1) CS+Ereversed: we aim to track the lingering trace of a previously threatening and experienced CS, even after verbal instructions that it would now be safe. Translated into our experimental conditions that would mean that we want to test whether CS+Ereversed (was threatening + experienced, is now safe) remains associated with its conditioning history in the reversal phase. (2) CS+Enonreversed: to detect memory traces in CS+Ereversed, we need to compare neural representations to another condition with the same conditioning history. CS+Enonreversed is the optimal choice here, since its meaning was identical to CS+Ereversed during the conditioning phase. Furthermore, because CS+Enonreversed remains threatening even in the reversal phase, the correlation r(CS+Ereversed, CS+Enonreversed) would indicate a lingering trace of prior CS–US experience in CS+Ereversed. (3) CS+Inonreversed: simply testing whether r(CS+Ereversed, CS+Enonreversed) was >0 would not control adequately; for example, baseline visual similarity between different CSs. CS+Inonreversed thus serves as an additional control condition, since it was also threatening in the conditioning phase, and remains threatening in the reversal phase as well, similar to CS+Enonreversed.
To test whether there was still a trace of prior CS–US experience, we therefore computed r(CS+Ereversed, CS+Enonreversed), and hypothesized that it would be larger than r(CS+Ereversed, CS+Inonreversed). This conservative comparison isolates the unique effect of prior experienced threat, while controlling for low-level visual stimulus features, the presence of threat in the conditioning phase, as well as threat/safety instructions. We tested this hypothesis using Bayesian paired t tests, r(CS+Ereversed, CS+Enonreversed) versus r(CS+Ereversed, CS+Inonreversed), separately for each ROI, and expected to find evidence for prior CS–US experience only in the right amygdala. Unexpectedly, we found evidence for prior CS–US experience in all brain regions assessed here (BF10 values > 3.25, ES values > 0.020), except the vmPFC (BF10 = 1.12, ES = 0.013) and right amygdala (BF10 = 0.44, ES = 0.014; Fig. 8). Thus, counter to our initial expectations, most threat-related brain regions showed evidence for the lingering effects of prior CS–US experience, even after verbal reversal instructions.
Figure 8.
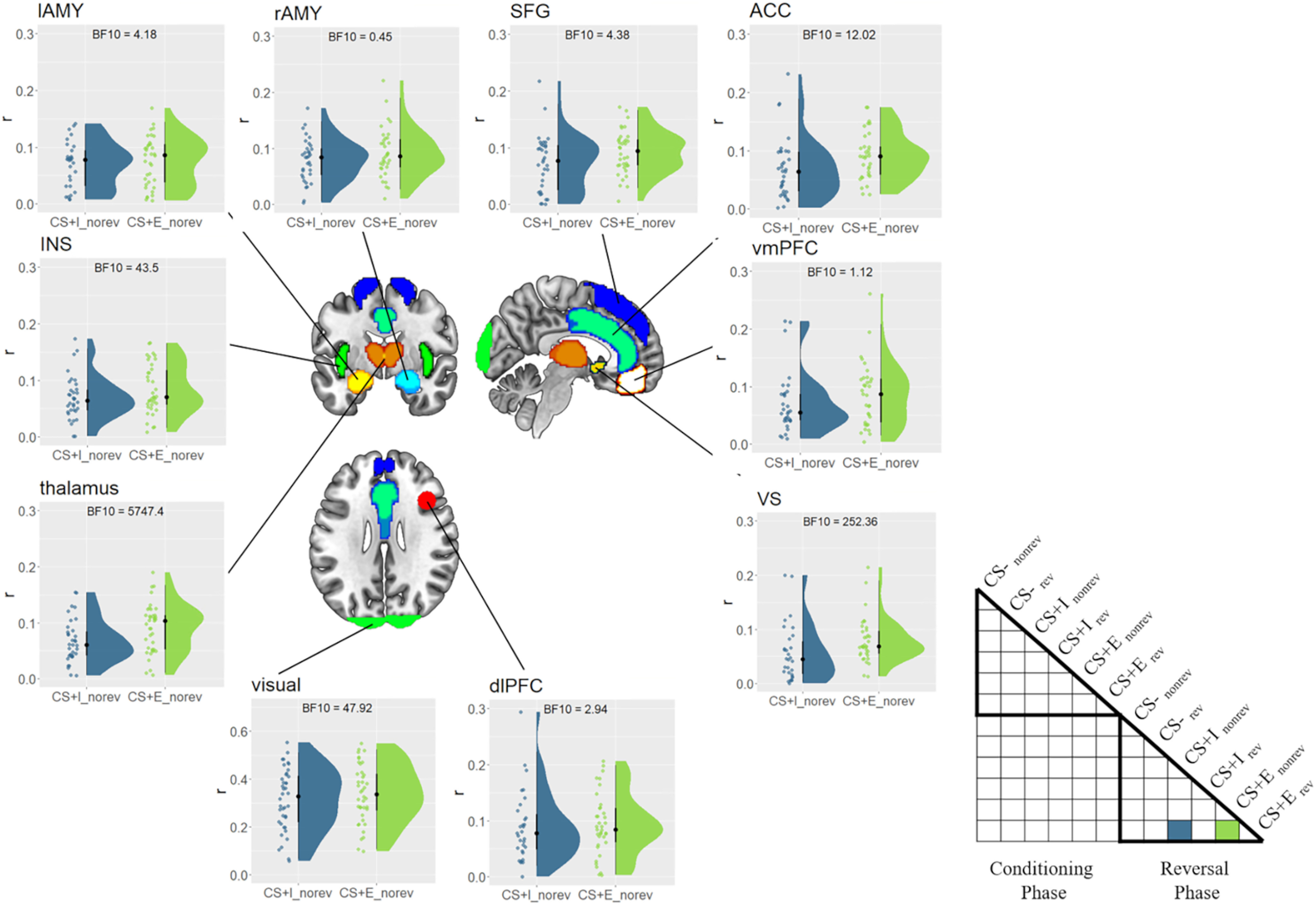
Effects of prior CS–US experience. Similarity between CS+Ereversed (safe) and CS+Inonreversed/CS+Enonreversed (threatening) in the reversal phase, separately for each ROI. Higher similarities for CS+Ereversed/nonreversed indicate a pavlovian trace. Raincloud plots depict the raw data as dots on the left side and the data distribution on the right side. The schematic RSM denotes which pairwise representational similarity measures were used in this analysis. CS = conditions stimulus, CS– = safe stimulus, CS+I = merely instructed stimulus, CS+E = instructed and experienced stimulus, rev = reversed, norev = not reversed.
In an additional post hoc test, we assessed whether these effects decreased over time. We reasoned that even if many fear-related brain regions showed lingering effects of prior CS–US experience, they might still differ in their duration (i.e., in some regions effects might vanish over time, while in others they might persist). We first used GLMblock to calculate r(CS+Ereversed, CS+Enonreversed) versus r(CS+Ereversed, CS+Inonreversed), separately for each of the three blocks in the reversal phase. We then entered these values into a one-factorial Bayesian ANOVA (block), separately for each ROI. We found evidence against any differences between blocks in all ROIs (BF10 values < 0.29, ES values < 0.023), except the rAMY, where evidence remained inconclusive (BF10 = 0.46, ES = 0.032). Thus, the effects of prior CS–US experience seem to be unaffected by presenting the CS without the US and seem to persist across time.
Exploratory analyses: model-based RSA of expectancy and fear ratings
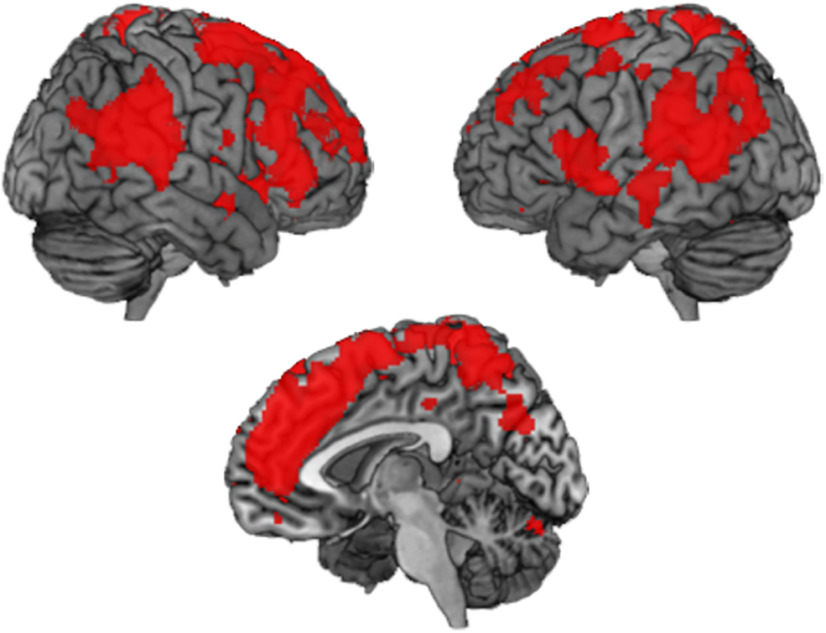
To explore the relation of neural voxel pattern responses and the behavioral ratings in more detail, we also performed an additional exploratory searchlight RSA. Here, we assessed to what degree neuronal activity is related to either US expectancy or fear ratings, respectively. We then tested whether any brain region is more strongly associated with US expectancy than with fear ratings, but we found no significant results (p < 0.05, FWE corrected). The opposite contrast, the fear rating > US expectancy rating, showed widespread results, however, with frontal, parietal, and temporal cortices, as well as insula, being more strongly related to fear than to US expectancy ratings (p < 0.05, FWE corrected; Fig. 9). This shows that most cortical brain regions we used in the ROI analyses are more closely related to fear ratings than they are related to US expectancy ratings, despite the fact that both types of ratings show largely similar behavioral results.
Figure 9.
Model-based searchlight RSA results. Brain regions more strongly associated with fear ratings than with expectancy ratings (p < 0.05, FWE corrected). RSA = representational similarity analysis, FWE = family-wise error rate.
Discussion
In this study, our main goal was to investigate the effects of prior CS–US experience on the effects of verbal reversal instructions during fear reversal. We expected verbal reversal instructions to fully explain neural responses after reversal in all brain regions, except the right amygdala. Here, we expected to find evidence for a pavlovian trace (i.e., a lingering representation of prior experienced CS–US pairings that goes counter to verbal instructions). Using representational similarity analysis, we found that verbal reversal instructions had a profound effect on CS representations. Surprisingly, although we found strong evidence for an effect of verbal reversal instructions and no evidence for lingering effects of experienced CS–US pairings on behavior, almost all fear-related brain regions included here showed evidence for a pavlovian trace.
The interaction of associative learning processes and verbal instructions received increased attention in recent years (for review, see Mertens et al., 2018). Much of the past research focused on understanding the effect of verbal instructions on associative learning, showing faster conditioning (Ugland et al., 2013; Atlas et al., 2016) and delayed extinction (Mertens and De Houwer, 2017) for verbally instructed CS–US pairings, highlighting the role of the dorsal ACC and dorsomedial PFC in verbally mediated fear learning (Mechias et al., 2010). Verbal reversal instructions have been shown to largely reverse acquired fear responses, including startle reflexes (Mertens and De Houwer, 2016; but see Sevenster et al., 2012). Some initial fMRI evidence further suggests that most fear-related brain regions are susceptible to verbal fear instructions, with the exception of the (right) amygdala (Atlas et al., 2016; Braem et al., 2017). Overall, verbal instructions are a powerful tool to alter previously experienced CS–US pairings quickly and efficiently.
Here, we asked the opposite question, however: how does prior CS–US experience affect the efficacy of verbal reversal instructions? Previous evidence from psychophysiological measures suggests that reversal effects can be fully explained through verbal instructions only, with no effect uniquely attributable to prior CS–US experience (Mertens and De Houwer, 2016). However, whether similar effects can be replicated for neural coding of fear-relevant stimuli remained unknown.
CS–US experience effects in the right amygdala and beyond
During initial fear learning, we found that both merely instructed and instructed plus experienced threats led to robust threat responses, albeit mostly in behavior and not in SCRs, and that each fear-relevant ROI dissociated between these two threatening CSs. After establishing that CS–US experience did affect initial fear learning, we used verbal instructions to reverse CS–US pairings and tested whether prior experience modulated the efficacy of these instructions. Please note that although we carefully controlled for US effects in the neural data, we cannot conclusively rule out that results in the conditioning phase might partially reflect responses to the US. Critically, this can be ruled out in the more important reversal phase, since no USs were presented here. As stated above, for most brain regions we expected verbal instructions to fully reverse CS–US pairings, with no residual effect attributable to prior CS–US experience. We hypothesized that the only exception would be the right amygdala, which has a key role in experience-based fear learning more generally (Öhman and Mineka, 2001; Critchley et al., 2002; Knight et al., 2009; Tabbert et al., 2011), and shows lingering threat representations for CS+Es in a static environment (Braem et al., 2017) and when CS+Es (but not CS+Is) were reversed multiple times in an experiment (Atlas et al., 2016). Here, we combined the strength of the latter two studies by directly comparing dynamic reversals of CS+E and CS+I within participants, similar to previous studies (Raes et al., 2014; Mertens and De Houwer, 2016), allowing us to clearly identify any lingering threat representations after verbal reversal instructions were received and to attribute such effects specifically to prior CS–US experience. Still, it should be noted that participants reported a weaker subjective fear response in this experiment than originally anticipated. Although it is difficult to pinpoint the source of this effect, the interleaved staircase US calibration procedure might have at least contributed. Still, we were able to show a neural dissociation between fear and US expectancy ratings, demonstrating that our procedure successfully induced a fear response. Future studies will have to carefully examine how subjective fear responses can be improved, which will likely lead to stronger neural effects as well.
Unexpectedly, and counter to previous findings, we found evidence for a pavlovian trace in almost all brain regions assessed in this study. We found that verbally instructing a CS–US reversal for experienced CSs (CS+Ereversed = previously CS+E, but now safe) led to neural representations being more similar to previously experienced and still threatening CSs (= CS+Enonreversed) than to previously instructed and still threatening CSs (= CS+Inonreversed). The latter condition served as a conservative baseline in this comparison, since the only difference between the compared conditions was the experience of CS–US pairings, keeping threat constant. This pattern of results was found in all ROIs assessed here, with the exception of the vmPFC, and, more interestingly, the right amygdala. Evidence in the latter region was inconclusive though, so that the current data does not allow us to draw strong conclusions about the presence of pavlovian traces in right amygdala.
One reason for this could be that a small subcortical region like the amygdala showed overall weak signal-to-noise ratios, making the detection of reliable voxel pattern responses difficult. Another reason could be our deliberate use of visually distinct but initially functionally identical CSs (e.g., CS+Ereversed and CS+Enonreversed). A region that closely monitors, traces, and represents experienced CS–US pairings might benefit more from separable, rather than similar neural pattern responses to these functionally identical CSs. That is, if the right amygdala kept track of different relevant CSs, it could be that it relies more on dynamically changing, separable neural representations, rather than arguably more inflexible, shared representations. Similarly, it has been shown that prefrontal cortex represents two separate items in working memory by using separable, orthogonal representational subspaces, which allows it to efficiently update or select either of them on demand (Panichello and Buschman, 2021). While it is important to emphasize that this reasoning is post hoc, this could explain why the right amygdala failed to show more similar pattern responses for r(CS+Ereversed, CS+Enonreversed) than for r(CS+Ereversed, CS+Inonreversed). This observation would also explain the different findings in previous studies (Atlas et al., 2016; Braem et al., 2017), which relied on measuring the effect of CS–US experience on neural responses within a single, visually and functionally identical CS.
CS–US experience and instruction validity
While the reasoning above might explain the absence of effects in the right amygdala, the widespread presence of prior CS–US experience effects in other cortical and subcortical brain regions is more difficult to reconcile with past findings. Our post hoc explanation for this unexpected finding is that the current task context might have encouraged participants to rely more heavily on past experiences in evaluating different CS than was the case in previous studies. The design of the merely instructed CS+s (CS+Is), which serve as a control condition in this and previous experiments, is critical here. Previously, CS+Is were implemented by replacing aversive USs with a visual placeholder stimulus (Raes et al., 2014; Mertens and De Houwer, 2016; Braem et al., 2017), with participants being instructed that the placeholder will be replaced with the actual US at some point in the experiment. Although this approach controls for the presence of reinforcement on CS+I trials, placeholders have disadvantages as well. They require a cover story, which participants might not believe, and likely induce additional, potentially interfering cognitive processes, such as expectations about how and when they will be replaced by the US. Therefore, we chose to omit placeholder stimuli in our study, and fully rely on verbal threat instructions only. This allowed us to omit cover stories and significantly reduce the complexity of already complex reversal instructions to participants. Additionally, it has been shown that verbal instructions can lead to consistent fear responses even in the absence of placeholders (Ugland et al., 2013), as also suggested by our fear and US expectancy ratings.
However, we believe that this seemingly subtle design choice potentially had significant effects on the weighting of CS–US experience. The instructions were not 100% valid in the beginning of the experiment (because not all CS+s were actually followed by a shock), as well after the reversal instructions (because no more shocks were given in the second reversal phase). If instructions were perceived as an unreliable source about the threat or safety of CSs, this could have led participants to rely more heavily on their prior experiences. Such an explanation would also indicate that what we originally designed as an analysis of pavlovian traces (i.e., lingering effects of prior CS–US experience) instead could be interpreted as an “instruction validity” effect, which has little to do with low-level fear-learning mechanisms (Öhman and Mineka, 2001). This could also explain why these widespread effects were also present in cortical brain regions commonly associated with verbal instruction implementation (Demanet et al., 2016; Bourguignon et al., 2018). However, systematic investigations will be needed to directly compare this assumed impact of placeholders on instruction validity and weighting or prior experience.
Conclusion
In sum, we demonstrated that CS–US experience showed no additional effect on self-reported fear and US expectancy ratings after reversal instruction, but substantially affected neural pattern responses, far beyond the amygdala. In relation to previous studies, our findings suggest that the effects of CS–US experience might be codependent on the experienced validity of prior instructions, opening up new avenues for future research on experience–instruction interactions in fear reversal learning. Finally, our results have implications for models of fear learning in the right amygdala, bringing important nuance to its role in CS–US experience tracking.
Footnotes
D.W. was supported by was supported by the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant 665501, the Flemish Science Foundation (FWO; Grant FWO.KAN.2019.0023.01), and the Special Research Fund of Ghent University. S.B. was supported by a European Research Council Starting Grant (European Union's Horizon 2020 research and innovation program, Grant 852570). C.G.-G. was funded by European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie Grant 835767, and the Spanish Ministry of Science and Innovation (Grant ID IJC2019-040208-I). J.D.H. was supported by Ghent University Grant BOF16/MET_V/002. M.B. was supported by an Einstein Strategic Professorship (Einstein Foundation Berlin), and the FWO (project G.0231.13).
The authors declare no competing financial interests.
References
- Andraszewicz S, Scheibehenne B, Rieskamp J, Grasman R, Verhagen J, Wagenmakers E-J (2015) An introduction to Bayesian hypothesis testing for management research. J Manag 41:521–543. 10.1177/0149206314560412 [DOI] [Google Scholar]
- Atlas LY (2019) How instructions shape aversive learning: higher order knowledge, reversal learning, and the role of the amygdala. Curr Opin Behav Sci 26:121–129. 10.1016/j.cobeha.2018.12.008 [DOI] [Google Scholar]
- Atlas LY, Phelps EA (2018) Prepared stimuli enhance aversive learning without weakening the impact of verbal instructions. Learn Mem 25:100–104. 10.1101/lm.046359.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Doll BB, Li J, Daw ND, Phelps EA (2016) Instructed knowledge shapes feedback-driven aversive learning in striatum and orbitofrontal cortex, but not the amygdala. eLife 5:e15192. 10.7554/eLife.15192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon NJ, Braem S, Hartstra E, De Houwer J, Brass M (2018) Encoding of novel verbal instructions for prospective action in the lateral prefrontal cortex: evidence from univariate and multivariate functional magnetic resonance imaging analysis. J Cogn Neurosci 30:1170–1184. 10.1162/jocn_a_01270 [DOI] [PubMed] [Google Scholar]
- Braem S, Houwer JD, Demanet J, Yuen KSL, Kalisch R, Brass M (2017) Pattern analyses reveal separate experience-based fear memories in the human right amygdala. J Neurosci 37:8116–8130. 10.1523/JNEUROSCI.0908-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa VD, Bradley MM, Lang PJ (2015) From threat to safety: instructed reversal of defensive reactions. Psychophysiology 52:325–332. 10.1111/psyp.12359 [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ (2002) Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron 33:653–663. 10.1016/s0896-6273(02)00588-3 [DOI] [PubMed] [Google Scholar]
- Demanet J, Liefooghe B, Hartstra E, Wenke D, De Houwer J, Brass M (2016) There is more into “doing” than “knowing”: the function of the right inferior frontal sulcus is specific for implementing versus memorising verbal instructions. Neuroimage 141:350–356. 10.1016/j.neuroimage.2016.07.059 [DOI] [PubMed] [Google Scholar]
- Etzel JA, Zacks JM, Braver TS (2013) Searchlight analysis: promise, pitfalls, and potential. Neuroimage 78:261–269. 10.1016/j.neuroimage.2013.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ (1994) Statistical parametric maps in functional neuroimaging: A general linear approach. Hum Brain Mapp 4:189–210. [Google Scholar]
- Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, Radua J (2016) Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry 21:500–508. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, Bandettini PA (2009) Neural substrates of explicit and implicit fear memory. Neuroimage 45:208–214. 10.1016/j.neuroimage.2008.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koban L, Jepma M, Geuter S, Wager TD (2017) What's in a word? How instructions, suggestions, and social information change pain and emotion. Neurosci Biobehav Rev 81:29–42. 10.1016/j.neubiorev.2017.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P (2008) Representational similarity analysis – connecting the branches of systems neuroscience. Front Syst Neurosci 2:4. 10.3389/neuro.06.004.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, et al. (2017) Don't fear “fear conditioning”: methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev 77:247–285. 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH (1971) Direct measurement of skin conductance: a proposal for standardization. Psychophysiology 8:656–672. 10.1111/j.1469-8986.1971.tb00501.x [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239. 10.1016/s1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. 10.1146/annurev.neuro.24.1.897 [DOI] [PubMed] [Google Scholar]
- Mechias M-L, Etkin A, Kalisch R (2010) A meta-analysis of instructed fear studies: implications for conscious appraisal of threat. Neuroimage 49:1760–1768. 10.1016/j.neuroimage.2009.09.040 [DOI] [PubMed] [Google Scholar]
- Mertens G, De Houwer J (2016) Potentiation of the startle reflex is in line with contingency reversal instructions rather than the conditioning history. Biol Psychol 113:91–99. 10.1016/j.biopsycho.2015.11.014 [DOI] [PubMed] [Google Scholar]
- Mertens G, De Houwer J (2017) Can threat information bias fear learning? Some tentative results and methodological considerations. J Exp Psychopathol 8:390–412. 10.5127/jep.060616 [DOI] [Google Scholar]
- Mertens G, Boddez Y, Sevenster D, Engelhard IM, De Houwer J (2018) A review on the effects of verbal instructions in human fear conditioning: empirical findings, theoretical considerations, and future directions. Biol Psychol 137:49–64. 10.1016/j.biopsycho.2018.07.002 [DOI] [PubMed] [Google Scholar]
- Nili H, Wingfield C, Walther A, Su L, Marslen-Wilson W, Kriegeskorte N (2014) A toolbox for representational similarity analysis. PLoS Comput Biol 10:e1003553. 10.1371/journal.pcbi.1003553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A, Mineka S (2001) Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychol Rev 108:483–522. 10.1037/0033-295x.108.3.483 [DOI] [PubMed] [Google Scholar]
- Olsson A, Phelps EA (2007) Social learning of fear. Nat Neurosci 10:1095–1102. 10.1038/nn1968 [DOI] [PubMed] [Google Scholar]
- Panichello MF, Buschman TJ (2021) Shared mechanisms underlie the control of working memory and attention. Nature 592:601–605. 10.1038/s41586-021-03390-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes AK, De Houwer J, De Schryver M, Brass M, Kalisch R (2014) Do CS-US pairings actually matter? A within-subject comparison of instructed fear conditioning with and without actual CS-US pairings. PLoS One 9:e84888. 10.1371/journal.pone.0084888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riberto M, Paz R, Pobric G, Talmi D (2022) The neural representations of emotional experiences are more similar than those of neutral experiences. J Neurosci 42:2772–2785. 10.1523/JNEUROSCI.1490-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmalz X, Biurrun Manresa J, Zhang L (2023) What is a Bayes factor? Psychol Methods 28:705–718. 10.1037/met0000421 [DOI] [PubMed] [Google Scholar]
- Sevenster D, Beckers T, Kindt M (2012) Instructed extinction differentially affects the emotional and cognitive expression of associative fear memory. Psychophysiology 49:1426–1435. 10.1111/j.1469-8986.2012.01450.x [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ (1999) Measuring anxiety and anger with the state-trait anxiety inventory (STAI) and the state-trait anger expression inventory (STAXI). In: The use of psychological testing for treatment planning and outcomes assessment, Ed 2 (Maruish ME, ed), pp 993–1021. Mahwah, NJ: Erlbaum. [Google Scholar]
- Tabbert K, Merz CJ, Klucken T, Schweckendiek J, Vaitl D, Wolf OT, Stark R (2011) Influence of contingency awareness on neural, electrodermal and evaluative responses during fear conditioning. Soc Cogn Affect Neurosci 6:495–506. 10.1093/scan/nsq070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugland CCO, Dyson BJ, Field AP (2013) An ERP study of the interaction between verbal information and conditioning pathways to fear. Biol Psychol 92:69–81. 10.1016/j.biopsycho.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Visser RM, Scholte HS, Kindt M (2011) Associative learning increases trial-by-trial similarity of BOLD-MRI patterns. J Neurosci 31:12021–12028. 10.1523/JNEUROSCI.2178-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser RM, Scholte HS, Beemsterboer T, Kindt M (2013) Neural pattern similarity predicts long-term fear memory. Nat Neurosci 16:388–390. 10.1038/nn.3345 [DOI] [PubMed] [Google Scholar]
- Walther A, Nili H, Ejaz N, Alink A, Kriegeskorte N, Diedrichsen J (2016) Reliability of dissimilarity measures for multi-voxel pattern analysis. Neuroimage 137:188–200. 10.1016/j.neuroimage.2015.12.012 [DOI] [PubMed] [Google Scholar]