Abstract
Streptococcus thermophilus Sfi6 produces a texturizing exopolysaccharide (EPS) consisting of a →3)[α-d-Galp-(1→6)]-β-d-Glcp-(1→3)-α-d-GalpNAc-(1→3)-β-d-Galp-(1→ repeating unit. We previously identified and analyzed a 14.5-kb gene cluster from S. thermophilus Sfi6 consisting of 13 genes responsible for its EPS production. Within this gene cluster, we found a central region of genes (epsE, epsF, epsG, and epsI) that showed similarity to glycosyltransferases. In this study, we investigated the sugar specificity of these enzymes. EpsE catalyzes the first step in the biosynthesis of the EPS repeating unit. It exhibits phosphogalactosyltransferase activity and transfers galactose onto the lipophilic carrier. The second step is fulfilled by EpsG, which transfers an α-N-acetylgalactosamine onto the first β-galactoside. The activity of EpsF was determined by characterizing the EPS produced by an S. thermophilus epsF deletion mutant. This EPS consisted of the monosaccharides Gal, Glc, and GalNAc in an approximately equimolar ratio, thus suggesting that epsF codes for the branching galactosyltransferase. epsI probably codes for the β-1,3-glucosyltransferase, since it is the only glycosyltransferase to which no gene has been assigned and it exhibits similarity to other β-glycosyltransferases. EpsE shows the conserved features of phosphoglycosyltransferases, whereas EpsF and EpsG exhibit the primary structure of α-glycosyltransferases, belonging to glycosyltransferase family 4, whose members are conserved in all major phylogenetic lineages, including the Archaea and Eukaryota.
Extracellular polysaccharides have a wide array of functions in bacteria and can play roles as varied as protection from desiccation or improvement of adherence, pathogenesis, or symbiosis (19, 20, 31, 32). They may take the form of capsules attached to the cell membrane or be secreted extracellularly. More commonly, in gram-negative bacteria they are present in the form of the O antigen of the lipopolysaccharide, and in gram-positive bacteria they occur as cell wall polysaccharides. Besides their biological role, polysaccharides are also of industrial interest because of their rheological properties. Exopolysaccharides (EPS) produced by lactic acid bacteria have in particular attracted the attention of the food industry because of their GRAS (generally regarded as safe) status. This has resulted in the elucidation of a large number of varied EPS structures from gram-positive Streptococcus (3, 5, 12), Lactococcus (7, 17), and Lactobacillus (8, 21–24, 26, 28, 37) strains.
While investigation of polysaccharide gene clusters in gram-negative bacteria began over 20 years ago (31, 36), research on those of gram-positive microorganisms has advanced only lately. Recent reports include the characterization of genes involved in polysaccharide biosynthesis from the pathogens Staphylococcus aureus and Streptococcus pneumoniae (11, 13, 16) and from the food microorganisms Streptococcus thermophilus and Lactococcus lactis (27, 33). The general organization of these clusters seems to be conserved: a central region with similarity to glycosyltransferase genes is flanked by two regions exhibiting similarity to genes involved in polymerization and export, and a putative regulatory region can be found at the beginning of each cluster. Even though this organization is not always conserved among genes involved in O-antigen synthesis, their homology points to similar biosynthetic pathways (10, 36). The repeating unit is first assembled by the sequential transfer of sugar residues onto a lipophilic carrier by specific glycosyltransferases. Unlike the other glycosyltransferases, the first glycosyltransferase does not catalyze a glycosidic linkage but transfers a sugar-1-phosphate onto a lipophilic anchor, such as undecaprenylphosphate. Subsequently, the completed repeating unit is exported and polymerized. In the case of cell surface polysaccharides, it is anchored to a cell envelope component while secreted polysaccharides are released.
Of the enzymes required for the biosynthesis of EPS, we were especially interested in the glycosyltransferases, because their sugar specificities determine the nature of the polysaccharide. Understanding and being able to predict their function are prerequisites for their use in carbohydrate bioengineering. However, even though a large number of polysaccharide gene clusters have been sequenced, only a very limited number of glycosyltransferases have been biochemically characterized (11, 34). Little is known about their sugar acceptor/donor specificities and their active centers.
Here we present a functional analysis of the S. thermophilus Sfi6 glycosyltransferase gene region, comprising the epsE-epsF-epsG-epsH-epsI genes, which are part of the previously characterized eps gene cluster (27, 29). Except for epsH, which shows homology to genes encoding O-acetyltransferases (2), all genes located in this region exhibit homology to glycosyltransferases involved in polysaccharide biosynthesis. By using a combination of biochemical and genetic approaches, we were able to shed light on the sugar specificity of the glycosyltransferases.
MATERIALS AND METHODS
Bacterial strains and media.
S. thermophilus Sfi6 (Nestlé culture collection accession no. NCC1971) was grown at 42°C in M17 broth (Difco Laboratories) supplemented with 1% (wt/vol) lactose. E. coli XL1-Blue (Stratagene), EC101 (15), and DH5α (1) were grown in Luria broth medium at 37°C (1). If required, the medium contained kanamycin (50 μg/ml) and/or ampicillin (100 μg/ml).
Construction of plasmids for glycosyltransferase assays and in vitro analysis of protein expression.
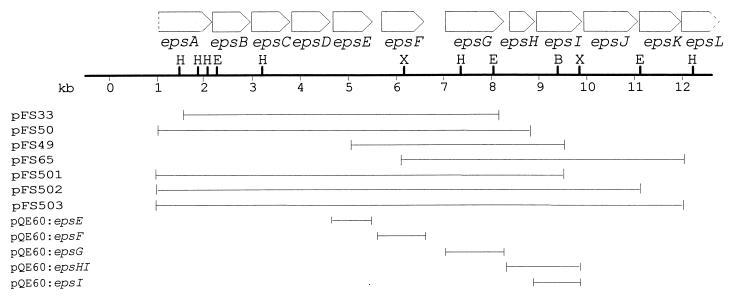
Plasmids pFS26, pFS30, pFS33, pFS49, pFS50, pFS65, and pFS80 were previously recovered from an S. thermophilus Sfi6 genomic library in λ-ZAP (27). Construction of the plasmids illustrated in Fig. 1 was performed as follows. Plasmids pFS502 and pFS503 were obtained by constructing the intermediary plasmid pFS501. pFS501 was constructed by cloning the 3.5-kb XmnI-NotI fragment of pFS49 into pFS50 previously cut with XmnI and NotI, which is located on the right side 3′ of the multiple cloning site of the pFS plasmids. Subsequently, to obtain pFS502, the central 3-kb EcoRI fragment of pFS65 was ligated into pFS501 partially digested with EcoRI. pFS503 was created by ligating the 2.7-kb BsgI-NotI fragment of pFS65 into pFS501 previously digested with BsgI and NotI. The series of plasmids derived from pQE60 (Qiagen) was constructed by amplifying the single glycosyltransferase genes with the high-fidelity Pfu polymerase (Stratagene) and cloning them into pQE60. The oligonucleotide primer pairs for the amplification of the different genes were designed in such a way that they included 18 bp of sequence of the 5′ or the 3′ end of the respective eps gene, a BamHI site (for the oligonucleotide primer complementary to the 3′ end of the gene) or an NcoI site (for the oligonucleotide primer complementary to the 5′ end, with the ATG of the start codon corresponding to the ATG of the NcoI site), and three additional, irrelevant nucleotides. Since the oligonucleotide primers contained the restriction sites NcoI and BamHI, respectively, the amplified PCR product could be digested with these enzymes and ligated into the previously NcoI- and BamHI-digested pQE60 vector. For the pFS and the pQE plasmid series, the plasmids were constructed in E. coli XL1-Blue and transformed into E. coli DH5α to assay the activity of the glycosyltransferases (11).
FIG. 1.
Schematic illustration of the plasmid constructs used for glycosyltransferase assays. The pFS series of plasmids is derived from pBK-CMV, whereas the pQE series is derived from the pQE60 vector. Restriction enzyme abbreviations are as follows: H, HindIII; E, EcoRI; B, BsgI; and X, XmnI. The construction of the plasmids is described in Materials and Methods.
Analysis of glycosyltransferase gene expression was performed with the E. coli T7 S30 extract system (Promega), which is an in vitro coupled T7 polymerase transcription-translation system. To reveal the synthesized proteins, the extract was incubated with [35S]methionine, the reaction mixture was loaded onto a sodium dodecyl sulfate–12% (wt/vol) polyacrylamide gel and subjected to autoradiography. Before the transcription-translation assay was performed, the plasmids were precipitated with 1/9 volume of 3 M sodium acetate and 2 volumes of ethanol and resuspended in RNase-free water. One microgram of each plasmid was employed for the reaction. The assay was carried out in accordance with the supplier’s instructions.
Isolation of glycosyltransferase-containing membranes.
Hydropathy plots of glycosyltransferases predicted a membrane location, and thus their activity was measured with isolated cell membranes as the enzyme source. A 200-ml Luria broth medium culture with the appropriate antibiotic (kanamycin for pFS plasmids and ampicillin for pQE plasmids) was started by the inoculation of 10 ml of an overnight culture, grown until it reached an optical density at 600 nm of 0.8, and induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h. Subsequently, the cells were harvested by centrifugation at 6,000 × g for 10 min at 4°C, washed in buffer A (50 mM Tris-acetate [pH 8.0], 1 mM dithiothreitol, 1 mM EDTA, 10% [vol/vol] glycerol), and centrifuged as before. The cells were resuspended in 10 ml of buffer A containing 1 mM phenylmethylsulfonyl fluoride and passed twice through a French press at 1,500 lb/in2. Unbroken cells were removed by a low-speed centrifugation (6,000 × g, 15 min, 4°C), and the membranes were collected from the supernatant by ultracentrifugation at 100,000 × g for 1 h. The pellet was resuspended in 2 ml of buffer A, aliquoted, and frozen at −80°C. The protein concentration in membranes was measured by the Lowry method in presence of 0.5% (wt/vol) sodium dodecyl sulfate (1) and was used to quantify the membrane yield. Typically this protocol yields a 10-mg/ml protein concentration.
Glycosyltransferase assay.
The glycosyltransferase assay was performed as described by Kolkman et al. (11). The reaction mixture contained 100 μl (≈1 mg) of membranes, 50 mM Tris acetate (pH 8.0), 10 mM MgCl2, 1 mM EDTA, and 1 μl (≈25 nCi) of UDP-[14C]glucose, UDP-[14C]galactose, or UDP-N-acetyl-[14C]galactosamine in a total volume of 150 μl. If unlabeled sugar nucleotides were added, they had a final concentration of 0.5 mM. The reaction was incubated at 10°C for 1 h and stopped by the addition of 2 ml of a chloroform-methanol mixture (2:1). The solution was vigorously vortexed and then incubated at room temperature for 30 min. Unincorporated sugar nucleotides were removed by extracting the organic phase three times with 0.4 ml of PSUP (15 ml of chloroform, 250 ml of methanol, 1.83 g of KCl, and 235 ml of H2O). The lower phase was vacuum dried, and the incorporated radioactivity was either counted in a scintillation counter and expressed as specific activity (in counts per minute per milligram of protein) or further analyzed by thin-layer chromatography (TLC).
Analysis of lipid-linked precursors by TLC.
The dried lipid-linked precursors were resuspended in 200 μl of 1-butanol and distributed into two tubes. One hundred microliters was subjected to mild acid hydrolysis by adding 100 μl of 50 mM trifluoroacetic acid (TFA) and heating the sample at 90°C for 20 min. The other 100 μl was subjected to strong acid hydrolysis by adding 4 M TFA and incubating the sample at 125°C for 1 h. The butanol-TFA mixtures were dried in a Speed Vac (SAVANT) and resuspended in 5 μl of a solution of carrier sugars in 40% (vol/vol) isopropanol (5 mg each of glucose, galactose, galactosamine, maltose, lactose, maltotriose, and maltotetraose/ml). The hydrolyzed precursors were loaded onto a 10- by 10-cm high-performance TLC silica gel 60 plate (Merck) and developed three times in chloroform-acetic acid-water (6:7:1). The TLC plate was autoradiographed with an LE-Transcreen screen (Kodak) and Biomax MS film (Kodak), with exposure for 16 to 24 h. The carrier sugars were visualized by spraying the plate with 5% (vol/vol) H2SO4 in ethanol and heating the plate at 100°C for 15 min.
Construction of an epsF deletion mutant of S. thermophilus Sfi6 (S. thermophilus Sfi6-55) and characterization of the EPS produced.
The deletion of epsF in S. thermophilus Sfi6 was achieved by a double-crossover mechanism, using the temperature-sensitive vector pGhost9 (15). One-kilobase fragments flanking the 5′ (oligonucleotide primers 5′-GACTCGAGTCTTTTGGTTACAGCCG and 5′-GAGCGGCCGCTCCACCACTCATCGCT) and the 3′ (oligonucleotide primers 5′-CAGCGGCCGCAGTGTAAGTTGTCAAATG and 5′-TAATCTGCAGTGCATCCATTTTCGCTG) regions of epsF were amplified with Pwo polymerase (Roche Molecular Biochemicals). The 5′ and the 3′ flanking fragments contained the restriction sites XhoI and NotI or NotI and PstI, respectively, and were joined in a trimolecular reaction by digestion with the appropriate enzymes and ligation into vector pGhost9 previously digested with XhoI and PstI. The ligation mixture was transformed into E. coli EC101, and a correctly ligated clone, pJN-M11, was selected. pJN-M11 was transformed into S. thermophilus as described previously (27), and the culture was plated at 32°C on M17 containing 1% (wt/vol) lactose and 2.5 μg of erythromycin/ml. The subsequent integration of pJN-M11 into the S. thermophilus Sfi6 genome (culturing at 42°C) and its resolution (culturing at 32°C in the absence of antibiotic) were performed as previously described (15). Deletion mutants obtained were screened by PCR with oligonucleotide primers flanking epsF, and six epsF deletion mutants were further confirmed by Southern hybridization and sequence determination of the epsE-epsG region.
One positive isolate, S. thermophilus Sfi6-55, was selected for biochemical characterization of its EPS. A 1-liter fermentation was carried out, and the EPS was isolated and purified as previously described (29). To determine the monosaccharide composition of the EPS, a 1-mg sample was hydrolyzed with 4 M TFA at 120°C for 1 h and lyophilized. Subsequently, the sample was resuspended in H2O at a concentration of 100 μg/ml and injected onto a CarboPac PA1 column (4 by 250 mm) of a Dionex LC carbohydrate system with amperometric detection (carbohydrate potentials on ED40). For comparison of the monosaccharide ratios, the same analysis was carried out in parallel with the EPS of S. thermophilus Sfi6.
Computational analysis of protein sequences.
Amino acid sequence homology searches were carried out in the National Center for Biotechnology Information databases with the advanced BLAST 2.0 (basic logical alignment search tool) software. Multiple alignments were performed with PILEUP from the Genetics Computer Group (GCG) Wisconsin Sequence Analysis Package. Hydrophobic cluster analysis (HCA) was performed with the HCA-plot program (Doriane informatique, Le Chesney, France). This program writes protein sequences on a duplicated α-helical net and circles clusters of hydrophobic amino acids (Ala, Val, Ile, Mat, Phe, and Gln). The plots were visually compared for similarity in their hydrophobic cluster patterns, with analysis limited to the predicted globular structures of the proteins. β-Strands and α-helices were deduced based on the observed association of specific hydrophobic cluster shapes with secondary structure.
RESULTS
EpsE has phosphogalactosyltransferase activity.
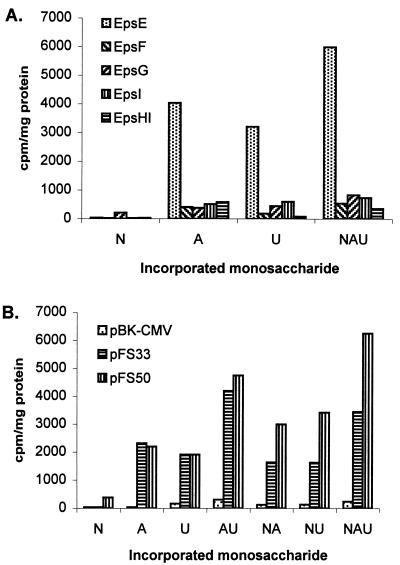
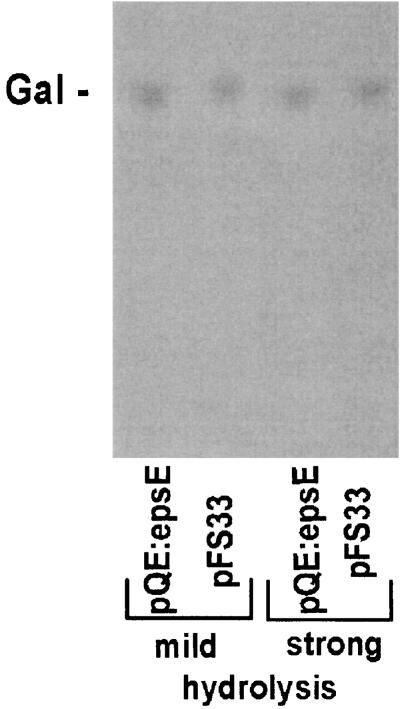
To test the activity of predicted glycosyltransferases, plasmids illustrated in Fig. 1 were constructed and expressed in E. coli DH5α. We previously reported that EpsE exhibits similarity to many phosphogalactosyltransferases and phosphoglucosyltransferases (27). This activity was confirmed by the incorporation studies shown in Fig. 2A. pQE60:epsE is able to promote the incorporation of Gal or Glc from labeled UDP-[14C]galactose and UDP-[14C]glucose into the lipophilic fraction. Since E. coli contains a UDP-glucose C4 epimerase (GalE), it is not possible to conclude whether this is due to a galactosyl- or glucosyltransferase activity, but the slightly (approximately 20%) higher incorporation attained with UDP-[14C]galactose suggests that it may be a galactosyltransferase. TLC analysis after weak and strong hydrolysis showed that this first sugar is galactose (Fig. 3, lanes 1 and 3), indicating that epsE codes for the phosphogalactosyltransferase initiating the biosynthesis of the repeating unit.
FIG. 2.
Incorporation of N-acetylgalactosamine (N), galactose (A), or glucose (U), or combinations thereof, from the respective sugar nucleotides into the membrane fraction by E. coli DH5α harboring the constructs shown in Fig. 1. (A) Expression of single glycosyltransferases EpsE, EpsF, EpsG, EpsI, and EpsHI via pQE60. Strain DH5α or DH5α with the vector alone incorporates very low levels of [14C]GalNAc (15 to 20 cpm/mg of protein) and between 100 and 500 cpm of [14C]galactose or [14C]glucose/mg of protein, depending on the membrane preparation and the type of experiment (single, double, or triple labeling). The values under 500 cpm/mg of protein are therefore not significant. (B) Coexpression of glycosyltransferases EpsE and EpsF (pFS33) and EpsE, EpsF, and EpsG (pFS50), respectively. pBK-CMV is the designation for the vector used for pFS33 and pFS50.
FIG. 3.
TLC analysis of lipid-linked precursors from E. coli DH5α harboring pQE:epsE or pFS33 after incubation with UDP-[14C]galactose. The standard sugar galactose is indicated as Gal. The bottom of the TLC image represents its origin.
EpsG has N-acetylgalactosaminyltransferase activity.
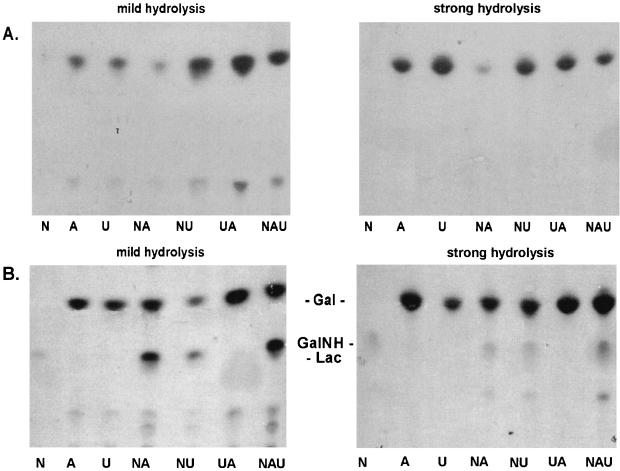
To investigate the function of the next glycosyltransferase, plasmid constructs pFS33 and pFS50 were utilized. Membranes from E. coli expressing pFS33 (containing only epsE and epsF) showed the same incorporation characteristics and TLC labeling pattern as pQE60:epsE when the glycosyltransferase assay was performed with UDP-[14C]galactose (Fig. 3). When the glycosyltransferase assay was performed with the labeled nucleotide sugars in all combinations, the only sugar incorporated was still Gal (Fig. 4A). Therefore, EpsF probably does not participate in the second step of the synthesis of the EPS repeating unit. On the other hand, membranes from E. coli expressing pFS50 (containing epsE, epsF, and epsG) showed incorporation of GalNAc from UDP-[14C]N-acetylgalactosamine (Fig. 2B): while levels of incorporation of Gal and Glc by pFS33 and pFS50 were similar (2,000 cpm/mg of protein for single labeling and 4,000 cpm/mg of protein for double labeling), the values were approximately twofold higher for pFS50 when labeled UDP-GalNAc was added to the reaction. The low level of GalNAc incorporation (400 cpm/mg of protein) from single UDP-GalNAc labeling was probably due to the presence of the lipid-linked Gal, produced by EpsE with endogenous UDP-Gal. Analysis of the incorporated material by TLC after mild hydrolysis revealed that a disaccharide, comigrating with lactose, had been produced only with UDP-[14C]N-acetylgalactosamine (Fig. 4B). Addition of UDP-[14C]galactose or UDP-[14C]glucose caused an enhancement of the disaccharide spot. Strong hydrolysis of the disaccharide showed that it consisted of Gal and GalNAc, which was revealed as galactosamine because of the breakage of the amide bond under conditions of strong hydrolysis. Since pFS33 and pFS50 differ only in the presence of epsG in pFS50 and epsG is functional without epsF (see below), the corresponding gene product accounts for the N-acetylgalactosaminyltransferase activity. This incorporation pattern is in agreement with the biosynthesis of the EPS repeating unit of the S. thermophilus Sfi6, in which an α-GalNAc is 1-3 linked to a β-Gal.
FIG. 4.
TLC analysis of lipid-linked precursors from E. coli DH5α harboring pFS33 (A) or pFS50 (B). Cell membranes of the recombinant E. coli cells were incubated with either UDP-[14C]galactose (A), UDP-[14C]glucose (U), UDP-[14C]N-acetylgalactosamine (N), or combinations thereof. The lipid-linked precursors were subjected to either mild or strong hydrolysis. Gal, GalNH, and Lac indicate the positions of the standard sugars galactose, galactosamine, and lactose, respectively. The bottom of the TLC image represents its origin.
Analysis of EpsF and EpsI activities and their expression.
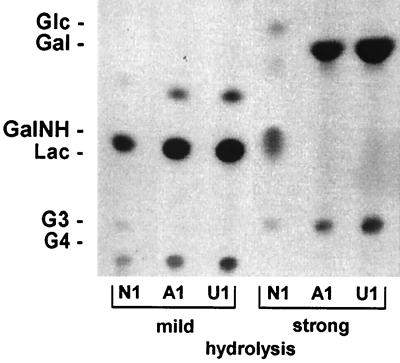
Since the activities of EpsE and EpsG could be determined biochemically, the analysis of the remaining glycosyltransferases was undertaken in a similar fashion. pFS502 and pFS503 were expressed in E. coli, and the glycosyltransferase activities were assayed as described above. As shown in Fig. 5, on the TLC autoradiograph of the labeled precursors subjected to mild hydrolysis, the Gal and the GalNAc-Gal intermediates were still present, but no spot could be detected in the region of tri- and tetrasaccharides (left three lanes). In the lanes in which the precursors were treated by strong hydrolysis, only the spots migrating to positions corresponding to Gal and GalNH appeared, and none migrated to the position corresponding to Glc, which would be the third incorporated sugar and would migrate to a position just above Gal (right three lanes). To evaluate whether the lack of activity was due to inefficient expression, we examined the epsF and epsI gene products in an in vitro transcription-translation assay. In an extract containing pFS30, clear protein bands in the region of the predicted molecular masses of EpsG (42.5 kDa), EpsB (28.0 kDa), EpsD (27.0 kDa), and EpsC (25.0 kDa), and EpsE (25.0 kDa) were detectable, but none were evident around 36.3 kDa, the predicted molecular mass of EpsF. Similarly, no distinct band could be detected for EpsI when plasmid pFS26 was employed (data not shown). Even though in vitro expression data does not always reflect the situation in vivo, these results strongly suggest that the lack of EpsF and EpsI activity in glycosyltransferase assays could be due to expression problems rather than inadequate assay conditions.
FIG. 5.
TLC analysis of lipid-linked precursors from E. coli DH5α harboring pFS502. Cell membranes of the recombinant E. coli cells were incubated with either UDP-[14C]galactose (A1), UDP-[14C]glucose (U1), or UDP-[14C]N-acetylgalactosamine (N1) and the other two sugar nucleotides (unlabeled). With membranes from E. coli DH5α harboring pFS503, the same pattern was obtained (data not shown). Gal, GalNH, and Lac indicate the positions of the standard sugars galactose, galactosamine, and lactose, respectively. The bottom of the TLC image represents its origin. The high-molecular-weight spots migrating below G4 probably represent unhydrolyzed glycolipids.
S. thermophilus Sfi6-55 produces an EPS composed of Gal, Glc, and GalNAc in an equimolar ratio.
Since we were unable to directly show the sugar specificity of EpsF expressed in E. coli, we took a genetic approach to investigate its role in EPS biosynthesis. An epsF deletion mutation was constructed in S. thermophilus Sfi6; the ATG start codon of epsG was placed at the position of the original start codon of epsF, thereby deleting the region between the epsF and the epsG start codons. This mutant had the same growth rate as the wild type and also produced a high-molecular-weight EPS. However, an EPS monosaccharide composition of Glc, Gal, and GalNAc in the molar ratio 1:1.30:0.90 was determined. Since the EPS from the wild-type strain S. thermophilus Sfi6 has a Glc/Gal/GalNAc ratio of 1:2:1, the approximately equimolar sugar ratio in the EPS of the epsF deletion mutant indicates that epsF encodes the branching α-1,6-galactosyltransferase. Furthermore, it shows that EpsG is able to perform its activity in the absence of EpsF, a fact that could not be entirely excluded by the TLC analysis of labeled precursors obtained with E. coli membranes expressing pFS50.
The GalNAc-Gal disaccharide is formed only if epsE and epsG are coexpressed.
In view of a future utilization of isolated glycosyltransferases in in vitro reactions for the directed biosynthesis of saccharides, we were interested in determining whether the biosynthesis of the GalNAc-Gal disaccharide could be accomplished if epsE and epsG were separately expressed and reconstituted for the glycosyltransferase reaction. As a source of epsE, pQE60:epsE or pFS33 was employed, while for epsG the pQE60:epsG or pFS65 construct was utilized. The membrane extracts of E. coli harboring pQE60:epsE and pQE:epsG or pFS33 and pFS65 were either just mixed, sonicated, or homogenized prior to the glycosyltransferase reaction with unlabeled UDP-Gal and UDP-[14C]N-acetylgalactosamine. The positive control for coexpression consisted of membrane extracts from E. coli harboring pFS50, with which the three treatments were carried out in parallel. None of the epsE/epsG combinations or experimental setups resulted in the incorporation of GalNAc from UDP-[14C]N-acetylgalactosamine, whereas membranes from E. coli(pFS50) gave a value of about 4,000 cpm/mg of protein.
DISCUSSION
In this study, we performed a functional analysis of the glycosyltransferases genes epsE, epsF, epsG, and epsI in the central region of the eps gene cluster of S. thermophilus Sfi6 (27). EpsE has a galactose-1-phosphate transferase activity that transfers the first galactose of the repeating unit onto the lipophilic carrier molecule. While in gram-negative bacteria the lipophilic carrier has been characterized as undecaprenylphosphate (36), it has not been shown that the same is true for gram-positive microorganisms. Since from preliminary experiments performed in our laboratory we know that EPS production in lactic acid bacteria is insensitive to bacitracin, one could consider alternative carriers (30). Phosphoglycosyltransferases exist either as two-domain enzymes, in which the C-terminal domain has the glycosyltransferase activity, or as one-domain glycosyltransferases, which correspond to the C-terminal domain of the two-domain enzymes. Even though there are indications that the N-terminal domain of the two-domain enzymes might be involved in keeping contact with the growing repeating unit in order to increase the rate of release of the repeating unit and facilitate undecaprenyl recycling, its function is not fully understood (14, 35). A similar enzyme that would carry out the corresponding function in microorganisms whose phosphoglycosyltransferases are lacking their N termini could not be found in protein databases.
The only sugar specificities that have been reported for EpsE-like enzymes are for galactose and glucose, regardless of whether they are composed of one or two domains. Thus, all four combinations are represented: RfbP and Cps14E of Salmonella typhimurium and S. pneumoniae have two domains and show galactosyl- (35) and glucosyltransferase (11) activity, respectively. Within the family of smaller, one-domain enzymes, EpsD from L. lactis (34) and GumD from Xanthomonas campestris (9) show glucosyltransferase activity and ExoY from Rhizobium meliloti (18) and EpsE from S. thermophilus exhibit galactosyltransferase activity. To identify regions that correlated with a sugar specificity, the sequences of the S. thermophilus, R. meliloti, and S. typhimurium galactosyltransferases and the L. lactis, X. campestris, and S. pneumoniae glucosyltransferases were aligned (Fig. 6). As reported previously (35), several regions, which probably interact with the lipid carrier and constitute the catalytic center, are conserved in all six enzymes. Surprisingly, though, except for a few residues conserved in galactosyl- or glucosyltransferases, no conserved sugar specificity motifs could be detected.
FIG. 6.
Multiple sequence alignment of three galactose-1-phosphate transferases (EpsE-Sth, ExoY-Rme, and RfbP-Sty) and three glucose-1-phosphate transferases (CpsE-Spn, EpsD-Lla, and GumD-Xca) involved in polysaccharide production. The glycosyltransferase designations are as follows: EpsE-Sth, EpsE from S. thermophilus (accession no. U40830); ExoY-Rme, ExoY from Rhizobium meliloti (accession no. Q02731); RfbP-Sty, RfbP from Salmonella typhimurium (accession no. P26406); CpsE-Spn, CpsE from S. pneumoniae (accession no. U409239); EpsD-Lla, EpsD from L. lactis (accession no. U49364); and GumD-Xca, GumD from X. campestris (accession no. S67820). Amino acids conserved in all six sequences are indicated by stars; the amino acid residues that are conserved with regard to sugar specificity are indicated by the letters A (for galactose specificity) and U (for glucose specificity).
The glycosyltransferase carrying out the second reaction of the EPS repeating unit was the α-1,3-N-acetylgalactosaminyltransferase encoded by epsG. Interestingly, transfer of the entire S. thermophilus Sfi6 eps gene cluster to L. lactis MG1363 resulted in an EPS with an altered composition and structure (29). The GalNAc residue had been replaced by a Gal, which suggested that EpsG might also have a weak galactosyltransferase activity which cannot be detected in vitro in E. coli.
As shown in an in vitro transcription-translation assay, EpsF and EpsI were not efficiently produced and their enzyme activities could not be directly measured when expressed in E. coli. We therefore took a genetic approach and created an epsF deletion mutation in S. thermophilus Sfi6. This resulted in the production of an altered EPS with nearly equimolar amounts of Glc, Gal, and GalNAc, indicating that epsF codes for the galactosyltransferase that adds the branching α-1,6-galactose onto the backbone. In conclusion, this leaves just EpsI without a functional assignment, but considering the fact that four glycosyltransferases are needed to produce a tetramer repeating unit, the remaining enzymatic activity would be the β-1,3-glucosyltransferase. This function is in agreement with the prediction conferred from its primary sequence analysis. As defined by HCA analysis, EpsI shows the β-glycosyltransferase motif typical of family 2 enzymes (4, 25).
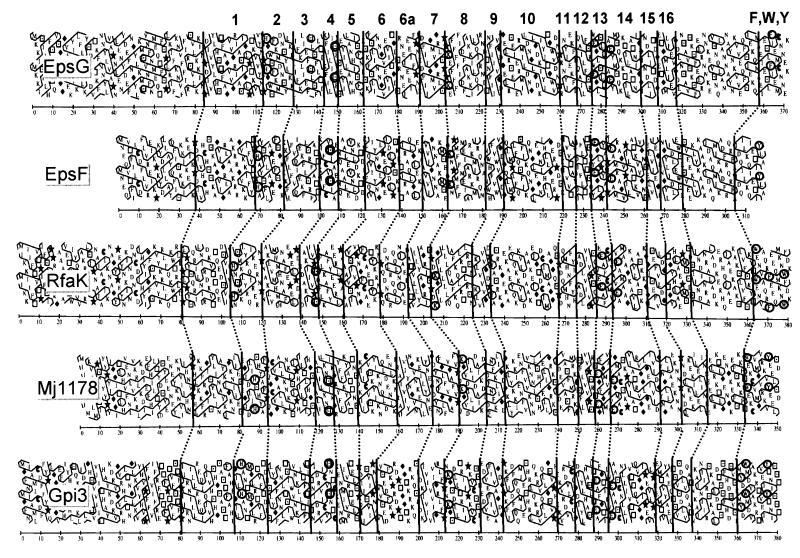
EpsF and EpsG belong to a broad family of α-glycosyltransferases with paralogs in gram-negative eubacteria (e.g., RfaK for LPS inner core synthesis in members of the family Enterobacteriaceae), archaea (e.g., putative proteins MJ1069, MJ1178, and MJ1059 from Methanococcus jannaschii), cyanobacteria (e.g., putative protein sll1971 from Synechocystis sp.), and eukaryotes (e.g., human Gpi1 and Saccharomyces cerevisiae Gpi3). There are no representatives within the mycoplasma. Given the relatively low level of similarity among these enzymes, one representative of each group was selected for the performance of a comparative HCA. Figure 7 illustrates that the overall hydrophobic domains matched very well with those reported for the α-mannosyltransferases (6), but domain 6 could be further subdivided. Interestingly, even though MJ1069 from M. jannaschii shows a low, but significant, 28.0% identity and 44.7% similarity to EpsG, it does not show the conserved EXXXXXXXE signature sequence which has been hypothesized as being the catalytic center (6). Taking into consideration the enzyme activities of EpsE, EpsF, and EpsG, the order of biosynthesis of the S. thermophilus Sfi6 EPS repeating unit is Gal, GalNAc, Glc, and the sidechain Gal. The fact that EpsE and EpsG could produce the GalNAc-Gal disaccharide only if they were coexpressed might indicate that the glycosyltransferases form an ordered biosynthetic complex to improve the processivity for the biosynthesis of the repeating unit.
FIG. 7.
HCA of α-glycosyltransferases EpsG and EpsF from S. thermophilus (accession no. U40830), RfaK from Salmonella typhimurium (accession no. P26470), Mj1178 from M. jannaschii (accession no. H64446), and Gpi3 from Saccharomyces cerevisiae (accession no. P32363). Mj1178 is a putative glycosyltransferase. Symbols representing amino acids are as follows: ⧫, glycine; ★, proline; □, threonine; and ⊡, serine. Vertical lines indicate the proposed corresponding domains of the proteins. The conserved segments are labeled 1 through 16 and correspond to segments proposed for α-mannosyltransferases (6), with a further segmentation of domain 6 into domains 6 and 6a. Conserved amino acids are circled: His in domain 2, Ser in domain 4, Lys in domain 8, Glu in domains 13 and 14, and aromatic amino acids (Phe, Trp, and Thy) in the C-terminal domain.
ACKNOWLEDGMENTS
We thank M. Kolkman for helpful advice on setting up the glycosyltransferase assays and B. Henrissat for assistance in interpreting the hydrophobic cluster analysis data for glycosyltransferases.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley and Sons; 1994. [Google Scholar]
- 2.Bhasin N, Albus A, Michon F, Livolsi P J, Park J S, Lee J C. Identification of a gene essential for O-acetylation of the Staphylococcus aureus type 5 capsular polysaccharide. Mol Microbiol. 1998;27:9–21. doi: 10.1046/j.1365-2958.1998.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.Bubb W A, Urashima T, Fujiwara R, Shinnai T, Ariga H. Structural characterisation of the exocellular polysaccharide produced by Streptococcus thermophilus OR 901. Carbohydr Res. 1997;301:41–50. doi: 10.1016/s0008-6215(97)00083-9. [DOI] [PubMed] [Google Scholar]
- 4.Campbell J A, Davies G J, Bulone V, Henrissat B. A classification of nucleotide-diphospho-sugar glycosyltransferases based on amino acid sequence similarities. Biochem J. 1997;326:929–939. doi: 10.1042/bj3260929u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doco T, Wieruszeski J M, Fournet B, Carcano D, Ramos P, Loones A. Structure of an exocellular polysaccharide produced by Streptococcus thermophilus. Carbohydr Res. 1990;198:313–321. doi: 10.1016/0008-6215(90)84301-a. [DOI] [PubMed] [Google Scholar]
- 6.Geremia R A, Petroni E A, Ielpi L, Henrissat B. Towards a classification of glycosyltransferases based on amino acid sequence similarities: prokaryotic α-mannosyltransferases. Biochem J. 1996;318:133–138. doi: 10.1042/bj3180133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruter M, Leeflang B R, Kuiper J, Kamerling J P, Vliegenthart J F. Structure of the exopolysaccharide produced by Lactococcus lactis subspecies cremoris H414 grown in a defined medium or skimmed milk. Carbohydr Res. 1992;231:273–291. doi: 10.1016/0008-6215(92)84025-n. [DOI] [PubMed] [Google Scholar]
- 8.Gruter M, Leeflang B R, Kuiper J, Kamerling J P, Vliegenthart J F. Structural characterisation of the exopolysaccharide produced by Lactobacillus delbrueckii subspecies bulgaricus rr grown in skimmed milk. Carbohydr Res. 1993;239:209–226. doi: 10.1016/0008-6215(93)84216-s. [DOI] [PubMed] [Google Scholar]
- 9.Katzen F, Ferreiro D U, Oddo C G, Ielmini M V, Becker A, Pühler A, Ielpi L. Xanthomonas campestris pv. campestris gum mutants: effects on xanthan biosynthesis and plant virulence. J Bacteriol. 1998;180:1607–1617. doi: 10.1128/jb.180.7.1607-1617.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenleyside W J, Whitfield C. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J Biol Chem. 1996;271:28581–28592. doi: 10.1074/jbc.271.45.28581. [DOI] [PubMed] [Google Scholar]
- 11.Kolkman M A, Wakarchuk W, Nuijten P J, van der Zeijst B A. Capsular polysaccharide synthesis in Streptococcus pneumoniae serotype 14: molecular analysis of the complete cps locus and identification of genes encoding glycosyltransferases required for the biosynthesis of the tetrasaccharide subunit. Mol Microbiol. 1997;26:197–208. doi: 10.1046/j.1365-2958.1997.5791940.x. [DOI] [PubMed] [Google Scholar]
- 12.Lemoine J, Chirat F, Wieruszeski J-M, Strecker G, Favre N, Neeser J-R. Structural characterization of the exocellular polysaccharides produced by Streptococcus thermophilus SFi39 and SFi12. Appl Environ Microbiol. 1997;63:3512–3518. doi: 10.1128/aem.63.9.3512-3518.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin W S, Cunneen T, Lee C Y. Sequence analysis and molecular characterization of genes required for the biosynthesis of type 1 capsular polysaccharide in Staphylococcus aureus. J Bacteriol. 1994;176:7005–7016. doi: 10.1128/jb.176.22.7005-7016.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu D, Cole R A, Reeves P R. An O-antigen processing function for Wzx (RfbX): a promising candidate for O-unit flippase. J Bacteriol. 1996;178:2102–2107. doi: 10.1128/jb.178.7.2102-2107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maguin E, Prévost H, Ehrlich S D, Gruss A. Efficient insertional mutagenesis in lactococci and other gram-positive bacteria. J Bacteriol. 1996;178:931–935. doi: 10.1128/jb.178.3.931-935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morona J K, Morona R, Paton J C. Characterization of the locus encoding the Streptococcus pneumoniae type 19F capsular polysaccharide biosynthetic pathway. Mol Microbiol. 1997;23:751–763. doi: 10.1046/j.1365-2958.1997.2551624.x. [DOI] [PubMed] [Google Scholar]
- 17.Nakajima H, Hirota T, Toba T, Itoh T, Adachi S. Structure of the extracellular polysaccharide from slime-forming Lactococcus lactis subsp. cremoris SBT 0495. Carbohydr Res. 1992;224:245–253. doi: 10.1016/0008-6215(92)84110-e. [DOI] [PubMed] [Google Scholar]
- 18.Reuber T L, Walker G C. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell. 1993;74:269–280. doi: 10.1016/0092-8674(93)90418-p. [DOI] [PubMed] [Google Scholar]
- 19.Roberts I S. Bacterial polysaccharides in sickness and in health. The 1995 Fleming Lecture. Microbiology. 1995;141:2023–2031. doi: 10.1099/13500872-141-9-2023. [DOI] [PubMed] [Google Scholar]
- 20.Roberts I S. The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol. 1996;50:285–315. doi: 10.1146/annurev.micro.50.1.285. [DOI] [PubMed] [Google Scholar]
- 21.Robijn G W, Thomas J R, Haas H, van den Berg D J, Kamerling J P, Vliegenthart J F. The structure of the exopolysaccharide produced by Lactobacillus helveticus 766. Carbohydr Res. 1995;276:137–154. doi: 10.1016/0008-6215(95)00171-o. [DOI] [PubMed] [Google Scholar]
- 22.Robijn G W, van den Berg D J, Haas H, Kamerling J P, Vliegenthart J F. Determination of the structure of the exopolysaccharide produced by Lactobacillus sake 0-1. Carbohydr Res. 1995;276:117–136. doi: 10.1016/0008-6215(95)00172-p. [DOI] [PubMed] [Google Scholar]
- 23.Robijn G W, Gutierrez Gallego R, van den Berg D J, Haas H, Kamerling J P, Vliegenthart J F. Structural characterization of the exopolysaccharide produced by Lactobacillus acidophilus LMG9433. Carbohydr Res. 1996;288:203–218. doi: 10.1016/s0008-6215(96)90799-5. [DOI] [PubMed] [Google Scholar]
- 24.Robijn G W, Wienk H L, van den Berg D J, Haas H, Kamerling J P, Vliegenthart J F. Structural studies of the exopolysaccharide produced by Lactobacillus paracasei 34-1. Carbohydr Res. 1996;285:129–139. doi: 10.1016/s0008-6215(96)90178-0. [DOI] [PubMed] [Google Scholar]
- 25.Saxena I M, Brown R M, Jr, Fevre M, Geremia R A, Henrissat B. Multidomain architecture of β-glycosyl transferases: implications for mechanism of action. J Bacteriol. 1995;177:1419–1424. doi: 10.1128/jb.177.6.1419-1424.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Staaf M, Widmalm G, Yang Z, Huttunen E. Structural elucidation of an extracellular polysaccharide produced by Lactobacillus helveticus. Carbohydr Res. 1996;291:155–164. doi: 10.1016/s0008-6215(96)00166-8. [DOI] [PubMed] [Google Scholar]
- 27.Stingele F, Neeser J-R, Mollet B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J Bacteriol. 1996;178:1680–1690. doi: 10.1128/jb.178.6.1680-1690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stingele F, Lemoine J, Neeser J-R. Lactobacillus helveticus Lh59 secretes an exopolysaccharide that is identical to the one produced by Lactobacillus helveticus TN-4, a presumed spontaneous mutant of Lactobacillus helveticus TY1-2. Carbohydr Res. 1997;302:197–202. doi: 10.1016/s0008-6215(97)00119-5. [DOI] [PubMed] [Google Scholar]
- 29.Stingele F, Vincent S J F, Faber E J, Newell J W, Kamerling J P, Neeser J-R. Introduction of the exopolysaccharide gene cluster from Streptococcus thermophilus Sfi6 into Lactococcus lactis MG1363: production and characterization of an altered polysaccharide. Mol Microbiol. 1999;32:1287–1295. doi: 10.1046/j.1365-2958.1999.01441.x. [DOI] [PubMed] [Google Scholar]
- 30.Stingele, F., and J.-R. Neeser. Unpublished results.
- 31.Sutherland I W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland I W. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–46. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 33.van Kranenburg R, Marugg J D, van Swam I I, Willem N, de Vos W M. Molecular characterization of the plasmid-encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol Microbiol. 1997;24:387–397. doi: 10.1046/j.1365-2958.1997.3521720.x. [DOI] [PubMed] [Google Scholar]
- 34.van Kranenburg R, van Swam I I, Marugg J D, Kleerebezem M, de Vos W M. Exopolysaccharide biosynthesis in Lactococcus lactis NIZO B40: functional analysis of the glycosyltransferase genes involved in synthesis of the polysaccharide backbone. J Bacteriol. 1999;181:338–340. doi: 10.1128/jb.181.1.338-340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Liu D, Reeves P R. C-terminal half of Salmonella enterica WbaP (RfbP) is the galactosyl-1-phosphate transferase domain catalyzing the first step of O-antigen synthesis. J Bacteriol. 1996;178:2598–2604. doi: 10.1128/jb.178.9.2598-2604.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whitfield C, Valvano M A. Biosynthesis and expression of cell-surface polysaccharides in gram-negative bacteria. Adv Microb Physiol. 1993;35:135–246. doi: 10.1016/s0065-2911(08)60099-5. [DOI] [PubMed] [Google Scholar]
- 37.Yamamoto Y, Murosaki S, Yamauchi R, Kato K, Sone Y. Structural study on an exocellular polysaccharide produced by Lactobacillus helveticus TY1-2. Carbohydr Res. 1994;261:67–78. doi: 10.1016/0008-6215(94)80006-5. [DOI] [PubMed] [Google Scholar]