Abstract
The initial enzymatic steps in anaerobic m-xylene oxidation were studied in Azoarcus sp. strain T, a denitrifying bacterium capable of mineralizing m-xylene via 3-methylbenzoate. Permeabilized cells of m-xylene-grown Azoarcus sp. strain T catalyzed the addition of m-xylene to fumarate to form (3-methylbenzyl)succinate. In the presence of succinyl coenzyme A (CoA) and nitrate, (3-methylbenzyl)succinate was oxidized to E-(3-methylphenyl)itaconate (or a closely related isomer) and 3-methylbenzoate. Kinetic studies conducted with permeabilized cells and whole-cell suspensions of m-xylene-grown Azoarcus sp. strain T demonstrated that the specific rate of in vitro (3-methylbenzyl)succinate formation accounts for at least 15% of the specific rate of in vivo m-xylene consumption. Based on these findings, we propose that Azoarcus sp. strain T anaerobically oxidizes m-xylene to 3-methylbenzoate (or its CoA thioester) via (3-methylbenzyl)succinate and E-(3-methylphenyl)itaconate (or its CoA thioester) in a series of reactions that are analogous to those recently proposed for anaerobic toluene oxidation to benzoyl-CoA. A deuterium kinetic isotope effect was observed in the (3-methylbenzyl)succinate synthase reaction (and the benzylsuccinate synthase reaction), suggesting that a rate-determining step in this novel fumarate addition reaction involves breaking a C-H bond.
Benzene, toluene, ethylbenzene, and the xylene isomers, collectively known as BTEX, are some of the most water-soluble constituents of gasoline (14). Due to improper handling and disposal of gasoline, BTEX are frequently found as soil and groundwater contaminants in the United States (33). BTEX have been known to be readily degraded by microorganisms under aerobic conditions (19). Under these conditions, well-characterized oxygenases catalyze the initial enzymatic step of BTEX degradation with molecular oxygen as a cosubstrate (19, 31). However, subsurface environments are frequently rendered anaerobic as a result of indigenous microorganisms consuming the available molecular oxygen (13). Under anaerobic conditions, bacteria face the biochemical challenge of activating aromatic hydrocarbons in the absence of molecular oxygen.
Within the past decade, substantial research has been conducted to elucidate the biochemical pathways of anaerobic BTEX degradation, with particular interest focused on the initial activation reactions (reviewed in references 22 and 23). Thus far, most of the research in this field has been conducted on anaerobic toluene degradation (reviewed in reference 23). The initial steps of anaerobic toluene degradation were recently elucidated by in vitro studies conducted with two denitrifying species, Thauera aromatica K172 (11) and Azoarcus sp. strain T (6) (which was previously identified as a Pseudomonas sp. [16]). These in vitro studies demonstrated that toluene is activated by addition to fumarate to form benzylsuccinate (BS) (6, 11). BS is subsequently oxidized to E-phenylitaconate (E-PI) (or its coenzyme A [CoA] thioester) (6) and benzoyl-CoA (6, 11). Benzoyl-CoA, a known central intermediate in anaerobic oxidation of numerous aromatic compounds, including toluene, is eventually oxidized to acetyl-CoA and carbon dioxide (22).
Compared to toluene, anaerobic mineralization of the xylene isomers does not appear to occur as readily and is not as well characterized. While more than 20 pure cultures have been isolated that mineralize toluene (reviewed in reference 23), only 5 pure cultures have been isolated that mineralize m-xylene (16–18, 21, 29), and only one pure culture has been isolated that mineralizes o-xylene (21). One pure culture has been reported to mineralize p-xylene, but no conclusive evidence such as carbon mass and electron balances was presented (32). An enrichment culture, however, has been shown to mineralize p-xylene (20).
Although a pathway for anaerobic m-xylene mineralization had not yet been established, recent findings suggested that m-xylene may be mineralized by a pathway analogous to the one demonstrated for toluene (6, 11). Metabolic studies conducted with Azoarcus sp. strain T demonstrated that m-xylene is oxidized to carbon dioxide (16) via 3-methylbenzoate (30) under denitrifying conditions. In addition, preliminary studies conducted with Azoarcus sp. strain T provided evidence suggesting that the 3-methyl homologs of BS and E-PI (or a closely related isomer) are formed during m-xylene metabolism (4, 30). Since BS (6, 11) and E-PI (6) had recently been demonstrated to be intermediates of anaerobic toluene oxidation to benzoyl-CoA (see above), we hypothesized that the 3-methyl homologs of BS and E-PI (or a closely related isomer) are transient intermediates of anaerobic m-xylene oxidation to 3-methylbenzoate (or its CoA thioester).
In this report, we demonstrate that permeabilized cells of m-xylene-grown Azoarcus sp. strain T catalyze the addition of m-xylene to fumarate to form (3-methylbenzyl)succinate (3-MeBS) (see Fig. 1) at specific activities that can account for in vivo anaerobic m-xylene mineralization in Azoarcus sp. strain T. We also demonstrate that 3-MeBS is oxidized to E-(3-methylphenyl)itaconate (E-3-MePI) (or a closely related isomer) and 3-methylbenzoate in the presence of succinyl-CoA and nitrate. Based on these findings, we propose that the initial reactions in anaerobic m-xylene mineralization in Azoarcus sp. strain T are the oxidation of m-xylene to 3-methylbenzoate (or 3-methylbenzoyl-CoA) via 3-MeBS and E-3-MePI (or E-3-MePI-CoA) (Fig. 1). Since the initial reactions demonstrated here for anaerobic m-xylene oxidation closely resemble those of anaerobic toluene oxidation (6, 11), we conducted preliminary biochemical studies to infer whether any of the analogous initial reactions in the m-xylene and toluene pathways are catalyzed by the same enzyme. The findings from these studies will be discussed.
FIG. 1.
Proposed initial reactions in anaerobic m-xylene oxidation in Azoarcus sp. strain T. Evidence for the proposed initial reactions is presented in the text.
MATERIALS AND METHODS
Chemicals.
The chemicals used in this study included m-xylene (≥99%; Aldrich Chemical Co., Inc. Milwaukee, Wis.); m-xylene-d6 (≥98 atom%; Isotech, Inc., Miamisburg, Ohio); toluene (99.8% high-performance liquid chromatography grade; Aldrich); toluene-d8 (≥99 atom%; Aldrich); o-xylene-d10 (>99 atom%; Aldrich); p-xylene-d10 (≥99 atom%; Aldrich); dl-benzylsuccinic acid (Sigma Chemical Co., St. Louis, Mo.); dl-3-MeBS (≥99%, custom synthesized; Radian International LLC, Austin, Tex.); E-PI (≥99%, custom synthesized; Radian); CoA, sodium salt (96%; Sigma); succinyl-CoA, sodium salt (92%; Sigma); ATP, disodium salt (grade I, 99%; Sigma); titanium(III) chloride (1.9 M solution in 2.0 M HCl; Aldrich); dl-dithiothreitol (DTT; 99%; Sigma). Initially, an authentic E-PI standard synthesized by Migaud et al. (27) was graciously provided by J. Tiedje, J. Frost, and coworkers (Michigan State University).
Growth of bacteria.
Azoarcus sp. strain T (DSM 9506; previously identified as a Pseudomonas sp.), a bacterium capable of mineralizing m-xylene and toluene under denitrifying conditions (16), was obtained from the Deutsche Sammlung von Mikroorganismen (Braunschweig, Germany). Cultures of Azoarcus sp. strain T were grown anaerobically in a bicarbonate-buffered mineral salts medium as described previously (6). m-Xylene or toluene was amended to the medium as the sole carbon and electron source, while nitrate was amended as the sole electron acceptor. During growth, neat m-xylene or toluene and sodium nitrate (from a 1 M stock solution) were repeatedly added to the cultures to avoid substrate depletion. Liquid concentrations of m-xylene and toluene were kept below 320 μM to avoid toxicity effects from the respective solvents, while that of nitrate was kept below 2 mM to avoid toxicity effects from nitrite. Liquid concentrations of m-xylene and toluene were calculated by using published data for Henry’s law constants (26).
Azoarcus sp. strain T was grown in 2.2-liter glass bottles (2 liters of anaerobic medium) sealed with polytetrafluoroethylene Mininert valves (Alltech Associates, Inc., Deerfield, Ill.). Cultures were incubated at room temperature (25°C) in an anaerobic glove box (Coy Laboratory Products, Inc., Grass Lake, Mich.) that contained an atmosphere of 80% N2, 10% H2, and 10% CO2. Cultures were grown exponentially to a final optical density at 600 nm of ca. 0.09 to 0.13.
Permeabilized cell assays.
Unless otherwise indicated, permeabilized cell assays were conducted with cells of Azoarcus sp. strain T grown under denitrifying conditions with m-xylene as the sole carbon and electron source. These assays were conducted in a similar fashion to that described previously (6). Briefly, cells from 2-liter batch cultures of Azoarcus sp. strain T in late exponential growth phase were harvested anaerobically by centrifugation (15,000 × g, 20 min, 4°C), washed once in degassed morpholinopropanesulfonic acid (MOPS) buffer (20 mM MOPS plus mineral salts as described previously [6], pH 7.2; designated as buffer A), resuspended in a final volume of 3 to 5 ml of buffer A, and then permeabilized by addition of Triton X-100 (2% [vol/vol] final concentration) for ca. 30 min. Assays were performed in 5-ml glass vials sealed with Mininert valves. The anoxic assay mixtures (final volume, 1 ml) contained buffer A, 200 μM titanium(III) chloride, permeabilized cells corresponding to 3 to 3.5 mg of protein, and particular substrates, depending on the assay. Reactions were started by addition of permeabilized cells, and mixtures were incubated for 1 h at room temperature in an anaerobic glove box while agitated on an orbital shaker. Reactions were ended by acidification with 1 M HCl to pH <2.
Reaction mixtures were then adjusted to ca. pH 7 and treated with 60 μg of DNase I for 30 min at 4°C. Following DNase treatment, the reaction mixtures, unless noted otherwise, were alkaline treated to hydrolyze any CoA thioesters that may have formed. The pH of these samples was adjusted to ≥13 with 5 M KOH. The alkaline-treated reaction mixtures, following incubation for 1.5 to 2 h at room temperature, and the non-alkaline-treated reaction mixtures, directly following the DNase I treatment, were then acidified to pH ≤2 with concentrated HCl and extracted three times with diethyl ether (Ultra Resi analyzed, distilled in glass; J. T. Baker, Inc., Phillipsburg, N.J.). The ether extracts were dried with anhydrous sodium sulfate, derivatized with ethereal diazomethane to convert carboxylic acids into methyl esters, exchanged into CH2Cl2 (Ultra Resi analyzed, distilled in glass; J. T. Baker), and analyzed by gas chromatography-mass spectrometry (GC/MS) (9).
Assays conducted to collect kinetic data were performed as described above with the following exceptions: (i) assay mixtures contained permeabilized cells corresponding to 0.7 to 0.9 mg of protein; (ii) they were conducted in 2.8-ml vials to minimize head-space volume; (iii) they were stopped at shorter defined time intervals (e.g., 5, 10, or 15 min); and (iv) they were not alkaline treated prior to being acidified and extracted.
Dialyzed cell extract assays.
m-Xylene-grown cells of Azoarcus sp. strain T were harvested anaerobically and washed in buffer A as described above. The washed cells were resuspended in a final volume of ca. 2 ml of buffer A that had been amended with 900 μg of DNase I. Cells were broken anoxically by four passages through a French pressure cell at 138 MPa. Unbroken cells and cell debris were removed by centrifugation (27,000 × g, 15 min, 4°C). The supernatant, defined as the cell extract, was dialyzed by using membrane tubing with a molecular mass cutoff of 12 to 14 kDa for ca. 16 h against 1 liter of anaerobic buffer (buffer A amended with 1 mM DTT).
Assays were performed and treated as described above for the permeabilized cell assays, except that the assay mixtures contained dialyzed cell extract corresponding to ca. 3 mg of protein instead of permeabilized cells.
Identification and quantification of reaction products by GC/MS.
BS, 3-MeBS, E-PI, benzoate, and 2-, 3-, and 4-methylbenzoate were identified as methyl esters based on comparisons of GC retention times and mass spectra to authentic standards and were quantified based on their respective response factors. The 2- and 4-methyl homologs of BS, and the 2-, 3-, and 4-methyl homologs of E-PI, none of which are commercially available, were tentatively identified based on mass spectral similarities to authentic BS and E-PI standards (dimethyl esters), respectively, and were semiquantified based on the response factors of BS and E-PI standards (dimethyl esters), respectively. BS and its methyl homologs were quantified based on the abundance of the tropylium ion (m/z 91, in unlabeled BS) and the methyltropylium ion (m/z 105, in unlabeled methyl BS homologs), respectively. E-PI and its methyl homologs and benzoate and its methyl homologs were quantified based on the abundance of their base peak. Recoveries of BS and 3-MeBS tested in the absence of permeabilized cells ranged from 75 to 90%.
Cell suspension studies.
Cells of m-xylene-grown Azoarcus sp. strain T were harvested anaerobically and washed in buffer A as described above. The washed cells were resuspended in a final volume of ca. 3 to 6 ml of buffer A. Assays were performed in 40-ml glass vials sealed with Mininert valves. The anoxic cell suspension mixture (total volume, 30 ml) contained buffer A, 200 μM titanium(III) chloride, ca. 200 μM m-xylene (ca. 7 μmol, total), 2.5 mM sodium nitrate, and whole cells corresponding to 4 to 6 mg of protein. The reaction was started by addition of cells. The reaction vials were incubated at room temperature in an anaerobic glove box while they were agitated on an orbital shaker. At defined time intervals, headspace samples were taken and analyzed for m-xylene by capillary GC and photoionization detection as described elsewhere (5), except that the analyses were conducted at the isothermal temperature of 110°C.
Protein determination.
Protein concentrations of cell suspensions were determined by using a modified Bradford assay (Bio-Rad Laboratories, Hercules, Calif.). Bovine serum albumin was used as the standard. Whole-cell suspensions were boiled for 15 min in 3 M NaOH prior to protein determination.
Sequence analysis of the 16S rRNA gene of strain T.
Strain T genomic DNA was isolated by the chloroform-phenol extraction method as described by Avery and Kaiser (3). The 16S rRNA gene was amplified by PCR with the set of primers fD1 and rD1 as described by Weisburg et al. (34), but modified by removing the linker sequences that contained restriction sites. The modified forward and reverse primers were 5′-AGAGTTTGATCCTGGCTCAG-3′ and 5′-AAGGAGGTGATCCAGCC-3′, respectively.
PCR was conducted according to standard methods and was carried out by using a GeneAmp PCR system 2400 (Perkin-Elmer Corp., Norwalk, Conn.). The amplified DNA fragment was purified by using the QIAquick PCR Purification kit protocol (Qiagen Inc., Chatsworth, Calif.). The purified PCR product was sequenced in both directions by the dideoxy chain termination method with labeled dideoxynucleoside triphosphates. Sequencing of the 16S rRNA gene was performed by the PAN facility (Stanford University, Stanford, Calif.), and the sequences were assembled with programs in the GCG software package (Wisconsin Package version 10.0; Genetics Computer Group, Madison, Wis.). The assembled sequence was analyzed by searching the DNA databases (GenBank, EMBL, and DDBJ) by using the BLAST (Basic Local Alignment Search Tool; release 2.0.8) server at the National Center for Biotechnology Information (1).
Nucleotide sequence accession number.
The 16S rRNA gene sequence of Azoarcus sp. strain T was deposited in the GenBank database under accession no. AF129465.
RESULTS
Phylogenetic position of strain T.
Sequence analysis of the 16S rRNA gene of strain T (previously identified as a Pseudomonas sp. [16]) revealed a close similarity to several Azoarcus species (data not shown). The closest known relative of strain T was Azoarcus evansii KB740 (99% identity), a denitrifying bacterium capable of oxidizing a variety of aromatic compounds under both aerobic and denitrifying conditions (2, 12). However, unlike strain T, A. evansii KB740 is unable to degrade toluene under denitrifying conditions (2), and its ability to degrade m-xylene has not been reported. Strain T, however, was found to be closely related (98 to 99% identity) to many Azoarcus spp. capable of degrading toluene (e.g., Azoarcus sp. strain ToN1 [29] and Azoarcus tolulyticus strains Td-3, 17, 19, and 21 [18]) and one Azoarcus sp. capable of degrading toluene and m-xylene (A. tolulyticus Td-15 [18]). To date, denitrifying bacteria capable of anaerobic alkylbenzene degradation appear to cluster in the two genera Azoarcus and Thauera, both of which are members of the β-subclass of the Proteobacteria (2, 23, 24, 35).
In vitro anaerobic oxidation of m-xylene to 3-methylbenzoate.
Under denitrifying conditions, Azoarcus sp. strain T oxidizes m-xylene to carbon dioxide (16) via 3-methylbenzoate as a transient intermediate (30). We used permeabilized cells of m-xylene-grown Azoarcus sp. strain T to investigate whether m-xylene is oxidized to 3-methylbenzoate via 3-MeBS and E-3-MePI (Fig. 1). Anoxic assays were conducted as described in Materials and Methods. Following incubation for 1 h, reaction products were acidified, derivatized to methyl esters, extracted, and then analyzed by GC/MS.
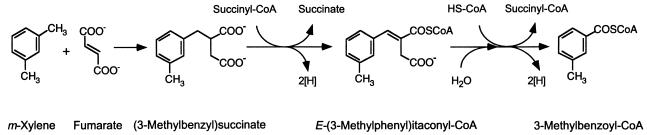
Permeabilized cells of Azoarcus sp. strain T were found to catalyze the addition of m-xylene to fumarate to form 3-MeBS. Assay mixtures amended with deuterium-labeled m-xylene (360 nmol) and fumarate (500 nmol) yielded ca. 190 nmol of deuterium-labeled 3-MeBS (Table 1). No other transformation products were detected in significant amounts (i.e., greater than 0.5 nmol) in these assays (Table 1). 3-MeBS was identified based on comparison of its GC retention time and mass spectrum to that of an authentic dl-3-MeBS standard. Figure 2 shows mass spectra of the dimethyl esters of an authentic dl-3-MeBS standard (Fig. 2A) and that of 3-MeBS-d6 (Fig. 2B) formed from m-xylene-d6 and fumarate by permeabilized cells.
TABLE 1.
Product yields from assays of permeabilized cells of m-xylene-grown Azoarcus sp. strain Ta
| Substrate added
|
Product (nmol)b
|
Product labeling | |||||
|---|---|---|---|---|---|---|---|
| Aromatic compound | Fumarate | Succinyl-CoA | Nitrate | 3-MeBS | E-3-MePI | 3-Methylbenzoate | |
| m-Xylene-d6 (360 nmol, total) | + | − | − | 190 | NDc | 0.3 | Deuterium labeled |
| m-Xylene-d6 (360 nmol, total) | + | + | + | 120 | 7 | 42 | Deuterium labeled |
| 3-MeBS (150 nmol, total) | − | + | + | 52 | 8 | 15d | |
Each anoxic assay mixture (total volume, 1 ml) contained titanium(III) chloride (200 μM) as a reductant and permeabilized cells (ca. 3 mg of protein). Initial concentrations of other substrates added are given as follows: fumarate, 500 μM; succinyl-CoA, 300 μM; and nitrate, 2 mM. A total of 360 nmol of m-xylene is equivalent to a liquid concentration of 170 μM. Assay mixtures were incubated for 1 h. Reaction products were extracted, derivatized to methyl esters, and analyzed by GC/MS.
3-MeBS and 3-methylbenzoate were identified by GC/MS by using authentic standards. E-3-MePI was tentatively identified based on mass spectral similarity to an authentic E-PI standard. Assays were conducted in duplicate. Product yields of duplicate assays were typically within 5 to 20% of each other. Product yields shown are averages of the duplicate assays, and are representative of other experiments conducted.
ND, not detected. The detection limit of E-3-MePI was less than 1 nmol.
Approximately 0.5 of the 15 nmol of 3-methylbenzoate detected in this assay may have been due to carryover from growth of the cells on m-xylene.
FIG. 2.
Mass spectra of dimethyl esters of 3-MeBS. (A) dl-3-MeBS standard. (B) 3-MeBS-d6 produced from m-xylene-d6 and fumarate by permeabilized cells of Azoarcus sp. strain T (Table 1). Although the exact location of the D atom abstracted from the m-xylene methyl carbon and retained in the succinyl moiety of 3-MeBS-d6 has not yet been determined, the D atom is depicted in Fig. 2B for illustrative purposes.
Recent in vitro studies of anaerobic toluene oxidation had found that when CoA and nitrate were amended to similar assays containing toluene and fumarate, toluene was transformed beyond BS to E-PI (6) and benzoyl-CoA (6, 11). Furthermore, when succinyl-CoA was substituted for CoA in such assays, higher product yields were obtained (8). Thus, by analogy, we supplemented the m-xylene- and fumarate-containing assays with succinyl-CoA and nitrate to investigate whether m-xylene is transformed beyond 3-MeBS to the 3-methyl homologs of E-PI and benzoate. As shown in Table 1, these assays yielded a product tentatively identified as E-3-MePI, and 3-methylbenzoate, in addition to 3-MeBS.
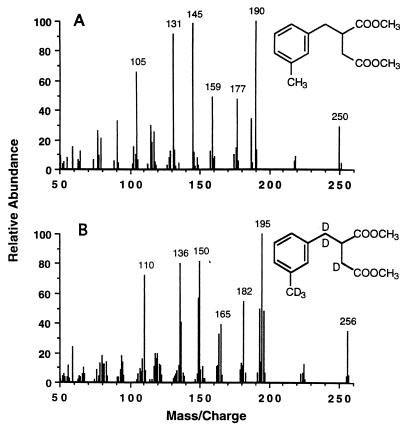
Although E-3-MePI is not commercially available, the reaction product detected in our assays was tentatively identified as E-3-MePI based on a strong mass spectral similarity to an authentic E-PI standard (dimethyl esters), and the identification of E-PI as a transformation product of toluene (27). Figure 3 shows mass spectra of the dimethyl esters of an E-PI standard (Fig. 3A) and the tentatively identified 3-methyl homolog of E-PI (Fig. 3B) formed from 3-MeBS by permeabilized cells. The similarities of the two mass spectra can be noted by comparing the mass/charge ratios and the relative intensities of the four major ions. Each of the four major ions in Fig. 3B (m/z 129, 188, 216, and 248) corresponds to one in Fig. 3A (m/z 115, 174, 202, and 234) with a similar relative intensity, but with an additional mass/charge ratio of 14, which corresponds to a methyl group (-CH3) minus an H atom.
FIG. 3.
Mass spectra of dimethyl esters of E-PI and its putative 3-methyl homolog. (A) E-PI acid standard. (B) E-3-MePI (or a closely related isomer) produced from 3-MeBS, succinyl-CoA, and nitrate by permeabilized cells of Azoarcus sp. strain T (Table 1).
There are four structurally similar isomers of E-3-MePI. They include (3-methylbenzyl)fumarate, (3-methylbenzyl)maleate, Z-(3-methylphenyl)itaconate, and E-3-MePI. These four isomers are probably indistinguishable by mass spectra, as was found for the homologous isomers benzylfumarate, benzylmaleate, Z-phenylitaconate, and E-PI (27). Based on the definitive identification of E-PI as a transformation product of toluene (27), we postulate that the 3-methyl homolog of E-PI is the m-xylene transformation product detected in our assays. Consequently, for the remainder of this article we will refer to the m-xylene transformation product as E-3-MePI for simplicity, knowing that it may be a closely related isomer.
To demonstrate that 3-MeBS is a transient intermediate of anaerobic m-xylene oxidation to 3-methylbenzoate, 3-MeBS was substituted for m-xylene and fumarate in assays amended with succinyl-CoA and nitrate. These assays yielded E-3-MePI and 3-methylbenzoate (Table 1), indicating that 3-MeBS is a true intermediate.
We also investigated the CoA-dependence of E-3-MePI and 3-methylbenzoate formation from m-xylene. In a preliminary study, we found that permeabilized cells catalyzed the formation of E-3-MePI and 3-methylbenzoate when succinyl-CoA was omitted from assays containing m-xylene, fumarate, and nitrate (data not shown). To test whether residual CoA (or CoA thioester) that might have been present in the permeabilized cells acted as a cosubstrate in this reaction, we reexamined the CoA dependence of E-3-MePI and 3-methylbenzoate formation in dialyzed cell extracts. A standard assay mixture containing m-xylene, fumarate, nitrate, and dialyzed cell extracts (ca. 3 mg of protein) yielded 3-MeBS, but neither E-3-MePI nor 3-methylbenzoate was detected (Table 2). When similar assay mixtures were supplemented with either succinyl-CoA or CoA plus ATP, the assays yielded E-3-MePI in addition to 3-MeBS (Table 2). However, when the assay mixture was supplemented with CoA in the absence of ATP, the assay yielded 3-MeBS, but not E-3-MePI (Table 2). Similarly, when 3-MeBS was substituted for m-xylene and fumarate in the standard assay mixture, E-3-MePI was formed in the presence of succinyl-CoA, but not in its absence (Table 2). These findings suggest that the formation of E-3-MePI requires the presence of succinyl-CoA or CoA plus ATP. This is contrary to findings from an analogous study conducted with toluene and dialyzed cell extracts of T. aromatica (11), whereby CoA in the absence of ATP was found to be sufficient for anaerobic toluene oxidation to benzoyl-CoA (11).
TABLE 2.
Product yields from assays of dialyzed cell extracts of m-xylene-grown Azoarcus sp. strain Ta
| Substrate added
|
Product (nmol)b
|
Product labeling | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Aromatic compound | Fumarate | Succinyl-CoA | CoA | ATP | Nitrate | 3-MeBS | E-3-MePI | 3-Methylbenzoate | |
| m-Xylene-d6 (360 nmol, total) | + | − | − | − | + | 200 | NDc | ND | Deuterium labeled |
| m-Xylene-d6 (360 nmol, total) | + | + | − | − | + | 73 | 85 | ND | Deuterium labeled |
| m-Xylene-d6 (360 nmol, total) | + | − | + | + | + | 67 | 49 | ND | Deuterium labeled |
| m-Xylene-d6 (360 nmol, total) | + | − | + | − | + | 130 | ND | ND | Deuterium labeled |
| 3-MeBS (150 nmol, total) | − | − | − | − | + | 110 | ND | ND | |
| 3-MeBS (150 nmol, total) | − | + | − | − | + | 64 | 40 | ND | |
Each anoxic assay mixture (total volume, 1 ml) contained DTT (1 mM) as a reductant and dialyzed cell extracts (ca. 3 mg of protein). Initial concentrations of other substrates added are given as follows: fumarate, 500 μM; succinyl-CoA, 300 μM; CoA, 300 μM; ATP, 2 mM; and nitrate, 2 mM. A total of 360 nmol of m-xylene is equivalent to a liquid concentration of 170 μM. Assay mixtures were incubated for 1 h. Reaction products were extracted, derivatized to methyl esters, and analyzed by GC/MS.
3-MeBS and 3-methylbenzoate were identified by GC/MS by using authentic standards. E-3-MePI was tentatively identified based on mass spectral similarity to an authentic E-PI standard. Assays were conducted in duplicate. Product yields of duplicate assays were typically within 5 to 20% of each other. Product yields shown are averages of the duplicate assays and are representative of other experiments conducted.
ND, not detected. The detection limits of E-3-MePI and 3-methylbenzoate were less than 1 nmol.
It should be noted that 3-methylbenzoate was not detected in any of the assays conducted with dialyzed cell extracts, even those supplemented with succinyl-CoA or CoA plus ATP (Table 2). This finding is consistent with observations from the toluene study conducted with dialyzed cell extracts of T. aromatica in which benzoate was only formed in assays containing a protein concentration greater than 20 mg/ml (11). Our assay, which was conducted with dialyzed cell extracts of Azoarcus sp. strain T, contained only ca. 3 mg of protein per ml.
Based on these dialyzed cell extract assays, it is apparent that a source of activated CoA (i.e., succinyl-CoA or CoA plus ATP) is required for anaerobic oxidation of 3-MeBS to E-3-MePI. To investigate whether the CoA esters of E-3-MePI and 3-methylbenzoate were formed, we conducted parallel experiments with m-xylene, fumarate, succinyl-CoA, nitrate, and permeabilized cells: one sample was subjected to alkaline treatment to hydrolyze any CoA thioester bonds that may have formed, while the other remained untreated. A comparison of the product yields from the alkaline-treated and untreated samples showed no significant differences (data not shown). A similar experiment was conducted with dialyzed cell extract, but again, no significant differences were observed in the respective product yields. One explanation why the CoA thioesters of E-3-MePI and 3-methylbenzoate were not detected may be that they are rapidly hydrolyzed under our assay conditions.
3-MeBS synthase reaction: retention of the abstracted H atom from the m-xylene methyl carbon in 3-MeBS.
Close inspection of the mass spectrum of deuterium-labeled 3-MeBS (dimethyl ester) (Fig. 2B) formed from m-xylene-d6 and fumarate revealed that the H atom abstracted from the methyl carbon of m-xylene during addition to fumarate is retained in the succinyl moiety of 3-MeBS. Retention of the abstracted H atom from m-xylene in 3-MeBS can be observed by comparing the mass/charge ratios of the molecular and methyltropylium ions of the unlabeled and labeled dimethyl esters of 3-MeBS (Fig. 2). For a more detailed explanation of how such a mass spectrum can be interpreted to illustrate the retention of the abstracted H atom in 3-MeBS, see Beller and Spormann (6). The H atoms abstracted from the methyl carbon of toluene (6), o-xylene (6), and p-xylene (data not shown) during analogous fumarate addition reactions were also found to be retained in the corresponding BS homologs, suggesting that the mechanism of these reactions may be similar.
Specific rate of in vitro 3-MeBS formation.
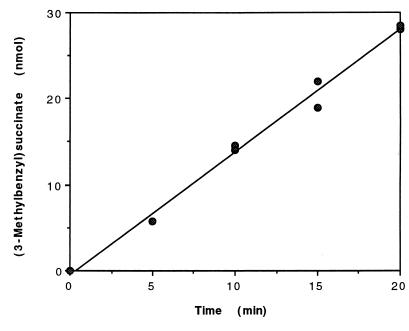
Five separate kinetic experiments were conducted to determine the specific rate of in vitro 3-MeBS formation in assay mixtures containing m-xylene-d6 (300 nmol, total; 200 μM, liquid concentration), fumarate (500 μM), and permeabilized cells of m-xylene-grown Azoarcus sp. strain T (ca. 0.8 mg of protein). The average specific rate of 3-MeBS formation was 1.5 nmol · min−1 · (mg of protein)−1 (standard deviation [SD] = 0.4). Figure 4 depicts the results from one such experiment.
FIG. 4.
Kinetics of 3-MeBS formation from m-xylene-d6 (300 nmol, total) and fumarate (500 μM) by permeabilized cells of Azoarcus sp. strain T (ca. 0.8 mg of protein). The data points at 10, 15, and 20 min are from duplicate assays. Fitting of the data by linear regression yielded a specific rate of 3-MeBS formation of 1.8 nmol · min−1 · (mg of protein)−1 (r2 = 0.990).
Primary kinetic isotope effect in the 3-MeBS synthase reaction.
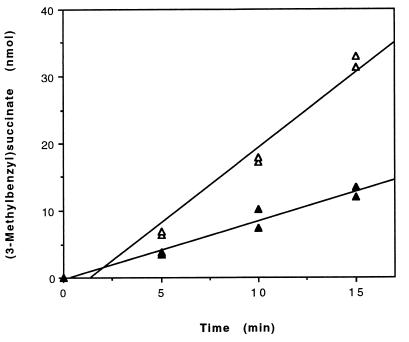
Since the 3-MeBS synthase reaction involves the transfer of an H atom from the methyl carbon of m-xylene to the product, 3-MeBS, we investigated whether a kinetic isotope effect is associated with this reaction. The specific rates of in vitro 3-MeBS formation from unlabeled m-xylene and from deuterium-labeled m-xylene (m-xylene-d6) were determined in two parallel experiments conducted with the same batch of permeabilized cells. Each experiment contained fumarate (500 μM), permeabilized cells (ca. 0.8 mg of protein), and either (i) unlabeled m-xylene (300 nmol, total; 200 μM, liquid concentration) or (ii) deuterium-labeled m-xylene (m-xylene-d6; 300 nmol, total; 200 μM, liquid concentration). The specific rates of 3-MeBS formation from unlabeled and deuterium-labeled m-xylene were found to be 3 and 1 nmol · min−1 · (mg of protein)−1, respectively (Fig. 5). Thus, 3-MeBS formation from unlabeled m-xylene was ca. 3 times faster than that from deuterium-labeled m-xylene. A kinetic isotope effect with a similar magnitude (kobs,H/kobs,D = ca. 3) was also observed in assays that initially contained equimolar concentrations of unlabeled m-xylene (150 nmol, total; 100 μM, liquid concentration) and deuterium-labeled m-xylene (m-xylene-d6; 150 nmol, total; 100 μM, liquid concentration), as well as fumarate (500 μM), and permeabilized cells (ca. 0.8 mg of protein) (data not shown).
FIG. 5.
Kinetics of 3-MeBS formation from fumarate (500 μM) and either unlabeled m-xylene (300 nmol, total) (▵) or m-xylene-d6 (300 nmol, total) (▴) by permeabilized cells of Azoarcus sp. strain T (ca. 0.8 mg of protein). Assays were conducted in parallel. The data points at 5, 10, and 15 min are from duplicate assays. Fitting of the data by linear regression yielded a specific rate of 3-MeBS formation of 3 nmol · min−1 · (mg of protein)−1 (r2 = 0.971) from unlabeled m-xylene and 1 nmol · min−1 · (mg of protein)−1 (r2 = 0.959) from m-xylene-d6. The yield of unlabeled 3-MeBS was corrected to account for carryover from growth of the cells on m-xylene. The amount of carryover was estimated to be ca. 0.5 nmol of 3-MeBS, which was the amount of unlabeled 3-MeBS observed in kinetic assays amended with deuterium-labeled m-xylene only.
Specific rate of in vivo m-xylene consumption.
To compare the specific rate of in vitro 3-MeBS formation with the specific rate of in vivo m-xylene consumption, four separate kinetic experiments were conducted with cell suspensions of m-xylene-grown Azoarcus sp. strain T. These experiments were amended with m-xylene (7 μmol, total; 200 μM, liquid concentration), nitrate (2 mM), and whole cells (4 to 6 mg of protein). The average specific rate of m-xylene consumption by whole-cell suspensions was 20 nmol · min−1 · (mg of protein)−1 (SD = 6). This range of rates is comparable to that calculated for m-xylene consumption by cells of Azoarcus sp. strain T growing under denitrifying conditions with m-xylene as the sole carbon and electron source (10 to 25 nmol · min−1 · (mg of protein)−1 at a doubling time of 27 h [data not shown]). It should be noted that the doubling time of Azoarcus sp. strain T growing under similar conditions has ranged from 16 to 27 h (data not shown). Variable growth rates for Azoarcus sp. strain T were also observed by Dolfing et al. (16).
In vitro anaerobic oxidation of toluene and o- and p-xylene to the corresponding benzoate homologs.
We investigated whether permeabilized cells of m-xylene-grown Azoarcus sp. strain T could transform methylbenzenes other than m-xylene. Each permeabilized cell assay was amended with fumarate, succinyl-CoA, nitrate, and one of the following methylbenzenes, toluene or o- or p-xylene. Assays amended with toluene yielded BS, E-PI, and benzoate (Table 3). Assays amended with p-xylene yielded products tentatively identified as the 4-methyl homologs of BS and E-PI, as well as 4-methylbenzoate (Table 3). And assays amended with o-xylene yielded the tentatively identified 2-methyl homologs of BS and E-PI (Table 3). 2-Methylbenzoate was detected in the o-xylene assays, but the concentration was too low to quantify (i.e., less than 1 nmol) (Table 3).
TABLE 3.
Product yields from assays of permeabilized cells of m-xylene-grown Azoarcus sp. strain T utilizing alternate substratesa
| Substrate added
|
Product (or respective methyl homolog) (nmol)b
|
Product labeling | |||||
|---|---|---|---|---|---|---|---|
| Aromatic compound | Fumarate | Succinyl-CoA | Nitrate | BS | E-PI | Benzoate | |
| Toluene-d8 (340 nmol, total) | + | + | + | 190 | 6 | 47 | Deuterium labeled |
| BS (150 nmol, total) | − | + | + | 98 | 7 | 6 | |
| o-Xylene-d10 (300 nmol, total) | + | + | + | 230 | 12 | NDc | Deuterium labeled |
| p-Xylene-d10 (360 nmol, total) | + | + | + | 110 | 8 | 70 | Deuterium labeled |
Each anoxic assay mixture (total volume, 1 ml) contained titanium(III) chloride (200 μM) as a reductant and permeabilized cells (ca. 3 mg of protein). Initial concentrations of other substrates added are given as follows: fumarate, 500 μM; succinyl-CoA, 300 μM; and nitrate, 2 mM. A total of 340 nmol of toluene, 300 nmol of o-xylene, and 360 nmol of p-xylene is equivalent to a liquid concentration of 160, 170, and 170 μM, respectively. Assays were incubated for 1 h. Reaction products were extracted, derivatized to methyl esters, and analyzed by GC/MS.
BS, E-PI, and benzoate were identified by GC/MS by using authentic standards. The 2- and 4-methyl homologs of BS and E-PI were tentatively identified based on mass spectral similarities to authentic standards of BS and E-PI, respectively. Product yields are averages of duplicate assays, except for the p-xylene assay. All of the assays shown in Table 3 were conducted with the same batch of cells.
ND, not detected. The detection limit of 2-methylbenzoate was less than 0.4 nmol. 2-Methylbenzoate was detected, but the concentration was too low to quantify.
An additional permeabilized cell assay was conducted with BS, succinyl-CoA, and nitrate to investigate whether m-xylene-grown cells could anaerobically oxidize BS to benzoate, as was shown for toluene-grown cells (6, 11). As indicated in Table 3, permeabilized cells of m-xylene-grown Azoarcus sp. strain T catalyzed the oxidation of BS to E-PI and benzoate.
In vitro anaerobic oxidation of m-xylene to 3-methylbenzoate by toluene-grown cells.
We investigated whether toluene-grown cells of Azoarcus sp. strain T could also transform m-xylene to 3-methylbenzoate via 3-MeBS and E-3-MePI. An assay mixture containing m-xylene-d6 (360 nmol, total; 170 μM, liquid concentration), fumarate (500 μM), succinyl-CoA (300 μM), nitrate (2 mM), and permeabilized cells of toluene-grown Azoarcus sp. strain T (ca. 3 mg protein) yielded the following deuterium-labeled products: 3-MeBS (150 nmol), E-3-MePI (15 nmol), and 3-methylbenzoate (40 nmol). When 3-MeBS (150 nmol) was substituted for m-xylene and fumarate in a similar assay mixture, 3-MeBS was transformed to E-3-MePI (20 nmol) and 3-methylbenzoate (15 nmol). These findings indicate that enzymatic activities for anaerobic oxidation of m-xylene to 3-methylbenzoate via 3-MeBS and E-3-MePI (Fig. 1) are also present in toluene-grown cells of Azoarcus sp. strain T.
DISCUSSION
We investigated the enzymatic steps in anaerobic m-xylene oxidation to 3-methylbenzoate in permeabilized cells and dialyzed cell extracts of m-xylene-grown Azoarcus sp. strain T. Based on the following experimental evidence, we propose that Azoarcus sp. strain T oxidizes m-xylene to 3-methylbenzoate (or its CoA thioester) via the transient intermediates, 3-MeBS and E-3-MePI (or its CoA thioester) (Fig. 1). Permeabilized cells of Azoarcus sp. strain T catalyzed the transformation of deuterium-labeled m-xylene to deuterium-labeled 3-MeBS by the addition of m-xylene-d6 to fumarate (Table 1 and Fig. 2B). Furthermore, when succinyl-CoA and nitrate were amended to assays containing m-xylene-d6 and fumarate, permeabilized cells catalyzed the transformation of m-xylene-d6 to E-3-MePI and 3-methylbenzoate in addition to 3-MeBS (Table 1). All three transformation products, 3-MeBS, E-3-MePI, and 3-methylbenzoate, were labeled with deuterium atoms originating from m-xylene-d6. Permeabilized cells also catalyzed the oxidation of 3-MeBS to E-3-MePI and 3-methylbenzoate (Table 1), suggesting that 3-MeBS is an intermediate in anaerobic m-xylene oxidation to 3-methylbenzoate.
Although we could not directly demonstrate the presence of the CoA thioesters of E-3-MePI and 3-methylbenzoate, circumstantial evidence suggests that they are formed. E-3-MePI (or its CoA thioester) formation was found to be dependent on a source of activated CoA (e.g., succinyl-CoA or CoA plus ATP) (Table 2), and the CoA thioester of benzoate was formed from toluene (6, 11) in a series of reactions analogous to those demonstrated for anaerobic m-xylene oxidation to 3-methylbenzoate (or 3-methylbenzoyl-CoA).
Assuming that the CoA thioesters of E-3-MePI and 3-methylbenzoate are formed, we postulate that 3-MeBS is oxidized to 3-methylbenzoyl-CoA via the following reactions. 3-MeBS is activated to its CoA thioester, which is subsequently oxidized to E-3-MePI-CoA via steps resembling β-oxidation of fatty acids. E-3-MePI-CoA is then oxidized to 3-methylbenzoyl-CoA via one of two pathways recently proposed for E-PI-CoA oxidation to benzoyl-CoA (6, 11).
Several lines of evidence suggest that the reactions demonstrated here for anaerobic m-xylene oxidation to 3-methylbenzoate (or 3-methylbenzoyl-CoA) (Fig. 1) are the initial steps in anaerobic m-xylene mineralization in Azoarcus sp. strain T. First, permeabilized cells of Azoarcus sp. strain T were found to transform 3-MeBS to 3-methylbenzoate, which is a known transient intermediate of anaerobic m-xylene mineralization in Azoarcus sp. strain T (30). Second, 3-MeBS and E-3-MePI have been tentatively identified by GC/MS as transformation products of an m-xylene-metabolizing culture of Azoarcus sp. strain T (4). Third, kinetic studies demonstrated that the specific rate of in vitro 3-MeBS formation (ca. 3 nmol · min−1 · [mg of protein]−1) (Fig. 5) can account for greater than 15% of the average specific rate of in vivo m-xylene consumption (ca. 20 nmol · [mg of protein]−1).
While 15% is significant in itself, it is a conservative estimate because it is based on a rate of 3-MeBS formation that is presumed to be at the low end of the range of rates representative of different cell batches. This assumption is based on the fact that the rate of 3-MeBS-d6 formation determined in a parallel experiment using the same batch of permeabilized cells was found to be 1.25 SDs less than the average rate determined from five separate experiments (1.5 nmol · min−1 · mg of protein−1 [SD = 0.4]). Furthermore, the 3-MeBS synthase activity is extremely oxygen sensitive. Considering these factors, our results suggest that the putative 3-MeBS synthase (or BS synthase) is present in Azoarcus sp. strain T at specific activities that are sufficient to account for in vivo anaerobic m-xylene mineralization. As anaerobic m-xylene mineralization is studied in more bacterial species, it will be interesting to observe whether the pathway demonstrated here (Fig. 1) is a general pathway for anaerobic m-xylene mineralization or whether it is unique to Azoarcus sp. strain T.
Although the prevalence of this pathway in other m-xylene-mineralizing bacteria is not yet known, findings from our study in combination with those from previous studies of toluene mineralization suggest that the fumarate addition reaction may be a general metabolic strategy for activating methylbenzenes in the absence of molecular oxygen. Toluene-mineralizing, denitrifying (6, 11, 28), and sulfate-reducing (7, 28) bacteria were shown to activate toluene by a fumarate addition reaction analogous to the one demonstrated for m-xylene. The mechanism of these fumarate addition reactions is not yet fully understood; however, they are believed to involve a glycyl radical (8, 15, 23, 25).
Observations of a kinetic deuterium isotope effect in the 3-MeBS synthase reaction (kobs,H/kobs,D = ca. 3) (Fig. 5) and the BS synthase reaction (conducted with toluene-d8 and fumarate; kobs,H/kobs,D = ca. 3) (data not shown) suggest that cleavage of a C-H bond contributes measurably to the overall rate of reaction. Based on a recently proposed radical reaction mechanism for the BS synthase reaction (8), one or two of the rate-determining steps in such fumarate addition reactions may be (i) abstraction of the H atom from the methyl carbon of m-xylene (or toluene) by the free radical-containing enzyme and/or (ii) abstraction of the H atom from the enzyme intermediate. However, it cannot be ruled out that introduction of deuterated methyl groups in m-xylene and toluene may have altered the rate-determining step(s) in the respective 3-MeBS and BS synthase reactions and thereby produced the observed kinetic isotope effect.
Since the reactions demonstrated here for anaerobic m-xylene oxidation to 3-methylbenzoate (or its CoA thioester) (Fig. 1) are analogous to those of anaerobic toluene oxidation to benzoyl-CoA (6, 11), we conducted preliminary studies to investigate whether any of the corresponding initial reactions in the oxidation of m-xylene and toluene may be catalyzed by the same enzyme. The catalytic activity and specificity of enzymes induced during anaerobic growth on m-xylene or toluene were characterized and compared in an attempt to differentiate between enzymes involved in the two pathways. However, regardless of whether Azoarcus sp. strain T was grown on m-xylene or toluene, permeabilized cells catalyzed both the 3-MeBS and the BS synthase reactions, and they did so at similar specific activities. The average specific rates of 3-MeBS-d6 formation by m-xylene-grown and toluene-grown cells were 1.5 nmol · min−1 · (mg of protein)−1 (this study) and 1.6 nmol · min−1 · (mg of protein)−1 (data not shown), respectively. The specific rates of BS-d8 formation by m-xylene-grown and toluene-grown cells were 4.1 nmol · min−1 · (mg of protein)−1 (data not shown) and 4.9 nmol · min−1 · (mg of protein)−1 (6), respectively.
Permeabilized cells of m-xylene-grown and toluene-grown Azoarcus sp. strain T also catalyzed an analogous fumarate addition reaction with o- or p-xylene as the substrate, forming the corresponding BS homolog (this study, data not shown, and reference 6). We also investigated whether m-xylene- and toluene-grown cells of Azoarcus sp. strain T could transform toluene and o- and m-xylene beyond the BS homologs to the corresponding E-PI and benzoate homologs. Again, regardless of whether cells of Azoarcus sp. strain T were grown on m-xylene or toluene, they catalyzed the same reactions. Permeabilized cells of m-xylene- and toluene-grown Azoarcus sp. strain T catalyzed the transformation of toluene and m-xylene to their respective homologs of BS, E-PI, and benzoate, and they catalyzed the transformation of o-xylene to the tentatively identified 2-methyl homologs of BS and E-PI (this study and reference 6).
Since no differences were observed in the specific activities or substrate specificities of the enzymes induced during anaerobic growth on m-xylene or toluene, the corresponding initial reactions in the proposed pathways of anaerobic m-xylene oxidation to 3-methylbenzoate (or 3-methylbenzoyl-CoA) and anaerobic toluene oxidation to benzoyl-CoA may be catalyzed by either (i) the same enzyme, (ii) distinct enzymes specific for each pathway, but induced during growth on either m-xylene or toluene, or, (iii) a combination of possibilities i and ii for the individual initial reactions. Molecular and genetic experiments in our laboratory are currently in progress to differentiate between these possibilities. Although genetic analysis is required to elucidate this matter, circumstantial evidence suggests that the same enzymes may catalyze the corresponding initial reactions in anaerobic oxidation of m-xylene and toluene to their respective benzoate (or benzoyl-CoA) homologs. Although T. aromatica is unable to grow on m-xylene, it too has the enzymatic capability to oxidize m-xylene to 3-methylbenzoate (or 3-methylbenzoyl-CoA), as demonstrated in dense suspensions of toluene-grown cells (10).
ACKNOWLEDGMENTS
Funding for this study was provided by the National Science Foundation (MCB-9733535 and MCB-9723312), by an OTL Research Incentive Fund (Stanford University), and by the U.S. Department of the Navy, under agreement N-47408-96-C-3342. Additional support was provided through fellowships to C.J.K. from the Stanford-NIH Graduate Training Program in Biotechnology and from Achievement Rewards for College Students (ARCs).
We thank J. Tiedje, J. Frost, J. Chee-Sanford, and M. Migaud (Michigan State University) for providing authentic E-PI. We thank Bettina Rosner for technical advice.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anders H-J, Kaetzke A, Kämpfer P, Ludwig W, Fuchs G. Taxonomic position of aromatic-degrading denitrifying Pseudomonad strains K 172 and KB 740 and their description as new members of the genera Thauera, as Thauera aromatica sp. nov., and Azoarcus, as Azoarcus evansii sp. nov., respectively, members of the beta subclass of the Proteobacteria. Int J Syst Bacteriol. 1995;45:327–333. doi: 10.1099/00207713-45-2-327. [DOI] [PubMed] [Google Scholar]
- 3.Avery L, Kaiser D. Construction of tandem genetic duplications with defined endpoints in Myxococcus xanthus. Mol Gen Genet. 1983;191:110–117. doi: 10.1007/BF00330897. [DOI] [PubMed] [Google Scholar]
- 4.Beller H R, Ding W-H, Reinhard M. Byproducts of anaerobic alkylbenzene metabolism useful as indicators of in situ bioremediation. Environ Sci Technol. 1995;29:2864–2870. doi: 10.1021/es00011a024. [DOI] [PubMed] [Google Scholar]
- 5.Beller H R, Grbić-Galić D, Reinhard M. Microbial degradation of toluene under sulfate-reducing conditions and the influence of iron on the process. Appl Environ Microbiol. 1992;58:786–793. doi: 10.1128/aem.58.3.786-793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beller H R, Spormann A M. Anaerobic activation of toluene and o-xylene by addition to fumarate in denitrifying strain T. J Bacteriol. 1997;179:670–676. doi: 10.1128/jb.179.3.670-676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beller H R, Spormann A M. Benzylsuccinate formation as a means of anaerobic toluene activation by sulfate-reducing strain PRTOL1. Appl Environ Microbiol. 1997;63:3729–3731. doi: 10.1128/aem.63.9.3729-3731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beller H R, Spormann A M. Analysis of the novel benzylsuccinate synthase reaction for anaerobic toluene activation based on structural studies of the product. J Bacteriol. 1998;180:5454–5457. doi: 10.1128/jb.180.20.5454-5457.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beller H R, Spormann A M, Sharma P K, Cole J R, Reinhard M. Isolation and characterization of a novel toluene-degrading sulfate-reducing bacterium. Appl Environ Microbiol. 1996;62:1188–1196. doi: 10.1128/aem.62.4.1188-1196.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biegert T, Fuchs G. Anaerobic oxidation of toluene (analogues) to benzoate (analogues) by whole cells and by cell extracts of a denitrifying Thauera sp. Arch Microbiol. 1995;163:407–417. doi: 10.1007/BF00272130. [DOI] [PubMed] [Google Scholar]
- 11.Biegert T, Fuchs G, Heider J. Evidence that anaerobic oxidation of toluene in the denitrifying bacterium Thauera aromatica is initiated by formation of benzylsuccinate from toluene and fumarate. Eur J Biochem. 1996;238:661–668. doi: 10.1111/j.1432-1033.1996.0661w.x. [DOI] [PubMed] [Google Scholar]
- 12.Braun K, Gibson D T. Anaerobic degradation of 2-aminobenzoate (anthranilic acid) by denitrifying bacteria. Appl Environ Microbiol. 1984;48:102–107. doi: 10.1128/aem.48.1.102-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champ D R, Gulens J, Jackson R E. Oxidation-reduction sequences in ground water flow systems. Can J Earth Sci. 1979;16:12–23. [Google Scholar]
- 14.Cline P V, Delfino J J, Rao P S C. Partitioning of aromatic constituents into water from gasoline and other complex solvent mixtures. Environ Sci Technol. 1991;25:914–920. [Google Scholar]
- 15.Coschigano P W, Wehrman T S, Young L Y. Identification and analysis of genes involved in anaerobic toluene metabolism by strain T1: putative role of a glycine free radical. Appl Environ Microbiol. 1998;64:1650–1656. doi: 10.1128/aem.64.5.1650-1656.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolfing J, Zeyer J, Binder-Eicher P, Schwarzenbach R P. Isolation and characterization of a bacterium that mineralizes toluene in the absence of molecular oxygen. Arch Microbiol. 1990;154:336–341. doi: 10.1007/BF00276528. [DOI] [PubMed] [Google Scholar]
- 17.Elmén J, Pan W, Leung S Y, Magyarosy A, Keasling J D. Kinetics of toluene degradation by a nitrate-reducing bacterium isolated from a groundwater aquifer. Biotechnol Bioeng. 1997;55:82–90. doi: 10.1002/(SICI)1097-0290(19970705)55:1<82::AID-BIT10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 18.Fries M R, Zhou J, Chee-Sanford J, Tiedje J M. Isolation, characterization, and distribution of denitrifying toluene degraders from a variety of habitats. Appl Environ Microbiol. 1994;60:2802–2810. doi: 10.1128/aem.60.8.2802-2810.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gibson D T, Subramanian V. Microbial degradation of aromatic hydrocarbons. In: Gibson D T, editor. Microbial degradation of organic compounds. New York, N.Y: Marcel Dekker, Inc.; 1984. pp. 181–252. [Google Scholar]
- 20.Häner A, Höhener P, Zeyer J. Degradation of p-xylene by a denitrifying enrichment culture. Appl Environ Microbiol. 1995;61:3185–3188. doi: 10.1128/aem.61.8.3185-3188.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harms G, Zengler K, Rabus R, Aeckersberg F, Minz D, Rosselló-Mora R, Widdel F. Anaerobic oxidation of o-xylene, m-xylene, and homologous alkylbenzenes by new types of sulfate-reducing bacteria. Appl Environ Microbiol. 1999;65:999–1004. doi: 10.1128/aem.65.3.999-1004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heider J, Fuchs G. Anaerobic metabolism of aromatic compounds. Eur J Biochem. 1997;243:577–596. doi: 10.1111/j.1432-1033.1997.00577.x. [DOI] [PubMed] [Google Scholar]
- 23.Heider J, Spormann A M, Beller H R, Widdel F. Anaerobic bacterial metabolism of hydrocarbons. FEMS Microbiol Rev. 1998;22:459–473. [Google Scholar]
- 24.Hurek T, Reinhold-Hurek B. Identification of grass-associated and toluene-degrading diazotrophs, Azoarcus spp., by analyses of partial 16S ribosomal DNA sequences. Appl Environ Microbiol. 1995;61:2257–2261. doi: 10.1128/aem.61.6.2257-2261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leuthner B, Leutwein C, Schulz H, Horth P, Haehnel W, Schiltz E, Schagger H, Heider J. Biochemical and genetic characterization of benzylsuccinate synthase from Thauera aromatica: a new glycyl radical enzyme catalysing the first step in anaerobic metabolism. Mol Microbiol. 1998;28:615–628. doi: 10.1046/j.1365-2958.1998.00826.x. [DOI] [PubMed] [Google Scholar]
- 26.Mackay D, Shiu W Y. A critical review of Henry’s law constants for chemicals of environmental interest. J Phys Chem Ref Data. 1981;10:1175–1199. [Google Scholar]
- 27.Migaud M E, Chee-Sanford J C, Tiedje J M, Frost J W. Benzylfumaric, benzylmaleic, and Z- and E-phenylitaconic acids: synthesis, characterization, and correlation with a metabolite generated by Azoarcus tolulyticus Tol-4 during anaerobic toluene degradation. Appl Environ Microbiol. 1996;62:974–978. doi: 10.1128/aem.62.3.974-978.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabus R, Heider J. Initial reactions of anaerobic metabolism of alkylbenzenes in denitrifying and sulfate-reducing bacteria. Arch Microbiol. 1998;170:377–384. [Google Scholar]
- 29.Rabus R, Widdel F. Anaerobic degradation of ethylbenzene and other aromatic hydrocarbons by new denitrifying bacteria. Arch Microbiol. 1995;163:96–103. doi: 10.1007/BF00381782. [DOI] [PubMed] [Google Scholar]
- 30.Seyfried B, Glod G, Schocher R, Tschech A, Zeyer J. Initial reactions in the anaerobic oxidation of toluene and m-xylene by denitrifying bacteria. Appl Environ Microbiol. 1994;60:4047–4052. doi: 10.1128/aem.60.11.4047-4052.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith M R. The biodegradation of aromatic hydrocarbons by bacteria. Biodegradation. 1990;1:191–206. doi: 10.1007/BF00058836. [DOI] [PubMed] [Google Scholar]
- 32.Su J-J, Kafkewitz D. Utilization of toluene and xylenes by a nitrate-reducing strain of Pseudomonas maltophilia under low oxygen and anoxic conditions. FEMS Microbiol Ecol. 1994;15:249–258. [Google Scholar]
- 33.U.S. Environmental Protection Agency. Underground motor fuel storage tanks: a national survey. EPA 560/5-86-013. U.S. Washington, D.C.: Environmental Protection Agency; 1986. [Google Scholar]
- 34.Weisburg W G, Barns S M, Pelletier D A, Lane D J. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou J, Fries M R, Chee-Sanford J C, Tiedje J M. Phylogenetic analyses of a new group of denitrifiers capable of anaerobic growth on toluene and description of Azoarcus tolulyticus sp. nov. Int J Syst Bacteriol. 1995;45:500–506. doi: 10.1099/00207713-45-3-500. [DOI] [PubMed] [Google Scholar]