Abstract
The hybrid yeast Zygosaccharomyces parabailii holds potential as a cell factory mainly because of its robustness in withstanding stressors that often characterize bio-based processes. However, a complex genome and a lack of gene editing tools hinder the capacity to engineer this yeast. In this work, we developed a CRISPR-Cas9 gene editing system for Z. parabailii that allows simultaneous disruption or deletion of both alleles of a gene. We evaluated four different gRNA expression systems consisting of combinations of tRNAs, tRNA and ribozyme or ribozymes as self-cleaving flanking elements and established that the most efficient systems used an RNA Pol II promoter followed by a 5’tRNA flanking the gRNA. This gRNA system was then used to construct a strain of Z. parabailii in which both alleles of DNL4 were inactivated and so relied on homologous recombination to repair double-stranded breaks. Our system can be used for gene inactivation in a wild-type strain and precise deletion with marker insertion in a dnl4 mutant. In some cases, we observed inter-chromosomal recombination around the site of the DSB that could cause loss of heterozygosity through gene conversion or deletion. Although an additional aspect that needs to be monitored during strain engineering, this phenomenon also offers opportunities to explore genome plasticity in hybrid yeasts.
Keywords: Zygosaccharomyces parabailii, CRISPR-Cas9, genome editing, hybrid yeast, gRNA expression system
A CRISPR-Cas9 gene editing system was developed for the hybrid yeast Z. parabailii that allows simultaneous disruption or deletion of both alleles of a gene.
Introduction
While yeasts have a long association with human activity, the number and diversity of species that find application are ever-increasing. Thus, while production of traditional foods and beverages continues to exploit yeasts such as Saccharomyces cerevisiae, Kluyveromyces marxianus, Kluyveromyces lactis, and Debaryomyces hansenii, modern biotechnology adds Komagataella phaffii, Yarrowia lipolytica, Rhodotorula toruloides, and others (Karaalioğlu and Yüceer 2021, Zhao et al. 2021, Bilal et al. 2022). As well as the pure species already mentioned, hybrid yeasts that derive from the union of two different parental species are especially significant (Gabaldón 2020). Domestication has often conferred traits such as the capacity to use specific substrates or withstand particular stresses, which can benefit industrial biotechnology exploitation. The better-known hybrid yeast is Saccharomyces pastorianus, which dates its origin to a hybridization event between S. cerevisiae and S. eubayanus, occurring about 500 hundred years ago in a German brewery (Raihofer et al. 2022, Hutzler et al. 2023). Other instances include hybrids between S. cerevisiae and S. kudriavzevi that are used to make some Belgian trappist beers (González et al. 2008), and hybrids between S. cerevisiae, S. kudriavzevi, S. uvarum are used in winemaking (Christine et al. 2007). Other genera in the Saccharomycetaceae also generate hybrids, with intraspecific hybrids of K. marxianus linked to dairy fermentations (Ortiz-Merino et al. 2017), and hybrids from the Zygosaccharomyces genus associated with the production of different fermented foods (Gibson et al. 2017, Solieri 2021, Sato and Ohnishi 2023). More specifically, Z. bailii and its hybrids Z. parabailii and Z. pseudobailii are traditionally associated with spoilage of highly acidic and high-sugar foods (Kuanyshev et al. 2017a); the traits that enable this are also attractive for the design of robust cell factories. However, the genetic basis of the ability of Z. bailii and Z. parabailii to withstand low pH, high osmotic pressure, high ethanol and weak organic acids concentrations are still largely to be described (Kuanyshev et al. 2017a, Palma et al. 2018, Palma and Sá-Correia 2019). To expand the industrial potential of Z. parabailii (including the generation of marker-free stable modified strains) as well as to understand the mechanisms underlying its unique characteristics, improved molecular tools are needed. Currently, the inability to obtain stable haploid strains is one of the key restrictions in this hybrid: to disrupt a gene function, two or more alleles of the gene, differing by 7% in the nucleotide sequence on average (Ortiz-Merino et al. 2017), should be simultaneously inactivated. Furthermore, the lack of available auxotrophic strains has forced researchers to rely on dominant markers. The development of genome-editing tools, such as CRISPR-Cas9, is a prerequisite to addressing the aforementioned engineering challenges. To date, while successful use of a CRISPR-Cas9 strategy for strain engineering was reported in the haploid species Z. bailii (Kuanyshev et al. 2021), no such system is available for the hybrid Z. parabailii. Indeed, the only hybrid yeast successfully edited using the CRISPR-Cas9 system to date is S. pastorianus (Gorter de Vries et al. 2017).
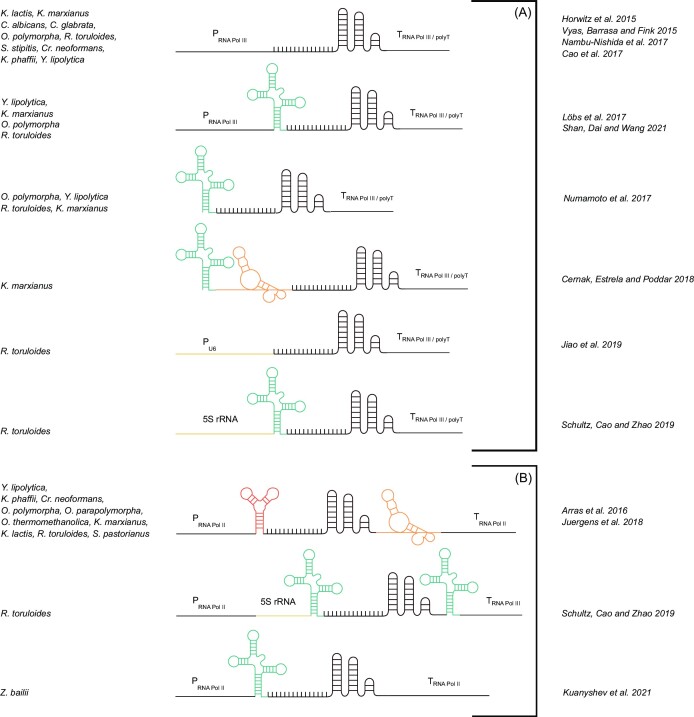
The increased adoption of non-traditional, or non-conventional, yeasts as cell factories has been greatly facilitated by the advent of CRISPR–Cas9 based genome-engineering as this tool opened up hitherto inaccessible genomes to modern genetic engineering and pathway remodeling (Stovicek et al. 2017). Distinct systems have been developed for different yeast species, but they all require a means of expression of high levels of a gRNA to guide Cas9 to the target site. This can be achieved using Pol III promoters such as SNR52 and RPR1 (Shan et al. 2021), T7 polymerase-dependent artificial promoters (Morse et al. 2018), or polymerase II (Pol II) promoters flanked with ribozyme cleaving systems or with tRNAs, which are also cleaved to yield a mature gRNA molecule (Shan et al. 2021). The range of systems that are possible is summarized in Fig. 1, with the details of which system has been applied in a particular yeast and of the cited references. Only recently a CRISPR-Cas9 system for the haploid species Z. bailii was developed (Kuanyshev et al. 2021). The authors reported the expression of the gRNA from a Pol II promoter along with tRNA, though full details of the 5’ and 3’ sequences flanking the gRNA were lacking in that study.
Figure 1.
Graphical representation of different gRNA expression systems used in various conventional and non-conventional yeasts. In red hammerhead ribozyme, orange HDV ribozyme, blue tRNA, and black the spacer sequence along with structural tRNA. (A) gRNAs designed for transcription by RNA Pol III. (B) gRNA designed for transcription by RNA Pol II. The right column indicates references for all the examples reported.
Considering that Z. parabailii is more robust than its haploid counterparts (Kuanyshev et al. 2016), and the need to better understand its specific interdependence of phenotype-genotype association, the goal of this study was to develop an efficient CRISPR-Cas9 system for genome editing. To determine the most efficient gRNA expression system in this hybrid yeast, we employed four different expression cassettes consisting of the spacer and structural RNA between self-cleaving elements, tRNAGly-tRNAAsp, tRNAGly-HDV (hepatitis D ribozyme), HH-tRNAGly (hammer head ribozyme) and HH-HDV, targeting ADE2 as a proof of concept. Similar to other non-Saccharomyces yeasts, Z. parabailii has poor homologous recombination (HR) efficiency relative to S. cerevisiae, and to overcome this limitation, we constructed a Z. parabailii dnl4 mutant using the previously optimized Cas9-gRNA system. With this system, efficiently targeted gene deletions were possible for the first time in this hybrid species. While analyzing mutants generated by CRISPR-Cas9, we observed a phenomenon where recombination between homologous chromosomes could lead to allelic exchange or large-scale loss of heterozygosity (LOH) via deletion. This phenomenon, on the one hand, causes challenges for strain engineering, but, on the other hand, it offers a route to explore possible mechanisms of genome plasticity in hybrid yeasts. For example, it would be possible to induce recombination of LOH at specific loci and then study how the genome adapts in terms of patterns of gene expression, compensatory genome changes, etc.
Materials and methods
Strains and growth conditions
Zygosaccharomyces parabailii ATCC60483 was used as the host strain for this work as its complete genome sequence is available (GCA_001984395.2) and the strain has a track record from previous studies (Suh et al. 2013, Kuanyshev et al. 2016, Ortiz-Merino et al. 2017, 2018). Other strains constructed over the course of this work are listed in Table 1. Yeast cultures were grown in YP medium (1% w v−1 yeast extract, 2% w v−1 peptone) with 2% w v−1 glucose (D) or fructose (F) as carbon source, or minimal synthetic medium (0.67% w v−1 YNB Biolife without amino acids) with 2% w v−1 glucose as carbon source. Solid media was prepared by adding 2% (w v−1) agar. Where required to detect the red color of ade2-disrupted strains, minimal media was supplemented with 10 mg L−1 adenine. Yeast cultures were routinely grown at 30°C in conical flasks (with a 1:5 ratio of medium to flask volume) on a rotary shaker set to 160 rpm. Where required hygromycin B (hyg), nourseothricin (clonNAT) and geneticin (G418) were added to YPF/YPD medium to a final concentration of 200 mg L−1, 100 mg L−1, and 200 mg L−1, respectively. Frozen stock cultures of mutants were prepared from overnight cultures by the addition of 20% (v v−1) glycerol, and aseptically stored at −80 °C.
Table 1.
Strains used in this study; all derived from Z. parabailii ATCC60483.
| Name of the strain | Genotype | Source |
|---|---|---|
| Z. parabailii ATCC60483 | WT | ATCC |
| Zp_ade2 | Δ/Δade2 | This study |
| Zp_dnl4 #1 | Δ/Δdnl4 | This study |
| Zp_dnl4 #2 | Δ/Δdnl4 | This study |
| Zp_dnl4#1_ade2 | Δ/Δdnl4 Δ/Δade2 | This study |
| Zp_dnl4#1_ade2_KAN | Δ/Δdnl4 ade2chr1::kanMX Δade2chr2 | This study |
| Zp_dnl4#1_ade2_NAT | Δ/Δdnl4 Δade2chr1 ade2chr2::natMX | This study |
Plasmid construction and design of gRNA expression cassettes
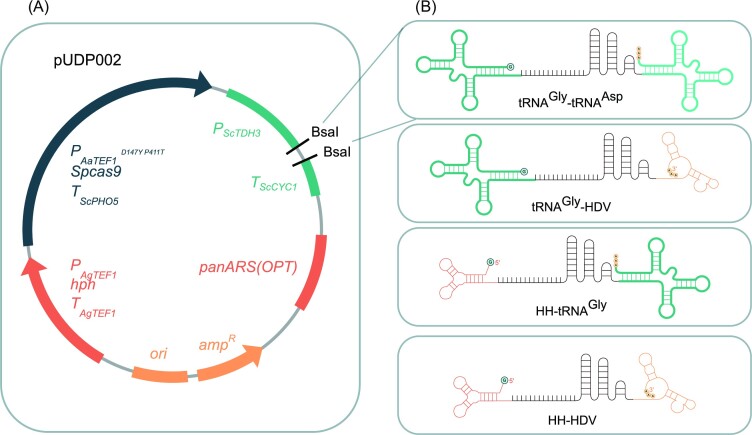
The broad-host-range Cas9/gRNA co-expression backbone plasmid pUDP002 (Juergens et al. 2018) was used. To integrate the synthetic gRNA constructs into pUDP002 they were flanked by inward-facing BsaI restriction sites generating sticky ends (underlined) ‘GGTCTCGCAAA’ and ‘ACAGCGAGACC’ at their 5’ and 3’ ends, respectively, compatible with BsaI-digested pUDP002 (Fig. 2). The final gRNA expression cassettes were synthesized as gene fragments with Twist Bioscience (Table 3).
Figure 2.
Graphical representation of (A) the empty plasmid backbone (pUDP002) and (B) with the addition of the structurally-different gRNAs, obtaining the pUDPZb series of gRNA plasmids (Table 2). The constructed gRNA cassettes were assembled by Golden Gate using the BsaI highlighted restriction sites, between the ScTDH3 promoter and ScCYC1 terminator; gRNA expression cassettes with the targeting spacer and structural RNA are between self-cleaving elements, tRNAGly and tRNAAsp, tRNAGly and HDV, HH-tRNAGly, HH-HDV.
Table 3.
Synthesized gRNA constructs. In gRNA construct sequences, bold sequences indicate the BsaI Type II restriction enzyme recognition site, while underlined sequences are the BsaI cutting site.
| Synthesized sequences | Sequence (5’-3’) |
|---|---|
| Self-cleaving elements | |
| HH | ATATTGCTGATGAGTCCGTGAGGACGAAACGAGTAAGCTCGTC |
| HDV | GGCCGGCATGGTCCCAGCCTCCTCGCTGGCGCCGGCTGGGCA ACATGCTTCGGCATGGCGAATGGGAC |
| tRNAGly | GCGCAAGTGGTTTAGTGGTAAAATTCATCGTTGCCATCGATGAGCCCCCGGTTCGATTCCGGG CTTGCGCA |
| tRNAAsp | TCCGTGATAGTTTAATGGTCAGAATGGGCGCTTGTCGCGTGCCAGATCGGGGTTCAATTCCCCGTCGCGGAG |
| Structural gRNA | GTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAA AAAGTGGCACCGAGTCGGTGC |
| gRNA constructs | |
| tRNAGly- ADE2 target—tRNAAsp | CCCTTTAAGGTCTCGCAAAGCGCAAGTGGTTTAGTGGTAAAATTCATCGTTGCCATCGATGAGCCCC CGGTTCGATTCCGGGCTTGCGCACAATATGATAGCGTCGTCCAGTTTTAGAGCTAGAAATAGCAAGT TAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTTTCCGTGATAGT TTAATGGTCAGAATGGGCGCTTGTCGCGTGCCAGATCGGGGTTCAATTCCC CGTCGCGGAGACAGCGAGACCCCCTTTAA |
| tRNAGly- ADE2 target—HDV | CCCTTTAAGGTCTCGCAAAGCGCAAGTGGTTTAGTGGTAAAATTCATCGTTGCCATCGATGAGCCCCC GGTTCGATTCCGGGCTTGCGCACAATATGATAGCGTCGTCCAGTTTTAGAGCTAGAAATAGCAAGTTA AAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCGGTGCTTTTGGCCGGCATGGTC CCAGCCTCCTCGCTGGCGCCGGCTGGGCAACATGCTTCGGCATGGCGAATGGGACACAGCGAGACCC CCTTTAA |
| HH—ADE2 target—tRNAAsp | CCCTTTAAGGTCTCGCAAAATATTGCTGATGAGTCCGTGAGGACGAAACGAGTAAGCTCGTCCAATAT GATAGCGTCGTCCAGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGA AAAAGTGGCACCGAGTCGGTGCTTTTGCGCAAGTGGTTTAGTGGTAAAATTCATCGTTGCCATCGATG AGCCCCCGGTTCGATTCCGGGCTTGCGCAACAGCGAGACCCCCTTTAA |
| HH- ADE2 target—HDV | CCCTTTAAGGTCTCGCAAAATATTGCTGATGAGTCCGTGAGGACGAAACGAGTAAGCTCGTCCAATAT GATAGCGTCGTCCAGTTTTAGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGA AAAAGTGGCACCGAGTCGGTGCTTTTGGCCGGCATGGTCCCAGCCTCCTCGCTGGCGCCGGCTGG GCAACATGCTTCGGCATGGCGAATGGGACACAGCGAGACCCCCTTTAA |
| tRNAGly- tRNAAsp-U1 | AAGGAAGCGTCTCACAAAGCGCAAGTGGTTTAGTGGTAAAATTCATCGTTGCCATCGATGAGCCCCC GGTTCGATTCCGGGCTTGCGCATGAGACCTCACACTTCACGGGTAGTATGGTAGGGTCTCCGTTTT AGAGCTAGAAATAGCAAGTTAAAATAAGGCTAGTCCGTTATCAACTTGAAAAAGTGGCACCGAGTCG GTGCTTTTTCCGTGATAGTTTAATGGTCAGAATGGGCGCTTGTCGCGTGCCAGATCGGGGTTCA ATTCCCCGTCGCGGAGACAGCGAGACGCTTCCAGGG |
The gRNA expression cassettes were composed of (i) 5’ self-cleaving element (HH or tRNAGly), followed by (ii) 20-nucleotide spacer, by the (iii) gRNA scaffold, and by a (iv) 3’ self-cleaving element (HDV, tRNAGly or tRNAAsp) (Fig. 2). All plasmids used in this study are described in Table 2 and the synthetic gRNA constructs are available in Table 3.
Table 2.
Plasmids used in this study.
| Name | Relevant characteristics | Source |
|---|---|---|
| pUDP002 | ori ampR panARS(OPT) AgTEF1p-hph-AgTEF1t ScTDH3p BsaI BsaI ScCYC1t AaTEF1p Spcas9-ScPHO5t | Gorter de Vries et al. 2017 |
| pUDPZb_ Gly_ADE2c_Asp | tRNAGly– ADE2 target—tRNAAsp cloned into pUDP002 | This study |
| pUDPZb_Gly_ADE2c_HDV | tRNAGly– ADE2 target—HDV cloned into pUDP002 | This study |
| pUDPZb_ HH_ADE2c_Gly | HH—ADE2 target—tRNAGly cloned into pUDP002 | This study |
| pUDPZb_ HH_ADE2c_HDV | HH—ADE2 target—HDV cloned into pUDP002 | This study |
| pSTBlue_repair chr1_kanMX | ori ampR panARS(OPT) US ADE2 AgTEF1p-kanMX-AgTEF1t DS ADE2 | This study |
| pSTBlue_repair chr2_NAT | ori ampR panARS(OPT) US ADE2 AgTEF1p-natMX-AgTEF1t DS ADE2 | This study |
| pZ3 | ori ampR panARS(OPT) AgTEF1p-kanMX-AgTEF1t | Branduardi et al. 2004 |
| pZ5 | ori ampR panARS(OPT) AgTEF1p-NAT-AgTEF1t | Branduardi et al. 2004 |
| pUDPZb_U1 | pUDP002 containing tRNAGly–BsaI restriction site– tRNAAsp | This study |
| pUDPZb_U1_DNL4 CT1 | DNL4 target1 inserted in the BsaI restriction site in pUDPZb_U1 | This study |
| PUDPZb_U1_DNL4 CT2 | DNL4 target2 inserted in the BsaI restriction site in pUDPZb_U1 | This study |
| PUDPZb_U1_DNL4 CT3 | DNL4 target3 inserted in the BsaI restriction site in pUDPZb_U1 | This study |
| PUDPZb_U1_DNL4 CT4 | DNL4 target4 inserted in the BsaI restriction site in pUDPZb_U1 | This study |
| PUDPZb_U1_DNL4 CT5 | DNL4 target5 inserted in the BsaI restriction site in pUDPZb_U1 | This study |
Sequences for the HH and HDV were taken from (Gao and Zhao 2014). When using HH as a 5’ self-cleaving element, the first six nucleotides were the reverse complement of the first six nucleotides of the spacer sequence. Sequences of tRNAs were identified from the genome of Z. parabailii (Ortiz-Merino et al. 2017) using the software tRNAscan-SE (Lowe and Eddy 1997). Among these sequences, tRNAGly and tRNAAsp were selected avoiding tRNAs with internal BsaI and Esp3I recognition sites. Different tRNAs for the 5’ and 3’ ends of the gRNA were selected to avoid undesired secondary structure formation. The structural gRNA sequence was taken from (Gorter de Vries et al. 2017). The 20-nucleotide spacer sequences were identified using the software sgRNAcas9 (Xie et al. 2014).
To design the universal gRNA expression cassette tRNA-tRNA-U1, the 20-nucleotide spacer sequence was replaced by a fragment with outward-facing BsaI restriction sites, for easy insertion of spacer sequences using Golden Gate Assembly and annealed oligonucleotides. This cassette was designed with flanking Esp3I recognition sites (CGTCTCacaaa at 5’ and acagcGAGACG at 3’), creating ends compatible with BsaI-digested pUDP002 (underlined). pUDPZb_U1 was obtained by BsaI restriction of pUDP002, Esp3I restriction of tRNA-tRNA-U1, purification of both fragments and ligation using Quick Ligase (Fig. S1).
Spacer sequences able to target both alleles of ADE2 or DNL4 genes were selected as follows. To inactivate ADE2, a spacer sequence already tested in the haploid yeast Z. bailii was used (Kuanyshev et al. 2021; Table 4). For the DNL4 disruption, five different spacer sequences targeting both alleles were designed and tested (Table 2). Before synthesis, the designed gRNA expression cassettes were checked for the correct secondary structure formation using SPOT-RNA (Singh et al. 2019). After the hybridization of oligonucleotides with the selected target sequences flanked by sticky ends compatible with the digested pUDPZb_U1 (CGCA at 5’ and GTTT at 3’), the hybridized nucleotides were assembled with the pUDPZb_U1 backbone using Golden Gate Assembly. Sanger sequencing confirmed the integration of target sequences in all the gRNA constructs.
Table 4.
Primers used in this study.
| Name | Sequence (5’-3’) |
|---|---|
| Construction of Z.parabailii repair fragment for ADE2 chromosome 1 copy | |
| Chr1_DS_fw | CTGGTCGCTATACTGTTTGATGTTTTCATATTTTGAACGTAC |
| Chr1_DS_rev | GGCTCGAGAAGCTTGTCGACGAATTCAGATTTATATCGTTGGATTTTTACTTGGG |
| Chr1_US_fw | GCGTTACGTATCGGATCCAGAATTCGTGATTGCCTATTCCTCCCATTTTTACTAGT |
| Chr1_US_rev | CTGGGCCTCCATGTCGACAAGAACAGAGGGTTTTGCG |
| kanMX_fw | CCCTCTGTTCTTGTCGACATGGAGGCCCAGAATAC |
| kanMX_rev | TATGAAAACATCAAACAGTATAGCGACCAGCATTC |
| Construction of Z.parabailii repair fragment for ADE2 chromosome 2 copy | |
| Chr2_DS_fw | CTGGTCGCTATACTGTGATGTTTTCATATTTTTTTGAGCGTAT |
| Chr2_DS_rev | GGCTCGAGAAGCTTGTCGACGAATTCAGATGAATTATATTGTTGGGTTTTTACTTGGA |
| Chr2_US_fw | GCGTTACGTATCGGATCCAGAATTCGTGATTCTAAAATAAATTTCCGTTTTACTAACCC |
| Chr2_US_rev | CTGGGCCTCCATGTCACCTATGCGACACCATCAG |
| NAT_ fw | TGGTGTCGCATAGGTGACATGGAGGCCCAGAATAC |
| NAT_ rev | AATATGAAAACATCACAGTATAGCGACCAGCATTC |
| Diagnostic primers for molecular analysis of ade2 mutants | |
| Fw_NHEJ_gRNA_Chr1 | CCAAAGAACTTTTCATCTCTGTCG |
| Rev_NHEJ_gRNA_Chr1 | GCGTATTTGGTCATTTTTTGTGGC |
| Fw_NHEJ_gRNA_Chr2 | GCCAAAGAACTTTTCCTCTCTCTCA |
| Rev_NHEJ_gRNA_Chr2 | GCATATTTAGCCATTCTTTGCGGT |
| Fw_HDR_Chr1_UP | AACTAGAACTCGATGGGCTA |
| Rev_HDR_Chr1_kanMX | TAAATCAGCATCCATGTTGG |
| Fw_HDR_Chr2_UP | CAACGAACAGTATTCTAGGACAC |
| Rev_HDR_Chr2_natMX | GTGTCGTCAAGAGTGGTAC |
| ARO10_Chr1_fw | CAACATAAGCTGTAGTTGTACCGGG |
| ARO10_Chr1_rv | TGAGAGAACCCCTCGTAATATTCTA |
| ARO10_Chr2_fw | CAGCATAAGCTGTAACTGTACCGGA |
| ARO10_Chr2_rv | AGAGGACCCCACACAATATTCTC |
| RGA1/RGA2_DS new_FW | TAGGCCAGCTTCAAAACC |
| RGA1/RGA2_DS new_rev | GGTTTTGAAGCTGGCCTA |
| UBP10 US_FW | GACAAGCTGATGACGCTGTA |
| UBP10_chr2_rev | CTTGAGGTGTACAAGCAGT |
| DIS3_chr2_fw | CACGATGTAGAATACAGACCA |
| DIS3_chr2_rev | TGGATCCAAAACAGACCTCAA |
| Diagnostic primers for gRNA insertion in pUDP002/pUDPZb_U1 | |
| pUDP002_GPDpro-F | CGGTAGGTATTGATTGTAATTCTG |
| pUDP002_CYC1 | GCGTGAATGTAAGCGTGAC |
| Diagnostic primers to confirm mutations in DNL4 | |
| DG_ct1 dnl4 chr2_Fw | GAACATAAATTTCTGAGCTGT |
| DG_ct1 dnl4 chr2_Rv | GTTTCTGTTAGTGTCAAACGAG |
| DG_cts dnl4 chr2_Fw | CGCCTCTACACATTAGAAAGC |
| DG_cts dnl4 chr2_Rv | GAAGTTTCCTCTTACAGGGAA |
| DG_ct1 dnl4 chr7_Fw | GAACATAAATTTCTGAACTGC |
| DG_ct1 dnl4 chr7_Rv | TCTGTTAGTGTCAAATGAT |
| DG_cts dnl4 chr7_Fw | TGCCTTTACACATTAGAAAACA |
| DG_cts dnl4 chr7_Rv | GAAGTTTCCCGTTACGGG |
| Golden gate assembly of DNL4 targets in pUDPZb_U1 | |
| DNL4_ct1_fw | CGCATGTATCCGATCTAAAGACTG |
| DNL4_ct1_Rv | AAACCAGTCTTTAGATCGGATACA |
| DNL4_ct2_fw | CGCAGCAATTGCAATGGGCTCTGA |
| DNL4_ct2_Rv | AAACTCAGAGCCCATTGCAATTGC |
| DNL4_ct3_fw | CGCAATTGTGATTGGAAGAACTCC |
| DNL4_ct3_Rv | AAACGGAGTTCTTCCAATCACAAT |
| DNL4_ct4_fw | CGCAAATCGCTAATGGCATTTCCC |
| DNL4_ct4_Rv | AAACGGGAAATGCCATTAGCGATT |
| DNL4_ct5_fw | CGCAAGCGGCACAAAGTTCAAGAC |
| DNL4_ct4_Rv | AAACGTCTTGAACTTTGTGCCGCT |
| ADE2 and DNL4 target sequences | |
| ADE2 common target | CAATATGATAGCGTCGTCCA |
| DNL4 Target 1 | TGTATCCGATCTAAAGACTG |
| DNL4 Target 2 | GCAATTGCAATGGGCTCTGA |
| DNL4 Target 3 | ATTGTGATTGGAAGAACTCC |
| DNL4 Target 4 | AATCGCTAATGGCATTTCCCA |
| DNL4 Target 5 | AGCGGCACAAAGTTCAAGAC |
Repair DNA fragments construction
Two different repair fragments were constructed for the targeted deletion of each ADE2 allele. Specific homology regions upstream and downstream of the two loci of ADE2 were selected and amplified from the gDNA of Z. parabailii. Further, two different dominant markers (kanMX for chromosome 1 and natMX for chromosome 2) were cloned between the homologous sequences. Both fragments were assembled using Gibson Assembly in the backbone plasmid pSTBlue-1.
The kanMX_Chr1 cassette was built by amplifying from the genome of Z. parabailii an upstream region (537 nt) and a downstream region (519 nt) using primers Chr1_US_fw/Chr1_US_rev and Chr1_DS_fw/Chr1_DS_rev. The dominant marker kanMX (coding for aminoglycoside phosphotransferase) was amplified from the plasmid pZ3 (Branduardi et al. 2004) using the primers kanMX_fw/kanMX_rev. The natMX_Chr2 cassette was built by amplifying from the genome of Z. parabailii an upstream region (539 nt) and a downstream region (522 nt) using primers Chr2_US_fw/Chr2_US_rev and Chr2_DS_fw/Chr2_DS_rev. The dominant marker natMX (coding for aminoglycoside phosphotransferase) was amplified from the plasmid pZ5 (Branduardi et al. 2004) using the primers NAT_fw/NAT_rev.
Positive clones were identified using blue/white screening and confirmed by sequencing.
To transform the repair fragments, linear DNA was amplified by PCR using Q5® High-Fidelity DNA Polymerase, the primers Chr1_US_fw/Chr1_DS_rev and Chr2_US_fw/Chr2_DS_rev and the previously constructed plasmids as a template. The PCR product was further purified, quantified, and used in the Z. parabailii transformation.
Yeast transformation
Zygosaccharomyces parabailii was transformed by using an optimized version of the LiAc/PEG/ss-DNA method (Branduardi et al. 2022). Overnight pre-cultures in YPD medium were used to inoculate a shake flask containing YPD medium to an initial OD660nm of 0.2. Cultures were then incubated at 30 °C until OD660nm of 0.8–1 was reached, harvested, and transformed with the DNA. 3 µg of purified plasmid was used in each transformation. To transform the repair fragments, double the molar amount was used compared to the plasmid, respectively, 1.45 µg and 1.31 µg of purified amplified DNA for kanMX_Chr1 and natMX_Chr2. After heat shock, cells were recovered in YPF medium supplemented with sorbitol (1 M) overnight and plated in selective rich media. Plates were kept at 30 °C for 3–4 days and transformants were further plated in minimum media with 10 mg L−1 adenine and incubated at 30 °C for 2 days and at 4°C for 3 days before assessing the percentage of red colonies. These plates were then replica plated in rich media with the addition of geneticin (G418) or nourseothricin (clonNAT) to assess the percentage of single and double insertion of repair fragments.
Molecular analysis
PCR amplification with Q5® High-Fidelity DNA Polymerase (New England Biolabs) was performed according to the manufacturer's instructions. Diagnostic PCRs were performed using WonderTaq polymerase (EuroClone S.p.A.). All primer sequences are shown in Table 4. DNA fragments obtained by PCR were separated by gel electrophoresis. Gel and PCR purifications were carried out using NucleoSpin® Gel and PCR Clean-up columns (Macherey-Nagel). Golden Gate Assembly was performed using BsaI-HF®v2 and T4 ligase (New England Biolabs) according to the manufacturer's recommendations (75 ng destination plasmid, amplicon fragment in a 2:1 molar ratio, 2.5 µL T4 ligase buffer, 500 U T4 ligase, 15 U BsaI-HFv2, water to 25 µL). All DNA sequencings were performed by Sanger sequencing. Escherichia coli strain DH5α was used for plasmid transformation, amplification, and storage.
To confirm the disruption of ADE2, red colonies were selected, and both alleles of the gene were amplified by colony PCR (using the primers Rw_NHEJ_gRNA_Chr1/Rev_NHEJ_gRNA_Chr1, Fw_NHEJ_gRNA_Chr2/Rev_NHEJ_gRNA_Chr2) and sequenced to confirm mutations in the target region.
For the selection of dnl4 double mutants, transformants were selected and both loci of DNL4 were amplified (using the primers DG_ct1 dnl4 chr2_Fw/DG_ct1 dnl4 chr2_Rv, DG_cts dnl4 chr2_Fw/DG_cts dnl4 chr2_Rv, DG_ct1 dnl4 chr7_Fw/DG_ct1 dnl4 chr7_Rv, DG_cts dnl4 chr7_Fw/DG_cts dnl4 chr7_Rv) and sequenced. Sequencing results were aligned to the original gene sequences retrieved from the Z. parabailii ATCC60483 genome. Colonies with confirmed disruption of both alleles of ADE2 and DNL4 were selected and stored for future purposes.
To confirm the correct integration of the repair fragments into the target allele in both wild-type and dnl4 mutants, diagnostic PCRs were performed on the colonies growing on either G418 or clonNAT using specific primers for each allele and antibiotic marker (Fw_HDR_Chr1_UP/Rev_HDR_Chr1_kanMX and Fw_HDR_Chr2_UP/Rev_HDR_Chr2_natMX).
Statistical analysis
Unless otherwise stated, results are presented either as one representative experiment of several or as the mean ± standard deviation (SD). A confidence interval of 95% was assumed for all statistical tests. One-way analysis of variance (ANOVA) was used for comparison of more than two means. No significant differences were assumed when P-value was higher than 0.05. Data were analysed and graphs were designed using GraphPad Prism 9.5 and Microsoft Office Excel.
Results and discussion
Efficient gRNA expression system for gene disruption in Z. parabailii
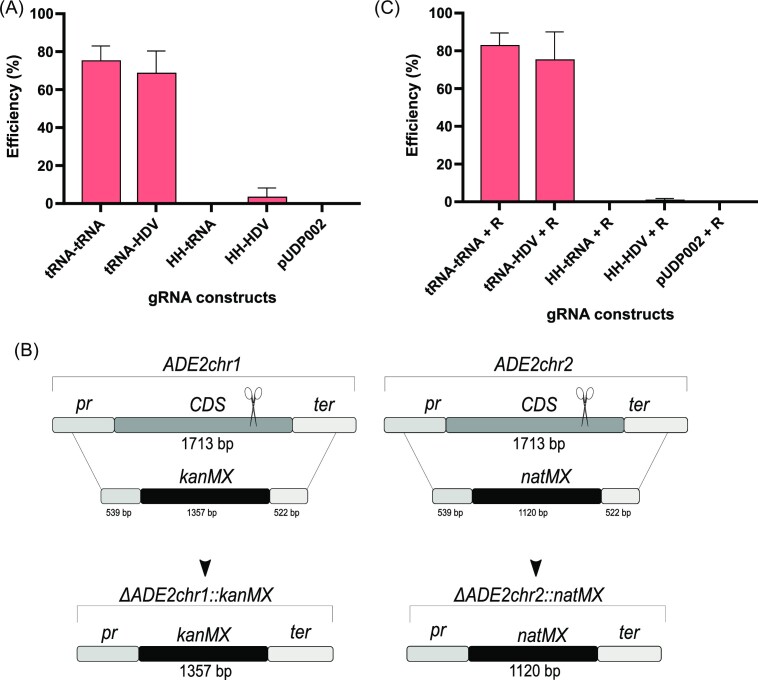
To evaluate the efficiency of different combinations of self-cleaving elements in gRNA constructs in Z. parabailii, we developed a CRISPR-Cas9 system based on the pUDP002 plasmid, which was shown to be effective in different yeast species (Juergens et al. 2018). This plasmid has the panARS element for replication in multiple yeasts, SpCas9 expressed from the Arxula adeninivorans TEF1 promoter (Gorter de Vries et al. 2017) and a cassette where a gRNA construct can be inserted between a ScTDH3 promoter and ScCYC1 terminator (Fig. 2). The target gene was ADE2 as its inactivation leads to intracellular accumulation of a red pigment and thus mutants are readily identified on agar plates (Ugolini and Bruschi 1996). Zygosaccharomyces parabailii carries two copies of ADE2, present on chromosome 1 (ADE2chr1) and chromosome 2 (ADE2chr2), that are 96% identical at the nucleotide level. Using combinations of two tRNAs (tRNAGly and tRNAAsp) and two ribozymes HH and HDV, we designed four different gRNA constructs and inserted these into the pUDP002 backbone (Fig. 2). To compare the efficiency of the four gRNA constructs, a synthetic construct comprising a common target sequence for the two ADE2 alleles of Z. parabailii ATCC60483, flanked by the different gRNA expression systems, was inserted into the pUDP002 backbone, creating four pUDPZb plasmids (Table 2). These constructs were transformed into Z. parabailii with selection for the pUDPZb plasmid. While transformation efficiency varied between replicate experiments, consistent patterns were seen (Table S1). The average number of transformants ranged from 5 to 469 (Table S1), with more transformants obtained from plasmids HH-tRNA and HH-HDV, which carried a HH ribozyme at the 5’ end, than plasmids tRNA-tRNA, and tRNA-HDV, which had a tRNAGly in that position. Between 5 and 52 transformants carrying each plasmid were screened to determine the efficiency of ADE2 inactivation, by the ratio of red (ade2) to white (ADE2) colonies (Fig. 3A). Whereas gRNA constructs carrying a 5’ self-cleaving tRNAGly gave rise to ade2 mutants at a frequency of 68%–75%, almost no ade2 mutants were generated by gRNA constructs using a 5’ HH ribozyme. No significant differences in efficiency were observed between having a tRNAAsp or an HDV ribozyme at the 3’ end of the gRNA construct.
Figure 3.
Evaluation of different gRNA constructs for the disruption of ADE2 in Z. parabailii. (A) Efficiency of red colonies obtained using Cas9 plasmids (pUDPZb) containing different gRNA constructs targeting ADE2. (B) DNA cassettes bearing kanMX or natMX and 500 bp homology arms, specific to each allele targeting the deletion of each of the two alleles of the gene. (C) Efficiency of red colonies obtained using Cas9 plasmids (pUDP002) containing different gRNA constructs targeting ADE2 with co-transformation of two DNA cassettes bearing a selective marker.
Parallel experiments were performed where we also included repair fragments in the transformation mix. These repair fragments were designed to integrate specifically at either the ADE2chr1 or the ADE2chr2 locus (Fig. 3B). Since the efficiency of homology-directed repair (HDR) is low in Z. parabailii, long (around 500 bp) homologous flanking arms specific for each allele were added to the 5’ and 3’ ends of the DNA repair fragments, which carried the kanMX or natMX hybrid genes, conferring resistance to G418 or clonNAT, respectively. When repair fragments were included in the transformations, the average number of transformants ranged from 7 to 698 (Table S2). The overall pattern of results was remarkably similar to the data obtained when no repair fragments were used (Fig. 3C). Notably, while the gRNA constructs carrying a 5’ self-cleaving tRNAGly gave rise to ade2 mutants at a frequency of 75–83%, almost no ade2 mutants were generated by gRNA constructs using a 5’ HH ribozyme. Next, we assessed whether the repair fragments had integrated into the target loci by replica plating ade2 mutants to determine whether the mutants had become G418 and/or clonNAT resistant, and if so, where the repair fragments integrated at the ADE2 loci (Table S2). We found that the efficiency of targeting either repair fragment was very low (less than 2% in all cases) and we had no instance where both repair fragments integrated at their respective ADE2 locus. These data indicated that, even in the presence of repair fragments, the ade2 mutations arose by NHEJ repair and the antibiotic resistance genes inserted into ectopic sites in the genome. Having established that efficient gRNA constructs needed a 5’ flanking tRNA, we designed the pUPDZb_U1 plasmid as an efficient system for cloning gRNA targets into a Cas9 delivery system. For the construction of this plasmid, pUDP002 was used as the backbone and a gRNA construct (tRNA-tRNA) was inserted between the ScTDH3 promoter and ScCYC1 terminator. This gRNA construct consists of a BsaI restriction site for the insertion of the DNA target (Fig. S1; Table 3), resulting in a universal Cas9 plasmid-bearing efficient tRNA system for the disruption of genes in Z. parabailii.
Construction of a Z. parabailii dnl4 strain
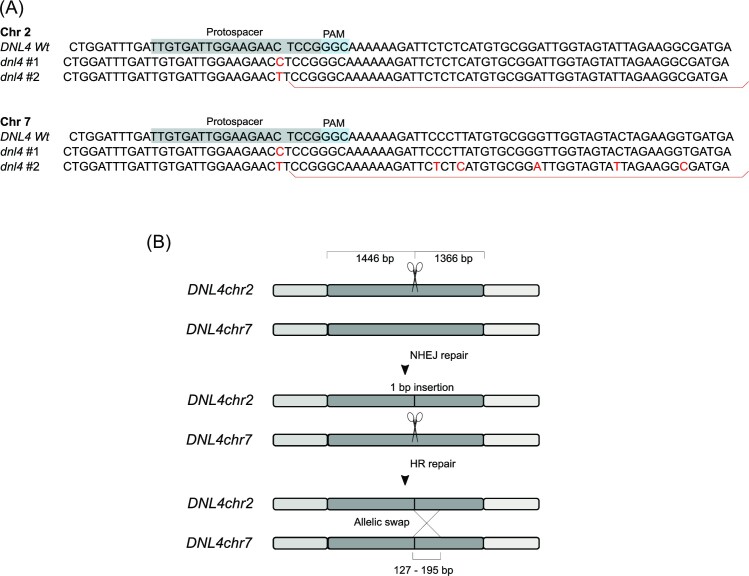
In most non-Saccharomyces yeasts, NHEJ is more efficient than HDR and to achieve efficient gene targeting, it is necessary to inactivate NHEJ by disruption of KU70, KU80, or DNL4. We chose to inactivate DNL4 in Z. parabailii as it has been shown from previous works in the literature that DNL4 deletion is the best way to eliminate multiple or random integrations and at the same time to maintain the same level of transformation efficiency (Choo et al. 2014, Rajkumar et al. 2019). In addition, the Ku70/Ku80 heterodimer has multiple roles in other yeast and its inactivation could have pleiotropic effects while Dnl4 has the sole role of NHEJ ligation (Polotnianka et al. 1998, Zahid et al. 2021). To proceed with DNL4 inactivation, we exploited the CRISPR-Cas9 system that we had developed. The Z. parabailii DNL4 alleles are 93% identical at the nucleotide level and located on chromosomes 2 and 7 (DNL4chr2, DNL4Chr7). To increase the chance of success, we designed and cloned five different common DNA targets for DNL4 (Table 4) into pUDPZb_U1. These were transformed into Z. parabailii (Table S3), transformants were screened by PCR, and sequenced to establish whether any double mutants has been obtained. Only target 3 generated any dnl4 mutants, and eight of these transformants were screened and showed a double DNL4 disruption by a nucleotide insertion in each DNL4 loci in the targeted region (Fig. 4A). The insertion of a single nucleotide occurred a few nucleotides before the PAM sequence, with an insertion of a cytosine in both DNL4chr2 and DNL4chr7 of mutant 1 and a thymine in both alleles of mutant 2 (Fig. 4A). Unexpectedly, the sequence in the mutant #2 DNL4chr7 allele matched that of the DNL4chr2 allele. We could identify a short (127–195 bp) region of allelic exchange between the two DNL4 loci that is due to the repair of the double-stranded break of the DNL4chr7 using the same region of DNL4chr2 as a template (Fig. 4B). The precise cross-over point could not be determined because the sequence is identical for part of the region. Allelic exchange during CRISPR-Cas9 mediated genome editing in yeast, leading to the loss of heterozygosity in a diploid yeast was previously described and explained as sequential events of cut and repair mechanisms after the double-strand break caused by the CRISPR-Cas9 system (Gorter de Vries et al. 2017).
Figure 4.
Z. parabailii dnl4 mutants 1 and 2 obtained using the pUDPZb plasmid. (A) Sequencing analysis of the DNL4 target regions of two mutants with the double inactivation. Insertions of nucleotides in red in the target region. Allele in chromosome 7 of mutant 2 shows allelic exchange with chromosome 2. (B) Representation of the repair mechanism observed in mutant 2: DNL4chr 2 is cut by Cas9 in the targeted sequence, and the double-strand break is repaired by NHEJ (as observed in most cases), leading to a frameshift mutation. Cas9 cuts DNL4chr7, and the first allele is used as a template for HDR, resulting in two disrupted alleles due to the same frameshift mutation; in chromosome 7 a hybrid protein is created.
Construction of targeted mutants in Z. parabailii dnl4
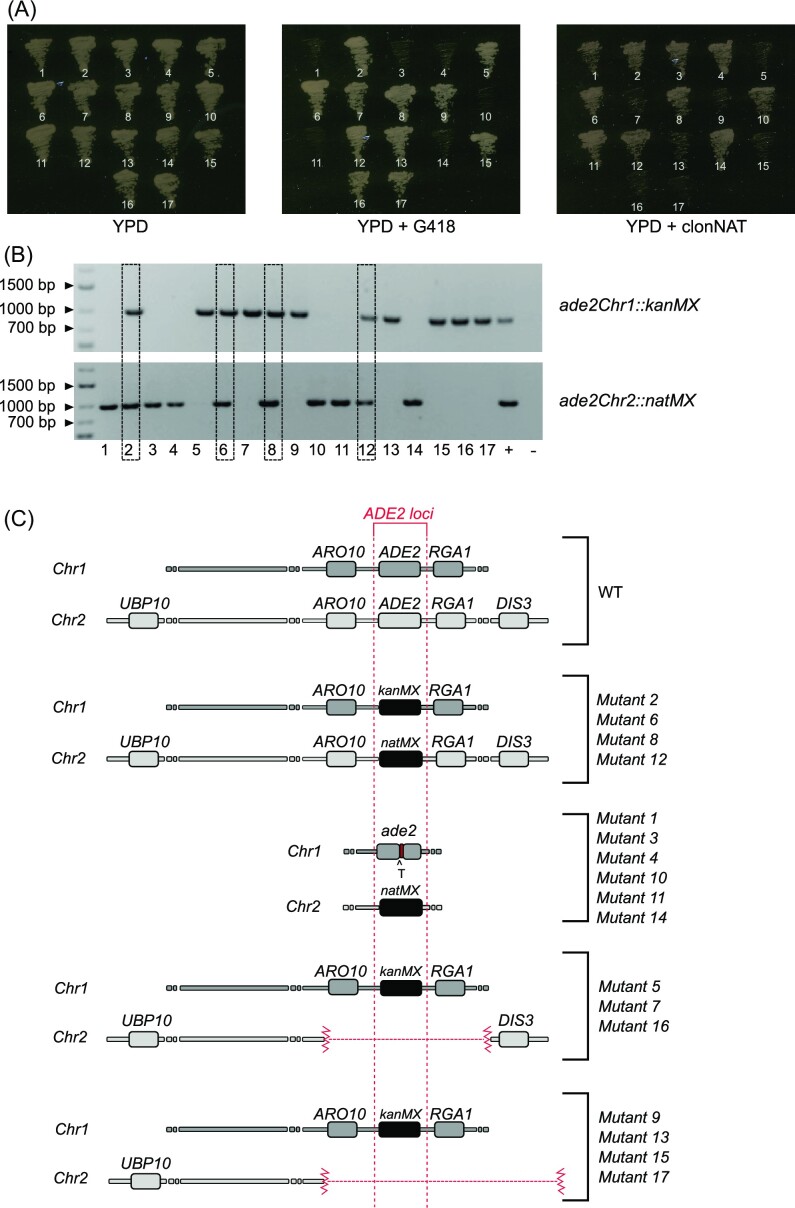
To assess whether the inactivation of DNL4 would lead to the anticipated increase in the efficiency of gene targeting by a repair fragment, independent transformation experiments were performed with the pUDPZb (tRNA-tRNA) targeting ADE2 with, and without, DNA repair fragments in both the wild-type and the dnl4 #1 strain (Table S4). As before (Fig. 3), transformants were screened to determine the nature of any ade2 mutations. Regarding the wild-type, out of thirty-one ade2 mutants tested, only three grew on G418 and one on clonNAT. PCR showed that only one of these had integrated kanMX at the ADE2chr1 locus and none had integrated natMX at the corresponding ADE2chr2 locus. This is in agreement with the previous experiments confirming that targeting repair fragments is inefficient in the wild-type strain. For the dnl4 mutant, 17 out of 27 transformants were red indicating the efficiency of 63% in generating ade2 mutants. These seventeen ade2 mutants were further analyzed by growth on G418 and clonNAT and then by PCR analysis to detect the insertion of DNA repair fragments. Of the 17 ade2 mutants, 10 and 11 grew on clonNat and G418, respectively (Fig. 5A). The molecular analysis showed that the eleven G418+ strains carried the kanMX gene at the ADE2chr1 locus and the ten clonNat+ strains carried natMX at the ADE2chr2 locus (Fig. 5B). Only four strains had the genotype ade2chr1::kanMX ade2chr2::natMX indicating integration of each repair fragment at the cognate locus (Fig. 5, lanes 2, 6, 8, 12). The remaining thirteen ade2 mutants had integrated either kanMX (Fig. 5, lanes 5, 7, 9, 13, 15, 16, 17) or natMX (Fig. 5, lanes 1, 3, 4, 10, 11, 14) at the correct ADE2 locus, but not both. These thirteen mutants were examined in more detail to determine what molecular events had taken place to generate these mutants, which by definition lacked functional Ade2, and so must have lesions at both ADE2 loci. The six ade2chr2::natMX strains all had a thymine insertion in the targeted region in ADE2chr1 before the PAM region. Thus, despite being in a dnl4 background, ADE2chr1 was inactivated by point mutation. The situation with the seven ade2chr1::kanMX strains was more complex, and efforts to amplify the ADE2chr2 locus were not successful. We found that all seven strains carried substantial chromosomal deletions of chromosome 2 that encompassed the ADE2chr2 locus. PCR amplifications in all seventeen mutants were performed to detect various genes upstream and downstream of the locus (Fig. S2) and identified two classes of mutants (Fig. 5C). Three of the mutants had a deletion of < 1.65 MB as the flanking genes UBP10 and DIS3 were present. The other four mutants appear to have lost an additional region downstream of DIS3 and probably are deleted to the right telomere of chr2.
Figure 5.
Phenotypic and molecular analysis of ade2 mutants in the Z. parabailii dnl4 mutant. (A) Seventeen ade2 mutants were patched onto YPD, YPD + G418 and YPD + clonNAT and growth evaluated. (B) Molecular assessment of the integration of the repair fragments with kanMX or natMX (∼1000 bp) into the targeted region of the ADE2 allele in chromosome 1 and chromosome 2, respectively. Mutants analyzed in lanes 2, 6, 8, and 12 have both fragments integrated into the targeted region. (C) Schematic view depicting the landscape around the ADE2 locus in chromosome 1 and chromosome 2 of the 17 Z. parabailii dnl4 ade2 mutants. For each mutant, different amplifications were performed targeting different up and downstream genes (UBP10, ARO10, RGA1, DIS3). The genes shown in each chromosome in the figure are the ones confirmed by amplification.
The frequent occurrence of large chromosomal deletions was striking and we wanted to investigate whether it was caused in some way by the dnl4 mutation. To do this, we selected four ade2 mutants that had been generated in the wild-type and performed the same type of PCR analysis as with the dnl4 ade2 mutants. The results, shown schematically in Fig. 6, found that each of the four mutants was generated differently. Mutant Z was the one mentioned earlier where the kanMX repair fragment correctly targeted ADE2chr1 and in this case, ADE2chr2 was disrupted by the insertion of thymine. Mutant X also inserted thymine at the ADE2chr2 locus but in this case, there was a large chromosomal deletion at the ADE2chr1 locus. Similarly, mutant Y carried a deletion on chromosome 1 and, this time, there was an 81 bp insertion that introduced a nonsense codon at the break site in ADE2chr2. Mutant W was more conventional with ADE2chr1 inactivated by insertion of a thymine and ADE2chr2 by insertion of a thymine and an adenine. This target-specific mutation of a nucleotide insertion is described as recurrent and generally predictable for mutations generated by the CRISPR-Cas9 system (Allen et al. 2018). The conclusion from this analysis is that the phenomenon of a mutation arising by deletion at the ADE2 locus can occur on either chromosome and is unrelated to the dnl4 mutation.
Figure 6.
Molecular analysis of the ADE2 loci of four random red colonies selected from the control transformation with the wild-type strain of Z. parabailii. Mutant W shows the insertion of one nucleotide in the target region in both loci and a potential short allelic exchange in ade2chr2. Mutant X—unable to amplify ade2chr1; nucleotide insertion in ade2chr2. Mutant Y—unable to amplify ade2chr1; 81 base insertion ade2chr2. Mutant Z—Insertion of kanMX cassette in ade2chr1; one base insertion in ade2chr2.
Conclusions
The primary aim of our study was to develop an efficient system for creating mutants of the hybrid yeast Z. parabailii. Although it was already possible to generate targeted mutants with traditional methods, low efficiency meant that repair fragments need very long flanking regions and large numbers of transformants must be screened. In this work, a CRISPR-Cas9 system was successfully constructed, and, for gene inactivation, we achieved efficiencies of 60%–80%. Different constructs were evaluated and it was determined that, in this yeast, gRNAs with a 5’ tRNA were far more efficient than those with a HH ribozyme in this position. Although inactivating mutations were readily generated, gene targeting remained very inefficient so a dnl4 mutant that should favor homology-dependent repair (HDR) over non-homologous end joining (NHEJ) repair was constructed. With Z. parabailii dnl4, it was possible to generate mutants where alleles on both chromosomes were inactivated by the targeted insertion of a repair fragment using a CRISPR-Cas9 system, with an HR efficiency of about 15%. In literature (Kuanyshev et al. 2021) a higher efficiency was reported (about 30%) but it refers to a haploid Z. bailii strain and therefore it is not comparable. The efficiency of the classical (non-Cas9 based) targeted gene-deletion in the hybrid yeast Z. parabailii is 1–5% for the first allele, and even lower for the second (Dato et al. 2010). In light of these considerations, our findings represent a major advance. However, in the present work, some unexpected inter-chromosomal recombination was observed so it remains necessary to use positive selection for both insertion events to ensure accurate strain construction. In summary, with the dnl4 mutant and the pUPDZb_U1 plasmid, a toolkit and protocol for manipulating and rewiring the genome of the hybrid yeast Z. parabailii is now available.
Diploid strains in general, and hybrid strains in particular, pose additional challenges for genome engineering. To date, for hybrid yeasts, efficient gene editing and de novo strain construction via CRISPR-Cas9 have only been demonstrated for S. pastorianus (Gorter de Vries et al. 2017, Mertens et al. 2019). Our work with Z. parabailii, therefore, provides some very useful insights into issues with engineering hybrid species. The frequency with which recombinations happened between chromosomes during mutagenesis came as a surprise. These recombinations occurred in all our transformations, at both the ADE2 locus and the DNL4 locus, whether repair fragments were present or not, and in the wild-type and dnl4 mutant, so we infer that it is a general phenomenon that is likely to take place whenever CRISPR-Cas9 is used in Z. parabailii. Although not widely observed, other studies in both yeast and mammalian cells suggest that this type of event is not unique to Z. parabailii. In diploid Saccharomyces strains, loss of heterozygosity by the exchange of sequences between the targeted allele and its homolog when single alleles were targeted using the CRISPR-Cas9 gene editing system was described (Gorter de Vries et al. 2019). Deletion of large sequences or chromosome arms in the targeted regions has also been reported as an effect of the use of CRISPR-Cas9 in mammalian cells (Kosicki et al. 2018). Further work is required to determine the factors that cause or exacerbate inter-chromosomal recombination as it is clearly undesirable when constructing strains for applications in biotechnology. It also makes it almost impossible to consider multiplexing as the large number of candidate mutants that would need to be screened would be prohibitive. The limited studies to date seem to indicate that an inter-chromosomal recombination is a diploid-related event, but it remains to be determined whether it is more prevalent in hybrids where the higher sequence divergence between sister chromosomes may have an impact. In fact, data from a recent study in S. pastorianus supports this idea as those authors observed less recombination in cases where divergent chromosomes were not present (Bennis et al. 2023). Although not central to this study, it is well known that the process of domestication often gives rise to polyploidization, aneuploidy and loss of heterozygosity, and it is interesting to speculate that similar forces may be at play here.
Yeast strain engineering has moved from the situation where only S. cerevisiae was amenable to molecule genetics to one where almost any yeast can now be considered a potential host. Despite this, the focus has remained on pure haploid species for ease of use. Our work demonstrates that genome editing systems can be put in place for diploid and more particularly hybrid, non-traditional yeasts. It also provides a template for the successful design and evaluation of the most efficient gRNA system. Despite this success, further work is required to understand the complete dynamics of the process, in particular the nature, frequency and parameters that govern types of mutation events, including those that give rise to loss of heterozygosity.
Supplementary Material
Contributor Information
Pooja Jayaprakash, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, Milano 20126, Italy; School of Microbiology, Environmental Research Institute, APC Microbiome Institute, SUSFERM Fermentation Centre, University College Cork, Cork T12 K8AF, Ireland.
Liliane Barroso, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, Milano 20126, Italy; Department of Genetics and Genome Biology, University of Leicester, Leicester LE1 7RH, UK.
Matteo Vajente, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, Milano 20126, Italy.
Letizia Maestroni, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, Milano 20126, Italy.
Edward J Louis, Department of Genetics and Genome Biology, University of Leicester, Leicester LE1 7RH, UK.
John P Morrissey, School of Microbiology, Environmental Research Institute, APC Microbiome Institute, SUSFERM Fermentation Centre, University College Cork, Cork T12 K8AF, Ireland.
Paola Branduardi, Department of Biotechnology and Biosciences, University of Milano-Bicocca, Piazza della Scienza 2, Milano 20126, Italy.
Author contributions
PJ, LB, MV, PB, and JM were involved in designing the experimental work plan. LB, MV, and PJ performed experimental work, analyzed data and wrote the manuscript. LM carried out the final editing of the manuscript and completed the reviewing process. PB, JM, and EJL provide inputs for the interpretation of results, performed data analysis and carried out the final editing of the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Funding
This project has received funding from the European Union's Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie Grant Agreement No. 764927. It was also partially supported by the MUSA—Multilayered Urban Sustainability Action—project, funded by the European Union—NextGenerationEU, under the National Recovery and Resilience Plan (NRRP) Mission 4 Component 2 Investment Line 1.5: Strengthening of research structures and creation of R&D ‘innovation ecosystems’, set up of ‘territorial leaders in R&D’.
References
- Allen F, Crepaldi L, Alsinet Cet al. Predicting the mutations generated by repair of Cas9-induced double-strand breaks. Nat Biotechnol. 2018;37:64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennis NX, Kostanjšek M, van den Broek Met al. Improving CRISPR-Cas9 mediated genome integration in interspecific hybrid yeasts. New Biotechnol. 2023;76:49–62. [DOI] [PubMed] [Google Scholar]
- Bilal M, Ji L, Xu Set al. Bioprospecting and biotechnological insights into sweet-tasting proteins by microbial hosts-a review. Bioengineered. 2022;13:9815–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branduardi P, Barroso L, Dato Let al. Molecular tools for leveraging the potential of the acid-tolerant yeast Zygosaccharomyces bailii as cell factory. Methods Mol Biol. 2022;2513:179–204. [DOI] [PubMed] [Google Scholar]
- Branduardi P, Valli M, Brambilla Let al. The yeast Zygosaccharomyces bailii: a new host for heterologous protein production, secretion and for metabolic engineering applications. FEMS Yeast Res. 2004;4:493–504. [DOI] [PubMed] [Google Scholar]
- Choo JH, Han C, Kim JYet al. Deletion of a KU80 homolog enhances homologous recombination in the thermotolerant yeast Kluyveromyces marxianus. Biotechnol Lett. 2014;36:2059–67. [DOI] [PubMed] [Google Scholar]
- Christine LJ, Marc L, Catherine Det al. Characterization of natural hybrids of Saccharomyces cerevisiae and Saccharomyces bayanus var. Uvarum. FEMS Yeast Res. 2007;7:540–9. [DOI] [PubMed] [Google Scholar]
- Dato L, Branduardi P, Passolunghi Set al. Advances in molecular tools for the use of Zygosaccharomyces bailii as host for biotechnological productions and construction of the first auxotrophic mutant. FEMS Yeast Res. 2010;10:894–908. [DOI] [PubMed] [Google Scholar]
- Gabaldón T. Hybridization and the origin of new yeast lineages. FEMS Yeast Res. 2020;20, doi: 10.1093/FEMSYR/FOAA040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhao Y. Self-processing of ribozyme-flanked rnas into guide rnas in vitro and in vivo for CRISPR-mediated genome editing. J Integr Plant Biol. 2014;56:343–9. [DOI] [PubMed] [Google Scholar]
- Gibson B, Geertman JMA, Hittinger CTet al. New yeasts-new brews: modern approaches to brewing yeast design and development. FEMS Yeast Res. 2017;17, doi: 10.1093/FEMSYR/FOX038. [DOI] [PubMed] [Google Scholar]
- González SS, Barrio E, Querol A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl Environ Microbiol. 2008;74:2314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter de Vries AR, Couwenberg LGF, Van Den Broek Met al. Allele-specific genome editing using CRISPR–Cas9 is associated with loss of heterozygosity in diploid yeast. Nucleic Acids Res. 2019;47:1362–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter de Vries AR, de Groot PA, van den Broek Met al. CRISPR-Cas9 mediated gene deletions in lager yeast Saccharomyces pastorianus. Microb Cell Fact. 2017;16:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorter de Vries AR, Pronk JT, Daran JMG. Industrial relevance of chromosomal copy number variation in Saccharomyces yeasts. Appl Environ Microbiol. 2017;83, doi: 10.1128/AEM.03206-16/ASSET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler M, Morrissey JP, Laus Aet al. A new hypothesis for the origin of the lager yeast Saccharomyces pastorianus. FEMS Yeast Res. 2023;23, doi: 10.1093/FEMSYR/FOAD023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens H, Varela JA, de Vries ARGet al. Genome editing in Kluyveromyces and Ogataea yeasts using a broad-host-range Cas9/gRNA co-expression plasmid. FEMS Yeast Res. 2018;18:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaalioğlu O, Yüceer YK. Nonconventional yeasts to produce aroma compounds by using agri-food waste materials. FEMS Yeast Res. 2021;21:63. [DOI] [PubMed] [Google Scholar]
- Kosicki M, Tomberg K, Bradley A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat Biotechnol. 2018;36:765–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuanyshev N, Adamo GM, Porro Det al. The spoilage yeast Zygosaccharomyces bailii: foe or friend?. Yeast. 2017a;34:359–70. [DOI] [PubMed] [Google Scholar]
- Kuanyshev N, Adamo GM, Porro Det al. The spoilage yeast Zygosaccharomyces bailii: Foe or friend?. Yeast. 2017a;34:359–70. [DOI] [PubMed] [Google Scholar]
- Kuanyshev N, Ami D, Signori Let al. Assessing physio-macromolecular effects of lactic acid on Zygosaccharomyces bailii cells during microaerobic fermentation. FEMS Yeast Res. 2016;16:58. [DOI] [PubMed] [Google Scholar]
- Kuanyshev N, Rao CV, Dien Bet al. Domesticating a food spoilage yeast into an organic acid-tolerant metabolic engineering host: lactic acid production by engineered Zygosaccharomyces bailii. Biotechnol Bioeng. 2021;118:372–82. [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens S, Gallone B, Steensels Jet al. Reducing phenolic off-flavors through CRISPR-based gene editing of the FDC1 gene in Saccharomyces cerevisiae x Saccharomyces eubayanus hybrid lager beer yeasts. PLoS One. 2019;14:e0209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse NJ, Wagner JM, Reed KBet al. T7 Polymerase expression of guide rnas in vivo allows exportable CRISPR-Cas9 editing in multiple yeast hosts. ACS Synth Biol. 2018;7:1075–84. [DOI] [PubMed] [Google Scholar]
- Ortiz-Merino RA, Kuanyshev N, Braun-Galleani Set al. Evolutionary restoration of fertility in an interspecies hybrid yeast, by whole-genome duplication after a failed mating-type switch. PLoS Biol. 2017;15:e2002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz-Merino RA, Kuanyshev N, Byrne KPet al. Transcriptional response to lactic acid stress in the hybrid yeast Zygosaccharomyces parabailii. Appl Environ Microbiol. 2018;84:2294–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M, Guerreiro JF, Sá-Correia I. Adaptive response and tolerance to Acetic acid in Saccharomyces cerevisiae and Zygosaccharomyces bailii: a physiological genomics perspective. Front Microbiol. 2018;9, doi: 10.3389/FMICB.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M, Sá-Correia I. Physiological genomics of the highly weak-acid-tolerant food spoilage yeasts of Zygosaccharomyces bailii sensu lato. Prog Mol Subcell Biol. 2019;58:85–109. [DOI] [PubMed] [Google Scholar]
- Polotnianka RM, Li J, Lustig AJ. The yeast ku heterodimer is essential for protection of the telomere against nucleolytic and recombinational activities. Curr Biol. 1998;8:831–5. [DOI] [PubMed] [Google Scholar]
- Raihofer L, Zarnow M, Gastl Met al. A short history of beer brewing. EMBO Rep. 2022;23:e56355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar AS, Varela JA, Juergens Het al. Biological parts for Kluyveromyces marxianus synthetic Biology. Front Bioeng Biotechnol. 2019;7, doi: 10.3389/FBIOE.2019.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Ohnishi Y. The chromosomal evolutionary lineage of the genus Zygosaccharomyces. FEMS Yeast Res. 2023;23, doi: 10.1093/FEMSYR/FOAD017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Dai Z, Wang Q. Advances and opportunities of CRISPR/cas technology in bioengineering non-conventional yeasts. Front Bioeng Biotechnol. 2021;9:942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Hanson J, Paliwal Ket al. RNA secondary structure prediction using an ensemble of two-dimensional deep neural networks and transfer learning. Nat Commun 2019 10:1. 2019;10:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solieri L. The revenge of Zygosaccharomyces yeasts in food biotechnology and applied microbiology. World J Microbiol Biotechnol 2021 37:6. 2021;37:1–22. [DOI] [PubMed] [Google Scholar]
- Stovicek V, Holkenbrink C, Borodina I. CRISPR/cas system for yeast genome engineering: advances and applications. FEMS Yeast Res. 2017;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh SO, Gujjari P, Beres Cet al. Proposal of Zygosaccharomyces parabailii sp. nov. And Zygosaccharomyces pseudobailii sp. nov., novel species closely related to Zygosaccharomyces bailii. Int J Syst Evol Microbiol. 2013;63:1922–9. [DOI] [PubMed] [Google Scholar]
- Ugolini S, Bruschi CV. The red/white colony color assay in the yeast Saccharomyces cerevisiae: epistatic growth advantage of white ade8-18, ade2 cells over red ade2 cells. Curr Genet. 1996;30:485–92. [DOI] [PubMed] [Google Scholar]
- Xie S, Shen B, Zhang Cet al. sgRNAcas9: a software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS One. 2014;9:e100448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahid S, El Dahan MS, Iehl Fet al. The multifaceted roles of Ku70/80. IJMS. 2021;22, doi: 10.3390/IJMS22084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Song B, Li Jet al. Rhodotorula toruloides: an ideal microbial cell factory to produce oleochemicals, carotenoids, and other products. World J Microbiol Biotechnol. 2021;38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.