Abstract
Background
Although polymyxin B has been in use since the late 1950s, there have been limited studies done to unravel its pharmacokinetics (PK) and pharmacodynamics (PD) index.
Methods
We determined, in neutropenic infected mice, the PK, plasma protein binding and PK/PD index best correlating with efficacy for Escherichia coli and Klebsiella pneumoniae strains.
Results
The pharmacokinetic profile showed non-linear PK; dose was significantly correlated with absorption rate and clearance. The inhibitory sigmoid dose–effect model for the fCmax/MIC index of E. coli fitted best, but was only modestly higher than the R2 of %fT>MIC and fAUC/MIC (R2 0.91–0.93). For K. pneumoniae the fAUC/MIC index had the best fit, which was slightly higher than the R2 of %fT>MIC and fCmax/MIC (R2 0.85–0.91). Static targets of polymyxin B fAUC/MIC were 27.5–102.6 (median 63.5) and 5.9–60.5 (median 11.6) in E. coli and in K. pneumoniae isolates, respectively. A 1 log kill effect was only reached in two E. coli isolates and one K. pneumoniae. The PTA with the standard dosing was low for isolates with MIC >0.25 mg/L.
Conclusions
This study confirms that fAUC/MIC can describe the exposure–response relationship for polymyxin B. The 1 log kill effect was achieved in the minority of the isolates whereas polymyxin B PK/PD targets cannot be attained for the majority of clinical isolates with the standard dosing regimen, indicating that polymyxin B may be not effective against serious infections as monotherapy.
Introduction
With the emergence of resistance and limited therapeutic options, the use of neglected and disused antibiotics becomes increasingly important. However, knowledge about pharmacokinetics (PK) and pharmacodynamics (PD) of these old antibiotics is mostly lacking or very limited. Polymyxin B is such an antibiotic, with activity against bacteria belonging to the largest antibiotic resistance threats according to the US CDC,1 including carbapenem-resistant Enterobacterales, Pseudomonas aeruginosa and Acinetobacter baumannii.
The use of polymyxin B fell out of favour in the mid-1970s, because of toxicity concerns, especially regarding nephrotoxicity and neurotoxicity,2 variability in exposure profiles and the narrow therapeutic window.3 Nowadays, it is increasingly used as a last-line antibiotic. The somewhat simpler PK of polymyxin B, compared with colistin, might be a therapeutic advantage, but nevertheless polymyxin B is currently not available in the EU.
Limited studies have investigated the PK/PD of polymyxin B, so optimal dosing regimens remain uncertain.3,4 Earlier in vitro and in vivo studies mainly focused on colistin PD in P. aeruginosa and A. baumannii isolates.5–7 In 2017, Landersdorfer et al.8 were the first to show in studies in mice that polymyxin B antimicrobial activity against Klebsiella pneumoniae best correlated with the ratio of the area under the unbound concentration–time curve to the MIC (fAUC/MIC). In addition, other in vitro studies using various Gram-negative species have suggested fAUC/MIC is relevant for polymyxin B,9,10 which is in line with results for colistin.11
In the present study, we investigated which PK/PD index is best correlated with efficacy of an Escherichia coli and a K. pneumoniae strain. Subsequently, we determined the magnitude of the PK/PD index needed for stasis and a 1 log kill in several E. coli and K. pneumoniae strains.
Materials and methods
Antibiotics and bacterial strains
Polymyxin B sulphate salt (lot. 117M4045V, 8926 units/mg; Sigma–Aldrich, Zwijndrecht, The Netherlands) and was reconstituted in normal sterile saline (0.9% NaCl, Baxter, Utrecht, The Netherlands). Solutions were freshly prepared for each experiment.
Four E. coli and five K. pneumoniae well-characterized clinical isolates with different resistance mechanisms were used (Table S1, available as Supplementary data at JAC Online).
In vitro susceptibility testing
The MIC of polymyxin B for each strain was determined in triplicate by broth microdilution, according to the CLSI guidelines.12,13 Median MIC values were reported and utilized in the PK/PD analysis.
In vivo thigh and lung infection models in neutropenic mice
Experiments were carried out in the Erasmus Laboratory Animal Science Centre in Rotterdam, The Netherlands in accordance with the EU Animal Directive 2010/63/EU 2010 directive14 (licence number AVD101002016702) as described before,15 with approval of the institutional Animal Welfare Body. Outbred female CD-1 mice obtained from Charles River Germany, weighing 20–25 g, 7–8 weeks old, were used in the experiments. The mice were housed under standard conditions with drink and feed supplied ad libitum.
Mice were rendered neutropenic by intraperitoneal injection of cyclophosphamide of 150 and 100 mg/kg, 4 days and 1 day before infection, respectively. On the day of infection, an overnight culture of the bacteria was diluted in fresh CAMHB and incubated at 37°C for 1 or 2 h depending on the growth rate of the strain. After incubation the inoculum was diluted in CAMHB or saline for the thigh and lung infection model, respectively, in order to obtain approximately 5.0 × 107 cfu/mL. Mice were then infected intramuscularly in each thigh (thigh infection model) or intranasally under isoflurane anaesthesia (lung infection model), with 0.05 mL of inoculum containing approximately 2.5 × 106 bacteria. Analgesia was given in the form of buprenorphine directly after infection, every 12 h.
PK of polymyxin B
Mice were injected subcutaneously with a 0.1 mL single dose of polymyxin B 2 h after infection (t = 0). The PK of polymyxin B ranging between 0.5 and 64 mg/kg were determined in two mice per dose level. Mice were separated into two groups, with four dose levels studied in the thigh infection model (0.5, 2, 8, 32 mg/kg) and four dose levels studied in the lung infection model (1, 4, 16, 64 mg/kg). At t = 0, two mice were humanely euthanized to determine the bacterial load in the infected organ at the start of infection. At 10 different timepoints, blood was collected under isoflurane anaesthesia, in K3E EDTA tubes (Sarstedt, Nümbracht, Germany) through orbital sinus bleeding, which was immediately followed by cervical dislocation. The collected blood samples were centrifuged immediately at 15 871 RCF for 5 min at 4°C. Plasma was stored at −80°C until analysis.
Bronchoalveolar lavage (BAL) was performed immediately after blood collection. The trachea was exposed by a ventral vertical incision in the neck for insertion of a cannula. Lungs were instilled two times with 1 mL of sterile saline, and the fluid was recovered immediately. The recovered lavages were pooled, directly placed on ice, and subsequently stored at −80°C.
Since the PK were analysed using a population PK model, which increases the power of the analysis, we used two mice per timepoint to detect outliers.
Protein binding
The binding of polymyxin B to plasma proteins was determined via ultrafiltration. Polymyxins have a tendency to non-specifically bind to different kinds of material, including ultrafiltration filters. Thus, to correct for polymyxin B loss on the filter, an experiment was performed in which ultrafiltrated (UF) phosphate buffer was spiked with multiple polymyxin B concentrations (ranging from 0.75 to 8 mg/L) and subsequently concentrations were measured. From these results the relationship between polymyxin B concentration post-UF and non-specific binding could be established.16 Using this relationship, the non-specific binding (NSB) for each measured polymyxin B UF concentration was calculated. Correction for the measured polymyxin B UF concentration using this NSB value was according to the following formula: polymyxin B UF Corrected Concentration = Measured polymyxin B UF Concentration × (1−NSB).
Epithelial lining fluid (ELF) concentration
By taking BAL fluid samples and a plasma sample at the same timepoint, the concentrations in ELF could be determined. The ratio of the urea concentration in BAL to the concentration in plasma was used to determine the ELF concentration.17 In short, polymyxin B concentration in ELF was calculated by multiplying the polymyxin B concentration in the BAL fluid by the ratio of the urea concentration in the plasma and BAL fluid: [polymyxin B]ELF = [polymyxin B]BAL × ([urea]Plasma/[urea]BAL). Urea concentrations were measured with an enzymatic assay (QuantiChrom urea assay kit; DIUR-100; BioAssay Systems, Hayward, CA, USA).
LC-MS/MS assay
Polymyxin B concentrations in plasma, BAL and ultrafiltrates were determined by a validated LC-MS/MS method,18 with a lower limit of quantification (LOQ) of 0.2 mg/L for plasma and 0.05 mg/L for BAL fluid and ultrafiltrates. In this assay, 0.1 mL of plasma or 0.05 mL of BAL fluid or ultrafiltrates was added to 0.05 mL of drug-free plasma, and was mixed with 0.75 mL of phosphate buffer (pH 7.2) and 0.01 mL of internal standard (colistin sulphate, Merck KGaA, Darmstadt, Germany) at 6.25 mg/L for plasma and 5 mg/L for BAL fluid and ultrafiltrates. The samples were briefly vortexed and then centrifuged at 3000 rpm for 5 min. The supernatants (0.8 mL) were loaded onto solid-phase extraction columns (Oasis HLB solid-phase extraction cartridges, 1 mL, 30 mg, Waters, Saint-Quentin-en-Yvelines, France), preconditioned with 1 mL of methanol and followed by 1 mL of water. Columns were washed with 1 mL of water and dried under nitrogen pressure. The analytes were eluted with 0.5% formic acid in methanol and they were evaporated at 45°C under a gentle nitrogen jet stream. The residues were dissolved in 0.1 mL of 0.1% formic acid in water and analysed by LC-MS/MS. The system included an Alliance Waters 2695 LC system module (Waters) coupled with an API Quattro Micro (Waters). Polymyxin B was analysed on an XBridge C18 column (5 µm, 2.1 × 150 mm; Waters). The mobile phase A consisted of 0.1% formic acid in water, and mobile phase B was 0.1% formic acid in acetonitrile. The gradient for mobile phases A and B were respectively set at 75% and 25% with a flow rate of 0.2 mL/min. Electrospray ionization in positive mode was used for the detection of polymyxin B. Ions were analysed in the multiple reaction monitoring, and the following transitions were inspected: m/z 602.1→101 for polymyxin B1, m/z 595.1→101 for polymyxin B2, m/z 585.1→101 for CSTA and m/z 578.1→101 for CSTB. Calibration curves of polymyxin B ranged from 0.1 to 10 mg/L for plasma and ultrafiltrates, and were quantified with a quadratic regression mode. The intraday variability was characterized at four levels (0.3, 1, 2.5 and 10 mg/L) with a precision and bias of <20% for the lowest level, and <15% for the others.
Pharmacokinetic modelling
A population PK model was developed using non-linear mixed-effects modelling (NONMEM, version 7.4.2 ICON Development Solutions, Ellicott City, MD, USA). The analysis was performed using the FOCE + I method on logarithmically transformed concentrations and data below the LOQ were omitted. Parameters were calculated for a virtual 1 kg mouse, resulting in pharmacokinetic parameters corresponding on a per kg base. In the first step a structural model was developed, using one- and two-compartment models. The absorption following subcutaneous injections was described using an absorption rate constant (ka). Typical values for central (Vc) and peripheral volume of distribution (Vp), CL and intercompartmental clearance (Q) were estimated. As bioavailability (F) could not be estimated, CL, Vc, Q, and Vp values corresponded to the ratios CL/F, Vc/F, Q/F and Vp/F, respectively. Addition of between-subject variability (BSV), described using an exponential model, was evaluated for each pharmacokinetic parameter. Residual variability was described using an additive error model for logarithmically transformed data. In the second step, relationships between pharmacokinetic parameters and potential covariates (infection site and polymyxin B dose) were investigated. The effect of dose on the parameters was modelled using a power function, effect of infection site with a linear function. Covariates were included using forward inclusion (P < 0.05) and backward elimination (P < 0.001). Minimum objective function values (OFVs), parameter precision, error estimates and visual inspection of the goodness-of-fit plots were considered for model selection. CIs around the final parameters were estimated by bootstrap with resampling (n = 1000) and fit to the data was evaluated using visual predictive checks (VPCs) and VPCs stratified for dose (n = 1000).
PD of polymyxin B
Dose-fractionation studies were performed for E. coli ATCC 25922 and K. pneumoniae 104 in the thigh infection model. Two hours after infection, treatment with polymyxin B was administered subcutaneously over 24 h at the following dosing regimens: 4–128 mg/kg q6h [total daily dose (TDD) 16–512 mg/kg]; 8–256 mg/kg q12h (TDD 16–512 mg/kg); 256–512 mg/kg q24h (TDD 256–512 mg/kg).
For dose–response studies, a 6 hourly dosing regimen of 1–32 mg/kg was studied in the thigh infection model. Mice were infected with E. coli ATCC 259222, 15, 51 or 71 or K. pneumoniae ATCC 43816, 17, 58 or 74.
For PD analysis, we used six doses in two animals, resulting in 12 data points. As a rule of thumb, 2n + 1 = 9 data points (n = model parameters, which in the case of the Emax model is 4; the Emax, Emin, ED50 and slope) are required in regression analysis.
Animals were humanely euthanized 24 h (t = 24 h) after the first dose, unless the welfare of the animals necessitated earlier termination, following animal welfare regulations. Excised thighs were transferred to a pre-cooled 14 mL polypropylene tube containing 2 mL of PBS and were homogenized using a T25 ULTRA-TURRAX (IKA-Werke GmbH & Co, Staufen, Germany). A 10-fold dilution series was prepared and 3 × 10 μL was plated per dilution. The following day, colonies were counted and the number of cfu per thigh was calculated.
The drug effect was determined by the difference between the log10 cfu values at t = 0 h (mean value of two mice) and t = 24 h (value of individual mice) expressed as Δlog10 cfu. The fAUC/MIC, fCmax/MIC and %fT>MIC were determined based on the simulated time–concentration profiles over 24 h as derived in MicLab 2.71 (MEDIMATICS, Maastricht, The Netherlands) using 200 points per dosing interval.
A sigmoid maximum effect (Emax) model was fitted to the PK/PD index versus Δlog10 cfu data by non-linear regression, and static or a 1 log or 2 log kill effect were determined using GraphPad Prism 8.0 (GraphPad, Inc., San Diego, CA, USA). A two-tailed unpaired t-test was performed in GraphPad Prism 8.0 to determine if there was a significant difference in the fAUC/MIC targets correlating with a static or a 1 log or 2 log decline, between E. coli and K. pneumoniae isolates.
PTA in humans
A previously published population PK model19 and Monte Carlo simulations was used to estimate the fAUCs of 5000 patients treated with 200 mg q24h (MicLab 2.71) taking into account protein-binding rates ranging from 36% to 98% as previously reported.20–23 The PTA was calculated for isolates with MICs ranging from 0.25 to 4 mg/L.
Results
Plasma protein binding
NSB was concentration dependent and log-linearly correlated with concentrations ranging from 77% ± 8.3% at 0.75 mg/L to 20% ± 0.6% at 8 mg/L. No concentration dependency was observed for polymyxin B and %fu (percent fraction unbound; mean ± SD) was determined to be 20% ± 4.0%. For the calculation of exposures to unbound compound [maximum concentration in plasma of the free fraction of drug (fCmax)] and fAUC, a single factor of 20% was used.
PK
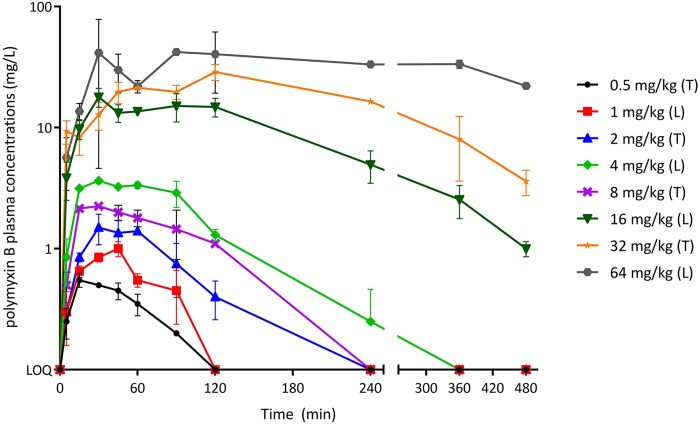
The pharmacokinetic profile of polymyxin B in mouse plasma after single subcutaneous administrations of 0.5–64 mg/kg is shown in Figure 1. The total peak plasma levels ranged from 0.6 to 68 mg/L, with detectable polymyxin B plasma levels up to 6 and 8 h after subcutaneous administration for doses up to 8 and 16–64 mg/kg, respectively. Low levels of polymyxin B in ELF were only detectable after a 64 mg/kg dose (data not shown); other concentrations in ELF were below the LOQ.
Figure 1.
Total plasma polymyxin B concentration versus time after single subcutaneous administrations of 2, 4, 8, 16, 32 or 64 mg/kg polymyxin B in infected neutropenic mice. Each data point represents two mice, with the mean ± SD plotted. Lower LOQ is 0.2 mg/L. T, thigh infection model; L, lung infection model. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Whatever the infection model, time to peak (Tmax) was ≤2 h. There were no significant differences in PK between thigh and lung models (Figure S1).
Pharmacokinetic modelling
The PK profile was described by a one-compartment model with first-order absorption. Inclusion of BSV on ka and Vc improved the model fit. CL and ka were significantly correlated with polymyxin B dose (Table 1 and Figure S2).
Table 1.
Parameter estimates and bootstrap analysis of the population PK model for polymyxin B
| Parameter | Description | Value | Bootstrap (5th/95th percentile) |
|---|---|---|---|
| ka (h−1) | Absorption rate constant for 10 mg/kg dose | 1.63 | 1.25/2.20 |
| V c/F (L/kg) | Central volume of distribution | 0.774 | 0.669/0.880 |
| CL/F (L/h/kg) | Clearance for 10 mg/kg dose | 0.384 | 0.348/0.424 |
| Dose on ka | Power on covariate effect of dose on ka | −0.407 | −0.485/−0.3217 |
| Dose on CL | Power on covariate effect of dose on CL | −0.26 | −0.322/−0.197 |
| BSV ka (%) | Inter individual variability on ka | 79 | 64/96 |
| BSV CL (%) | Inter-individual variability on clearance | 45 | 20/58 |
| Residual error | Variance of the log additive residual error | 0.093 | 0.040/0.167 |
The covariate polymyxin B dose (in mg/kg) is added as follows: CL = 0.384 × (DOSE/10)−0.26 and ka = 1.63 × (DOSE/10)−0.407. Bootstrap is presented as 90-percentile range of 475 successful resampling runs.
The goodness-of-fit plots showed good agreement between observations and model predictions. The final model was validated using bootstrap and VPC (Figure S3). No effect on PK was found for infection site.
In the PK model the dose per administration is a covariate on CL as well as ka, which means that total drug exposure of the same TDDs differs between the q6h and q12h intervals.
Dose fractionation study
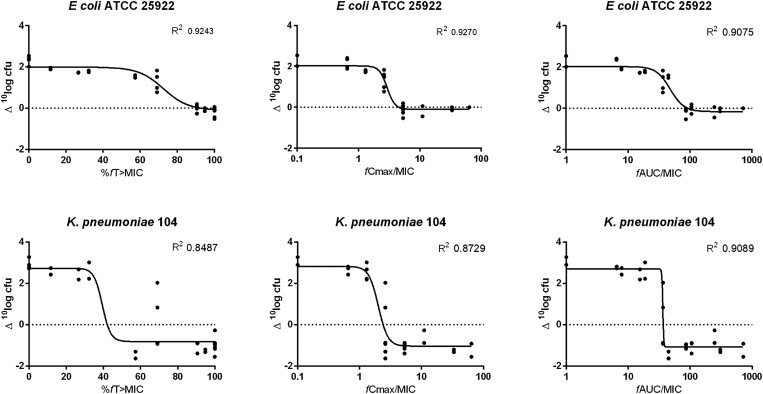
In dose fractionation studies the average bacterial burden at start of treatment (t = 0) was 1.5 × 107 (range: 1.2–2.0 × 107) cfu/thigh. Polymyxin B doses >64 mg/kg were not tolerated. The relationship between the bacterial load and fAUC/MIC, fCmax/MIC and %fT>MIC for E. coli ATCC 25922 and K. pneumoniae 104 is shown in Figure 2. The inhibitory sigmoid dose–effect model for fCmax/MIC of E. coli fitted best (R2 0.9270), but was only modestly higher than %fT>MIC (R2 0.9243) and fAUC/MIC (R2 0.9075). For K. pneumoniae 104, fAUC/MIC had the best fit (R2 0.9089), which was slightly higher than the R2 values of 0.8729 for fCmax/MIC and 0.8487 for %fT>MIC.
Figure 2.
Dose-fractionation studies. Relationships after polymyxin B treatment, where the bacterial burden, expressed as Δlog10 cfu, is plotted against %fT>MIC, fCmax/MIC and fAUC/MIC for E. coli ATCC 25922 and K. pneumoniae 104. Each data point represents a single thigh; the solid line represents the fit of the inhibitory sigmoid dose–effect model; the dotted line indicates the point at which there is no change from the burden at the start of treatment.
Determination of the PK/PD index linked to efficacy
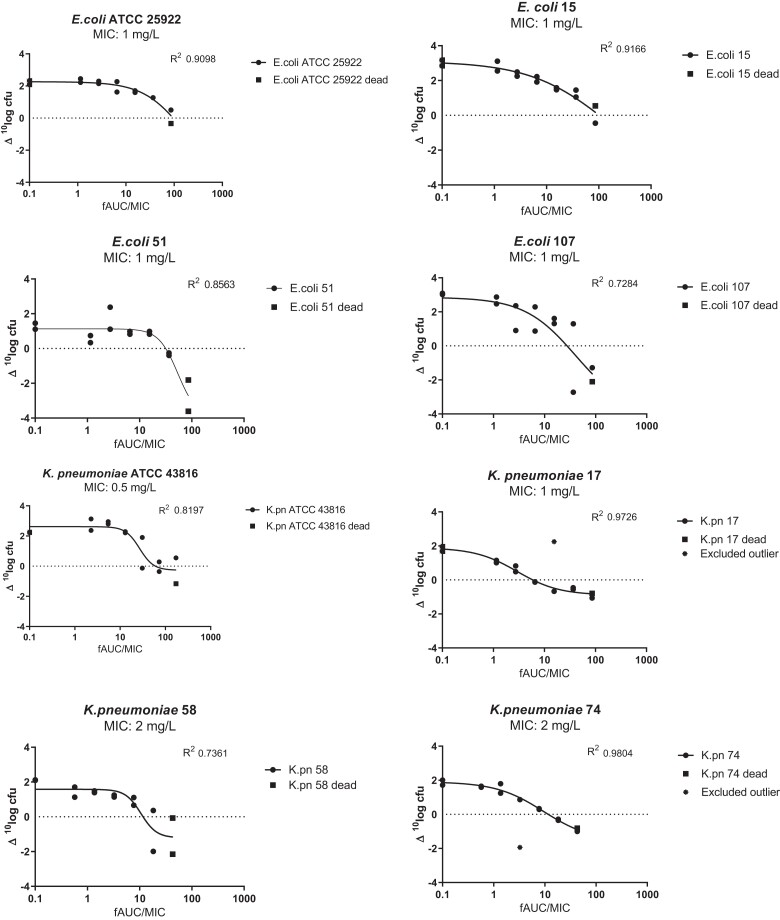
The dose–response curves for four E. coli and four K. pneumoniae strains with q6h dosing regimens are shown in Figure 3. At start of treatment, the average bacterial load was 1.5 × 107 (range: 4.0 × 106–3.3 × 107) cfu/thigh. A static effect was achieved in 5/8 isolates (static effect was observed in 1/2 mice for E. coli ATCC 25922 and 15, and K. pneumoniae ATCC 43816) whereas a 1 log kill effect was observed in 3/8 isolates (E. coli 51 and 107 and K. pneumoniae 58), and 2 log kill effect in 1/8 isolates (E. coli 51). The exposure–response relationships of the eight strains were well described by the Emax model (R2 > 0.7) and static, 1 log and 2 log cfu reduction effects were calculated. The fAUC/MIC correlating with a static, 1 log and 2 log kill effect for the individual strains is presented in Table 2. The fAUC/MIC correlating with a static effect in the thigh infection model was not statistically different for the E. coli compared with the K. pneumoniae strains (the two-tailed P value equals 0.1294; unpaired t-test). In mice that received the highest dosing regimen, 32 mg/kg q6h (TDD 128 mg/kg), the last (fourth) administration of polymyxin B was not tolerated in 2/4 mice.
Figure 3.
Relationship between the bacterial load in the thighs, expressed as Δlog10 cfu, and the fAUC/MIC after 24 h of polymyxin B treatment in a 6 hourly dosing interval in the neutropenic mouse thigh infection model for four E. coli and four K. pneumoniae strains. Each data point represents a single thigh; the solid line represents the fit of the inhibitory sigmoid dose–effect model; the dotted line indicates the point at which there is no change from the burden at the start of treatment.
Table 2.
Target values of fAUC/MIC for stasis, 1 and 2 log decline in cfu in the neutropenic E. coli and K. pneumoniae thigh infection model for polymyxin B
| Target values of fAUC/MIC | ||||||
|---|---|---|---|---|---|---|
| Bacterial isolate | MIC (mg/L) or data measure |
Bacterial burden at start of therapy (log10 cfu/thigh)a | Increase in bacterial burden at 24 h in untreated control animals (Δlog10 cfu/thigh)a | static effect | 1 log kill | 2 log kill |
| E coli ATCC 25922 | 1 | 7.6 | 2.2 | 95.3 | NA | NA |
| E. coli 15 | 1 | 7.6 | 3.0 | 102.6 | NA | NA |
| E. coli 51 | 1 | 6.8 | 1.3 | 31.6 | 46.8 | 65.3 |
| E. coli 107 | 1 | 7.1 | 3.1 | 27.5 | 54.5 | NA |
| K. pneumoniae ATCC 43816 | 0.5 | 7.1 | 2.2 | 60.5 | NA | NA |
| K. pneumoniae 17 | 1 | 7.2 | 1.8 | 5.9 | NA | NA |
| K. pneumoniae 58 | 2 | 7.0 | 2.1 | 11.6 | 25.2 | NA |
| K. pneumoniae 74 | 2 | 7.1 | 1.9 | 11.6 | NA | NA |
| E. coli | average | 7.3 | 2.4 | 64.3 | — | — |
| median | 7.3 | 2.6 | 63.5 | — | — | |
| SD | 0.3 | 0.7 | 34.8 | — | — | |
| K. pneumoniae | average | 7.1 | 2.0 | 22.4 | — | — |
| median | 7.1 | 2.0 | 11.6 | — | — | |
| SD | 0.04 | 0.2 | 22.1 | — | — | |
NA, not achieved.
Mean of two mice.
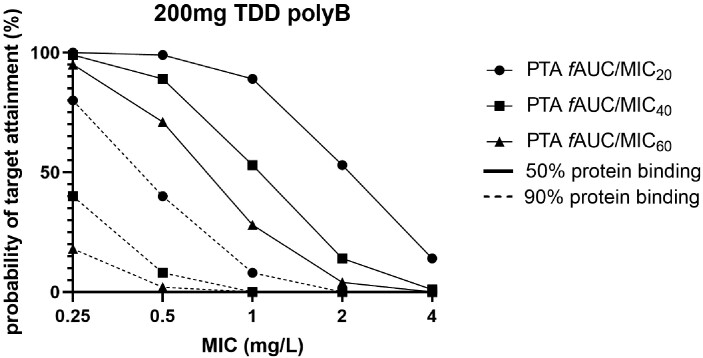
PTA in humans
As reported values for human protein binding in literature showed a wide range, the PTA for the 200 mg clinical dose q24h (Figure 4) was performed taking into account a protein binding of 50% and 90%. In addition, the PK/PD targets for fAUC/MIC of 20, 40 and 60 were analysed in order to include a range for effects from stasis to 2 log kill observed for E. coli and K. pneumoniae. Important differences were detected between the two degrees of protein binding. Only for strains with MIC of 0.25 mg/L and when low protein binding (50%) was taken into account, the PTAs reached in this analysis were >95%.
Figure 4.
PTA for strains with increasing MICs. Both 50% and 90% protein binding, and fAUC/MIC targets of 20, 40 and 60 were taken into account.
Discussion
Overall, we found the PK/PD index of polymyxin B to best correlate with efficacy to be fAUC/MIC. Dose–response studies showed that stasis was achieved at fAUC/MIC values of 27.5–102.6 (median 63) for E. coli isolates and 5.9–60.5 (median 11.6) for K. pneumoniae. A 1 log reduction was only achieved in two E. coli isolates and one K. pneumoniae isolate.
The PK profile in mice showed non-linear PK with decreasing absorption rate and clearance with increasing doses. Non-linearity in clearance and absorption rate was already shown by Landersdorfer et al.,8 albeit described by a different function than in the current work (Emax versus power). Polymyxin B levels in ELF were very low, indicating low penetration in lungs after subcutaneous administration. These findings add to previous studies, in which subcutaneous administration to lung-infected mice did not result in killing of K. pneumoniae and A. baumanni8,24 and where relatively low polymyxin B levels were found in ELF.
Generally, fAUC/MIC is described as the best indicator for efficacy for other polymyxins.7–9 In our study, the fAUC/MIC inhibitory sigmoid dose–effect model showed a slightly better fit than the %fT>MIC and fCmax/MIC for K. pneumoniae 104. In contrast to K. pneumoniae 104, %fT>MIC showed a marginally better fit than fAUC/MIC in the E. coli ATCC 25922 thigh infection model. Other studies where fAUC/MIC was reported as the assumed driver for efficacy also reported only small differences in goodness of fit of the different PK/PD indices.5,8,10
Stasis was found for all four E. coli and K. pneumoniae isolates, with fAUC/MIC targets for E. coli being higher than those for K. pneumoniae [median (range) 63 (27.5–102.6) versus 11.6 (5.9–60.5), respectively]. A 1 log kill was found only for 2/4 E. coli isolates in a range of 46.8–54.5 (median 50.6) and 1/4 K. pneumoniae isolate (25.2 fAUC/MIC). No 2 log kill effect was found for any isolate. When comparing fAUC/MIC targets, Landersdorfer et al.8 reported targets between 1.2 and 13.5 for a static effect and 3.7 and 28.0 for 1 log kill for three K. pneumoniae isolates. Our fAUC/MIC targets in four K. pneumoniae isolates fell in the range of 5.9–60.5 (median 11.6) for a static effect and was 25.2 for a 1 log reduction effect (one isolate). The differences in targets can partially be explained by differences found in unbound fraction. There is considerable inter-strain variation between target values found for the fAUC/MIC, especially for K. pneumoniae. This variation cannot be explained by differences between characteristics in bacterial growth. Overall, the fAUC/MIC targets for E. coli seem to be higher compared with K. pneumoniae. While both species belong to the Enterobacterales, it is known from urinary tract infections that there is a clinical difference between the two species, with E. coli causing more severe infections compared with K. pneumoniae.25 The difference in virulence might also explain the difference in values found in current study.
Protein binding of polymyxin B is known to be high. However, a difference was found between the current study (unbound fraction 20%) compared with previous studies (8.6% for polymyxin B,8 8.4% for colistin5), which affects the PTA. In the present study, ultrafiltration was used for determining the unbound fraction, whilst ultracentrifugation was used in the other studies. With the ultrafiltration method, correction for the NSB to the ultrafiltration membranes was needed. In addition, differences in mouse plasma used for determination of polymyxin B protein binding may (partly) explain the discrepancy. Plasma of infected mice treated with polymyxin B was used, which likely contains polymyxin B metabolites as well, while spiked pooled plasma was used in the other studies. But instead of being methodology-related, differences in unbound fractions may rather reflect the difficulty of handling polymyxin B.
In clinical studies, the protein binding of polymyxin B is much higher than reported in mice, with protein binding in plasma up to 99% for polymyxin B.22,26,27 This applies to colistin as well.5,28 Therefore, the PK/PD targets based on the unbound fraction are necessary for translating PK/PD data of polymyxin B to the clinic.
To determine whether these targets are achieved in humans, the PTAs for the 200 mg q24h dose were calculated. It is clear that the degree of protein binding is essential herein and more clarity on the protein binding in human plasma is needed before an optimal dosing regimen can be recommended, since for a highly protein-bound drug a small error on the fu would yield important PTA differences. Data on the WT distributions for polymyxin B are also lacking on the EUCAST website but MIC90 values in previous studies were ≤0.5–1 mg/L,29 which is higher than the lowest MIC 0.25 mg/L with PTA >95%. Furthermore, the 1 log kill effect, which is associated with clinical response in serious infections, was achieved in only 38% of isolates. EUCAST has currently no clinical breakpoints for polymyxin B; however, the United States Committee on Antimicrobial Susceptibility Testing (USCAST) has a susceptibility/resistance breakpoint for Enterobacterales of ≤2/≥4 mg/L. Given the data currently available, the targets might not be reached in all patients. This might contribute to the fact that polymyxin B has been associated with therapeutic failure previously.30,31
In conclusion, this study confirms that fAUC/MIC describes the exposure–response relationship for polymyxin B. The 1 log kill effect was achieved in the minority of the isolates whereas polymyxin B PK/PD targets cannot be attained for the majority of clinical isolates with the standard dosing regimen, indicating that polymyxin B monotherapy may be not effective against serious infections.
Supplementary Material
Acknowledgements
We thank Marian ten Kate for technical assistance.
Contributor Information
Aart van der Meijden, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Vincent Aranzana-Climent, INSERM U1070, CHU de Poitiers et Université de Poitiers, Poitiers, France.
Heleen van der Spek, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Rotterdam, The Netherlands.
Brenda C M de Winter, Department of Hospital Pharmacy, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; CATOR, Center for Antimicrobial Treatment Optimization Rotterdam, Rotterdam, The Netherlands; Rotterdam Clinical Pharmacometrics Group, Rotterdam, The Netherlands.
William Couet, INSERM U1070, CHU de Poitiers et Université de Poitiers, Poitiers, France.
Joseph Meletiadis, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; Clinical Microbiology Laboratory, Attikon University General Hospital, Medical School, National and Kapodistrian University of Athens, Athens, Greece.
Anouk E Muller, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; CATOR, Center for Antimicrobial Treatment Optimization Rotterdam, Rotterdam, The Netherlands; Department of Medical Microbiology, Haaglanden MC, The Hague, The Netherlands.
Sanne van den Berg, Department of Medical Microbiology and Infectious Diseases, Erasmus MC, University Medical Center, Rotterdam, The Netherlands; CATOR, Center for Antimicrobial Treatment Optimization Rotterdam, Rotterdam, The Netherlands.
Funding
This work was supported by the Joint Programming Initiative on Antimicrobial Resistance (JPIAMR) and ZonMw under research grant ‘CO-ACTION’.
Transparency declarations
J. Meletiadis has received research funding from Astellas, Gilead, MSD and Pfizer. All other authors: nothing to declare.
Supplementary data
Table S1 and Figures S1–S3 are available as Supplementary data at JAC Online.
References
- 1. CDC . Antibiotic Resistance Threats in the United States, 2019. 2019. https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. [Google Scholar]
- 2. Koch-Weser J, Sidel VW, Federman EBet al. . Adverse effects of sodium colistimethate. Manifestations and specific reaction rates during 317 courses of therapy. Ann Intern Med 1970; 72: 857–68. 10.7326/0003-4819-72-6-857 [DOI] [PubMed] [Google Scholar]
- 3. Avedissian SN, Liu J, Rhodes NJet al. . A review of the clinical pharmacokinetics of polymyxin B. Antibiotics (Basel) 2019; 8: 31. 10.3390/antibiotics8010031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zavascki AP, Goldani LZ, Li Jet al. . Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J Antimicrob Chemother 2007; 60: 1206–15. 10.1093/jac/dkm357 [DOI] [PubMed] [Google Scholar]
- 5. Cheah SE, Wang J, Nguyen VTet al. . New pharmacokinetic/pharmacodynamic studies of systemically administered colistin against Pseudomonas aeruginosa and Acinetobacter baumannii in mouse thigh and lung infection models: smaller response in lung infection. J Antimicrob Chemother 2015; 70: 3291–7. 10.1093/jac/dkv267 [DOI] [PubMed] [Google Scholar]
- 6. Dudhani RV, Turnidge JD, Coulthard Ket al. . Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother 2010; 54: 1117–24. 10.1128/AAC.01114-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lin YW, Zhou QT, Cheah SEet al. . Pharmacokinetics/pharmacodynamics of pulmonary delivery of colistin against Pseudomonas aeruginosa in a mouse lung infection model. Antimicrob Agents Chemother 2017; 61: e02025-16. 10.1128/AAC.02025-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Landersdorfer CB, Wang J, Wirth Vet al. . Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J Antimicrob Chemother 2018; 73: 462–8. 10.1093/jac/dkx409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagihara M, Housman ST, Nicolau DPet al. . In vitro pharmacodynamics of polymyxin B and tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 2014; 58: 874–9. 10.1128/AAC.01624-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tam VH, Schilling AN, Vo Get al. . Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 2005; 49: 3624–30. 10.1128/AAC.49.9.3624-3630.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bergen PJ, Bulitta JB, Forrest Aet al. . Pharmacokinetic/pharmacodynamic investigation of colistin against Pseudomonas aeruginosa using an in vitro model. Antimicrob Agents Chemother 2010; 54: 3783–9. 10.1128/AAC.00903-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. CLSI . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically—Tenth Edition: M07-A10. 2015. [Google Scholar]
- 13. CLSI . Performance Standards for Antimicrobial Susceptibility Testing—Twenty-Sixth Edition: M100. 2016. [Google Scholar]
- 14. EU . Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. 2010. https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF.
- 15. Melchers MJ, Teague J, Warn Pet al. . Pharmacokinetics and pharmacodynamics of murepavadin in neutropenic mouse models. Antimicrob Agents Chemother 2019; 63: e01699-18. 10.1128/AAC.01699-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang C, Williams NS. A mass balance approach for calculation of recovery and binding enables the use of ultrafiltration as a rapid method for measurement of plasma protein binding for even highly lipophilic compounds. J Pharm Biomed Anal 2013; 75: 112–7. 10.1016/j.jpba.2012.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Louie A, Fregeau C, Liu Wet al. . Pharmacodynamics of levofloxacin in a murine pneumonia model of Pseudomonas aeruginosa infection: determination of epithelial lining fluid targets. Antimicrob Agents Chemother 2009; 53: 3325–30. 10.1128/AAC.00006-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gobin P, Lemaitre F, Marchand Set al. . Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob Agents Chemother 2010; 54: 1941–8. 10.1128/AAC.01367-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kubin CJ, Nelson BC, Miglis Cet al. . Population pharmacokinetics of intravenous polymyxin B from clinical samples. Antimicrob Agents Chemother 2018; 62: e01493-17. 10.1128/AAC.01493-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Surovoy YA, Burkin MA, Galvidis IAet al. . Comparative polymyxin B pharmacokinetics in patients receiving extracorporeal membrane oxygenation. J Antimicrob Chemother 2022; 77: 1379–84. 10.1093/jac/dkac021 [DOI] [PubMed] [Google Scholar]
- 21. Kwa AL, Lim TP, Low JGet al. . Pharmacokinetics of polymyxin B1 in patients with multidrug-resistant Gram-negative bacterial infections. Diagn Microbiol Infect Dis 2008; 60: 163–7. 10.1016/j.diagmicrobio.2007.08.008 [DOI] [PubMed] [Google Scholar]
- 22. Abodakpi H, Gohlke J, Chang KTet al. . Analytical and functional determination of polymyxin B protein binding in serum. Antimicrob Agents Chemother 2015; 59: 7121–3. 10.1128/AAC.01815-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandri AM, Landersdorfer CB, Jacob Jet al. . Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57: 524–31. 10.1093/cid/cit334 [DOI] [PubMed] [Google Scholar]
- 24. Bowers DR, Cao H, Zhou Jet al. . Assessment of minocycline and polymyxin B combination against Acinetobacter baumannii. Antimicrob Agents Chemother 2015; 59: 2720–5. 10.1128/AAC.04110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hyun M, Lee JY, Kim HAet al. . Comparison of Escherichia coli and Klebsiella pneumoniae acute pyelonephritis in Korean patients. Infect Chemother 2019; 51: 130–41. 10.3947/ic.2019.51.2.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li YQ, Chen KF, Ding JJet al. . External evaluation of published population pharmacokinetic models of polymyxin B. Eur J Clin Pharmacol 2021; 77: 1909–17. 10.1007/s00228-021-03193-y [DOI] [PubMed] [Google Scholar]
- 27. Zavascki AP, Goldani LZ, Cao Get al. . Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 2008; 47: 1298–304. 10.1086/592577 [DOI] [PubMed] [Google Scholar]
- 28. Nation RL, Garonzik SM, Li Jet al. . Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62: 552–8. 10.1093/cid/civ964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of gram-negative pathogens: results from the SENTRY antimicrobial surveillance program (2006–09). J Antimicrob Chemother 2011; 66: 2070–4. 10.1093/jac/dkr239 [DOI] [PubMed] [Google Scholar]
- 30. Fang J, Li H, Zhang Met al. . Efficacy of ceftazidime-avibactam versus polymyxin B and risk factors affecting clinical outcomes in patients with carbapenem-resistant Klebsiella pneumoniae infections a retrospective study. Front Pharmacol 2021; 12: 780940. 10.3389/fphar.2021.780940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dubrovskaya Y, Chen TY, Scipione MRet al. . Risk factors for treatment failure of polymyxin B monotherapy for carbapenem-resistant Klebsiella pneumoniae infections. Antimicrob Agents Chemother 2013; 57: 5394–7. 10.1128/AAC.00510-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.