Abstract
Introduction
FIGO 2018 IIIC remains controversial for the heterogeneity of its prognoses. To ensure a better management of cervical cancer patients in Stage IIIC, a revision of the FIGO IIIC version classification is required according to local tumor size.
Material and methods
We retrospectively enrolled cervical cancer patients of FIGO 2018 Stages I–IIIC who had undergone radical surgery or chemoradiotherapy. Based on the tumor factors from the Tumor Node Metastasis staging system, IIIC cases were divided into IIIC‐T1, IIIC‐T2a, IIIC‐T2b, and IIIC‐(T3a+T3b). Oncologcial outcomes of all stages were compared.
Results
A total of 63 926 cervical cancer cases were identified, among which 9452 fulfilled the inclusion criteria and were included in this study. Kaplan–Meier pairwise analysis showed that: the oncology outcomes of I and IIA were significantly better than of IIB, IIIA+IIIB, and IIIC; the oncology outcome of IIIC‐(T1‐T2b) was significantly better than of IIIA+IIIB and IIIC‐(T3a+T3b); no significant difference was noted between IIB and IIIC‐(T1‐T2b), or IIIC‐(T3a+T3b) and IIIA+IIIB. Multivariate analysis indicated that, compared with IIIC‐T1, Stages T2a, T2b, IIIA+IIIB and IIIC‐(T3a+T3b) were associated with a higher risk of death and recurrence/death. There was no significant difference in the risk of death or recurrence/death between patients with IIIC‐(T1‐T2b) and IIB. Also, compared with IIB, IIIC‐(T3a+T3b) was associated with a higher risk of death and recurrence/death. No significant differences in the risk of death and recurrence/death were noted between IIIC‐(T3a+T3b) and IIIA+IIIB.
Conclusions
In terms of oncology outcomes of the study, FIGO 2018 Stage IIIC of cervical cancer is unreasonable. Stages IIIC‐T1, T2a, and T2b may be integrated as IIC, and it might be unnecessary for T3a/T3b cases to be subdivided by lymph node status.
Keywords: cervical cancer, Stage IIIC, International Federation of Gynecology and Obstetrics staging (FIGO staging), oncology outcome, T‐staging
FIGO 2018 Stage IIIC of cervical cancer is unreasonable. Stages IIIC‐T1, T2a, and T2b may be integrated as IIC.

Abbreviations
- DFS
disease‐free survival
- FIGO
International Federation of Gynecology & Obstetrics
- OS
overall survival
Key message.
FIGO 2018 Stage IIIC is unreasonable from the perspective of oncological outcomes ‐IIIC‐T1, T2a, and T2b should be integrated as IIC, and it is unnecessary for T3a/T3b cases to be subdivided by lymph node status.
1. INTRODUCTION
For cervical cancer patients, lymph node (LN) status is widely accepted as a pivotal predictor of survival. 1 , 2 With improvements in global medical technology and the recognition of LN metastasis in cervical cancer prognosis, the newly updated 2018 FIGO (the International Federation of Gynecology & Obstetrics) staging system saw the first‐ever inclusion of LN status. 3 According to the revised staging guidelines, patients with positive pelvic or para‐aortic LN identified via imaging or pathology are designated as Stage IIIC. However, the rationality of this Stage IIIC classification remains controversial. 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 The major dissenting view postulates that it ignores the heterogeneity of local tumor factors in cervical cancer patients with LN metastasis; hence, it cannot accurately predict the oncology outcomes of such patients. 8 , 9 , 10 , 11 , 12 , 13
Our team's previous study based on the Clinical Diagnosis and Treatment for Cervical Cancer in China (Four‐C) database compared the 5‐year overall survival (OS) and disease‐free survival (DFS) rates of patients with Stage III cervical cancer and showed that the survival outcomes of Stage IIIC are better than those of Stage IIIB, comparable to those of Stage IIIA, and associated with local tumor size. 11 Other studies revealed that cervical cancer cases with smaller tumor diameters have significantly better prognosis than IIIA and IIIB cases, even those with LN metastasis. 12 , 13 , 14
To ensure the better management of cervical cancer patients in Stage IIIC, we believe that revision of the FIGO IIIC version classification is required according to local tumor size. Therefore, here we divided Stage IIIC patients according to tumor factors from the Tumor Node Metastasis (TNM) staging system and compared the 5‐year OS and DFS rates of all eight stages: I, IIA, IIB, IIIA+IIIB, IIIC‐T1, IIIC‐T2a, IIIC‐T2b, and IIIC‐(T3a+T3b), aiming to demonstrate specific improvement.
2. MATERIAL AND METHODS
2.1. Data source
Cases in the present study were selected from the Four‐C database (International Clinical Trials Registry Platform Search Port, CHiCTR1800017778), a cervical cancer‐specialized disease database (n = 63 926) that includes consecutive patients with cervical cancer from 47 hospitals in mainland China treated between January 2004 and December 2018. The specific collection methods refer to the previous study. 15 , 16 This study was approved by the Institutional Review Board of Nanfang Hospital Affiliated with Southern Medical University (NFEC‐2017‐135). All procedures in this study involving human participants were performed in accordance with the Declaration of Helsinki (revised in 2013). As the study used previously collected clinical data, the need for informed consent was waived.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) age 18 years or older; (b) cervical cancer diagnosed by cervical biopsy; (c) squamous cell carcinoma, adenocarcinoma, or adenosquamous carcinoma based on histology; (d) therapeutic strategies that adhered to the National Comprehensive Cancer Network (NCCN) Guidelines version 1.2022, including (i) abdominal surgery: no preoperative treatments; Q‐M type B/C radical hysterectomy + pelvic lymphadenectomy ± para‐aortic lymphadenectomy and adjuvant postoperative treatment performed in accordance with Sedlis standards; and (ii) radical chemoradiotherapy: either radiotherapy: external irradiation + afterloading/brachytherapy (radiotherapy dose higher than 45 Gy) or chemotherapy: including paclitaxel + carboplatin, paclitaxel + other platinum, platinum + 5‐fluorouracil, platinum + other, etc., which were used according to guidelines and drug instructions; and (e) data available on survival outcomes.
Patients were excluded if they had the following special conditions: pregnancy; cervical stump cancer; additional malignant tumors; or a history of pelvic surgery.
2.3. Stage IIIC cases restaged according to local tumor size
Tumor factors of the TNM staging system of the latest American Joint Committee on Cancer 17 were used to classify the IIIC cases into four substages: IIIC‐T1, IIIC‐T2a, IIIC‐T2b, and IIIC‐(T3a+T3b).
2.4. Observational indicators
We selected 5‐year OS and DFS rates as the study end points. OS was defined as the time from diagnosis to death of any cause or the date of last follow up. DFS was defined as the time from diagnosis to death, recurrence, or date of last follow up.
2.5. Statistical analysis
Continuous variables are expressed as mean ± standard deviation (SD), while categorical variables are presented as number and proportion (%). Student's t test was used to compare continuous variables and Fisher's exact test or the chi‐squared test was used to compare categorical variables. The Kaplan–Meier curves were used to estimate survival outcomes, and pairwise log‐rank tests were used to used to compare these among the eight groups. With patient demographics and tumor histological characteristics entered in the Cox proportional hazards models, calculations of hazard ratios (HR) and 95% confidence intervals (CI) were used to evaluate the survival associations between stages. All hypotheses were two‐tailed, and values of p < 0.05 were considered statistically significant. All statistical analyses were conducted using IBM SPSS statistics version 25.0 (SPSS).
2.6. Ethics statement
The establishment of the Clinical Diagnosis and Treatment for Cervical Cancer in China (Four C) Database was approved by the Ethics Committee of the Southern Hospital of Southern Medical University (no. FEC‐2017‐ 135) on August 21, 2017. The clinical research registration number is CHiCTR1800017778 (International Clinical Trials Registry Platform Search Port, http://apps.who.int/trialsearch/), registered on 2018‐08‐14.
3. RESULTS
3.1. Data filtering process
Among the 63 926 cases collected in the Four‐C database, 9452 cases were included in the present study. Based on the T principles of the TNM staging system, IIIC cases (n = 2660) were divided into four substages: IIIC‐T1, IIIC‐T2a, IIIC‐T2b, and IIIC‐(T3a+T3b). The median follow‐up period was 39 months: 48 months for Stage I (n = 3189), 48 months for Stage IIA (n = 1093), 32 months for Stage IIB (n = 1232), 31 months for Stage IIIA+IIIB (n = 1278), 43 months for Stage IIIC‐T1 (n = 865), 38 months for Stage IIIC‐T2a (n = 587), 28 months for Stage IIIC‐T2b (n = 436), and 27 months for Stage IIIC‐(T3a+T3b) (n = 772). A selection schematic is presented in Figure 1.
FIGURE 1.
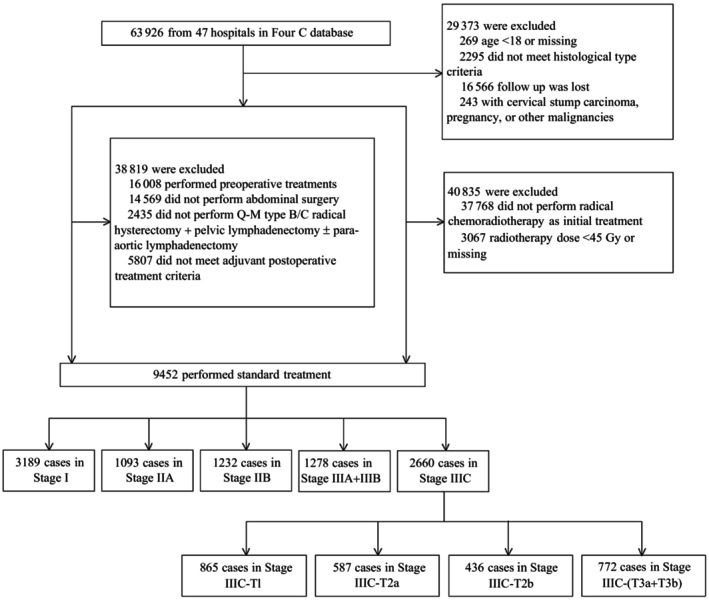
Flow diagram of patient recruitment and exclusion processes.
3.2. Patients' clinical and pathological characteristics and survival analysis
The patients' clinicopathological characteristics across the eight stages are shown in Table 1. There were statistically significant differences in age and histological type among the stages.
TABLE 1.
Clinicopathological characteristics of patients by stage.
| Stage | Age (mean ± SD) | Histological type, n (%) | ||
|---|---|---|---|---|
| Squamous cell carcinoma | Adenocarcinoma | Adenosquamous | ||
| I (n = 3189) | 46.70 ± 9.410 | 2803 (87.9) | 328 (10.3) | 58 (1.8) |
| IIA (n = 1093) | 52.91 ± 9.384 | 1047 (95.8) | 31 (2.8) | 15 (1.4) |
| IIB (n = 1232) | 56.21 ± 10.326 | 1194 (96.9) | 27 (2.2) | 11 (0.9) |
| IIIA+IIIB (n = 1278) | 55.57 ± 10.438 | 1237 (96.8) | 31 (2.4) | 10 (0.8) |
| IIIC‐T1 (n = 865) | 46.77 ± 9.018 | 751 (86.8) | 80 (9.2) | 34 (3.9) |
| IIIC‐T2a (n = 587) | 49.93 ± 9.557 | 533 (90.8) | 38 (6.5) | 16 (2.7) |
| IIIC‐T2b (n = 436) | 54.61 ± 10.946 | 422 (96.8) | 11 (2.5) | 3 (0.7) |
| IIIC‐(T3a+T3b) (n = 772) | 54.51 ± 10.935 | 747 (96.8) | 20 (2.6) | 5 (0.6) |
| p value | <0.001 | <0.001 | ||
Abbreviation: SD, standard deviation.
In the Kaplan–Meier analysis, the pairwise log‐rank tests across the eight stages showed that: (a) Stage I and IIA disease exhibited significantly better OS and DFS rates than Stages IIB and IIIA+IIIB and the IIIC substages; (b) Stage IIIC‐(T1‐T2b) disease exhibited significantly better OS and DFS rates than Stage IIIA+IIIB and IIIC‐(T3a+T3b) (p < 0.005) and similar OS and DFS rates to Stage IIB; (c) Stage IIIC‐(T3a+T3b) disease exhibited significantly worse OS and DFS rates than Stage IIIC‐(T1‐T2b) and similar OS and DFS rates to Stage IIIA+IIIB; (d) Stage IIC‐T1 disease exhibited significantly better OS and DFS rates than Stage IIIC‐T2a. Stage IIC‐T1 disease exhibited a significantly better OS rate than but a similar DFS rate to that of Stage IIIC‐T2b. Stage IIIC‐T2a disease exhibited similar OS and DFS rates to those of Stage IIIC‐T2b. To be specific, 5‐year OS and DFS rates of each stage and the corresponding survival curves are presented in Figure 2. Values of p in the pairwise Kaplan–Meier analysis of 5‐year OS and DFS rates are presented in Tables 2 and 3.
FIGURE 2.
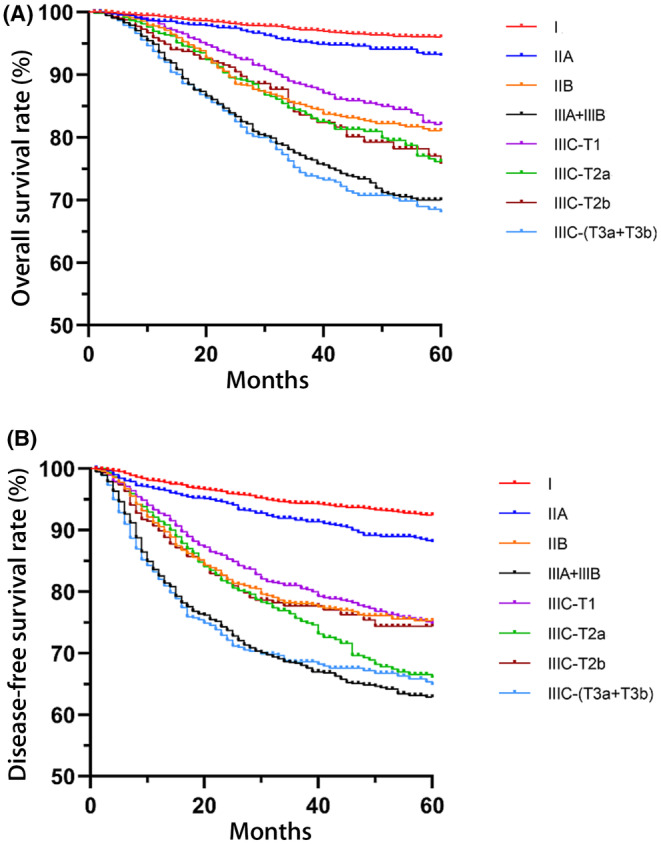
The 5‐year overall survival and disease‐free rates of the eight disease stages. (A) Overall survival rates of the eight disease stages. (B) Disease‐free survival rates of the eight disease stages described with different colored lines described with different colored lines.
TABLE 2.
The 5‐year overall survival (OS) and disease‐free survival (DFS) rates of the eight stages.
| Stage | 5‐year OS (%) | 5‐year DFS (%) |
|---|---|---|
| I | 96.0 | 92.4 |
| IIA | 93.0 | 88.0 |
| IIB | 81.1 | 75.2 |
| IIIA+IIIB | 70.0 | 62.7 |
| IIIC‐T1 | 82.1 | 74.7 |
| IIIC‐T2a | 76.1 | 65.9 |
| IIIC‐T2b | 75.8 | 73.7 |
| IIIC‐(T3a+T3b) | 68.0 | 64.8 |
TABLE 3.
Pairwise log‐rank tests of the 5‐year overall survival rates across the eight stages.
| Stage (p value) | I | IIA | IIB | IIIA+IIIB | IIIC‐T1 | IIIC‐T2a | IIIC‐T2b | IIIC‐(T3a+T3b) |
|---|---|---|---|---|---|---|---|---|
| I | – | – | – | – | – | – | – | – |
| IIA | 0.001 | – | – | – | – | – | – | – |
| IIB | <0.001 | <0.001 | – | – | – | – | – | – |
| IIIA+IIIB | <0.001 | <0.001 | <0.001 | – | – | – | – | – |
| IIIC‐T1 | <0.001 | <0.001 | 0.131 | <0.001 | – | – | – | – |
| IIIC‐T2a | <0.001 | <0.001 | 0.226 | 0.001 | 0.012 | – | – | – |
| IIIC‐T2b | <0.001 | <0.001 | 0.381 | 0.006 | 0.031 | 0.978 | – | – |
| IIIC‐(T3a+T3b) | <0.001 | <0.001 | <0.001 | 0.463 | <0.001 | <0.001 | 0.002 | – |
Cox multivariate survival analyses were performed of the substages IIIC, IIB, and IIIA+IIIB (Table 4). The result confirmed the results of the above Kaplan–Meier pairwise analyses as follows. Compared with IIIC‐T1, T2a, and T2b, Stage IIIA+IIIB was associated with a higher risk of death and recurrence/death. Compared with IIIC‐T1, T2a, and T2b, IIC‐(T3a+T3b) was associated with a higher risk of death and recurrence/death. There was no significant difference in the risk of death and recurrence/death between IIIC‐(T1‐T2b) and IIB. Compared with IIB, IIIC‐(T3a+T3b) was associated with a higher risk of death and recurrence/death. There was no significant difference in the risk of death and recurrence/death between IIIC‐(T3a+T3b) and IIIA+IIIB. (Table 5).
TABLE 4.
Pairwise log‐rank tests of the 5‐year disease‐free survival rates across the eight stages.
| Stage (p value) | I | IIA | IIB | IIIA+IIIB | IIIC‐T1 | IIIC‐T2a | IIIC‐T2b | IIIC‐(T3a+T3b) |
|---|---|---|---|---|---|---|---|---|
| I | – | – | – | – | – | – | – | – |
| IIA | <0.001 | – | – | – | – | – | – | – |
| IIB | <0.001 | <0.001 | – | – | – | – | – | – |
| IIIA+IIIB | <0.001 | <0.001 | <0.001 | – | – | – | – | – |
| IIIC‐T1 | <0.001 | <0.001 | 0.411 | <0.001 | – | – | – | – |
| IIIC‐T2a | <0.001 | <0.001 | 0.031 | 0.004 | 0.004 | – | – | – |
| IIIC‐T2b | <0.001 | <0.001 | 0.498 | 0.001 | 0.170 | 0.403 | – | – |
| IIIC‐(T3a+T3b) | <0.001 | <0.001 | <0.001 | 0.867 | <0.001 | 0.014 | 0.002 | – |
TABLE 5.
Cox multivariate survival analysis results.
| 5‐year OS | 5‐year DFS | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
|
IIIC‐T1 (HR, 1) |
IIB | 1.154 | 0.899–1.483 | 0.262 | 1.135 | 0.928–1.390 | 0.218 |
| IIIA+IIIB | 1.967 | 1.564–2.474 | <0.001 | 1.853 | 1.537–2.234 | <0.001 | |
| IIIC‐(T3a+T3b) | 2.135 | 1.663–2.742 | <0.001 | 1.835 | 1.491–2.257 | <0.001 | |
|
IIIC‐T2a (HR, 1) |
IIB | 0.841 | 0.655–1.080 | 0.174 | 0.826 | 0.674–1.011 | 0.064 |
| IIIA+IIIB | 1.431 | 1.138–1.800 | 0.002 | 1.346 | 1.116–1.625 | 0.002 | |
| IIIC‐(T3a+T3b) | 1.551 | 1.208–1.993 | 0.001 | 1.334 | 1.083–1.643 | 0.007 | |
|
IIIC‐T2b (HR, 1) |
IIB | 0.867 | 0.643–1.170 | 0.352 | 0.918 | 0.719–1.174 | 0.497 |
| IIIA+IIIB | 1.476 | 1.112–1.959 | 0.007 | 1.498 | 1.186–1.892 | 0.001 | |
| IIIC‐(T3a+T3b) | 1.602 | 1.186–2.164 | 0.002 | 1.480 | 1.151–1.901 | 0.002 | |
|
IIIC‐(T3a+T3b) (HR, 1) |
IIB a | 0.541 | 0.435–0.673 | <0.001 | 0.621 | 0.517–0.746 | <0.001 |
| IIIA+IIIB | 0.922 | 0.759–1.121 | 0.415 | 1.012 | 0.856–1.197 | 0.889 | |
Abbreviations: CI, confidence interval; DFS, disease‐free survival; HR, hazard ratio; OR, odds ratio; OS, overall survival.
To unify the expressions, we recalculated the data using Stage IIB as reference (HR, 1) (5‐year OS: HR, 1.843; 95% CI, 1.482–2.292; p < 0.001; 5‐year DFS: HR, 1.619; 95% CI, 1.347–1.946, p < 0.001).
4. DISCUSSION
Considering the adverse effect of regional LN metastasis on the survival of patients with cervical cancer, the FIGO 2018 staging system incorporated imaging and pathological factors of LN into Stage IIIC. 3 However, one idea worth considering is that LN status alone is insufficient for determining the clinical characteristics of Stage IIIC patients and may not be appropriate in the clinical setting. Some recent studies explored the rationality of Stage IIIC from the perspective of oncology prognosis and demonstrated that the survival rate of women with positive LN (Stage IIIC) disease was superior to that of those with Stage IIIA and IIIB tumors. 5 , 8 , 18 , 19 , 20 , 21
In several studies, the effect of local tumor size and extent on Stage IIIC outcomes was further explored according to the T classification of the TNM staging system. Consistent with our results, these studies indicated that primary cervical mass was an independent survival risk factor for patients with Stage IIIC disease.
Shin et al 20 examined the new FIGO staging system's prognostic ability using data from the Korean National Cancer Registry (2010–2015). A survival analysis revealed that patients with Stage IIIC1/IIIC2 disease had better survival than those with Stage IIIA/IIIB disease (p < 0.001) and demonstrated significant differences in the survival curves among T1, T2, and T3 (p < 0.001). However, only 502 IIIC cases were included in the study, no specific inclusion criteria were presented, and only the OS rate was studied. Matsuo et al 8 examined data from the SEER database (1988–2014). Among a Stage III cohort (n = 11 733), Stage IIIC1 was independently associated with improved cause‐specific survival compared with Stage IIIB disease (adjusted HR 0.79; 95% CI 0.74–0.85; p < 0.001). Survival of Stage IIIC1 disease significantly differed based on T stage, (5‐year rates: 74.8% for T1; 58.7% for T2; 39.3% for T3). Information about para‐aortic LN metastasis was not available in the study; hence, there was only a single IIIC1 cohort. Bai et al 21 performed a survival analysis of 242 patients with early cervical cancer and LN‐positive disease to determine its prognostic factors. That study reported that T stage was an independent prognostic factor for DFS and OS and that patients with Stage T2 disease had a poorer prognosis than those with Stage T1 disease (T1 vs. T2, 5‐year OS: 86% vs. 58%, p = 0.001; 5‐year DFS: 80% vs. 20%, p = 0.007). However, it was a single‐center retrospective study that included only early‐stage cervical cancer patients (T1/T2) who received adjuvant radiotherapy.
Compared with previous studies, the current study was more comprehensive, larger, and used strict inclusion criteria. It used data from the Four‐C database of 9452 patients treated at 47 hospitals based on the latest NCCN Guideline, including 2260 cases of FIGO 2018 Stage IIIC1 or IIIC2 disease. We particularly excluded cases with preoperative treatment or underdosage of radiotherapy according to the NCCN guidelines. Laparoscopy cases were also excluded to rule out deviation, because Bai et al 21 reported that in women with early cervical cancer, the DFS and OS rates after minimally invasive radical hysterectomy were lower than those after open radical hysterectomy.
The univariate and multivariate analyses performed in our study revealed that the oncological outcomes of women with Stages IIIC‐(T1‐T2b) disease were better than those with Stages IIIA+IIIB and IIIC‐(T3a+T3b) but not significantly different from those with Stage IIB. The oncological outcomes of Stage IIIC‐(T3a+T3b) were significantly worse than those of Stage IIB but not significantly different from those of Stage IIIA+IIIB. To our knowledge, our study is the first to propose a specific improvement version for Stage IIIC of the 2018 FIGO staging system from the perspective of oncological outcomes. To be specific, Stage IIIC‐T1, T2a, and T2b should be integrated as Stage IIC, and it is unnecessary for Stage IIIC to be distinguished in terms of LN status in T3a/T3b cases because the major therapies and prognoses in Stages IIIC‐(T3a+T3b) and IIIA+IIIB are approximately the same.
Furthermore, our new findings may inform treatment options for patients with Stage IIIC disease, which have long been controversial in clinical practice. In the NCCN version 1.2022 guidelines, radical chemoradiotherapy was uniformly recommended in Stage IIIC disease. However, Ramirez et al 22 insisted that the 5‐year survival of IIIC‐T1 patients after radical hysterectomy with concurrent chemoradiotherapy was up to 90%, comparable to the findings of the studies of Huang et al 23 and Kim et al. 24 Hence, for patients with LN metastasis and a low local T stage, adjuvant treatment after radical surgery is reasonable. The results of this study also suggest a heterogeneity of Stage IIIC prognoses. It seems unreasonable to administer the same treatment to patients with significantly different prognoses. Despite being a country with a high cervical cancer rate, China lacks chemoradiation resources. Given the findings of the above studies, patients with a low local tumor stage (T stage) and positive LNs may still have the opportunity to receive surgery as the initial treatment. The above‐mentioned findings support the incorporation of cases treated with abdominal surgery followed by adjuvant postoperative treatment performed in accordance with Sedlis standards in the present study. However, we still cannot rule out the possibility that the choice of the treatment modality may affect the result. Further randomized controlled studies are needed to instruct feasible treatment in patients with Stage IIIC disease.
Our study has the following limitations. First, it was a retrospective study involving inherent biases that could not be avoided. Second, there might be differences in preoperative imaging findings and medical record report writing standards at different hospitals, resulting in a lack of clinical data. Moreover, this study did not distinguish the positive LN identified via imaging (r) or pathology (p), while the pathological diagnosis of LN metastasis usually has higher accuracy than imaging diagnoses. 25 , 26 A well‐powered randomized controlled trial is needed to verify the results of this study.
As the present study shows, node‐positive patients were heterogeneous. Except for LN states and tumor size, parametrial involvement, tumor–stroma ratio (TSR) and the tumor involvement have been shown to be independent predictors of survival. 27 , 28 , 29 , 30 , 31 Chang et al 27 and Park et al 28 reported that parametrial involvement was an independent risk factor for survival in patients with lymph node metastasis after radical hysterectomy for early‐stage cervical cancer. Zong et al's 29 study included a series of patients with pure squamous cell carcinoma of the cervix with lymph node metastases, and found that the TSR was an independent predictor of these patients' survival. Lindegaard et al 30 , 31 validated the T‐score (a ranked ordinal scale to assign points for the degree of tumor involvement of eight locations: cervix, left parametrium, right parametrium, vagina, corpus uteri, bladder, ureter, and rectum), which outperformed the FIGO 2018 staging system for both survival and local control. Given these factors, further clinical trials are warranted to develop a more nuanced staging schema incorporating local tumor factors based on the current study.
5. CONCLUSION
We suggest that FIGO 2018 Stage IIIC of cervical cancer is inadequate as it unites heterogeneous tumors with different prognosis based only on the presence of LN metastasis. An introduction of tumor size as follows will be valuable for cervical cancer cases with LN metastasis and tumor size equal to Stage IIIC‐T1, T2a, and T2b could be integrated as Stage IIC. Furthermore, it might be unnecessary for cases with T3a/T3b to be subdivided by LN status. Incorporation of these suggestion needs to be tested in prospective studies for verification.
AUTHOR CONTRIBUTIONS
HD contributed to conceptualization, methodology, investigation, formal analysis, ad writing—original draft. HL contributed to data curation, investigation, and writing—original draft. SK contributed to visualization, and investigation. HZ, BC, and LW contributed to resources and supervision. PLi, YW, and WW contributed to software and validation. PLiu contributed to visualization and writing—review and editing. CC contributed to conceptualization, funding acquisition, resources, supervision, and writing—review and editing.
FUNDING INFORMATION
This study received funding from the National Science and Technology Support Program of China (grant no. 2014BAI05B03) and the Science and Technology Plan of Guangzhou (grant no. 201508020264).
CONFLICT OF INTEREST STATEMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
ACKNOWLEDGMENTS
We thank Min Hao (The Second Hospital of Shanxi Medical University), Wuliang Wang (The Second Affiliated Hospital of Zhengzhou University), Shan Kang (The Fourth Hospital of Hebei Medical University), Bin Ling (China–Japan Friendship Hospital), Lixin Sun and Hongwei Zhao (Shanxi Cancer Hospital), Jihong Liu and Lizhi Liang (Sun Yat‐sen University Cancer Center), Lihong Lin and Yu Guo (Anyang Tumor Hospital), Li Wang (The Affiliated Tumor Hospital of Zhengzhou University), Weidong Zhao (Anhui Provincial Cancer Hospital), Yan Ni (The Yuncheng Central Hospital of Shanxi Province), Wentong Liang and Donglin Li (Guizhou Provincial People's Hospital), Jianxin Guo (Daping Hospital, The Third Military Medical University), Shaoguang Wang (The Affiliated Yantai Yuhuangding Hospital of Qingdao University), Xuemei Zhan and Mingwei Li (Jiangmen Central Hospital), Weifeng Zhang (Ningbo Women & Children's Hospital), Peiyan Du (The Affiliated Cancer Hospital and Institute of Guangzhou Medical University), Ziyu Fang (Liuzhou Workers' Hospital), Rui Yang (Shenzhen Hospital of Peking University), Long Chen (Qingdao Municipal Hospital), Encheng Dai and Ruilei Liu (Linyi People's Hospital), Yuanli He and Mubiao Liu (Zhujiang Hospital, Southern Medical University), Jilong Yao and Zhihua Liu (Shenzhen Maternity & Child Health Hospital), Xueqin Wang (The Fifth Affiliated Hospital of Southern Medical University), Anwei Lu (Maternal and Child Health Hospital of Guiyang Province), Shuangling Jin (Peace Hospital affiliated to Changzhi Medical College), Yan Xu (Guangzhou Pan Yu Central Hospital), Ben Ma (Guangzhou First People's Hospital), Zhonghai Wang (Shenzhen Nanshan People's Hospital), Lin Zhu (The Second Hospital of Shandong University), Hongxin Pan (The Third Affiliated Hospital of Shenzhen University), Qianyong Zhu (No. 153 Center Hospital of Liberation Army/Hospital No. 988 of the Chinese People's Liberation Army Joint Support Force), Dingyuan Zeng and Zhong Lin (Maternal and Child Health Care Hospital of Liuzhou), Xiaohong Wang (Laiwu People's Hospital/Jinan City People's Hospital), and Bin Zhu (The Affiliated Yiwu Women and Children Hospital of Hangzhou Medical College).
Duan H, Li H, Kang S, et al. Rationality of FIGO 2018 IIIC restaging of cervical cancer according to local tumor size: A cohort study. Acta Obstet Gynecol Scand. 2023;102:1045‐1052. doi: 10.1111/aogs.14612
Huimin Li, Shan Kang contributed equally to this work.
REFERENCES
- 1. Kim SI, Kim TH, Lee M, et al. Lymph node ratio is a strong prognostic factor in patients with early‐stage cervical cancer undergoing minimally invasive radical hysterectomy. Yonsei Med J. 2021;62:231‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo Q, Zhu J, Wu Y, et al. Validation of the prognostic value of various lymph node staging systems for cervical squamous cell carcinoma following radical surgery: a single‐center analysis of 3,732 patients. Ann Transl Med. 2020;8:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bhatla N, Aoki D, Sharma DN, Sankaranarayanan R. Cancer of the cervix uteri: 2021 update. Int J Gynaecol Obstet. 2021;155(Suppl 1):28‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yan DD, Tang Q, Chen JH, Tu YQ, Lv XJ. Prognostic value of the 2018 FIGO staging system for cervical cancer patients with surgical risk factors. Cancer Manag Res. 2019;11:5473‐5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ayhan A, Aslan K, Bulut AN, et al. Is the revised 2018 FIGO staging system for cervical cancer more prognostic than the 2009 FIGO staging system for women previously staged as IB disease? Eur J Obstet Gynecol Reprod Biol. 2019;240:209‐214. [DOI] [PubMed] [Google Scholar]
- 6. Balaya V, Guani B, Magaud L, et al. Validation of the 2018 FIGO classification for cervical cancer: Lymphovascular space invasion should Be considered in IB1 stage. Cancers (Basel). 2020;12:3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang X, Guo C, Liu S, Guo J, Hua K, Qiu J. A novel prognostic nomogram utilizing the 2018 FIGO staging system for cervical cancer: a large multicenter study. Int J Gynaecol Obstet. 2021;155:86‐94. [DOI] [PubMed] [Google Scholar]
- 8. Matsuo K, Machida H, Mandelbaum RS, Konishi I, Mikami M. Validation of the 2018 FIGO cervical cancer staging system. Gynecol Oncol. 2019;152:87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shim SH, Lee SW, Park JY, et al. Risk assessment model for overall survival in patients with locally advanced cervical cancer treated with definitive concurrent chemoradiotherapy. Gynecol Oncol. 2013;128:54‐59. [DOI] [PubMed] [Google Scholar]
- 10. Fleming ND, Frumovitz M, Schmeler KM, et al. Significance of lymph node ratio in defining risk category in node‐positive early stage cervical cancer. Gynecol Oncol. 2015;136:48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Li Z, Duan H, Guo J, et al. Discussion on the rationality of FIGO 2018 stage IIIC for cervical cancer with oncological outcomes: a cohort study. Ann Transl Med. 2022;10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshida K, Jastaniyah N, Sturdza A, et al. Assessment of parametrial response by growth pattern in patients with International Federation of Gynecology and Obstetrics Stage IIB and IIIB cervical cancer: analysis of patients from a prospective, multicenter trial (EMBRACE). Int J Radiat Oncol Biol Phys. 2015;93:788‐796. [DOI] [PubMed] [Google Scholar]
- 13. Grigsby PW, Massad LS, Mutch DG, et al. FIGO 2018 staging criteria for cervical cancer: impact on stage migration and survival. Gynecol Oncol. 2020;157:639‐643. [DOI] [PubMed] [Google Scholar]
- 14. Logsdon MD, Eifel PJ. Figo IIIB squamous cell carcinoma of the cervix: an analysis of prognostic factors emphasizing the balance between external beam and intracavitary radiation therapy. Int J Radiat Oncol Biol Phys. 1999;43:763‐775. [DOI] [PubMed] [Google Scholar]
- 15. Zhang XR, Li ZQ, Sun LX, et al. Cohort profile: Chinese cervical cancer clinical study. Front Oncol. 2021;11:690275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li W, Liu P, Zhao W, et al. Effects of preoperative radiotherapy or chemoradiotherapy on postoperative pathological outcome of cervical cancer‐from the large database of 46,313 cases of cervical cancer in China. Eur J Surg Oncol. 2020;46:148‐154. [DOI] [PubMed] [Google Scholar]
- 17. Torous VF, Oliva E. On the new (version 9) American Joint Committee on cancer tumor, node, metastasis staging for cervical cancer‐a commentary. Cancer Cytopathol. 2021;129:581‐582. [DOI] [PubMed] [Google Scholar]
- 18. Wright JD, Matsuo K, Huang Y, et al. Prognostic performance of the 2018 International Federation of Gynecology and Obstetrics Cervical Cancer Staging Guidelines. Obstet Gynecol. 2019;134:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vengaloor Thomas T, Reddy KK, Gandhi S, et al. Stage migration in cervical cancer using the FIGO 2018 staging system: a retrospective survival analysis using a single‐institution patient cohort. Cureus. 2021;13:e19289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shin W, Ham TY, Park YR, Lim MC, Won YJ. Comparing survival outcomes for cervical cancer based on the 2014 and 2018 International Federation of Gynecology and Obstetrics staging systems. Sci Rep. 2021;11:6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bai Y, Rong L, Hu B, Ma X, Wang J, Chen H. The combination of T stage and the number of pathologic lymph nodes provides better prognostic discrimination in early‐stage cervical cancer with lymph node involvement. Front Oncol. 2021;11:764065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramirez PT, Frumovitz M, Pareja R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895‐1904. [DOI] [PubMed] [Google Scholar]
- 23. Huang H, Feng YL, Wan T, et al. Effectiveness of sequential chemoradiation vs concurrent chemoradiation or radiation alone in adjuvant treatment after hysterectomy for cervical cancer: the STARS phase 3 randomized clinical trial. JAMA Oncol. 2021;7:361‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim SW, Chun M, Ryu HS, et al. Long‐term results of early adjuvant concurrent chemoradiotherapy for high‐risk, early stage uterine cervical cancer patients after radical hysterectomy. BMC Cancer. 2017;17:297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B, Gao S, Li S. A comprehensive comparison of CT, MRI, positron emission tomography or positron emission tomography/CT, and diffusion weighted imaging‐MRI for detecting the lymph nodes metastases in patients with cervical cancer: a meta‐analysis based on 67 studies. Gynecol Obstet Invest. 2017;82:209‐222. [DOI] [PubMed] [Google Scholar]
- 26. Luo Q, Luo L, Tang L. A network meta‐analysis on the diagnostic value of different imaging methods for lymph node metastases in patients with cervical cancer. Technol Cancer Res Treat. 2018;17:1533034617742311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chang H, Wang M, Liu Y, Wu Y. Parametrial involvement and decreased survival of women with FIGO stage IIIC1 cervical cancer. J Gynecol Oncol. 2023;34. doi: 10.3802/jgo.2023.34.e46. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, . Further stratification of risk groups in patients with lymph node metastasis after radical hysterectomy for early‐stage cervical cancer. Gynecol Oncol. 2010;117:53‐58. [DOI] [PubMed] [Google Scholar]
- 29. Zong L, Zhang Q, Kong Y, et al. The tumor‐stroma ratio is an independent predictor of survival in patients with 2018 FIGO stage IIIC squamous cell carcinoma of the cervix following primary radical surgery. Gynecol Oncol. 2020;156:676‐681. [DOI] [PubMed] [Google Scholar]
- 30. Lindegaard JC, Petric P, Lindegaard AM, Tanderup K, Fokdal LU. Evaluation of a new prognostic tumor score in locally advanced cervical cancer integrating clinical examination and magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2020;106:754‐763. [DOI] [PubMed] [Google Scholar]
- 31. Lindegaard JC, Petric P, Schmid MP, et al. Prognostic implications of uterine cervical cancer regression during chemoradiation evaluated by the T‐score in the multicenter EMBRACE I study. Int J Radiat Oncol Biol Phys. 2022;113:379‐389. [DOI] [PubMed] [Google Scholar]


