Abstract
Allogeneic hematopoietic cell transplantation can cure patients with high-risk leukemia through graft-versus-leukemia (GVL) effects, the process by which malignant leukemic cells are cleared by donor-derived immune cells from the graft. The problem of harnessing GVL effects while controlling inflammation and host-organ damage linked with graft-versus-host disease (GVHD) has been the most formidable hurdle facing allogeneic hematopoietic cell transplantation. This powerful, curative-intent therapy remains among the most toxic treatments in the hematologist’s armamentarium due to the combined risks of GVHD-related morbidity, infections, and leukemia relapse. In this issue of the JCI, Li, Wang, et al. report that T cell Stat3 deficiency can extricate GVL effects from GVHD through tissue-specific programmed death-ligand 1/programmed cell death protein 1–dependent (PD-L1/PD-1-dependent) bioenergetic alterations that blunt harmful T cell effects in GVHD target organs, while preserving their beneficial antitumor activity in lymphohematopoietic tissues.
In search of the transplantation holy grail
In patients with blood cancers, the goal of allogeneic hematopoietic cell transplantation is to unleash curative graft-versus-leukemia (GVL) effects while minimizing morbidity from graft-versus-host disease (GVHD) (1). It has been challenging to disentangle the benefits of GVL from the harms of GVHD, as both are mediated by alloreactive donor T cells recognizing foreign tissue antigens. Patients receiving autologous grafts or T cell–depleted allogeneic transplants have lower GVHD rates, but higher risks of relapse. Patients who receive donor lymphocyte infusions can achieve remission of recurrent disease, but with a risk of enhanced GVHD. Current GVHD prophylactic strategies involve global immunosuppression whereby reduced GVHD severity in target tissues (e.g., gut, liver, skin, and lung) is achieved at the increased cost of leukemia relapse and impaired antiinfective immunity.
The STAT3 pathway relays signals from multiple cytokines, including IL-6, IL-17, IL-21, and IL-23, with an impact on T cell differentiation and autoimmune disorders, making it an ideal candidate for a role in alloimmunity (2). Early research on STAT3 found reduced GVHD-related mortality in mice treated with an inhibitor of STAT3 phosphorylation (2). Stat3 inactivation in CD4+ T cells prevented chronic sclerodermatous GVHD in mice and limited donor T cell accumulation in vivo while expanding Tregs and decreasing numbers of inflammatory Th17 cells (3). In other work, Stat3 deletion preserved natural Tregs and expanded induced Tregs (iTregs) to protect from acute GVHD (4). Pharmacological inhibition of phosphorylated STAT3 in human Tregs also prevented skin graft rejection in immunodeficient mice and reduced xenogeneic GVHD (5).
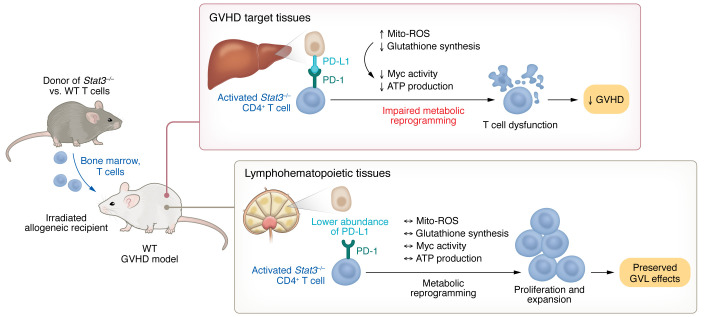
In this issue of the JCI, Li, Wang, et al. describe how Stat3 deficiency in donor T cells maintained potent GVL activity while preventing GVHD (6). In a major histocompatibility complex–mismatched mouse model of severe GVHD, the authors demonstrated protection from GVHD with Stat3-deficient T cells, but only in the presence of intact programmed death-ligand 1/programmed cell death protein 1 (PD-L1/PD-1) signaling. Stat3 inactivation in T cells enhanced PD-L1/PD-1–mediated inhibition of metabolic pathways in alloreactive T cells, thus interfering with metabolic reprogramming and leading to T cell dysfunction specifically in GVHD target organs (Figure 1). Because PD-L1 was more abundant in GVHD target tissues compared with lymphohematopoietic organs where hematologic malignancies mostly reside, Stat3 deficiency had tissue-specific effects that blunted GVHD in target organs, but maintained potent GVL activity. Importantly, studies comparing transplants with low doses of control and Stat3–/– donor T cells revealed a mild decrease in the GVL effects of Stat3-deficient T cells when assessed on a per cell basis. However, recipients tolerated much higher doses of Stat3-deficient T cells due to GVHD protection, thus preserving potent overall GVL activity. Past work from these authors also identified STAT3-dependent expansion and differentiation of pathogenic PSGLloCD4+ T cells in the lung and liver of recipients with chronic GVHD (7). Altogether, Stat3 inactivation had divergent effects at different body sites, thereby controlling GVHD but preserving GVL effects in lymphohematopoietic organs — one potential path toward the transplantation holy grail.
Figure 1. STAT3 deficiency prevents GVHD in target organs while preserving GVL activity in lymphohematopoietic tissues.
In GVHD target organs, activated T cells undergo metabolic reprogramming to sustain inflammatory T cell responses via the glutathione pathway. However, in the absence of STAT3, PD-1 signaling triggers high levels of Mito-ROS and decreased glutathione synthesis, which inhibits Myc activity and ATP production to impair metabolic reprogramming. These changes lead to T cell dysfunction, limiting T cell expansion and effector functions in target tissues, thus preventing GVHD. The lower abundance of PD-L1 in the spleen and other lymphohematopoietic tissues leads to lower PD-1 signaling, which allows metabolic reprogramming that supports T cell expansion and preserves GVL activity.
Target tissue PD-L1 regulates GVHD
Li, Wang, et al. showed that GVHD prevention achieved by Stat3 loss in T cells required PD-1 signaling in donor T cells mediated by PD-L1 in recipient tissues (6). Stat3 deficiency reduced glutathione synthesis and increased mitochondrial ROS (mito-ROS) in a PD-1–dependent manner (Figure 1). The antioxidant glutathione pathway is critical for metabolic reprogramming to sustain inflammatory T cell responses after initial T cell activation (8). Consistently, mito-ROS accumulation and decreased glutathione in Stat3-deficient alloreactive T cells were associated with decreased Myc expression and activity, decreased ATP production, and evidence of T cell dysfunction in GVHD target tissues (6). Yet it remains unclear where precisely Stat3 deficiency intersects with PD-1 signaling or its downstream consequences in T cells.
PD-1 blockade can accelerate GVHD in mice and humans (9, 10). The authors from the same research group previously speculated that differential binding of PD-L1 to PD-1 in GVHD target tissues and CD80 in lymphohematopoietic tissues contributed to GVHD protection alongside preservation of GVL (11, 12). Differential expression of PD-L1 in GVHD target organs and lymphohematopoietic tissue was proposed as a mechanism to explain how GVHD was prevented in Stat3-deficient T cell recipients, while GVL was largely preserved. Indeed, genetic or pharmacologic inhibition of PD-L1/PD-1 signaling erased GVHD protection from Stat3 inactivation (6). PD-L1/PD-1 signaling blockade revealed distinct PD-L1/PD-1–dependent alterations in Stat3-deficient T cell metabolism between GVHD target organs and lymphohematopoietic tissue. These observations fit with work from others identifying the importance of tissue-resident, locally maintained donor-derived T cells in GVHD (13).
The tissue-specific bioenergetic consequences of Stat3 deletion should also be considered as part of evolving work on metabolic control of GVHD. While others focused on glycolysis or other bioenergetic pathways in GVHD (14, 15), Li, Wang, et al. reported differential T cell metabolic profiles in spleen and GVHD target organs upon Stat3 loss (6). Specifically, Stat3-deficient T cells showed decreased glutathione/Myc pathway activity and ATP production in the liver, but not in the spleen. These findings should prompt caution in the interpretation of T cell metabolic findings when assessed only in lymphoid organs rather than also in target tissues.
Tregs or not Tregs
Despite prior findings suggesting a role for Tregs in GVHD protection upon T cell Stat3 deficiency, Li, Wang, et al. found no impact of Treg depletion on GVHD severity or survival in their model (6). Prior work showed that anti-CD25–mediated Treg depletion reversed protection from Stat3 deficiency in a mouse model of chronic sclerodermatous GVHD, where anti-CD25 treatment resulted in delayed, organ-specific GVHD in recipients of Stat3-deficient T cells (3). Other groups connected Tregs with Stat3 deficiency’s protection from GVHD based on destabilization of natural Tregs and reduced iTreg differentiation from naive CD4+ T cells (4). Finally, human pSTAT3–inhibited iTregs reduced skin graft rejection and protected from xenogeneic GVHD based on a metabolic shift from oxidative phosphorylation to glycolysis, increasing their suppressive function (5). Further work is needed to explain the divergent role reported for Tregs upon STAT3 loss, although unique features of STAT3-targeting methods and individual transplantation models could be at play.
While the Li, Wang, et al. report explored metabolic consequences of Stat3 deficiency, an important future direction should include identifying key ligands and receptors upstream of STAT3 signaling in GVHD. In past work, these authors speculated that IL-6 and IL-21 could act upstream of STAT3 signaling in pathogenic CXCR5–PD-1hi helper T cells implicated in chronic GVHD pathogenesis (16). It is unclear in their current work what might act upstream of STAT3 (6). Preclinical data showed that IL-6 inhibition reduced GVHD while allowing robust GVL, making IL-6 a candidate for acting upstream of STAT3 signaling in this model (17). However, benefits of IL-6 antagonism in GVHD prevention were not substantiated in patients (18). Thus, it is possible that STAT3 integrates IL-6 signaling with other pathways during GVHD pathogenesis.
STAT3 as therapeutic target for GVHD prophylaxis
Going beyond genetics as a discovery approach, Li, Wang, et al. explored a pharmacological strategy of targeted STAT3 degradation with translational potential (6). They tested a STAT3-selective proteolysis-targeting chimera (PROTAC) degrader, called SD-36, that was developed as an anticancer treatment. SD-36 mediates potent inhibition of signaling by monomeric and dimeric STAT3 without affecting other STAT molecules (19). The STAT3 degrader prevented GVHD when used to pretreat donor T cells ex vivo and also administered to recipients on days 0 and 3 of transplantation, a remarkably short overall treatment duration (6). The high therapeutic activity of transient STAT3 inhibition contrasts with the suggestion that STAT3 activity was essential to sustaining the pathogenic activity of tissue-resident T cells mediating GVHD. However, it is possible that STAT3 activity was particularly relevant for GVHD pathogenesis during seeding of target tissues by T cells early after transplantation. This observation dovetails with findings demonstrating the impact of other transient interventions during the early posttransplant period, such as Notch inhibition, for long-term GVHD control (20). In the future, questions about the feasibility of STAT3 targeting will need to be addressed, including those regarding on-target toxicities of STAT3 inhibition, optimal duration of treatment, and impact on GVL. Concomitant benefits of targeting STAT3 could include direct anticancer effects in STAT3-driven malignancies.
Conclusions and future directions
T cell Stat3 deficiency protected mice from GVHD while maintaining potent GVL responses (6). The relative separation between GVHD and GVL achieved by Stat3 deficiency relied on PD-L1/PD-1 signaling. PD-L1 expression in GVHD target tissues conferred protection from GVHD that was not shared in lymphohematopoietic tissue, where donor T cells are likely to encounter hematological neoplasms. PD-1 signaling in Stat3-deficient T cells resulted in inhibition of the glutathione/Myc pathway, culminating in T cell dysfunction. The upstream signals and downstream pathways connecting STAT3 and PD-1 remain subject to further investigation. Furthermore, the role of Tregs appears unresolved given divergent data between Li, Wang, et al. and prior work about the effects of anti-CD25 treatment on GVHD after transplantation with Stat3-deficient T cells. Altogether, Li, Wang, et al. propose STAT3 degradation as an attractive strategy for controlling GVHD while maintaining potent GVL activity as well as an interesting concept in which tissue-specific T cell dysfunction explains the distinct effects of STAT3 loss on harmful and beneficial T cell responses (6).
Acknowledgments
Research on GVHD in the Maillard laboratory has been supported by funding from the National Institute of Allergy and Infectious Diseases (R01-AI091627) and the Leukemia and Lymphoma Society (TRP 6583-20). JDB is supported by an American Society of Transplantation and Cellular Therapy (ASTCT) New Investigator Award and a Doris Duke Foundation Physician-Scientist Fellowship.
Version 1. 08/01/2023
Electronic publication
Footnotes
Conflict of interest: IM has received research funding from Genentech and Regeneron and is a member of Garuda Therapeutics’ Scientific Advisory Board.
Copyright: © 2023, Brandstadter et al. This is an open access article published under the terms of the Creative Commons Attribution 4.0 International License.
Reference information: J Clin Invest. 2023;133(15):e172251. https://doi.org/10.1172/JCI172251.
Contributor Information
Joshua D. Brandstadter, Email: Joshua.Brandstadter@pennmedicine.upenn.edu.
Riley Outen, Email: Riley.Outen@pennmedicine.upenn.edu.
Ivan Maillard, Email: imaillar@pennmedicine.upenn.edu.
References
- 1.Negrin RS. Graft-versus-host disease versus graft-versus-leukemia. Hematology Am Soc Hematol Educ Program. 2015;2015:225–230. doi: 10.1182/asheducation-2015.1.225. [DOI] [PubMed] [Google Scholar]
- 2.Lu SX, et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood. 2008;112(13):5254–5258. doi: 10.1182/blood-2008-03-147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radojcic V, et al. STAT3 signaling in CD4+ T cells is critical for the pathogenesis of chronic sclerodermatous graft-versus-host disease in a murine model. J Immunol. 2010;184(2):764–774. doi: 10.4049/jimmunol.0903006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurence A, et al. STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft-versus-host disease. Immunity. 2012;37(2):209–222. doi: 10.1016/j.immuni.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walton K, et al. Metabolic reprogramming augments potency of human pSTAT3-inhibited iTregs to suppress alloreactivity. JCI Insight. 2020;5(9):e13643. doi: 10.1172/jci.insight.136437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, et al. Donor T cell STAT3 deficiency enables tissue PD-L1–dependent prevention of graft-versus-host disease while preserving graft-versus-leukemia activity. J Clin Invest. 2023;133(15):e165723. doi: 10.1172/JCI165723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong X, et al. Tissue-resident PSGL1loCD4+ T cells promote B cell differentiation and chronic graft-versus-host disease-associated autoimmunity. J Clin Invest. 2021;131(1):e13546. doi: 10.1172/JCI135468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mak TW, et al. Glutathione primes T cell metabolism for inflammation. Immunity. 2017;46(6):1089–1689. doi: 10.1016/j.immuni.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 9.Blazar BR, et al. Blockade of programmed death-1 engagement accelerates graft-versus-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol. 2003;171(3):1272–1277. doi: 10.4049/jimmunol.171.3.1272. [DOI] [PubMed] [Google Scholar]
- 10.Haverkos BM, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017;130(2):221–228. doi: 10.1182/blood-2017-01-761346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni X, et al. PD-L1 interacts with CD80 to regulate graft-versus-leukemia activity of donor CD8+ T cells. J Clin Invest. 2017;127(5):1960–1977. doi: 10.1172/JCI91138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song Q, et al. Tolerogenic anti-IL-2 mAb prevents graft-versus-host disease while preserving strong graft-versus-leukemia activity. Blood. 2021;137(16):2243–2255. doi: 10.1182/blood.2020006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sacirbegovic F, et al. Graft-versus-host disease is locally maintained in target tissues by resident progenitor-like T cells. Immunity. 2023;56(2):369–385. doi: 10.1016/j.immuni.2023.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nguyen HD, et al. Metabolic reprogramming of alloantigen-activated T cells after hematopoietic cell transplantation. J Clin Invest. 2016;126(4):1337–1352. doi: 10.1172/JCI82587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, et al. Targeting glycolysis in alloreactive T cells to prevent acute graft-versus-host disease while preserving graft-versus-leukemia effect. Front Immunol. 2022;13:751296. doi: 10.3389/fimmu.2022.751296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong X, et al. Trafficking between clonally related peripheral T-helper cells and tissue-resident T-helper cells in chronic GVHD. Blood. 2022;140(25):2740–2753. doi: 10.1182/blood.2022016581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khuat LT, et al. Increased efficacy of dual proinflammatory cytokine blockade on acute GVHD while maintaining GVT effects. Blood. 2021;138(24):2583–2588. doi: 10.1182/blood.2021011216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy GA, et al. A phase 3 double-blind study of the addition of tocilizumab vs placebo to cyclosporin/methotrexate GVHD prophylaxis. Blood. 2021;137(14):1970–1979. doi: 10.1182/blood.2020009050. [DOI] [PubMed] [Google Scholar]
- 19.Bai L, et al. A potent and selective small-molecule degrader of STAT3 achieves complete tumor regression in vivo. Cancer Cell. 2019;36(5):498–511. doi: 10.1016/j.ccell.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tkachev V, et al. Notch signaling drives intestinal graft-versus-host disease in mice and nonhuman primates. Sci Transl Med. 2023;15(702):eadd1175. doi: 10.1126/scitranslmed.add1175. [DOI] [PMC free article] [PubMed] [Google Scholar]