Abstract
Saccharomyces cerevisiae cells lacking the regulatory subunit of casein kinase 2 (CK-2), encoded by the gene CKB1, display a phenotype of hypersensitivity to Na+ and Li+ cations. The sensitivity of a strain lacking ckb1 is higher than that of a calcineurin mutant and similar to that of a strain lacking HAL3, the regulatory subunit of the Ppz1 protein phosphatase. Genetic analysis indicated that Ckb1 participates in regulatory pathways different from that of Ppz1 or calcineurin. Deletion of CKB1 increased the salt sensitivity of a strain lacking Ena1 ATPase, the major determinant for sodium efflux, suggesting that the function of the kinase is not mediated by Ena1. Consistently, ckb1 mutants did not show an altered cation efflux. The function of Ckb1 was independent of the TRK system, which is responsible for discrimination of potassium and sodium entry, and in the absence of the kinase regulatory subunit, the influx of sodium was essentially normal. Therefore, the salt sensitivity of a ckb1 mutant cannot be attributed to defects in the fluxes of sodium. In fact, in these cells, both the intracellular content and the cytoplasm/vacuole ratio for sodium were similar to those features of wild-type cells. The possible causes for the salt sensitivity phenotype of casein kinase mutants are discussed in the light of these findings.
As for many cell types, sodium cations are rather toxic for yeast cells, and consequently, the maintenance of suitable intracellular concentrations of Na+ is a strong requirement for survival (see reference 42 for a review). Intracellular sodium levels are the result of influx and efflux processes that are subjected to regulation. Saccharomyces cerevisiae actively extrudes sodium through the Na+-ATPase encoded by the gene ENA1, the first member of the ENA (also called PMR2) locus (13, 18, 40, 47). ENA1 is barely expressed under normal growth conditions, but its expression is sharply increased by osmotic and saline (sodium or lithium) stresses, as well as by alkaline pH (13, 23, 25). As a consequence, cells lacking ENA1 are highly sensitive to sodium and lithium. Several components of the regulatory network that controls ENA1 expression have been identified in the last few years. Interestingly, this regulation involves phospho-dephosphorylation mechanisms. For instance, the Ser/Thr protein phosphatase PP2B (calcineurin) is needed for full response to sodium stress (25, 28). On the other hand, the Ppz1 protein phosphatase represses ENA1 expression through a mechanism that is independent from that of calcineurin. This repression of ENA1 results in phosphatase mutants that are hypertolerant to sodium (32). Recent work has shown that HAL3, initially identified as a halotolerant determinant that influences ENA1 expression (11), is a negative regulatory subunit of Ppz1 and thus defines a novel regulatory pathway (8). Hal1, a conserved salt-induced protein (14), has been defined as an effector of ENA1 expression (39). Recently, HAL8 and HAL9 have been determined to be genes encoding putative transcriptional activators of the ENA1 response to salt stress (24).
In S. cerevisiae the uptake of K+ and Na+ is mediated by the Trk1-Trk2 transport system, being the Trk1 function predominant under normal growth conditions (12, 19, 20, 36). The TRK system discriminates between Na+ and K+, thus preventing the entry of an excess of Na+ when the levels of the cation in the medium are too high. Therefore, a proper functioning of this cation uptake system ought to be important for salt tolerance, as demonstrated by the observation that trk1 trk2 mutants are hypersensitive to sodium ions (17, 19). In addition, intracellular sequestration of sodium can also be an efficient method of improving salt tolerance, and confinement of Na+ in the vacuole has been proposed as a mechanism that reduces the cytosolic levels of this cation (19). It has been documented that the putative Na+-H+ exchanger encoded by the gene NHX1 is involved in the vacuolar compartmentalization of sodium ions (29, 30).
Therefore, sodium homeostasis in yeast appears to be a complex process, still poorly understood at the molecular level. Casein kinase 2 (CK-2) has been proposed as an additional component of this regulatory system. CK-2 is a highly conserved Ser/Thr protein kinase that has also been related to cell polarity and cell cycle progression (for a recent review, see reference 16). In yeast, CK-2 is an oligomer composed of two related catalytic subunits (α and α′), encoded by the genes CKA1 and CKA2 (6, 31), and two regulatory polypeptides (β and β′), encoded by the genes CKB1 and CKB2 (5, 37), respectively. In order to survive, yeast cells require at least one of the catalytic subunits (31). On the contrary, the regulatory subunits do not appear to be necessary for growth under normal conditions. Interestingly, deletion of either CKB1 or CKB2 results in the same phenotype of hypersensitivity to Na+ and Li+ (5). The effect of the mutations is not additive and does not affect the tolerance to potassium cations (5).
Our laboratories are interested in the analysis of the role of protein phosphorylation in the regulation of salt tolerance in yeast cells. Therefore, to gain insight into the mechanism responsible for the role of CK-2 in yeast biology, we undertook a genetic and biochemical study of the effects of the absence of Ckb1 on the different cell processes that affect the sensitivity to Na+ and Li+. Our results indicate that CK-2, in contrast with recently reported data, does not regulate the influx or the efflux of sodium, thus suggesting that this kinase might be involved in the regulation of a putative target for sodium toxicity.
MATERIALS AND METHODS
Strains and growth conditions.
Escherichia coli NM522 and DH5α were used as hosts for DNA cloning. Bacterial cells were grown at 37°C in Luria-Bertani medium containing 50 μg of ampicillin per ml, when needed, for plasmid selection. Yeast cells were grown at 28°C in yeast extract-peptone-dextrose (YPD) medium or, when indicated, in synthetic minimal (SD) or complete minimal medium (43). The relevant genotypes of the strains described in this work can be found in Table 1.
TABLE 1.
Yeast strains used in this work
| Strain | Relevant genotype | Source or reference |
|---|---|---|
| DBY746 | MATα ura3-52 leu2-3,112 his3-Δ1 trp1-Δ239 | A. Rodríguez-Navarro |
| JA30 | DBY746 ppz1::ura3 | 32 |
| JA40 | DBY746 cnb1::HIS3 | 32 |
| RH16.6 | DBY746 ena1–ena4::LEU2 | 18 |
| EDN1 | DBY746 ckb1::TRP1 | This work |
| EDN3 | DBY746 ckb1::HIS3 | This work |
| EDN4 | DBY746 hal3::LEU2 | This work |
| EDN6 | DBY746 hal1::LEU2 | This work |
| EDN11 | DBY746 ppz1::ura3 ckb1::TRP1 | This work |
| EDN21 | DBY746 hal3::LEU2 ckb1::TRP1 | This work |
| EDN22 | DBY746 cnb1::HIS3 ckb1::TRP1 | This work |
| EDN25 | RH16.6 ckb1::HIS3 | This work |
| W303.1A | MATa ade-2-2 his3-11,15 leu2-3,112 ura3-1 trp1-1 | |
| W59 | W301.1A trk1::LEU2 TRK2 | 22 |
| WΔ3 | W301.1A trk1::LEU2 trk2::HIS3 | 22 |
| EDN42 | W301.1A ckb1::TRP1 | This work |
| EDN44 | WΔ3 ckb1::TRP1 | This work |
Recombinant DNA techniques, gene disruptions, and plasmids.
E. coli and S. cerevisiae cells were transformed by standard techniques as previously described (8). Restriction reactions, DNA ligations, and other standard recombinant DNA techniques were carried out as described previously (41). Gene disruptions were performed as follows. Disruption of PPZ1 and CNB1 was as described in reference 32. Disruption of CKB1 with the HIS3 marker was made by integration of plasmid pAPB17 linearized by digestion with EcoRI (5). To disrupt CKB1 with the marker TRP1, plasmid pAPB17 was digested with XhoI and SacI and the insert (about 1.0 kbp) was cloned into plasmid pRS304. This plasmid was linearized as described above and used to transform yeast cells. Disruption of the genes HAL1 and HAL3 with the LEU2 marker was performed in manners similar to those described in references 14 and 11, respectively.
To achieve high levels of expression of Hal1 and Hal2, the corresponding open reading frames were cloned into high-copy-number vectors carrying the PMA1 promoter, as previously described (26, 39).
β-Galactosidase measurements.
To evaluate the effect of the ckb1 mutation on ENA1 expression, wild-type DBY746 and EDN1 (ckb1Δ) cells were transformed with the multicopy plasmid pKC201 (1, 7), which contains ENA1 sequences from −1385 to +35 (relative to the starting initiating Met), fused to lacZ. Cells (5 ml) were grown to an optical density at 660 nm of 0.5 to 1.0, solid NaCl was added to achieve a final concentration of 0.75 M, and growth was resumed for 60 min. Cells were then centrifuged, and β-galactosidase activity was measured as described in reference 38.
Determination of cation influx and efflux.
For influx experiments, cells grown in SD medium were potassium starved by incubation in the minimal ammonium-phosphate medium (35). After 5 h, cells were harvested and resuspended in buffer containing RbCl or LiCl (50 mM). Samples were taken at regular time intervals, filtered immediately, and treated for determination of intracellular ion content.
For determination of efflux rate, cells were grown in SD medium up to optical density at 660 nm of 0.3 to 0.4 and then LiCl or NaCl (100 mM) was added. Growth was resumed for 3 h in order to load the cells with the cation. After this time, cells were harvested and resuspended in buffer {10 mM MES [2-(N-morpholino)ethanesulfonic acid] brought to pH 5.8 with Ca(OH)2 and containing 0.1 mM MgCl2 and 2% glucose}, supplemented with 10 mM KCl to trigger the efflux process. Samples were taken at regular time intervals, filtered, and treated for determination of intracellular ion content.
The intracellular ion content of the cells was determined as previously described (34, 36). Briefly, samples of cells were filtered, washed with 20 mM MgCl2, and treated with acid and the cations were analyzed by atomic absorption spectrophotometry.
Other methods.
Salt sensitivity assays were performed with freshly prepared YPD plates containing different concentrations of the compound (drop tests) or with liquid cultures as described in reference 32. Measurement of proton fluxes were performed as described previously (2), except that cells were grown in YPD medium. Differential extraction of potassium and sodium ions from the cytoplasm and vacuole was essentially achieved as previously described (10), with minor modifications, including a treatment of the cells with 0.1 mg of digitonin per ml for 5 min.
RESULTS
CK-2 regulates sodium tolerance by a mechanism independent from that of calcineurin and Ppz1.
It has been reported that deletion of CKB1 results in a phenotype of sensitivity to sodium and lithium ions (5). To evaluate the potency of this phenotype, we deleted the CKB1 gene in the DBY746 background and compared the sensitivities to sodium and lithium of the ckb1Δ mutant with those of cells lacking other genes known to be involved in salt sensitivity, such as the regulatory subunit of calcineurin (CNB1), HAL1, and HAL3. Strains with mutations in the HAL1 gene displayed a very weak salt sensitivity phenotype. Deletion of CKB1 resulted in a phenotype that was stronger than that of calcineurin mutants and almost as strong as that of cells lacking HAL3 (not shown). Dose-response experiments performed with SD liquid cultures showed that the tolerance to lithium ions of a ckb1Δ mutant was reduced by about 30% compared to the tolerance of the wild-type strain (50% inhibitory concentration 18 mM versus 26 mM).
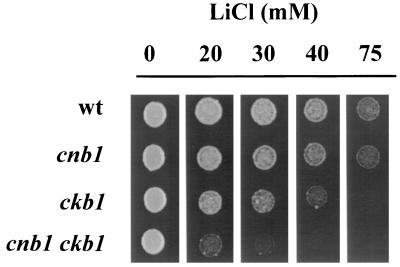
Because CKB1 encodes a regulatory subunit of a protein kinase, we considered it interesting to test the possibility of genetic interaction between this gene and the pathways defined by the calcineurin and Ppz1 phosphatase genes. To this end, we disrupted the CKB1 gene in cells lacking CNB1, the gene encoding the regulatory subunit of calcineurin, and tested the sensitivity of these cells to Li+. As shown in Fig. 1, lack of Ckb1 resulted in an additional increase in sensitivity to lithium cations, indicating that the kinase and the phosphatase do not share a common regulatory pathway.
FIG. 1.
Additive effects of the calcineurin and ckb1 mutations. Strains DBY746 (wild type [wt]), JA40 (cnb1), EDN1 (ckb1), and EDN22 (cnb1 ckb1) were plated on YPD plates containing the indicated concentrations of LiCl. Plates were incubated at 28°C, and growth was scored after 2 days.
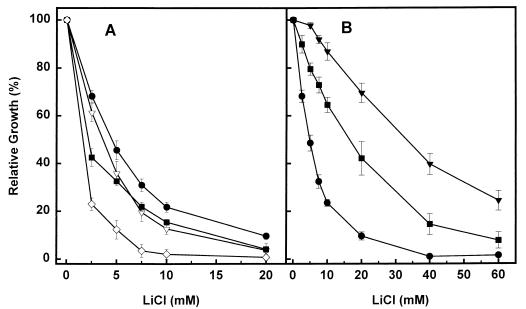
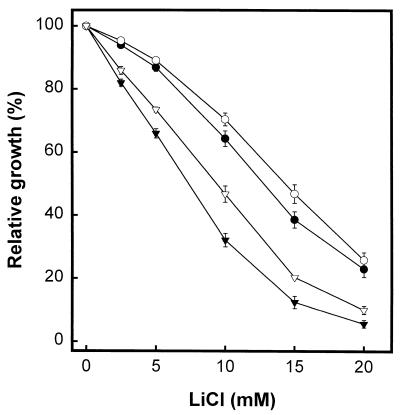
Disruption of the protein phosphatase Ppz1 resulted in increased salt tolerance. As shown in Fig. 2, the absence of Ckb1 decreased the tolerance of a ppzlΔ strain, as would be expected if Ckb1 and Ppz1 regulate independent pathways. Hal3 has been defined as a regulatory subunit of Ppz1, thus placing Ppz1 and Hal3 in the same regulatory pathway. To confirm our observation, we generated a ckb1 hal3 double mutant and analyzed its sensitivity to lithium ions. As shown in Fig. 2, the ckb1 hal3 double mutant was more sensitive than a single hal3 or ckb1 deletion mutant.
FIG. 2.
The effect of CK-2 on salt tolerance is not mediated by the Hal3/Ppz1 pathway. (A) YPD medium containing the indicated concentrations of LiCl was inoculated (initial A660, 0.007) with wild-type strain DBY746 (●) or its derivatives EDN1 (ckb1) (▿), EDN4 (hal3) (■), and EDN22 (ckb1 hal3) (◊). Cultures were grown for 18 h, and the densities of the cultures were then measured. Relative growth was calculated as the ratio between growth in the presence and growth in the absence of added salts and expressed as a percentage. (B) Cultures of DBY746 (●), JA30 (ppz1) (▾), and EDN11 (ppz1 ckb1) (■) cells were grown as indicated above. Data are means ± standard errors of the means of results from four independent experiments.
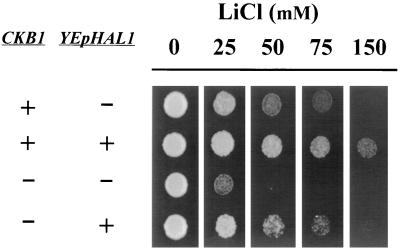
Both calcineurin and Ppz1 are known to affect sodium tolerance by regulating the expression of ENA1, a gene encoding the Na+-ATPase which represents the major mechanism for Na+ efflux in budding yeast. HAL1 had been defined as a gene that, when it is expressed in multicopy numbers, was able to increase ENA1 expression. We considered that if Ckb1 was placed downstream of Hal1, high levels of Hal1 would not confer sodium tolerance to the mutant. However, as shown in Fig. 3, high-copy-number expression of HAL1 clearly increased the tolerance of a ckb1Δ strain, indicating that the effect of Hal1 is independent of the presence of Ckb1.
FIG. 3.
High-copy-number expression of HAL1 increases salt tolerance in a ckb1 background. Strains DBY746 (CKB1) and EDN1 (ckb1) were transformed with the high-copy-number plasmid pRS699-HAL1 (denoted YEpHAL1) (+) or the empty plasmid YEplac195 (−). Positive cultures were plated on YPD plates containing the indicated concentrations of LiCl, and growth was monitored as described in the legend to Fig. 1.
Analysis of the sodium efflux mechanisms in a ckb1 mutant.
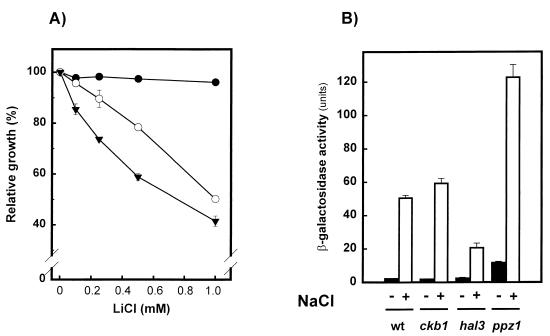
Because of the relatively strong phenotype of the ckb1 mutation, we decided to explore in a systematic way the possible effect of Ckb1 on the expression of ENA1 and, therefore, on sodium efflux. To this end, we disrupted the CKB1 gene in an RH16.6 strain that lacks the ENA1-ENA4 gene cluster. This strain has a very reduced sodium efflux, and therefore it is highly sensitive to Li+ and Na+. Interestingly, the deletion of CKB1 further increased the sensitivity to Li+ of the ena1-ena4 mutant (Fig. 4A). This result was somewhat unexpected because it indicated that, in contrast to preliminary published data (16), the function of CK-2 does not involve the Ena1 ATPase. To confirm this possibility, we transformed wild-type and ckb1 strains with plasmid pKC201, which bears the entire ENA1 promoter fused to β-galactosidase. The cells were stressed with 0.75 M NaCl for 1 h, and the β-galactosidase activity was measured. As shown in Fig. 4B, cells lacking Ckb1 showed a response essentially identical to that of wild-type cells whereas, under the same conditions, hal3 and ppz1 mutants displayed decreased (hal3) and increased (ppz1) responses, respectively, as previously described (11, 32). Therefore, our data did not support the notion that CK-2 is an effector of ENA1 transcription and suggested that cation efflux might not be affected in Cbk1-deficient yeast cells. This possibility was directly tested by loading wild-type, cnb1Δ, and ckb1Δ cells with lithium and measuring the efflux of this cation. As shown in Fig. 5, whereas the cation efflux of calcineurin mutants was reduced (as previously described), the efflux of the ckb1Δ strain was essentially identical to that of wild-type cells. Therefore, from our data it can be concluded that the increased sensitivity of the ckb1 mutant to sodium and lithium cannot be attributed to a reduced efflux of these cations.
FIG. 4.
The ckb1 mutation is additive to those of ENA1 to ENA4 and does not alter the expression of the ATPase. (A) Strains DBY746 (●), RH16.6 (ena1 to ena4) (○), and EDN25 (ena1 to ena4 ckb1) (▾) were tested for LiCl sensitivity in liquid cultures as described for Fig. 2. Data are means ± standard errors of the means of results from four independent experiments. (B) DBY746 (wild type [wt]), EDN1 (ckb1), EDN4 (hal3), and JA30 (ppz1) were transformed with the multicopy plasmid pKC201, which allows expression of the β-galactosidase protein from the ENA1 promoter. Cells were grown as described in Materials and Methods, and β-galactosidase activity was measured in permeabilized cells treated with (+) or without (−) 0.75 M NaCl for 60 min. Data are means ± standard errors of the means of results from 16 to 18 independent experiments performed with five independent clones (DBY746 and EDN1) or eight independent experiments performed with four independent clones (EDN4 and JA30).
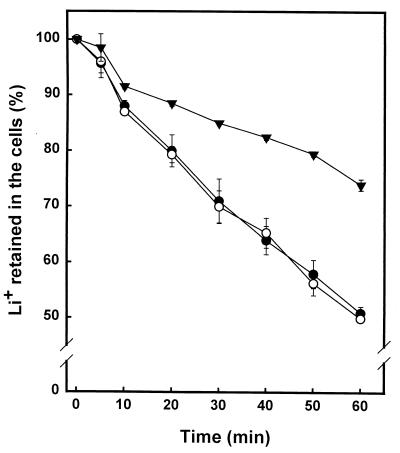
FIG. 5.
Measurement of the efflux of lithium cations in ckb1 cells. Wild-type DBY746 (●), as well as EDN1 (ckb1) (○) and JA40 (cnb1) (▾), cells were loaded with LiCl for 3 h and washed, and the efflux of Li+ was monitored as described in Materials and Methods. Data are means ± standard errors of the means of results from three independent experiments.
Mutation of CKB1 does not alter sodium or potassium influx.
Changes in the influx of sodium and potassium, mediated by the TRK system, can be responsible for salt sensitivity phenotypes. To test the possibility that CK-2 affects the TRK system, we introduced the ckb1 deletion in a strain lacking TRK1 and TRK2. When this strain was tested for sodium sensitivity, we observed that it was more sensitive than the trk double mutant (Fig. 6). This result supported the notion that CK-2 is required for normal salt resistance in trk1 trk2 cells and suggests that the TRK system is not regulated by CK-2. In fact, we have measured Li+ and Rb+ influx (the latter being used as an analog of K+ for transport experiments) in wild-type and ckb1 cells (Fig. 7). The time course of the uptake of these cations showed that the initial velocities of influx were virtually identical in both strains but that, as expected, it was dramatically reduced in a trk1 mutant, which is defective in high-affinity potassium transport. Consequently, the salt sensitivity phenotype of the ckb1 mutant cannot be attributed to changes in the influx of these cations. Because changes in proton efflux can affect salt tolerance, we determined this parameter in wild-type and ckb1 cells, obtaining values of 15 ± 2 and 13 ± 1.5 nmol of H+/mg (dry weight) of cells. Therefore, our results indicate that the ckb1 mutation does not modify proton pumping.
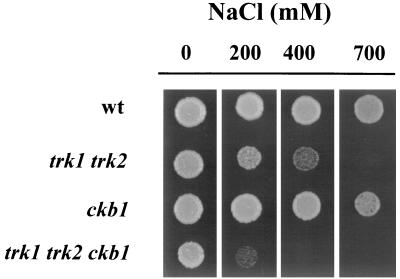
FIG. 6.
The deletion of CKB1 increases the salt sensitivity of a trk strain. The CKB1 gene was disrupted in the wild-type (wt) strain W303.1A and in its isogenic strain WΔ3 (trk1 trk2) to yield EDN42 and EDN44, respectively. The sensitivities to NaCl of these strains were tested on plates as described in the legend to Fig. 1.
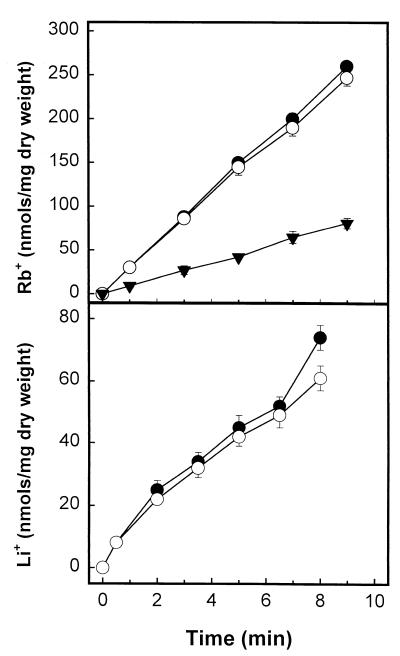
FIG. 7.
Influx of Li+ and Rb+ in a ckb1 strain. Potassium-starved wild-type W303.1A (●) and EDN1 (ckb1) (○) were incubated with RbCl (upper panel) or LiCl (lower panel), and the influxes of these cations were determined as described in Materials and Methods. Data from strain W59 (trk1) (▾), which is known to have a decreased potassium transport, is included for comparison. Data are means ± standard errors of the means of results from four independent experiments.
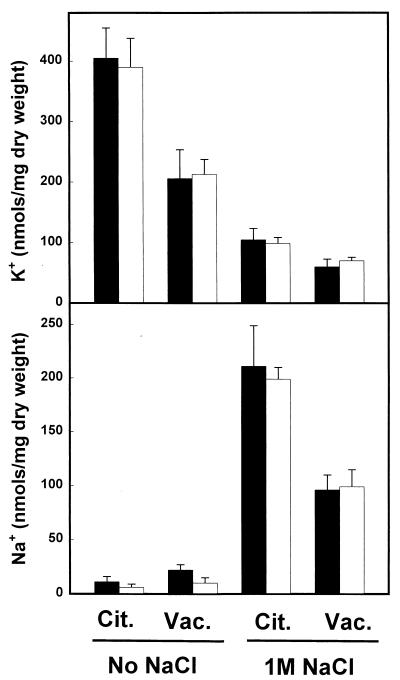
The data presented so far indicate that the mutation of CKB1 does not alter the normal influx and efflux of Na+ and K+. Consistently with this evidence, we have observed that, after the cells were challenged with a range of NaCl concentrations (from 0.25 to 1 M), the intracellular contents of Na+ and K+, as well as the Na+/K+ ratio, were virtually identical in wild-type cells and ckb1 mutants (data not shown). Finally, we have examined the possibility that CK-2 is somehow involved in the process of sequestration of sodium into the vacuole. To this end, we measured the cytoplasmic and vacuolar contents for Na+ and K+, before and after 6 h of incubation of the cells with 1 M NaCl. As shown in Fig. 8, the intracellular distributions of both cations were very much alike in wild-type and ckb1 cells.
FIG. 8.
Cytoplasmic and vacuolar distributions of sodium and potassium ions in wild-type and ckb1 yeast cells. Wild-type (filled bars) and EDN1 (ckb1) (open bars) cells were grown on YPD medium and incubated for 6 h with or without 1 M NaCl. The contents of Na+ and K+ in the cytoplasm (Cit.) and the vacuole (Vac.) were determined as described in Materials and Methods. Data are means ± standard errors of the means of results from three experiments.
The fact that mutation of Ckb1 affects Na+ and Li+ tolerance in the absence of increased levels of these cations drew our attention to the HAL2 (also called MET22) gene, which codes for an Na+- and Li+-sensitive phosphohydrolase identified as a putative target for the toxicities of these cations. Overexpression of HAL2 in a wild-type background resulted in a relatively weak increase in salt tolerance. As shown in Fig. 9, high levels of Hal2 also increase the tolerance of a ckb1 strain, indicating that the regulatory subunit of CK-2 does not mediate Hal2 function.
FIG. 9.
Overexpression of HAL2 increases the Li+ tolerance of a ckb1 strain. Wild-type DBY746 (circles) and EDN1 (ckb1) (triangles) cells were transformed with plasmid pRS699-HAL2 (open symbols) or the empty plasmid YEplac185 (filled symbols), and their sensitivities to LiCl were measured in liquid cultures as described for Fig. 2. Data are means ± standard errors of the means of results from four independent experiments.
DISCUSSION
The finding that the mutation of the regulatory subunits of CK-2 (CKB1 and CKB2) results in a phenotype of sensitivity to sodium and lithium ions (5) raised the key question of what type of cellular process, relevant for cation tolerance, involves this kinase. Because of the equivalence in potency and the lack of an additive phenotype, the disruption of only one gene, CKB1, was chosen as a working model. Our data indicated that the potency of the mutation is relatively strong, thus suggesting that this cellular process is highly relevant for salt tolerance. In S. cerevisiae, a key factor for salt tolerance is the proper function of the major determinant for sodium efflux, the Ena1 Na+-ATPase (13, 19, 47). In addition, the expression of the ENA1 gene is regulated by mechanisms involving phospho-dephosphorylation reactions. Therefore, it was reasonable to assume that the function of CK-2 is related to that of the Hal3 and Ppz1 (8) or the calcineurin (25) regulatory system. However, our data clearly show that CK-2 participates in a mechanism that is independent from the mentioned phosphatases. In addition, we demonstrate that the function of Hal1, which, when expressed at high levels, results in increased ENA1 expression (39), does not require Ckb1.
While the above-mentioned results are still compatible with the notion that CK-2 regulates a novel Ena1-regulatory pathway, we provide here biochemical and genetic data demonstrating that the function of Ckb1 in salt tolerance does not involve Ena1 and that the expression of the ENA1 ATPase gene is not altered by the absence of Ckb1. These conclusions are in sharp contrast with recently published data suggesting that the kinase regulates the transcription of the ENA1 gene (45). Tenny and Glover (45) derived their conclusions essentially from experiments with a β-galactosidase reporter system (similar to what is shown in our Fig. 4B). Although, at this moment, we cannot account for these contradictory results, it is worth noting that the experimental conditions differed in a number of circumstances, including strain background and concentration (0.4 instead 0.75 M) and time of exposure to NaCl (30 min instead 60 min). However, we performed β-galactosidase experiments after stressing the cells for different times with 0.4 M NaCl and still could not find differences between wild-type and ckb1 strains. As an additional proof for the involvement of Ena1 in the mechanism of action of the kinase, Tenny and Glover invoked the fact that the overexpression of ENA1 suppresses the salt sensitivity of the ckb1 mutants. However, it seems evident that overexpression of Ena1 would result in active extrusion of sodium and lithium cations and, probably, sequestration in intracellular compartments (4). This overexpression would also alleviate the salt sensitivity phenotype of an Ena1-independent mutation, simply by reducing the cytosolic amount of the cations. Furthermore, our analysis of cation efflux clearly shows that the output of sodium or lithium ions is not modified by the absence of Ckb1. In contrast, and consistently with reported data (25), a cnb1 mutant (which has a weaker salt sensitivity phenotype), shows a clear-cut decrease in efflux rate. This decrease can be considered further evidence against an involvement of ENA1 in CK-2 function. We feel that our conclusions are further strengthened by the fact that they are sustained by a combination of both genetic and biochemical evidence. A consequence of the independence of Ckb1 and Ena1 is that the function of CK-2 would also be independent from that of the SOP1 and SOP2 gene products, which have been shown to require ENA1 for function (21). In addition, from our efflux data, one might expect that the absence of Ckb1 would not affect the function of the Nha1 antiporter, a protein that under specific circumstances also plays a role in sodium efflux (3, 33, 44).
A very relevant finding regarding the role of CK-2 in salt tolerance is that the absence of Ckb1 does not increase the intracellular sodium content or alter the intracellular Na+/K+ ratio. These facts are in agreement with our findings that the lack of Ckb1 has very little effect on Li+ and Rb+ uptake and that the ckb1 mutation shows, as far as sodium tolerance is concerned, an additive effect on the trk1 trk2 mutation. Our data also rule out the possibility that the absence of Ckb1 alters the ability of the cell to reduce the cytoplasmic levels of sodium cations through vacuolar sequestration.
Therefore, our findings define a scenario in which ckb1 cells are substantially more sensitive to sodium and lithium than wild-type cells in the absence of an increased intracellular cation content. A reasonable hypothesis would be that the absence of Ckb1 results in increased sensitivity to sodium and lithium cations of an important component of the cellular machinery. This component would be a direct or an indirect target for CK-2 phosphorylation, being the dephosphorylated salt-sensitive protein and, therefore, a cellular target for salt toxicity.
So far, only Hal2/Met22 has been characterized through genetic and biochemical methods as a target for lithium toxicity (15, 26, 27). Hal2 degrades adenosine 3′, 5′-bisphosphate (pAp) and 3′-phosphoadenosine, 5′-phosphosulphate (pApS). These compounds are intermediates of the sulfate assimilation pathway, which is needed mainly for the synthesis of sulfur-containing amino acids (see references 46 for a review). Overexpression of HAL2 increases Li+ tolerance because this enzyme is inhibited by Li+, and pApS is highly toxic for yeast. While at this moment we cannot rule out the possibility that the salt sensitivity phenotype of ckb1 mutants is related to alterations in the sulfate uptake pathway, several lines of reasoning suggest that this does not occur through the regulation of Hal2. For instance, Hal2 does not appear to contain consensus sequences for CK-2 phosphorylation. We show here that overexpression of HAL2 still increases Li+ tolerance even in the absence of Ckb1 and that the ckb1 mutant has a rather strong phenotype but that the hal2 deletion has almost no effect on salt tolerance (15). Finally, hal2Δ mutants display an auxotrophy for methionine (15), presumably because Met supplementation greatly reduces the need for sulfate intake and, hence, pAp and pApS formation, whereas we have found that ckb1 mutants grow well in the absence of Met (data not shown). It has been recently suggested that RNA processing might be a target function for lithium toxicity and that this would be the result of the existence of several Li+-sensitive components displaying synergistic toxicity (9). Certain components would be inhibited by an excess of pAp or pApS (as a result of Hal2 inhibition), whereas others, such as the RNase MRP ribonucleoprotein, would be directly inhibited by lithium ions. A conceivable hypothesis would be that one of the latter components is phosphorylated by CK-2 and that the absence of Ckb1 would yield a dephosphorylated protein, hypersensitive to Li+ and Na+ ions.
ACKNOWLEDGMENTS
We thank C. V. Glover for the CKB1 disruption cassettes, A. Rodríguez-Navarro for the RH16.6 strain, R. Haro for the W59 and WΔ3 strains, and R. Serrano for the HAL1 and HAL2 plasmids. The skillful technical help of Anna Vilalta and Mireia Zaguirre is acknowledged.
This work was supported by grants PB95-0663 and PB95-0976 from the Dirección General de Investigación Científica y Técnica, Spain, to J.A. and J.R., respectively; by an Ajut de Suport als Grups de Recerca de Catalunya (SGR97-127) from the Generalitat de Catalunya to J.A.; and by grant BIO4-CT97-2210 from the European Union to J.R. E.d.N. is the recipient of a predoctoral fellowship from the Ministerio de Educación y Cultura, Spain.
REFERENCES
- 1.Alepuz P M, Cunningham K W, Estruch F. Glucose repression affects ion homeostasis in yeast through the regulation of the stress-activated ENA1 gene. Mol Microbiol. 1997;26:91–98. doi: 10.1046/j.1365-2958.1997.5531917.x. [DOI] [PubMed] [Google Scholar]
- 2.Balcells L, Martín R, Ruiz M C, Gómez N, Ramos J, Ariño J. The Pzh1 protein phosphatase and the Spm1 protein kinase are involved in the regulation of the plasma membrane H+-ATPase in fission yeast. FEBS Lett. 1998;435:241–244. doi: 10.1016/s0014-5793(98)01082-5. [DOI] [PubMed] [Google Scholar]
- 3.Bañuelos M A, Sychrová H, Bleykasten-Grosshans C, Souciet J L, Potier S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology. 1998;144:2749–2758. doi: 10.1099/00221287-144-10-2749. [DOI] [PubMed] [Google Scholar]
- 4.Benito B, Quintero F J, Rodríguez-Navarro A. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: conditions for phosphorylation from ATP and Pi. Biochim Biophys Acta. 1997;1328:214–226. doi: 10.1016/s0005-2736(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 5.Bidwai A P, Reed J C, Glover C V. Cloning and disruption of CKB1, the gene encoding the 38-kDa beta subunit of Saccharomyces cerevisiae casein kinase II (CKII). Deletion of CKII regulatory subunits elicits a salt-sensitive phenotype. J Biol Chem. 1995;270:10395–10404. doi: 10.1074/jbc.270.18.10395. [DOI] [PubMed] [Google Scholar]
- 6.Chen-Wu J L, Padmanabha R, Glover C V. Isolation, sequencing, and disruption of the CKA1 gene encoding the alpha subunit of yeast casein kinase II. Mol Cell Biol. 1988;8:4981–4990. doi: 10.1128/mcb.8.11.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham K W, Fink G R. Calcineurin inhibits VCX1-dependent H+/Ca2+ exchange and induces Ca2+ ATPases in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Nadal E, Clotet J, Posas F, Serrano R, Gómez N, Ariño J. The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppzlp Ser/Thr protein phosphatase. Proc Natl Acad Sci USA. 1998;95:7357–7362. doi: 10.1073/pnas.95.13.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dichtl B, Stevens A, Tollervey D. Lithium toxicity in yeast is due to the inhibition of RNA processing enzymes. EMBO J. 1997;16:7184–7195. doi: 10.1093/emboj/16.23.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunn T, Gable K, Beeler T. Regulation of cellular calcium by yeast vacuoles. J Biol Chem. 1994;269:7273–7278. [PubMed] [Google Scholar]
- 11.Ferrando A, Kron S J, Rios G, Fink G R, Serrano R. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol Cell Biol. 1995;15:5470–5481. doi: 10.1128/mcb.15.10.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaber R F, Styles C A, Fink G R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988;8:2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garciadeblás B, Rubio F, Quintero F J, Bañuelos M A, Haro R, Rodríguez-Navarro A. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet. 1993;236:363–368. doi: 10.1007/BF00277134. [DOI] [PubMed] [Google Scholar]
- 14.Gaxiola R, de Larrinoa I F, Villalba J M, Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser H U, Thomas D, Gaxiola R, Montrichard F, Surdin-Kerjan Y, Serrano R. Salt tolerance and methionine biosynthesis in Saccharomyces cerevisiae involve a putative phosphatase gene. EMBO J. 1993;12:3105–3110. doi: 10.1002/j.1460-2075.1993.tb05979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glover C V. On the physiological role of casein kinase II in Saccharomyces cerevisiae. Prog Nucleic Acid Res Mol Biol. 1998;59:95–133. doi: 10.1016/s0079-6603(08)61030-2. [DOI] [PubMed] [Google Scholar]
- 17.Gómez M J, Luyten K, Ramos J. The capacity to transport potassium influences sodium tolerance in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1996;135:157–160. doi: 10.1111/j.1574-6968.1996.tb07982.x. [DOI] [PubMed] [Google Scholar]
- 18.Haro R, Garciadeblás B, Rodríguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991;291:189–191. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- 19.Haro R, Bañuelos M A, Quintero F J, Rubio F, Rodríguez-Navarro A. Genetic basis of sodium exclusion and sodium tolerance in yeast. A model for plants. Physiol Plant. 1993;89:868–874. [Google Scholar]
- 20.Ko C H, Gaber R F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1991;11:4266–4273. doi: 10.1128/mcb.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larsson K, Böhl F, Sjöström I, Akhtar N, Strand D, Mechler B M, Grabowski R, Adler L. The Saccharomyces cerevisiae SOP1 and SOP2 genes, which act in cation homeostasis, can be functionally substituted by the Drosophila lethal(2)giant larvae tumor suppressor gene. J Biol Chem. 1998;273:33610–33618. doi: 10.1074/jbc.273.50.33610. [DOI] [PubMed] [Google Scholar]
- 22.Madrid R, Gómez M J, Ramos J, Rodríguez-Navarro A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J Biol Chem. 1998;273:14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- 23.Márquez J A, Serrano R. Multiple transduction pathways regulate the sodium-extrusion gene PMR2/ENA1 during salt stress in yeast. FEBS Lett. 1996;382:89–92. doi: 10.1016/0014-5793(96)00157-3. [DOI] [PubMed] [Google Scholar]
- 24.Mendizabal I, Ríos G, Mulet J M, Serrano R, de Larrinoa I F. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998;425:323–328. doi: 10.1016/s0014-5793(98)00249-x. [DOI] [PubMed] [Google Scholar]
- 25.Mendoza I, Rubio F, Rodríguez-Navarro A, Pardo J M. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J Biol Chem. 1994;269:8792–8796. [PubMed] [Google Scholar]
- 26.Murguía J R, Belles J M, Serrano R. A salt-sensitive 3′(2′),5′-bisphosphate nucleotidase involved in sulfate activation. Science. 1995;267:232–234. doi: 10.1126/science.7809627. [DOI] [PubMed] [Google Scholar]
- 27.Murguía J R, Belles J M, Serrano R. The yeast HAL2 nucleotidase is an in vivo target of salt toxicity. J Biol Chem. 1996;271:29029–29033. doi: 10.1074/jbc.271.46.29029. [DOI] [PubMed] [Google Scholar]
- 28.Nakamura T, Liu Y, Hirata D, Namba H, Harada S, Hirokawa T, Miyakawa T. Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nass R, Cunningham K W, Rao R. Intracellular sequestration of sodium by a novel Na+/H exchanger in yeast is enhanced by mutations in the plasma membrane H+-ATPase. Insights into mechanisms of sodium tolerance. J Biol Chem. 1997;272:26145–26152. doi: 10.1074/jbc.272.42.26145. [DOI] [PubMed] [Google Scholar]
- 30.Nass R, Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J Biol Chem. 1998;273:21054–21060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabha R, Chen-Wu J L, Hanna D E, Glover C V. Isolation, sequencing, and disruption of the yeast CKA2 gene: casein kinase II is essential for viability in Saccharomyces cerevisiae. Mol Cell Biol. 1990;10:4089–4099. doi: 10.1128/mcb.10.8.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Posas F, Camps M, Ariño J. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J Biol Chem. 1995;270:13036–13041. doi: 10.1074/jbc.270.22.13036. [DOI] [PubMed] [Google Scholar]
- 33.Prior C, Potier S, Souciet J L, Sychrová H. Characterization of the NHA1 gene encoding a Na+/H+-antiporter of the yeast Saccharomyces cerevisiae. FEBS Lett. 1996;387:89–93. doi: 10.1016/0014-5793(96)00470-x. [DOI] [PubMed] [Google Scholar]
- 34.Prista C, Almagro A, Loureiro-Dias M C, Ramos J. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl Environ Microbiol. 1997;63:4005–4009. doi: 10.1128/aem.63.10.4005-4009.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ramos J, Contreras P, Rodríguez-Navarro A. A potassium transport mutant of Saccharomyces cerevisiae. Arch Microbiol. 1985;143:88–93. [Google Scholar]
- 36.Ramos J, Alijo R, Haro R, Rodríguez-Navarro A. TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae. J Bacteriol. 1994;176:249–252. doi: 10.1128/jb.176.1.249-252.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reed J C, Bidwai A P, Glover C V. Cloning and disruption of CKB2, the gene encoding the 32-kDa regulatory beta′-subunit of Saccharomyces cerevisiae casein kinase II. J Biol Chem. 1994;269:18192–18200. [PubMed] [Google Scholar]
- 38.Reynolds A, Lundblad V, Dorris D, Keaveney M. Yeast vectors and assays for expression of cloned genes. In: Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1997. pp. 13.6.1–13.6.6. [DOI] [PubMed] [Google Scholar]
- 39.Ríos G, Ferrando A, Serrano R. Mechanisms of salt tolerance conferred by overexpression of the HAL1 gene in Saccharomyces cerevisiae. Yeast. 1997;13:515–528. doi: 10.1002/(sici)1097-0061(199705)13:6<515::aid-yea102>3.3.co;2-o. [DOI] [PubMed] [Google Scholar]
- 40.Rudolph H K, Antebi A, Fink G R, Buckley C M, Dorman T E, LeVitre J, Davidow L S, Mao J I, Moir D T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 41.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 42.Serrano R. Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int Rev Cytol. 1996;165:1–52. doi: 10.1016/s0074-7696(08)62219-6. [DOI] [PubMed] [Google Scholar]
- 43.Sherman F, Fink G R, Hicks J B. Laboratory course manual for methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 44.Sychrová H, Ramírez J, Peña A. Involvement of Nha1 antiporter in regulation of intracellular pH in Saccharomyces cerevisiae. FEMS Microbiol Lett. 1999;171:167–172. doi: 10.1111/j.1574-6968.1999.tb13428.x. [DOI] [PubMed] [Google Scholar]
- 45.Tenney K A, Glover C V. Transcriptional regulation of the S. cerevisiae ENA1 gene by casein kinase II. Mol Cell Biochem. 1999;191:161–167. [PubMed] [Google Scholar]
- 46.Thomas D, Surdin-Kerjan Y. Metabolism of sulfur amino acids in S. cerevisiae. Microbiol Mol Biol Rev. 1997;61:503–532. doi: 10.1128/mmbr.61.4.503-532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland J, Nitsche A M, Strayle J, Steiner H, Rudolph H K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995;14:3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]