Abstract
Introduction:
Selective serotonin reuptake inhibitors (SSRIs) and, to a lesser extent, serotonin-norepinephrine reuptake inhibitors (SNRIs) are the cornerstone of pharmacotherapy for children and adolescents with anxiety and depressive disorders. These medications alleviate symptoms and restore function for many youths; however, they are associated with a distinct adverse effect profile, and their tolerability may complicate treatment or lead to discontinuation. Yet, SSRI/SNRI tolerability has received limited attention in the pediatric literature.
Methods:
This review examines the early- (e.g., activation, gastrointestinal symptoms, sedation) and late-emerging (e.g., weight gain) adverse effects of SSRIs and some SNRIs in pediatric patients.
Results:
We provide a framework for discussing SSRI/SNRI tolerability with patients and their families and describe the pharmacologic basis, course, and predictors of adverse events in youth. Strategies to address specific tolerability concerns are presented. For selected adverse events, using posterior simulation of mean differences over time, we describe their course based on Physical Symptom Checklist measures in a prospective, randomized trial of anxious youth aged 7–17 years who were treated with sertraline (n = 139) or placebo (n = 76) for 12 weeks in the Child/Adolescent Anxiety Multimodal Study (CAMS).
Main Results:
In CAMS, the relative severity/burden of total physical symptoms (p < 0.001), insomnia (p = 0.001), restlessness (p < 0.001), nausea (p = 0.002), abdominal pain (p < 0.001), and dry mouth (p = 0.024) decreased from baseline over 12 weeks of sertraline treatment, raising the possibility that these symptoms are transient. No significant changes were observed for sweating (p = 0.103), constipation (p = 0.241), or diarrhea (p = 0.489). Finally, we review the antidepressant withdrawal syndrome in children and adolescents and provide guidance for SSRI discontinuation, using pediatric pharmacokinetic models of escitalopram and sertraline—two of the most used SSRIs in youth.
Conclusion:
SSRI/SNRIs are associated with both early-emerging (often transient) and late-emerging adverse effects in youth. Pharmacokinetically-informed appraoches may address some adverse effects and inform SSRI/SNRI discontinuation strategies.
Keywords: activation; antidepressant; CAMS; selective serotonin reuptake inhibitor (SSRI, SRI); side effects
1 ∣. INTRODUCTION
Antidepressants, including selective serotonin reuptake inhibitors (SSRIs), are the first-line pharmacologic treatment for children and adolescents with depressive,1 anxiety,2 and obsessive–compulsive disorders—the most prevalent psychiatric disorders in youth.3,4 These medications—as monotherapy or combined with psychotherapy—decrease symptoms and improve functioning.5-7 However, some youth experience adverse effects during treatment with SSRIs and other antidepressant medications.8-11 Understanding antidepressant-associated adverse effects could substantially improve outcomes, while understanding the relationship between risk factors for adverse effects and pharmacokinetic and pharmacodynamic processes could help clinicians to predict adverse effects and use pharmacologically informed strategies to manage them.
Despite several examinations of antidepressant tolerability and discontinuation in youth,9,12-14 only two meta-analyses have examined the risks of specific adverse effects in children and adolescents.6,15 In our recent meta-analysis of SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) in youth, discontinuation due to adverse effects was similar to relative risk estimates derived from recent network meta-analyses that focused primarily on efficacy and compared multiple medication classes (e.g., SSRIs, SNRIs, tricyclic antidepressants [TCAs], benzodiazepines, and α2 agonists).5,7 Across disorders, SSRIs are more likely to produce activation, abdominal pain, sedation/drowsiness, and adverse effect-related discontinuation compared to placebo, and SSRI tolerability is similar in pediatric patients with obsessive compulsive disorder (OCD) and anxiety disorders as well as depressive disorders.15 Beyond these two meta-analyses, there is limited guidance and no recent synthesis of current data, despite clinicians being ethically and medicolegally compelled to discuss medication side effects/adverse effects with patients and their parents.
For discontinuation of antidepressant medications, few studies have examined wash-out, and those that have included discontinuation have not reported taper or discontinuation-related adverse effects. Moreover, most current studies that have examined discontinuation in children or adolescents either examined antidepressants with nonlinear kinetics and long half-lives (e.g., fluoxetine)16 or included only a single time point post discontinuation (e.g., duloxetine, escitalopram). By contrast, emerging data in adults with depressive and anxiety disorders suggest the potential benefit of slower or hyperbolic discontinuation.17,18
We provide a narrative review and describe the most common antidepressant-related adverse effects in youth.19 Then, using publicly available data from the largest trial of an SSRI in pediatric patients with anxiety disorders (CAMS),20 we examined the course of specific physical symptoms that overlap with symptoms that are frequently evaluated as side effects. Finally, we provide guidance for managing these adverse effects and discontinuing antidepressant medications in children and adolescents.
2 ∣. METHODS
2.1 ∣. Narrative review
For this unstructured, narrative review, searches were conducted in PubMed, PsychINFO, and the abstracts from the Annual Meeting of the American Academy of Child & Adolescent Psychiatry (AACAP) (2016–2022). Search results were compiled and reviewed to provide an overview of the current knowledge regarding antidepressant-related adverse effects and their management as well as discontinuation strategies. For the PubMed search (inception through October 18, 2022), we used the following search strategy (adolescent* OR children OR pediatric OR youth) AND (selective serotonin reuptake inhibitor OR SSRI OR selective serotonin norepinephrine reuptake inhibitor OR SNRI OR selective serotonin norepinephrine reuptake inhibitor OR fluoxetine OR fluvoxamine OR citalopram OR escitalopram OR fluoxetine OR paroxetine OR venlafaxine OR desvenlafaxine OR duloxetine OR vortioxetine OR vilazodone) AND (side effect* OR adverse event* OR tolerability OR taper* OR discontin*). The results of the search were then manually limited, and the references of selected studies and review articles were searched for additional references. The physician authors, both board-certified child and adolescent psychiatrists with a combined 30 years of clinical experience, also provided guidance on the management of side effects and discussed proposed side effect selection with other practicing child and adolescent psychiatrists with at least 5 years of clinical practice (n = 11), psychiatric nurse practitioners (n = 3), and board-certified clinical pharmacists (n = 3) (see acknowledgments).
2.2 ∣. Illustrative presentation of the temporal course of physical symptoms in a prospectively treated sample of SSRI-treated youth
Many symptoms that are frequently reported as side effects fluctuate contemporaneously with antidepressant treatment in pediatric patients. However, most trials report the presence or absence of these items (e.g., abdominal pain, diarrhea, and headache), which fails to capture their temporal stability/resolution. As an illustrative example and to provide a rare glimpse into the resolution/stability of items, which may be seen by clinicians as side effects, we used publicly available data from the largest trial of an SSRI in pediatric patients with anxiety disorders (CAMS).20
In CAMS, patients, aged 7–17 years, with generalized separation and/or social anxiety disorders (N = 488), were randomized 2:2:2:1 to cognitive behavioral therapy (CBT), sertraline, CBT + sertraline, or placebo and were treated for 12 weeks. The primary efficacy measures were improvement in the pediatric anxiety rating scale (PARS) and Clinical Global Impression-Severity and Clinical Global Impression-Improvement (CGI-I) scores, which were assessed at baseline and every 4 weeks by independent evaluators. To be eligible, patients had to have substantial impairment and an IQ ≥80. Participants could have comorbid psychiatric disorders of lesser severity than their primary anxiety disorder. Additionally, patients with attention deficit-hyperactivity disorder (ADHD) could receive stable doses of a stimulant; however, those who were receiving other psychoactive medications and who had psychiatric diagnoses that made participation in the study clinically inappropriate (i.e., current major depressive or substance-use disorder; unmedicated ADHD; or a lifetime history of bipolar, psychotic, or pervasive developmental disorders) or those deemed to be a risk to themselves or others were excluded. Additional exclusion criteria included unstable medical conditions, anxiety-related school refusal, inadequate response to two adequate SSRI trials, or an adequate trial of CBT.
In this sample of sertraline-treated youth (n = 139) who were assessed at baseline, week 4, week 8, and week 12 (or early termination), we examined specific physical symptoms extracted from the physical symptom checklist. With an uninformative prior (Uniform or conjugate Normal-Gamma with large prior variance), the posterior distribution of the mean at each time point is a Student-t (ST) distribution. The difference in means at each time point, for each physical symptom score (e.g., insomnia, nausea), which reflects frequency and severity from baseline, was therefore computed using Monte Carlo (MC) simulation draws from an ST distribution for the mean at each time point, , where = number of observations in period , = the sample mean symptom score in period , and with = standard deviation of the sample in period . The posterior for the difference in means was then computed by subtracting the baseline posterior MC sample from the posterior MC sample for each other time point. Mean differences, 95% credible intervals (CrI), and Bayesian p-values were computed from the simulation draws. All computations were carried out using Julia 1.7, and statistical significance was defined as a tail posterior probability (p-value) <0.05.
2.3 ∣. Pharmacokinetic modeling and antidepressant dosing simulation
Concentration-time curves were generated for an adolescent female (age 14 years) to illustrate both variation in exposure for sertraline and escitalopram—two SSRIs approved for pediatric use. Additionally, concentration–time curves were generated to visualize medication exposure during SSRI tapering. For pharmacokinetic models, pediatric volumes of distribution (VD), clearance, and bioavailability were extracted from previously published data for sertraline and escitalopram. Then, based on common sertraline and escitalopram dosing strategies, plasma SSRI concentrations were modeled with allometric scaling using MwPharm (MediWare BV, version 3.82) as:
For escitalopram models, parameters included metabolic clearance 33 L/h/70 kg, V1 17.5 L/kg lean body mass, and ka = 0.8 h−1. For sertraline models, parameters included clearance 200 L/h/70 kg, V1 110 L/kg lean body mass, and ka = 0.8 h−1.
3 ∣. SSRI-RELATED ADVERSE EFFECTS IN CHILDREN AND ADOLESCENTS
3.1 ∣. Time course of adverse effects
Clinical trials of antidepressants in youth rarely examine the time course of adverse effects. Yet, clinicians are aware that some side effects emerge early and resolve quickly (e.g., activation, gastrointestinal symptoms). In contrast, other side effects are late-emerging (e.g., weight gain) or persistent (e.g., sexual dysfunction). For acutely emerging side effects such as gastrointestinal symptoms, dynamic physiologic relationships may mitigate these effects. For example, nausea—which emerges early—may relate to acute increases in serotonergic tone, increasing gastrointestinal motility. The resolution of these effects may relate to desensitization of enteric serotonergic receptors. Discussing the temporal course of the side effects and distinguishing between persistent and transient side effects is critical. Ensuring that patients are aware not only of side effects but the tendency of some side effects to be transient is important and should be part of discussions with patients and their families. For many, knowing that a side effect is likely transient, as opposed to persistent, may significantly influence the patient and family's anxiety or fears related to medication.
3.2 ∣. Relationship between adverse effects and symptoms of the disorder being treated
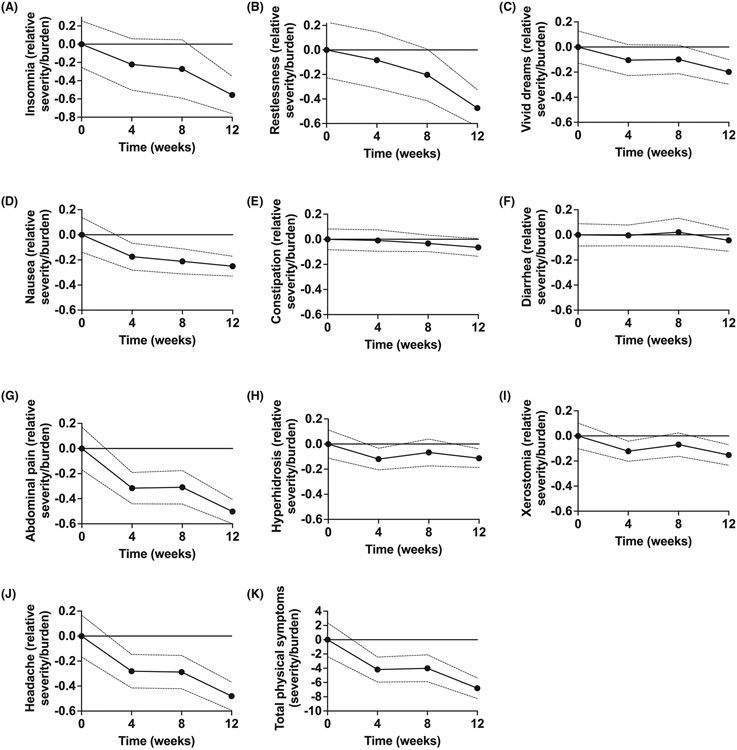
Given the overlap of potential adverse effects of pharmacotherapy and physical and somatic symptoms of depression or anxiety,21-23 which are associated with patient age, family characteristics, and endogenous factors, it is critical to assess symptoms before beginning treatment. Several tools are available to clinicians to assist with characterizing baseline symptoms, including the physical symptom checklist. This 46-item inventory assesses “how much you have been bothered by each condition during the PAST WEEK” by specific symptoms. Anchors range from “not at all” (0) to “very much” (3), and this instrument has been used in large prospective trials of SSRIs in children and adolescents.20,24 Consistent with the possibility that overall physical symptoms are related to the disorder being treated, in CAMS, we observed that total physical symptom scores decreased in youth who received sertraline (n = 139) but not in those who received placebo at week 4 (sertraline: p = 0.006; placebo: p = 0.686) and week 8 of the trial (sertraline: p = 0.011; placebo: p = 0.106) although, at week 12, total physical symptoms significantly improved (compared with baseline) in both groups (sertraline: p < 0.001; placebo: p = 0.001) (Figure 1). However, this finding does not preclude the possibility that some SSRI-related adverse effects emerge early and then disappear before the first assessment of physical symptoms (week 4).
FIGURE 1.
Physical Symptoms in SSRI-treated Children and Adolescents. Physical symptoms (A–J) generally decrease over time in sertraline-treated children and adolescents (n = 139) during a prospective, double-blind placebo-controlled trial. Similarly, total physical symptoms (K) significantly decrease over time in sertraline-treated youth. Points represent the change from baseline in mean burden score on the physical symptom checklist (PSC) at baseline, week 4, week 8, and week 12, and dotted lines represent the 95% credible interval (CrI).
3.3 ∣. Side effects and antidepressant pharmacokinetics
Many early-emerging adverse effects (e.g., activation) and some late-emerging adverse effects have been related to pharmacokinetic factors, and these are increasingly linked to variation in cytochrome activity. In considering these relationships in children and adolescents, clinicians will do well to recall that cytochrome (CYP) activity varies across development25 and that genetic variation in these enzymes significantly impacts exposure (area under the curve [AUC]), maximum concentration (), and half life (t½) for several commonly used SSRIs in youth.26 Further, in considering potential pharmacokinetically relevant adverse events, it is essential to consider interactions and phenoconversion. For example, treatment with a strong CYP2D6 inhibitor such as paroxetine or fluoxetine can reduce CYP2D6 activity to levels seen in poor metabolizers.
Additionally, drug–drug interactions, including with illicit substances such as cannabis or cannabidiol (CBD), are relevant to many adolescents, given the increasing use of these substances. Both CBD and tetrahydrocannabinol (THC) are moderate to strong inhibitors of cytochrome enzymes and interact with SSRIs and increase SSRI plasma concentrations. In a small study of es/citalopram-treated adolescents/young adults aged 17–24 years, CBD significantly increased citalopram plasma concentration27, and in pharmacokinetic models of adolescents treated with sertraline or escitalopram, CBD and THC increase sertraline and es/citalopram and . Finally, it is noteworthy that a recent examination of the Food and Drug Administration Adverse Event Reporting System database revealed that co-administration of CBD and CYP2C19-metabolized SSRIs increased the risk of some SSRI-related side effects (e.g., diarrhea, dizziness, and fatigue), which may relate to SSRI concentrations.28
3.4 ∣. Discussion of adverse events with parents and youth
Potential side effects should be discussed with patients and their families before beginning any medication. Importantly, these discussions vary considerably among clinicians.29 Some present all side effects, whereas others discuss the most common or serious potential side effects. Some clinicians may discuss the probability of side effects in addition to their temporal course (see above). Still other clinicians and package inserts provided with medications present the rates of side effects across trials.
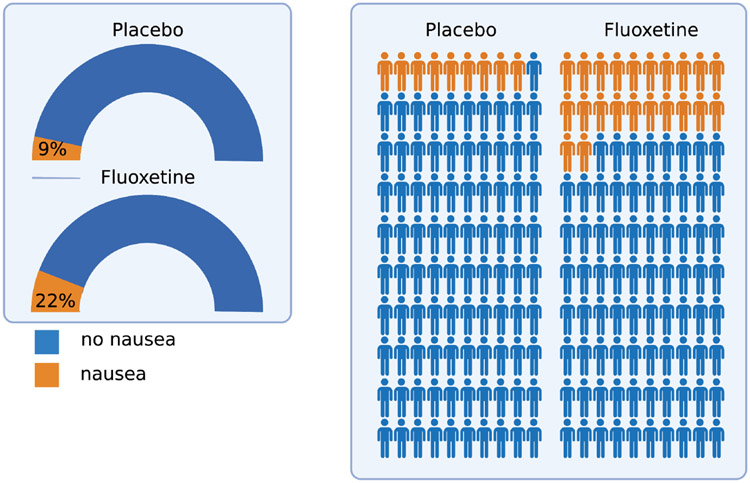
How risk is presented—whether absolute risk, expected frequencies, odds, or odds ratios—may confuse patients and their families.30 Importantly, the multiple ways results can be reported contribute to this problem. For example, consider a trial in which 6% of youth receiving placebo reported headaches compared with 7% of those receiving medication. There is an absolute difference of 1% (or 1 out of 100), but the relative risk is 1.18 (or an 18% increase). Presenting this “risk” as a 20% increase in risk associated with antidepressant-treated youth potentially misrepresents both an overall low risk and an infrequent event. Currently, in package inserts, reactions are classified as frequent (>1/100), infrequent (between 1/100 and 1/1000), and rare (≤1/1000). In reviewing the package insert for fluoxetine, 22% of patients who received fluoxetine reported nausea (631 of 2869 patients) compared with 9% of those who received placebo (151 of 1673). This represents a 133% larger incidence of nausea in patients who received fluoxetine compared with those who received placebo, a 22% incidence or an additional 13 out of 100 patients experiencing nausea with fluoxetine compared to placebo. The odds of this side effect (2.4:1) is also misleading. We would argue that context is missing for this generally transient and relatively mild side effect. Finally, visual approaches to representing these data may be more helpful, including dials, gauges, and personographs (Figure 2).
FIGURE 2.
Alternative Approaches to Visualizing Adverse Effects. In contrast to presenting relative rates, odds ratio, or bar graphs, adverse effects can be visually presented relative to the population of antidepressant-treated patients and the comparison group to provide more context. Modified gauges (left) and a pictogram (right) show the incidence of nausea (based on rates in the package insert) for fluoxetine-treated patients across registration trials. Figure created using BioRender.com
4 ∣. EARLY-EMERGING SIDE EFFECTS
4.1 ∣. Gastrointestinal symptoms
Gastrointestinal symptoms may be associated with SSRI treatment in pediatric patients,13 but are also influenced by the presence and severity of the underlying condition being treated.21 Yet gastrointestinal symptoms generally improve with treatment, whether psychopharmacologic or psychotherapeutic.21 Mechanistically, SSRI-associated gastrointestinal symptoms are related to central and peripheral actions (e.g., direct action at serotonin receptors in the gut that modulate gastrointestinal motility). However, assessments of these symptoms in the literature are complicated by significant heterogeneity in the reporting and categorizing of gastrointestinal symptoms (e.g., nausea vs. dyspepsia vs. nausea+abdominal pain vs. abdominal pain). A recent meta-analysis of SSRI-treated pediatric patients with anxiety disorders and OCD found that SSRIs (compared with placebo) were associated with greater abdominal pain (p < 0.001). In contrast, SNRIs only differed from placebo in terms of the likelihood of nausea (p < 0.001).15 In general, similar rates of nausea, abdominal pain, and other gastrointestinal symptoms are reported in trials of SSRIs in depressive, anxiety, and obsessive–compulsive disorders.15
The temporal progression of gastrointestinal symptoms in pediatric SSRI trials has not been systematically assessed, although ongoing trials are evaluating this. However, in the largest trial of an SSRI in youth with anxiety disorders, CAMS,20 the frequency/severity of increased abdominal pain, as assessed by the Physical Symptom Checklist, significantly decreased by week 4 in SSRI-treated youth (p = 0.004) and remained significantly improved at weeks 8 and 12 (p = 0.006 and p < 0.001, respectively) (Figure 1). Abdominal pain also improved in patients receiving placebo; though this difference did not emerge until week 8 (p = 0.027). For nausea, SSRI-treated patients had improvement at week 8 and 12 (p = 0.016 and p = 0.002, respectively), although at week 4, the improvement failed to reach the 5% significance threshold (p = 0.053). In contrast, in patients who received placebo, nausea was unchanged from baseline at weeks 4, 8, and 12 (p = 0.332, p = 0.187, and p = 0.209, respectively) (Figure 1). Of note, this potentially differs from the package insert data presented for other SSRIs (Figure 2), which are based on counts rather than repeated measures over time. The frequency and severity of bloating did not change over time in patients who received sertraline (p = 0.863, p = 0.461, p = 0.233 at weeks 4, 8, and 12) or placebo (p = 0.575, p = 0.782, p = 0.569 at weeks 4, 8, and 12). Diarrhea severity/frequency did not significantly change in sertraline-treated youth (p = 0.944, p = 0.776, p = 0.489 at weeks 4, 8, and 12); diarrhea severity/frequency also did not change significantly over time in youth who received placebo except for the average severity rating at week 8 (p = 0.813, p = 0.012, p = 0.090 at weeks 4, 8, and 12). Finally, constipation did not significantly change in patients who received sertraline (p = 0.872, p = 0.546, p = 0.241 at weeks 4, 8, and 12) or placebo (p = 0.897, p = 0.335, p = 0.638 at weeks 4, 8, and 12) (Figure 1).
4.2 ∣. Insomnia in SSRI-treated youth
In randomized, controlled trials of youth with anxiety, depressive, and obsessive–compulsive disorders, insomnia occurs in between 8% and 19% and pediatric patients; however, baseline rates of insomnia are often inconsistently reported in these trials, and types of insomnia (e.g., initial, middle, and terminal insomnia) may vary based on the disorder being treated. In a recent meta-analysis of youth with anxiety and related disorders, SSRIs were associated with treatment-emergent insomnia. In our follow-up meta-analysis, which examined the risk of insomnia for specific SSRIs in youth with anxiety and obsessive–compulsive disorders, sertraline was associated with twice the likelihood of insomnia compared with placebo (Relative Risk: 1.94). Still, the other SSRIs did not differ from placebo. In CAMS, the frequency/severity of insomnia, as assessed by the Physical Symptom Checklist, significantly decreased in SSRI-treated patients by week 12 (p = 0.001) but did not differ from baseline at week 4 (p = 0.256) or week 8 (p = 0.197) (Figure 1). In contrast, placebo did not affect insomnia frequency/severity.
SSRI-related insomnia may relate to non-serotonergic effects, given that SSRIs differ in their effects on other monoamines. For example, more dopaminergic SSRIs may be associated with more insomnia in adults. Also, consistent with this possibility, sleep disturbances increase from SSRIs to SNRIs to tricyclic antidepressants (TCAs) to monoamine oxidase inhibitors (MAOIs), which parallels the decreasing specificity of these agents in terms of monoaminergic effects.
Insomnia is transient for many patients and, when persistent, is best managed through one of several behavioral or psychopharmacologic approaches. For youth with treatment-emergent insomnia, a first intervention is timing the SSRI administration so that the maximum concentration () occurs during the day rather than in the evening. In other words, ensuring that the dosing of the SSRI is in the morning rather than in the afternoon or evening can be helpful for many patients. Additionally, SSRI-related insomnia has been associated with daytime fatigue/tiredness in children and adolescents.15 This underscores the shared etiology of these two “sleep-related” adverse effects and may provide evidence against their being etiologically separate adverse effects.
After ensuring the appropriate SSRI dosing and administration timing (e.g., morning), healthy sleep practices are the first-line behavioral treatment. They may be combined with cognitive behavioral therapy for insomnia (CBT-I).31 Further, exclude other primary causes of dyssomnia (e.g., obstructive sleep apnea, iron deficiency anemia) before pursuing pharmacotherapy because pharmacotherapy could mask an underlying disorder.31 Regarding the relatively limited evidence for psychopharmacologic interventions for sleep difficulties in children, a large survey of child and adolescent psychiatrists suggested that medications were considered for one in four youth with insomnia. Among these interventions are melatonin, antihistamines, zolpidem, zaleplon, and trazodone.31 However, in SSRI-treated youth, trazodone may be particularly problematic. In the Treatment of SSRI-Resistant Depression in Adolescents (TORDIA) study,32 when trazodone was combined with antidepressants that inhibit CYP2D6 (e.g., fluoxetine, paroxetine), none of the trazodone-treated patients improved with regard to depressive symptoms.33 This finding—which has been replicated in a separate cohort of depressed adolescents34—may relate to CYP2D6 interactions and the accumulation of methyl-chloro-piperazine. This trazodone metabolite is associated with dysphoria, irritability, and depression.
4.3 ∣. Tiredness, fatigue, and sedation
Tiredness, fatigue, and sedation overlap considerably, and their prevalence varies across prospective trials of SSRIs in youth.35-38 Further, like insomnia, treatment-emergent drowsiness, fatigue, and sedation overlap with core symptoms of depressive and anxiety disorders, making their association with treatment difficult to discern. Meta-analyses of SSRIs in youth with anxiety and obsessive–compulsive disorders suggested that fluoxetine and fluvoxamine are associated with more treatment-emergent sedation compared with placebo.39
SSRI-related tiredness may relate to medication-specific receptor pharmacology, although this has been difficult to assess in pediatric trials of these medications. For example, paroxetine and citalopram (and potentially escitalopram at high doses) are antihistaminergic, which may contribute to greater treatment-emergent tiredness and sedation in some trials of adults; however, this is confounded by general tolerability concerns for paroxetine in pediatric patients and the relative scarcity of prospective pediatric trials of citalopram. Like other potential SSRI-related adverse effects, tiredness, fatigue, and sedation syndromically overlap with core symptoms of anxiety and depressive disorders. As such, these symptoms should be systematically assessed before beginning pharmacotherapy.
To address SSRI-related tiredness, fatigue, or sedations, clinicians may leverage medication-specific pharmacokinetics. For most SSRIs, absorption is rapid, and occurs shortly following administration. Clinicians may change to evening administration for youth with significant treatment-emergent tiredness, fatigue, and sedation; however, this strategy should incorporate differences in absorption. For example, suppose a child is experiencing escitalopram-related tiredness. In that case, the administration might be optimal in the early evening to avoid daytime somnolence after morning administration.
For a child experiencing sertraline- or escitalopram-related fatigue and sedation, dosing might be at dinner, given that the time to maximum concentration () for the two medications is 4 h and nearly 8 h, respectively, for the two medications. Also, taking the medication without food (sine cibum) will hasten absorption and thus change and although these effects are less pronounced with most SSRIs. If these strategies are ineffective and there are no significant concerns related to sleep hygiene, clinicians could consider changing to an SSRI with a lower risk of treatment-emergent tiredness, fatigue, and sedation (e.g., fluoxetine).
4.4 ∣. Activation in SSRI-treated youth
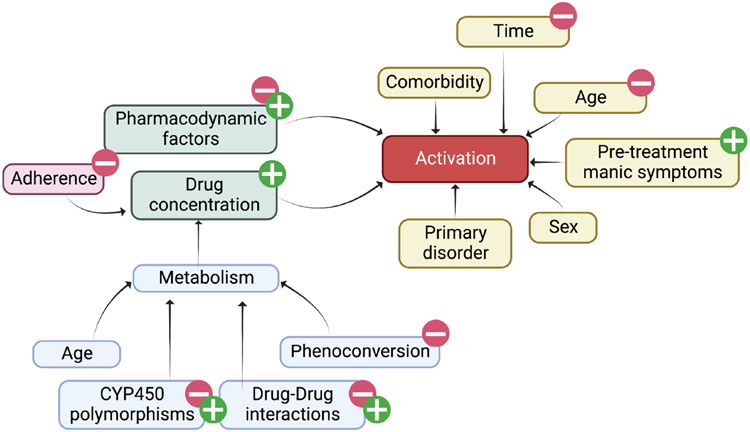
First described in the 1990s and early 2000s,12 SSRI-related activation represents a constellation of symptoms consisting of hyperactivity, irritability, insomnia, disinhibition, restlessness, hyperactivity,14 and anxiety. In adults, this syndrome—termed “antidepressant-induced jitteriness/anxiety syndrome”—has been described in adults (see Sinclair et al. (2009) for a systematic review of this syndrome in antidepressant-treated adults).40 Importantly, these symptoms overlap with one another and with core symptoms of the disorders that are the focus of SSRI treatment. Activation emerges early in treatment or following dose titration and generally resolves with dose reduction or discontinuation of the medication.14,41 Also consistent with a pharmacokinetic component to risk for SSRI-related activation, the resolution is related to the onset and may be longer with SSRIs that have longer half-lives, such as fluoxetine.41 To date, several studies have examined activation-related AEs in SSRI-treated youth. In the first report of activation in a prospective, double-blind, placebo-controlled study of children and adolescents, fluvoxamine was associated with activation in 10 of 22 participants (45%) compared with 1 of 23 (4%) patients who received placebo.9 In this study, most activation-related adverse events occurred by the fourth week of treatment, and patients with greater fluvoxamine concentrations—not dose—had higher activation rates. Though pharmacogenetic testing was not included in this study, higher fluvoxamine concentrations have been associated with CYP2D6 poor metabolizer status,42 and the Clinical Pharmacogenetics Implementation Consortium recommends reduced fluvoxamine doses in CYP2D6 poor metabolizers.43 In a second study, fluoxetine exposure was indirectly associated with greater activation.44 In a third study, in adolescents with generalized anxiety disorder treated with escitalopram, activation was associated with greater escitalopram (p = 0.04) and in patients for whom pharmacokinetic sampling was completed.24 A fourth study, a large retrospective study of pediatric patients treated with escitalopram/citalopram, revealed that activation-related symptoms were more likely in slower CYP2C19 metabolizers.45 Importantly, the role of pharmacodynamic genes in the development of activation is unclear, although it is likely that pharmacodynamic factors interact with pharmacokinetic factors to produce activation (Figure 3). Finally, with regard to the CAMS trial, there is no “activation” item on the Physical Symptom Checklist, and activation was examined separately.13 The “restlessness” and “agitation” items significantly improved by week 12 in both sertraline-treated patients (p < 0.001) and in those who received placebo (p = 0.001). However, compared with baseline, no significant changes were observed at weeks 4 or 8.
FIGURE 3.
Functional Causal Diagram of Activation in Selective Serotonin Reuptake Inhibitor-Treated Youth. Activation, a constellation of symptoms including insomnia, restlessness, irritability, and mild impulsivity, (red) is influenced by both pharmacologic (green), and clinical (beige) factors as well as adherence. Additional factors that influence medication metabolism are shown in blue. Figure created using BioRender.com
In general, management of SSRI-related activation involves dose reduction and a slower titration. Interestingly, one study examined “slow” and “standard” sertraline titration in children and adolescents with OCD, and there were no differences in activation rates between the two groups, but the study was unable to adjust for differences in metabolism and weight that are now known to produce differences in medication exposure. Currently, when patients experience activation at higher doses of an SSRI, there is evidence41 that decreasing the total daily dose or stopping the medication can effectively reduce activation. Also, because activation may directly relate to higher blood concentrations, switching to an extended-release formulation may help reduce the peak concentration of the medication (i.e., ). If pharmacogenetic testing is available, consider slower titration and lower target doses to avoid activation in poor metabolizers of the medication (e.g., CYP2C19 poor metabolizers for escitalopram and CYP2D6 poor metabolizers for fluvoxamine).
5 ∣. PERSISTENT OR LATE-EMERGING ADVERSE EFFECTS
5.1 ∣. Antidepressant-related hyperhidrosis
Increased sweating has been evaluated meta-analytically in adults (N = 28,544) treated with “second generation antidepressants,” and both SNRIs and SSRIs have similar risk ratios compared with placebo (SNRI risk ratio: 3.17, 95% confidence interval (CI): 2.63–3.82; SSRI risk ratio: 2.93, 95% CI: 2.46–3.47).46 However, in this meta-analysis, all antidepressants, except fluvoxamine, bupropion, and vortioxetine, were significantly associated with hyperhidrosis, and the risk was related to the affinity for the dopamine transporter. To date, similar meta-analytic evaluations have not been completed in SSRI-treated pediatric patients, although hyperhidrosis occurs in SSRI-treated children and adolescents. In the largest trial of SSRIs in pediatric patients with anxiety disorders, CAMS, the frequency/severity of increased sweating assessed by the Physical Symptom Checklist significantly decreased in SSRI-treated patients. By week 4, sweating did not significantly change from baseline in either sertraline-treated youth (p = 0.099, p = 0.400, p = 0.103 at weeks 4, 8, and 12) or those who received placebo (p = 0.327, p = 0.113, p = 0.078 at weeks 4, 8, and 12) (Figure 1).
Management of SSRI-related hyperhidrosis in children and adolescents is primarily based on an adaptation of strategies that have been successful in adults.46 In general, strategies have included using low-dose glycopyrrolate to attenuate cholinergic effects. Glycopyrrolate is available in oral formulations, including an orally dissolvable tablet. Importantly, unlike other anticholinergics, glycopyrrolate has a limited ability to cross the blood–brain barrier, which may produce fewer unwanted central nervous system (CNS) anticholinergic effects compared with other anticholinergics that are commonly used to address psychotropic adverse effects in children and adolescents. However, glycopyrrolate has relatively poor bioavailability in youth47 and may be associated with constipation, nasal congestion, and urinary retention. Topical agents have been used in pediatric populations with primary hyperhidrosis and include glycopyrronium tosylate (2.4%), a topical anticholinergic approved in the United States for primary axillary hyperhidrosis in patients ≥9 years.48 Additionally, in the case of antidepressant-related palmar-plantar hyperhidrosis, clinicians may consider aluminum salts (e.g., 12.5% aluminum chloride hexahydrate or 20% aluminum zirconium salts), which precipitate ions that obstruct eccrine ducts and in doing so block the movement of sweat to the skin surface.49 Finally, while oral oxybutynin may be used for the treatment of primary hyperhidrosis in youth, we do not recommend it given its tertiary structure, which allows it to penetrate the CNS more easily and produce neurocognitive adverse effects.
5.2 ∣. Antidepressant-related Headaches
Headaches are commonly reported in antidepressant-treated youth; however, headaches and headache syndromes are also more common in pediatric patients with depressive and anxiety disorders.21 Headaches associated with SSRIs and SNRIs may relate to myriad pre- and post-synaptic serotonergic effects. It is noteworthy that the triptans—5-HT1B/1D agonists and TCAs are often used as abortifacients for headaches and prophylaxis, respectively. In one meta-analysis of headaches in antidepressant-treated adults, SSRIs were not associated with headaches. However, among individual SSRIs, this effect was nearly significant (Relative risk: 1.06, CI: 1.00 to 1.13), and the effect was significant for escitalopram.50 Additionally, neither SNRIs (Relative risk: 0.97, CI: 0.88 to 1.06) nor second-generation antidepressants (e.g., bupropion, vilazodone, vortioxetine) were associated with a significant increase in headaches over the course of the trial. However, bupropion was associated with more headaches compared with placebo. In this analysis, there was no significant difference in the relative risk of headache with second-generation antidepressants and no difference based on whether the trials were conducted in depressed or anxious adults. In meta-analyses of antidepressants in pediatric patients with anxiety and OCD, headaches do not occur more commonly than placebo.15 In a meta-analysis of side effects in children and adolescents treated with antidepressants, headaches were not associated with SSRI or SNRI treatment, and the risk was not different between the two.15 Finally, in the largest trial of SSRIs in pediatric patients with anxiety disorders, CAMS, headache frequency/severity, as assessed by the Physical Symptom Checklist, significantly decreased in SSRI-treated patients. By week 4, headaches were improved compared with baseline (mean difference: −0.28, CrI: −0.415 to −0.147, p = 0.011) and remained decreased relative to baseline (12-week mean difference: −0.48, CrI: −0.593 to −0.368, p < 0.001, Figure 1) whereas placebo did not affect headaches (12-week mean difference from baseline: −0.17, CrI: −0.366 to 0.033, p = 0.254). This effect did not differ between males and females (p = 0.249).
The limited data related to headaches in children and adolescents treated with antidepressants illustrate the importance of context for interpreting side effects. Headaches may improve over the course of active treatment, but not placebo. However, most trials and United States Food and Drug Administration (FDA)-required registration trials require reporting the emergence of side effects—including headaches—at any point during the trial. Standard approaches to examining headaches in antidepressant-treated youth underscore the importance of assessing the baseline frequency and severity of these headaches, which is uncommon in clinical trials.
5.3 ∣. Vivid dreams
Vivid dreams have been reported with multiple antidepressants, a phenomenon that may relate to disruption in REM sleep. TCAs and MAOIs generally increase total sleep time and decrease wake time after an individual falls asleep; however, SSRIs have the opposite effect as a class.51 In young, healthy adults who recorded dreams over 30 days, treatment with SSRIs decreased REM sleep and increased the intensity of dreaming experience upon initiation and during discontinuation. Dream reports during SSRI treatment contained more visual terms, which increased during discontinuation, potentially reflecting “cholinergic rebound from serotonergic suppression [during treatment].”52 Finally, in CAMS, vivid dreams significantly decreased in SSRI-treated patients. By week 12, vivid dreams/nightmares were improved compared to baseline (mean difference: −0.20, CrI: −0.296 to −0.101, p = 0.016) but were similar to baseline at week 4 (p = 0.250) and week 8 (p = 0.258). In youth who received placebo, the frequency/severity of vivid dreams/nightmares was similar to baseline (p = 0.492, p = 0.079, p = 0.158 at weeks 4, 8, and 12) (Figure 1).
5.4 ∣. SSRI-related xerostomia
Dry mouth has received less attention than any other potential SSRI or SNRI-related side effect. Rates of xerostomia are higher in patients treated with TCAs compared with SSRIs, and in one study of antidepressant-treated adults, TCAs reduced salivary flow by nearly 60%, whereas SSRIs reduce salivary flow by approximately 30%.53 Proposed treatments for dry mouth, regardless of pharmacologic etiology, include lozenges, sprays, mouth rinses, gels, oils, and chewing gum, which broadly fall into two categories: saliva stimulants and saliva substitutes. Recent systematic reviews for the treatment of xerostomia have failed to identify any topical therapies as effective for symptomatic relief, although oxygenated glycerol triester (OGT) saliva substitute spray is more effective than an aqueous electrolyte spray, and chewing gums appear to increase saliva production in those with residual secretory capacity and may be preferred by patients, but there is no evidence that gum is better or worse than saliva substitutes.53 Saliva substitutes have been used in pediatric patients with multiple disorders and are a reasonable first-line intervention. In CAMS, xerostomia significantly decreased in SSRI-treated patients and those who received placebo at week 12 (p = 0.024 for sertraline and p = 0.022 for placebo). However, xerostomia did not differ from baseline in sertraline-treated patients or those who received placebo at weeks 4 or 8 (ps at both time points≥0.068 for sertraline and p≥0.091 for placebo) (Figure 1). The observation that xerostomia decreased in both groups is interesting; however, the failure to observe an effect may have been related to lower rates of xerostomia being present in this sample or to the etiologic heterogeneity of xerostomia (e.g., anxiety-related, medication-related) as well as the significant subjectivity of sensations of dry mouth.
5.5 ∣. Considerations related to suicidal thinking and behavior in antidepressant-treated youth
In 2004, a boxed warning was added to all “antidepressants” warning of suicidal thinking and behavior in children, adolescents, and adults. This significantly decreased the prescription of antidepressants to children, adolescents, and young adults54 and produced considerable trepidation among clinicians.29 However, the findings from this initial fixed-effects model have not been replicated in most6,7,15,55 meta-analyses or recent prospective controlled trials.20,24,56,57 That said, accumulating data suggest that multiple factors influence the likelihood of treatment-emergent suicidality, including specific medication (e.g., venlafaxine, paroxetine),7,58,59 baseline suicidality, clinical factors,58 and the type of disorder being treated.55 Further, assessing suicidal thinking in these short-term studies is difficult given that some high-risk groups (e.g., prior history or recent history of suicide attempt, substance use disorders) are often excluded from pediatric clinical trials of antidepressant medications.
Recent well-designed cohort studies have provided new data related to suicidality. One large population-based cohort study (N = 538,577) of individuals—including children, adolescents, and young adults—who began SSRIs, linked multiple Swedish national registers using unique personal identifiers to examine suicidal behavior in the 365 days prior to initiating an SSRI and in the 365 days following SSRI initiation.60 This analysis also stratified by age and included a 6–17-year-old cohort and an 18–24-year-old cohort. The risk of suicidal behavior was highest in the month prior to initiating SSRIs and then decreased following initiation of SSRIs.60 This finding, in a very large cohort with repeated assessments, underscores the possibility raised in other naturalistic studies that suicidality is confounded by the disorder being treated and by comorbidity, which complicates causal linking. In the CAMS sample, suicidality was assessed separately from the physical symptom checklist (PSC), although no significant associations were identified.13
5.6 ∣. Antidepressant-associated weight gain
Data concerning weight gain in SSRI-treated youth have produced mixed results. This may be related to changes in appetite associated with the underlying disorder being treated. Additionally, these effects are difficult to detect given age- and sex-related developmental shifts in weight and growth trajectory and the short-term nature of most clinical trials. However, several studies of SNRIs suggest that in adolescents, SNRIs produce weight loss early in treatment,57,61 although this abates over the course of longer-term treatment. For SSRI-treated youth, weight gain can emerge early or late and may persist. The two SSRIs associated with the most weight gain in prospective studies of youth with anxiety and depressive disorders are paroxetine and citalopram.62 Additionally, for escitalopram and citalopram, weight gain may be associated with CYP2C19 metabolism in pediatric patients.45 In one prospective study of adolescents (and young adults) that examined SSRI-related weight gain over more than a year of follow-up, SSRI treatment and dose were associated with increases in body mass index (BMI), fat mass index, and changes in lean BMI z-scores. Also, in this cohort, increased body composition measures were greatest in those treated with citalopram and escitalopram, and smaller effects were observed for fluoxetine. In contrast, sertraline was not associated with significant changes in body composition measures.62
The mechanism for SSRI-related weight gain is unclear. However, the past half-century of experience with multiple classes of antidepressants suggests that TCAs and MAOIs are most likely to cause weight gain, whereas as classes, the SNRIs and SSRIs are less likely to cause weight gain. Within the specific classes, data in adults suggest that this may be related to antihistamine effects63 and 5-HT2C receptor activity.64
5.7 ∣. Antidepressant-related sexual dysfunction
Sexual side effects are common in antidepressant-treated adults and contribute to medication discontinuation and nonadherence. These sexual side effects (e.g., decreased libido, genital anesthesia, erectile dysfunction, delayed ejaculation, loss of lubrication, and anorgasmia) degrade quality of life and compound the decreased sexual functioning related to depression and anxiety. However, very little attention has been paid to these side effects in youth65,66 and, similarly, little attention has been paid to sexual function in adolescents with depressive and anxiety disorders.67 In one 2 years, prospective study of antidepressant-treated adolescents aged 15–20 years (N = 263) who were assessed within 1 month of beginning an SSRI, depression severity, but not anxiety severity, was associated with worse sexual functioning (arousal, orgasm, and pleasure). Additionally, lower total scores {β = −0.13, p ≤ 0.001) and lower arousal, orgasm, and pleasure subscale scores (all β = −0.03, p ≤ 0.003) using the Changes in Sexual Functioning Questionnaire (CSFQ) were noted. Additionally, in this sample, higher SSRI doses were associated with more anorgasmia.67
Sexual side effects may relate to antidepressant-specific pharmacology (e.g., anticholinergic effects and nitric oxide synthase inhibition), and SSRI-related sexual dysfunction persists in up to 80% of adults. Dose reduction can help some patients treated with very high doses; however, dose reductions of up to 50% are needed for some adults to improve sexual dysfunction. This approach to managing sexual dysfunction in adolescents would be consistent with prospective studies of adolescents, which have found greater anorgasmia in adolescents treated with higher doses of SSRIs. Yet, unless they are poor metabolizers or concomitantly prescribed inhibiting medications, reducing the dose by this much puts patients at risk for subtherapeutic antidepressant exposure, and sexual side effects could persist. Other strategies include switching antidepressants and adjunctive antidepressants (with a lower risk for sexual dysfunction); however, the risk of sexual dysfunction is generally inferred from studies of adults, given the relatively limited data in the pediatric population.67
6 ∣. ANTIDEPRESSANT WITHDRAWAL SYNDROME AND ANTIDEPRESSANT DISCONTINUATION
Withdrawal symptoms have been described with SSRIs and SNRIs in children, adolescents, and adults over the past several decades and generally emerge when antidepressants are discontinued abruptly, although withdrawal symptoms can occur with missed doses and, in some patients, following significant dose reductions. These symptoms can include gastrointestinal and flu-like symptoms, dysesthesias, dyssomnia, increasing anxiety, agitation, or irritability and must be distinguished from recrudescence of symptoms associated with the disorder being treated.68,69 Antidepressant withdrawal has been evaluated from both pharmacokinetic and pharmacodynamic perspectives. In general, pharmacodynamic aspects of antidepressant withdrawal have focused on the 5-HT transporter, SERT.70 However, withdrawal may relate to pharmacokinetic factors; withdrawal symptoms are more common with antidepressants with shorter half-lives (e.g., paroxetine) than antidepressants with longer half-lives (e.g., fluoxetine).71,72 Further, in one 4 weeks study of adults treated with fluoxetine, sertraline, or paroxetine, double-blind placebo-controlled discontinuation for 5–8 days resulted in significantly fewer “discontinuation-emergent events than either sertraline-treated or paroxetine-treated patients (p < 0.001).”72 Given that the half-life may be 50% shorter for ultrarapid metabolizers than normal metabolizers,73 withdrawal symptoms may be more common in ultrarapid metabolizers. Finally, although antidepressant withdrawal symptoms relate to pharmacokinetic and pharmacodynamic factors, other processes have been implicated in both adult studies and in preclinical studies and include glutamatergic effects, altered binding at glutamatergic receptors, increases in synaptic GABA, changes in the density of post-synaptic NMDA receptors, and BDNF expression.
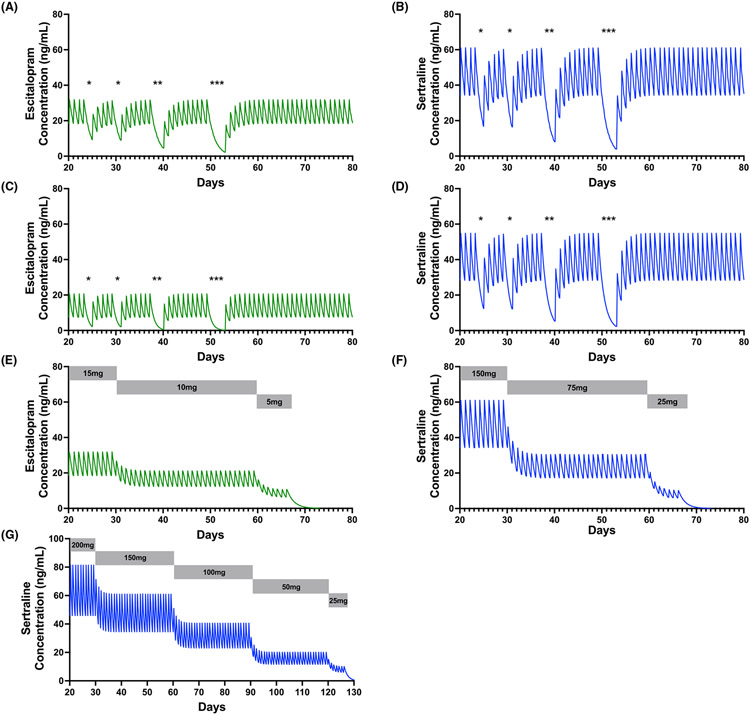
The general approach to managing antidepressant withdrawal is ensuring (i) adherence and (ii) slow discontinuation when stopping an antidepressant is necessary.74 Regarding adherence, pharmacokinetic modeling studies suggest significant decreases in serum concentrations of several antidepressants used in children and adolescents (e.g., sertraline and escitalopram), even following one or two missed doses.26 These effects may be accentuated in youth with increased CYP2C19 metabolism. For example, in a 14-year-old adolescent normal (Figure 4A) or ultrarapid (Figure 4C) metabolizer, missing two or more consecutive doses of escitalopram significantly decreases AUC and decreases CMIN to nearly 0. Similar effects can be seen when the adolescent is treated with sertraline 150 mg daily (Figure 4B,D). Slow discontinuation has been consistently recommended; however, few studies have evaluated slow versus rapid discontinuation of antidepressants, and a recent systematic review could not provide guidance on rates of discontinuation on the emergence of withdrawal symptoms in adults.17 Further, significant pharmacologic heterogeneity often complicates these studies. As an example, one prospective study of slow (2 weeks) versus rapid (3 days) discontinuation of adults treated with multiple SSRIs and venlafaxine did not find differences in the two discontinuation approaches; however, patients treated with short half-life antidepressants had significantly greater increases in discontinuation and depressive symptoms than those stopping fluoxetine. A similar study in adults also observed significantly more withdrawal symptoms in paroxetine compared with fluoxetine-treated patients during a double-blind, placebo-controlled taper over 5–8days.72 Taken together, the extant prospective studies in adults suggest that knowledge of SSRI pharmacology and the pharmacokinetics of the antidepressant needs to be considered when considering the length of the taper.
FIGURE 4.
Plasma selective serotonin reuptake inhibitor concentrations with missed doses and during hyperbolic discontinuation. The effect of missed doses (*) can be seen in an adolescent treated with 15 mg daily of escitalopram (A) and 150 mg daily of sertraline (B) who is a normal metabolizer for CYP2C19. However, these effects are amplified in the same adolescent treated with escitalopram 15 mg daily (C) or sertraline 150 mg daily (D), if she is an ultrarapid CYP2C19 metabolizer. Hyperbolic discontinuation of escitalopram (E) and sertraline (F and G) is shown with dosing shown in gray bars. Pharmacokinetic models were developed for a 14-year-old female weighing 52 kg with a height of 162 cm. Green curves represent escitalopram, and blue curves represent sertraline
Recently, hyperbolic discontinuation has been proposed as an alternative to more rapid discontinuations.18 This approach leverages the hyperbolic relationship between SSRI concentration and receptor occupancy. These dose reductions, which occur as a fixed percentage, produce exponential decreases in total dose as opposed to a linear reduction.18 This approach is based mainly on PET imaging data of serotonin transporter occupancy by SSRIs in adults and supports the notion that hyperbolically reducing SSRI doses decreases serotonin transporter inhibition linearly. This approach also recommends tapering more slowly, which is frequently done in the discontinuation components of most clinical trials, and drops to doses lower than typical “therapeutic minimums.”18 The ANTidepressants to prevent reLapse in dEpRession (ANTLER) trial75 examined discontinuation in adults with major depressive disorder who were treated with either citalopram 20 mg, sertraline 100 mg, fluoxetine 20 mg, or mirtazapine 30 mg daily. This study used a modified version of hyperbolic discontinuation. Patients received half the dose of their original medication (citalopram 10 mg, sertraline 50 mg, or mirtazapine 15 mg), “followed by a quarter of the dose for a month taken as half the dose and placebo on alternate days and then taking placebo for the remainder of the study” (except for fluoxetine which was taken as 20 mg every other day for the last month).75 The Discontinuation-Emergent Signs and Symptoms Scale was used to examine discontinuation symptoms, and despite tapering, patients who continued antidepressants and those who tapered had similar scores at week 6, but scores significantly increased and differed, between the maintenance and discontinuation group at week 12. Thereafter, discontinuation symptoms continued to decrease. However, the study did not examine differences in the discontinuation rates among the specific antidepressants.75
For tapering in pediatric patients, using a typical taper, a patient taking sertraline 200 mg would be reduced to 100 mg, then to 50 mg, and then to 25 mg (i.e., 50% dose reduction at each titration point). However, we have tended to use even slower discontinuation strategies shown in Figure 4E-G for two common “target doses” of sertraline and escitalopram. Importantly, even with discontinuation of longer half-life SSRIs (e.g., fluoxetine), the single prospective discontinuation trial in adolescents suggests that the highest risk of symptomatic recurrence occurs within the first 2 months following discontinuation.76 Therefore, following the discontinuation of antidepressants in youth, monthly monitoring for several months may represent a reasonable approach.
The decision to discontinue an antidepressant should consider the adolescent's psychosocial milieu.33 Antidepressants may be discontinued during lower stress periods, recognizing the importance of incorporating factors such as school or separation-related events (e.g., leaving for summer camp) into the decision to stop an antidepressant. Additionally, clinicians should consider the type, frequency, and duration of psychotherapy. Long-term data in youth with major depressive disorder and anxiety disorders suggest more rapid improvement associated with combined therapy (i.e., SSRI + psychotherapy) relative to antidepressant treatment or psychotherapy alone.77 A patient who has successfully engaged in psychotherapy during the course of pharmacologic treatment or a patient with a more rapid response to acute treatment (more likely with combined treatment) might require a briefer psychopharmacologic intervention. However, no prospective, randomized controlled trials have evaluated this possibility.
Although debate remains regarding the optimal duration of antidepressant treatment for youth with major depressive disorder or anxiety disorders, the overarching goal of treatment is obvious: remission. Any discussion regarding treatment discontinuation is predicated on the patient having achieved remission of depressive or anxiety symptoms; remission status indicates clinical relief is linked to functional recovery.74 Current data suggest that 9–12 months of SSRI treatment is recommended for pediatric patients with major depressive disorder. For children and adolescents with generalized separation and social anxiety disorders, 6–9 months of SSRI treatment may be sufficient.78,79 Many clinicians extend treatment to 12 months based on extrapolation of data from adults with anxiety disorders.80 Such extended treatment periods may decrease the risk of long-term morbidity and recurrence; however, the goal of treatment is ultimately remission rather than the duration of antidepressant pharmacotherapy.
7 ∣. CONCLUSION
Our understanding of antidepressant tolerability and the management of antidepressant-related adverse events remains underdeveloped; however, significant advances have been made over the past several decades. We now have a greater understanding of how developmental pharmacology underlies some side effects and how the risk for specific side effects varies among specific antidepressant medications and between antidepressant classes. Importantly and consistent with the analyses presented herein, many physical symptoms improve in SSRI-treated children and adolescents. As such, it is critical that baseline symptoms be assessed using either unstructured, semistructured approaches, or structured physical symptom inventories (e.g., physical symptom checklists).
In general, early emerging side effects (e.g., activation, gastrointestinal symptoms, sedation) can be managed by modifying exposure and potentially considering individual differences in medication metabolism. However, with early-emerging side effects, it is critical to have evaluated baseline symptoms before initiating pharmacotherapy, given the syndromic overlap with the disorder being treated.
Management is more nuanced for late-emerging adverse effects (e.g., weight gain). Weight gain—for SSRIs--may relate, in part, to medication metabolism45 and the specific medication being used.62 In such cases, switching, perhaps with cross titration, represents a reasonable strategy. However, for some antidepressants, such as duloxetine, the effects on weight are more complicated and require a dynamic management approach. For example, duloxetine is associated with initial weight loss, which normalizes during longer-term treatment.57 Thus, monitoring or changing diet may represent a better strategy for patients experiencing duloxetine-related weight loss.
The antidepressant withdrawal syndrome in children and adolescents has been poorly described, and pharmacokinetic risk factors for discontinuation are inadequately described in pediatric patients compared with adults. Yet, pharmacokinetic factors likely subtend the risk of developing withdrawal symptoms in youth and must be considered in approaches to SSRI discontinuation. Approaches to antidepressant discontinuation are largely based on strategies used in adults, and studies are urgently needed to understand how differences in cytochrome ontogeny and pharmacogenetic variation affect discontinuation in youth. Finally, how symptoms should be monitored after antidepressant discontinuation is unclear, although a single study in pediatric patients suggests that the highest risk of relapse occurs within the first 3 months after discontinuing SSRIs, even for long-half-life SSRIs.
In sum, side effects of antidepressant medications vary considerably and are influenced by developmental pharmacology. Our nascent understanding of side effect prevalence is incomplete at best, and our understanding of their temporal stability and course has not been systematically evaluated. Frequently, these side effects are reported as being present during a trial, which fails to capture the temporal stability and resolution of many side effects. These side effects—which relate to pharmacokinetic and pharmacodynamic processes, can be categorized as early-emerging and late-emerging. Managing antidepressant-related side effects should involve collaboration with the patient and family and may include specific strategies (e.g., dose reduction, change in administration time), adjunctive medications, or cross-titration to another antidepressant.
ACKNOWLEDGMENTS
The authors thank Rachel Ballard, MD, Melissa DelBello, MD, Sergio Delgado, MD, Heather Dlugosz, MD, Nicole Jederlinik, DO, Brooks Keeshin, MD, Brian Kurtz, MD, Molly McVoy, MD, James McCracken, MD, Thuhung Vu, MD, and John Walkup, MD in addition to Stephani Stancil, APRN, PhD, Erin Delgado, APRN, Ashley Cunningham, APRN, Lara Picard, PharmD, Kristina Reinstatler, PharmD, and Nicole Romstadt, PharmD for helpful discussions related to side effects of antidepressants.
FUNDING INFORMATION
Support was received from the Eunice Kennedy Schriver National Institute of Child Health and Development (NICHD) through Grant R01HD098757 and the National Institute of Mental Health (NIMH) through Grant K23MH106037 and from the Yung Family Foundation. This work was supported by the Yung Family Foundation (Drs. Strawn and Mills), the National Institutes of Child Health and Development (R01HD098757 [Strawn, Ramsey] and R01HD099775 [Strawn]) and the National Institutes of Health Clinical and Translational Science Award (CTSA) program (UL1TR001425, Strawn) and Patient-Centered Outcomes Research Institute (PCORI, Strawn), and the National Institute of Mental Health of the National Institutes of Health (F31MH132265, Poweleit).
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Number: R01HD098757, R01HD099775; National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR001425; Patient-Centered Outcomes Research Institute, Grant/Award Number: 2019C1-16328; National Institutes of Health; National Institute of Mental Health; National Institutes of Health
Footnotes
CONFLICT OF INTEREST
Dr. Strawn has received research support from the Yung Family Foundation, the National Institutes of Health (NIMH/NIEHS), the National Center for Advancing Translational Sciences, the Patient-Centered Outcomes Research Institute (PCORI), and Abbvie. He has received material support from Myriad Health and royalties from three texts (Springer). Dr. Strawn serves as an author for UpToDate and is an Associate Editor for Current Psychiatry and has provided consultation to the FDA, Cereval and IntraCellular Therapeutics. Views expressed within this article represent those of the authors and are not intended to represent the position of NIMH, the National Institutes of Health (NIH), or the Department of Health and Human Services. Additionally, the views, statements and opinions in this article are solely the responsibility of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
REFERENCES
- 1.Strawn JR, Dobson ET, Giles LL. Primary pediatric care psychopharmacology: focus on medications for ADHD, depression, and anxiety. Curr Probl Pediatr Adolesc Health Care. 2017;47(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strawn JR, Lu L, Peris TS, Levine A, Walkup JT. Research review: pediatric anxiety disorders - what have we learnt in the last 10 years? J Child Psychol Psychiatry. 2021;62(2):114–139.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beesdo-Baum K, Knappe S. Developmental epidemiology of anxiety disorders. Child Adolesc Psychiatr Clin N Am. 2012;21(3):457–478. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locher C, Koechlin H, Zion SR, et al. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents. JAMA Psychiatry. 2017;74(10):1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strawn JR, Welge JA, Wehry AM, Keeshin BR, Rynn MA. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: a systematic review and meta-analysis. Depress Anxiety. 2015;32(3):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dobson ET, Bloch MH, Strawn JR. Efficacy and tolerability of pharmacotherapy for pediatric anxiety disorders: a network meta-analysis. J Clin Psychiatry. 2019;80(1):17r12064. [DOI] [PubMed] [Google Scholar]
- 8.Reid AM, McNamara JPH, Murphy TK, et al. Side-effects of SSRIs disrupt multimodal treatment for pediatric OCD in a randomized-controlled trial. J Psychiatr Res. 2015;71:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinblatt SP, DosReis S, Walkup JT, Riddle MA. Activation adverse events induced by the selective serotonin reuptake inhibitor fluvoxamine in children and adolescents. J Child Adolesc Psychopharmacol. 2009;19(2):119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramsey LB. Cyp2C19 influence on Escitalopram efficacy and tolerability in youth with anxiety and depression. J Am Acad Child Adolesc Psychiatry. 2018;57(10):S301. [Google Scholar]
- 11.Zuckerman ML, Vaughan BL, Whitney J, et al. Tolerability of selective serotonin reuptake inhibitors in thirty-nine children under age seven: a retrospective chart review. J Child Adolesc Psychopharmacol. 2007;17(2):165–174. [DOI] [PubMed] [Google Scholar]
- 12.Safer DJ, Zito JM. Treatment-emergent adverse events from selective serotonin reuptake inhibitors by age group: children versus adolescents. J Child Adolesc Psychopharmacol. 2006;16(1–2):159–169. [DOI] [PubMed] [Google Scholar]
- 13.Rynn MA, Walkup JT, Compton SN, et al. Child/adolescent anxiety multimodal study: evaluating safety. J Am Acad Child Adolesc Psychiatry. 2015;54(3):180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luft MJ, Lamy M, DelBello MP, McNamara RK, Strawn JR. Antidepressant-induced activation in children and adolescents: risk, recognition and management. Curr Probl Pediatr Adolesc Health Care. 2018;48(2):50–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills JA, Strawn JR. Antidepressant tolerability in pediatric anxiety and obsessive-compulsive disorders: a Bayesian hierarchical modeling meta-analysis. J Am Acad Child Adolesc Psychiatry. 2020;59(11):1240–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165(4):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Leeuwen E, van Driel ML, Horowitz MA, et al. Approaches for discontinuation versus continuation of long-term antidepressant use for depressive and anxiety disorders in adults. Cochrane Database Syst Rev. 2021;4(4):CD013495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bernstein GA, Massie ED, Thuras PD, Perwien AR, Borchardt CM, Crosby RD. Somatic symptoms in anxious-depressed school refusers. J Am Acad Child Adolesc Psychiatry. 1997;36(5):661–668. [DOI] [PubMed] [Google Scholar]
- 18.Horowitz MA, Taylor D. Tapering of SSRI treatment to mitigate withdrawal symptoms - Authors' reply. Lancet Psychiatry. 2019;6(7):562–563. [DOI] [PubMed] [Google Scholar]
- 19.Connolly SD, Bernstein GA. Practice parameter for the assessment and treatment of children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2007;46(2):267–283. [DOI] [PubMed] [Google Scholar]
- 20.Walkup JT, Albano AM, Piacentini J, et al. Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med. 2008;359(26):2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crawley SA, Caporino NE, Birmaher B, et al. Somatic complaints in anxious youth. Child Psychiatry Hum Dev. 2014;45(4):398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ginsburg GS, Riddle MA, Davies M. Somatic symptoms in children and adolescents with anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1179–1187. [DOI] [PubMed] [Google Scholar]
- 24.Strawn JR, Mills JA, Schroeder H, et al. Escitalopram in adolescents with generalized anxiety disorder: a double-blind, randomized, placebo-controlled study. J Clin Psychiatry. 2020;81(5):20m13396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koukouritaki SB, Manro JR, Marsh SA, et al. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308(3):965–974. [DOI] [PubMed] [Google Scholar]
- 26.Strawn JR, Poweleit EA, Uppugunduri CRS, Ramsey LB. Pediatric therapeutic drug monitoring for selective serotonin reuptake inhibitors. Front Pharmacol. 2021;12:749692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anderson LL, Doohan PT, Oldfield L, et al. Citalopram and Cannabidiol: In vitro and In vivo evidence of pharmacokinetic interactions relevant to the treatment of anxiety disorders in Young people. J Clin Psychopharmacol. 2021;41(5):525–533. [DOI] [PubMed] [Google Scholar]
- 28.Vaughn SE, Strawn JR, Poweleit EA, Sarangdhar M, Ramsey LB. The impact of marijuana on antidepressant treatment in adolescents: clinical and pharmacologic considerations. J Pers Med. 2021;11(7):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tulisiak AK, Klein JA, Harris E, et al. Antidepressant prescribing by pediatricians: a mixed-Methods analysis. Curr Probl Pediatr Adolesc Health Care. 2017;47(1):15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spiegelhalter D Introducing The Art of Statistics: How to Learn from Data. Numeracy [Internet]. 2020. [cited 2023 Jan 11];13(1). Available from: https://scholarcommons.usf.edu/numeracy/vol13/iss1/art7 [Google Scholar]
- 31.Skoch SH. Pediatric insomnia: treatment. Current Psychiatry [Internet]. 2022. [cited 2023 Jan 11];21(1). Available from: https://www.mdedge.com/psychiatry/article/250339/sleep-medicine/pediatric-insomnia-treatment [Google Scholar]
- 32.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299(8):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamseddeen W, Clarke G, Wagner KD, et al. Treatment-resistant depressed youth show a higher response rate if treatment ends during summer school break. J Am Acad Child Adolesc Psychiatry. 2011;50(11):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sultan MA, Courtney DB. Adjunctive trazodone and depression outcome in adolescents treated with serotonin Re-uptake inhibitors. J Can Acad Child Adolesc Psychiatry. 2017;26(3):233–240. [PMC free article] [PubMed] [Google Scholar]
- 35.Pediatric OCD Treatment Study (POTS) Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the pediatric OCD treatment study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. [DOI] [PubMed] [Google Scholar]
- 36.Wagner KD, Ambrosini P, Rynn M, et al. Efficacy of sertraline in the treatment of children and adolescents with major depressive disorder: two randomized controlled trials. JAMA. 2003;290(8):1033–1041. [DOI] [PubMed] [Google Scholar]
- 37.Keller MB, Ryan ND, Strober M, et al. Efficacy of paroxetine in the treatment of adolescent major depression: a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2001;40(7):762–772. [DOI] [PubMed] [Google Scholar]
- 38.Wagner KD, Berard R, Stein MB, et al. A multicenter, randomized, double-blind, placebo-controlled trial of paroxetine in children and adolescents with social anxiety disorder. Arch Gen Psychiatry. 2004;61(11):1153–1162. [DOI] [PubMed] [Google Scholar]
- 39.Hamill Skoch S, Mills JA, Ramsey L, Strawn JR. Letter to the editor: sleep disturbances in selective serotonin reuptake inhibitor-treated youth with anxiety disorders and obsessive compulsive disorder-a Bayesian hierarchical modeling meta-analysis. J Child Adolesc Psychopharmacol. 2021;31(5):387–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinclair LI, Christmas DM, Hood SD, et al. Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry. 2009;194(6):483–490. [DOI] [PubMed] [Google Scholar]
- 41.Wilens TE, Biederman J, Kwon A, et al. A systematic chart review of the nature of psychiatric adverse events in children and adolescents treated with selective serotonin reuptake inhibitors. J Child Adolesc Psychopharmacol. 2003;13(2):143–152. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki Y, Sugai T, Fukui N, et al. CYP2D6 genotype and smoking influence fluvoxamine steady-state concentration in Japanese psychiatric patients: lessons for genotype-phenotype association study design in translational pharmacogenetics. J Psychopharmacol. 2011. Jul;25(7):908–914. [DOI] [PubMed] [Google Scholar]
- 43.Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics implementation consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Therapeut. 2015;98(2):127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birmaher B, Axelson DA, Monk K, et al. Fluoxetine for the treatment of childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry. 2003;42(4):415–423. [DOI] [PubMed] [Google Scholar]
- 45.Aldrich SL, Poweleit EA, Prows CA, Martin LJ, Strawn JR, Ramsey LB. Influence of CYP2C19 metabolizer status on Escitalopram/citalopram tolerability and response in youth with anxiety and depressive disorders. Front Pharmacol. 2019;19(10):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beyer C, Cappetta K, Johnson JA, Bloch MH. Meta-analysis: risk of hyperhidrosis with second-generation antidepressants. Depress Anxiety. 2017;34(12):1134–1146. [DOI] [PubMed] [Google Scholar]
- 47.Rautakorpi P, Manner T, Ali-Melkkilä T, Kaila T, Olkkola K, Kanto J. Pharmacokinetics and oral bioavailability of glycopyrrolate in children. Pharmacol Toxicol. 1998;83(3):132–134. [DOI] [PubMed] [Google Scholar]
- 48.Hebert AA, Glaser DA, Green L, et al. Long-term efficacy and safety of topical glycopyrronium tosylate for the treatment of primary axillary hyperhidrosis: post hoc pediatric subgroup analysis from a 44-week open-label extension study. Pediatr Dermatol. 2020;37(3):490–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Remington C, Ruth J, Hebert AA. Primary hyperhidrosis in children: a review of therapeutics. Pediatr Dermatol. 2021;38(3):561–567. [DOI] [PubMed] [Google Scholar]
- 50.Telang S, Walton C, Olten B, Bloch MH. Meta-analysis: second generation antidepressants and headache. J Affect Disord. 2018;236:60–68. [DOI] [PubMed] [Google Scholar]
- 51.Tribl GG, Wetter TC, Schredl M. Dreaming under antidepressants: a systematic review on evidence in depressive patients and healthy volunteers. Sleep Med Rev. 2013;17(2):133–142. [DOI] [PubMed] [Google Scholar]
- 52.Pace-Schott EF, Gersh T, Silvestri R, Stickgold R, Salzman C, Hobson JA. SSRI treatment suppresses dream recall frequency but increases subjective dream intensity in normal subjects. J Sleep Res. 2001;10(2):129–142. [DOI] [PubMed] [Google Scholar]
- 53.Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 2011;7(12):CD008934. [DOI] [PubMed] [Google Scholar]
- 54.Lu CY, Zhang F, Lakoma MD, et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ. 2014;18(348):g3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297(15):1683–1696. [DOI] [PubMed] [Google Scholar]
- 56.Durgam S, Chen C, Migliore R, Prakash C, Edwards J, Findling RL. A phase 3, double-blind, randomized, placebo-controlled study of Vilazodone in adolescents with major depressive disorder. Paediatr Drugs. 2018;20(4):353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Strawn JR, Prakash A, Zhang Q, et al. A randomized, placebo-controlled study of duloxetine for the treatment of children and adolescents with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(4):283–293. [DOI] [PubMed] [Google Scholar]
- 58.Brent DA, Emslie GJ, Clarke GN, et al. Predictors of spontaneous and systematically assessed suicidal adverse events in the treatment of SSRI-resistant depression in adolescents (TORDIA) study. Am J Psychiatry. 2009;166(4):418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cipriani A, Zhou X, Giovane CD, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. The Lancet Elsevier. 2016;388(10047):881–890. [DOI] [PubMed] [Google Scholar]
- 60.Lagerberg T, Fazel S, Sjölander A, Hellner C, Lichtenstein P, Chang Z. Selective serotonin reuptake inhibitors and suicidal behaviour: a population-based cohort study. Neuropsychopharmacology. 2022;47(4):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rynn MA, Riddle MA, Yeung PP, Kunz NR. Efficacy and safety of extended-release venlafaxine in the treatment of generalized anxiety disorder in children and adolescents: two placebo-controlled trials. Am J Psychiatry. 2007;164(2):290–300. [DOI] [PubMed] [Google Scholar]
- 62.Calarge CA, Mills JA, Janz KF, Burns TL, Coryell WH, Zemel BS. Body composition in adolescents during treatment with selective serotonin reuptake inhibitors. Pediatrics. 2017;140(1):e20163943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26(10):1673–1677. [DOI] [PubMed] [Google Scholar]
- 64.Blumenthal SR, Castro VM, Clements CC, et al. An electronic health records study of long-term weight gain following antidepressant use. JAMA Psychiat. 2014;71(8):889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Scharko AM. Selective serotonin reuptake inhibitor-induced sexual dysfunction in adolescents: a review. J Am Acad Child Adolesc Psychiatry. 2004;43(9):1071–1079. [DOI] [PubMed] [Google Scholar]
- 66.Levine A, McGlinchey E. Assessing sexual symptoms and side effects in adolescents. Pediatrics. 2015;135(4):e815–e817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Deumic Shultz E, Mills JA, Ellingrod VL, Bishop JR, Calarge CA. Sexual functioning in adolescents with major depressive disorder: a prospective study. J Clin Psychiatry. 2021;82(6):21m13892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schatzberg AF, Haddad P, Kaplan EM, et al. Serotonin reuptake inhibitor discontinuation syndrome: a hypothetical definition. Discontinuation Consensus Panel J Clin Psychiatry. 1997;58(Suppl 7):5–10. [PubMed] [Google Scholar]
- 69.Fava GA, Gatti A, Belaise C, Guidi J, Offidani E. Withdrawal symptoms after selective serotonin reuptake inhibitor discontinuation: a systematic review. Psychother Psychosom. 2015;84(2):72–81. [DOI] [PubMed] [Google Scholar]
- 70.Shapiro BB. Subtherapeutic doses of SSRI antidepressants demonstrate considerable serotonin transporter occupancy: implications for tapering SSRIs. Psychopharmacology (Berl). 2018;235(9):2779–2781. [DOI] [PubMed] [Google Scholar]
- 71.Tint A, Haddad PM, Anderson IM. The effect of rate of antidepressant tapering on the incidence of discontinuation symptoms: a randomised study. J Psychopharmacol. 2008;22(3):330–332. [DOI] [PubMed] [Google Scholar]
- 72.Rosenbaum JF, Fava M, Hoog SL, Ascroft RC, Krebs WB. Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87. [DOI] [PubMed] [Google Scholar]
- 73.Strawn JR, Poweleit EA, Ramsey LB. CYP2C19-guided Escitalopram and sertraline dosing in pediatric patients: a pharmacokinetic modeling study. J Child Adolesc Psychopharmacol. 2019;29(5):340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hathaway EE, Walkup JT, Strawn JR. Antidepressant treatment duration in pediatric depressive and anxiety disorders: how long is long enough? Curr Probl Pediatr Adolesc Health Care. 2018;48(2):31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lewis G, Marston L, Duffy L, et al. Maintenance or discontinuation of antidepressants in primary care. N Engl J Med. 2021;385(14):1257–1267. [DOI] [PubMed] [Google Scholar]
- 76.Emslie GJ, Kennard BD, Mayes TL, et al. Continued effectiveness of relapse prevention cognitive-behavioral therapy following fluoxetine treatment in youth with major depressive disorder. J Am Acad Child Adolesc Psychiatry. 2015;54(12):991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strawn JR, Mills JA, Suresh V, Peris TS, Walkup JT, Croarkin PE. Combining selective serotonin reuptake inhibitors and cognitive behavioral therapy in youth with depression and anxiety. J Affect Disord. 2022;298(Pt A):292–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swan AJ, Kendall PC, Olino T, et al. Results from the child/adolescent anxiety multimodal longitudinal study (CAMELS): functional outcomes. J Consult Clin Psychol. 2018;86(9):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ginsburg GS, Becker-Haimes EM, Keeton C, et al. Results from the child/adolescent anxiety multimodal extended long-term study (CAMELS): primary anxiety outcomes. J Am Acad Child Adolesc Psychiatry. 2018;57(7):471–480. [DOI] [PubMed] [Google Scholar]
- 80.Rickels K, Etemad B, Khalid-Khan S, Lohoff FW, Rynn MA, Gallop RJ. Time to relapse after 6 and 12 months' treatment of generalized anxiety disorder with venlafaxine extended release. Arch Gen Psychiatry. 2010;67(12):1274–1281. [DOI] [PubMed] [Google Scholar]