Abstract
The genus Saccharomyces consists of several species divided into the sensu stricto and the sensu lato groups. The genomes of these species differ in the number and organization of nuclear chromosomes and in the size and organization of mitochondrial DNA (mtDNA). In the present experiments we examined whether these yeasts can exchange DNA and thereby create novel combinations of genetic material. Several putative haploid, heterothallic yeast strains were isolated from different Saccharomyces species. All of these strains secreted an a- or α-like pheromone recognized by S. cerevisiae tester strains. When interspecific crosses were performed by mass mating between these strains, hybrid zygotes were often detected. In general, the less related the two parental species were, the fewer hybrids they gave. For some crosses, viable hybrids could be obtained by selection on minimal medium and their nuclear chromosomes and mtDNA were examined. Often the frequency of viable hybrids was very low. Sometimes putative hybrids could not be propagated at all. In the case of sensu stricto yeasts, stable viable hybrids were obtained. These contained both parental sets of chromosomes but mtDNA from only one parent. In the case of sensu lato hybrids, during genetic stabilization one set of the parental chromosomes was partially or completely lost and the stable mtDNA originated from the same parent as the majority of the nuclear chromosomes. Apparently, the interspecific hybrid genome was genetically more or less stable when the genetic material originated from phylogenetically relatively closely related parents; both sets of nuclear genetic material could be transmitted and preserved in the progeny. In the case of more distantly related parents, only one parental set, and perhaps some fragments of the other one, could be found in genetically stabilized hybrid lines. The results obtained indicate that Saccharomyces yeasts have a potential to exchange genetic material. If Saccharomyces isolates could mate freely in nature, horizontal transfer of genetic material could have occurred during the evolution of modern yeast species.
The genus Saccharomyces includes numerous species (2). These have been divided into sensu stricto and sensu lato groups (11). The sensu stricto group includes S. cerevisiae, S. bayanus, S. pastorianus, and S. paradoxus, and the sensu lato group includes S. exiguus, S. castellii, S. unisporus, S. dairenensis, S. servazzii, and S. kluyveri (11). Yeasts belonging to the sensu stricto group are interfertile and represent sister species (15). Several natural hybrids, which are a product of interspecific crosses within the group, have been described (9, 12). On the other hand, sensu lato yeasts represent a far more heterogenous group of not well characterized yeasts (10). In general, a good definition of what a yeast species is still lacking.
The genome of S. cerevisiae has been studied intensively, and the complete sequence has been determined. S. cerevisiae has 16 chromosomes (for a review, see reference 4), and the mitochondrial DNA (mtDNA) molecule varies in size from 75 to 85 kb (3a). A part of the S. cerevisiae genome represents an ancient duplication (23). Other Saccharomyces sensu stricto yeasts exhibit a karyotype and genome size similar to those of S. cerevisiae (22). The gene organization, including the gene order, seems to be rather conserved among the sensu stricto yeasts. Therefore, the chromosomes are believed to be at least partially homologous (7, 20). mtDNA of sensu stricto yeasts contains many guanine-cytosine clusters, and the size is greater than 60 kb. S. paradoxus has a 75-kb mtDNA molecule, and S. pastorianus and S. bayanus have a 65-kb one (19). The coding parts of the mitochondrial genes within the sensu stricto group show less than 5% sequence diversity (5).
Sensu lato yeasts generally contain a smaller number of chromosomes (22). The two extremes are S. kluyveri, which has 7 chromosomes, and S. exiguus, which is believed to contain 16 (17). The four remaining sensu lato species lie between these two extremes. S. dairenensis and S. castellii display 9 bands in pulsed-field gel electrophoresis analysis, and S. servazzii and S. unisporus display 12 (17). Sensu lato yeasts’ mtDNAs do not contain an excess of guanine-cytosine clusters and are smaller than 50 kb, i.e., 48 kb for S. dairenensis, 29 kb for S. servazzii and S. unisporus, 26 kb for S. castellii, and 23 kb for S. exiguus (19). The gene order varies a lot, and the coding regions of the mitochondrial genes within the sensu lato group show 10 to 20% sequence diversity (17a). Hence, each Saccharomyces species has a characteristic karyotype (17) and also a specific restriction pattern of its mtDNA (19).
In principle, the definition of a yeast species should be based on the concept of genetic isolation, as is the case for plants and animals. This means that members of the same species are interfertile, whereas genetically isolated species are not. However, so far interfertility among Saccharomyces yeasts has not been examined in detail except among Saccharomyces sensu stricto species. As mentioned before, sensu stricto yeasts can mate and generate viable hybrids. The best-described example of a yeast hybrid is the lager brewer’s yeast, S. pastorianus (synonym, S. carlsbergensis), which arose upon a mating event between baker’s yeast, S. cerevisiae, and an unknown yeast belonging to the S. bayanus complex. This hybrid is an allotetraploid displaying chromosomes from both parents, whereas its mtDNA molecule was inherited from the non-S. cerevisiae parent (9, 19). However, two other native hybrids have been described recently also (12). Thus, two genomes can coexist in the same cell, and it is likely that sensu stricto yeasts may be able to exchange their genetic material in nature. Several years ago it was reported that S. cerevisiae and S. kluyveri could recognize each other’s pheromones and could generate hybrids with a decreased viability. However, these hybrids were not studied in detail (1, 13). Because genetic tools, such as the availability of haploid and auxotrophic strains, were poorly developed for non-S. cerevisiae yeasts, other approaches were used to define species among these yeasts. A major advance in our present understanding of yeast systematics has resulted from studies on DNA relatedness (10) and from nucleic acid sequence comparisons (8, 11). However, assessing phylogenetic relationships by using sequence analyses of short pieces of DNA makes sense only if the tested yeasts are genetically isolated and of monophyletic origin.
Interspecific mating has previously been performed successfully only between sensu stricto yeasts, and only the phenotypes of the resulting hybrids were analyzed (15). So far only limited information on mating spores among sensu lato yeasts exists (16). In this study, we report that several Saccharomyces yeasts can mate at low frequency and produce viable hybrids. If such a horizontal transfer of genetic material occurs in nature, modern yeast species may contain DNA of polyphyletic origin. Thus, sequencing studies alone may sometimes be misleading when used for the determination of phylogenetic relationships among different yeasts.
MATERIALS AND METHODS
Strains.
The yeast strains used in this project are listed in Table 1. Several are heterothallic and apparently also haploid. Some of the heterothallic non-S. cerevisiae strains were selected by testing isolates from various collections for pheromone secretion. Some homothallic strains were mutated by ethyl methanesulfonate (EMS) mutagenesis or gene disruption to acquire heterothallic strains.
TABLE 1.
Strains used in this study
| Strain numbera | Species or strain | Mating type | Genotype or description | Reference or sourcec |
|---|---|---|---|---|
| Y169 | S. cerevisiae | MATa | lys1 | This laboratory |
| Y170 | S. cerevisiae | MATα | lys1 | This laboratory |
| Y184 | S. cerevisiae | MATα | ade1 his1 | This laboratory |
| Y185 | S. cerevisiae | MATa | ade1 his1 | This laboratory |
| Y266 | S. cerevisiae | MATα | lys1 ρ− | Y170 |
| Y267 | S. cerevisiae | MATα | lys1 ρ− | Y170 |
| Y166 | S. bayanus | Diploid | Homothallic, ura3-1 | MCYC 623-6C (R. Borts) |
| Y244 | S. bayanus | MATα | ura3− G418R | Y166 transformed with P161 |
| Y245 | S. bayanus | MATa | ura3− G418R | Y166 transformed with P161 |
| Y327 | S. exiguus | MATα | Spontaneous ho− | CBS 2141 |
| Y328 | S. exiguus | MATa | Spontaneous ho− | CBS 2141 |
| Y339 | S. exiguus | MATα | his− | Y327 |
| Y345 | S. exiguus | MATa | arg− | Y328 |
| Y188 | S. castellii | Diploid | Homothallic | CBS 4310 |
| Y239 | S. castellii | MATa | Induced ho− | Y188 |
| Y257 | S. castellii | MATa | met− | Y239 |
| Y323 | CBS 6463b | MATa | Spontaneous ho−, ρ− | CBS 6463 |
| Y344 | CBS 6463 | MATa | ura3− ρ− | Y323 |
| Y280 | Hybrid | Hybrid | Prototroph | Y244 × Y169 |
| Y500 | Hybrid | Hybrid | Prototroph | Y257 × Y245 |
| Y507 | Hybrid | Hybrid | Prototroph, ρ− | Y184 × Y344 |
| MT502 | S. cerevisiae | MATa | sst2-1 met1 leu2-3,112 his3 can1 | M. T. Hansen |
| MT503 | S. cerevisiae | MATα | sst2-1 met1 leu2-3,112 his3 can1 | M. T. Hansen |
| MT504 | S. cerevisiae | MATα | leu2-3,112 gal2 sst1 | V. McKay |
| MT505 | S. cerevisiae | MATa | leu2-3,112 gal2 sst1 | V. McKay |
Y, J. Piskur collection; MT, T. Nilsson-Tillgren collection.
CBS 6463 (Y323) was previously classified as S. dairenensis, but it actually represents a novel species (17b).
MCYC, Microbiology Collection of Yeast Cultures, Madrid, Spain; CBS, Centraalbureau voor Schimmelculture, Delft, Holland.
Media for routine growth.
The yeast strains were grown at 25°C in the following media. YPD medium consisted of 1% yeast extract, 1% Bacto Peptone, and 2% glucose; minimal medium (SD) consisted of 1% succinic acid, 0.6% NaOH, 0.67% yeast nitrogen base (Difco) without amino acids, and 2% glucose; sporulation medium consisted of 1% potassium acetate, 0.1% Bacto Yeast Extract, and 0.05% glucose. When necessary, these media were solidified with 2% agar.
EMS mutagenesis.
The ho− mutations and auxotrophic markers were introduced by EMS mutagenesis. One milliliter of stationary-phase yeast culture (either homothallic spores or prototrophic vegetative cells) was exposed to EMS (25 μl for Y188 and Y239, 30 μl for Y327 and Y328, and 35 μl in the case of Y323). A 200-μl portion of cells was transferred immediately to 8 ml of 5% sodium thiosulfate to quench the reaction (tube 1), but the rest of cells were incubated at 30°C for 45 min and 200 μl of cells was transferred to tubes 2 and 3 containing 8 ml of 5% thiosulfate. Finally, the rest of the cells were exposed to EMS in the same manner for 2 h (tubes 4 and 5).
Cells from tubes 1, 2, and 4 were plated on YPD medium to determine the percentages of surviving cells. The chemical treatment was considered efficient when survival was lower than 50%. Tubes 3 and 5 were centrifuged (3,000 × g for 4 min), and the cells were washed twice with sterile water. Thereafter, the cells were resuspended in 1 ml of YPD medium and incubated at 25°C for 60 min. The suspension was spread onto YPD plates (ca. 200 cells/plate), and after 3 days of incubation, the plates were replicated onto either sporulation medium (when screening for ho− mutants) or SD (when screening for auxotrophs). Putative auxotrophs were further examined to identify the induced auxotrophic markers.
Halo assay.
When pheromones from yeast cells of the opposite mating type are present in the medium, haploid heterothallic yeast cells undergo G1 phase arrest. Such cells do not divide further. To test for pheromone secretion, approximately 105 cells of a haploid S. cerevisiae tester strain (MT502, MT503, MT504, or MT505) were spread as a lawn on a YPD plate. MT502 and MT505 were used as a tester for α factor secretion, whereas MT503 and MT504 were used as a tester for a factor secretion. Then various yeast colonies were transferred as patches to such plates. Clear zones around patches after overnight incubation indicated inhibition of growth of the tester strain.
Induction of heterothallism.
The diploid S. castellii strain Y188 was sporulated, and separation of spores was achieved in the following way. The asci were resuspended in 200 μl of Zymolyase 100T at a concentration of 0.2 mg/ml and incubated while shaking for 30 min at 25°C. Approximately 100 μl of sterile glass beads was then added, and the tube was incubated for another 30 min. After addition of 0.2 ml of 0.1% Triton X-100, the tube was vortexed for 1 min. Spore separation was checked under a microscope before spores were washed twice with sterile sodium phosphate buffer, pH 7.0. Thereafter the spores were mutagenized with 25 μl of EMS by the procedure described above.
The treated cells were grown on YPD medium and replicated onto sporulation medium, and after several days they were checked for sporulation under UV light. In Saccharomyces yeasts, a sporulated culture exhibits fluorescence when excited by UV light at 305 nm. Colonies which were not fluorescent were checked for sporulation by microscopy. Colonies which did not give rise to spores were assumed to be either haploids mutated in the HO gene or diploids mutated in a gene necessary for the sporulation process. In the next step such mutants were checked for secretion of pheromones by using the S. cerevisiae tester strains. A halo around a colony was taken as evidence that the strain exhibited sexual activity and presumably was heterothallic and haploid.
Disruption of the HO gene.
The homothallic, diploid S. bayanus strain Y166 was transformed with the plasmid P161, which carries a part of the S. cerevisiae HO gene. The plasmid was constructed in the following way. The initial vector was the pCH217 plasmid (from Chris Harfield, University of Leicester, United Kingdom), which contains a 2-kb insert of the ATP1 gene conferring resistance to geneticin, G418R, at a concentration of 150 mg/liter (see also reference 18). The ATP1 gene is flanked on both sides by several unique restriction sites, e.g., PstI and BamHI, which both map 3′ from the ATP1 gene. A 0.75-kb PstI-BglII fragment of the S. cerevisiae HO gene originating from a plasmid carrying the complete HO gene (14) was inserted into pCH217, which had previously been digested with PstI and BamHI. The newly constructed plasmid was named P161. The 0.75-kb HO fragment carries BamHI and Eco47III restriction sites, which are present as unique sites in P161. For transformation purposes P161 was cut with BamHI or Eco47III and introduced into a culture containing spores of Y166 by the usual yeast transformation procedure. The plasmid P161 was expected to be inserted into the HO gene and generate two defective copies of this gene. Colonies resistant to geneticin were examined for the structure of the HO region by Southern analysis and for secretion of pheromones. Diploid transformed cells were sporulated, and haploid lines were obtained after dissection of tetrads.
Mass mating test.
The auxotrophic parental strains were grown independently in liquid YPD medium overnight. Stationary-phase a and α cells of different mutants, corresponding to approximately 0.5 × 108 cells, were mixed together and then dropped onto YPD plates. After several hours of incubation at 25°C, the yeasts were checked by microscopy for the formation of zygotes. When zygotes were detected, approximately 107 cells were taken from the YPD plate and plated onto SD, or SD plus additives, in order to screen for viable hybrids.
Selection procedure.
When both parents were auxotrophs, viable hybrids were selected on the selective SD medium. Cells were grown for 3 to 5 days at 25°C. The parental strains were also incubated on SD medium in order to check for revertants.
DAPI staining for nucleus detection.
Suspensions of cells containing zygotes were stained with DAPI (4′,6-diamidino-2-phenylindole), which causes DNA to fluoresce bluish-white. Approximately 105 cells were added to 20 μl of DAPI solution (1 μg of DAPI per 1 ml of H2O) and boiled for 5 min. Under a fluorescence microscope it was possible to determine whether the two parental nuclei in zygotes had fused. This method indicated whether zygotes were homo- or heterokaryons.
Analysis of nuclear chromosomes.
Chromosomes of various putative hybrids were isolated and separated by pulsed-field gel electrophoresis (contour-clamped homogeneous electric field [CHEF] gel electrophoresis). The usual conditions for preparation of chromosomal DNA from the Saccharomyces yeasts were followed (17). A CHEF-DR II apparatus (Bio-Rad, Richmond, Calif.) was used for separation of chromosomes. The electrophoresis gels had an agarose concentration of 1%, and the electrophoresis buffer was 0.5× TBE (10× TBE is 1.0 M Tris, 0.9 M boric acid, and 0.01 M EDTA) cooled to 14°C. Electrophoresis was carried out at 150 V for 8 h with a switching time of 240 s and then for 10 h with a switching time of 160 s followed by 14 h with a switching time of 90 s and finally 6 h with a switching time of 60 s. After electrophoresis, the gels were stained with ethidium bromide for visualization of chromosomal DNA.
Analysis of mtDNA.
mtDNA was isolated according to a fast procedure described by Defontaine et al. (3). In some cases, mtDNA was isolated by the CsCl method (19). mtDNA was cut with restriction enzymes, and fragments were separated on a gel. Mitochondrial fragments were visualized with ethidium bromide, or the gel was blotted onto a membrane and the mtDNA bands were visualized by hybridization with a labelled probe based on CsCl-purified mtDNA.
RESULTS
Construction of haploid heterothallic strains.
Two yeast strains belonging to the same Saccharomyces species can be crossed successfully if they are of opposite mating types. A cross can occur between homothallic spores or by crossing heterothallic strains. In the present study it was possible to obtain, isolate, or construct strains belonging to the sensu lato yeasts that were maters and that carried recessive auxotrophic mutations. The strains were tested for secretion of pheromones on the Saccharomyces tester strains. All strains used in this study could arrest S. cerevisiae in the G1 phase (Table 2). These strains were used in mass mating experiments between different species.
TABLE 2.
Secretion of pheromones among Saccharomyces strains
| Strain | Species or strain | Pheromone secretiona on:
|
Mating type | |||
|---|---|---|---|---|---|---|
| MT502 (MATa) | MT503 (MATα) | MT504 (MATα) | MT505 (MATa) | |||
| Y184 | S. cerevisiae | +++ | X | X | + | MATα |
| Y185 | S. cerevisiae | X | +++ | + | X | MATa |
| Y169 | S. cerevisiae | X | +++ | + | X | MATa |
| Y170 | S. cerevisiae | +++ | X | X | + | MATα |
| Y244 | S. bayanus | +++ | X | X | + | MATα |
| Y245 | S. bayanus | X | +++ | + | X | MATa |
| Y339 | S. exiguus | +++ | + | X | + | MATα |
| Y345 | S. exiguus | X | +++ | X | X | MATa |
| Y257 | S. castellii | X | +++ | + | X | MATa |
| Y344 | CBS 6463 | X | +++ | X | X | MATa |
Symbols: X, no response; +, weak signal; +++, strong signal.
S. castellii heterothallic strains were isolated after EMS mutagenesis on the Y188 diploid strain as described above. Y239 secreted only one type of pheromone (Table 2). Furthermore, a recessive mutation was induced in this strain, and therefore this strain probably is a haploid. The resulting methionine-requiring strain, Y257, was used in mass mating experiments.
S. bayanus heterothallic strains were isolated after disruption of the HO gene in the Y166 strain. The P161 plasmid, which was cut with Eco47III, recombined into the HO gene in such a way as to generate two mutant copies of the HO gene (data not shown). The crossovers most likely took place between two regions originating from two different species, i.e., S. cerevisiae and S. bayanus. Apparently, the homology was sufficiently high to promote homologous recombination. When transformed Y166 colonies sporulated, haploid strains, such as Y244 and Y245, each secreting only one type of pheromone, were obtained upon tetrad dissection (Table 2). Transformation of sensu lato strains with P161 was not successful, possibly because homology between the S. cerevisiae sequences and the target sequences was too low.
S. exiguus strains Y339 and Y345, as well as CBS 6463 (strain Y344), were obtained from different yeast collections as natural isolates of heterothallic strains. Two of these three strains secreted one type of pheromone, but Y339 secreted strongly α-like, and weakly a-like, pheromone (Table 2). Recessive mutations were induced successfully in these strains, and thus they also are likely to be haploid.
The strains described above were then tested for genetic stability of the induced mutations. All strains listed in Table 1 gave a very low frequency of revertants, i.e., less than 10−7, and were therefore suitable for mating experiments. Table 2 shows that all heterothallic strains secreted sexual pheromones that could be recognized by the hypersensitive S. cerevisiae tester strains. Thus, the action of these pheromones is not restricted to only one species.
Mating.
The strains described above were then tested for their ability to undergo further steps in the mating process, i.e., abilities to fuse and produce zygotes. Various crosses were performed, and the presence of different cell types, especially zygotes, was checked by microscopy (Fig. 1 and Table 3). Zygotes appeared at various frequencies after strains were exposed to one another for 5 to 8 h.
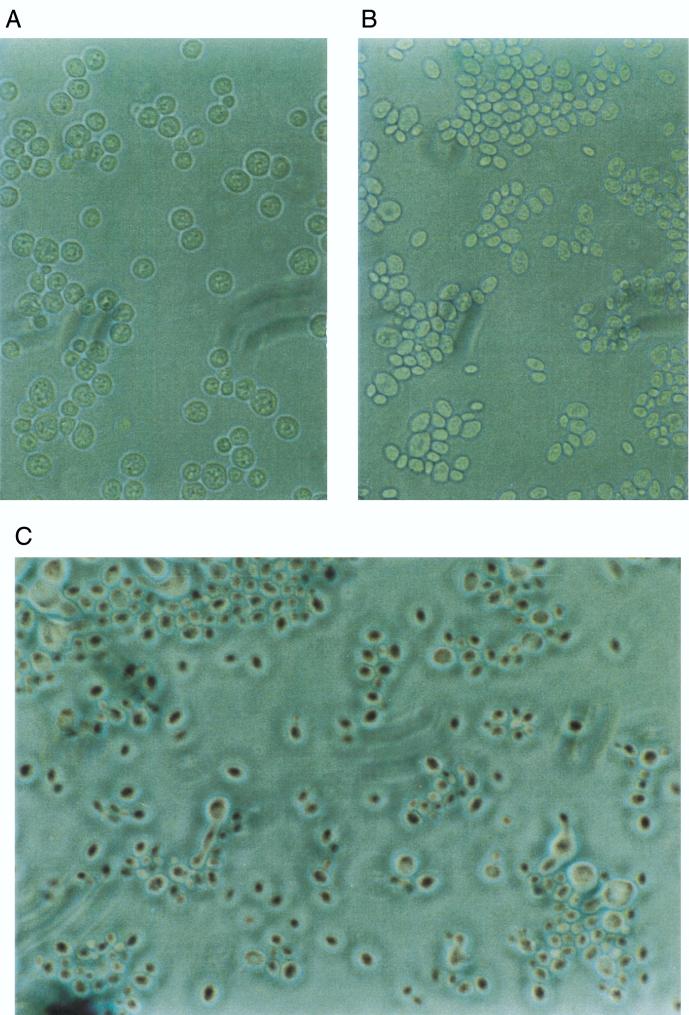
FIG. 1.
Microscopic observation of interspecific zygotes obtained after a cross between S. cerevisiae (Y184) and S. exiguus (Y345). (A) Parental strain Y184. (B) Parental strain Y345. Note that no cells in the mixture have a zygote-like shape. (C) A mixture in which the two parental strains had been in contact for 6 h. Both parental cells and cells with a zygote-like shape are visible.
TABLE 3.
Appearance of zygotes in intra- and interspecies crosses
| Crossa | Mating type | Frequency of zygotesb | Presence of homo- or heterokaryonsc |
|---|---|---|---|
| A | |||
| Y184 × Y185 | MATα × MATa | 0.40 | n.p. |
| Y169 × Y170 | MATa × MATα | 0.30 | n.p. |
| Y184 × Y169 | MATα × MATa | 0.22 | n.p. |
| Y244 × Y245 | MATα × MATa | 0.30 | n.p. |
| B (Y339 × Y345) | MATα × MATa | 0.05 | n.p. |
| C | |||
| Y184 × Y245 | MATα × MATa | 0.26 | n.p. |
| Y185 × Y244 | MATa × MATα | 0.33 | n.p. |
| Y169 × Y244 | MATa × MATα | 0.33 | n.p. |
| Y170 × Y245 | MATα × MATa | 0.30 | n.p. |
| D | |||
| Y184 × Y345 | MATα × MATa | 0.08 | Homokaryons |
| Y184 × Y257 | MATα × MATa | 0.10 | Homokaryons |
| Y184 × Y344 | MATα × MATa | 0.05 | Homokaryons |
| Y185 × Y339 | MATa × MATα | 0.09 | n.p. |
| Y185 × Y257 | MATa × MATa | 0.19 | n.p. |
| Y185 × Y344 | MATa × MATa | 0.15 | n.p. |
| E | |||
| Y345 × Y257 | MATa × MATa | 0.03 | Homokaryons |
| Y345 × Y344 | MATa × MATa | 0.15 | Homokaryons |
| Y257 × Y344 | MATa × MATa | 0.07 | Homokaryons |
A, intraspecific crosses within the sensu stricto group; B, intraspecific crosses within the sensu lato group; C, interspecific crosses between yeasts belonging to the sensu stricto group; D, interspecific crosses between yeasts belonging to the sensu stricto and sensu lato groups; E, interspecific crosses between yeasts belonging to the sensu lato group.
Note that the frequency of zygotes is estimated on approximately 1,000 cells.
n.p., not performed.
Within the sensu stricto group, mating occurred at high frequencies (ca. 22 to 40% of cells in the mating mix were present as zygotes). Intra- and interspecific crosses gave a similar frequency of zygotes (Table 3, crosses A and C). Homocrosses, i.e., mating between yeasts having the same mating response on the tester strains, did not result in any zygotes. In short, the mating between S. cerevisiae and S. bayanus was very efficient and mating type dependent. S. cerevisiae was also successfully crossed with all sensu lato isolates. However, the frequency of zygotes appeared to be lower than that for crosses within the sensu stricto group. Most of the time, the appearance of zygotes was mating type dependent but some homocrosses were also observed, e.g., Y185 × Y257 and Y185 × Y344 (Table 3, cross D).
In the case of sensu lato yeasts, zygotes were obtained only from homocrosses. In the case of crosses between yeasts having the opposite mating type, such as Y339 × Y257 or Y339 × Y344, no zygotes were observed. In the sensu lato group, the interspecific crosses were apparently not mating type dependent. Note that these yeasts were tested several times on S. cerevisiae tester strains (Table 2), and they secreted only one type of pheromones as recognized by S. cerevisiae. The hybrid nature of some putative hybrids resulting from homocrosses was confirmed by chromosome analysis (see later sections). Similar homocrosses were observed and reported previously among wild-type yeast isolates (21). In general, frequencies of zygotes were lower in the case of interspecific crosses among sensu lato yeasts than in other crosses. However, these differences between zygote frequencies are difficult to interpret, because the strains used are perhaps not perfectly heterothallic, i.e., may have a leaky ho− allele, or they may secrete different amounts of pheromones. Lower frequencies may be a consequence of variations in the structure of pheromones belonging to different species. These experiments clearly demonstrated that heterothallic yeasts belonging to the genus Saccharomyces can recognize each other and that the initial steps of mating can take place among different species.
Karyogamy.
Zygotes from a few interspecific crosses were examined to ascertain the number of nuclei per cell. After paired parental strains were incubated for 5 to 8 h the cells were DAPI stained and examined by fluorescence microscopy. If they contained one nucleus they were designated homokaryons, and if two parental nuclei were present in each cell they were designated heterokaryons. After 6 h the zygotes possessed only one nucleus, and no heterokaryons were found (Table 3). Therefore, fusion of nuclei occurred almost immediately upon generation of interspecific hybrid zygotes.
Frequency of viable hybrids.
In the following experiment, various yeasts were crossed and selection for viable hybrids was undertaken. Crosses were performed between strains of different species carrying auxotrophic markers and cells plated on selective medium. In most cases, the mixture contained a substantial proportion of zygotes (see also Table 3). Note that when a hybrid colony appeared on the selective medium, the putative hybrid had already undergone a large number of divisions. Results showing a number of putative hybrids are presented in Table 4. Some crosses, such as Y184 × Y339, Y339 × Y257, Y339 × Y344, Y184 × Y257, and Y184 × Y345, which provided only a single viable hybrid or no hybrids, are not shown in Table 4. However, later on some of these hybrids, which appeared at a very low frequency, were also analyzed for the structure of their genomes. In all cases, the frequency of viable hybrids was far lower than the frequency of zygotes observed under the microscope. In the case of an intraspecific cross between two S. cerevisiae strains, most zygotes seem to have given viable diploids (Table 4). However, even in the case of crosses between S. cerevisiae and S. bayanus, which are relatively closely related, only ca. one-tenth of the observed zygotes seem to have given viable hybrid colonies. The frequency of viable hybrids was several orders of magnitude lower in the case of crosses employing sensu lato yeasts. Note that these sensu lato yeasts are not very closely related to each other or to S. cerevisiae (11). Apparently, a majority of zygotes which appeared during the first hours after mating were not stable and did not give rise to viable prototrophic progeny.
TABLE 4.
Frequency of viable interspecific hybrids
| Crossa | No. of putative hybrids on SDb | Frequency of putative viable hybridd |
|---|---|---|
| A | ||
| Y185 × Y170 | ∼104 | 32 × 10−2 |
| Y184 × Y245 | 1,000 | 3.8 × 10−2 |
| Y185 × Y244 | 1,500 | 4.5 × 10−2 |
| Y169 × Y244 | 1,500 | 4.5 × 10−2 |
| Y170 × Y245 | 1,000 | 3.3 × 10−2 |
| B | ||
| Y185 × Y339 | 6c | 6.7 × 10−6 |
| Y184 × Y344 | 50c | 10−4 |
| Y185 × Y344 | 70c | 4.7 × 10−5 |
| C | ||
| Y345 × Y257 | ∼103 | ∼3.3 × 10−3 |
| Y345 × Y344 | ∼103 | ∼6.7 × 10−4 |
| Y257 × Y344 | 5 + 50e | 7.8 × 10−5 |
A, crosses within the sensu stricto group; B, crosses between yeasts belonging to the sensu stricto and sensu lato groups; C, crosses between yeasts belonging to the sensu lato group. Note that only interspecific crosses, which gave viable hybrids, are shown in this table.
All of the parental strains were tested for revertants on SD, and the reversion rate was shown to be lower than 10−7 for each of them. Therefore, when a colony appeared on SD, it was likely to be a hybrid because only approximately 107 parental cells, including zygotes, were plated on the selective plate.
Small colonies.
Frequencies of putative viable hybrids are the ratio between the number of colonies on SD plates and the presumed number of hybrids plated out.
Five large and 50 small colonies.
Analysis of the hybrid progeny.
Some of the putative hybrids which formed colonies on minimal medium were analyzed for the organization of their genomes. Chromosomal DNA was extracted and separated by pulsed-field gel electrophoresis, and the patterns were compared to those of the parental strains. Also mtDNA molecules were purified and subjected to restriction analysis. The patterns obtained were compared to those of the parental strains. Results are shown in Tables 5 and 6 and in Fig. 2. Hybrids obtained from the crosses between S. cerevisiae and S. bayanus always contained both parental chromosomes (Tables 5 and 6 and Fig. 2). Only occasionally was a single chromosome band belonging to one parental set missing or did it exhibit a changed size (data not shown). Apparently, the hybrid situation was genetically stable and both parental chromosomes could cohabit within the same nucleus. However, if both parental strains contained mtDNA, then only the S. cerevisiae mtDNA molecule was detected among the progeny (Table 6). Only when the S. cerevisiae parent was a petite mutant, and thus did not contain any functional mtDNA, was the S. bayanus mtDNA molecule transmitted to the progeny (Table 6). In principle a hybrid could contain, propagate, and express either of the two parental mtDNA molecules.
TABLE 5.
Analysis of the hybrid progeny
| Cross | Hybrid no. | Nuclear pattern | Mitochondrial pattern | Growth ona:
|
|
|---|---|---|---|---|---|
| YPD medium | SD | ||||
| Y184 × Y245 | 1–10 | S. cerevisiae and S. bayanus | S. cerevisiae | +++ | +++ |
| Y169 × Y244 | 1–10 | S. cerevisiae and S. bayanus | S. cerevisiae | +++ | +++ |
| Y185 × Y339 | 1–6 | S. exiguus | S. exiguus | + | + |
| Y184 × Y344 | 1–20 | CBS 6463 | ρ− | + | +/−b |
| Y345 × Y257 | 1–10 | S. exiguus | S. exiguus | +++ | + |
| 11–20 | S. castellii | S. castellii | + | + | |
| Y345 × Y344 | 1–10 | S. exiguus | S. exiguus | + | + |
| 11–20 | CBS 6463 | ρ− | + | + | |
| Y184 × Y344 | Y507 | CBS 6463 plus one band from S. cerevisiae | ρ− | + | +/− |
| Y257 × Y245 | Y500 | Mixed parental and new bands | S. bayanus | +++ | +++ |
+++, very good growth; −, no growth; +/−, very weak growth; +, weak growth.
Most of these hybrids had reduced viability on any medium, and they were difficult to propagate.
TABLE 6.
Sensu stricto crosses
| Cross | Characteristics | Nuclear patterna | Mitochondrial patterna |
|---|---|---|---|
| Y244 × Y169 | S. cerevisiae ρ+ × S. bayanus ρ+ | Both parental chromosomes | S. cerevisiae |
| Y245 × Y170 | S. cerevisiae ρ+ × S. bayanus ρ+ | Both parental chromosomes | S. cerevisiae |
| Y266 × Y245 | S. cerevisiae ρ− × S. bayanus ρ+ | Both parental chromosomes | S. bayanus |
| Y267 × Y245 | S. cerevisiae ρ− × S. bayanus ρ+ | Both parental chromosomes | S. bayanus |
Over 100 hybrids from each cross were checked for their nuclear and mitochondrial genome.
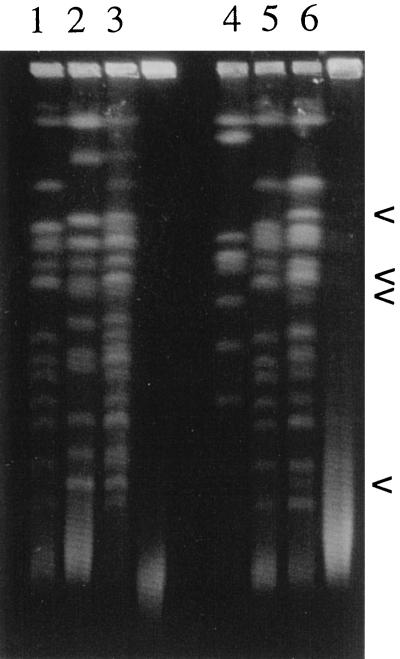
FIG. 2.
Karyotypes of two interspecific hybrids and their parental strains. Lanes: 1, S. bayanus; 2, S. cerevisiae; 3, hybrid between S. bayanus and S. cerevisiae, Y280; 4, S. castellii; 5, S. bayanus; 6, hybrid between S. bayanus and S. castellii, Y500. The interspecific hybrid produced by crossing yeasts belonging to the sensu stricto group shown in lane 3 displays both sets of parental chromosomes, whereas the interspecific hybrid produced by crossing a sensu stricto yeast and a sensu lato yeast shown in lane 6 displays most of the chromosomes from the sensu stricto parent, an extra chromosome band originating from the sensu lato parent, and some bands of a modified size or an unknown origin (arrows).
In hybrids for which at least one of the parents belonged to the sensu lato group, the situation regarding the viability and genome structure was different from that in the previous case. Most hybrid colonies which appeared on selective medium had low viability when transferred to fresh selective medium. In addition, many of these hybrids showed poor growth even on rich medium. For example, hybrids originating from the cross between S. cerevisiae and CBS 6463 were initially represented by small colonies, which were subsequently difficult to propagate even on YPD medium. Only some of the hybrids could be propagated sufficiently to allow analyses of their nuclear and mitochondrial genomes. In general, only one parental set of chromosomes was found in hybrids of sensu stricto and sensu lato yeast species (Table 5). This chromosomal set was always accompanied by the mtDNA molecule having the same parental origin as the chromosomes (Table 5). It is interesting to point out that in some crosses, such as Y345 × Y257 or Y257 × Y344, the resulting hybrids had one set of chromosomes, but originating from either one parent or the other. Only rarely were additional chromosome bands, in addition to one parental set, observed in some hybrids, such as Y500 and Y507. Apparently such chromosomal bands were inherited from the other parent, or their origin could not be deduced (Fig. 2). Sensu stricto-to-sensu stricto hybrids could sporulate at a very low frequency, and a majority of spores were not viable. A majority of sensu stricto-to-sensu lato and sensu lato-to-sensu lato hybrids could not sporulate; for example, Y500 and Y507 did not sporulate.
DISCUSSION
The genus Saccharomyces contains a number of species which differ from one another in various phenotypic characteristics (2). Among the most polymorphic characteristics are the number, size, and organization of nuclear chromosomes and the size and organization of mtDNA (17, 19, 22). DNA relatedness studies and gene sequencing provided the first avenues for understanding the evolutionarary relationships among modern Saccharomyces yeasts (8, 11). So far, sequence comparisons have been performed on genes coding for rRNA and because of technical reasons not on other genes (for a review, see reference 10). So far our understanding of evolutionary relationships has been based on the assumption that each of the modern species is monophyletic and genetically isolated from other species. If so, then the sequence of any piece of the genome should reflect the evolutionary history of the whole genome, as well as a phylogenetic relationship with other species. However, if horizontal transfer of genetic material still operates among species, then analysis of a single gene and comparison of it with other genes may lead to misleading information about evolutionary relationships.
The present project has established a laboratory model for studying horizontal gene transfer among Saccharomyces yeasts. For this purpose, different mating-competent yeasts were prepared. Some of these strains, such as Y339, Y345, and Y344, were isolated upon screening natural isolates from different collections. Some others, such as Y244, Y245, and Y257, were constructed by gene disruption or mutagenesis.
S. cerevisiae strains sensitive to pheromones were able to recognize sexual factors secreted by any of the putative haploid, heterothallic strains of the tested Saccharomyces yeast species (Table 2). When these yeasts were exposed to each other in many combinations, interspecific zygotes were detected (Fig. 1 and Table 3). Therefore, no absolute, interspecific barriers exist in the mating process among the Saccharomyces yeasts. Apparently, different species could recognize each others’ pheromones, cells fused, and karyogamy took place (Tables 2 and 3). Interspecific hybrids were generated and existed for at least a short time. While mating between cells of yeasts from different species was possible, it happened with a lower frequency than intraspecific mating between cells of strains of the same species, e.g., S. cerevisiae (Table 3). In general, the less related the species were, the lower the frequency of zygotes was (Table 3). The S. cerevisiae mating type system was followed in crosses where at least one sensu stricto yeast was involved, i.e., MATα cells from one species mated with MATa cells from another, and vice versa (Table 3). In a number of crosses, particularly when sensu lato species were crossed, cells classified as MATa of one species mated with MATa cells from another species, or MATα cells mated with MATα cells (Table 3). This observation is difficult to explain. However, bisexual behavior of Saccharomyces yeasts has been reported earlier (21). Nevertheless, the most interesting result of these crosses is that hybrids were generated at all.
Saccharomyces sensu stricto yeasts are believed to have homologous chromosomes; i.e., the order of genes is largely preserved among different species (7, 20). Interestingly, viable hybrids between two sensu stricto yeasts, S. cerevisiae and S. bayanus, displayed both sets of parental chromosomes (Tables 5 and 6 and Fig. 2). These hybrids were stable and could in general propagate themselves through mitosis during many generations without undergoing any apparent rearrangements of their nuclear genome. It seems that both parental sets of chromosomes were compatible and could coexist in the same cell (Fig. 3). On the other hand, mtDNA was inherited from only one parent. If both parental mtDNA molecules were present in the zygotes, the S. cerevisiae mtDNA molecules outcompeted the S. bayanus mtDNA molecule and were preferentially transmitted to the progeny. When the S. cerevisiae parent did not have a functional mtDNA molecule, the S. bayanus mtDNA molecule was transmitted successfully and persisted among the progeny (Table 6). Thus, the hybrid nuclear background can accommodate either of the parental mtDNA molecules. However, the frequency of viable hybrids, i.e., the frequency of zygotes able to go through mitosis and generate a yeast colony, was lower in the case of interspecific crosses than in the case of intraspecific crosses (Table 4). A great part of zygotes obtained upon interspecific mating did not give viable hybrids. Apparently, a weak species barrier exists between S. cerevisiae and S. bayanus. In any case, natural hybrids of sensu stricto yeasts exist in nature (6, 12), and interspecific matings may have contributed to the polymorphism observed among sensu stricto yeasts.
FIG. 3.
Schematic illustration of mating events within the genus Saccharomyces. (A) A hybrid produced by crossing two sensu stricto yeasts contains chromosomes from both parents but mtDNA from only one parent. In the case of a sensu stricto-to-sensu lato cross (B) or a sensu lato-to-sensu lato cross (C), predominantly only one parental set, plus some fragments from the other parent, are observed.
Members of the sensu lato group have from 7 to 16 chromosomes, which so far are only poorly characterized. Preservation of gene order seems to be limited (17b). Furthermore, mtDNAs vary in size, in gene order, and also in their introns (17a, 19). In interspecific crosses, S. cerevisiae could give zygotes upon mating with several other yeast strains belonging to the sensu lato group. However, interspecific zygotes produced by mating between yeasts belonging to the sensu stricto and the sensu lato groups appeared at a frequency several times lower than in the case of crosses within the sensu stricto group (Table 3). Only a small fraction of these zygotes developed further and gave rise to viable hybrids. A similar situation existed in the sensu lato-to-sensu lato crosses (Table 4). In general, putative hybrids inherited nuclear DNA and mtDNA from only one of the two parents. In some cases, putative hybrids contained a complete set of chromosomes from one parent, as well as extra chromosomes from the other parent (Fig. 2 and 3). Moreover, sometimes novel chromosome bands appeared. These results indicate that both parental sets of chromosomes were not compatible within one cell; subsequently, during early mitotic divisions, one set of chromosomes was wholly or partially lost (Fig. 3). Apparently, at most a limited number of genes from one of the parents was retained as a separate chromosome or perhaps by integration into one or more of the chromosomes of the other parent (Fig. 3).
In conclusion, interspecific hybrids could be obtained by crossing yeasts belonging to the genus Saccharomyces. However, genome integrity and stability seem to differ in hybrids produced by crossing closely and less closely related yeasts. In the case of a close phylogenetic relationship, i.e., within the sensu stricto group, interspecific zygotes can be obtained at a high frequency, and some of them can give rise to stable hybrids possessing both sets of parental chromosomes. Thus, such parental genomes can coexist. In the case of lesser phylogenetic and structural relationships, as in crosses between sensu stricto and sensu lato yeasts or crosses between different sensu lato yeasts, interspecific zygotes appeared with low frequency, and only a very small fraction of them resulted in interspecific hybrids. In these cases, the two parental sets of chromosomes seem not to be compatible, and extensive rearrangements occur. It appears that similar organization between two parental sets of chromosomes correlates with the coexistence of two parental chromosome sets in the nuclei of hybrid cells and their stability.
The results described above prove that horizontal transfer of genetic material is possible among the modern species of the genus Saccharomyces. If this mechanism operated also in the past, then the modern genomes may not have a simple monophyletic origin. Thus, when sequence analysis is used to determine the relationship among Saccharomyces yeasts, several independent genes should be studied.
ACKNOWLEDGMENTS
This work was supported by grants from the Danish Natural Science Research Council (SNF), the Carlsberg Foundation, the Plasmid Foundation, and the Novo Nordisk Foundation. Gaelle Marinoni and Martine Manuel acknowledge training support from the EU Erasmus/Socrates program and the Technical University of Denmark.
Judita Gartner is acknowledged for help in isolation of heterothallic mutants. Torsten Nilsson-Tillgren, Albert Kahn, and Gennadi Naumov are acknowledged for their comments on this work.
REFERENCES
- 1.Barker E R, Miller M W. Some properties of Saccharomyces kluyveri. Antonie van Leeuwenhoek. 1969;35:159–171. doi: 10.1007/BF02219126. [DOI] [PubMed] [Google Scholar]
- 2.Barnett J A. The taxonomy of the genus Saccharomyces Meyen ex Reess: a short review for non-taxonomists. Yeasts. 1992;8:1–23. [Google Scholar]
- 3.Defontaine A, Lecocq F M, Hallet J N. A rapid miniprep method for the preparation of yeast mitochondrial DNA. Nucleic Acids Res. 1991;19:185. doi: 10.1093/nar/19.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.de Zamaroczy M, Bernardi G. Sequence organization of the mitochondrial genome of yeast—a review. Gene. 1985;37:1–17. doi: 10.1016/0378-1119(85)90252-5. [DOI] [PubMed] [Google Scholar]
- 4.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philippsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:546–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 5.Groth C. Saccharomyces sensu stricto yeasts: characterization of mitochondrial DNA. Masters Thesis. Denmark: University of Copenhagen; 1998. [Google Scholar]
- 6.Groth, C., J. Hansen, and J. Piskur. A natural chimeric yeast containing genetic material from three species. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 7.Hunter N, Chambers S R, Louis E J, Borts R H. The mismatch repair system contributes to meiotic sterility in an interspecific yeast hybrid. EMBO J. 1996;15:1726–1733. [PMC free article] [PubMed] [Google Scholar]
- 8.James S A, Cai J, Roberts I N, Collins M D. A phylogenetic analysis of the genus Saccharomyces based on 18S rRNA gene sequences: description of Saccharomyces kunashirensis sp. nov. and Saccharomyces martinae sp. nov. Int J Syst Bacteriol. 1997;47:453–460. doi: 10.1099/00207713-47-2-453. [DOI] [PubMed] [Google Scholar]
- 9.Kielland-Brandt M, Nilsson-Tillgren T, Gjermansen C, Holmberg S, Pedersen M B. Genetics of brewing yeasts. In: Rose A H, Wheals E, Harrison J S, editors. The yeasts. Vol. 6. London, United Kingdom: Academic Press; 1995. pp. 223–254. [Google Scholar]
- 10.Kurtzman C P, Fell J W. The yeasts: a taxonomic study. 4th ed. Amsterdam, The Netherlands: Elsevier; 1998. [Google Scholar]
- 11.Kurtzman C P, Robnett C J. Phylogenetic relationships among species of Saccharomyces, Debaryomyces and Schwanniomyces determined from partial ribosomal RNA sequences. Yeast. 1991;7:61–72. doi: 10.1002/yea.320070107. [DOI] [PubMed] [Google Scholar]
- 12.Masneuf I, Hansen J, Groth C, Piskur J, Dubourdieu D. New hybrids between Saccharomyces sensu stricto yeast species found among wine and cider production strains. Appl Environ Microbiol. 1998;64:3887–3892. doi: 10.1128/aem.64.10.3887-3892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough J, Herskowitz I. Mating pheromones of Saccharomyces kluyveri: pheromone interactions between Saccharomyces kluyveri and Saccharomyces cerevisiae. J Bacteriol. 1979;138:146–154. doi: 10.1128/jb.138.1.146-154.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meiron H, Nahon E, Raveh D. Identification of the heterothallic mutation in HO-endonuclease of S. cerevisiae using HO/ho chimeric genes. Curr Genet. 1995;28:367–373. doi: 10.1007/BF00326435. [DOI] [PubMed] [Google Scholar]
- 15.Naumov G I. Genetic identification of biological species in the Saccharomyces sensu stricto complex. J Ind Microbiol. 1996;17:295–302. [Google Scholar]
- 16.Naumov G I, Naumova E S, Marinoni G, Piskur J. Genetic analysis of S. castellii, S. exiguus and S. martiniae yeasts. Genetika. 1998;34:565–568. [PubMed] [Google Scholar]
- 17.Petersen, R. F., T. Nilsson-Tillgren, and J. Piskur. Karyotypes of Saccharomyces sensu lato species. Int. J. Syst. Bacteriol., in press. [DOI] [PubMed]
- 17a.Petersen R F. Genome dynamics and evolution of the mitochondrial and nuclear genomes in Saccharomyces sensu lato species. Ph.D. Thesis. Denmark: University of Copenhagen; 1998. [Google Scholar]
- 17b.Piskur, J. Unpublished data.
- 18.Piskur J. The transmission disadvantage of yeast mitochondrial intergenic mutants is eliminated in the mgt1 (cce1) background. J Bacteriol. 1997;179:5614–5617. doi: 10.1128/jb.179.17.5614-5617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piskur J, Smole S, Groth C, Petersen R F, Pedersen M B. Structure and genetic stability of the mitochondrial genomes vary among yeasts of the genus Saccharomyces. Int J Syst Bacteriol. 1998;48:1015–1024. doi: 10.1099/00207713-48-3-1015. [DOI] [PubMed] [Google Scholar]
- 20.Ryu S L, Murooka Y, Kaneko Y. Genomic reorganization between two sibling yeast species, Saccharomyces bayanus and Saccharomyces cerevisiae. Yeast. 1996;12:757–764. doi: 10.1002/(sici)1097-0061(19960630)12:8<757::aid-yea970>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 21.Spencer J F T, Spencer D M. Apparent bisexual behavior of yeast strains obtained from hybridization of industrial yeasts of the genus Saccharomyces with auxotrophic diploids. Antonie van Leeuwenhoek. 1977;43:245–254. doi: 10.1007/BF02313752. [DOI] [PubMed] [Google Scholar]
- 22.Vaughan-Martini A, Martini A, Cardinali G. Electrotypic karyotyping as a taxonomic tool in the genus Saccharomyces. Antonie van Leeuwenhoek. 1993;63:145–156. doi: 10.1007/BF00872389. [DOI] [PubMed] [Google Scholar]
- 23.Wolfe K H, Shields D C. Molecular evidence for an ancient duplication of the entire yeast genome. Nature. 1997;387:708–713. doi: 10.1038/42711. [DOI] [PubMed] [Google Scholar]