Abstract
Purpose
To assess the inhibitory effect of acupuncture on pain symptoms in migraine models, and to further summarize the potential mechanisms of acupuncture in regulating hyperalgesia in the treatment of migraine.
Materials and Methods
Literature search in databases such as China National Knowledge Infrastructure (CNKI), PubMed, and Web of Science (WOS) etc. The quality was evaluated by the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) bias risk assessment tool and Collaborative Approach to Meta-analysis and Review of Animal Data from Experimental Studies (CAMARADES) checklist. Meta-analyses were performed using Stata 17.0 software.
Results
Twenty-one studies involving 489 animals were identified. The qualitative score ranged from 3 to 9 points. Facial mechanical withdrawal threshold (FMWT) and paw mechanical withdrawal threshold (PMWT) measured by Von Frey filaments were selected as major outcomes, and serum calcitonin gene-related peptide (CGRP) levels measured by ELISA were selected as secondary outcome. Meta-analysis results revealed that true acupuncture (TA) group significantly increased FMWT, PMWT and CGRP compared to model group. TA group showed superior effect in FMWT, PMWT relative to sham acupuncture (SA) group. Subgroup analysis results showed that high risk of bias scores may be responsible for the high heterogeneity of FMWT; additionally, CGRP analysis suggests that acupoint selection and blood collection sites may be sources of heterogeneity. In the treatment of migraine pain symptoms, the underlying mechanism of acupuncture treatment is either the regulation of hyperalgesia and neurotransmitters, or the reduction of inflammatory factors.
Conclusion
The results indicate that TA treatment effectively increased the pain threshold and reduced hyperalgesia in migraine rats. In summary, our study highlights the potential of TA as an effective treatment for migraine, but further investigation is required to fully comprehend its mechanism of action and optimize its clinical application.
Keywords: migraine, acupuncture, hyperalgesia, systematic review, meta-analysis
Introduction
Migraine is a chronic neurological disorder which is characterized by attacks of moderate or severe unilateral throbbing and pulsating headaches.1 According to the latest Global Burden of Disease (GBD) study in 2019, migraine directly affects more than 1 billion individuals worldwide, and Migraine is a highly disabling neurological disorder, which remains second among the world’s causes of disability, and first among young women.2–4 Meantime, it was the second leading cause of disability among men and women across all age groups.4 The most characteristic symptoms associated with migraine include photophobia, phonophobia and cutaneous allodynia.5 Cutaneous allodynia is a sensation of pain or discomfort in response to a normal, non-noxious stimulus that develops in 50–70% of migraineurs, and it is believed to result from central sensitization of the trigeminovascular pain pathway.6,7 The pain associated with migraine is a major cause of its accompanying disability and can impact almost every aspect of daily living.8,9
Patients are unsatisfied with commonly used migraine drugs because of the insufficient control of pain and the disturbing adverse events.10–13 As a safe non-pharmacological therapy, the role of acupuncture in reducing the frequency of migraine attacks and reducing the intensity of pain has been demonstrated in clinical trials and systematic reviews.14–16 Although confirmed to be a generally safe and effective therapy, the mechanisms underlying the analgesia effect of acupuncture on migraine have not been fully clarified. Animal studies investigating the mechanism underlying acupuncture’s analgesic effect in migraine have developed rapidly over the last decade. Facial mechanical withdrawal threshold, paw mechanical withdrawal threshold and calcitonin gene-related peptide (CGRP) were commonly used in previous studies as pain indicators.17–19 However, the efficacy and mechanisms of acupuncture in the treatment of experimentally induced migraine (either nitroglycerin injection or neurostimulation-induced animal models) have not been systematically investigated yet.
Pre-clinical systematic reviews identify where there is a need for further “basic” research, preclude unnecessary study replication, and can contribute to both “reduction” and “refinement” in animal experimentation.20 Furthermore, a systematic review based on animal data can not only provide methodological and experimental design references for animal experiments but also provide information for future clinical research directions, so as to clearly clarify the potential therapeutic mechanism and clinical efficacy of acupuncture. Therefore, we systematically reviewed and meta-analyzed the analgesia effect of acupuncture in migraine rat models, and the mechanism of the analgesia effect of acupuncture on migraine was also discussed.
Materials and Methods
Material and Methods
This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines21 and the protocol of this systematic review and meta-analysis was published on the PROSPERO (registration no. CRD42022333841).
Search Strategy
China National Knowledge Infrastructure (CNKI), VIP Database for Chinese Technical Periodicals (VIP), WANFANG Database (WF), Chinese biomedical literature service system (Sino Med), PubMed, Web of Science (WOS), and Embase were all searched between January 2013 and April 2022. The search terms and basic search strategy were as follows: (“animal study” OR “rat” OR “mice” OR “mechanism” OR “preclinical study”) AND (“migraine” OR “migraine disorders”) AND (“acupuncture” OR “acupuncture therapy” OR “electroacupuncture” OR “acupoints”). Table 1 displayed detailed search strategies. Reference lists of eligible studies and previous reviews were also reviewed to identify further eligible studies.
Table 1.
Search Strategy in PubMed
| Steps | Search |
|---|---|
| #1 | “Migraine disorders” [MeSH] OR “migraine” [Ti/Ab] Filters: from 2013–2022 |
| #2 | “Acupuncture” [MeSH] OR “acupuncture therapy” [Ti/Ab] OR “electroacupuncture” [Ti/Ab] OR “acupoints” [Ti/Ab] Filters: from 2013–2022 |
| #3 | “Rats” [MeSH] OR “mice” [MeSH] OR “animal study” [Ti/Ab] OR “mechanism” [Ti/Ab] OR “preclinical study” [Ti/Ab] Filters: from 2013–2022 |
| #4 | #1 AND #2 AND #3 |
Inclusion Criteria
Types of Subjects. Rat model of migraine which induced by nitroglycerin or inflammatory soup or dural electrical stimuli.
Types of Interventions. Only true acupuncture (TA) was included, which covers both manual-acupuncture and electroacupuncture.
Types of Controls. Migraine model group or sham acupuncture (SA) group.
Types of Outcomes. Mechanical withdrawal threshold measured by Von Frey filaments including facial mechanical withdrawal threshold (FMWT), paw mechanical withdrawal threshold (PMWT), 50% facial mechanical withdrawal threshold (50% FMWT) and 50% paw mechanical withdrawal threshold (50% PMWT), serum calcitonin gene-related peptide (CGRP) levels, measured by ELISA.
Types of Studies Included. All controlled experiments of the effect of acupuncture in a rat model of migraine were included.
Exclusion Criteria
Other acupuncture techniques including laser acupuncture, auricular acupuncture, acupoint injection, etc. Acupuncture therapies combined with other forms of acupuncture (such as auricular acupuncture, acupoint injection, moxibustion, etc.), traditional Chinese medicine or western medication.
Studies which does not include migraine model group or SA group.
Studies did not use mechanical withdrawal threshold or serum CGRP levels as outcomes.
Case reports, clinical trials, cross-over studies, reviews, meta-analyses, or meeting abstracts.
Duplicate publications.
Study Selection
Before selecting articles, all reviewers got professional training to comprehend the review’s aim and procedure. The search strategy was created by two reviewers (SSQ and LL), who also searched the databases. The results of the search were saved and duplicates were removed using EndNote software (version X9). Two reviewers (ZMD and LY) independently evaluated and screened the studies using the inclusion and exclusion criteria. A third reviewer was consulted to resolve disagreements. The exclusion reasons of studies were documented.
Data Extraction
The data was extracted and proofread independently by two reviewers (SSQ and LL). The following information was extracted: the first author’s last name, year of publication, animal strain, gender, migraine modeling methodology, number of animals in intervention or control groups, acupuncture manipulation methods (acupoints, electrical stimulator parameters, depth, sessions and retention time), the underlying mechanism of acupuncture, outcome measures, data of mean outcome and standard deviation. If only a chart was used to display the results, the data was gathered by using the software GetData Graph Digitizer22,23 to accurately measure the values. We attempted to contact the authors to collect the detailed data if the meta-analysis data was missing. Disagreements during data cross-checking were settled by consulting a third reviewer.
Risk of Bias Assessment
Systematic Review Centre for Laboratory animal Experimentation (SYRCLE) risk of bias tool was used to evaluate the risk of bias of the included studies by two independent investigators (ZMD and LY) as previously reported.24 Disagreements were settled through consensus or consultation with a third investigator (SMS). The ten domains evaluated as low, unclear or high risk of bias were sequence generation, baseline characteristics, allocation concealment, random housing, blinding of participants/personnel, random outcome assessment, blinding of the assessor, incomplete outcome data, selective outcome reporting and other bias. Blinding of participants/personnel was considered “not applicable” as proper blinding of the acupuncture giver was not possible.
Quality of Evidence
The methodological quality was assessed by using the CAMARADES 10-item checklist:16 (1) peer-reviewed journal; (2) temperature control; (3) animals were randomly allocated; (4) blind established model; (5) blinded outcome assessment; (6) reporting of animals excluded from analysis; (7) appropriate animal model; (8) calculation of sample size; (9) statement of compliance with animal welfare regulations; (10) possible conflicts of interest. One point was assigned to each of the ten items on the scale. Each study was given a quality score ranging from zero to ten. The greater the score, the higher the quality of the article. The average of the quality scores was then calculated. Two reviewers (SSQ and LL) assessed the risk of bias, and any disagreement was arbitrated by a third reviewer.
Statistical Analysis
We synthesized the data if at least three studies reported the same outcome’s results, so we evaluated five outcomes separately (FMWT, PMWT, 50% FMWT, 50% PMWT and serum CGRP levels). First, we performed a meta-analysis of studies that compared the TA group to the model group or the SA group. Meta-analyses were performed using Stata 17.0 software. The continuous outcome results with same unit in this study were described as weighted mean difference (WMD). The heterogeneity of included studies was evaluated by I2. If the heterogeneity was not obvious (P>0.1; I2≤50%), the fixed effect models were adopted. If the heterogeneity was obvious (P≤0.1; I2≤50%), the random effect models were used. Sensitivity analyses were carried out by removing one study at a time from the pooled studies. Subgroup analysis was conducted for the following factors: acupoint selection, acupuncture sessions, duration of each treatment, risk of bias assessment. Serum CGRP level data were also analyzed for subgroup analyses of blood collection sites. If at least ten studies are included in a meta-analysis of the outcomes, funnel plots were used to assess the possibility of publication bias.
Results
Search Results
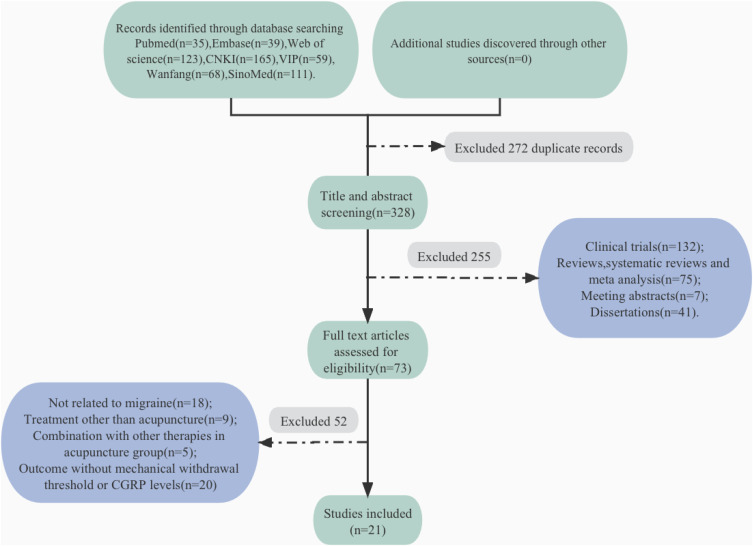
Figure 1 illustrates the process of inclusion and exclusion. A total of 600 potentially relevant records were identified from seven databases, after removing duplicates, 328 articles remained. We screened the titles and the abstracts of 328 remaining records, and 255 records were excluded. Full-text screening was performed for the remaining 73 studies, of which 52 were excluded. As a result, this review included a total of 21 studies.
Figure 1.
Flow diagram of study selection process.
Abbreviations: CNKI, China National Knowledge Infrastructure; VIP, VIP Database for Chinese Technical Periodical; Sino Med, Chinese biomedical literature service system.
Study Characteristics
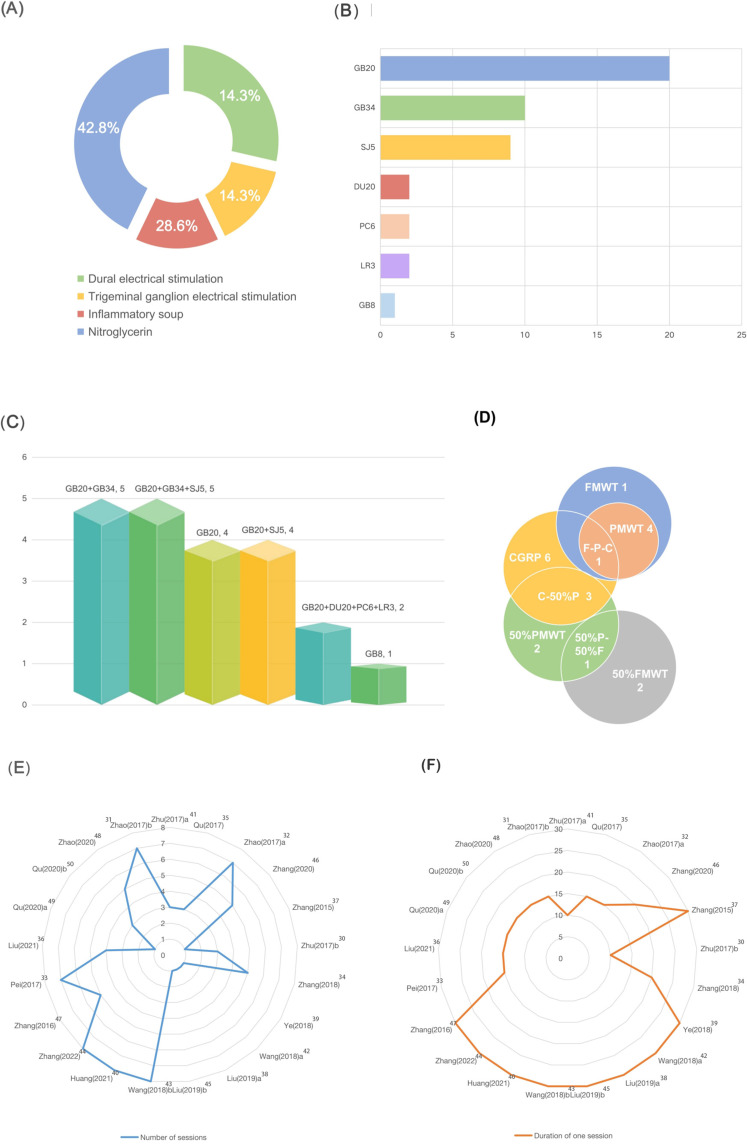
The 21 studies included 489 rats, 192 in the acupuncture group and 297 in the control groups. Except for two studies using Wistar rats, Sprague Dawley rats were applied. Of these, 16 studies using male rats and 5 studies using both male and female rats. Of the migraine models used in these studies, 6 used a dural electrical stimulation-induced migraine model, 3 used electrical stimulation of the trigeminal ganglia (TG), 9 used a nitroglycerin-induced migraine model and 3 used an inflammatory soup-induced migraine model. Fourteen studies mentioned the method to determine whether the migraine model was successfully established. Acupuncture was used in 21 studies. Five studies selected acupoints Fengchi (GB20) and Yanglingquan (GB34). Five studies used acupoints Fengchi (GB20), Yanglingquan (GB34) and Waiguan (SJ5). Four studies chose acupoint Fengchi (GB20) only. Four studies selected Fengchi (GB20) and Waiguan (SJ5). Two studies selected Fengchi (GB20), Baihui (DU20), Neiguan (PC6) and Taichong (LR3). One study only selected acupoint Shuaigu (GB8). The treatment course varied from 10 min to 30 min, and the number of sessions varied from 1 to 8. Of the 21 studies, 2 used manual acupuncture and the rest used EA. Studies using EA all mentioned stimulus parameter. Nineteen studies used disperse-dense waves. Most frequently used disperse-dense wave was 2/15 Hz, and most frequently used current intensity was 0.5–1mA. All studies used migraine model groups or SA groups as control groups. Outcome measures were consisted of FMWT (7 studies), PMWT (5 studies), 50% FMWT (3 studies), 50% PMWT (6 studies) and serum CGRP levels (10 studies). Study characteristics are shown in Table 2 and Figure 2.
Table 2.
Characteristics of the 21 Included Studies
| Study (Year) | Animals (Species, Sex) | Modeling Method | Control Group (n) | Intervention Group | Outcome Measurement | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Method (n) | Stimulator Parameters | Acupoints | Acupuncture Session, Retention Time | Insertion Depth | ||||||
| Zhu (2017) a | SD rats, male | Dural electrical stimulation | Migraine group (8) SA group (8) |
EA (8) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20, GB34 | 3 sessions; 10 min |
2–3 mm | FMWT | [25] |
| Qu (2017) | SD rats, male | Dural electrical stimulation | Migraine group (6) SA group (6) |
EA (6) | Alternating frequency of 2/15 Hz; 0.5–1mA intensity |
GB20, GB34 | 3 sessions; 10 min |
GB20: 8 mm; GB34: 5 mm | FMWT PMWT |
[26] |
| Zhao (2017) a | SD rats, male | Dural electrical stimulation | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20, GB34 | 7 sessions; 15 min |
NM | FMWT | [27] |
| Zhang (2020) | SD rats, male | Trigeminal ganglion electrical stimulation | Migraine group (10) | EA (10) | Alternating frequency of 2/15 Hz; 1mA intensity |
GB20, SJ5 | 5 sessions; 20min |
2–3mm | FMWT | [28] |
| Zhang (2015) | SD rats, male | Trigeminal ganglion electrical stimulation | Migraine group (10) | EA (10) | Alternating frequency of 2/15 Hz; 1mA intensity |
GB20, SJ5 | 1 session; 30min |
2–3mm | CGRP | [29] |
| Zhu (2017) b | SD rats, male | Nitroglycerin | Migraine group (8) | EA (8) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20 | 3 sessions; 10 min |
2–3mm | FMWT PMWT |
[30] |
| Zhang (2018) | SD rats, male | Nitroglycerin | Migraine group (5) | EA (5) | Alternating frequency of 2/15 Hz; 1mA intensity |
GB20, SJ5 | 5 sessions; 20min |
2–3mm | CGRP | [31] |
| Ye (2018) | SD rats, both male and female | Nitroglycerin | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 10/50 Hz; 0.5–1 mA intensity |
GB20, GB34, SJ5 | 1 session; 30 min |
GB20: 2–3mm; SJ5: 1mm; GB34: 6mm | 50% PMWT | [32] |
| Wang (2018) a | SD rats, both male and female | Nitroglycerin | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 2/100 Hz; NM |
GB20, GB34, SJ5 | 1 session; 30 min |
3–10mm | 50% PMWT | [33] |
| Liu (2019) a | SD rats, both male and female | Nitroglycerin | Migraine group (10) | EA (10) | Alternating frequency of 10/50 Hz; 0.5–1 mA intensity |
GB20, GB34, SJ5 | 1 session; 30 min |
1–6mm | 50% PMWT | [34] |
| Liu (2019) b | SD rats, both male and female | Nitroglycerin | Migraine group (10) | EA (10) | Alternating frequency of 10/50 Hz; 0.5–1 mA intensity |
GB20, GB34, SJ5 | 1 session; 30 min |
1–6mm | 50% PMWT | [35] |
| Wang (2018) b | Wistar rats, | Nitroglycerin | Migraine group (8) | MA (8) | / | GB20, DU20, PC6, LR3 | 8 sessions; 30min |
1–2mm | CGRP | [36] |
| Huang (2021) | Wistar rats, | Nitroglycerin | Migraine group (8) | MA (8) | / | GB20, DU20, PC6, LR3 | 8 sessions; 30min |
1–2mm | CGRP | [37] |
| Zhang (2022) | SD rats, both male and female | Nitroglycerin | Migraine group (10) | EA (10) | Alternating frequency of 10/50 Hz; 0.5–1 mA intensity |
GB20, GB34, SJ5 | 8 sessions; 30 min |
1–6mm | 50% PMWT | [38] |
| Zhang (2016) | SD rats, male | Trigeminal ganglion electrical stimulation | Migraine group (8) | EA (8) | Alternating frequency of 2/15 Hz; 1mA intensity |
GB20, SJ5 | 5 sessions; 30min |
2–3mm | CGRP | [39] |
| Pei (2017) | SD rats, male | Dural electrical stimulation | Migraine group (16) SA group (16) |
EA (16) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20 | 7 sessions; 15 min |
2–3mm | FMWT PMWT |
[40] |
| Liu (2021) | SD rats, male | Inflammatory soup | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 2/15 Hz; 1 mA intensity |
GB20, GB34 | 4 sessions; 15 min |
1.5 mm | 50% FMWT 50% PMWT |
[41] |
| Qu (2020) a | SD rats, male | Inflammatory soup | Migraine group (7) SA group (7) |
EA (7) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB8 | 1 session; 15 min |
8mm | 50% FMWT | [42] |
| Qu (2020) b | SD rats, male | Inflammatory soup | Migraine group (8) SA group (8) |
EA (8) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20 | 3 sessions; 15 min |
8mm | 50% FMWT | [43] |
| Zhao (2020) | SD rats, male | Dural electrical stimulation | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20, GB34 | 5 sessions; 15 min |
2–3mm | FMWT PMWT |
[44] |
| Zhao (2017) b | SD rats, male | Dural electrical stimulation | Migraine group (10) SA group (10) |
EA (10) | Alternating frequency of 2/15 Hz; 0.5–1 mA intensity |
GB20 | 7 sessions; 15 min |
2–3mm | FMWT PMWT |
[49] |
Notes: The first column uses the combination of the author’s last name and the year of publication of the article as the pronoun for the article, and the suffix a or b is used to distinguish it if the name is repeated; In addition, what is not mentioned in the article is unknown in the table with/denotation.
Abbreviations: SD, Sprague Dawley; SA, sham acupuncture; EA, electroacupuncture; MA, manual acupuncture; FMWT, facial mechanical withdrawal threshold; PMWT, paw mechanical withdrawal threshold; 50% FMWT, 50% facial mechanical withdrawal threshold; 50% PMWT, 50% paw mechanical withdrawal threshold; NM, not mentioned; GB20, Fengchi; GB34, Yanglignquan; GB8, Shuaigu; SJ5, Waiguan.
Figure 2.
Characteristics of the 21 included studies.
Notes: (A) Schematic diagram of the proportion of different animal models in the included article. (B) Number of different acupoints selected for the treatment of migraine model animals in the included articles. (C) The proportion of combinations of different acupoints in the included articles. (D) Statistics on the number of times different outcomes were used in the included articles. (E) The duration of treatment compared between included articles. (F) The duration of a single treatment compared between included articles.
Abbreviations: FMWT, facial mechanical withdrawal threshold; PMWT, paw mechanical withdrawal threshold; 50% FMWT, 50% facial mechanical withdrawal threshold; 50% PMWT, 50% paw mechanical withdrawal threshold; GB8, Shuaigu; LR3, Taichong; PC6, Neiguan; DU20, Baihui; SJ5, Waiguan; GB34, Yanglignquan; GB20, Fengchi.
Risk of Bias and Quality Assessment
Ten items were evaluated according to the SYRCLE risk of bias tool.15 The blinding of participants was considered not applicable as mentioned above. We found that 14 studies described detailed sequence generation, 7 studies only mentioned randomness without describing specific random methods. All the studies mentioned that each group was similar at baseline. All the studies stated random housing of the animals and were thought to have low risk of selective outcome reporting and other bias. Allocation concealment and randomly selecting animals for outcome evaluation were unclear in included studies. Eleven studies successfully blinded the outcome evaluators or not blinded but did not affect their outcome measures. Nineteen studies were free of incomplete outcome data, and two studies were influenced by incomplete outcome data, primarily due to model failure, but they did not specify whether the incomplete outcome data was imputed using appropriate methods. The score of risk of bias assessment ranged from 4 to 7 (mean 5.86) (Table 3). Ten items were evaluated according to the CAMARADES checklist.45 All the 21 studies used appropriate animal model and were peer-reviewed. Nineteen studies included statements describing temperature control. Only 2 studies mentioned blinding in animal model construction, and only one study reported calculations of sample sizes (via G power software). Four studies reported animals excluded from analysis. Fifteen studies reported compliance with animal welfare regulations and six declared potential conflicts of interest. The quality score of the studies ranged from 3 to 9 (mean 5.71) (Table 4).
Table 3.
Risk of Bias Assessment According to the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) Tool
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Scores | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhu (2017) a | H | L | ? | L | NA | ? | ? | L | L | L | 5 | [25] |
| Qu (2017) | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [26] |
| Zhao (2017) a | H | L | ? | L | NA | ? | ? | ? | L | L | 4 | [27] |
| Zhang (2020) | H | L | ? | L | NA | ? | ? | ? | L | L | 5 | [28] |
| Zhang (2015) | H | L | ? | L | NA | ? | ? | ? | L | L | 5 | [29] |
| Zhu (2017) b | L | L | ? | L | NA | ? | ? | ? | L | L | 5 | [30] |
| Zhang (2018) | L | L | ? | L | NA | ? | L | ? | L | L | 6 | [31] |
| Ye (2018) | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [32] |
| Wang (2018) a | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [33] |
| Liu (2019) a | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [34] |
| Liu (2019) b | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [35] |
| Wang (2018) b | L | L | ? | L | NA | ? | L | ? | L | L | 6 | [36] |
| Huang (2021) | L | L | ? | L | NA | ? | L | ? | L | L | 6 | [37] |
| Zhang (2022) | L | L | ? | L | NA | ? | ? | L | L | L | 6 | [38] |
| Zhang (2016) | L | L | ? | L | NA | ? | L | L | L | L | 7 | [39] |
| Pei (2017) | L | L | ? | L | NA | ? | L | L | L | L | 7 | [40] |
| Liu (2021) | H | L | ? | L | NA | ? | L | L | L | L | 6 | [41] |
| Qu (2020) a | L | L | ? | L | NA | ? | L | L | L | L | 7 | [42] |
| Qu (2020) b | L | L | ? | L | NA | ? | L | L | L | L | 7 | [43] |
| Zhao (2020) | H | L | ? | L | NA | ? | ? | L | L | L | 5 | [44] |
| Zhao (2017) b | H | L | ? | L | NA | ? | L | L | L | L | 6 | [49] |
Notes: (1) Generation of animal allocation sequence was random; (2) each group was similar or was at adjusted at baseline; (3) the allocation was adequately concealed; (4) animals were housed at random; (5) both animal breeders and researchers were blinded for the intervention of each animal received; (6) animals were selected randomly for outcome evaluation; (7) outcome evaluator was blinded; (8) the incomplete outcome data were absolutely addressed; (9) reports of the research were free of selective outcome reporting; (10) study was evidently free of other potential issues which may cause bias.
Abbreviations: L, low-risk bias; H, high risk bias; ?, unclear; NA, not applicable.
Table 4.
Quality Assessment of Included Studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Scores | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zhu (2017) a | Y | Y | Y | N | N | Y | Y | N | Y | N | 6 | [25] |
| Qu (2017) | Y | Y | Y | N | N | N | Y | N | N | N | 4 | [26] |
| Zhao (2017) | Y | Y | Y | N | N | N | Y | N | N | N | 4 | [27] |
| Zhang (2020) | Y | Y | Y | N | Y | N | Y | N | Y | N | 5 | [28] |
| Zhang (2015) | Y | N | Y | N | Y | N | Y | N | Y | N | 5 | [29] |
| Zhu (2017) b | Y | N | Y | N | N | N | Y | N | N | N | 3 | [30] |
| Zhang (2018) | Y | Y | Y | N | Y | N | Y | N | Y | N | 6 | [31] |
| Ye (2018) | Y | Y | Y | N | N | N | Y | N | Y | N | 5 | [32] |
| Wang (2018) | Y | Y | Y | N | N | N | Y | N | N | N | 4 | [33] |
| Liu (2019) a | Y | Y | Y | N | N | N | Y | N | Y | N | 5 | [34] |
| Liu (2019) b | Y | Y | Y | N | N | N | Y | N | Y | N | 5 | [35] |
| Wang (2018) b | Y | Y | Y | N | Y | N | Y | N | Y | N | 6 | [36] |
| Huang (2021) | Y | Y | Y | N | Y | N | Y | N | N | N | 5 | [37] |
| Zhang (2022) | Y | Y | Y | N | N | N | Y | N | N | N | 4 | [38] |
| Zhang (2016) | Y | Y | Y | N | Y | N | Y | Y | Y | Y | 8 | [39] |
| Pei (2017) | Y | Y | Y | N | Y | N | Y | N | Y | Y | 7 | [40] |
| Liu (2021) | Y | Y | Y | N | Y | Y | Y | Y | Y | N | 8 | [41] |
| Qu (2020) a | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 | [42] |
| Qu (2020) b | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | 9 | [43] |
| Zhao (2020) | Y | Y | Y | N | N | N | Y | N | Y | Y | 6 | [44] |
| Zhao (2017) | Y | Y | Y | N | Y | N | Y | Y | Y | Y | 8 | [49] |
Notes: Studies fulfilling the criteria of the following: (1) peer-reviewed journal; (2) temperature control; (3) animals were randomly allocated; (4) blind established model; (5) blinded outcome assessment; (6) reporting of animals excluded from analysis; (7) appropriate animal model; (8) calculation of sample size; (9) statement of compliance with animal welfare regulations; (10) possible conflicts of interest.
Abbreviations: Y, yes; N, no.
Results of Meta-Analysis
FMWT
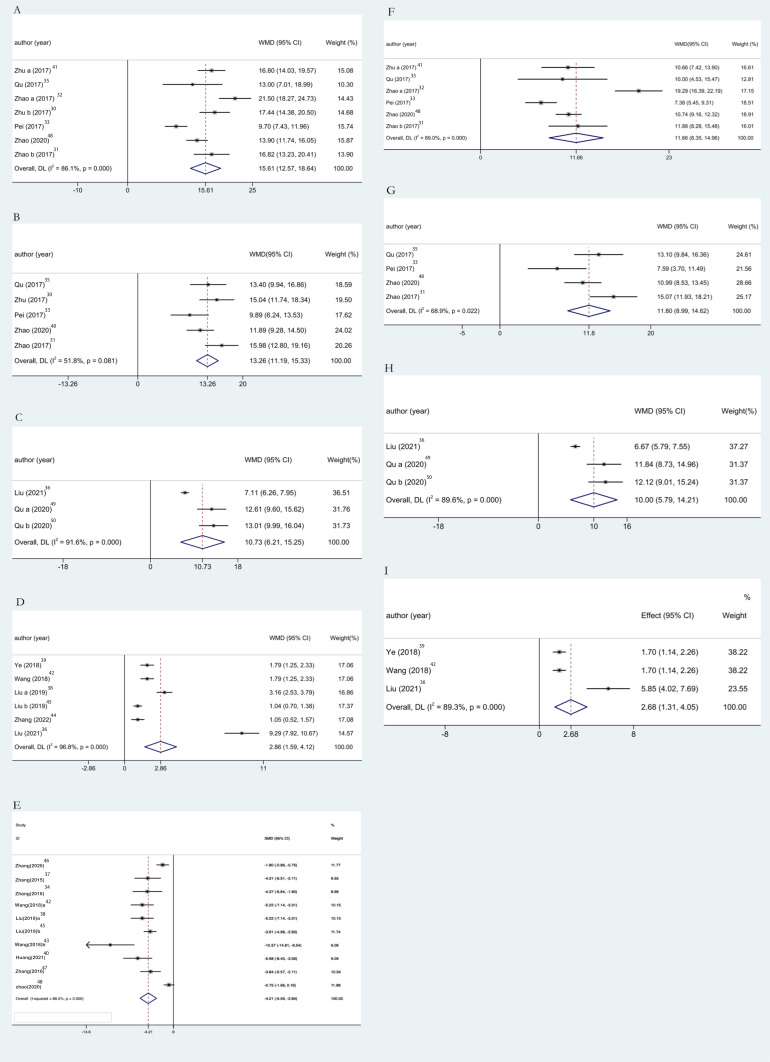
Seven studies including 196 animals conducted the meta-analysis of FMWT value. Compared with the migraine model group, TA group showed a mean increase of FMWT for 15.61g with significant heterogeneity in a pooled analysis of 7 studies (Figure 3A) (WMD 15.61, 95% CI: 12.57 to 18.64, P<0.0001; heterogeneity: chi2: 43.12, df: 6 (P<0.0001), I2= 86.1%, random-effects model). Compared with the SA group, TA group showed a mean increase of FMWT for 11.66g with statistically significant heterogeneity in a pooled analysis of 6 studies (Figure 3F) (WMD 11.66, 95% CI: 8.35 to 14.96, P<0.0001; heterogeneity: chi2: 45.32, df: 5 (P<0.0001), I2= 89.0%, random-effects model). The above results showed that acupuncture can effectively inhibit hyperalgesia while significantly increased FMWT, and its effect value is larger than that of the model group and the SA group, and it can participate in pain suppression more effectively.
Figure 3.
Results of meta-analysis.
Notes: Pool results of acupuncture-treated migraine model rats versus migraine model rats on (A) FMWT, (B) PMWT, (C) 50% FMWT, (D) 50% PMWT and (E) CGRP; and the results of migraine model rats treated with sham-acupuncture on (F) FMWT, (G) PMWT, (H) 50% FMWT and (I) 50% PMWT.
Abbreviations: WMD, weighted mean difference; SMD, standard mean difference.
PMWT
Five studies including 142 animals conducted the meta-analysis of PMWT value. Compared with the migraine model group, TA group showed a mean increase of PMWT for 13.26g in a pooled analysis of 5 studies (Figure 3B) (WMD 13.26, 95% CI: 11.19 to 15.33, P<0.0001; heterogeneity: chi2: 8.29, df: 4 (P=0.081), I2= 51.8%, random-effects model). Compared with the SA group, TA group showed a mean increase of PMWT for 11.8g in a pooled analysis of 4 studies (Figure 3G) (WMD 11.80, 95% CI: 8.99 to 14.62, P<0.0001; heterogeneity: chi2: 9.65, df: 3 (P=0.022), I2= 68.9%, random-effects model). The results showed that TA could significantly improve PMWT and effectively inhibit hyperalgesia.
50% FMWT
Three studies including 72 animals conducted the meta-analysis of 50% FMWT value. Compared with the migraine model group, TA group showed a mean increase of 50% FMWT for 10.73g with significant heterogeneity in a pooled analysis of 3 studies (Figure 3C) (WMD 10.73, 95% CI: 6.21 to 15.25, P<0.0001; heterogeneity: chi2: 23.8, df: 2 (P<0.0001), I2= 91.6%, random-effects model). Compared with the SA group, TA group showed a mean increase of 50% FMWT for 10g with statistically significant heterogeneity in a pooled analysis of 3 studies (Figure 3H) (WMD 10.95% CI: 5.79 to 14.21, P<0.0001; heterogeneity: chi2: 19.27, df: 2 (P<0.0001), I2= 89.6%, random-effects model). In summary, TA significantly increased 50% FMWT compared to the model group and the SA group.
50% PMWT
Six studies including 170 animals conducted the meta-analysis of 50% PMWT value. Compared with the migraine model group, TA group showed a mean increase of 50% PMWT for 2.86g with significant heterogeneity in a pooled analysis of 6 studies (Figure 3D) (WMD 2.86, 95% CI: 1.59 to 4.12, P<0.0001; heterogeneity: chi2: 158.13, df: 5 (P<0.0001), I2= 96.8%, random-effects model). Compared with the SA group, TA group showed a mean increase of 50% PMWT for 2.68g with statistically significant heterogeneity in a pooled analysis of 3 studies (Figure 3I) (WMD 2.68, 95% CI: 1.31 to 4.05, P<0.0001; heterogeneity: chi2: 18.76, df: 2 (P<0.0001), I2=89.3%, random-effects model). It can be seen that TA significantly increased 50% PMWT compared with the model group and the SA group.
Serum CGRP Levels
The combined synthesized finding of 10 studies including 190 animals conducted the meta-analysis of serum CGRP levels. Compared with the migraine model group, TA group showed a mean decrease of serum CGRP levels for 4.58 pg/mL, with significant heterogeneity in a pooled analysis of 10 studies (Figure 3E) (WMD −10.692, 95% CI:-13.089 to −8.295, P<0.0001; heterogeneity: chi2:191.50, df:9 (P<0.0001), I2=95.3%, random-effects model). Briefly, the significant reduction in serum CGRP levels as an objective indicator after TA suggests that acupuncture can effectively inhibit hyperalgesia and participate in the process of pain inhibition.
Sensitivity Analysis and Subgroup Analysis
Significant heterogeneity (I2>50%) was observed in FMWT, PMWT, 50% FMWT, 50% PMWT and CGRP. Thus, random-effects model was applied. To confirm the robustness of our findings, a sensitivity analysis will be conducted based on the different levels of bias of the included studies. A sensitivity analysis was performed for each of the combined data, analysis for FMWT / PMWT / 50% FMWT / 50% PMWT / CGRP revealed that the results did not change significantly when any one study was excluded (Figures S1–S5). We stratified the included studies based on factors to investigate potential factors that influenced the outcome measures, as shown in Tables S1–S6. Due to the small number of studies, subgroup analyses of 50% FMWT comparing TA group and SA group, 50% FMWT comparing TA group and migraine model group, and 50% PMWT comparing TA group and SA group were not performed. The heterogeneity decreased in risk of bias assessment (5 scores: I2=55.7%, P<0.0001; 6 scores: I2=13.0%, P<0.0001) after subgroup analysis of FMWT between TA group and model group, the same results are found in the TA group and the SA group (5 scores: I2=0.00%, P<0.0001; 6 scores: I2=0.00%, P<0.0001). Besides, the subgroup analysis of CGRP between TA group and model group shows that the heterogeneity decreased in acupoint selection (GB20+GB34+SJ5: I2=75.3%, P<0.0001; GB20+DU20+PC6+LR3: I2=0.00%, P<0.0001), although heterogeneity remained significant. It is worth noting that we conducted a subgroup analysis of CGRP for blood collection sites, and the results showed that heterogeneity decreased significantly in blood collection sites (Jugular vein: I2=88.7%, P<0.0001; Abdominal aorta: I2=75.3%, P<0.0001; Abdominal veins: I2=0.00%, P<0.0001) suggesting that differences in blood collection sites may be the source of heterogeneity. Therefore, the risk of bias might be cause of heterogeneity for FMWT; acupoint selection and the blood collection sites may be responsible for the high heterogeneity of CGRP. However, we could not find the causes of heterogeneity for PMWT and 50% PMWT between different groups.
Assessment of Publication Bias
Since there were fewer than 10 included studies for most outcome measure, we did not perform publication bias analysis for all data.46 We assessed only serum CGRP levels, funnel plots of included studies were made to find the publication bias. The results of Egger regression analysis showed that the funnel graph was asymmetric (t=−2.57, P=0.033<0.05), suggesting publication bias (Figure S6).
Proposed Mechanisms
Twenty-one included studies provided detailed descriptions of possible therapeutic mechanisms of acupuncture in the rat model of migraine. Table 5 summarizes the proposed mechanisms.
Table 5.
Potential Mechanism of Included Studies
| Study | Potential Mechanism | Reference |
|---|---|---|
| Zhu (2017) a | Up-regulated 5-HT1B receptor mRNA and protein in TNC and TG tissue of migraine rats | [25] |
| Qu (2017) | Up-regulated 5-HT1F receptor mRNA and protein in TNC, TG and RMg tissue of migraine rats | [26] |
| Zhao (2017) a | Up-regulated 5-HT1D receptor mRNA and protein in TNC and TG tissue of migraine rats | [27] |
| Zhang (2020) | Inhibited miR-34a-5p expression in TG, increase SIRT1 expression, down-regulated IL-1β/ COX-2, reduce PGE2 synthesis, reduce CGRP released from peripheral terminals of migraine rats | [28] |
| Zhang (2015) | Up-regulated CB1 in TG tissue, down-regulated CGRP serum levels of migraine rats | [29] |
| Zhu (2017) b | NM | [30] |
| Zhang (2018) | Inhibited trigeminal nerve-vascular activation by the kynurate pathway, reduced serum CGRP levels of migraine rats | [31] |
| Ye (2018) | Down-regulated NO and ET-1 levels in serum | [32] |
| Wang (2018) | Up-regulated 5-HT in serum, down-regulated CGRP and SP in serum | [33] |
| Liu (2019) a | Down-regulated NO, ET and CGRP levels in serum | [34] |
| Liu (2019) b | Down-regulated CGRP and SP in serum | [35] |
| Wang (2018) b | Inhibited serum inflammatory factors (CGRP, IL-1β, SP TNF-α) | [36] |
| Huang (2021) | Down-regulated 5-HT, CGRP, SP and ADORA1 in migraine rats | [37] |
| Zhang (2022) | Down-regulated IL-6, TNF-α positive cells in brainstem and CGRP mRNA expression in STN | [38] |
| Zhang (2016) | Up-regulated CB1 receptor expression and inhibited TGES-induced changes in IL-1β, COX2, PGE2 and CGRP. | [39] |
| Pei (2017) | Down-regulated 5-HT7R positive cells and protein expression in the PAG, RMg and TNC | [40] |
| Liu (2021) | Down-regulated 5-HT7R positive cells, mRNA expression and protein expression by regulating PKA and ERK 1/2 in TG and TNC | [41] |
| Qu (2020) a | Reduced C-fiber evoked WDR neuronal discharges of the TCC and exerted a DNIC effect | [42] |
| Qu (2020) b | Decreased the discharge rate of neurons in the TCC and regulated the activity state of TCC neurons | [43] |
| Zhao (2020) | Inhibited dural mast cells, macrophages, and serum inflammatory factors (CGRP, IL-1β, IL-6, TNF-α, BDNF and COX-2 in plasma levels) | [44] |
| Zhao (2017) | Inhibited CGRP protein expression in the TG, TNC and VMTN. Inhibited CGRP positive cells in the TG. | [49] |
Abbreviations: TG, trigeminal ganglia; TNC, trigeminal nucleus caudalis; NM, not mentioned; NO, nitric oxide; CB1, cannabinoid receptor 1; PEG2, serum prostaglandin E2; ET-1, endothelin-1; CGRP, calcitonin gene-related peptide; SP, substance P; 5-HT, 5-hydroxytryptamine; ET, endothelin; IL-6, interleukin 6; TNF-α, tumor necrosis factor-alpha; PAG, periaqueductal grey; RMg, raphe magnus nucleus; PKA, phosphorylation of protein kinase A; ERK 1/2, extracellular signal-regulated kinase 1/2; WDR, wide dynamic range; TCC, trigeminocervical complex; DNIC, diffuse noxious inhibitory controls; IL-1β, interleukin 1β; STN, spinal trigeminal nucleus; BDNF, brain derived neurotrophic factor; COX2, cyclooxygenase-2; VMTN, ventroposterior medial thalamic nucleus; ADORA1, Adenosine A1 Receptor.
Discussion
Summary of Findings
Medication treatment is the main treatment for migraine, but its long-time use may increase potential complications. In this study, we included 21 experiments to evaluate whether acupuncture treatment is an effective option for treating migraine, and discuss the mechanism of the analgesia effect of acupuncture on migraine. The review shows that treatment with acupuncture reveal significant increase in FMWT, PMWT, 50% FMWT and 50% PMWT levels when compared to the model group and SA group (P<0.001). This review also indicates that compared to the model group, the levels of CGRP were significantly inhibited in the group treated with acupuncture. The same positive results from previous meta-analyses conducted by Chen47 which indicated that acupuncture could significantly alleviated the decrease in paw and facial withdrawal thresholds and significantly lessened the increase in the levels of serum CGRP, thus showing results in migraine treatment. All the above results suggested that acupuncture could suppress pain symptoms in a rat model of migraine and exerted effective analgesic effects.
Sources of Heterogeneities
The result of sensitivity analysis did not decrease heterogeneity in FWMT between the TA group and model group (I2= 86.1%), FMWT between TA group and the SA group (I2= 89.0%); PMWT between TA group and model group (I2= 51.8%), PMWT between TA group and SA group (I2= 68.9%), 50% FMWT between TA group and model group (I2= 91.6%), 50% FMWT between TA group and SA group (I2= 89.6%), 50% PMWT between TA group and model group (I2= 96.8%), 50% FMWT between TA group and SA group (I2=89.3%) and CGRP between TA group and model group (I2= 95.3%). To further identify sources of high heterogeneity, we performed subgroup analyses from four factors: acupoint selection, acupuncture sessions, treatment course, risk of bias assessment. Previous studies have reported that serum CGRP levels vary depending on where the blood samples were collected.48 Thus, we performed a separate subgroup analysis of CGRP on the site of blood collection. Interestingly, after subgroup analyses, we found that the factor that may reduce heterogeneity turned out to be risk of bias. For CGRP between the TA group and the model group, the acupoint selection and blood collection sites could reduce the heterogeneity. Unfortunately, we were unable to find a perfect explanation for the high heterogeneity of PMWT and 50% PMWT. Therefore, other confounding factors cannot be ruled out as sources of their heterogeneity. In addition, due to the limited number of studies, subgroup analyses of 50% FMWT comparing TA group and SA group, 50% FMWT comparing TA group and model group, and 50% PMWT comparing TA group and SA group were not performed.
Potential Mechanism
The underlying molecular mechanisms of acupuncture for migraine covered in this article are summarized as follows (except for one article that does not explicitly mention mechanisms30): (1) Improve hyperalgesia. Acupuncture attenuated the expression of CGRP in the trigeminovascular system ascending pathway (TG, TNC, ventral posterior nucleus of thalamus);49 regulates the activation of 5-HT receptors in the trigeminovascular system descending pathways (PAG, RMg, TNC),27,40 inhibits the expression of c-Fos and CamkII,31 and indirectly inhibits the nociceptive signaling to the trigeminal vascular system (TVS). In addition, 5-HT receptor activation in the TNC can inhibit head-face central sensitization, and 5-HT receptor activation in the RMg can inhibit plantar central sensitization.26,41 In addition, CB1 receptor activation can inhibit the release of CGRP from peripheral branch of trigeminal nerve, thereby inhibiting TVS activation and preventing pain signal influx.29 (2) Regulates vasoactivity-related neurotransmitters. First, acupuncture can regulate vasoactive substances and neurotransmitters such as 5-HT, CGRP, SP, ADORA1, ETC,25,32–34,37 and has a good regulatory effect on migraine caused by the trigeminal vascular system; Second, acupuncture can also maintain the normal function of cerebral vasomotor by regulating the balance of endothelin-1 and nitric oxide.32 (3) Decreases inflammatory factor levels. Acupuncture can inhibit the expression of CGRP, SP, IL-1, IL-6 and TNF-α, reduce the release of inflammatory substances,35,36,38 inhibit neurogenic inflammation, and thus exert analgesic effect. In addition, acupuncture can also inhibit the expression of miR-34a-5p in the trigeminal ganglion and increase the expression of SIRT1 to inhibit the IL-1β/COX2/PGE2 inflammatory pathway.28 Or by activating CB1 and downregulating IL-1β/COX2 inflammatory response signaling.39 It has also been reported that inhibition of dural mast cells, macrophages, and serum inflammatory factors may be one of the mechanisms involved in acupuncture treatment’s effect on migraine.44 (4) Regulates neuronal discharges. Acupuncture could reduce the C-fibers evoked wide dynamic range (WDR) neuronal discharges of the trigeminocervical complex (TCC) in migraine.42,43 In conclusion, acupuncture can exert analgesic effects by improving pain sensitization, regulating the expression of related neurotransmitters, and reducing inflammatory factor levels, thereby improving pain symptoms in migraine rats.
Methodological Interpretations
The qualitative score evaluating by SYRCLE’s guideline24 and CAMARADES checklist45 ranging from 3 to 9 points indicated that the quality of the evidence included varied. Based on this assessment, it suggests that we should be cautious about the interpretation given by the results. The methodological weakness of the included studies is summarized below: (1) Although all 21 included studies reported the use of random allocation, only 14 explicitly mentioned the details of allocation. (2) In general, acupuncture is difficult to blind investigators, but it can be performed on the assessor of the outcome. The use of this approach was mentioned in only half of the 21 studies, but fortunately blinding the investigators was not a key issue in animal experiments, and blinding in outcome assessment was recommended to increase confidence in the results. (3) Explaining the animal allocation process and the randomness of the outcome assessment of animal selection can significantly improve the persuasiveness of the results, which unfortunately has not been reported on by any project. (4) One-third of the literature did not clearly describe how incomplete outcome data were processed. A high-quality study should be designed to be strictly standard, clearly reporting on the use of randomization and blinding, as well as how to handle data appropriately, and how conflicts of interest can be avoided. Notably, quality issues reported in studies are prevalent in preclinical studies. The transparency of the report needs to be improved, and the authors of the report should refer to tools such as the SYRCLE guidelines to improve the completeness and transparency of the report content to ensure the quality of the literature.
Implication for Further Studies
Animal experiments are an indispensable link for researchers to alleviate existing diseases and explore new disease treatments and play an important role in the development of medical research. The quality of animal experimental design is directly related to the transformation process from preclinical basic research to clinical research. Our results suggest that acupuncture raises the pain threshold and improves pain symptoms in migraine rats. However, due to the variable quality of the included literature, the results of this meta-analysis need to be interpreted with caution. We have summarized several potential mechanisms of acupuncture in the treatment of pain symptoms in rats with migraine, but more rigorous, well-designed, large-sized animal studies are needed to confirm this result in anticipation of a more convincing explanation. The details of acupuncture varied among the included studies, which may have been a major source of heterogeneity. At the same time, there is no clear standard for judging the success of pain modeling, and the way to judge the success of modeling through behavioral performance seems to be easily influenced by subjective consciousness. We recommend that the standardization of model making be required for better follow-up research. In addition, depending on the choice of acupoints, the outcome data obtained by acupuncture in rats with migraine may vary. Differences in needle depth, sessions, and single treatment time are difficult to form a uniform even in clinical studies. However, it is worth noting that when we analyzed serum CGRP levels, there was extremely high heterogeneity in the results, and we have not yet identified a source of heterogeneity. Previous literature has reported that differences in the location of blood sample collection will affect the results of serum CGRP levels.48 Therefore, it is reasonable to assume that the source of the high heterogeneity in our analysis of serum CGRP may be due to differences in the location of blood sample collection. Studies have shown that extracranial circulation causes dilution of neurotransmitters, in future studies, researchers can select jugular venous blood as a target sample to study neurotransmitters released by the trigeminal nerve.
Limitations
Conclusions based on the included literature may have the following limitations: (1) Our search only included articles in Chinese and English, and articles published in other languages may be omitted, which may give rise to selective bias. (2) The total number of studies and sample sizes were relatively small, the methodological quality was low, and the risk of bias was relatively high, thus reducing persuasiveness and reliability. (3) Some of the data is not presented directly in digital form, and needs to be extracted from the images published in the article. Data accuracy decreases due to image distortion. However, fortunately, all groups in the same image were affected and did not cause excessive bias. (4) Reasons for the high heterogeneity of some results were not found, and it is difficult to explain the high heterogeneity of the results. (5) Due to the limited number of included studies, we did not test all results for publication bias, only articles with CGRP as an outcome were analyzed and suggested publication bias, and the absence of publication bias could not be ruled out. Therefore, the interpretation of this study should take into account the above limitations.
Conclusion
Based on the results of our systematic review and meta-analysis, acupuncture can effectively increase FMWT, PMWT, 50% FMWT, 50% PMWT, while reducing serum CGRP levels, and when compared with the model group and SA group, the TA group shows a superior effect, significantly increases the pain threshold, and inhibits hyperalgesia. This review provides evidence of acupuncture could upregulate the pain threshold and inhibited serum CGRP levels in migraine rats. Although the included studies showed a high heterogeneity, the beneficial effect of acupuncture on analgesia seems to be unaffected. Poor methodological quality, high risk of bias and heterogeneity might reduce persuasiveness of positive results. However, to further elucidate these results, future studies with larger sample sizes and more rigorous designs are necessary. Additionally, the current research only scratches the surface by focusing primarily on identifying changes in indicators. Future research should aim to deepen our understanding of the specific mechanisms underlying the therapeutic effects of acupuncture on acute or chronic migraine. This could involve exploring these mechanisms using advanced techniques such as optogenetics and patch-clamp technology. In summary, our study highlights the potential of TA as an effective treatment for migraine, but further investigation is required to fully comprehend its mechanism of action and optimize its clinical application.
Funding Statement
This study was supported by the China National Natural Science Foundation (Nos.82274664, 82004486).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
- 1.Zhang N, Robbins MS. Migraine. Ann Intern Med. 2023;176(1):ITC1–ITC16. doi: 10.7326/AITC202301170 [DOI] [PubMed] [Google Scholar]
- 2.Tepper SJ, Cirillo J, Kim E, et al. The temporal trend of placebo response in migraine prevention from 1990 to 2021: a systematic literature review and meta-analysis with regression. J Headache Pain. 2023;24(1):54. doi: 10.1186/s10194-023-01587-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashina M, Buse DC, Ashina H, et al. Migraine: integrated approaches to clinical management and emerging treatments. Lancet. 2021;397(10283):1505–1518. doi: 10.1016/S0140-6736(20)32342-4 [DOI] [PubMed] [Google Scholar]
- 4.Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting the burden: the global campaign against headache. Migraine remains second among the world’s causes of disability, and first among young women: findings from GBD2019. J Headache Pain. 2020;21(1):137. doi: 10.1186/s10194-020-01208-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dodick DW. Migraine. Lancet. 2018;391(10127):1315–1330. doi: 10.1016/S0140-6736(18)30478-1 [DOI] [PubMed] [Google Scholar]
- 6.Misra UK, Kalita J, Bhoi SK. Allodynia in migraine: clinical observation and role of prophylactic therapy. Clin J Pain. 2013;29(7):577–582. doi: 10.1097/AJP.0b013e31826b130f [DOI] [PubMed] [Google Scholar]
- 7.Louter MA, Bosker JE, van Oosterhout WPJ, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain. 2013;136(Pt 11):3489–3496. doi: 10.1093/brain/awt251 [DOI] [PubMed] [Google Scholar]
- 8.Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache. 2018;58(Suppl 1):17–32. doi: 10.1111/head.13301 [DOI] [PubMed] [Google Scholar]
- 9.Stovner LJ, Nichols E, Steiner TJ. Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):954–976. doi: 10.1016/S1474-4422(18)30322-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katsuki M, Kashiwagi K, Kawamura S, Koh A. The efficacy of Japanese Herbal Kampo Medicine as an acute and prophylactic medication to treat chronic daily headache and medication overuse headache:-single arm retrospective study. Cureus. 2022;14(5):e25419. doi: 10.7759/cureus.25419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen P, Saxena PR, Dahlöf C. Ergotamine in the acute treatment of migraine: a review and European consensus. Brain. 2000;123(Pt 1):9–18. doi: 10.1093/brain/123.1.9 [DOI] [PubMed] [Google Scholar]
- 12.Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. 2018;15(2):274–290. doi: 10.1007/s13311-017-0592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy AJ, Gandhi S, Bhola R, Goadsby PJ. Intravenous dihydroergotamine for inpatient management of refractory primary headaches. Neurology. 2011;77(20):1827–1832. doi: 10.1212/WNL.0b013e3182377dbb [DOI] [PubMed] [Google Scholar]
- 14.Ishiyama S, Shibata Y, Ayuzawa S, Matsushita A, Matsumura A, Ishikawa E. The modifying of functional connectivity induced by peripheral nerve field stimulation using electroacupuncture for migraine: a prospective clinical study. Pain Med. 2022;23(9):1560–1569. doi: 10.1093/pm/pnac048 [DOI] [PubMed] [Google Scholar]
- 15.Linde K, Allais G, Brinkhaus B. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;2016(6):Cd001218. doi: 10.1002/14651858.CD001218.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis: a randomized clinical trial. JAMA Intern Med. 2017;177(4):508–515. doi: 10.1001/jamainternmed.2016.9378 [DOI] [PubMed] [Google Scholar]
- 17.Ma BF, Williams JP, Zhang JF, Wang RG, Guo J, An JX. Electroacupuncture alleviates thalamic pain in rats by suppressing ADCY1 protein upregulation. Pain Physician. 2022;25(4):E629–E640. [PubMed] [Google Scholar]
- 18.Jiang M, Chen X, Zhang L, et al. Electroacupuncture suppresses glucose metabolism and GLUT-3 expression in medial prefrontal cortical in rats with neuropathic pain. Biol Res. 2021;54(1):24. doi: 10.1186/s40659-021-00348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trento MMS, Moré AOO, Duarte ECW, Martins DF. Peripheral receptors and neuromediators involved in the antihyperalgesic effects of acupuncture: a state-of-The-art review. Pflugers Arch. 2021;473(4):573–593. doi: 10.1007/s00424-020-02503-0 [DOI] [PubMed] [Google Scholar]
- 20.Murphy SP, Murphy AN. Pre-clinical systematic review. Neurochem. 2010;115(4):805. doi: 10.1111/j.1471-4159.2010.06998.x [DOI] [PubMed] [Google Scholar]
- 21.McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–396. doi: 10.1001/jama.2017.19163 [DOI] [PubMed] [Google Scholar]
- 22.Wang R, Yao Q, Chen W. Stem cell therapy for Crohn’s disease: systematic review and meta-analysis of preclinical and clinical studies. Stem Cell Res Ther. 2021;12(1):463. doi: 10.1186/s13287-021-02533-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dun RL, Lan TY, Tsai J, et al. Protective effect of melatonin for renal ischemia-reperfusion injury: a systematic review and meta-analysis. Front Physiol. 2021;12:791036. doi: 10.3389/fphys.2021.791036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14(43). doi: 10.1186/1471-2288-14-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu Y, Pei P, Liu L, Zhao L, Qu Z, Wang L. Regulating effect of 5-HT_(1B) receptors in migraine rat model electrical stimulating dura mater via electroacupuncture. World Chin Med. 2017;12(12):3058–3062. [Google Scholar]
- 26.Qu Z, Pei P, Liu L, Zhao L, Wang L. Effects of electrical targeting of serotonin 1F receptor mRNA and protein expression in the brain of rats with migraine in remission. J Trand Chin Med. 2017;58(02):157–161. doi: 10.13288/j.11-2166/r.2017.02.016 [DOI] [Google Scholar]
- 27.Zhao P, Liu L, Pei P, Qu Z, Zhu Y, Wang L. Effect of electroacupuncture on 5- HT_(1D) receptor expression in migraine rats. Jilin J Chin Med. 2017;37(07):711–714. [Google Scholar]
- 28.Zhang H, He S, Zong D, Zhang X, Luo J, Zheng J. Effect of electricity on the miR-34a-5p/SIRT1 pathway in trigeminal ganglion in migraine rats. Acupunct Res. 2020;45(11):868–874. doi: 10.13702/j.1000-0607.200378 [DOI] [PubMed] [Google Scholar]
- 29.Zhang H, Hu Y, Wu J, Zheng H. Effect of electro-acupuncture on expression of trigeminal ganglion CB1 receptor, CGRP and serum concentrations of CGRP in rats with migraine. CJTCMP. 2015;30(09):3297–3300. [Google Scholar]
- 30.Zhu Y, Pei P, Liu L. Behavioral observation of electro-acupuncture on rat model with Nitroglycerin migraine. Beijing J Trad Chin Med. 2017;36(4):317–320. doi: 10.16025/j.1674-1307.2017.04.009 [DOI] [Google Scholar]
- 31.Zhang H, He S, Chen G, Zhang F, Pan X, sheng L. Inhibiting influence of electroacupuncture on Nitroglycerin-induced changes in serum CGRP levels and c-Fos and CamKII expression in migraine rats induced by Nitroglycerin through kynurenine pathway. Hubei J TCM. 2018;40(01):13–16. [Google Scholar]
- 32.Ye Y, Liu Q, Kang L, et al. Effect of electroacupuncture at specific acupoints of shaoyang meridians on serum NO and ET-1 levels and 50% PWT in rats with migraine. J Human Univ Chin Med. 2018;38(12):1453–1457. [Google Scholar]
- 33.Wang Q, Liu Q, Ye Y, et al. Effects of electroacupuncture on serum related neurotransmitters and 50% foot reduction threshold in rats with migraine. Lishizhen Med Materia Medica Res. 2018;29(11):2802–2804. [Google Scholar]
- 34.Liu Q, Ye Y, Wang Q, Liu W. Effects of electro-acupuncture at specific acupoint on Shaoyang Meridian in different periods on behavior, 50% paw withdrawal threshold and serum ET, NO, CGRP contents in migraine rats. J Chin Trad Med. 2019;34(11):5428–5432. [Google Scholar]
- 35.Liu Q, Ye Y, Wang Q, Liu A, Chen J, He X. Effects of pre-electroacupuncture specific acupoint on shaoyang meridians on 50%PWT and serum CGRP and SP content in migraine rats. J Emerg Trad Chin Med. 2019;28(04):572–575. [Google Scholar]
- 36.Wang M, Yu X, Geng W, et al. Effect of manual acupuncture preconditioning in behavior and contents of serum CGRP, SP, IL-1β and TNF-α levels in migraine rats. Acupunct Res. 2018;43(06):375–379. doi: 10.13702/j.1000-0607.170415 [DOI] [PubMed] [Google Scholar]
- 37.Huang S, Wang S, Wang M, Han J, Yang D. Effects of acupuncture therapy of soothing liver and regulating shen on expression of vasoactive substances and adenosine A1 receptor in migraine rats. J Basic Chin Med. 2021;27(04):579–582+622. doi: 10.19945/j.cnki.issn.1006-3250.2021.04.015 [DOI] [Google Scholar]
- 38.Zhang Y, Song B, He Y, Yang L, Fu L, Liu A. Effect of acupuncture on levels of brainstem IL-6, TNF-α and spinal trigeminal nucleus CGRP in migraine rats. Chin J Inf Tradit Chin Med. 2022;29(01):59–64. doi: 10.19879/j.cnki.1005-5304.202105018 [DOI] [Google Scholar]
- 39.Zhang H, He S, Hu Y, Zheng H. Antagonism of cannabinoid receptor 1 attenuates the anti-inflammatory effects of electroacupuncture in a rodent model of migraine. Acupunct Med. 2016;34(6):463–470. doi: 10.1136/acupmed-2016-011113 [DOI] [PubMed] [Google Scholar]
- 40.Pei P, Liu L, Zhao LP, et al. Electroacupuncture exerts an anti-migraine effect via modulation of the 5-HT7 receptor in the conscious rat. Acupunct Med. 2019;37(1):47–54. doi: 10.1136/acupmed-2017-011410 [DOI] [PubMed] [Google Scholar]
- 41.Liu L, Xu X, Qu Z, et al. Determining 5HT7R’s involvement in modifying the antihyperalgesic effects of electroacupuncture on rats with recurrent migraine. Front Neurosci. 2021;15:668616. doi: 10.3389/fnins.2021.668616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qu Z, Liu L, Yang Y, Zhao L, Zhu Y. Electro-acupuncture inhibits C-fiber-evoked WDR neuronal activity of the trigeminocervical complex: neurophysiological hypothesis of a complementary therapy for acute migraine modeled rats. Brain Res. 2020;1730:146670. doi: 10.1016/j.brainres.2020.146670 [DOI] [PubMed] [Google Scholar]
- 43.Qu Z, Liu L, Zhao L, Xu X, Li Z. Prophylactic electroacupuncture on the upper cervical segments decreases neuronal discharges of the trigeminocervical complex in migraine-affected rats: an in vivo extracellular electrophysiological experiment. J Pain Res. 2020;13:25–37. doi: 10.2147/JPR.S226922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao L, Liu L, Xu X, Qu Z, Zhu Y. Electroacupuncture inhibits hyperalgesia by alleviating inflammatory factors in a rat model of migraine. J Pain Res. 2020;13:75–86. doi: 10.2147/JPR.S225431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macleod MR, O’Collins T, Howells DW, Donnan GA. Pooling of animal experimental data reveals influence of study design and publication bias. Stroke. 2004;35(5):1203–1208. doi: 10.1161/01.STR.0000125719.25853.20 [DOI] [PubMed] [Google Scholar]
- 46.Ahmed I, Sutton AJ, Riley RD. Assessment of publication bias, selection bias, and unavailable data in meta-analyses using individual participant data: a database survey. BMJ. 2012;344:d7762. doi: 10.1136/bmj.d7762 [DOI] [PubMed] [Google Scholar]
- 47.Chen Y, Liu Y, Zhao S, Li B, Sun J, Liu L. Therapeutic applications and potential mechanisms of acupuncture in migraine: a literature review and perspectives. Front Neurosci. 2022;16:1022455. doi: 10.3389/fnins.2022.1022455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu L, Pei P, Wang L. Progression of the mechanism study on experimental migraine treated with acupuncture in rat model. Chin Acup Moxib. 2016;36(03):331–336. doi: 10.13703/j.0255-2930.2016.03.029 [DOI] [PubMed] [Google Scholar]
- 49.Zhao L, Pei P, Qu Z, Zhu Y, Wang L, Wang L-P. Electroacupuncture at Fengchi (GB20) inhibits calcitonin gene-related peptide expression in the trigeminovascular system of a rat model of migraine. Neural Regen Res. 2017;12(5):804–811. doi: 10.4103/1673-5374.206652 [DOI] [PMC free article] [PubMed] [Google Scholar]