Abstract
Ferroptosis may improve the efficacy of tumor treatment, according to recent evidences. This study is to explore value of histone deacetylases 1 (HDAC1), ATP binding cassette subfamily B member 1 and ferroptosis-related proteins as potential predictive biomarkers. Eighty-two women who received neoadjuvant chemotherapy (NAC) confirmed breast cancer was included. Immunohistochemistry staining of HDAC1, ATP binding cassette subfamily B member 1 and ferroptosis-related proteins was performed in core needle biopsy and tumor resection tissue. Univariate and multivariate logistic regression were conducted to explore the potential biomarkers for breast cancer undergoing NAC. There was a weak positive correlation of HDAC1 level before and after NAC with imaging outcome (R = 0.390, P < .001). The expression of HDAC1 and glutathione peroxidase 4 before NAC was an independent predictor of imaging efficacy (OR = 7.633, CI 1.831–31.821, P < .001; OR = 0.700, CI 0.505–0.971, P < .05, respectively). HDAC1 and Glutathione peroxidase 4 may act as a new predictive biomarker for NAC in breast cancer. And personalized treatment can be provided based on them.
Keywords: ABCB1, breast cancer, ferroptosis, GPX4, HDAC1, neoadjuvant chemotherapy, xCT
1. Introduction
The role of neoadjuvant chemotherapy (NAC) in treating early-stage, locally advanced breast cancer has grown in recent years, but some patients still fail to respond to treatment due to drug resistance.[1,2] It is important to evaluate the response to NAC accurately in order to determine the effect of systemic therapies on breast cancer biology, the prognosis, and further treatment options. The advantages of biomarkers include the ability to detect early damage at low levels in a sensitive and accurate manner, providing clinicians with early warnings and helping them diagnose more easily.[3] However, there are no biomarkers available for the clinical assessment of NAC efficacy.
Anthracyclines, such as doxorubicin and epirubicin, and taxanes are generally used in NAC.[4] In breast cancer cells, the drug efflux system can decrease the concentration of these drugs and cause drug resistance.[5] ATP binding cassette subfamily B member 1 (ABCB1) can increase drug efflux and was identified as an ABC transporter associated with clinical chemotherapy resistance.[6] Recent studies have shown that expression changes of histone deacetylases and the overexpression of ABC transporters are probably associated with expression changes of the ferroptosis-related proteins. Ferroptosis is a programmed cell death by reactive oxygen species (ROS)-activated lipid peroxidation and is an iron-dependent, non-apoptotic form of cell death.[7] A great deal of attention has been paid to ferroptosis-related genes in breast cancer over the past few years.[8] Glutathione (GSH), glutathione peroxidase 4 (GPX4), and the light chain of the glutamate-cysteine reverse transporter protein gene name SLC7A11 (xCT) have been shown to be central regulatory genes for ferroptosis.[9] According to Kennedy et al[10], inhibition of xCT and GPX4 promoted ferroptosis in cancer cells. Recently, a study showed that GPX might predict better pathological outcomes for breast cancer patients receiving NAC.[11] Hence, it is worthwhile to investigate ferroptosis-related proteins predictive role in NAC efficacy. M Ines et al found that histone deacetylases inhibitors (HDACIs) could specifically inhibit xCT transporter protein expression, suggesting that HDACIs could enhance ferroptosis in cancer cells.[12] A study showed that ABCB1-mediated resistance to docetaxel in ovarian cancer could be reversed by an xCT inhabitor.[13]The use of HDAC and ABCB1 as cancer treatment targets is currently being studied by many researchers. However, there are very few studies examining the predictive value of these proteins for the efficacy of NAC in breast cancer.
The purpose of this study is to investigate the correlation between the expression of xCT, GPX4, ABCB1, Histone deacetylases 1 (HDAC1) in breast cancer patients before NAC and the change in expression in the pre-NAC and post-NAC and the efficacy. The correlation between the expression of GPX4 and HDAC1 before NAC and the efficacy is verified in this study.
2. Materials and methods
2.1. Study population
In this retrospective study, we recruited 82 women who received NAC (Neoadjuvant chemotherapy regimens, http://links.lww.com/MD/J345, Supplemental Digital Content) at Fujian Provincial Hospital with histologically proven breast cancer from January 2017 to January 2022.There had been no prior treatment related to oncology for any of them. In all patients, core needle biopsy and tumor resection tissue were retained (except for 5 patients who achieved complete response (CR) following chemotherapy whose tumor resection specimens were missing). These patients met the following inclusion criteria: Female patients with pathological diagnostic results confirming breast cancer, clinical stage cT2 to 3, N0 to 3, M0; All patients were confirmed with invasive breast cancer by puncture biopsy; Treated with NAC and could actively cooperate with standard treatment. The exclusion criteria were: Incomplete clinical data; Patients who were allergic to chemotherapy drugs or patients who could not tolerate chemotherapy and surgery due to serious organic diseases; Patients with previously received antitumor treatments; Patients who did not receive NAC but underwent direct surgical resection; Patients who did not undergo surgery after NAC. The TNM staging method was referred to the American Joint Committee on Cancer 8th edition TNM staging system for breast cancer. The neoadjuvant treatment protocol after enrollment is detailed in the Supplemental Digital Content, http://links.lww.com/MD/J345. All patients signed informed consent forms. The study was approved by the Institutional Research Ethics Committee of Fujian Provincial Hospital (approval number # K2019-01-047).
2.2. Data collection
The following baseline data were collected: age, menopausal status, clinical stage T, clinical stage N, estrogen receptor (ER), progesterone receptor (PR), Human epidermal growth factor receptor 2 (HER2), and Ki-67. Among these, a positive staining for ER/PR was defined as having nuclear staining in 1% of tumor cells, while HER2 positivity was defined as either an immunohistochemistry (IHC)3 + or a FISH-amplification of the gene.[14] The cutoff value for Ki-67 is 30%,[15] with greater than or equal to the cutoff value indicating strong expression and less than the cutoff value indicating low expression. Molecular typing criteria refer to the St. Gallen Expert Consensus[16]: Luminal A-like (ER-positive and/or PR-positive, HER2-negative and Ki-67 < 15%), Luminal B-like (ER-positive and/or PR-positive, HER2-negative and Ki-67 ≥ 15% or HER2-positive), HER2-enriched (ER-negative and PR-negative, and HER2-positive) and triple-negative (ER-negative, PR-negative and HER2-negative). The cutoff values of the expression of HDAC1, xCT, GPX4, and ABCB1 before NAC were calculated according to the principle of minimal P value: 10, 7, 7, and 6, respectively.
Both before and after treatment, mammography was performed. Outcomes were separated into 2 categories: imaging efficacy and pathological efficacy. According to the criteria of RECIST v1., imaging outcomes were assessed as: CR was defined as the removal of all target lesions; partial response (PR) was defined as a 30% reduction in the sum of the longest diameter of target lesions; progressive disease (PD) was defined as a 20% rise in the sum of the longest diameters of target lesions or the emergence of new lesions; stable disease was defined as tumor alterations between PR and PD. Miller-Payne (MP) classification was used to assess pathological efficacy, and the results were classified based on the proportion of tumor cell reduction: MP1 grade (no significant reduction or no change in tumor cells), MP2 grade (tumor cell reduction < 30%), MP3 grade (tumor cell reduction 30% to 90%), MP4 grade (tumor cell reduction > 90%), MP5 grade (tumor cells complete disappearance, ductal carcinoma-in situ component may be present). RECIST v.1.1 criteria were used to assess stable disease and PD for poor imaging response group and CR and PR for good imaging response group; the Miller-Payne system was used to assess grades I-II for poor pathological response group and grades III to IV for good pathological response group.
2.3. Bioinformatics analysis
On the UALCAN website (http://ualcan.path.uab.edu/index.html), we predicted the relationship between HDAC1, xCT, GPX4, ABCB1 expression levels in breast cancer and overall survival (OS).
To assess the effect of HDAC1, xCT, GPX4, and ABCB1 expression on survival indices under NAC conditions, The relapse-free survival (RFS) survival analysis was performed by using the Kaplan–Meier Plotter (http://kmplot.com/analysis/index.php?p = service) and selecting NAC from the website option.
2.4. IHC
In this study, immunohistochemical assays were performed using the EliVisionTM super 2-step assay kit (Fuzhou Maishin Biotechnology Development Co., Ltd.). To detect the expression of HDAC1, xCT, GPX4 and ABCB1, the primary antibodies used were as follows: mouse anti-HDAC1 antibody (1:100, Immunoway, USA), rabbit anti-xCT antibody (1:100, Proteintech, USA), mouse anti-GPX4 antibody (1:100, Proteintech, USA) and rabbit anti-ABCB1 antibody (1:100, Immunoway, USA). Slides were incubated with primary antibody overnight at 4°C, then washed and incubated with high-sensitive enzyme-labeled anti-mouse/rabbit IgG polymer (1:1, MXB Biotechnologies) for 20 minutes at room temperature. Negative controls were treated identically for the rest in the absence of primary antibody. semiquantitative immune response score (IRS, Remmele score) was used to evaluate the staining intensity and deposition range of each index. Multiplying the dyeing intensity with the score of the deposition range is the IRS score. Five fields of view were randomly selected under high magnification (×200) to score the percentage of positive cells and the degree of positive cell staining. The product of the 2 scores was the expression level of the assay. The sections were observed and scored by 2 pathologists who were double-blinded to the patients data, and if 2 physicians disputed the results of the same pathology section, a third physician scored the results.[17]
2.5. Statistical analysis
HDAC1, xCT, GPX4, and ABCB1 expression levels in different clinicopathological characteristics before NAC were evaluated using the chi-square test or Fisher exact test analysis. Chi-square test or Fisher exact test was used to evaluate difference betweent xCT, GPX4 and ABCB1 expression levels and HDAC1 expression levels before NAC. Paired-samples t-test or Wilcoxon signed-rank test was used to analyze the differences in expression of each study index in the pre-NAC and post-NAC. The correlation ship between expression level and each index was analyzed by Spearman correlation test. The correlations between expression of ferroptosis-related protein and HDAC1 was analyzed by Spearman correlation test. Univariate analysis was used to analyze the differences in different clinicopathological characteristics, expression levels of each index before NAC and changes in expression of each index in the pre-NAC and post-NAC in the 2 efficacy groups. Two-tailed test or Mann–Whitney U test for all continuous variable and chi-square test or Fisher exact test for categorical variables were performed to identify candidate covariates. All potential confounders with P values < .1 were then included in the multivariate logistic regression model based on a stepwise forward method to select significant predictors of imaging and pathological outcome. Variables with P values < .05 were considered as independent risk factors. A 2-tailed P value < .05 was used in all of this study to indicate a statistically significant difference. All data were statistically analyzed by SPSS 26.0.
3. Results
3.1. Baseline features
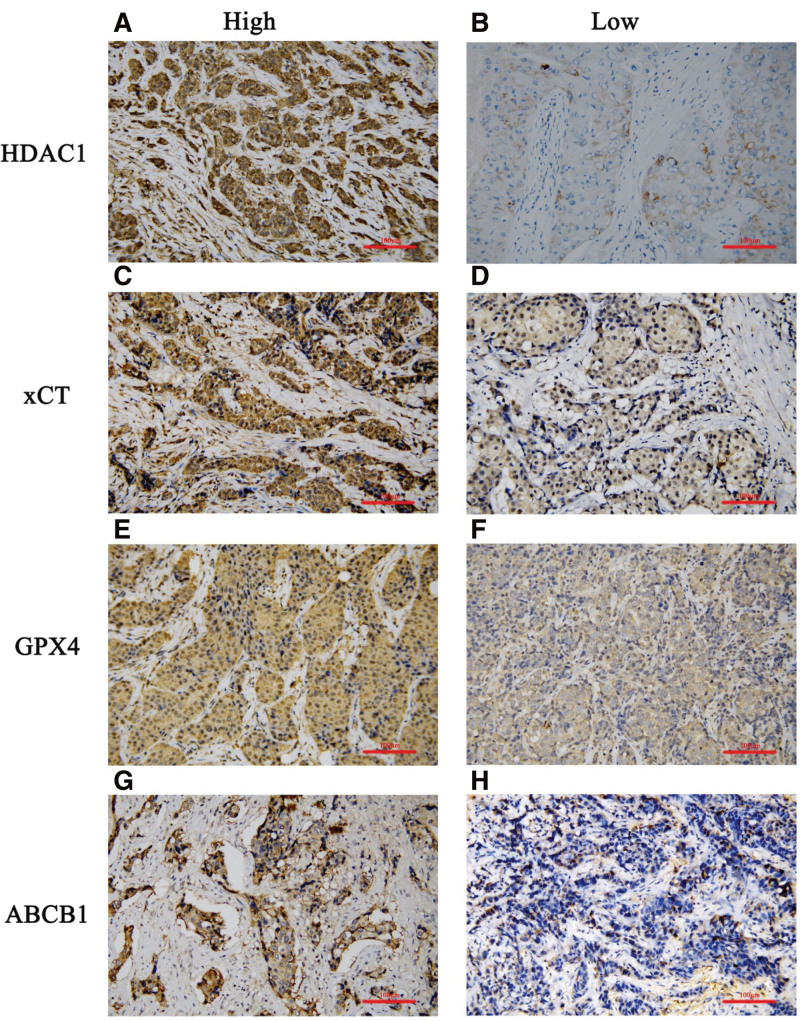
HDAC1, xCT, GPX4, and ABCB1 were expressed mainly in the cytoplasm. The representative IHC staining images are shown in Figure 1. There are no significant differences in the expression of HDAC1, xCT, GPX4, and ABCB1 in different clinicopathological features before NAC (P > .05). Only GPX4 expression before NAC in different lymph node status (P < .05) were statistically significant (Table S1, Supplemental Digital Content, http://links.lww.com/MD/J346).
Figure 1.
Representative IHC images. High expression of histone deacetylases 1 (HDAC1) (A), glutamate-cysteine reverse transporter protein (xCT, gene name SLC7A11) (C), glutathione peroxidase 4 (GPX4) (E), and P- glycoprotein (MDR1/ABCB1) (G) protein in cancer tissues in the pre- neoadjuvant chemotherapy and post- neoadjuvant chemotherapy. Low expression of HDAC1(B), xCT (D), GPX4(F), and ABCB1 (H) protein in cancer tissues in the pre- neoadjuvant chemotherapy and post- neoadjuvant chemotherapy (at x200 magnification. Scale bar = 200 μm). ABCB1 = ATP binding cassette subfamily B member 1, IHC = Immunohistochemistry, xCT = The light chain of the glutamate-cysteine reverse transporter protein, gene name SLC7A11.
3.2. Survival ananlysis
To investigate the potential role of HDAC1, xCT, GPX4, and ABCB1 in breast cancer, UALCAN database and Kaplan–Meier Plotter database were used. On the UALCAN database, the expression of in breast cancer and OS of breast cancer patients were significantly correlated, and the survival of breast cancer patients with low expression of xCT was greater than that of patients with high expression (P < .05, respectively, Figure S1 B, Supplemental Digital Content, http://links.lww.com/MD/J347); statistically significant relationship between HDAC1, GPX4, and ABCB1 expression levels and OS (P > .05, P > .05 and P > .05, respectively, Figure S1 A, C, and D, Supplemental Digital Content, http://links.lww.com/MD/J347).
On the Kaplan–Meier Plotter database, we observed a positive correlation of low expression of xCT with RFS in breast cancer after NAC (P < .05, Figure S2 B, Supplemental Digital Content, http://links.lww.com/MD/J348), and a negative correlation of low expression of GPX4 with RFS (P < .001, Figure S2 C, Supplemental Digital Content, http://links.lww.com/MD/J348). There was no correlation of the expression of HDAC1 and ABCB1 with RFS. (P > .05, P > .05, respectively, Figure S2 A and D, Supplemental Digital Content, http://links.lww.com/MD/J348). As a result, it is possible to hypothesize that after NAC, low expression of xCT and high expression of GPX4 are associated with a better prognosis.
3.3. Expression changes in the pre-NAC and post-NAC.
In order to explore the relationship between ferroptosis-related proteins, ABCB1 and HDAC1 and the efficacy of NAC in breast cancer patients, the expression changes of them pre- NAC and post-NAC were examined (Figure S2, S3, S4, S5, and S6, Supplemental Digital Content, http://links.lww.com/MD/J348). In the imaging efficacy group, it was shown in Table 1 that the expression of xCT and GPX4 significantly decreased both in poor and good response groups after NAC. Increase of the expression of HDAC1 after NAC in the poor response group associated with no statistically significance (P > .05), while the expression of HDAC1 in the good response group after NAC showed a significantly decrease (P < .001). The expression of ABCB1 after NAC increased both in poor and good response groups (P > .05, P > .05, respectively).
Table 1.
Expression changes in the pre- neoadjuvant chemotherapy and post- neoadjuvant chemotherapy.
| Protein | Total (n = 82) | P value | Imaging efficacy | Pathological efficacy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Poor response (n = 16, 19.5%) | P value | Good response (n = 66, 80.5%) | P value | Poor response (n = 17, 20.7%) | P value | Good response (n = 65, 79.3%) | P value | ||||
| HDAC1 | Before NAC | 9.00 (6.00–12.00) | <.001 | 7.50 (6.00–9.00) | .530 | 10.20 (6.75–12.00) | <.001 | 8.00 (6.00–12.00) | .117 | 10.20 (6.00–12.00) | <.001 |
| After NAC | 4.00 (1.00–6.00) | 7.50 (5.25–12.00) | 3.00 (0.50–6.00) | 6.00 (2.50–8.50) | 3.50 (1.00–6.00) | ||||||
| xCT | Before NAC | 8.00 (6.00–9.00) | <.001 | 7.88 ± 2.39 | .019 | 8.00 (6.00–9.00) | <.001 | 7.88 ± 2.55 | .003 | 7.67 ± 2.29 | <.001 |
| After NAC | 3.00 (2.00–6.00) | 5.06 ± 4.43 | 3.00 (2.00–5.25) | 4.65 ± 4.17 | 3.74 ± 2.82 | ||||||
| GPX4 | Before NAC | 8.00 (6.00–8.00) | <.001 | 8.00 (8.00–8.00) | .001 | 8.00 (6.00–8.00) | <.001 | 7.80 ± 1.59 | <.001 | 8.00 (6.00–8.00) | <.001 |
| After NAC | 2.00 (0.00 (3.00) | 2.00 (1.25–4.00) | 2.00 (0.00–3.00) | 2.06 ± 1.82 | 2.00 (0.00–3.50) | ||||||
| ABCB1 | Before NAC | 3.00 (1.00–3.00) | .012 | 3.13 ± 2.16 | .104 | 3.00 (1.00–3.00) | .049 | 2.94 ± 2.44 | .295 | 3.00 (1.50–3.00) | .016 |
| After NAC | 3.00 (0.00–8.00) | 5.13 ± 3.93 | 3.00 (0.00–8.00) | 4.06 ± 3.60 | 3.00 (0.00–8.00) | ||||||
Data are given as mean ± standard deviation if calculated using the paired t test, and as median (interquartile range) if calculated using the Wilcoxon signed-rank test.
ABCB1 = ATP binding cassette subfamily B member 1, GPX4, glutathione peroxidase 4, HDAC1 = histone deacetylases 1, NAC = neoadjuvant chemotherapy, xCT = glutamate-cysteine reverse transporter protein.
In the pathological efficacy group, it was shown in Table 1 that the expression of xCT and GPX4 significantly decreased both in poor and good response groups after NAC. Increase of the expression of HDAC1 after NAC in the poor response group associated with no statistically significance (P > .05,), while the expression of HDAC1 in the good response group after NAC showed a significantly decrease (P < .001). The expression of ABCB1 after NAC increased both in poor and good response groups (P > .05, P < .05, respectively).
3.4. Correlations between the expression changes of HDAC1, xCT, GPX4 and ABCB1 in the pre-NAC and post-NAC and therapeutic effect
To futher investigate the corelationship between HDAC1, xCT, GPX4 and ABCB1 in the pre-NAC and post-NAC and therapeutic effect, the Spearman rank correlation coefficient test was conducted. There was a weak positive correlation of the expression of HDAC1 before and after NAC in the imaging efficacy group (R = 0.390, P < .001, Table 2). A lower expression of HDAC1 in the pre- NAC and post-NAC was associated with a better imaging outcome. Table 2 shows that the rest were not statistically significant.
Table 2.
Correlations between the expression changes in pre- and post- neoadjuvant chemotherapy and therapeutic effect.
| Protein | Imaging efficacy | Pathological efficacy | ||
|---|---|---|---|---|
| r | P value | r | P value | |
| Expression Change of HDAC1 | 0.390 | <.001 | 0.138 | .215 |
| Expression Change of xCT | 0.063 | .576 | 0.029 | .798 |
| Expression Change of GPX4 | < 0.001 | 1.000 | −0.034 | .763 |
| Expression Change of ABCB1 | 0.090 | .424 | 0.006 | .959 |
Correlations between the expression changes of RAC1, HDAC1, xCT, GPX4 and ABCB1 in the pre- and post- NAC and therapeutic effect.
ABCB1 = ATP binding cassette subfamily B member 1, GPX4 = glutathione peroxidase 4, HDAC1 = histone deacetylases 1, xCT = glutamate-cysteine reverse transporter protein.
3.5. Difference between expression of ferroptosis-related protein and HDAC1 before NAC
The discrepancy of expression of GPX4 and xCT in different expression levels of HAC1 was shown in Table S2, Supplemental Digital Content, http://links.lww.com/MD/J349 (P < .05, P < .001, respectively).
3.6. Correlations between expression change of ferroptosis-related protein and HDAC1/ABCB1 in the pre-NAC and post-NAC.
The Spearman rank correlation coefficient test was conducted for futher investigation of the correlationship between ferroptosis-related protein and HDAC1/ABCB1 in the pre-NAC and post-NAC. Result of correlations between expression change of ferroptosis-related protein and HDAC1 in the pre-NAC and post-NAC was shown in Table 3, and 4. In poor pathological and imaging response groups, a positive correlation exists between ABCB1 expression and HDAC1 expression (R = 0.692, P < .01; R = 0.591, P < .05; respectively). In good response group, a positive correlation of xCT, GPX4, ABCB1 expression respectively with HDAC1 expression (P < .001).
Table 3.
Correlations between expression change of ferroptosis-related protein and HDAC1 in the pre- neoadjuvant chemotherapy and post- neoadjuvant. chemotherapy.
| Protein | Expression change of HDAC1 | |||
|---|---|---|---|---|
| Poor pathological response | Good pathological response | |||
| r | P value | r | P value | |
| Expression change of xCT | −0.385 | .127 | 0.495 | <.001 |
| Expression change of GPX4 | 0.171 | .511 | 0.435 | <.001 |
| Expression change of ABCB1 | 0.692 | .002 | 0.433 | <.001 |
ABCB1 = ATP binding cassette subfamily B member 1, GPX4 = glutathione peroxidase 4, HDAC1 = histone deacetylases 1, NAC = neoadjuvant chemotherapy, xCT = glutamate-cysteine reverse transporter protein.
Table 4.
Correlations between expression change of ferroptosis-related protein and HDAC1 in the pre- neoadjuvant chemotherapy and post- neoadjuvant.
| Protein | Expression change of HDAC1 | |||
|---|---|---|---|---|
| Poor imaging response | Good imaging response | |||
| r | P value | r | P value | |
| Expression change of xCT | −0.381 | .146 | 0.491 | <.001 |
| Expression change of GPX4 | 0.184 | .494 | 0.481 | <.001 |
| Expression change of ABCB1 | 0.591 | .016 | 0.428 | <.001 |
ABCB1 = ATP binding cassette subfamily B member 1, GPX4 = glutathione peroxidase 4, HDAC1 = histone deacetylases 1, NAC = neoadjuvant chemotherapy, xCT = glutamate-cysteine reverse transporter protein.
Result of correlations between expression change of ferroptosis-related protein and ABCB1 in the pre-NAC and post-NAC was shown in Table S3, S4, Supplemental Digital Content, http://links.lww.com/MD/J350. In poor pathological response group, a negative correlation exists between xCT expression and ABCB1expression (r = −0.549, P < .01). In good response group, a positive correlation of xCT, GPX4, HDAC1 expression respectively with ABCB1 expression (P < .01).
3.7. Univariate and multivariate logistic regression
To explore the potetial biomarkers for breast cancer undergoing NAC, univariate and multivariate logistic regression were conducted. Result of univariate analysis was shown in Table 5. In the imaging efficacy group, as a categorical variable, it is statistically significant to observe a difference in HDAC1 level before NAC between poor and good responses (P < .01), while there was no significant difference between the imaging efficacy and the expressions of xCT, GPX4 and ABCB1. As a continuous variable, only HDAC1 was statistically different between groups (P < .05), while the rest were not statistically different. A multivariate logistic regression analysis was conducted involving 3 variables. Table 6 shows the selected covariates and regression results after adjusting for these covariates, including Expression of HDAC1 before NAC (OR:7.633, CI: 1.831–31.821, P < .01), GPX4 before NAC (OR: 0.700, CI: 0.505–0.971, P < .05).
Table 5.
Relationships of clinical characteristics with therapeutic effect by univariate analysis.
| Characteristics | Imaging efficacy | Pathological efficacy | ||||
|---|---|---|---|---|---|---|
| Poor response (n = 16, 19.5%) |
Good response (n = 66, 80.5%) |
P value | Poor response (n = 17, 20.7%) |
Good response (n = 65, 79.3%) |
P value | |
| Age | .332 | .862 | ||||
| Age < 35 | 2 (12.5) | 8 (12.1) | 2 (11.8) | 8 (12.3) | ||
| 35 ≤ Age ≤ 50 | 5 (31.3) | 34 (51.5) | 7 (41.2) | 32 (49.2) | ||
| Age > 50 | 9 (56.3) | 24 (36.4) | 8 (47.1) | 25 (38.5) | ||
| Menopausal Status | .267 | .164 | ||||
| Premenopausal | 7 (43.8) | 39 (59.1) | 7 (41.2) | 39 (60) | ||
| Postmenopausal | 9 (56.3) | 27 (40.9) | 10 (58.8) | 26 (40) | ||
| Clinical T stage | .179 | .132 | ||||
| T2 | 13 (81.3) | 42 (63.6) | 14 (82.4) | 41 (63.1) | ||
| T3 | 3 (18.8) | 24 (36.4) | 3 (17.6) | 24 (36.9) | ||
| Clinical N Stage | .642 | .163 | ||||
| N0 | 7 (43.8) | 30 (45.5) | 5 (29.4) | 32 (49.2) | ||
| N1 | 4 (25) | 17 (25.8) | 6 (35.3) | 15 (23.1) | ||
| N2 | 4 (25) | 9 (13.6) | 5 (29.4) | 8 (12.3) | ||
| N3 | 1 (6.3) | 10 (15.2) | 1 (5.9) | 10 (15.4) | ||
| ER Status | .753 | .644 | ||||
| ER negative | 6 (37.5) | 22 (33.3) | 5 (29.4) | 23 (35.4) | ||
| ER positive | 10 (62.5) | 44 (66.7) | 12 (70.6) | 42 (64.6) | ||
| PR Status | 1.000 | 1.000 | ||||
| PR negative | 15 (93.8) | 61 (92.4) | 16 (94.1) | 60 (92.3) | ||
| PR positive | 1 (6.3) | 5 (7.6) | 1 (5.9) | 5 (7.7) | ||
| Her2 Status | .213 | .137 | ||||
| Her2 negative | 14 (87.5) | 46 (69.7) | 15 (88.2) | 45 (69.2) | ||
| Her2 positive | 2 (12.5) | 20 (30.3) | 2 (11.8) | 20 (30.8) | ||
| Molecular subtypes | .180 | .936 | ||||
| Luminal A-like | 1 (6.3) | 5 (7.6) | 1 (5.9) | 5 (7.7) | ||
| Luminal B-like | 9 (56.3) | 39 (59.1) | 11 (64.7) | 37 (56.9) | ||
| Her2 enriched | 0 (0) | 10 (15.2) | 1 (5.9) | 9 (13.8) | ||
| Triple negative | 6 (37.5) | 12 (18.2) | 4 (23.5) | 14 (21.5) | ||
| Characteristics | Imaging efficacy | Pathological efficacy | ||||
| Poor response (n = 16, 19.5%) |
Good response (n = 66, 80.5%) |
P value | Poor response (n = 17, 20.7%) |
Good response (n = 65, 79.3%) |
P value | |
| Ki-67 | .249 | .346 | ||||
| Ki-67 < 30 | 7 (43.8) | 19 (28.8) | 7 (41.2) | 19 (29.2) | ||
| Ki-67 ≥ 30 | 9 (56.3) | 47 (71.2) | 10 (58.8) | 46 (70.8) | ||
| Expression of HDAC1 before NAC | .007 | .073 | ||||
| Low | 13 (81.3) | 29 (43.9) | 12 (70.6) | 30 (46.2) | ||
| High | 3 (18.8) | 37 (56.1) | 5 (29.4) | 35 (53.8) | ||
| Expression of xCT before NAC | .452 | .132 | ||||
| Low | 4 (25.0) | 23 (34.8) | 3 (17.6) | 24 (36.9) | ||
| High | 12 (75.0) | 43 (65.2) | 14 (82.4) | 41 (63.1) | ||
| Expression of GPX4 before NAC | .213 | .539 | ||||
| Low | 2 (12.5) | 20 (30.3) | 3 (17.6) | 19 (29.2) | ||
| High | 14 (87.5) | 46 (69.7) | 14 (82.4) | 46 (70.8) | ||
| Expression of ABCB1 before NAC | .368 | .384 | ||||
| Low | 13 (81.3) | 60 (90.9) | 14 (82.4) | 59 (90.8) | ||
| High | 3 (18.8) | 6 (9.1) | 3 (17.6) | 6 (9.2) | ||
| HDAC1 before NAC | 7.50 (6.00–9.00) | 10.20 (7.00–12.00) | .030 | 8.00 (6.00–12.00) | 10.20 (6.00–12.00) | .501 |
| xCT before NAC | 8.00 (6.50–9.00) | 8.00 (6.00–9.00) | .826 | 8.00 (7.00–9.00) | 8.00 (6.00–9.00) | .605 |
| GPX4 before NAC | 8.00 (8.00–8.00) | 8.00 (6.00–8.00) | .082 | 8.00 (8.00–8.00) | 8.00 (6.00–8.00) | .378 |
| ABCB1 before NAC | 3.00 (1.00–4.50) | 3.00 (1.00–3.00) | .575 | 2.00 (1.00–3.00) | 3.00 (2.00–3.00) | .742 |
ABCB1 = ATP binding cassette subfamily B member 1, ER = estrogen receptor, GPX4 = glutathione peroxidase 4, HDAC1 = histone deacetylases 1, HER2 = human epidermal growth factor receptor 2, NAC = neoadjuvant chemotherapy, PR = partial response, PR = progesterone receptor, xCT = glutamate-cysteine reverse transporter protein.
Table 6.
Independent predictor of imaging efficacy by multivariate analysis.
| Coefficient | OR (95% CI) | P value | |
|---|---|---|---|
| Expression of HDAC1 before NAC | 2.032 | 7.633 (1.831–31.821) | .005 |
| GPX4 before NAC | −0.357 | 0.700 (0.505–0.971) | .032 |
| Constant | 3.462 | / | / |
ABCB1 = ATP binding cassette subfamily B member 1, GPX4 = glutathione peroxidase 4, HDAC1 = histone deacetylases 1, NAC = neoadjuvant chemotherapy, xCT = glutamate-cysteine reverse transporter protein.
In the pathological efficacy group, it was clearly shown in Table 5 that none of the variables were statistically different. Only 1 value was included in multivariate logistic regression for further analysis. The results suggested that there was no statistical significance between any of the variables and pathological efficacy.
4. Discussion
Research into ferroptosis-related genes in breast cancer patients, especially those receiving NAC, is limited at present. Ferroptosis may improve the efficacy of tumor treatment, according to recent evidences.[18,19] As part of this study, we revealed the value of GPX4 and HDAC1 before NAC as an independent predictor of imaging efficacy. Based on these curing effect prediction biomarkers, personalized treatment can be provided.
Reportedly, it is possible for Paclitaxel to induce ferroptosis, which can also happen in NAC.[20] Given the established role of ferroptosis, we explored whether ferroptosis- related proteins can act as new biomarkers for breast cancer receiving NAC. In this study, there was a significant reduction after NAC in the expression of GPX4 and xCT both in poor and good response groups. Repordly, it has been also demonstrated by Sha et al[11] that pCR rates of breast cancer patients who received NAC increase with GPX4 decrease. Ferroptosis is a programmed cell death by lipid peroxidation activated by ROS.[7] GSH is a potent ROS scavenger, and its synthetic raw material, cystine, is transported into the cell via xCT. After synthesizing GSH in the cell, cystine exerts antioxidant effects through GPX4, an important enzyme that protects membranes from lipid peroxidation.[21] According to Kennedy et al[10], inhibition of xCT and GPX4 promoted ferroptosis in cancer cells. This study also found that the expression of GPX4 before NAC was a negative independent predictor of imaging efficacy. The lower GPX4 level before NAC, the better the imaging outcome. Coincidently with the results of this study, Xiang Song et al[22] showed that inhibition of GPX4 expression increased ROS levels in triple-negative breast cancer cells, thereby promoting ferroptosis to increase anticancer efficacy.
Futhermore, available reports suggest that the induction of ferroptosis was synergistically increased by HDACIs treatment.[23,24] HDAC1 is a histone deacetylase found in mammals that affects tumor proliferation, metastasis, differentiation and invasion.[25,26] Currently, histone deacetylase (HDAC) protein has emerged as an attractive therapeutic target in many cancers, including breast cancer.[25,27] Thus, we futher explored whether HDAC1 can act as a new biomarker for breast cancer receiving NAC. According to the results of this study, the expression of HDAC1 before NAC were an independent predictor of imaging efficacy. It has been shown that higher levels of HDAC1 are associated with accelerated proliferation of breast cancer cells. Our findings differ from that reports and suggest that HDAC1 activation in breast cancer patients before NAC has better imaging efficacy. On the 1 hand, the experiment of report is conducted in vitro. In addition, it is not a prediction of the efficacy of NAC for breast cancer. Our findings can provide a new biomarker for clinical personised strategy. On the other hand, Hideki Kawai et al[28] found that, TRIM46-mediated degradation of HDAC1 leads to the upregulation of BRCA1 and the chemical resistance of breast cancer cells.[29] It supports our findings that HDACIs may be not required in breast cancer patients whose HDAC1 levels are high before NAC.
Alternatively, it was found in this study that decrease of expression of HDAC1 after NAC correlates with better imaging result. Study shows that the use of HDAC inhibitors (HDACIs) can increase ROS levels in cancer cells.[5]Further exploring the association between poor imaging response and HDAC, we found that a tendency for HDAC1 expression to increase after NAC, as demonstrated in poor response groups, was also associated with increase of ABCB1 expression after NAC. As a key component of breast cancer chemoresistance, ABCB1 plays an important role.[30] It has been shown that downregulation of xCT enhances ROS-induced P-glycoprotein (MDR1/ABCB1) overexpression and drug resistance in MCF-7 breast cancer cells.[31,32] Based on these reports, our study also found that xCT decreased with ABCB1 increased after NAC in poor response groups. In addition, it is suggested that HDACIs induce increased ferroptosis in breast cancer cells by disrupting the structure of GPX4 when ferroptosis inducers are induced by combined application of HDACIs.[33]Our study found that a positive correlation of the change of HDAC expression with that of ABCB1 exprssion. In numerous studies, upregulation of ABC transporters is often observed following treatment with HDAC inhibitors, particularly increased expression of P-glycoprotein.[34] Consequently, both HDAC and ABCB1 inhibitors should be used in poor response after NAC. However, study shows that HDAC1 was significantly underexpressed in Luminal A and Luminal B compared with HER2 and TNBC.[35]Therefore it is possible that molecular subtypes of breast cancer has an effect on the prognosis of HDAC.
This study has some shortcomings. First, a small sample size was used in this study, since only 82 patients were included, and the study period was short. Hence, future studies require an expanded study sample and extended follow-up periods. Additionally, all breast cancer subtypes were included in this study, and we were not able to perform subgroup analyses of HDAC1, xCT, GPX4, or ABCB1 in 1 subtype or 1 chemotherapy regimen due to the limitation of the number of cases. But the results of this study still provide a basis for further analysis. Finally, this study found that expression of HDAC1and GPX4 before NAC was associated with the clinical outcome of NAC in breast cancer patients. And to the best of our knowledge, it is the first time that HDAC1 has been shown to predict response to NAC in breast cancer patients, which may provide a new scheme for clinical decision-making. However, no further studies and analyses on its mechanism of action are available, so further studies are still needed.
5. Conclusion
According to this study, the expression of HDAC1, xCT, and GPX4 in breast cancer were markedly downwardly revised during NAC. HDAC1 and GPX4 may act as a new predictive biomarker for NAC in breast cancer, and it may be useful in shortlisting potential candidates and ascertaining chemotherapy protocols. In order to demonstrate these possible mechanisms, more research is needed.
Acknowledgments
We would like to thank the patients who participated in this study.
Author contributions
Conceptualization: Hong Sun, Ying Lin.
Formal analysis: Jia Liu, Xiaohan Zheng, Yiming Wang.
Writing – original draft: Jia Liu, Xiaohan Zheng, Yiming Wang.
Writing – review & editing: Jiaqin Cai, Xiaoxia Wei.
Supplementary Material
Abbreviations:
- ABCB1
- ATP binding cassette subfamily B member 1
- CR
- complete response
- ER
- estrogen receptor
- GPX4
- glutathione peroxidase 4
- GSH
- glutathione
- HDAC
- histone deacetylase
- HDAC1
- histone deacetylases 1
- HDACIs
- histone deacetylases inhibitors
- HER2
- human epidermal growth factor receptor 2
- IHC
- immunohistochemistry
- MP
- Miller-Payne
- NAC
- neoadjuvant chemotherapy
- OS
- overall survival
- PD
- progressive disease
- PR
- partial response
- PR
- progesterone receptor
- RFS
- relapse-free survival
- ROS
- reactive oxygen species
- xCT
- The light chain of the glutamate-cysteine reverse transporter protein, gene name SLC7A11
Supplemental Digital Content is available for this article.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
This study was supported in part by grants from the Natural Science Foundation of Fujian (No. 2022J011004) and the Fujian provincial health technology project (No.2022CXB001). The Natural Science Foundation of Fujian, China (No. 2021J01397); Fujian provincial health technology project (No.2022GGA010); the Young and Middle-aged Backbone Research Fund of Fujian Provincial Health Care Commission (2020GGA010), and the Natural Science Foundation of Fujian Province (2020J011106).
The authors have no conflicts of interest to disclose.
How to cite this article: Sun H, Lin Y, Liu J, Zheng X, Wang Y, Cai J, Wei X. The effect of ferroptosis - related proteins and histone deacetylases1 on neoadjuvant chemotherapy in breast cancer. Medicine 2023;102:30(e34444).
Contributor Information
Hong Sun, Email: sunhong7777@fjmu.edu.cn.
Ying Lin, Email: linying0323@163.com.
Jia Liu, Email: liujia@fjmu.edu.cn.
Xiaohan Zheng, Email: 1027102880@qq.com.
Yiming Wang, Email: Aug0805@163.com.
Jiaqin Cai, Email: jiaqincai@163.com.
References
- [1].Montemurro F, Nuzzolese I, Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother. 2020;21:1071–82. [DOI] [PubMed] [Google Scholar]
- [2].Korde LA, Somerfield MR, Carey LA, et al. Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol. 2021;39:1485–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hristova VA, Chan DW. Cancer biomarker discovery and translation: proteomics and beyond. Expert Rev Proteomics. 2019;16:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].An J, Peng C, Tang H, et al. New advances in the research of resistance to neoadjuvant chemotherapy in breast cancer. Int J Mol Sci . 2021;22:9644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chaudhary LN. Early stage triple negative breast cancer: management and future directions. Semin Oncol. 2020;47:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engle K, Kumar G. Cancer multidrug-resistance reversal by ABCB1 inhibition: a recent update. Eur J Med Chem. 2022;239:114542. [DOI] [PubMed] [Google Scholar]
- [7].Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang Z, Qiu X, Yan Y, et al. Evaluation of ferroptosis-related gene AKR1C1 as a novel biomarker associated with the immune microenvironment and prognosis in breast cancer. Int J General Med. 2021;14:6189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sui S, Xu S, Pang D. Emerging role of ferroptosis in breast cancer: new dawn for overcoming tumor progression. Pharmacol Ther. 2022;232:107992. [DOI] [PubMed] [Google Scholar]
- [10].Kennedy L, Sandhu JK, Harper ME, et al. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10:1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sha R, Xu Y, Yuan C, et al. Predictive and prognostic impact of ferroptosis-related genes ACSL4 and GPX4 on breast cancer treated with neoadjuvant chemotherapy. EBioMedicine. 2021;71:103560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wolf IM, Fan Z, Rauh M, et al. Histone deacetylases inhibition by SAHA/vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci Rep. 2014;4:6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou HH, Chen X, Cai LY, et al. Erastin reverses ABCB1-mediated docetaxel resistance in ovarian cancer. Front Oncol. 2019;9:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- [15].Nielsen TO, Leung SCY, Rimm DL, et al. Assessment of Ki67 in breast cancer: updated recommendations from the international Ki67 in breast cancer working group. J Natl Cancer Inst. 2021;113:808–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Curigliano G, Burstein HJ, E PW, et al. De-escalating and escalating treatments for early-stage breast cancer: the St. Gallen international expert consensus conference on the primary therapy of early breast cancer 2017. Annal Oncol. 2019;30:1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li F, Zhao Y, Wei L, et al. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol Ther. 2018;19:695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Xu H, Ye D, Ren M, et al. Ferroptosis in the tumor microenvironment: perspectives for immunotherapy. Trends Mol Med. 2021;27:856–67. [DOI] [PubMed] [Google Scholar]
- [19].Sun LL, Linghu DL, Hung MC. Ferroptosis: a promising target for cancer immunotherapy. Am J Cancer Res. 2021;11:5856–63. [PMC free article] [PubMed] [Google Scholar]
- [20].Lv C, Qu H, Zhu W, et al. Low-dose paclitaxel inhibits tumor cell growth by regulating glutaminolysis in colorectal carcinoma cells. Front Pharmacol. 2017;8:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Battaglia AM, Chirillo R, Aversa I, et al. Ferroptosis and cancer: mitochondria meet the “iron maiden” cell death. Cells. 2020;9:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Song X, Wang X, Liu Z, et al. Role of GPX4-mediated ferroptosis in the sensitivity of triple negative breast cancer cells to gefitinib. Front Oncol. 2020;10:597434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Oliveira T, Hermann E, Lin D, et al. HDAC inhibition induces EMT and alterations in cellular iron homeostasis to augment ferroptosis sensitivity in SW13 cells. Redox Biol. 2021;47:102149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fan F, Liu P, Bao R, et al. A Dual PI3K/HDAC inhibitor induces immunogenic ferroptosis to potentiate cancer immune checkpoint therapy. Cancer Res. 2021;81:6233–45. [DOI] [PubMed] [Google Scholar]
- [25].Brunmeir R, Lagger S, Seiser C. Histone deacetylase HDAC1/HDAC2-controlled embryonic development and cell differentiation. Int J Dev Biol. 2009;53:275–89. [DOI] [PubMed] [Google Scholar]
- [26].Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31. [DOI] [PubMed] [Google Scholar]
- [27].Damaskos C, Garmpis N, Valsami S, et al. Histone deacetylase inhibitors: an attractive therapeutic strategy against breast cancer. Anticancer Res. 2017;37:35–46. [DOI] [PubMed] [Google Scholar]
- [28].Kawai H, Li H, Avraham S, et al. Overexpression of histone deacetylase HDAC1 modulates breast cancer progression by negative regulation of estrogen receptor alpha. Int J Cancer. 2003;107:353–8. [DOI] [PubMed] [Google Scholar]
- [29].Zhang Z, Liu X, Li L, et al. SNP rs4971059 predisposes to breast carcinogenesis and chemoresistance via TRIM46-mediated HDAC1 degradation. EMBO J. 2021;40:e107974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Modi A, Roy D, Sharma S, et al. ABC transporters in breast cancer: their roles in multidrug resistance and beyond. J Drug Target. 2022;30:927–47. [DOI] [PubMed] [Google Scholar]
- [31].Ge C, Cao B, Feng D, et al. The down-regulation of SLC7A11 enhances ROS induced P-gp over-expression and drug resistance in MCF-7 breast cancer cells. Sci Rep. 2017;7:3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wang H, Chi CH, Zhang Y, et al. Effects of histone deacetylase inhibitors on ATP-binding cassette transporters in lung cancer A549 and colorectal cancer HCT116 cells. Oncol Lett. 2019;18:63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Miyamoto K, Watanabe M, Boku S, et al. xCT inhibition increases sensitivity to vorinostat in a ROS-dependent manner. Cancers (Basel). 2020;12:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ni X, Li L, Pan G. HDAC inhibitor-induced drug resistance involving ATP-binding cassette transporters (Review). Oncol Lett. 2015;9:515–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guo Q, Cheng K, Wang X, et al. Expression of HDAC1 and RBBP4 correlate with clinicopathologic characteristics and prognosis in breast cancer. Int J Clin Exp Path. 2020;13:563–72. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.