Abstract
Nasopharyngeal carcinoma (NPC) is the most common malignant neoplasm of the nasopharynx. Despite improvements in the clinical treatment strategies for NPC, NPC patients usually have poor survival rates because of late diagnosis, tumor metastasis, and recurrence. Therefore, the identification of potential diagnostic and prognostic markers for NPC is imperative. We investigated the differential expression of cell adhesion-related genes (gene ontology:0003779) and tumorigenesis-related genes (GSE12452) in patients with NPC. The correlations between synaptopodin-2 (SYNPO2) immune expression and clinicopathological features were analyzed using Pearson chi-square test. Multivariate analysis was performed using Cox proportional hazards model. SYNPO2 expression was significantly higher in NPC tumor tissues than in nontumor tissues. High SYNPO2 expression was significantly associated with the advanced disease stage (P = .006). Univariate analysis showed that high expression of SYNPO2 was associated with poor disease-specific survival, distal metastasis-free survival, and local recurrence-free survival in patients with NPC. Notably, our multivariate analysis demonstrated that high SYNPO2 expression was substantially correlated with inferior disease-specific survival (hazard ratio = 1.968, P = .012) and local recurrence-free survival (hazard ratio = 3.386, P = .001). Overall, our findings reveal that SYNPO2 may aid in the development of potential prognostic biomarkers for NPC patients.
Keywords: cell adhesion-related genes, nasopharyngeal carcinoma (NPC), SYNPO2
1. Introduction
Nasopharyngeal carcinoma (NPC) is the most common malignant neoplasm arising in the mucosa of the nasopharynx.[1] It has a mainly geographic distribution, with approximately 81% of new cases occurring in Southeast Asia.[2] Its high frequency renders it a significant public health problem with a considerable impact on individual and social economies.[3] NPC is primarily treated with surgery, radiation therapy (RT), chemotherapy, and concurrent chemoradiotherapy [4–6] depending on the disease stage. RT is the primary treatment for stage I to II NPC, with corresponding 5-year overall survival rates of up to 95%. However, in the early stages of NPC, symptoms are often absent or similar to those of other clinical diseases; therefore, early diagnosis becomes very difficult, and NPC is often discovered at an advanced stage.[7–9] In addition, many patients with NPC undergoing treatment develop tumor metastasis and recurrence, resulting in poor survival outcomes. Therefore, defining potential therapeutic targets for improving the precision of prognostic prediction in NPC is imperative.
In clinical settings, distant metastasis and recurrence are significant factors that lead to low survival rates and poor prognosis in various cancer types, including NPC.[10–12] Abundant evidence indicates that the first step in distant metastasis is cancer cell migration away from the primary tumor.[11,13,14] Furthermore, substantial evidence has revealed that cell adhesion may play an essential role in cell migration, which is believed to be the origin of distant metastasis.[15–17] In this study, we analyzed the expression profiles of cell adhesion-related genes (gene ontology [GO]:0003779) and tumorigenesis-related genes in the NPC transcriptome (GSE12452). We found that several cell adhesion-related genes were differentially expressed in NPC tumors compared to nontumor cells, and synaptopodin-2 (SYNOP2) had the highest differential expression in NPC tumors. SYNOP2 regulates actin binding and -bundling activities and is involved in the regulation of cell migration. Several studies have suggested that SYNOP2 is expressed in different types of cancer, including breast cancer,[18] ovarian cancer,[19] prostate cancer,[20] and melanoma.[21] Moreover, studies have confirmed that SYNOP2 is involved in tumor growth, migration, and invasion, thus promoting tumor metastasis. For example, SYNOP2 enhances the assembly of the peripheral actin bundle and promotes PC3 prostate cancer cell migration.[22] Notably, low expression of SYNOP2 correlated with an unfavorable prognosis in patients with breast cancer.[18] According to the initial findings, SYNOP2 can have both favorable and unfavorable effects, and dysregulated SYNOP2 could be regarded as a double-edged sword, depending on the type of cancer. Nevertheless, the role of SYNOP2 in NPC still needs to be fully understood, and evidence supporting its biological role of SYNOP2 in NPC is scarce.
Accordingly, we conducted this study to assess SYNOP2 expression in NPC patients. Thus, we report for the first time the associations of SYNOP2 with clinicopathological factors and prognosis in NPC. Our observations suggest that SYNOP2 expression levels were positively correlated with NPC patients. In addition, different SYNOP2 expression levels are associated with poor survival in NPC patients. Our findings may provide new diagnostic markers and therapeutic targets for the prognostic prediction of NPC.
2. Materials and methods
2.1. Analysis of expression profile from publicly available NPC transcriptome dataset
We selected mRNA expression datasets from the gene expression omnibus database (National Center for Biotechnology Information, Bethesda, MD), from which we obtained and analyzed the transcriptome dataset (GSE12452). The GSE12452 dataset included 31 NPC tissues and 10 nonneoplastic nasopharyngeal mucosal epithelial tissue samples. Using Nexus Expression 3 software (BioDiscovery, USA) and the Affymetrix Human Genome U133 Plus 2.0 Array terrace to obtain the original files, we calculated the expression degrees of genes by probe combinations. The probe combination was resolved without preselection or filtration. Through an inspected comparative analysis, genes that were expressed in the dataset were intersected to identify the most significant genes. We searched for genes with significant differential expression (log2 ratio > 2, P < .01), focusing on genes related to cell adhesion (GO:0003779).
2.2. Patients and tumor specimens
In this study, paraffin-embedded tissue blocks were retrieved from 124 patients with NPC diagnosed between January 1993 and December 2002. The use of formalin-fixed NPC tissues was ratified by the Institutional Review Board (IRB) of Chi Mei Medical Center (IRB10302013). These patients exhibited no indication of remote metastasis at initial diagnosis. Following the present World Health Organization classification, the histological subtypes were reappraised by 2 pathologists. Tumor staging was conducted based on the literature and reevaluated using the 7th American Joint Committee on Cancer (AJCC) system.
2.3. Immunohistochemical (IHC) staining
Formalin-fixed, paraffin-embedded tissue blocks from NPC cases with primary histopathological diagnoses were then subjected to IHC staining. The tissues were sectioned into 4-μm sections, mounted on cohesive slides (SuperFrost Plus, Thermo Scientific), baked for 50 minutes at 60°C to dewax, and cleared in xylene. Before IHC staining, the sections were dipped in absolute ethanol and xylene, called hydration, and then cleaned in distilled water. Next, 0.3% hydrogen peroxide was added to 95% ethanol for 5 minutes. Endogenous peroxidase was blocked, and the slides were cleaned with distilled water. For antigen retrieval, the slides were brood in an antigen retrieval solution (citrate buffer, pH 6.0) and boiled for 4 minutes at 125°C. The slides were laid in distilled water, and then Tris-buffered saline (TBS, Dako, Denmark) was added to the sections and incubated for 3 minutes followed by a washing step. Primary antibodies (Anti- synaptopodin-2 [SYNPO2]; polyclonal, company: Novus Biologicals, dilution:1: 100) were diluted, and the secondary reagent HRP polymer was brooded for 30 minutes at room temperature. After washing, the slides were developed for 10 minutes at room temperature using chromogen diaminobenzidine. They were then counterstained with Mayer hematoxylin (Histolab), mounted with Pertex (Histolab), and prepared for assessment and interpretation.
2.4. Statistical analysis
We used the chi-square test to assess the statistical significance between the clinicopathological parameters and SYNPO2 expression. Data analysis was performed using SPSS software (version 14). The endpoints analyzed were local recurrence-free survival (LRFS), disease-specific survival (DSS), and distant metastasis-free survival (DMeFS). These measures were calculated from the date of RT to the date of the event. Multivariate analysis was performed using Cox proportional hazards model. All tests were 2-sided, and a P value < .05 was considered statistically significant.
3. Results
3.1. SYNPO2 has significantly upregulated in cell adhesion-related genes in NPC
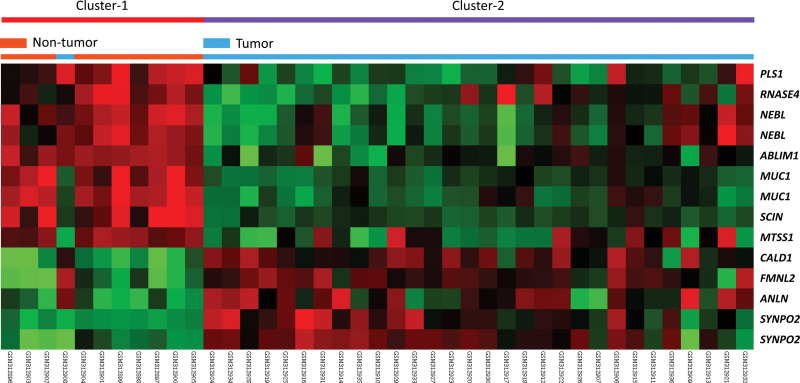
To disclose potential genes associated with cell adhesion and tumorigenesis in NPC, we analyzed the GSE12452 dataset in the gene expression omnibus database and specialized in differentially expressed genes related to cell adhesion. In the transcriptome of nasopharyngeal carcinoma (GSE12452) public database analysis, we found that many cell adhesion-related genes were significantly differentially expressed in NPC tissues (n = 31) versus non-NPC tissues (n = 10) (Fig. 1). We noted that SYNPO2 exhibited the highest fold change in expression between NPC tumor and nontumor tissues (log2 ratio = 2.618 and 2.2709, respectively; P < .0001; Table 1). Based on bioinformatic information, we focused on SYNPO2 expression to explore whether it is associated with clinicopathological factors and prognosis in NPC.
Figure 1.
Heatmap of differentially expressed genes between NPC patients and non-NPC tissues from the GSE12452 dataset, focusing on cell adhesion-related genes (GO: 0003779). The green color represents genes with lower levels of expression, and the red color represents genes with high levels of expression. NPC = nasopharyngeal carcinoma, GO = gene ontology.
Table 1.
Summary of significant differentially expressed genes related to cell adhesion (GO: 0003779) and associated with tumorigenesis of NPC in the transcriptome of nasopharyngeal carcinoma (GSE12452).
| Probe | Comparing tumor to nontumor | Gene Symbol | Gene Name | Molecular Function | |
|---|---|---|---|---|---|
| Comparison log ratio | Comparison P value | ||||
| 1552365_at | −3.0827 | <.0001 | SCIN | scinderin | actin binding, actin filament binding, calcium ion binding, phosphatidylinositol binding, phosphatidylinositol-4;5-bisphosphate binding, phosphatidylserine binding |
| 200965_s_at | −2.2188 | <.0001 | ABLIM1 | actin binding LIM protein 1 | actin binding, metal ion binding, protein binding, zinc ion binding |
| 203037_s_at | −2.0587 | <.0001 | MTSS1 | metastasis suppressor 1 | actin binding, actin monomer binding, receptor binding |
| 203961_at | −2.5083 | <.0001 | NEBL | nebulette | actin binding, metal ion binding, structural constituent of muscle, zinc ion binding |
| 203962_s_at | −2.1109 | <.0001 | NEBL | nebulette | actin binding, metal ion binding, structural constituent of muscle, zinc ion binding |
| 205190_at | −2.3329 | <.0001 | PLS1 | plastin 1 (I isoform) | actin binding, actin filament binding, calcium ion binding, structural constituent of cytoskeleton |
| 207847_s_at | −2.2289 | <.0001 | MUC1 | mucin 1; cell surface associated | actin binding, hormone activity |
| 212077_at | 2.0059 | <.0001 | CALD1 | caldesmon 1 | actin binding, calmodulin binding, myosin binding, tropomyosin binding |
| 213397_x_at | −2.1895 | <.0001 | RNASE4 | ribonuclease; RNase A family; 4 | DNA binding, actin binding, copper ion binding, endonuclease activity, heparin binding, hydrolase activity, nuclease activity, nucleic acid binding, pancreatic ribonuclease activity, protein binding, rRNA binding, receptor binding, ribonuclease activity |
| 213693_s_at | −3.8805 | <.0001 | MUC1 | mucin 1; cell surface associated | actin binding, hormone activity |
| 222608_s_at | 2.3379 | <.0001 | ANLN | anillin; actin binding protein | actin binding |
| 225720_at | 2.2709 | <.0001 | SYNPO2 | synaptopodin-2 | actin binding, protein binding |
| 225895_at | 2.618 | <.0001 | SYNPO2 | synaptopodin-2 | actin binding, protein binding |
| 226184_at | 2.1456 | <.0001 | FMNL2 | formin-like 2 | Rho GTPase binding, actin binding, transcription factor activity |
Selection criteria: Log2 ratio>=+/−2 and P < .0001.
GO = gene ontology, NPC = nasopharyngeal carcinoma, SYNPO2 = synaptopodin-2.
3.2. Associations between SYNPO2 expression and the clinicopathological parameters in NPC
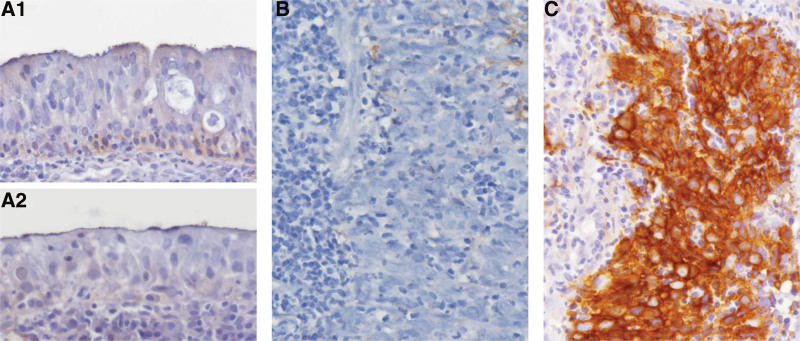
To elucidate the relationship between SYNPO2 immuno expression and the clinicopathological parameters of NPC, we included 124 patients with NPC, of whom 98 and 26 were aged ≥ 60 and < 60 years, respectively, and 95 and 29 were men and women, respectively. Immunohistochemical staining revealed that pseudostratified respiratory mucosa (Fig. 2A, upper) and that with squamous metaplasia (Fig. 2A, lower) barely expressed SYNPO2. Nasopharyngeal carcinoma with low SYNPO2 expression (Fig. 2B) was at a significantly lower stage. High SYNPO2 expression in nasopharyngeal carcinoma (Fig. 2C) was associated with a higher stage. These results indicate that higher expression of SYNPO2 was significantly correlated with late AJCC stage (P = .006) but not with sex, age, primary tumor (T), lymph node metastasis, histological grade, or Epstein–Barr virus (EBV)-encoded small RNA expression (Table 2). Overall, these results suggest that SYNPO2 expression is significantly associated with the AJCC stage in NPC.
Figure 2.
Immunohistochemical staining of SYNPO2. (A) Pseudostratified respiratory mucosa (upper) and that with squamous metaplasia (lower) barely express SYNPO2. The nasopharyngeal carcinoma showing low SYNPO2 expression (B) is of a significantly lower stage. High SYNPO2 expression in nasopharyngeal carcinoma (C) is associated with a higher stage. SYNPO2 = synaptopodin-2.
Table 2.
Associations between SYNPO2 expression and other important clinicopathological variables.
| Parameters | Category | SYNPO2 Exp. | P value | |
|---|---|---|---|---|
| Low | High | |||
| Gender | Male | 46 | 49 | .524 |
| Female | 16 | 13 | ||
| Age (yr) | <60 yr | 51 | 47 | .378 |
| >=60 yr | 11 | 15 | ||
| Primary tumor (T) | T1–T2 | 44 | 36 | .133 |
| T3–T4 | 18 | 26 | ||
| Nodal status (N) | N0–N1 | 33 | 23 | .071 |
| N2–N3 | 29 | 39 | ||
| Stage | I–II | 26 | 12 | .006* |
| III–IV | 36 | 50 | ||
| Histological grade | Keratinizing | 4 | 1 | .089 |
| Non-keratinizing | 31 | 23 | ||
| Undifferentiated | 27 | 38 | ||
| EBER | Negative | 0 | 1 | .315 |
| Positive | 62 | 61 | ||
SYNPO2 = synaptopodin-2.
statistically significant.
3.3. High expression of SYNPO2 was a positive correlation with poor prognosis in NPC
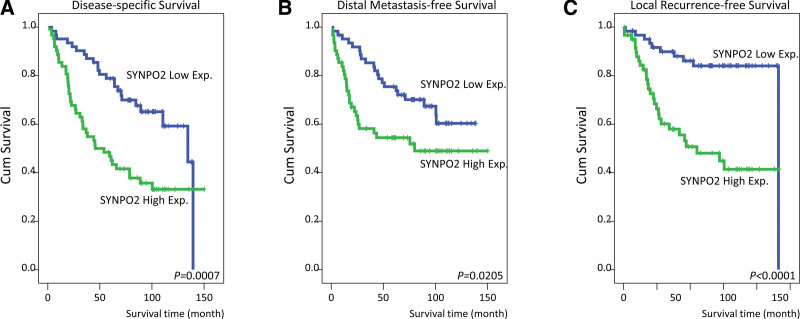
We also evaluated whether SYNPO2 expression was associated with survival outcomes in NPC. Our univariate analysis (Table 3) demonstrated that DSS was significantly correlated with the primary tumor (P = .0425), nodal status (P = .0002), AJCC stage (P = .0010), and SYNPO2 expression (P = .0007, Fig. 3A). In addition, DMFS was associated with the primary tumor (P = .0063), nodal status (P = .0096), AJCC stage (P = .0068), and SYNPO2 expression (P = .0205, Fig. 3B). We also observed that LRFS was positively associated with the primary tumor (P = .0252), nodal status (P = .0122), AJCC stage (P = .0028), and SYNPO2 expression (P < .0001; Fig. 3C). In particular, our multivariate survival analysis (Table 4) demonstrated that advanced AJCC stage (III–IV) was closely correlated with poor DSS (hazard ratio [HR] = 2.452, 95% confidence interval [CI] = 1.225–4.911, P = .011), DMeFS (HR = 2.290, 95% CI = 1.091–4.805, P = .028), and LRFS (HR = 2.761, 95% CI = 1.055–7.223, P = .039). Furthermore, high SYNPO2 expression was associated with poor DSS (HR = 1.968, 95% CI = 1.161–3.335, P = .012) and LRFS (HR = 3.386, 95% CI = 1.618–7.088, P = .001) but was not significantly associated with DMeFS. Therefore, SYNPO2 could be a significant biomarker and predictive factor for the prognosis of patients with NPC.
Table 3.
Univariate log-rank analyses.
| Parameters | Category | No. of case | DSS | DMeFS | LRFS | |||
|---|---|---|---|---|---|---|---|---|
| No. of event | P value | No. of event | P value | No. of event | P value | |||
| Gender | Male | 95 | 48 | .8104 | 39 | .5524 | 31 | .3313 |
| Female | 29 | 15 | 11 | 7 | ||||
| Age (yr) | <60 yr | 98 | 52 | .5857 | 42 | .4146 | 30 | .8804 |
| >=60 yr | 26 | 11 | 8 | 8 | ||||
| Primary tumor (T) | T1–T2 | 80 | 34 | .0425* | 25 | .0063* | 19 | .0252* |
| T3–T4 | 44 | 29 | 25 | 19 | ||||
| Nodal status (N) | N0–N1 | 56 | 19 | .0002* | 17 | .0096* | 12 | .0122* |
| N2–N3 | 68 | 44 | 33 | 26 | ||||
| Stage | I–II | 38 | 10 | .0010* | 9 | .0068* | 5 | .0028* |
| III–IV | 86 | 53 | 41 | 33 | ||||
| Histological grade | Keratinizing/Non-keratinizing | 47 | 24 | .4120 | 17 | .2155 | 16 | .9323 |
| Undifferentiated | 77 | 39 | 33 | 22 | ||||
| EBER | Negative | 1 | 1 | .0567 | 1 | .0923 | 0 | .7314 |
| Positive | 123 | 62 | 49 | 38 | ||||
| SYNPO2 Exp. | Low Exp. (H-score < median) | 62 | 23 | .0007* | 21 | .0205* | 10 | <.0001* |
| High Exp. (H-score>=median) | 62 | 40 | 29 | 28 | ||||
DSS = disease-specific surviva, DMeFS = distal metastasis-free survival, LRFS = local recurrence-free survival, SYNPO2 = synaptopodin-2.
statistically significant.
Figure 3.
The Kaplan–Meier Plotter analysis of the association of SYNPO2 in NPC patients’ survival rates. High expressions of SYNPO2 are associated with poor (A) disease-specific survival (DSS) (P = .0007), (B) distal metastasis-free survival (DMeFS) (P = .0205), and (C) local recurrence-free survival (LRFS) (P < .0001). NPC = nasopharyngeal carcinoma. SYNPO2 = synaptopodin-2.
Table 4.
Multivariate survival analyses.
| Parameter | Category | DSS | DMeFS | LRFS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| H.R | 95% CI | P value | H.R | 95% CI | P value | H.R | 95% CI | P value | ||
| Stage | I–II | 1 | - | .011* | 1 | - | .028* | 1 | .039* | |
| III–IV | 2.452 | 1.225–4.911 | 2.290 | 1.091–4.805 | 2.761 | 1.055–7.223 | ||||
| SYNPO2 Exp. | Low Exp. | 1 | - | .012* | 1 | - | .106 | 1 | - | .001* |
| High Exp. | 1.968 | 1.161–3.335 | 1.611 | 0.904–2.871 | 3.386 | 1.618–7.088 | ||||
CI = confidence interval, DSS = disease-specific survival, DMeFS = distal metastasis-free survival, LRFS = local recurrence-free survival, SYNPO2 = synaptopodin-2.
statistically significant.
3.4. Comprehensive analysis of all SYNPO2-associated genes and GO biological process enrichment
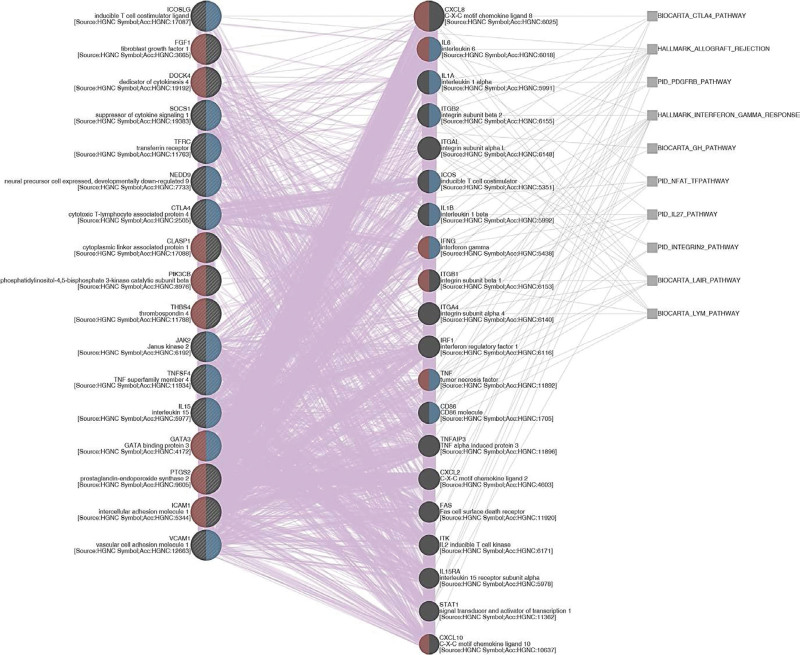
To evaluate the gene correlation networks of SYNPO2, we analyzed SYNPO2 coexpression genes using the TCGA database. Our results presented the top 200 positive correlations or negative correlations with SYNPO2 (Table S1, Supplemental Digital Content, http://links.lww.com/MD/J336). After compiling our SYNPO2 positively or negatively associated gene list, we performed GO enrichment using the Gene Ontology Resource (http://geneontology.org/). GO analysis showed that the biological process enrichment terms of SYNPO2 positive correlations were mainly significantly associated with positive regulation of cellular processes (GO:0048522; Fold Enrichment = 1.71; P value = 1.63E-08; FDR = 8.49E-05), multicellular organism development (GO:0007275; Fold Enrichment = 1.84; P value = 1.15E-08; FDR = 9.00E-05), and positive regulation of biological process (GO:0048518; Fold Enrichment = 1.68; P value = 5.99E-09; FDR = 9.38E-05). (Table S2, Supplemental Digital Content, http://links.lww.com/MD/J337). Moreover, to link all genes that were implicated in the most significant GO term (positive regulation of cellular processes) to each other, a weighted network was constructed by a web interface GeneMANIA (https://genemania.org/). We observed that positive regulation of cell migration and positive regulation of cell adhesion are 2 of the prominent functions predicted. The genes that were involved in the 2 functions mentioned above include NEDD9, CLASP1, cytotoxic T-lymphocyte-associated protein 4 (CTLA4), DOCK4, ICOSLG, FGF1, SOCS1, TFRC, PIK3CB, JAK2, THBS4, TNFSF4, IL15, PTGS2, ICAM1, GATA3, and VCAM1. Specifically, we connected these genes to each other and identified several distinguished pathways, including lymphocyte, interferon gamma (IFN-γ) response, and CTLA4 (Fig. 4). However, the GO biological process enrichment terms of SYNPO2 negatively associated genes did not meet the criteria (FDR > 0.05) (Table S3, Supplemental Digital Content, http://links.lww.com/MD/J338). Collectively, these results help us better understand the SYNPO2-associated regulatory biological process network in patients with NPC.
Figure 4.
Gene coexpression network. A weighted network that correlates all genes to each other was constructed by a web interface GeneMANIA. Red and blue semicircles symbolize genes that are involved in positive regulation of cell migration and positive regulation of cell adhesion, respectively. Purple and gray lines represent coexpression and pathways, correspondingly.
4. Discussion
NPC is one of the most common head and neck cancers and is a major public health threat.[23] NPC has distinct variations in race, geographical location, and familial epidemiological environment.[2] Clinical statistics indicate that NPC is uncommon in the Western world but widespread in certain parts of Southeast Asia, the Middle East, and North Africa.[24] It can be classified into 3 subtypes according to histological grade: non-keratinizing undifferentiated carcinoma (60–65%), non-keratinizing differentiated carcinoma (10–15%), and keratinizing squamous cell carcinoma (20–25%).[25,26] Recent large-scale studies have indicated that EBV is the primary etiological agent in the pathogenesis of NPC, and circulating fragments of EBV-derived DNA have been detected in more than 95% of patients with advanced NPC.[27–29] Although EBV infection is positively associated with NPC, only a minority of individuals with EBV infection develop NPC.[30,31] These results suggest that other risk factors may play significant roles in NPC pathogenesis. For example, genetics, environmental factors, salt-cured food, nitrite and nitrosamine intake, tobacco, and alcohol consumption may play a significant role in NPC development.[2] Owing to the unique location and proximity of the nasopharynx to critical neurovascular structures, surgical resection is difficult; consequently, RT is usually the primary treatment.[32,33] Although recent advances in therapy have been applied to prolong survival rates, many patients with NPC undergoing treatment still develop tumor metastasis and rapid tumor recurrence, resulting in poor survival outcomes. Identifying novel biological markers that are available for the diagnosis of NPC is imperative to overcome the limitations of conventional treatment for recurrent and metastatic NPC.
Our study is the first to demonstrate an association between SYNPO2 and adverse clinical outcomes in NPC. We detected SYNPO2 from genes related to cell adhesion (GO:0003779) and the GSE12452 dataset. In this dataset, we found that SYNPO2 was significantly highly expressed in NPC patient tissues compared with that in nontumor tissues. Furthermore, high SYNPO2 immuno expression was positively correlated with poor prognosis in NPC patients. SYNPO2 is a protein-coding gene that is involved in the regulation of cell migration. A recent study suggested that SYNPO2 promotes tumor metastasis through calcineurin-induced nuclear-cytoplasmic translocation in hepatocellular carcinoma cells and is positively associated with recurrence.[34] Another study reported that SYNPO2 enhances the assembly of peripheral actin bundles to promote prostate cancer cell migration.[22] These results suggest that SYNPO2 acts as an oncogene in hepatocellular carcinoma and prostate cancer.
Notably, some evidence indicates that SYNPO2 may be a tumor suppressor gene in several cancer types. Liu et al[18] reported that SYNPO2 inhibits YAP/TAZ activity to inhibit triple-negative breast cancer metastasis. In addition, inhibition of SYNPO2 expression promotes the PI3K/Akt/mTOR signaling pathway, which promotes cell migration/invasion in different breast cancer cell lines.[35] Under hypoxic conditions, SYNPO2 inhibits cell migration and the epidermal–mesenchymal transition process in colorectal cancer through the YAP-KLF5 signaling pathway.[36] Moreover, some studies have indicated that promoter methylation of SYNPO2 causes SYNPO2 gene expression inhibition and correlates with poor survival outcomes in melanoma patients,[21] bladder cancer,[37] and colon cancer.[38] Overall, these studies reveal that SYNPO2 may have many biological functions in different cancer types because it can act as either an oncogene or a tumor suppressor gene. Our results indicate that SYNPO2 can be considered an oncogene and that high expression of SYNPO2 is significantly correlated with poor prognosis in patients with NPC.
In clinical practice, the treatment of distant metastasis is a significant problem that has existed for a long time in NPC, and understanding the metastasis mechanism of NPC is beneficial for the treatment of patients. Moreover, we know that the SYNPO2 gene is associated with cell adhesion; thus, we also wanted to know how SYNPO2 participates in NPC cell adhesion. In this study, we analyzed SYNPO2 top 200 positively or negatively associated genes and used these associated genes to identify GO biological process enrichment terms correlated with SYNPO2. Our analysis found that the GO biological process terms for genes that were significantly positively correlated with SYNPO2 were mainly associated with cellular processes. More specifically, positive regulation of cell migration and positive regulation of cell adhesion were 2 significant functions predicted by the GeneMANIA prediction server. Given the associations between SYNPO2 and cell adhesion, this observation is perhaps unsurprising because the cellular process-mediated regulatory function includes the regulation of actin filament severing, cell activation, and cell adhesion. Note worthily, several unrevealed pathways, including lymphocyte, IFN-γ response, and CTLA4, were identified (Fig. 4). Recently, immunotherapy has inspired innovation in the clinical management of many tumors, including NPC.[39] IFN-γ has been indicated to upregulate programmed death ligand 1 expression and escape the immune surveillance in NPC.[40] CTLA4, primarily expressed by T cells, functions as a negative regulator of the antitumor effect of T cells. Additionally, tumor overexpression of CTLA4 has also been correlated with poor prognosis in patients with NPC.[41] Therefore, we surmise that SYNPO2 may be one of the triggers of immune evasion, resulting in cancer recurrence and metastasis, eventually leading to poor survival outcomes in NPC patients. However, whether NPC patients with SYNPO2 overexpression could predict good response to immunotherapy needs further investigation. According to our univariate analysis, the primary tumor, lymph node metastasis, AJCC stage, and high SYNPO2 expression were identified as prognostic factors for DSS, DMeFS, and LRFS in patients with NPC. Moreover, our multivariate survival analysis using the Cox proportional hazard model identified that AJCC stage and high SYNPO2 expression were independent prognostic factors for DMeFS and LRFS in patients with NPC.
However, our study has some limitations. First, this was a retrospective, single-center cohort study. Therefore, future studies should analyze larger cohorts to confirm our results. Second, we could not clarify the regulatory mechanism of SYNPO2 or candidate molecules in SYNPO2-mediated poor prognosis in patients with NPC; thus, future research should determine the roles of SYNPO2 in tumor progression in NPC as well as the underlying signaling pathway involved in the regulation of SYNPO2.
5. Conclusion
In conclusion, this is the first study to report that SYNPO2 may be a significant prognostic biomarker in NPC. Our results demonstrated that SYNPO2 was highly expressed in NPC patients and significantly correlated with the clinicopathological characteristics of these patients. In particular, high SYNPO2 expression was associated with poor DSS, DMeFS, and LRFS in NPC patients. Our findings support a new potential target for SYNPO2 as a diagnostic and prognostic marker in patients with NPC.
Acknowledgments
The authors are grateful for the kind help received from the Translational Research Laboratory of Human Cancers, Department of Medical Research, Chi Mei Medical Center.
Author contributions
Conceptualization: Shih-Lun Chang, Hong-Yue Lai, Sung-Wei Lee.
Formal analysis: Shih-Lun Chang, Ching-Chieh Yang, Hong-Yue Lai, Hsin-Hwa Tsai, Cheng-Fa Yeh, Sung-Wei Lee, Nai-Wen Kang, Wen-Bin Wu, Tzu-Ju Chen.
Funding acquisition: Wen-Bin Wu, Tzu-Ju Chen.
Investigation: Shih-Lun Chang, Ching-Chieh Yang, Hsin-Hwa Tsai, Cheng-Fa Yeh, Yu-Hsuan Kuo, Nai-Wen Kang, Wen-Bin Wu, Tzu-Ju Chen.
Methodology: Shih-Lun Chang, Ching-Chieh Yang, Hong-Yue Lai, Hsin-Hwa Tsai, Cheng-Fa Yeh, Yu-Hsuan Kuo, Tzu-Ju Chen.
Resources: Sung-Wei Lee, Yu-Hsuan Kuo, Nai-Wen Kang, Wen-Bin Wu, Tzu-Ju Chen.
Supervision: Wen-Bin Wu, Tzu-Ju Chen.
Validation: Shih-Lun Chang, Ching-Chieh Yang, Hong-Yue Lai, Hsin-Hwa Tsai, Cheng-Fa Yeh, Sung-Wei Lee, Yu-Hsuan Kuo, Nai-Wen Kang.
Visualization: Shih-Lun Chang, Ching-Chieh Yang, Hsin-Hwa Tsai, Cheng-Fa Yeh, Wen-Bin Wu, Tzu-Ju Chen.
Writing – original draft: Shih-Lun Chang, Wen-Bin Wu.
Writing – review & editing: Hong-Yue Lai, Tzu-Ju Chen.
Supplementary Material
Abbreviations:
- AJCC
- American Joint Committee on Cancer
- CI
- confidence interval
- CTLA4
- cytotoxic T-lymphocyte-associated protein 4
- DMeFS
- distal metastasis-free survival
- DSS
- disease-specific survival
- EBV
- Epstein–Barr virus
- GO
- gene ontology
- HR
- hazard ratio
- IHC
- immunohistochemical
- IFN-γ
- interferon gamma
- LRFS
- local recurrence-free survival
- NPC
- nasopharyngeal carcinoma
- RT
- radiation therapy
- SYNPO2 =
- synaptopodin-2
As a rule, every participant signed an informed consent before enrolling in the biobank. This study and its use of NPC tissues de-identified from the biobank were approved by the Ethics Committee and Institutional Review Board of Chi Mei Medical Center (IRB10302013) and followed the ethical guidelines of the Helsinki Declaration and the regulations of the government.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplemental Digital Content is available for this article.
The authors have no funding and conflicts of interest to disclose.
This work was supported by Chi Mei Medical Center (CMFHR11050 and CMJF11002) and by China Medical University (CMU111-N-23).
How to cite this article: Chang S-L, Yang C-C, Lai H-Y, Tsai H-H, Yeh C-F, Lee S-W, Kuo Y-H, Kang N-W, Wu W-B, Chen T-J. SYNPO2 upregulation is an unfavorable prognostic factor for nasopharyngeal carcinoma patients. Medicine 2023;102:30(e34426).
Contributor Information
Shih-Lun Chang, Email: c3224710@ms16.hinet.net.
Ching-Chieh Yang, Email: cleanclear0905@gmail.com.
Hong-Yue Lai, Email: golddigger815@yahoo.com.tw.
Hsin-Hwa Tsai, Email: livelychord@yahoo.com.tw.
Cheng-Fa Yeh, Email: u802091@gmail.com.
Sung-Wei Lee, Email: lisungwei@hotmail.com.
Yu-Hsuan Kuo, Email: a10901@mail.chimei.org.tw.
Nai-Wen Kang, Email: wnk119@gmail.com.
Wen-Bin Wu, Email: wenbin@mail.fju.edu.tw.
References
- [1].Almomani MH, Zulfiqar H, Nagalli S. Nasopharyngeal carcinoma (NPC, Lymphoepithelioma). Treasure Island (FL): StatPearls; 2022. [PubMed] [Google Scholar]
- [2].Chang ET, Ye W, Zeng YX, et al. The evolving epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2021;30:1035–47. [DOI] [PubMed] [Google Scholar]
- [3].Fan HC, Chen CY, Hsu YC, et al. Increased risk of incident nasopharyngeal carcinoma with exposure to air pollution. PLoS One. 2018;13:e0204568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen YP, Ismaila N, Chua MLK, et al. Chemotherapy in combination with radiotherapy for definitive-intent treatment of stage II–IVA nasopharyngeal carcinoma: CSCO and ASCO guideline. J Clin Oncol. 2021;39:840–59. [DOI] [PubMed] [Google Scholar]
- [5].Zhang LL, Xu F, Song D, et al. Development of a nomogram model for treatment of nonmetastatic nasopharyngeal carcinoma. JAMA Netw Open. 2020;3:e2029882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kou J, Zhang LL, Yang XL, et al. Development of a nomogram model for treatment of elderly patients with locoregionally advanced nasopharyngeal carcinoma. J Pers Med. 2021;11:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tabuchi K, Nakayama M, Nishimura B, et al. Early detection of nasopharyngeal carcinoma. Int J Otolaryngol. 2011;2011:638058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li XY, Jia GD, Sun XS, et al. Intensive local radiotherapy is associated with better local control and prolonged survival in bone-metastatic nasopharyngeal carcinoma patients. Front Oncol. 2020;10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhi-Qiang W, Qi M, Ji-Bin L, et al. The long-term survival of patients with III-IVb stage nasopharyngeal carcinoma treated with IMRT with or without Nimotuzumab: a propensity score-matched analysis. BMC Cancer. 2019;19:1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chen C, Wu JB, Jiang H, et al. A prognostic score for nasopharyngeal carcinoma with bone metastasis: development and validation from multicenter. J Cancer. 2018;9:797–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fares J, Fares MY, Khachfe HH, et al. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liao W, He J, Gou Q, et al. Local treatment of metastases plus systemic chemotherapy on overall survival of patients with metastatic nasopharyngeal carcinoma. Head Neck. 2021;43:2423–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Clark AG, Vignjevic DM. Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol. 2015;36:13–22. [DOI] [PubMed] [Google Scholar]
- [14].Pachmayr E, Treese C, Stein U. Underlying mechanisms for distant metastasis - molecular biology. Visc Med. 2017;33:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Janiszewska M, Primi MC, Izard T. Cell adhesion in cancer: beyond the migration of single cells. J Biol Chem. 2020;295:2495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Darvishi B, Boroumandieh S, Majidzadeh AK, et al. The role of activated leukocyte cell adhesion molecule (ALCAM) in cancer progression, invasion, metastasis and recurrence: a novel cancer stem cell marker and tumor-specific prognostic marker. Exp Mol Pathol. 2020;115:104443. [DOI] [PubMed] [Google Scholar]
- [17].Massague J, Ganesh K. Metastasis-initiating cells and ecosystems. Cancer Discov. 2021;11:971–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu J, Ye L, Li Q, et al. Synaptopodin-2 suppresses metastasis of triple-negative breast cancer via inhibition of YAP/TAZ activity. J Pathol. 2018;244:71–83. [DOI] [PubMed] [Google Scholar]
- [19].Ma L, Zhang W, Ding Z, et al. Association of a common variant of SYNPO2 gene with increased risk of serous epithelial ovarian cancer. Tumour Biol. 2017;39:1010428317691185. [DOI] [PubMed] [Google Scholar]
- [20].Kai F, Duncan R. Prostate cancer cell migration induced by myopodin isoforms is associated with formation of morphologically and biochemically distinct actin networks. FASEB J. 2013;27:5046–58. [DOI] [PubMed] [Google Scholar]
- [21].Gao L, van den Hurk K, Nsengimana J, et al. Prognostic significance of promoter hypermethylation and diminished gene expression of SYNPO2 in melanoma. J Invest Dermatol. 2015;135:2328–31. [DOI] [PubMed] [Google Scholar]
- [22].Kai F, Fawcett JP, Duncan R. Synaptopodin-2 induces assembly of peripheral actin bundles and immature focal adhesions to promote lamellipodia formation and prostate cancer cell migration. Oncotarget. 2015;6:11162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu L, Li C, Pan L. Nasopharyngeal carcinoma: a review of current updates. Exp Ther Med. 2018;15:3687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Dai W, Zheng H, Cheung AK, et al. Genetic and epigenetic landscape of nasopharyngeal carcinoma. Chin Clin Oncol. 2016;5:16. [DOI] [PubMed] [Google Scholar]
- [25].Sinha S, Gajra A. Nasopharyngeal cancer. Treasure Island (FL): StatPearls; 2022. [Google Scholar]
- [26].Jicman Stan D, Niculet E, Lungu M, et al. Nasopharyngeal carcinoma: a new synthesis of literature data (review). Exp Ther Med. 2022;23:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Richardo T, Prattapong P, Ngernsombat C, et al. Epstein-Barr virus mediated signaling in nasopharyngeal carcinoma carcinogenesis. Cancers (Basel). 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Coghill AE, Wang CP, Verkuijilen S, et al. Evaluation of nasal and nasopharyngeal swab collection for the detection of Epstein-Barr virus in nasopharyngeal carcinoma. J Med Virol. 2018;90:191–5. [DOI] [PubMed] [Google Scholar]
- [29].Li YQ, Khin NS, Chua MLK. The evolution of Epstein-Barr virus detection in nasopharyngeal carcinoma. Cancer Biol Med. 2018;15:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu LT, Liang YJ, Guo SS, et al. Identifying distinct risks of treatment failure in nasopharyngeal carcinoma: study based on the dynamic changes in peripheral blood lymphocytes, monocytes, N classification, and plasma Epstein-Barr virus DNA. Head Neck. 2022;44:34–45. [DOI] [PubMed] [Google Scholar]
- [31].Kim KY, Le QT, Yom SS, et al. Clinical utility of Epstein-Barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2017;98:996–1001. [DOI] [PubMed] [Google Scholar]
- [32].Blanchard P, Biau J, Huguet F, et al. Radiotherapy for nasopharyngeal cancer. Cancer Radiother. 2022;26:168–73. [DOI] [PubMed] [Google Scholar]
- [33].Sharma V, Kumar A, Chaudhary A. Locoregional radiotherapy in metastatic nasopharyngeal cancer. JAMA Oncol. 2021;7:310–1. [DOI] [PubMed] [Google Scholar]
- [34].Gao J, Zhang HP, Sun YH, et al. Synaptopodin-2 promotes hepatocellular carcinoma metastasis via calcineurin-induced nuclear-cytoplasmic translocation. Cancer Lett. 2020;482:8–18. [DOI] [PubMed] [Google Scholar]
- [35].Xia E, Zhou X, Bhandari A, et al. Synaptopodin-2 plays an important role in the metastasis of breast cancer via PI3K/Akt/mTOR pathway. Cancer Manag Res. 2018;10:1575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].OuYang C, Xie Y, Fu Q, et al. SYNPO2 suppresses hypoxia-induced proliferation and migration of colorectal cancer cells by regulating YAP-KLF5 axis. Tissue Cell. 2021;73:101598. [DOI] [PubMed] [Google Scholar]
- [37].Alvarez-Mugica M, Cebrian V, Fernandez-Gomez JM, et al. Myopodin methylation is associated with clinical outcome in patients with T1G3 bladder cancer. J Urol. 2010;184:1507–13. [DOI] [PubMed] [Google Scholar]
- [38].Esteban S, Moya P, Fernandez-Suarez A, et al. Diagnostic and prognostic utility of methylation and protein expression patterns of myopodin in colon cancer. Tumour Biol. 2012;33:337–46. [DOI] [PubMed] [Google Scholar]
- [39].Xu JY, Wei XL, Wang YQ, et al. Current status and advances of immunotherapy in nasopharyngeal carcinoma. Ther Adv Med Oncol. 2022;14:17588359221096214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fang W, Zhang J, Hong S, et al. EBV-driven LMP1 and IFN-γ up-regulate PD-L1 in nasopharyngeal carcinoma: implications for oncotargeted therapy. Oncotarget. 2014;5:12189–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Huang PY, Guo SS, Zhang Y, et al. Tumor CTLA-4 overexpression predicts poor survival in patients with nasopharyngeal carcinoma. Oncotarget. 2016;7:13060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.