Abstract
The objective was to investigate the potential cardiac arrhythmia-related target proteins and molecular mechanisms underlying the anti-arrhythmic effects of Sophora flavescens using network pharmacology and molecular docking. The bioactive ingredients and related target proteins of S flavescens obtained from the Traditional Chinese medicine systems pharmacology data platform, and gene names for target proteins were obtained from the UniProt database. Arrhythmia-related genes were identified by screening GeneCards and Online Mendelian inheritance in man databases. A Venn diagram was used to identify the key arrhythmia-related genes that are potentially targeted by the bioactive ingredients of S flavescens. Furthermore, CytoScape 3.7.2 software was used to construct an “ingredient-target” network diagram and the “drug-ingredient-target-disease” network diagram. We performed gene ontology and Kyoto encyclopedia of genes and genomes enrichment analysis in the Metascape database and performed the docking analysis using CB-Dock software. We identified 45 main bioactive ingredients, from S flavescens and 66 arrhythmia-related target proteins. Gene ontology and Kyoto encyclopedia of genes and genomes pathway enrichment analysis showed that these targets were related to the chemical carcinogenesis-receptor activation signaling pathway, lipid and atherosclerosis signaling pathway, and fluid shear stress and atherosclerosis signaling pathway. Molecular docking showed that the target protein had good binding power with the main active components of the compound of S flavescens. Our study demonstrated the synergistic effects of multiple bioactive components of S flavescens on multiple arrhythmia-related target proteins and identified potential therapeutic mechanisms underlying the anti-arrhythmic effects of S flavescens, providing new clinical ideas for arrhythmia treatment.
Keywords: cardiac arrhythmia, molecular docking, network pharmacology, Sophora flavescens, traditional Chinese medicine
1. Introduction
Cardiac arrhythmia is defined as an irregular or abnormal heartbeat wherein the frequency and/or rhythm of the heartbeat is either slower or faster than normal. It is caused by abnormal excitation of the sinus node or excitation generated outside the sinus node and/or slow, blocked, or irregular conduction of electrical activity through the conduction channels, probably due to impairment in the origin and/or conduction of cardiac activity.[1] Arrhythmias are an important group of cardiovascular diseases that can develop alone or in association with other cardiovascular diseases. A major symptom of abnormal cardiac activity in cardiac arrhythmias is palpitations. These can occur at any age and are most commonly observed in subjects with acquired arrhythmias and heart diseases such as coronary artery disease, cardiomyopathy, myocarditis, and wind heart disease. Palpitations are more common in subjects with heart failure or acute myocardial infarction and in subjects with hereditary arrhythmias, including those with long QT syndrome, short QT syndrome, and Brugada syndrome, which are caused by genetic mutations in the channels. Palpitations are also reported in healthy individuals or patients with vegetative dysfunction and arrhythmias and are caused by etiologies such as electrolyte or endocrine disorders, anesthesia, hypothermia, thoracic or cardiac surgery, drug effects and central nervous system disorders. The current clinical management strategies for subjects with arrhythmias include drug therapies and surgical treatment. However, the current treatment of arrhythmias is suboptimal because of adverse effects or side effects of the available anti-arrhythmic drugs and limitations of surgical treatment.[2] Traditional Chinese medicine (TCM) is a novel avenue for the treatment of cardiac arrhythmias because of its holistic treatment concept and its multi-target, multi-channel, and multi-link effects on functions and characteristics, which have been confirmed by network pharmacology. This provides a new research paradigm for transforming TCM from an empirical to an evidence-based medicine system.[3]
Sophora flavescens, also known as Ku Shen in Chinese, has been widely used in TCM for nearly 2 thousand years. It was described in 200 A.D. in the first Chinese medical classic, Shen-Nung’s Pen-Ts’ao.[4] Active components from S flavescens including picrasidine, oxymatrine, and flavonoids have been isolated and characterized by modern pharmacological studies, and are associated with antitumor, antiviral, anti-inflammatory, hepatoprotective, anti-arrhythmic, analgesic, antipyretic, anti- anaphylactic, and antiasthma properties.[5]
Network pharmacology (NP) is a new paradigm that integrates network biology and pharmacology to systematically understand the mechanisms underlying the effect of drugs on complex disease networks. NP is based on the complex interactions between complex networks of disease-related genes and drug targets. NP is used to elucidate potential mechanisms by which herbal medicines act at a molecular level by its ability to generate complex interaction networks between the target biomolecules (for example, proteins) and the bioactive compounds.[6] NP is also used to estimate the pharmacological activity of the active drug compounds on multiple protein targets[7] and can be used to unravel the multi-target framework of various small molecule drugs.[8] NP has been used to investigate mechanisms of action for therapeutically active compounds in the ayurvedic herbs (ancient traditional Indian Medical System) including the phytochemicals of Piper longum and Tephrosia purpurea.[9,10] NP is considered as a next generation drug discovery method for traditional drugs.[10] Molecular docking is an essential tool in structural molecular biology and modern pharmacology that uses computer-aided drug design to predict the major binding sites of ligands in the target proteins.[11] The target proteins and underlying mechanisms of action have not yet been elucidated for S flavescens in the treatment of arrhythmias. Therefore, in this study, we used network pharmacology and molecular docking techniques to investigate the potential arrhythmia-related target proteins and the mechanisms of action for the active components of S flavescens in the treatment of arrhythmias.
2. Materials and methods
2.1. Screening active ingredients of S flavescens
We searched the Traditional Chinese Medicine Systems Pharmacology (TCMSP, http://tcmspw.com/tcmspsearch.php) database[12] using “Ku Shen” as the keyword and extracted all the active ingredients of S flavescens. We used oral bioavailability (OB) and drug-likeness (DL) as screening conditions because they represent important evaluation indices in the process of absorption, distribution, metabolism, and excretion during drug development.[13] Active ingredients with OB ≥ 30% and DL ≥ 0:18 were selected for further investigation.[14]
2.2. Screening of target proteins of the active ingredients of S flavescens
We searched “Related Targets” in the TCMSP database with “Kushen” as the keyword to identify potential proteins targeted by the active ingredients of S flavescens.[15] Then, the genes corresponding to the target proteins were identified using the UniProt (https://www.uniprot.org/) database.
2.3. Identification of target genes related to arrhythmia
We searched the GeneCards (https://www.genecards.org/) and Online Mendelian Inheritance in Man (https://www.omim.org) databases[14] with “arrhythmia” as the keyword to identify potential target genes associated with arrhythmia. In addition, the UniProt database (https://www.uniprot.org/)[16] was used to identify the target proteins. The overlapping target proteins for the active ingredients of S flavescens and arrhythmia were visualized using the VENNY 2.1 tool (https://bioinfogp.cnb.csic.es/tools/venny/).
2.4. Construction of the protein-protein interaction network
We analyzed protein-protein interactions (PPI) between the overlapping target proteins by constructing the PPI network using the Search Tool for the Retrieval of Interacting Genes/Proteins database version 11.5[17] by setting “Homo sapiens” as the species. The minimum interaction score was set as 0.4 to identify protein-protein interactions with high confidence. A visual comprehensive network drug-ingredient-target-disease was built using the Cytoscape 3.7.2 software (https://cytoscape.org/)[18] based on the interactions between the active ingredients of S flavescens, intersection target genes, and disease of interest (arrhythmia).
2.5. Gene ontology (GO) and Kyoto encyclopedia of genes and genomes (KEGG) pathway enrichment analysis
We performed GO and KEGG pathway enrichment analysis[19] in the Metascape database using an adjusted P value < .01 as a threshold parameter to deiermine the potential molecular mechanisms underlying the anti-arrhythmic effects of S flavescens.
2.6. Molecular docking of active ingredients with key target proteins
We selected “Homo Sapiens” as the species in the protein data bank database (https://www.rcsb.org/)[19] and used screen resolutions of 2.0 to 2.5 and 2.5 to 3.0 to extract the protein data bank files of key target proteins in the S flavescens-arrhythmia target network graph, which was based on degree scores. We then searched the ZeroDesigner DrugSci (ZeroDesigner DrugSci Is Not Commercial) database (http://zinc.docking.org/)[20] to extract the MOL2 files (readable formats by many chemoinformatics/bioinformatics software packages). of the top 3 active ingredients of S flavescens. The top 3 target proteins were docked with the top 3 bioactive ingredients of S flavescens using the CB-Dock (http://clab.labshare.cn/cb-dock/php/index.php) database,[21] and their binding affinities were estimated based on the Vina score[22] values. This study did not involve human or animal subjects, and thus, no ethical approval was required.
3. Results
3.1. Identification of the bioactive ingredients of S flavescens
We searched the TCMSP database with “kushen” as the keyword and identified 113 types of chemical ingredients including 45 potential bioactive ingredients using OB ≥ 30% and DL ≥ 0:18 as screening parameters (Table 1). Then, these potential active compounds were screened using degree ≥ 5 (from the TCMSP database) as a threshold parameter and the following 12 potential active ingredients of S flavescens were confirmed with potential anti-arrhythmic properties: Quercetin, Luteolin, Phaseolin, Formononetin, (2R)-5,7-dihydroxy-2-(4-hydroxyphenyl) chroman-4-1,8-isopentenyl-kempferol, Matrine, Wighteone, Glyceollin, iermine, Kushenin, Maackiain Table 2 shows their PubChem compound identifier numbers, molecular formulas, as well as degree, betweenness, and closeness centrality values of these 12 bioactive ingredients.
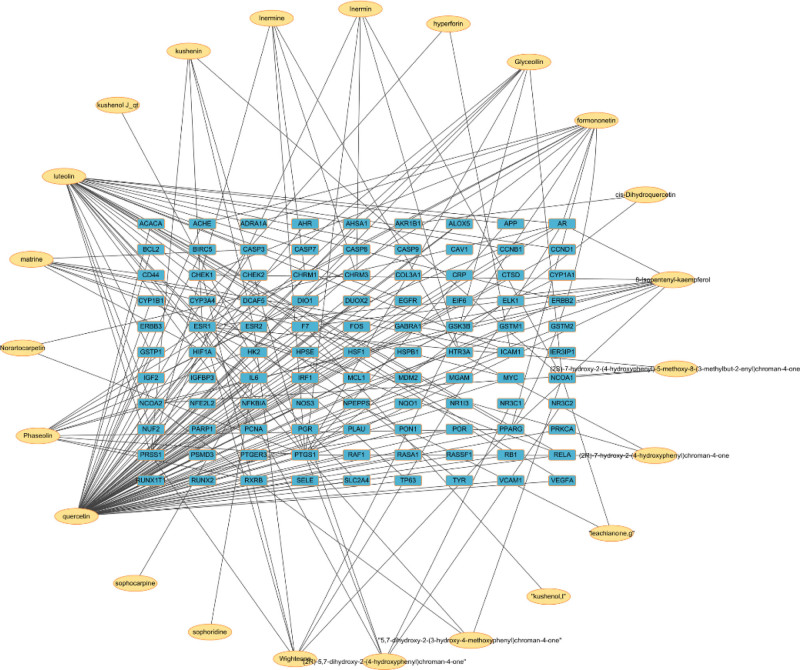
Figure 1.
Network of the main bioactive components and the target proteins of Sophora flavescens. Each oval shape (yellow) represents one of the main bioactive ingredients of Sophora flavescens; each rectangle (blue) represents a target protein.
Table 2.
The main topological parameters of the main bioactive components of Sophora flavescens.
| PubChem | Name | Degree | Betweenness | Closeness centrality |
|---|---|---|---|---|
| 5,280,343 | Quercetin | 77 | 0.771886 | 0.633507853 |
| 5,280,445 | Luteolin | 28 | 0.177609 | 0.418685121 |
| 5,280,378 | Formononetin | 12 | 0.043008 | 0.36119403 |
| 91,572 | Phaseolin | 11 | 0.045741 | 0.36119403 |
| 129,716,399 | 8-Isopentenyl-kaempferol | 10 | 0.026302 | 0.36119403 |
| 91,466 | Matrine | 8 | 0.050532 | 0.312661499 |
| 5,281,814 | Wighteone | 7 | 0.010436 | 0.346704871 |
| 162,807 | Glyceollin | 7 | 0.013967 | 0.352769679 |
| 667,495 | (2R)-5,7-dihydroxy -(4-hydroxyphenyl) chroman-4-one |
5 | 0.050099 | 0.34870317 |
| 91,510 | Iermine | 5 | 0.014139 | 0.344729345 |
Table 1.
Characteristics of the bioactive ingredients of Sophora flavescens with anti-arrhythmic properties.
3.2. Identification of the target proteins/genes of S flavescens and construction of an active ingredient-target network
We identified 963 potential target proteins for the 45 major bioactive ingredients of S flavescens using the TCMSP platform. The gene names for these 963 target proteins were identified using the UniProt database, total of 99 targets were obtained after deleting the duplicate target names. Then, the Cytoscape 3.7.2 software was used to construct a network between the bioactive ingredients and the corresponding target proteins as shown in Figure 1. The Cytoscape 3.7.2 software automatically hid the bioactive ingredients and the target protein nodes with low correlation and only represented those with significant correlation. The highest degree score in the network analysis was 77 for quercetin. The Betweenness and Closeness centrality values for quercetin were 0.7719 and 0.6335, respectively. Cytoscape analysis predicted that quercetin was the most important bioactive ingredient of S flavescens with anti-arrhythmic properties followed by luteolin, frontopontine, phaseolin, 8-isopentenyl-kempferol, and matrine (Table 2). According to the TCMSP database, iermine was one of the main bioactive ingredients of S flavescens. However, the network analysis showed that the connection and mediator degrees of iermine were low and did not show any significant interaction with the target proteins. Therefore, iermine was excluded from the network diagram.
3.3. Identification of arrhythmia-related target genes
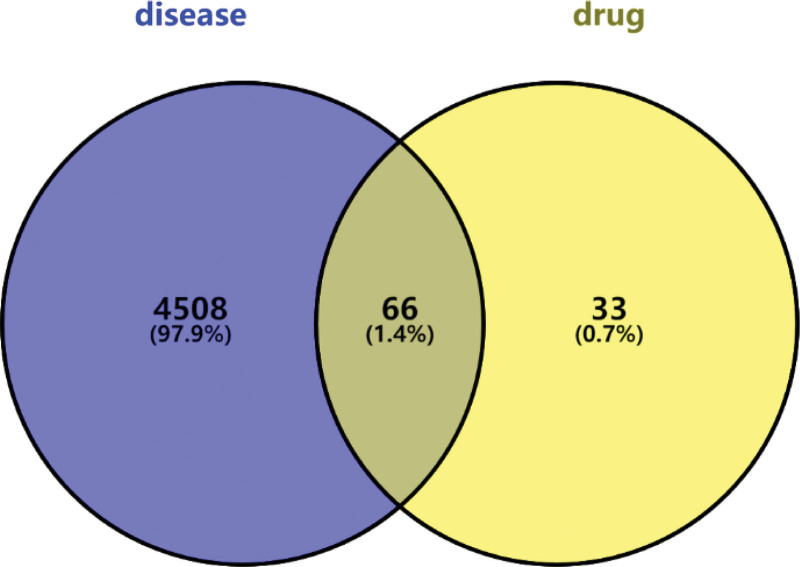
We searched the GeneCards and Online Mendelian Inheritance in Man databases using the keyword “nephrotic syndrome” and identified 4574 genes. Sixty-six intersecting target proteins were identified by overlapping the target proteins of S flavescens with the target proteins of arrhythmia using the Venn diagram (Fig. 2).
Figure 2.
Venn diagram of the Sophora flavescens target proteins (n = 4574) and the atherosclerotic target proteins (n = 99). As shown, blue section represents the Sophora flavescens target proteins (n = 4508), yellow section represents atherosclerotic target proteins (n = 33), and the gray overlapping section at the center represents the potential target proteins of the bioactive ingredients of Sophora flavescens in the treatment of arrhythmia (n = 66).
3.4. Identification of the arrhythmia-related target proteins of the bioactive ingredients and construction of the drug-ingredient-target-disease network
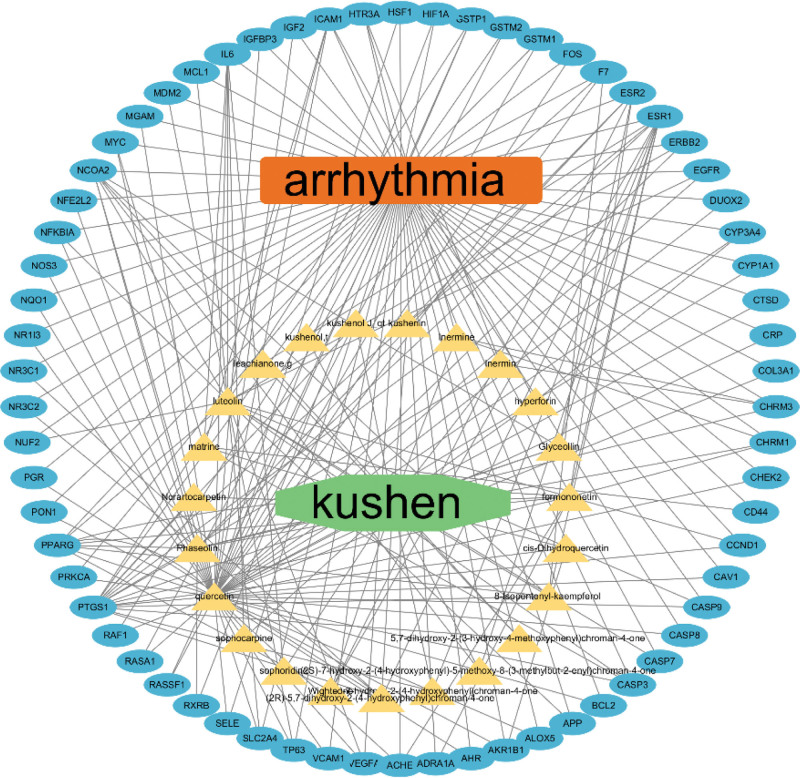
The Cytoscape 3.7.2 software was used to construct the drug-ingredient-target-disease network (Fig. 3) that included 4 components, namely, the drug (kushen; separate node in green), bioactive ingredients (The bioactive ingredient of a drug is defined as a chemical compound with specific molecular and structural formulas.) (yellow triangle nodes), target genes (blue oval nodes), and the disease (arrhythmia; separate node in orange). This network showed the potential mechanisms by which Kushen alleviated arrhythmia through interactions between the bioactive ingredients and their target genes. As shown in Table 3, that IL-6 was the most important target for S flavescens in regulating arrhythmias with a connectivity of 90, a median of 0.14 and a tightness of 0.7778. Epidermal growth factor receptor (EGFR), caspase-3 (CASP3), ESR1, MYC, PPARG, VEGFA, HIF1A, CCND1, and ERBB2 were also relatively important targets.
Figure 3.
Drug - active component - Target gene - disease network. The green cylinder represents the drug (Kushen); orange rectangle represents the disease (arrhythmia); yellow triangles represent the main bioactive components of Sophora flavescens; blue ovals represent the arrhythmia target proteins.
Table 3.
The main topological parameters of the main Sophora flavescens- atherosclerosis target network proteins.
| Target | Degree | Betweenness | Closeness centralities |
|---|---|---|---|
| IL-6 | 90 | 0.141713 | 0.777778 |
| EGFR | 82 | 0.042565 | 0.732558 |
| CASP3 | 80 | 0.043925 | 0.732558 |
| ESR1 | 80 | 0.039289 | 0.724138 |
| MYC | 80 | 0.062172 | 0.724138 |
| PPARG | 78 | 0.0685 | 0.715909 |
| VEGFA | 78 | 0.035429 | 0.715909 |
| HIF1A | 76 | 0.060418 | 0.707865 |
| CCND1 | 64 | 0.013289 | 0.663158 |
| ERBB2 | 62 | 0.016036 | 0.649485 |
CASP3 = caspase-3, EGFR = epidermal growth factor receptor.
3.5. Construction of protein-protein interaction network with the arrhythmia-related protein targets of S flavescens
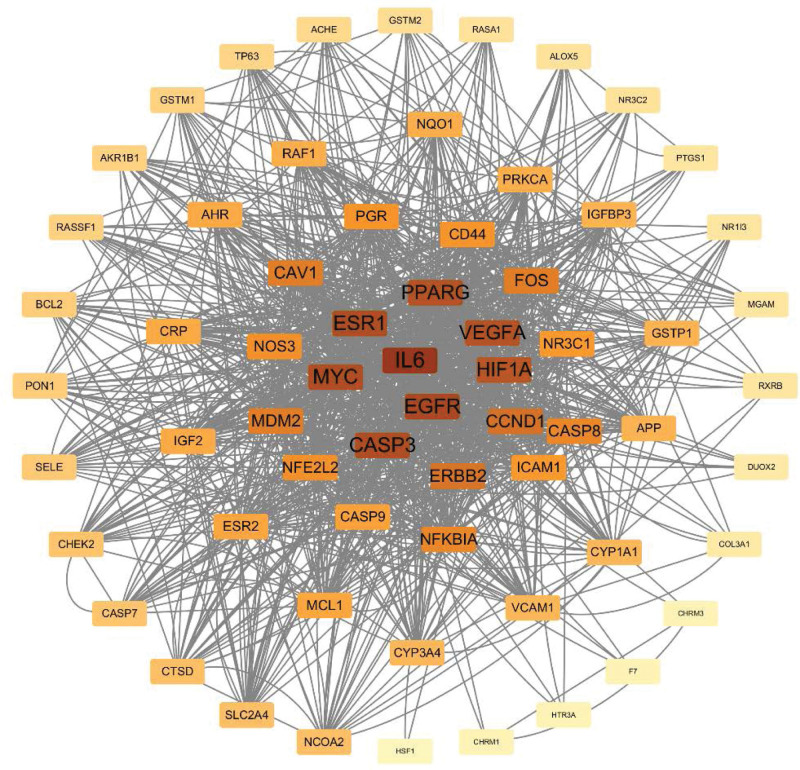
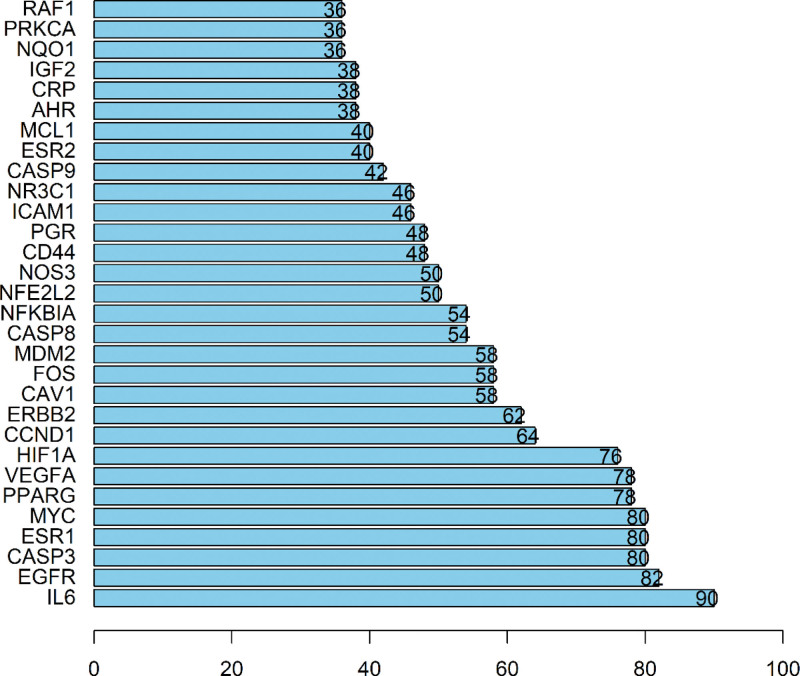
The 66 overlapping target proteins were imported into the Search Tool the Retrieval of Interaction Gene database to construct a PPI network. As shown in Figure 4, the PPI network included 64 nodes and 1152 edges. Nodes with higher degree value were denoted with darker fill color and were closer to the center. The target proteins were ranked from high to low according to the degree values. The top 5 proteins with highest degree values were IL-6, EGFR, CASP3, ESR1, and MYC (Fig. 5). We predicted that these were the 5 critical target proteins of the bioactive ingredients of S flavescens in the treatment of arrhythmia.
Figure 4.
Protein-protein interaction (PPI) network diagram shows the arrhythmia-related target proteins of Sophora flavescens. The PPI network included 64 nodes and 1152 edges. Nodes with higher degree value were denoted with darker fill color and were closer to the center.
Figure 5.
Ranking of the 30 core target proteins arranged according to the degree value.
3.6. GO and KEGG pathway enrichment analysis of the target proteins
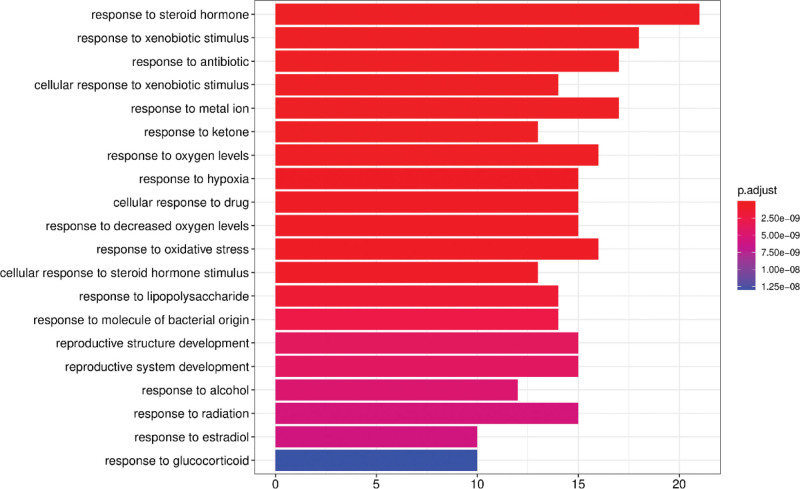
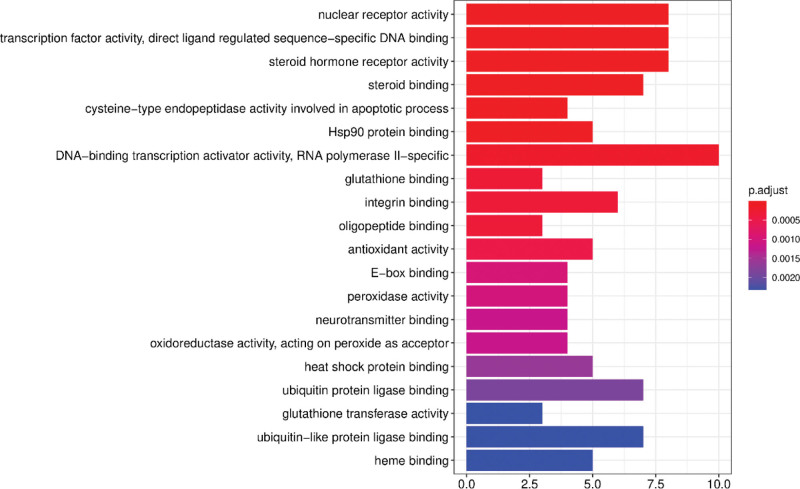
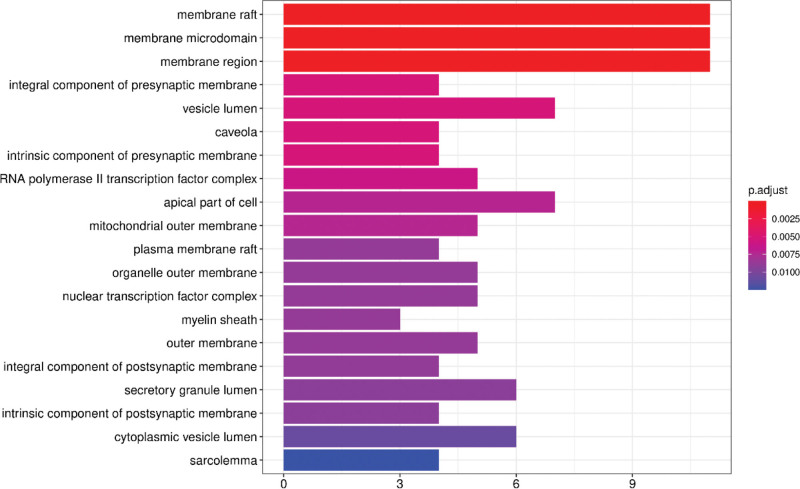
We then performed GO and KEGG pathway analysis of the intersecting target genes using the Metascape database with P < .01 as the threshold parameter to identify the potential anti-arrhythmic mechanisms of the bioactive ingredients of S flavescens. The functional enrichment analysis showed that the predicted target genes were mainly enriched in 1106 biological processes (BPs), 36 cellular components (CC), and 88 molecular functions (MF). The top BPs included response to steroid hormone, response to xenobiotic stimulus, response to antibiotic, cellular response to xenobiotic stimulus, response to metal ion, response to oxygen levels, and response to oxidative stress (Fig. 6). The top MF included DNA − binding transcription activator activity, RNA polymerase II − specific, nuclear receptor activity, transcription factor activity, direct ligand regulated sequence − specific DNA binding, steroid hormone receptor activity, and steroid binding (Fig. 7). The top CC included membrane rafts, membrane microdomains, and membrane regions (Fig. 8).
Figure 6.
Biological process (BP) enrichment analysis results for the arrhythmia-related target proteins of Sophora flavescens.
Figure 7.
Molecular function (MF) enrichment analysis results of the arrhythmia-related target proteins of Sophora flavescens.
Figure 8.
Cell component (CC) enrichment analysis of the arrhythmia-related target proteins of Sophora flavescens.
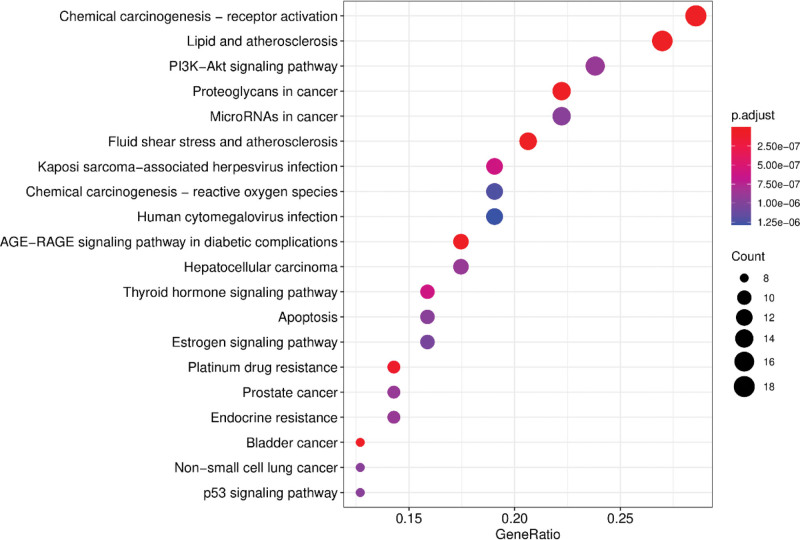
The KEGG pathway enrichment analysis of the predicted target genes was also performed in the Metascape database. The top 20 KEGG pathways based on their P values are shown in Figure 9. These included pathways related to chemical carcinogenesis − receptor activation, lipid, and atherosclerosis, PI3K-Akt signaling, proteoglycans in cancer, microRNAs in cancer, and fluid shear stress and atherosclerosis. These results suggested that the therapeutic effects of the bioactive ingredients of S flavescens to alleviate cardiac arrhythmias involved multiple biological processes, molecular functions, cellular components and signaling pathways.
Figure 9.
KEGG pathway enrichment analysis of the arrhythmia-related target proteins of Sophora flavescens. KEGG = Kyoto encyclopedia of genes and genomes.
3.7. Molecular docking analysis of key bioactive ingredients with key target proteins
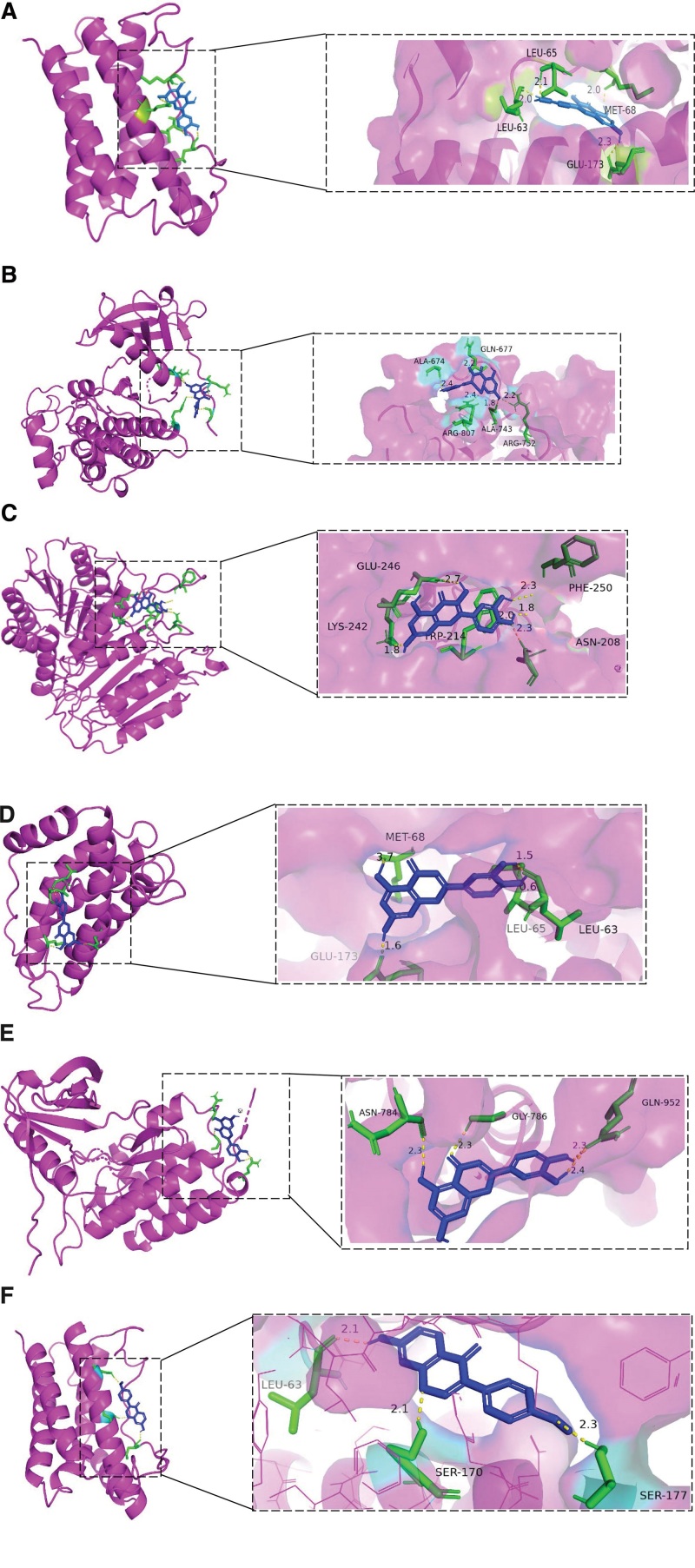
The top 3 core target proteins (IL-6, EGFR, and CASP3) based on the PPI network and the top 3 bioactive compounds of S flavescens were selected for the molecular docking analyses. The docking energy scores are shown in Table 4. In general, interactions with lower docking energy are associated with higher binding affinity. The minimum binding energies of the 3 key pharmacodynamic components to the 3 target proteins were <0. The binding docking energy scores were all lower than − 5 kcal/mol. This suggested that the interactions between the predicted core target proteins and the bioactive components of S flavescens were stable. The molecular docking results are shown in Figure 10. The structures of the compounds are represented by sticks; the protein surface structures are represented by different colors; and the hydrogen bonds are shown by the yellow dashed lines.
Table 4.
Molecular docking results for the core bioactive components of Sophora flavescens and the corresponding target proteins.
| Bioactive components |
Binding energies (kJ/mol) | ||
|---|---|---|---|
| IL-6 (541, 472) | EGFR (1956) | CASP3 (836) | |
| Quercetin | −6.74 | −5.8 | −5.59 |
| Luteolin | −6.73 | −4.74 | −6.14 |
| Formononetin | −6.37 | −5.25 | −6.35 |
CASP3 = caspase-3, EGFR = epidermal growth factor receptor.
* The numbers in parentheses are the protein-PDB ID used for docking.
Figure 10.
(A–C) Molecular docking diagrams show the interactions of quercetin with (A) IL-6, (B) EGFR, and (C) CASP3 proteins. (D, E) Molecular docking diagrams show the interactions of luteolin with (D) IL-6 and (E) EGFR. (F) Molecular docking diagram shows the interaction of formononetin with IL-6. EGFR = epidermal growth factor receptor. CASP3 = caspase-3.
4. Discussion
Heart arrhythmias are categorized as palpitations in Chinese medicine and have been recorded in ancient TCM books including Su Wen - Zhi Zhen Yao Da Lun and Ling Shu - Ben Shen, in which they have been described as “heart tantalizingly moving,” “heart frightened,” “heart is frightened,” and “the heart wants to move.”[23] These records are consistent with the manifestations of cardiac arrhythmia. S flavescens has bitter and cold properties. “The Shen Nong Ben Cao Jing” classic describes S flavescens as bitter, cold, and nontoxic, and is recommended for treating heart and abdominal gas, obstruction and accumulation, jaundice, drowning with residual leachate, expelling water, removing carbuncles and swelling, terrifying the eyes, and stopping tears.[24]
Several studies have investigated the pharmacological effects of the bioactive ingredients of S flavescens. The main bioactive ingredients of S flavescens are alkaloids, flavonoids, triterpenoid saponins, lignans, phenolic acids, and low quantities of phenylpropanoids,[25] which have antiviral, anti-inflammatory, antitumor, and immunomodulatory properties. Previous studies have shown that the S flavescens significantly alleviate ventricular arrhythmias that are induced by aconitine, ligation of the anterior descending branch of the left coronary artery, and ischemia-reperfusion in rats[26,27]; moreover, they prevent chloroform-induced ventricular fibrillation in mice by shortening the potentiation time.[28] In this study, we systematically investigated the bioactive ingredients in S flavescens, their potential target genes, and the underlying molecular mechanisms of action for the treatment of cardiac arrhythmias by using a network pharmacology approach. Our aim was to investigate the potential clinical value of S flavescens for the treatment of cardiac arrhythmias, identify potential gene targets, and provide a scientific basis for future clinical studies.
In this study, we used the network pharmacology method to construct a network diagram of the bioactive components of S flavescens and the potential target genes. This approach was used to investigate the potential therapeutic mechanisms of the bioactive compounds of S flavescens for the treatment of cardiac arrhythmias. The key bioactive ingredients of S flavescens were quercetin, luteolin, frontopontine, phaseolin, 8-isopentenyl-Kempferol and matrine. Previous studies have shown that these bioactive compounds are associated with pharmacological properties. Quercetin is a flavonoid that downregulates Toll-like receptor 4 and nuclear transcription factor-κB p65 expression levels in the serum of hypertensive rats; it also decreases blood pressure and improves ventricular remodeling by downregulating the Toll-like receptor 4 protein and mRNA levels in the myocardial tissues of hypertensive rats, thereby reducing vascular dysfunction and structural changes in the vasculature.[29–32] Quercetin suppressed aberrantly high Cx43 expression in the middle cerebral artery of spontaneously hypertensive rats and ameliorated myotonia.[33] Quercetin also reduced blood pressure in the renal hypertensive rats by decreasing the concentration of intracellular free calcium ions.[34] Chekalina et al[35] analyzed the effects of quercetin on the central hemodynamic and myocardial ischemic parameters in patients with stable coronary heart disease and reported significant reduction in the number of premature ventricular contractions based on 24-hour ambulatory ECG monitoring. Their findings demonstrated the cardioprotective properties of quercetin in coronary heart disease.
Lignans (3’, 4’, 5, 7-tetrahydroxyflavones) are natural flavonoids with antitumor, antioxidant, and anti-inflammatory properties. Subjects with metabolic syndrome treated with supplements containing lignans and chlorogenic acid show significant improvement in liver function and cardiometabolic parameters such as weight, waist circumference, lipids, and liver transaminases.[36] In one study, lignocaine upregulated the expression levels of liver X receptor alpha gene (LxRα) in the diet-induced obesity model mice, thereby improving hypercholesterolemia and abnormal glucose tolerance; it also increased the expression levels of LxRα-dependent proteins in the HepG2 cells; moreover, lignocaine regulated serum lipid levels by increasing the expression levels of LxRα, ATP transport cassette transporter G1, and type B type 1 scavenger receptor, which play a significant role in the cholesterol efflux mechanism, thereby reducing the incidence of arrhythmias.[37]
Formononetin is an isoflavone that protects H9c2 cardiomyocytes from hypoxic injury by reducing creatine kinase and lactate dehydrogenase activity, and the levels of malondialdehyde.[38] Phaseolin is a plant protein with antitoxin, antioxidant, and antigenotoxic properties that decreased pro-inflammatory mediators and increased anti-inflammatory mediators by reducing NO synthesis and inducible nitric oxide synthase expression.[39] Excessive inflammatory response plays a significant role in several chronic inflammation-related diseases including cardiovascular diseases. Phaseolin exerts anti-inflammatory effects in the RAW264.7 cells and the zebrafish larvae by downregulating nuclear transcription signaling pathway.[40] 8-Prenyl kaempferol is a flavonoid product found in S flavescens with antibacterial, anti-inflammatory, antiviral, antitumor, and anti-arrhythmic effects. Previous studies showed that the isopentenyl group in 8-Prenyl kaempferol was responsible for the antibacterial and anti-inflammatory effects.[41]
Oxymatrine is another component from S flavescens that improves myocardial energy metabolism and reverses ventricular remodeling in the heart failure model mice with viral myocarditis by protecting mitochondrial function, thereby reducing cardiomyocyte apoptosis.[42] Alkaloids from S flavescens significantly decrease adriamycin-induced cardiotoxicity in mice by reducing oxidative stress and apoptosis.[43] They also significantly alleviate high glucose-induced cardiomyocyte injury.[44] Cardiac fibrosis is mainly characterized by excessive production and deposition of extracellular matrix, which impairs contractility of the cardiomyocytes and adversely affects normal electrical conduction and causes arrhythmias. Alkaloids from S flavescens reduce trans-differentiation of the fibroblasts and cardiac fibrosis by regulating ribosomal protein S5 and inhibiting p38 phosphorylation.[45] This suggests that alkaloids from S flavescens may be used to prevent arrhythmias. Picrasidine also reduces susceptibility to atrial fibrillation in mice by inhibiting atrial fibrosis.[46] Alkaloids from S flavescens protect against cardiac hypertrophy by downregulating cytokines such as insulin-like growth factor-1 and transforming growth factor-β1, which promote enlargement of the cardiac cells, fibroblast proliferation, and subsequent cardiac hypertrophy by activating specific protein kinases.[47]
The anti-arrhythmic effects of bittersweet are related to the inhibition of various potassium currents and prolongation of the action potential duration. The inhibition of rapidly activates delayed rectifier potassium current by bittersweet is comparable with the effects of propranolol, a β-blocker, but weaker than quinidine, amiodarone, and RP58866 (a benzopyran derivative and a class III anti-arrhythmic drug); it is less likely to induce prolongation of the QT interval and arrhythmias.[48–50] Yi et al[51] showed concentration-dependent pharmacological effects of matrine on the sodium ion channel currents in the guinea pig ventricular myocytes. Matrine reduced the occurrence of ventricular arrhythmia after infarction by sustaining the action potential and maintaining the ion channels.
Our initial screening identified 4s 574 target genes of S flavescens including 66 target genes that were associated with cardiac arrhythmia. The core target genes were IL-6, EGFR, CASP3, ESR1, and MYC. IL-6 and related cytokines play a role in left ventricular remodeling by inducing cardiomyocyte hypertrophy and apoptosis through upregulation of the antiapoptotic protein, B-cell lymphoma extra-large cells.[52] Exogenous IL-6 induced cardiomyocyte apoptosis in a rat model of myocardial ischemia-reperfusion injury by promoting inducible nitric oxide synthase activity.[53] In the sterile pericarditis rat model of postoperative atrial fibrillation, IL-6 neutralization ameliorated in vivo atrial inflammation and fibrosis and atrial fibrillation susceptibility, as well as the frequency of atrial ectopy, and atrial fibrillation with a reentrant pattern ex vivo.[54] EGFR promoted recovery from trauma by accelerating the aggregation and movement of endothelial cells and fibroblasts to the site of trauma, β-adrenergic receptor-mediated EGFR transactivation reduced cardiomyocyte apoptosis by activating ERK1/2 and Akt.[55] CASP3 is a cysteine-aspartate protease that plays a central role in apoptosis.[56] CASP3 plays a significant role in the pathogenesis of atrial structural remodeling during atrial fibrillation.[57] ESR1 encodes the estrogen receptor α. Estrogen receptors play an important role in fibroblast activation.[58] Estrogen affects blood circulation and can increase the risk of neurological and cardiovascular diseases.[58] Estrogen receptors mediate the cardioprotective function of estrogen by regulating the transcription of the downstream genes and activation of the MAPK signaling pathway.[58] MYC is an essential transcription factor for cardiac development and function, the ability of Myc to rescue Mycn deficiency during cardiogenesis and the involvement of Cell Competition and cardiomyocyte replacement in this rescue, Myc is able to mimic Mycn function, rescue Mycn-deficient cells and promote the elimination of Mycn-deficient cells to restore a viable heart.[59] For there exist the problem of lacking of clinical trials of target genes that were associated with cardiac arrhythmia, Further investigations are necessary to further confirm the the underlying regulatory mechanisms involved in cardiac arrhythmia.
We further investigated the mechanisms underlying the anti-arrhythmic effects of S flavescens by performing GO and KEGG pathway enrichment analysis of the target genes. The results showed enrichment of BPs, MF, and CC terms such as response to exogenous cellular stimuli, oxidative stress, response to foreign stimuli, response to antibiotics, response to steroid hormones, response to oxygen levels, membrane rafts, membrane microstructure domains, membrane regions, DNA binding Transcription activator activity, RNA polymerase specific, nuclear receptor activity, transcription factor activity, direct ligand regulation of sequence-specific DNA binding, steroid hormone receptor, and steroid binding. We also observed enrichment of KEGG pathways related to chemical carcinogenesis-receptor activation signaling pathway, lipid and atherosclerosis signaling pathway, and fluid shear stress and atherosclerosis signaling pathway. The shear stress-mediated signaling pathways are involved in endothelial dysfunction and cardiovascular diseases. Fluid shear stress and atherosclerotic signaling pathways are mainly related to the flow properties of blood components including the plasma and the blood cells. Lower blood flow shear is associated with the formation of atherosclerotic plaques, plaque rupture, and endothelial damage, whereas higher blood flow shear is required for the normal functioning of the vascular endothelium and protection of the intima.[60]
Pathophysiological changes in the heart including coronary atherosclerotic heart disease, myocardial infarction, and heart failure causes acidification of the myocardial microenvironment resulting in adverse changes in the myocardial electrophysiological properties and is manifested in the form of arrhythmia.[61] Further investigations are necessary to further confirm the potential clinical value of S flavescens in the treatment of cardiac arrhythmias and the underlying regulatory mechanisms involved in the process.
Molecular docking results showed that the minimum binding energy was <0 for quercetin, luteolin, and formononetin, which were the 3 key pharmacological components of S flavescens with anti-arrhythmic properties. The lower minimum binding energy implies stronger binding of the pharmacological molecule to the target protein. Furthermore, binding energy of these 3 key bioactive ingredients of S flavescens with IL-6 and CASP3 proteins was less than -5.0 kJ/mol. This suggested that the prediction results of the network pharmacological screening of the target proteins for the bioactive ingredients of S flavescens were reliable because they showed significantly high binding affinities.
5. Conclusion
This study systematically analyzed the mechanism of action of S flavescens in the potential treatment of cardiac arrhythmias using network pharmacology and molecular docking techniques. Our results demonstrated synergistic multi-component, multi-target, and multi-pathway effects of the bioactive ingredients of S flavescens in the potential treatment of cardiac arrhythmias. Our study demonstrated that significant clinical value of multiple bioactive ingredients of S flavescens for the treatment of arrhythmias. However, further clinical and basic scientific research is required to confirm our findings.
Acknowledgments
We are very grateful for the contributions of the TCMSP database, UniProt database, GeneCards and OMIM databases. as well as all colleagues involved in the study.
Author contributions
Data curation: Jinwei Li.
Formal analysis: Jinwei Li.
Writing – original draft: Yuyun Zhai.
Writing – review & editing: Quan Zhang.
Abbreviations:
- BP
- biological processes
- CASP3
- caspase-3
- CC
- cellular components
- DL
- drug-likeness
- EGFR
- epidermal growth factor receptor
- GO
- gene ontology
- KEGG
- Kyoto encyclopedia of genes and genomes
- LxRα
- liver X receptor alpha gene
- MF
- molecular functions
- NP
- network pharmacology
- OB
- oral bioavailability
- PPI
- protein-protein interaction
- TCM
- traditional Chinese medicine
- TCMSP
- traditional Chinese medicine systems pharmacology
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no conflicts of interest to disclose.
How to cite this article: Zhai Y, Li J, Zhang Q. Network pharmacology and molecular docking analyses of the potential target proteins and molecular mechanisms underlying the anti-arrhythmic effects of Sophora Flavescens. Medicine 2023;102:30(e34504).
Contributor Information
Yuyun Zhai, Email: 1062687340@qq.com.
Jinwei Li, Email: 1920171453@qq.com.
References
- [1].Deng Y, Chen H, Wu Q, et al. Discussion on the mechanism of action of Ganong in the intervention of arrhythmias based on network pharmacology. Mod Chin Mater Med. 2020;22:1485–93. [Google Scholar]
- [2].Tisdale JE, Chung MK, Campbell KB, et al. Drug-induced arrhythmias: a scientific statement from the American Heart Association. Circulation. 2020;142:e214–33. [DOI] [PubMed] [Google Scholar]
- [3].Niu M, Zhang S, Zhang B, et al. Interpretation of guidelines for network pharmacology evaluation methods. Chin Herb Med. 2021;52:4119–29. [Google Scholar]
- [4].He X, Fang J, Huang L, et al. Sophora flavescens Ait: traditional usage, phytochemistry and pharmacology of an important traditional Chinese medicine. J Ethnopharmacol. 2015;172:10–29. [DOI] [PubMed] [Google Scholar]
- [5].Su L. Research progress on chemical composition and pharmacological effects of matrine. Chem Eng. 2021;3503:58–61. [Google Scholar]
- [6].Zhang R, Zhu X, Bai H, et al. Network pharmacology databases for traditional Chinese medicine: review and assessment. Front Pharmacol. 2019;10:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choudhary N, Singh V. Multi-scale mechanism of antiviral drug-alike phytoligands from Ayurveda in managing COVID-19 and associated metabolic comorbidities: insights from network pharmacology. Mol Divers. 2022;26:2575–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Choudhary N, Singh V. Insights about multi-targeting and synergistic neuromodulators in Ayurvedic herbs against epilepsy: integrated computational studies on drug-target and protein-protein interaction networks. Sci Rep. 2019;9:10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Choudhary N, Choudhary S, Kumar A, et al. Deciphering the multi-scale mechanisms of Tephrosia purpurea against polycystic ovarian syndrome (PCOS) and its major psychiatric comorbidities: studies from network pharmacological perspective. Gene. 2021;773:145385. [DOI] [PubMed] [Google Scholar]
- [10].Choudhary N, Singh V. A census of P. longum’s phytochemicals and their network pharmacological evaluation for identifying novel drug-like molecules against various diseases with a special focus on neurological disorders. PLoS One. 2018;13:e0191006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Morris GM, Lim-Wilby M. Molecular docking. Methods Mol Biol. 2008;443:365–82. [DOI] [PubMed] [Google Scholar]
- [12].Lu J, Yan J, Yan J, et al. Network pharmacology-based research into the effect and mechanism of Xijiao Dihuang decoction against sepsis. Biomed Pharmacother. 2020;122:109777. [DOI] [PubMed] [Google Scholar]
- [13].Feipeng G, Luxin X, Beili C, et al. Exploration of Ziziphi Spinosae Semen in treating insomnia based on network pharmacology strategy. Evid Based Complement Alternat Med. 2021;2021:9888607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Amberger JS, Hamosh A. Searching Online Mendelian Inheritance in Man (OMIM): a knowledgebase of human genes and genetic phenotypes. Curr Protoc Bioinformatics. 2017;58:1.2.1–1.2.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ma JX, Ye M, Ma K, et al. Network pharmacology-based strategy for predicting active ingredients and potential targets of Coptis chinensis Franchin polycystic ovary syndrome. Evid Based Complement Alternat Med. 2021;2021:6651307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].UniProt Consortium. UniProt: the universal protein knowledgebase in 2021. Nucleic Acids Res. 2021;49:D480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Szklarczyk D, Gable AL, Nastou KC, et al. The STRING database in 2021: customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49:D605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shannon P, Markiel A, Ozier O, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kanehisa M, Goto S, Sato Y, et al. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Irwin JJ, Shoichet BK. ZINC--a free database of commercially available compounds for virtual screening. J Chem Inf Model. 2005;45:177–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Bobrowski TM, Korn DR, Muratov EN, et al. ZINC express: a virtual assistant for purchasing compounds annotated in the ZINC database. J Chem Inf Model. 2021;61:1033–6. [DOI] [PubMed] [Google Scholar]
- [22].Trott O, Olson AJ. AutoDock vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yi Y, Qingqi W. On the inspiration of the theory of “the heart is the master of blood” in the Yellow Emperor’s classic of internal medicine for the diagnosis and treatment of coronary heart disease. Chin J Tradit Chin Med. 2017;32:2397–401. [Google Scholar]
- [24].National Pharmacopoeia Commission. Pharmacopoeia of the Chinese Republic of China (Part I). Beijing: China Medical Science and Technology Press; 2015. [Google Scholar]
- [25].Zhang F, Ma Y, Gao H, et al. Research progress on the chemical composition of matrine. Chin J Exp Formulary. 2014;20:205–14. [Google Scholar]
- [26].Zhang B, Wang N, Hong N, et al. Effect of matrine on experimental arrhythmias. Pharmacol Clin Pract Tradit Chin Med. 1987;3(Suppl):97. [Google Scholar]
- [27].Huang C, Xie S, Huang S. Experimental study on the anti-arrhythmic effect of matrine. J Dalian Med Univ. 2002;24:177–9. [Google Scholar]
- [28].Xu Q. Anti-arrhythmia and experimental study of matrine and oxidized matrine (abstract). Shaanxi New Med. 1981;10:58. [Google Scholar]
- [29].Nunes KP, de Oliveira AA, Mowry FE, et al. Targeting toll-like receptor 4 signaling pathways: can therapeutics pay the toll for hypertension. Br J Pharmacol. 2019;176:1864–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bomfim GF, Echem C, Martins CB, et al. Toll-like receptor 4 inhibition reduces vascular inflammation in spontaneously hypertensive rats. Life Sci. 2015;122:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jiang H, Qu P, Wang J, et al. Effect of NF-κB inhibitor on Toll-like receptor 4 expressions in the left ventricular myocardium in two-kidney-one-clip hypertensive rats. Eur Rev Med Pharmacol Sci. 2018;22:3224–33. [DOI] [PubMed] [Google Scholar]
- [32].Zhou X, Liu J, Li J. Effect and mechanism of quercetin on blood pressure, intestinal flora, and ventricular remodeling in spontaneously hypertensive rats. Nat Prod Res. 2020;32:1449–55. [Google Scholar]
- [33].Hou X, Qin X, Zhang M. Effect of quercetin on expression of mid-cerebral arterial tone and crevice connexin 43 in spontaneously hypertensive rats. Chin J Clin Pharmacol. 2018;34:2821–4. [Google Scholar]
- [34].Hou G, Qin X, Hou X, et al. Study on the hypotensive effect and mechanism of quercetin on rats with renal hypertension. J Cardiovasc Cerebrovasc Dis Integr Tradit Chin West Med. 2016;14:137–9. [Google Scholar]
- [35].Chekalina NI, Shut SV, Trybrat TA, et al. Effect of quercetin on parameters of central hemodynamics and myocardial ischemia in patients with stable coronary heart disease. Wild Lek. 2017;70:707–11. [PubMed] [Google Scholar]
- [36].Castellino G, Nikolic D, Magán-Fernández A, et al. Supplement containing chlorogenic acid and luteolin improved hepatic and cardiometabolic parameters in subjects with metabolic syndrome: a 6-month randomized, double-blind, placebo-controlled study. Nutrients. 2019;11:2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Park HS, Lee K, Kim SH, et al. Luteolin improves hypercholesterolemia and glucose intolerance through LXRα-dependent pathway in diet-induced obese mice. J Food Biochem. 2020;44:e13358. [DOI] [PubMed] [Google Scholar]
- [38].Yu X, Su C, Wang X, et al. Protective effects of kaempferol and formononetin on hypoxic damage to H9c2 cells. Liaoning J Tradit Chin Med. 2020;47:154–6. [Google Scholar]
- [39].Chen M, Ding Y, Tong Z. Efficacy and safety of sophora flavescens (kushen) based traditional Chinese medicine in the treatment of ulcerative colitis: clinical evidence and potential mechanisms. Front Pharmacol. 2020;11:603476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hwang SJ, Song YS, Lee HJ. Phaseolin attenuates lipopolysaccharide-induced inflammation in RAW 264.7 cells and zebrafish. Biomedicines. 2021;9:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim JH, Cho IS, So YK, et al. Kushenol A and 8-prenylkaempferol, tyrosinase inhibitors, derived from Sophora flavescens. J Enzyme Inhib Med Chem. 2018;33:1048–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang Y, Pan Y, Lv R, et al. Effect of matrine oxide in improving ventricular remodeling in mice with viral myocarditis. Chinese J Mod Med. 2019;29:1–7. [Google Scholar]
- [43].Hu C, Tang Q, Zhang N, et al. Effect of matrine on lipopolysaccharide-induced inflammatory response and oxidative stress of human umbilical vein endothelial cells. Chin J Biomed Eng. 2018;24:305–10. [Google Scholar]
- [44].Hu C, Tang Q. Effect of matrine on high sugar induced H9C2 cardiomyocyte damage. Chin J Geriatr Cardiovasc Cerebrovasc Dis. 2022;22:180–4. [Google Scholar]
- [45].Zhang X, Hu C, Zhang N, et al. Matrine attenuates pathological cardiac fibrosis via RPS5/p38 in mice. Acta Pharmacol Sin. 2021;42:573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ma J, Ma S, Yin C, et al. Matrine reduces susceptibility to postinfarct atrial fibrillation in rats due to antifibrotic properties. J Cardiovasc Electrophysiol. 2018;29:616–27. [DOI] [PubMed] [Google Scholar]
- [47].Wei S, Zhou J, Gan M, et al. The improving effect of matrine on cardiac function and left ventricular remodeling in rats with cardiac hypertrophy. Chin J Clin Pharmacol. 2017;33:338–42. [Google Scholar]
- [48].Zhou YH, Xu CQ, Shan HL, et al. Inhibition of matrine on potassium currents in guinea pig ventricular myocytes. Chin J Pharmacol Toxicol. 2007;21:167–73. [Google Scholar]
- [49].Shudong N, Zelin H, Yinghui Z, et al. Comparison of effects of traditional antiarrhythmic drugs and matrine on rapid delayed rectifier potassium current in rabbit ventricular myocytes. China J Mod Med. 2010;20:1609–13. [Google Scholar]
- [50].Chaoqian X, Deli D, Zhimin D, et al. Comparison of antiarrhythmic effects of matrine and berbamine with amiodarone and RP58866. Acta Pharm Sin. 2004;39:691–4. [PubMed] [Google Scholar]
- [51].Yi W, Hanqing T, Xiaohua L. Effects of Sophora flavescens alkaloids on sodium channel currents in guinea pig ventricular myocytes. Chin J Exp Formulary. 2013;19:199–202. [Google Scholar]
- [52].Wollert KC, Drexler H. The role of interleukin-6 in the failing heart. Heart Fail Rev. 2001;6:95–103. [DOI] [PubMed] [Google Scholar]
- [53].Yaoita H, Kawaguchi M, Maehara K, et al. IS061: interleukin-6 induces apoptosis of cardiomyocytes via inducible nitric oxide synthase action in rat myocardial reperfusion injury. Jpn Circ J. 1997;61:34. [Google Scholar]
- [54].Liao J, Zhang S. Interleukin-6-mediated-Ca2+ handling abnormalities contributes to atrial fibrillation in sterile pericarditis rats. Front Immunol. 2021;12:758157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grisanti LA, Talarico JA, Carter RL, et al. β-Adrenergic receptor-mediated transactivation of epidermal growth factor receptor decreases cardiomyocyte apoptosis through differential subcellular activation of ERK1/2 and Akt. J Mol Cell Cardiol. 2014;72:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Yang B, Ye D, Wang Y. Caspase-3 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2013;17:255–63. [DOI] [PubMed] [Google Scholar]
- [57].Luo Z, Yan C, Yu P, et al. CASP3 genetic variants and susceptibility to atrial fibrillation in Chinese Han population. Int J Cardiol. 2015;183:1–5. [DOI] [PubMed] [Google Scholar]
- [58].Puzianowska-Kuźnicka M. ESR1 in myocardial infarction. Clin Chim Acta. 2012;413:81–7. [DOI] [PubMed] [Google Scholar]
- [59].Muñoz-Martín N, Sierra R, Schimmang T, et al. Myc is dispensable for cardiomyocyte development but rescues Mycn-deficient hearts through functional replacement and cell competition. Development. 2019;146:dev170753. [DOI] [PubMed] [Google Scholar]
- [60].Na R, Wang X, Ding H, et al. Differential analysis of expression of shear force, MMP-9 and CRP in traditional Chinese medicine and clinical carotid artery blood flow of normal blood pressure and hypertension. Med Rev. 2019;25:4747–51. [Google Scholar]
- [61].Wan Z, Zhenwei P, Tie-Ming F, et al. The effect of Sophora flavescens base on rapid delayed rectifier potassium current in ischemic ventricular myocytes. Chin J Pharmacol. 2008:322–6. [Google Scholar]