Abstract
Background: The population of individuals affected by breast cancer is growing, and with advances in cancer treatment implemented into usual care, there is an urgent need to improve the recognition, monitoring and treatment of therapy-induced adverse effects. This study aims to explore the use of an in-app electronic questionnaire to assess and monitor chemotherapy-related symptoms in early breast cancer patients treated with perioperative chemotherapy. Method: Between December 2019 and June 2021, 72 female study participants used the mobile app Centrum Chorób Piersi UCK and completed an in-app questionnaire about the 14 most common chemotherapy-related symptoms. Replies including symptoms with a critical value triggered automatic email alerts to the nursing team. Results: Acceptance of the study was higher among younger women and patients originating from rural areas, while possible digital exclusion among patients >60 years was observed during the enrolment process. A total of 55 participants completed the electronic questionnaire at least once and generated 553 responses with 1808 specific problems reported. Fatigue (n = 428) was the most common problem, and fever (n = 5) the least reported problem. A total of 21 participants triggered alerts with responses containing symptoms with critical value assessment (n = 89). Significant negative correlation was observed between the number of responses and time from the first chemotherapy administration; however, the number of responses was not determined by any sociodemographic or medical factors. Significant positive correlations were identified between the number of communicated problems and participants’ age. The usage of our electronic symptom assessment questionnaire decreased substantially after the period of active encouragement during the study enrolment. Conclusions: Not all societies are ready for innovative eHealth solutions. Patients’ age should be carefully considered when app-based interventions are introduced to usual cancer care. Additional support is suggested for older patients to improve their awareness and participation in eHealth interventions. More research involving older participants is needed to explore and address their particular needs and perspectives on eHealth solutions.
Keywords: chemotherapy, early breast cancer, eHealth, mobile app, symptom management, symptom reporting, ePROM
1. Introduction
Breast cancer is the most prevalent cancer diagnosis and the second most frequent cause of cancer-related deaths among Polish women. According to the Polish National Cancer Registry in 2020, 23.8% of primary cancer diagnoses in women were breast cancers. While the population of people diagnosed with breast cancer in Poland is growing [1] and advances in cancer treatment are being implemented into usual care [2], there is an urgent need to improve the recognition, monitoring and treatment of therapy-induced adverse effects. Electronic patient reported outcome measures (ePROMs) such as mobile and web applications (apps) offer the opportunity to address particular unmet needs of cancer patients. They can be utilised for screening, diagnostic, therapeutic and educational purposes [3]. Various in-app questionnaires facilitate capturing patient-reported outcomes, such as symptom burden, physical function, mental status and quality of life [4,5,6,7,8]. Implementing electronic monitoring of treatment side effects into routine breast cancer care decreases the chemotherapy-induced symptom burden, and improves symptom management, self-efficacy and quality of life [8,9,10,11,12,13]. Moreover, several studies exploring mobile apps encouraging physical activity among breast cancer patients demonstrated improved quality of life and reduced fatigue and distress levels [14,15,16]. Additionally, the results of research testing eHealth solutions during the COVID-19 pandemic not only confirmed that remote monitoring and management of treatment-related symptoms reduce the symptom burden and improve the quality of life, but also limit unplanned healthcare utilisation, decreasing demand on healthcare systems and improve patients’ symptom experience, including perception of the frequency, intensity, distress, and meaning of symptoms [6]. Furthermore, eHealth solutions have been identified as empowering, improving patients’ involvement in the continuum of cancer care [17,18].
The primary objective of this study was to analyse the results of using an in-app electronic questionnaire to assess and monitor chemotherapy-related symptoms in patients treated for early-stage breast cancer.
2. Materials and Methods
2.1. Study Design and Participants
This study assessed the use of a mobile app used by Polish early breast cancer patients treated with perioperative chemotherapy. The study was conducted in the Breast Cancer Chemotherapy Day Unit of the University Clinical Centre (UCC) in Gdańsk, Poland, between December 2019 and June 2021. Approval from the Independent Bioethics Committee for Scientific Research at the Medical University of Gdańsk (NKBBN/642/2019, NKBBN/642-534/2020) was obtained before the study initiation.
Patients who met the following inclusion criteria were eligible for participation in this study: referral for perioperative chemotherapy for early-stage breast cancer (all types), possession of their own smart device, and an ability to navigate the device and download mobile applications independently; these patients completed a signed informed consent form. Patients with metastatic breast cancer and those treated for other types of cancers were excluded from the study.
Perioperative chemotherapy was categorised as neoadjuvant or adjuvant with further division into specific treatment regimens (Table 1). Anthracycline-taxane regimens included combinations of doxorubicin and cyclophosphamide with paclitaxel +/− carboplatin or monoclonal antibodies (trastuzumab, pertuzumab), whereas anthracycline or taxane-based chemotherapy consisted of doxorubicin or docetaxel combined with cyclophosphamide, or combinations of paclitaxel, carboplatin and monoclonal antibodies.
Table 1.
Medical characteristics of study participants.
| Breast cancer type: | ||
| HER2+ | 21 | 29.2% |
| luminal A | 12 | 16.6% |
| luminal B | 19 | 26.4% |
| TNBC | 20 | 27.8% |
| Breast cancer stage: | ||
| IA | 11 | 15.3% |
| IB | 3 | 4.2% |
| IIA | 27 | 37.5% |
| IIB | 14 | 19.4% |
| IIIA | 6 | 8.3% |
| IIIB | 6 | 8.3% |
| IIIC | 5 | 6.9% |
| Chemotherapy setting: | ||
| neoadjuvant | 53 | 74% |
| adjuvant | 19 | 26% |
| Chemotherapy regimen: | ||
| anthracycline + taxane-based: | 58 | 80.6% |
| neoadjuvant: | 48 | 66.7% |
| 12xPXL>>4xAC | 15 | 20.8% |
| 12xPXL>>4xddAC | 15 | 20.8% |
| 12xPXL+carboplatin>>4xddAC | 7 | 9.7% |
| 4xddAC>>12xPXL + T | 5 | 6.9% |
| 4xAC>>12xPXL + T | 6 | 8.3% |
| adjuvant: | 10 | 13.9% |
| 12xPXL>>4xAC | 6 | 8.3% |
| 12xPXL>>4xddAC | 1 | 1.4% |
| 4xAC>>12xPXL + T | 1 | 1.4% |
| 4xddAC>>12xPXL | 1 | 1.4% |
| 4xddAC>>12xPXL + T | 1 | 1.4% |
| anthracycline- or taxane-based: | 14 | 19.4% |
| neoadjuvant: | 5 | 6.9% |
| 12xPXL | 1 | 1.4% |
| 4xddAC | 1 | 9.7% |
| 12xPXL + carboplatin + T + P | 3 | 4.2% |
| adjuvant: | 9 | 12.5% |
| 12xPXL + T | 4 | 5.6% |
| 4xTC | 4 | 5.6% |
| 12xPXL | 1 | 1.4% |
| Number of chemotherapy administrations: | ||
| 16 | 56 | 77.8% |
| 12 | 10 | 13.9% |
| 7 * | 1 | 1.4% |
| 4 | 5 | 6.9% |
| Treatment with G-CSF: | ||
| yes | 33 | 46% |
| no | 39 | 54% |
| Coexisting medical conditions: | ||
| no | 36 | 50% |
| yes: | 36 | 50% |
| single condition | 24 | 33.3% |
| multiple conditions | 12 | 16.7% |
* patient’s decision to change treating hospital. AC—doxorubicin-cyclophosphamide, dd—dose dense, G-CSF—granulocyte colony stimulating factors, P—pertuzumab, PXL—paclitaxel, T—trastuzumab, TC—docetaxel-cyclophosphamide, TNBC—triple negative breast cancer.
2.2. Measures
During the pre-treatment preparation process, participants were asked to download the free mobile app Centrum Chorób Piersi UCK, which contains an electronic questionnaire (ePROM) for monitoring chemotherapy-related adverse effects. Nursing staff collected medical data (cancer stage and phenotype, setting of chemotherapy (preoperative vs. postoperative), type of cytotoxic drugs used, number of cycles, coexisting medical conditions and use of granulocyte colony-stimulating factors) from patients’ health records. Sociodemographic details (age, sex, place of residence, education, employment, economic and marital status) were self-reported by participants at the end of the patient satisfaction survey.
Enrolled patients were instructed by the breast cancer nurse (BCN) to complete the questionnaire weekly and on occasions when they experienced distressing symptoms. To ensure participants’ safety, it was emphasised that in the case of an emergency or sudden health deterioration, they must seek medical help through emergency services or an appointment with a physician or nurse, as appropriate. Similar information was displayed on the questionnaire’s summary screen before submission.
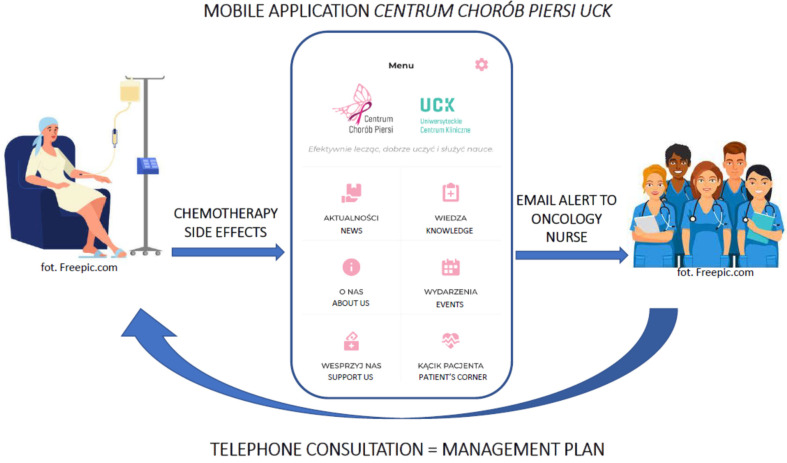
The questionnaire consists of 14 questions about the most common chemotherapy-related adverse events (Supplementary Table S1). The symptom severity assessment scale used in the questionnaire is based on the Common Terminology Criteria for Adverse Events v.4 [19] with its own modifications. Participants defined relevant symptoms on a 5-point scale, where 0 meant no problem at all, 1—mild, 2—moderate, 3—severe and 4—debilitating problem. The application did not allow omitted questions or free-text responses. Reports generated in connection to patients’ in-app activity were closely monitored by the BCN. Figure 1 demonstrates a simplified patient–breast care team communication process via the in-app questionnaire.
Figure 1.
Patient–breast care team communication process via in-app questionnaire.
Replies including symptoms with critical value (≥3, apart from fever, which activated alerts when rated as 1 or above) triggered automatic email alerts to the nursing team. Participants were informed that after triggering an alert they will be contacted by the BCN within one working day during office hours. Nursing interventions performed in response to app alerts were recorded in patients’ documentation. Every intervention began with a telephone consultation leading to further advice as necessary, and the types and outcomes of the recommended interventions were analysed. Additionally, to evaluate patients’ opinions on the application, including the electronic questionnaire, participants were asked to complete a paper survey (Appendix A) after finishing the last chemotherapy cycle or after the physician’s decision to terminate the treatment. The proprietary survey consisted of 10 questions relating to: the difficulty level of using the in-app questionnaire, safety during treatment, satisfaction from received care, sense of control, well-being during treatment, hospital admissions and free text option for additional participant suggestions.
2.3. Data Analysis
Data were managed with Microsoft Excel software and statistical analyses were performed with free RStudio software, version 4.2.2 (R Core Team, 2021) under the terms of the General Public License. Medians were used to report the central tendency, as the data were not normally distributed. Nonparametric statistical methods were used to analyse the results of this study due to the small population and non-normally distributed outcome data. An α level of 0.05 was set for all tests. Correlations were assessed with the ρ-Spearman’s correlation coefficient and its significance test. The Mann–Whitney U test was used to describe differences in continuous variables between two groups and the Kruskal–Wallis test for multiple (>2) groups. Post hoc Wilcoxon tests were performed to determine which groups were significantly different. For comparison between two categorical variables, Chi-square tests (for values > 5) and Exact Fisher’s test (for values < 5) were used. Additionally, Wilcoxon Rank sum tests were performed to check differences between the median number of reported problems and two selected qualitative characteristics. Benjamini–Hochberg p-value correction for multiple comparisons was used for all performed tests.
3. Results
3.1. Enrolment
Between December 2019 and December 2020, 93 early-stage breast cancer patients were assessed for eligibility. A total of 20 women refused, and a single eligible man was excluded from the analysis by the research team, resulting in 72 women being included in the study.
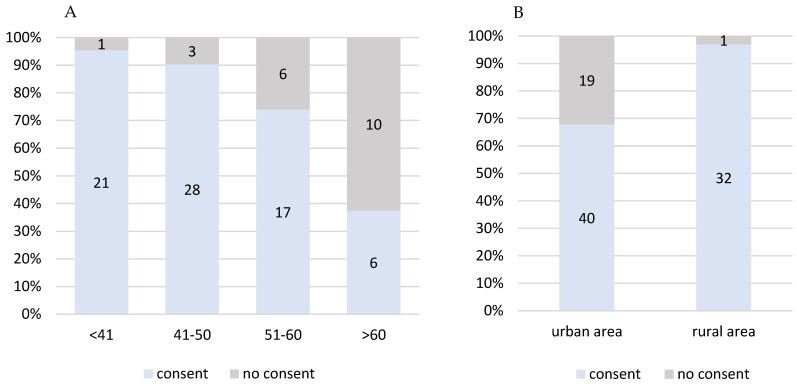
Acceptance of the study was higher among younger women and patients originating from rural areas (Figure 2A,B). Although the reasons for refusal were not systematically collected, the most frequently observed explanations included difficulties with navigating through mobile apps, no access to a smartphone or limited ability to use it.
Figure 2.
Study acceptance (A) in different age groups (p = 0.00006), (B) based on place of residence (p = 0.00114).
3.2. Participants’ Characteristics
Medical and sociodemographic characteristics of study participants are presented in the following table (Table 2).
Table 2.
Sociodemographic characteristics of study participants.
| Age | Median | Range |
|---|---|---|
| 46 | (34–70) | |
| Frequency (n) | Percentage (%) | |
| Total | 72 | 100% |
| Gender: | ||
| female | 72 | 100% |
| male | 0 | 0% |
| Marital status: | ||
| married | 52 | 72.2% |
| single | 12 | 16.7% |
| divorced | 3 | 4.2% |
| in relationship | 3 | 4.2% |
| widow | 2 | 2.8% |
| Place of residence: | ||
| city > 50,000 | 27 | 37.5% |
| city < 50,000 | 14 | 19.4% |
| rural area | 31 | 43.1% |
| Education: | ||
| higher | 39 | 54.2% |
| secondary | 17 | 23.6% |
| vocational | 16 | 22.2% |
| Employment status: | ||
| employed | 63 | 87.5% |
| unemployed | 5 | 6.9% |
| retired | 4 | 5.6% |
| Economic status (self-assessed): | ||
| satisfactory | 58 | 80.6% |
| unsatisfactory | 14 | 19.4% |
Medical conditions reported by participants included: hypothyroidism (n = 20), hypertension (n = 7), asthma (n = 4), endometriosis (n = 3), obesity (n = 3), varicose veins (n = 3), coronary disease (n = 1), irritable bowel syndrome (n = 1), glaucoma (n = 1), nephrolithiasis (n = 1), epilepsy (n = 1) and rheumatoid arthritis (n = 1). Details of concomitant medication were not collected for the purpose of this study.
3.3. Participant Engagement with the App and Questionnaire
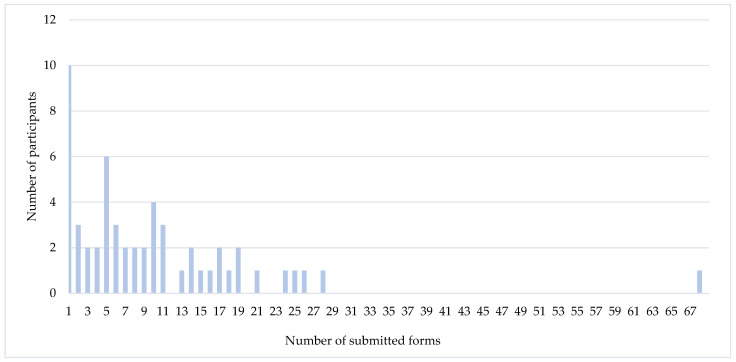
Data collection lasted from December 2019 to June 2021. During the study, 55 (76%) participants completed the electronic questionnaire at least once and of those 23 (42%)–10 or more times. Patients who used the electronic questionnaire and those who submitted no responses did not differ in terms of sociodemographic or medical characteristics (not reported). The number of responses generated by those who completed the questionnaire ranged from 1 to 68, median 7 (Figure 3).
Figure 3.
Responses generated during the study.
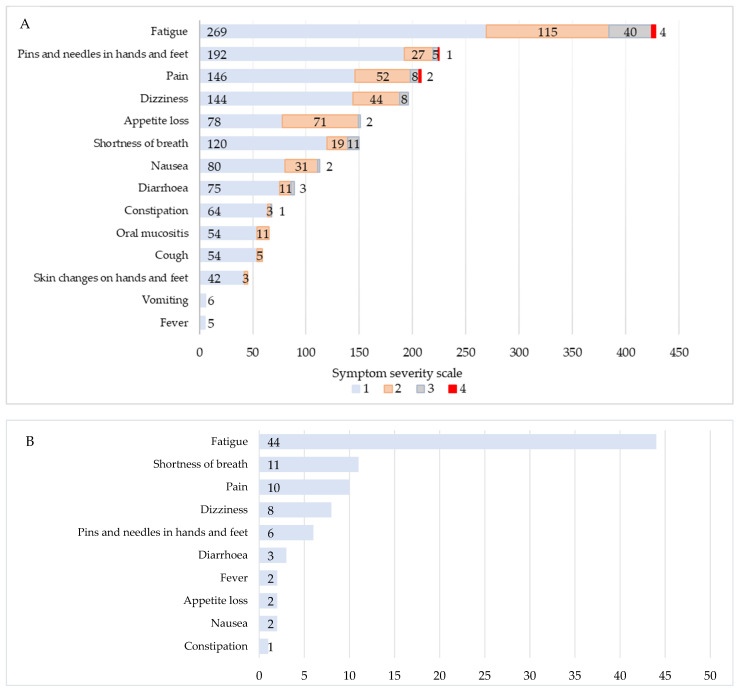
Only 6 (11%) participants filled in the questionnaire as instructed by the BCN; others chose to complete it only when experiencing problematic side-effects. Overall, 553 responses were collected and some referred to more than one symptom, resulting in 1808 reported problems (Figure 4A). Fatigue (n = 428) was generally the most frequently reported problem, followed by the sensation of pins and needles in hands and feet (n = 226), pain (n = 208) and dizziness (n = 196). The least common problems reported included vomiting (n = 6) and fever (n = 5). 1329 (73.5%) symptoms were mild, 392 (21.7%)—moderate, 80 (4.4%)—severe and 7 (0.4%)—debilitating.
Figure 4.
Summary of (A) specific symptoms reported during the study, (B) specific symptoms with critical value assessment.
A total of 21 (29%) participants triggered alerts with responses containing symptoms with critical value assessment. Overall, 58 responses with values over the predefined critical threshold were collected, of which 21 (36%) referred to more than one symptom with a critical value (Figure 4B). Altogether, there were no statistically significant differences in sociodemographic or medical characteristics between patients who triggered alerts and the remaining participants.
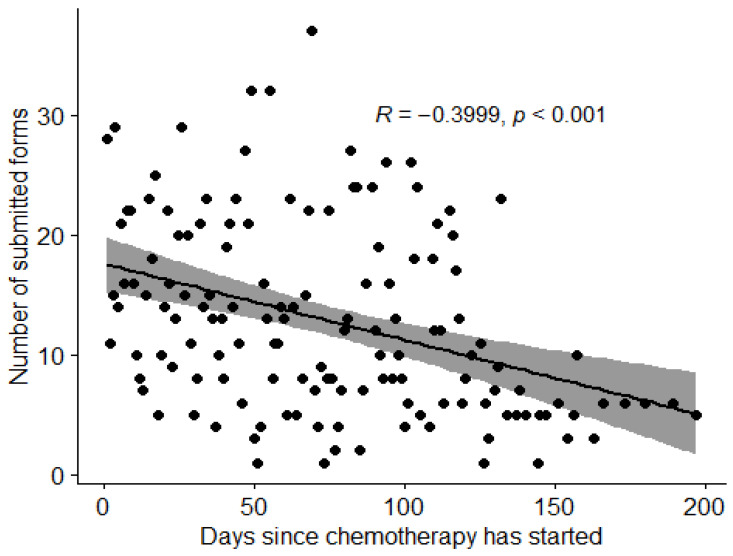
Although there were no statistically significant differences in the number of generated responses in relation to sociodemographic details such as age, place of residence, education level, employment, economic or marital status, and medical factors including breast cancer stage, chemotherapy setting, chemotherapy regimen, number of treatment cycles, coexisting medical conditions or use of G-CSFs, a statistically significant negative correlation was observed between the number of responses and time from the first chemotherapy administration (Figure 5).
Figure 5.
Distribution of responses collected during study period.
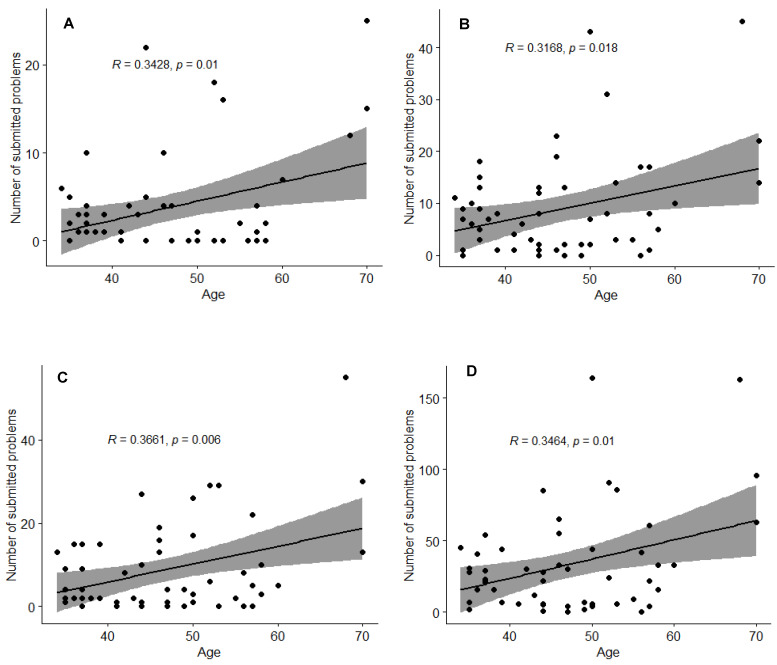
For the purposes of analysis of correlations identified between communicated problems and specific participant characteristics, problems were arranged into five categories: fatigue, dizziness, gastrointestinal problems (appetite loss, nausea, diarrhoea, constipation, oral mucositis, vomiting), respiratory problems (shortness of breath, cough) and others (pain, skin changes on hands and feet, sensation of pins and needles in hands and feet, fever). Detailed responses collected during the study and grouped accordingly are presented in Supplementary Table S2 and Figure 6.
Figure 6.
Correlations between age and (A) respiratory problems, (B) gastrointestinal problems, (C) other problems, (D) all problems reported.
The participants’ age was found to be the only factor determining the number of reported problems. Statistically significant positive correlations observed between age and the number of reported specific problems are presented in Figure 6A–D.
Additionally, factors including employment status, marital status and stage of the breast cancer were initially found to be significant in the Kruskal–Wallis test; however, further analysis (Wilcoxon signed-rank tests) found these to be statistically insignificant (p > 0.05) (Supplementary Table S2). Separate sensitivity analyses (not reported) confirmed that results are not affected by the inclusion of a single male participant or the exclusion of participants who visibly exaggerated the number of reports (Figure 3).
All participants completed the patient satisfaction survey. The majority (89%) of respondents assessed using the in-app questionnaire for symptoms reported it to be very easy to use. Moreover, most of them believed that the possibility of reporting symptoms via an electronic questionnaire not only improved their safety and well-being during treatment, but also made them feel in control of the situation in which they found themselves. Most participants felt satisfied with the support they received in response to the submitted forms. More than half of respondents thought that using the side-effects-reporting module improved the treatment of bothersome symptoms. According to the vast majority of participants, the overall care and support provided by medical staff met their expectations, with only a small percentage feeling that their expectations were not met. Ten participants expressed their suggestions of adding extra questions about nail problems (n = 4), cardiac complications (n = 1) and other unspecified unincluded symptoms (n = 5). Detailed responses to specific questions are presented in Table 3.
Table 3.
Detailed responses from patients’ satisfaction survey.
| 1. In your opinion, how difficult it is to use the in-app side-effects-reporting module? | |||||
| Answer | Very easy | Rather easy |
Difficult
to say |
Rather difficult | Very difficult |
| N (%) |
64 (89) |
6 (8) |
2 (3) |
0 | 0 |
| 2. In your opinion, did the possibility of using the in-app side-effects-reporting module improve your safety during chemotherapy? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
44 (61) |
26 (36) |
2 (3) |
0 | 0 |
| 3. Are you satisfied with the support you received in response to submitted answers? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
42 (58) |
10 (14) |
20 (28) |
0 | 0 |
| 4. Did you feel in control of the situation, when using the in-app side-effects-reporting module? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
30 (42) |
26 (36) |
16 (22) |
0 | 0 |
| 5. In your opinion, did the possibility of using the in-app side effects-reporting-module improve your well-being during chemotherapy? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
37 (51) |
32 (45) |
3 (4) |
0 | 0 |
| 6. In your opinion, did the use of the in-app side-effects-reporting module improve the treatment of bothersome side effects of chemotherapy? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
22 (30) |
23 (32) |
26 (36) |
1 (1) |
0 |
| 7. How do you assess the waiting time for the reaction of the medical staff in response to submitted answers about feeling unwell? | |||||
| Answer | Very short | Rather short | Difficult to say |
Rather long | Very long |
| N (%) |
22 (30) |
25 (35) |
25 (35) |
0 | 0 |
| 8. Did the support provided to you by the medical staff meet your expectations? | |||||
| Answer | Definitely yes | Rather yes | Difficult to say |
Rather no | Definitely no |
| N (%) |
56 (78) |
11 (15) |
3 (4) |
2 (3) |
0 |
| 9. Did you require hospital admission due to exacerbation of chemotherapy-related side effects? | |||||
| Answer | Yes | No | |||
| N (%) |
5 (7) |
67 (93) |
|||
| 10. Is there anything that you would like to change in the in-app side-effects-reporting module? | |||||
| Answer | Yes | No | |||
| N (%) |
10 (14) |
62 (86) |
|||
3.4. Nursing Interventions
During the study, 58 nursing interventions (telephone consultations) were performed in response to app alerts generated by 21 participants. All interventions provided psychological support and reassurance combined with additional guidance as required. Topics covered during telephone consultations included: physical activity and sleep (74%), pain management (24%), drug compliance (16%) and dietary needs (10%). A total of 64% of nursing interventions related to single problem reports and 36% to alerts about multiple problems; mostly consisting of combinations of severe fatigue, pain, shortness of breath and dizziness. Overall, five (7%) patients in 23 interventions were advised to use emergency care services due to severe dyspnoea (n = 10), dizziness (n = 8), diarrhoea (n = 3) and fever (n = 2); however, no hospital admissions were required. The number of follow-up contacts was flexible and tailored to the patient’s needs; nevertheless, they were not analysed within this study.
3.5. Post-Study Observations
After the end of the study, we continued to provide new patients with information about the possibility of reporting chemotherapy-related symptoms via the mobile app. In the follow-up process of the study, mobile application reports were monitored for an additional year. Unexpectedly, the use of the mobile app for symptom reporting dropped substantially after the active study ended, resulting in only two patients using the in-app questionnaire to report treatment-related side effects over this period.
4. Discussion
High acceptance of digital tools in cancer care is widely recognised [3,7,9,20,21,22]. Nevertheless, there are still societies that prefer the traditional way of communication. Specialist breast cancer nurse-led interventions, mostly telephone consultations, tailored to patients’ requirements are part of standard care for all breast cancer patients treated in UCC Gdańsk, where this study was conducted. Complex nursing interventions can significantly reduce the symptom burden [23,24], and symptom-focused education can enable patients to administer self-care more effectively [25,26]. With already well-established patient–nurse communication standards, the uptake of an additional tool to report treatment-related side effects by breast cancer patients was poor, especially after the period of active recruitment to the study. Moreover, possible digital exclusion among older (>60 yrs) patients was observed during the enrolment process, resulting in their lower interest in study participation (Figure 2), albeit the rationale for study refusal was not recorded. Similar digital inequalities between age groups were discussed in multiple studies involving participants with other diseases such as diabetes [27], irritable bowel syndrome [28], asthma and COPD [29], heart failure [30] and mental illnesses [31], and age was found to be a significant factor that influences the use of eHealth. However, although patients older than 60 years of age were generally less interested in participating in our study, once enrolled, they used the in-app questionnaire for symptoms reporting with comparable patterns of reporting, and overall, a pattern of reporting more problems than younger participants (Supplementary Table S2, Figure 6).
Adherence to the Centrum Chorób Piersi UCK app decreased with individual progress throughout chemotherapy (Figure 5). Similar observations were made in another study in early breast cancer patients [32], where the authors suggested that the reason for dropping engagement was an improvement in understanding the nature of particular side effects and the development of coping strategies; therefore, patients did not feel reporting symptoms to still be necessary. App adherence in cancer care has been explored in another study [20], where predictors of adherence were evaluated; similarly to our findings, no significant correlations were identified.
The prevalence of particular symptoms reported during our project is consistent with other studies. Fatigue is known to be the most frequent and distressing symptom for breast cancer patients [33,34,35,36]. In our study, fatigue accounted for the majority of overall problems reported, and triggers activating alerts to the BCNs. Dyspnoea was the second most common reason triggering alerts. Compared with another study testing a similar app solution [37], the average number of alerts per patient in our series was similar (2.8 vs. 2.7). However, in contrast to research led by Basch et al. [38], the proportion of individual symptoms triggering alerts in our study was higher (89/1808, 4.9% vs. 1431/84212, 1.7%), while the type of triggers remained consistent, fatigue, dyspnoea and pain being the most common severe or debilitating problems. Multiple studies examined factors related to fatigue; however, results are ambiguous. Several reports [39,40,41], found no significant relationship between fatigue and age. Other studies [42,43] have observed younger patients experiencing higher levels of fatigue, which was also associated with working while in treatment. In contrast, the present study suggests that older patients are at a higher risk of experiencing cancer-related fatigue. The breast cancer stage and number of chemotherapy cycles were insignificant in our investigation, while other studies [36,44,45] indicate that patients with more advanced nonmetastatic breast cancer and those receiving more treatments are at higher risk of suffering from cancer-related fatigue. The number of responses and specific problems communicated during our study were not determined by education level, marital, employment or economic status. Nevertheless, another study [21] demonstrated that married and cohabitating participants generated more reports than those living alone. The same study evaluated patients’ perceptions of using the app, and in line with the results of our study, demonstrate that the possibility of reporting treatment side effects in real-time created feelings of assurance and safety. Moreover, other research that explored the effect of eHealth apps on patient satisfaction during treatment confirmed that the use of mobile health apps could improve patient experience and overall health outcomes [22]. Recently published results of the PreCycle trial [46] present the successful utilisation of the interactive app (CANKADO), that works without any intervention by healthcare professionals to significantly delay the deterioration of quality of life among patients treated for metastatic breast cancer. The study reveals the next generation of ePRO monitoring and management, opening further discussion for patient empowerment and involvement in the continuum of cancer care.
Limitations of the Study
The present study has some limitations. Our project was performed in a single centre with a limited number of participants due to the COVID-19 pandemic. To explore participants’ perception of the app, we used a proprietary survey, instead of a standardised tool; however, we achieved a 100% completion rate with overall positive feedback. Another potential drawback is that the study design did not involve automatic reminders for participants to complete the in-app questionnaire, resulting in low app adherence (e.g., only 6% of participants completed the questionnaire as instructed).
5. Conclusions
Although successful use of ePROMs for monitoring treatment-related adverse events has been described in many settings, the results of this study suggest a possible lack of trust and/or understanding of eHealth tools among Polish patients treated for early-stage breast cancer. Our findings suggest that patients older than 60 years of age find it difficult to engage with mobile technology and eHealth solutions. On the other hand, this is the population that, according to our research, is at a higher risk of experiencing not only cancer-related fatigue, but also other problems caused by the treatment. To improve patient engagement and understanding of eHealth solutions, it is essential that patients are invited to and involved in the fundamental stages of creating innovative app-based interventions. With age being a significant factor in determining the number of problems experienced during chemotherapy, we suggest that additional support be provided to older patients to enhance their awareness of the beneficiary potential of eHealth interventions. More research involving older participants is needed to explore and address their particular needs and perspectives on eHealth solutions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11142114/s1.
Appendix A. Patient’s Satisfaction Survey
| “Patient’s Satisfaction Survey” | |||||||||
| 1. In your opinion, how difficult it is to use the in-app side-effects-reporting module? | |||||||||
| Very easy | Rather easy | Difficult to say | Rather difficult | Very difficult | |||||
| 2. In your opinion, did the possibility of using the in-app side-effects-reporting module improve your safety during chemotherapy? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 3. Are you satisfied with the support you received in response to submitted answers? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 4. Did you feel in control of the situation, when using the in-app side-effects-reporting module? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 5. In your opinion, did the possibility of using the in-app side-effects-reporting module improve your well-being during chemotherapy? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 6. In your opinion, did the use of the in-app side-effects-reporting module improve the treatment of bothersome side effects of chemotherapy? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 7. How do you assess the waiting time for the reaction of the medical staff in response to submitted answers about feeling unwell? | |||||||||
| Very short | Rather short | Difficult to say | Rather long | Very long | |||||
| 8. Did the support provided to you by the medical staff meet your expectations? | |||||||||
| Definitely yes | Rather yes | Difficult to say | Rather no | Definitely no | |||||
| 9. Did you require hospital admission due to exacerbation of chemotherapy-related side effects? | |||||||||
| no | yes | ||||||||
| 10. Is there anything that you would like to change in the in-app side-effects-reporting module? Please write below: | |||||||||
| Age: | Sex: | ||||||||
|
Place of residence: |
city > 50.000
|
city < 50.000
|
rural area
|
||||||
|
Education: |
primary
|
vocational
|
lower secondary
|
secondary
|
higher
|
||||
|
Marital status: |
in relationship
|
divorced
|
widowed
|
married
|
single
|
||||
|
Employment status: |
working
|
unemployed
|
retired
|
student/pupil
|
|||||
|
Economic status: |
satisfactory
|
unsatisfactory
|
|||||||
Author Contributions
Conceptualization, G.S. and E.S.; methodology, G.S. and E.S.; formal analysis, A.K., M.P. and G.S.; investigation, G.S. and E.S; data curation, G.S. and A.K.; writing—original draft preparation, G.S.; writing—review and editing, E.S., G.S., A.K. and M.P.; visualization, G.S.; supervision, E.S.; project administration, G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of the Medical University of Gdańsk (Project identification codes: NKBBN/642/2019 approved 3 December 2019, NKBBN/642-534/2020 approved 17 September 2020).
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.National Institute of Oncology—National Research Institute. Wojciechowska K.U., Barańska I., Michałek P., Olasek M., Miklewska J.A.D. Cancer in Poland in 2020. National Institute of Oncology—National Research Institute; Warsaw, Poland: 2022. pp. 5–90. [Google Scholar]
- 2.Cardoso F., Kyriakides S., Ohno S., Penault-Llorca F., Poortmans P., Rubio I.T., Zackrisson S., Senkus E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019;30:1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 3.Jongerius C., Russo S., Mazzocco K., Pravettoni G. Research-Tested Mobile Apps for Breast Cancer Care: Systematic Review. JMIR Mhealth Uhealth. 2019;7:e10930. doi: 10.2196/10930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denis F., Yossi S., Septans A.-L., Charron A., Voog E., Dupuis O., Ganem G., Pointreau Y., Letellier C. Improving Survival in Patients Treated for a Lung Cancer Using Self-Evaluated Symptoms Reported Through a Web Application. Am. J. Clin. Oncol. 2017;40:464–469. doi: 10.1097/COC.0000000000000189. [DOI] [PubMed] [Google Scholar]
- 5.Putranto D., Rochmawati E. Mobile Applications for Managing Symptoms of Patients with Cancer at Home: A Scoping Review. Int. J. Nurs. Pract. 2020;26:e12842. doi: 10.1111/ijn.12842. [DOI] [PubMed] [Google Scholar]
- 6.Mooney K., Iacob E., Wilson C.M., Lloyd J., Nielson H., Ward J.H. Randomized Trial of Remote Cancer Symptom Monitoring during COVID-19: Impact on Symptoms, QoL, and Unplanned Health Care Utilization. J. Clin. Oncol. 2021;39:12000. doi: 10.1200/JCO.2021.39.15_suppl.12000. [DOI] [Google Scholar]
- 7.Aapro M., Bossi P., Dasari A., Fallowfield L., Gascón P., Geller M., Jordan K., Kim J., Martin K., Porzig S. Digital Health for Optimal Supportive Care in Oncology: Benefits, Limits, and Future Perspectives. Support. Care Cancer. 2020;28:4589–4612. doi: 10.1007/s00520-020-05539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kearney N., Mccann L., Norrie J., Taylor L., Gray P., Mcgee-Lennon M., Sage M., Miller M., Maguire R. Evaluation of a Mobile Phone-Based, Advanced Symptom Management System (ASyMS © ) in the Management of Chemotherapy-Related Toxicity. Support. Care Cancer. 2008;17:437–444. doi: 10.1007/s00520-008-0515-0. [DOI] [PubMed] [Google Scholar]
- 9.Suchodolska G., Senkus E. Mobile Applications for Early Breast Cancer Chemotherapy-Related Symptoms Reporting and Management: A Scoping Review. Cancer Treat. Rev. 2022;105:102364. doi: 10.1016/j.ctrv.2022.102364. [DOI] [PubMed] [Google Scholar]
- 10.Fjell M., Langius-Eklöf A., Nilsson M., Wengström Y., Sundberg K. Reduced Symptom Burden with the Support of an Interactive App during Neoadjuvant Chemotherapy for Breast Cancer—A Randomized Controlled Trial. Breast. 2020;51:85–93. doi: 10.1016/j.breast.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grašič Kuhar C., Gortnar Cepeda T., Kovač T., Kukar M., Ružić Gorenjec N., Kuhar C.G., Cepeda T.G., Kovač T., Kukar M., Gorenjec N.R. Mobile App for Symptom Management and Associated Quality of Life During Systemic Treatment in Early Stage Breast Cancer: Nonrandomized Controlled Prospective Cohort Study. JMIR mHealth uHealth. 2020;8:e17408. doi: 10.2196/17408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egbring M., Far E., Roos M., Dietrich M., Brauchbar M., Kullak-Ublick G.A., Trojan A. A Mobile App to Stabilize Daily Functional Activity of Breast Cancer Patients in Collaboration with the Physician: A Randomized Controlled Clinical Trial. J. Med. Internet Res. 2016;18:e238. doi: 10.2196/jmir.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singleton A.C., Raeside R., Hyun K.K., Partridge S.R., Di Tanna G.L., Hafiz N., Tu Q., Tat-Ko J., Sum S.C.M., Sherman K.A., et al. Electronic Health Interventions for Patients with Breast Cancer: Systematic Review and Meta-Analyses. J. Clin. Oncol. 2022;68:2257–2271. doi: 10.1200/JCO.21.01171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Çınar D., Karadakovan A., Erdoğan A.P. Effect of Mobile Phone App-Based Training on the Quality of Life for Women with Breast Cancer. Eur. J. Oncol. Nurs. 2021;52:101960. doi: 10.1016/j.ejon.2021.101960. [DOI] [PubMed] [Google Scholar]
- 15.Chung I.Y., Jung M., Park Y.R., Cho D., Chung H., Min Y.H., Park H.J., Lee M., Lee S.B., Chung S., et al. Exercise Promotion and Distress Reduction Using a Mobile App-Based Community in Breast Cancer Survivors. Front. Oncol. 2019;9:1505. doi: 10.3389/fonc.2019.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cairo J., Williams L., Bray L., Goetzke K., Perez A.C. Evaluation of a Mobile Health Intervention to Improve Wellness Outcomes for Breast Cancer Survivors. J. Patient-Centered Res. Rev. 2020;7:313. doi: 10.17294/2330-0698.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Groen W.G., Kuijpers W., Oldenburg H.S., Wouters M.W., Aaronson N.K., van Harten W.H. Empowerment of Cancer Survivors Through Information Technology: An Integrative Review. J. Med. Internet Res. 2015;17:e270. doi: 10.2196/jmir.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuijpers W., Groen W.G., Loos R., Oldenburg H.S.A., Wouters M.W.J.M., Aaronson N.K., van Harten W.H. An Interactive Portal to Empower Cancer Survivors: A Qualitative Study on User Expectations. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2015;23:2535–2542. doi: 10.1007/s00520-015-2605-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.U.S. Department of Health And Human Services . In: Definitions. National Institutes of Health; National Cancer Institute, editor. Volume 2009 Qeios; London, UK: 2020. Common Terminology Criteria for Adverse Events. [Google Scholar]
- 20.Armbruster C., Knaub M., Farin-Glattacker E., von der Warth R. Predictors of Adherence to Cancer-Related MHealth Apps in Cancer Patients Undergoing Oncological or Follow-Up Treatment—A Scoping Review. Int. J. Environ. Res. Public Health. 2022;19:13689. doi: 10.3390/ijerph192013689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crafoord M.-T., Fjell M., Sundberg K., Nilsson M., Langius-Eklof A. Engagement in an Interactive App for Symptom Self-Management during Treatment in Patients with Breast or Prostate Cancer: Mixed Methods Study. J. Med. Internet Res. 2020;22:e17058. doi: 10.2196/17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu C., Hu Y., Xie J., Fu Q., Leigh I., Governor S., Wang G. The Use of Mobile Health Applications to Improve Patient Experience: Cross-Sectional Study in Chinese Public Hospitals. JMIR mHealth uHealth. 2018;6:e126. doi: 10.2196/mhealth.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coolbrandt A., Wildiers H., Aertgeerts B., Van der Elst E., Laenen A., Dierckx de Casterlé B., van Achterberg T., Milisen K. Characteristics and Effectiveness of Complex Nursing Interventions Aimed at Reducing Symptom Burden in Adult Patients Treated with Chemotherapy: A Systematic Review of Randomized Controlled Trials. Int. J. Nurs. Stud. 2014;51:495–510. doi: 10.1016/j.ijnurstu.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Brown T., Cruickshank S., Noblet M. Specialist Breast Care Nurses for Support of Women with Breast Cancer. Cochrane Database Syst. Rev. 2021;2:CD005634. doi: 10.1002/14651858.CD005634.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams P.D., Williams K., Lafaver-Roling S., Johnson R., Williams A.R. An Intervention to Manage Patient-Reported Symptoms during Cancer Treatment. Clin. J. Oncol. Nurs. 2011;15:253–258. doi: 10.1188/11.CJON.253-258. [DOI] [PubMed] [Google Scholar]
- 26.Aranda S., Jefford M., Yates P., Gough K., Seymour J., Francis P., Baravelli C., Breen S., Schofield P. Impact of a Novel Nurse-Led Prechemotherapy Education Intervention (ChemoEd) on Patient Distress, Symptom Burden, and Treatment-Related Information and Support Needs: Results from a Randomised, Controlled Trial. Ann. Oncol. 2012;23:222–231. doi: 10.1093/annonc/mdr042. [DOI] [PubMed] [Google Scholar]
- 27.AshaRani P.V., Jue Hua L., Roystonn K., Siva Kumar F.D., Peizhi W., Ying Jie S., Shafie S., Chang S., Jeyagurunathan A., Boon Yiang C., et al. Readiness and Acceptance of EHealth Services for Diabetes Care in the General Population: Cross-Sectional Study. J. Med. Internet Res. 2021;23:e26881. doi: 10.2196/26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bossuyt P., Pouillon L., Bonnaud G., Danese S., Peyrin-biroulet L. E-Health in Inflammatory Bowel Diseases: More Challenges than Opportunities ? Dig. Liver Dis. 2017;49:1320–1326. doi: 10.1016/j.dld.2017.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Hofstede J., de Bie J., van Wijngaarden B., Heijmans M. Knowledge, Use and Attitude toward EHealth among Patients with Chronic Lung Diseases. Int. J. Med. Inform. 2014;83:967–974. doi: 10.1016/j.ijmedinf.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 30.Anglada-Martínez H., Rovira-Illamola M., Martin-Conde M., Sotoca-Momblona J.M., Codina-Jané C. MHealth Intervention to Improve Medication Management in Chronically Ill Patients: Analysis of the Recruitment Process. Postgrad. Med. 2016;128:427–431. doi: 10.1080/00325481.2016.1170580. [DOI] [PubMed] [Google Scholar]
- 31.Wootton B.M., Titov N., Dear B.F., Spence J., Kemp A. The Acceptability of Internet-Based Treatment and Characteristics of an Adult Sample with Obsessive Compulsive Disorder: An Internet Survey. PLoS ONE. 2011;6:e20548. doi: 10.1371/journal.pone.0020548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Handa S., Okuyama H., Yamamoto H., Nakamura S., Kato Y. Effectiveness of a Smartphone Application as a Support Tool for Patients Undergoing Breast Cancer Chemotherapy: A Randomized Controlled Trial. Clin. Breast Cancer. 2020;20:201–208. doi: 10.1016/j.clbc.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Ruiz-Casado A., Álvarez-Bustos A., de Pedro C.G., Méndez-Otero M., Romero-Elías M. Cancer-Related Fatigue in Breast Cancer Survivors: A Review. Clin. Breast Cancer. 2021;21:10–25. doi: 10.1016/j.clbc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 34.Servaes P., Verhagen C., Bleijenberg G. Fatigue in Cancer Patients during and after Treatment: Prevalence, Correlates and Interventions. Eur. J. Cancer. 2002;38:27–43. doi: 10.1016/S0959-8049(01)00332-X. [DOI] [PubMed] [Google Scholar]
- 35.Álvarez-Bustos A., de Pedro C.G., Romero-Elías M., Ramos J., Osorio P., Cantos B., Maximiano C., Méndez M., Fiuza-Luces C., Méndez-Otero M., et al. Prevalence and Correlates of Cancer-Related Fatigue in Breast Cancer Survivors. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer. 2021;29:6523–6534. doi: 10.1007/s00520-021-06218-5. [DOI] [PubMed] [Google Scholar]
- 36.Hajj A., Chamoun R., Salameh P., Khoury R., Hachem R., Sacre H., Chahine G., Kattan J., Rabbaa Khabbaz L. Fatigue in Breast Cancer Patients on Chemotherapy: A Cross-Sectional Study Exploring Clinical, Biological, and Genetic Factors. BMC Cancer. 2022;22:16. doi: 10.1186/s12885-021-09072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Furlong E., Darley A., Fox P., Buick A., Kotronoulas G., Miller M., Flowerday A., Miaskowski C., Patiraki E., Katsaragakis S., et al. Adaptation and Implementation of a Mobile Phone-Based Remote Symptom Monitoring System for People With Cancer in Europe. JMIR Cancer. 2019;5:e10813. doi: 10.2196/10813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Basch E., Kris M.G., Scher H.I., Hudis C.A., Sabbatini P., Rogak L., Atkinson T.M., Chou J.F., Dulko D., Sit L., et al. Symptom Monitoring With Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Jong N., Courtens A.M., Abu-Saad H.H., Schouten H.C. CE TEST: Fatigue in Patients With Breast Cancer Receiving Adjuvant Chemotherapy: A Review of the Literature. Cancer Nurs. 2002;25:298–299. doi: 10.1097/00002820-200208000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Mast M.E. Correlates of Fatigue in Survivors of Breast Cancer. Cancer Nurs. 1998;21:136–142. doi: 10.1097/00002820-199804000-00007. [DOI] [PubMed] [Google Scholar]
- 41.Haghighat S., Akbari M.E. Factors Predicting Fatigue in Breast Cancer Patients. Support. Care Cancer. 2003;11:533–538. doi: 10.1007/s00520-003-0473-5. [DOI] [PubMed] [Google Scholar]
- 42.Bower J.E., Ganz P.A., Desmond K.A., Rowland J.H., Meyerowitz B.E., Belin T.R. Fatigue in Breast Cancer Survivors: Occurrence, Correlates, and Impact on Quality of Life. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 43.Woo B., Dibble S.L., Piper B.F., Keating S.B., Weiss M.C. Differences in Fatigue by Treatment Methods in Women with Breast Cancer. Oncol. Nurs. Forum. 1998;25:915–920. [PubMed] [Google Scholar]
- 44.Donovan K.A., Jacobsen P.B., Andrykowski M.A., Winters E.M., Balducci L., Malik U., Kenady D., McGrath P. Course of Fatigue in Women Receiving Chemotherapy and/or Radiotherapy for Early Stage Breast Cancer. J. Pain Symptom Manag. 2004;28:373–380. doi: 10.1016/j.jpainsymman.2004.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sultan A., Choudhary V., Parganiha A. Worsening of Rest-Activity Circadian Rhythm and Quality of Life in Female Breast Cancer Patients along Progression of Chemotherapy Cycles. Chronobiol. Int. 2017;34:609–623. doi: 10.1080/07420528.2017.1286501. [DOI] [PubMed] [Google Scholar]
- 46.Harbeck N., Fasching P.A., Wuerstlein R., Degenhardt T., Lüftner D., Kates R.E., Schumacher J., Räth P. Signi Fi Cantly Longer Time to Deterioration of Quality of Life Due to CANKADO PRO-React EHealth Support in HR D HER2 L Metastatic Breast Cancer Patients Receiving Palbociclib and Endocrine Therapy: Primary Outcome Analysis of the Multicenter Randomized. Ann. Oncol. 2023;34:660–669. doi: 10.1016/j.annonc.2023.05.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.