Abstract
Following the waning severity of COVID-19 due to vaccination and the development of immunity, the current variants of SARS-CoV-2 often lead to mild upper respiratory tract infections (MURTIs), suggesting it is an appropriate time to review the pathogenesis and treatment of such illnesses. The present article reviews the diverse causes of MURTIs and the mechanisms leading to symptomatic illness. Different symptoms of MURTIs develop in a staggered manner and require targeted symptomatic treatment. A wide variety of remedies for home treatment is available, including over-the-counter drugs and plant-derived substances. Recent pharmacological research has increased the understanding of molecular effects, and clinical studies have shown the efficacy of certain herbal remedies. However, the use of subjective endpoints in these clinical studies may suggest limited validity of the results. In this position paper, the importance of patient-centric outcomes, including a subjective perception of improved well-being, is emphasized. A best practice approach for the management of MURTIs, in which pharmacists and physicians create an improved multi-professional healthcare setting and provide healthcare education to patients, is proposed. Pharmacists act as first-line consultants and provide patients with remedies, considering the individual patient’s preferences towards chemical or plant-derived drugs and providing advice for self-monitoring. Physicians act as second-line consultants if symptoms worsen and subsequently initiate appropriate therapies. In conclusion, general awareness of MURTIs should be increased amongst medical professionals and patients, thus improving their management.
Keywords: common cold, coronavirus, cough, COVID-19 pandemic, holistic health, mild upper respiratory tract infections, pharmacist, rhinitis, rhinovirus, sleep
Introduction
This position paper addresses the treatment of mild upper respiratory tract infections (MURTIs) and provides recommendations for best practice. When the SARS-CoV-2 pandemic hit, infectious respiratory diseases were brought into an unprecedented, generalized focus, with COVID-19 dominating news, politics and our daily lives. COVID-19 is a potentially life-threatening disease, affecting many organ systems and requiring intensive care in many patients.1,2 However, patients may also experience milder forms of COVID-19 with flu-like symptoms1,3,4 and the evolution of the virus as well as increased immunity of general populations have led to a decline in hospitalizations and deaths during the year 2022.2 Today, SARS-CoV-2 variants often lead to MURTIs, suggesting that a review of our knowledge of MURTIs is desirable and a reassessment of treatment options is required. Unmet needs and appropriate healthcare provision will also be evaluated. Background information on the cause and outcomes of MURTIs provides the context for discussion of treatment.
MURTIs are complex viral diseases of the upper airways caused by a diversity of viruses, including multiple strains of rhinovirus, coronavirus, influenza virus, parainfluenza virus, respiratory syncytial virus, adenovirus, enterovirus and other viruses (Table 1). In total, it is estimated that >200 viral strains are causative for MURTIs.1,5 Viral incubation typically lasts 2–4 days.6 Depending on the viral trigger, MURTIs can manifest as a common cold – a self-limiting disease in immunocompetent patients, usually lasting about 2 weeks. The multiple symptoms related to MURTIs (Table 2) are not directly caused by the viruses but by an immune-mediated response to them,7,8 and include typical nasal congestion, cough, sore throat and malaise, which causes a strong negative effect on general well-being.9
Table 1.
Common cold-causing viruses.
| Virus | The estimated annual proportion of cases |
|---|---|
| Rhinoviruses | 30–50% |
| Coronavirusesa | 15–30% |
| Influenza viruses | 5–15% |
| Respiratory syncytial virus | 5% |
| Parainfluenza viruses | 5% |
| Adenoviruses | <5% |
| Enteroviruses | <5% |
| Metapneumovirus | Unknown |
| Unknown | 20–30% |
Table 2.
Common symptoms of common cold, influenza and COVID-19.
| Common cold | Influenza | COVID-19a | Seasonal allergies | |
|---|---|---|---|---|
| Gradual onset of symptoms | Abrupt onset of symptoms | Symptoms range from mild to severe | Abrupt onset of symptoms | |
| Length of symptoms | <14 days | 7–14 days | 7–25 days | Several weeks |
| Sneezing | Common | No | No | Common |
| Runny or stuffy nose | Common | Sometimes | Rare | Common |
| Sore throat | Common | Sometimes | Sometimes | Sometimes (mild) |
| Cough | Common (mild) | Common (dry) | Common (dry) | Rare (dry) |
| General aches, pains | Common | Common | Sometimes | No |
| Loss of taste or smell | Sometimes | Sometimes | Common | Rare |
| Fever | Rare | Common | Common | No |
| Shortness of breath | No | No | Sometimes | No |
| Wheezing | No | Sometimes | Rare | Sometimes |
Sleep is a vital process for maintaining homeostasis and the quality of human life.10 Nasal congestion and cough often affect a patient’s ability to sleep well.11,12 Lack of sleep has been found to increase the susceptibility to infectious diseases and stressors, including pathogens altering sleep patterns or disrupting sleep.13–19 Considering the above-listed symptoms, the quality of life (QoL) of affected persons is often severely impaired, leading them to self-medicate or seek medical attention.
The large number of viral strains involved in MURTIs means that the development of effective vaccines is complex as respiratory viruses frequently mutate to escape immunity8 but important public health messages can help prevent or reduce infections.20,21 In most cases, antibiotics are inappropriate, as they do not influence the natural history of viral MURTIs but contribute to antibiotic resistance22–27 and possible side effects such as gastrointestinal symptoms and allergies. Drugs for symptomatic treatment of different manifestations of MURTIs are available, including analgesics, decongestants, antihistamines, herbal remedies or essential oils for inhalation, oral and transdermal application.28,29 Many of these over-the-counter (OTC) drugs have proven efficacy in improving symptoms and QoL in both randomized controlled trials (RCTs)30 and clinical use.31
In summary, MURTIs are amongst the most widespread infectious diseases.5,32,33 On average, adults have two to four MURTIs, whilst children have six to eight MURTIs per year,5 accounting for millions of lost working and school days5 and relevant direct costs per episode.34
Pathophysiology and current management of MURTIs
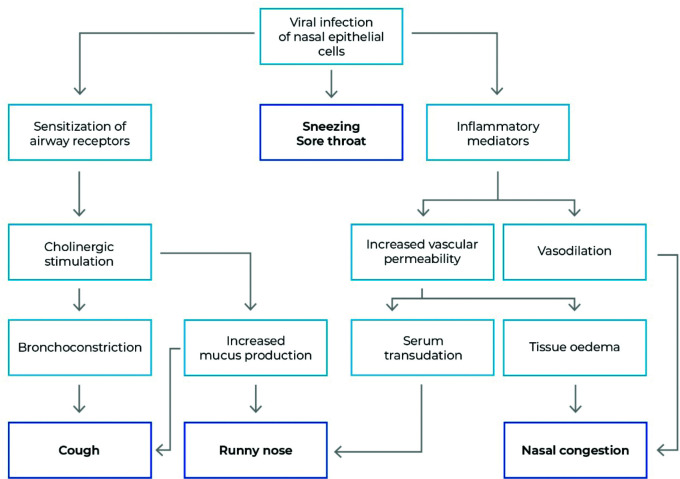
Clinical symptoms of the common cold, flu and other MURTIs are caused by an immune response to the viruses7,8,35,36 (Figure 1). Typically, the onset of symptoms is staggered (Figure 2).37 Symptoms of MURTIs include sore throat, nasal congestion, nasal discharge, sneezing, headache, cough and general malaise (Table 2).38 Symptom triggers include bradykinin release into the pharyngeal area as a cause for sore throat;39 cytokine release causing breakdown of muscle tissue to facilitate protein release to support responsiveness leading to general aches, headaches and pain as well as fever;40–44 bradykinin and histamine release cause nasal congestion due to vasal dilation;39,45,46 and excess mucus production and inflammatory mediators47,48 sensitize and stimulate sensory nerve endings, inducing cough, which starts around 48 hours after the onset of symptoms and usually persists well beyond all other symptoms (Table 2).37 Because the viruses do not directly cause the symptoms, it is difficult to distinguish the common cold, influenza and mild COVID-19 based on clinical presentation only, though some differences are observed (Table 2).1,3,4,49
Figure 1.
Immune response-mediated symptoms of MURTIs.
The symptoms of mild upper respiratory tract infections (MURTIs) are caused by the immune response to viral agents. They vary in incidence and severity but are generally similar regardless of the causative viral strain, including common cold-causing viruses (see Table 1), influenza viruses and SARS-CoV-2 (see Table 2).
Based on data from Eccles et al.120
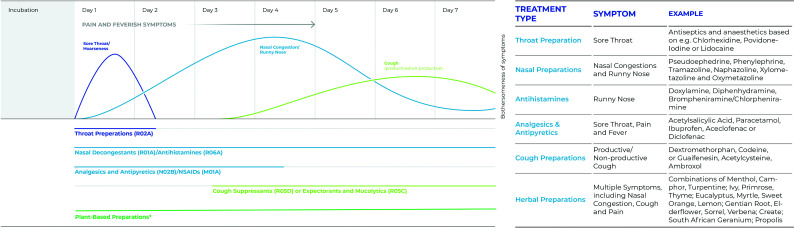
Figure 2.
Phases of common cold and suitable therapeutic agents.
Sore throat, nasal congestion and cough are the most burdensome symptoms of mild upper respiratory tract infections. Symptoms develop in a staggered manner (left panel). Targeted symptomatic treatment may require staggered use of drugs from different therapeutic classes (R02A, R01A, R06A, N02B, M01A, R05D, R05C based on the WHO ATC classification system). The use of single or combined plant-derived substances may provide a (co)treatment option with overlapping effects, decreasing the number of different pharmaceutical products required for the treatment of a common cold episode (list of examples, right panel). *See refs.36,38,53,59,61,62,65–68,70–73,81,115 Adapted from Witek et al.37
In addition, patient QoL is often severely affected by sleep disturbances caused by MURTI symptoms.50 Immunity and sleep are bi-directionally related. Sleep increases lymphocyte proliferation and neutrophil function, whilst sleep deprivation increases complement activation and inflammatory gene expression. In a study with 22,726 participants, lack of sleep (i.e. <5 hours per day) or diagnosed sleep disorders increased the susceptibility to infectious diseases by 1.8-fold to 2.2-fold.13 Vice versa, stressors like pathogens alter sleep patterns or disrupt sleep,14 contributing to a vicious circle in patients with MURTIs. Despite the high socio-economic burden,5,34 awareness towards MURTIs was low prior to the COVID-19 pandemic, and significant patient information gaps and concerns have been identified.51 Self-medication, though common, is typically not discussed with general practitioners. Furthermore, as discussed in greater detail later, the use of antibiotics is still widespread despite not being indicated in uncomplicated acute viral diseases. Therefore, awareness towards improved management of MURTIs should be raised.
Treatment of MURTIs is purely symptomatic and can speed up recovery52–54 and mitigate symptoms. A variety of OTC solutions, home remedies, food supplements or plant-derived substances, which can be taken orally, topically or via inhalation, are available28,29,55 and proven to soothe symptoms by influencing different functional triggers within the molecular network of symptom development (Figures 1 and 2). Intense research over the last decades has not only led to a better understanding of these triggers but also of the molecular processes of a diversity of pharmacologically active substances, thus allowing for more holistic approaches with adapted and well-targeted therapies.
Nasal decongestants, mainly sympathomimetics, constrict nasal blood vessels;56 antitussives centrally or topically reduce the frequency and/or intensity of coughs and include opium alkaloids and derivatives;57 expectorants increase the production of fluid in the lungs to reduce the viscosity of mucus, whilst mucolytics break down disulfide bonds in the mucus.55 First-generation antihistamines block both histaminic and muscarinic receptors and, in contrast to second-generation antihistamines, pass the blood–brain barrier, exerting their effects on the cough centre located in the brainstem. With their anticholinergic properties, they disrupt nerve signals that trigger mucus secretion and sneezing.58 Analgesics can provide relief from pain-associated common cold symptoms and may be antipyretic.31
In addition, pharmacologically active plant-based molecules are also treatment options, including systemic use, topical application or inhalation of aromatics such as menthol, eucalyptus, camphor, thyme, ivy, primrose, African geranium, gentian root, elderflower and vervain herb, which have long been well-established treatments for respiratory tract illnesses. As an example, so-called inhaled therapeutic vapours are widely used to improve breath, reduce cough, improve alertness and improve sleep during a common cold.36
In this respect, a recent systematic review59 provided an overview of pharmacological studies showing that aromatic compounds derived from natural plant extracts, such as menthol, eucalyptus or camphor, have multifaceted modulating effects on transient receptor potential channel (TRP) ‘cough’ receptors and/or the nasal TRP channels, such as TRPM8 and TRPA1,59,60 and therefore have the potential to target multiple symptoms during the course of MURTIs (Figure 2):38,59,61 Clinically, the modulation of TRP receptors results in a significant reduction of coughing, as shown in three single-blind crossover studies (total n=104 with induced cough),62 where a combination of menthol, camphor, eucalyptus, turpentine oil, cedar leaf oil, myristica oil and thymol significantly reduced the frequency of coughing more effectively than single components. Further, the application of this combination significantly reduced the time taken for the feeling of nasal cooling and, importantly, the time to nasal decongestion compared with a petrolatum control (reviewed in detail by Smith and Matthews36). Nasal airflow also influences TRP channels,38,61 and the absence of nasal airflow is associated with the feeling of stuffiness. Interestingly, as recently described in a systematic review by Stinson et al.,59 some of these plant-derived aromatic substances have been shown to have a diversity of medicinal properties not restricted to the management of MURTIs, including antipyretic, analgesic, anti-inflammatory or antibacterial effects (Table 3).59,63,64
Table 3.
Medicinal properties of plant-based substances.
| Menthol | Camphor | Eucalyptus oil | Turpentine oil | Thymol | Cedar leaf oil | Nutmeg oil | |
|---|---|---|---|---|---|---|---|
| Plant | Mentha x piperita (Peppermint) and other members of the mint family | Cinnamomum camphora (Camphor laurel) | Eucalyptus globulus (Tasmanian blue gum) and other members of the eucalyptus family | Pinus Pinaster (Maritime pine) and other members of the pine family | Thymus vulgaris | Thuja orientalis (Arbor vitae) and other members of the Cupressaceae family | Myristica fragrans (Fragrant nutmeg) |
| Medicinal properties described | |||||||
| Antibacterial | ✓ | – | ✓ | – | – | ✓ | – |
| Analgesic | ✓ | ✓ | ✓ | – | – | – | ✓ |
| Anti-inflammatory | – | ✓ | ✓ | ✓ | – | – | ✓ |
| Antioxidant | – | – | ✓ | ✓ | – | ✓ | ✓ |
| Antiviral | – | – | – | ✓ | – | ✓ | – |
| Antimicrobial | – | – | ✓ | ✓ | ✓ | – | ✓ |
| Antitussive | ✓ | ✓ | – | – | ✓ | – | – |
| Antipyretic | – | – | – | – | ✓ | – | – |
| Expectorant | – | ✓ | – | ✓ | ✓ | – | – |
| Sedative | – | – | – | – | ✓ | – | – |
| Cooling effect | ✓ | – | – | – | – | – | – |
| Counter irritant | – | ✓ | – | – | – | – | – |
✓ = Medicinal properties most relevant to the management of cold symptoms.
Adapted from Stinson et al.59
The clinical efficacy of a combination of cowslip, gentian root, black elder, sorrel and common vervain was tested in patients with acute viral rhinosinusitis (n=386)65 in a placebo-controlled, double-blind, randomized trial and led to a significant and clinically relevant improvement of the investigator-assessed symptom score on day 15.65 Furthermore, a combination of gentian root, primula flower, sorrel, elderflower and common vervain was shown to be safe and efficacious in patients with acute rhinosinusitis in phase IIb/III and phase III placebo-controlled clinical trials (total n=589).66
Wagner et al.67 and Kardos68 provided summaries of the efficacy of plant-based cough remedies. A preparation of Andrographis paniculata (creat or green chiretta) was tested against placebo in five RCTs and versus echinacea and bromhexine in a sixth study (total n=807),67 and was shown to significantly improve cough-related symptoms in all but one small pilot study.67,69 Four RCTs using a preparation based on ivy, primrose and thyme (total n=1428)67 showed strong evidence for the beneficial effect of this combination, which not only reduced the frequency and severity of cough but also facilitated secretolysis.67,70 Furthermore, oral treatment with a syrup containing thyme and ivy was shown to significantly reduce the Bronchitis Severity Score and cough severity and to improve health-related QoL in an observational, prospective, uncontrolled study (n=730).71 A meta-analysis of randomized, placebo-controlled trials assessing the effects of a Pelargonium sidoides extract (South African geranium), including 11 trials and 2195 patients,72 showed a reduced burden, earlier remission of cough and an increase in disease-associated QoL. Furthermore, patients treated with the geranium extract felt able to resume normal daily routines sooner than those treated with pacebo.72 Several studies on the effect of a mixture of eucalyptus oil, sweet orange oil, myrtle oil, and lemon oil on coughing have been published. For example, Gillissen et al. reported significantly superior effects to placebo in coughing-related endpoints and sleep disturbances (n=413).53 Fürst et al. reviewed clinical trials conducted with this mixture in China and confirmed its efficacy in the treatment of respiratory tract diseases in a Chinese patient population.73
In this light, an oral spray based on propolis, traditionally used to maintain oral cavity and upper respiratory tract health due to its antimicrobial and anti-inflammatory properties, was tested in a randomized, double-blind, placebo-controlled clinical study in adults with MURTIs (n=122). Resolution from MURTIs was observed 2 days earlier in propolis extract-treated patients versus placebo-treated patients.74
Not all studies successfully demonstrated the efficacy of essential oils in objective endpoints or lasting superiority over placebo controls, but they still reported efficacy in subjective endpoints, including the nasal sensation of airflow and quality of sleep.38,75–77 Assessment of patient-centric outcomes is part of evidence-based medical research and should thus not be disregarded; symptomatic relief perceived by the individual patient is considered beneficial for well-being, stress reduction, and sleep quality9,78 and is considered the most important endpoint in a self-limiting disease. Even in RCTs of asthma, interstitial lung disease and chronic obstructive pulmonary disease, the FDA and EMA require patient-related outcomes (i.e. QoL Questionnaires) as coprimary or secondary endpoints. Given the importance of sleep for overall survival and individual well-being, treatment of sleep disorders and increasing quality of sleep are essential to improving immunity15,78; thus, further studies and focus are required on holistic health approaches in the therapy of MURTIs.
In conclusion, relief of nasal congestion and cough is key to breathing appropriately, which is not only important for sleep quality but also relevant for well-being. This may further lead to reduced susceptibility to infection and faster recovery, highlighting the importance of these therapeutic targets and holistic approaches, and acknowledging the significance of patient-centric efficacy outcomes.
Unmet needs in public health messages
Despite the high number of patients affected by and costs associated with MURTIs, many have regarded these diseases as nothing more than a slight nuisance, adding to the somewhat neglected status of MURTIs. With the pandemic, a new public and medical focus towards MURTIs has developed. As previously stated, clinical symptoms may overlap between the common cold, influenza and mild COVID-19, making identification of the infecting agent difficult (Table 2). Diagnostic testing may be required depending on local regulations or at the health professional’s recommendation based on risk factors.
Whilst MURTIs are usually not severe or life-threatening diseases in immunocompetent patients, immunocompromised patients are at risk of developing lower respiratory tract infections, including potentially fatal conditions like bronchiolitis obliterans syndrome in transplant patients15 and pneumonia.79 Viral MURTIs may also cause exacerbation of pre-existing respiratory conditions such as asthma,80 chronic obstructive pulmonary disease,81 allergic rhinitis,80 chronic rhinosinusitis,82 interstitial lung diseases83 and breathing disturbances during sleep.84
In the paediatric patient population, the use of OTC cough and cold medications often lacks support from well-designed, contemporary research and proof of efficacy.85–88 However, OTC products for the treatment of MURTIs are widely used, increasing the trend of abuse and potential toxicity,85,86,88 which may even cause fatalities if used in very young children and/or in overdose.89
Vaccine development is complex due to the sheer number of and frequent mutations in the viral strains involved, the latter making it impossible to develop complete natural immunity.8 It is unlikely that effective vaccines will become available in the near to mid-term future for the majority of common cold-causing viral strains. Effective prophylactic measures, for example, wearing masks,90,91 strongly depend on the individual’s compliance and capability and may not be suitable for some patient populations.
Finally, yet importantly, a holistic medical approach allows for centring the attention of medical professionals on the individual patient’s needs, preferences and well-being, thus triggering compliance, increasing security, and reducing psychosocial stress and chronic disease.92 Stress, as described, can negatively influence immunity and could contribute to the worsening or prolongation of the symptoms of MURTIs.93
In summary, awareness towards MURTIs has increased, but the pandemic has also increased people’s insecurity about how to behave and where to seek medical attention and fear of viral infection.94,95 Thus, new guidance from medical professionals, including a holistic, patient-centric approach, is needed to guide treatment that is medically justified as well as endorsed by the patient.
Recommendations for best practice
The pandemic has put a new focus on MURTIs in the general population as well as in politics, media and medical professionals. However, does this imply a lasting paradigm shift in the management of MURTIs?
Prophylactic measures recommended or required by regulations to fight the COVID-19 pandemic included testing, public measures such as curfews, lockdowns and reduced crowd densities in shops and events, as well as personal measures such as wearing masks and improved hand hygiene. Whilst these measures were in place and/or still voluntarily endorsed by many, the incidence of the common cold and influenza as well as of bacterial respiratory infections and chronic obstructive pulmonary disease exacerbations was significantly reduced.96–98 Prior to the pandemic, the use of face masks to prevent the spreading or catching of diseases was uncommon in Europe and the United States. It will be interesting to study if there is a lasting effect on the general population’s behaviour once incidences of the common cold and influenza rise to pre-pandemic levels. Such a lasting change in awareness regarding the effectiveness of personal hygiene measures against common infectious diseases might include the voluntary use of face masks as well as further embracing a shift in attitudes towards working places and educational institutions: will people return to a habit of going to work or school sick? Will colleagues accept the presence of team members suffering from (and potentially spreading) respiratory infections?
The pandemic has also put a new focus on the importance of pharmacists as first-line health consultants. MURTIs are the main cause of consultation in both primary care and community pharmacy. With long opening hours and short anticipated waiting times, pharmacies provide low-threshold access to medication advice and play an important role as the primary point of contact with a healthcare professional as patients consult the pharmacist for health advice, health literacy and social support.99,100 During the COVID-19 pandemic, a wide range of pharmaceutical interventions was provided, and expanded powers were granted to pharmacies, enhancing their role in a multi-professional healthcare setting.101 Furthermore, approximately 20% of consultations with doctors could be dealt with by other professionals like pharmacists, which would save 1 out of every 5 hours of medical doctor time that could be devoted to other activities or patients.102
Enhancing the role of pharmacists as first-line consultants within applicable local legal boundaries could further reduce the use of antibiotics in patients with the common cold. Pharmacists are highly educated, giving advice regarding symptomatic treatment for viral upper and lower respiratory tract infections. Patients may erroneously believe that antibiotics could aid in the fast relief of symptoms. However, antibiotics were shown to be ineffective in changing the natural history of common colds and are not indicated in uncomplicated MURTIs, as shown in a number of RCTs and observational studies.22–27,103 Still, 41% of all antibiotic prescriptions are for respiratory conditions,104 which is one of the most important contributions to antibiotic resistance, whilst several scientific societies advise against antibiotic use in MURTIs.105–107
Symptomatic therapies versus subjective endpoints?
Despite accumulating clinical evidence and a better understanding of the mode of action of available pharmacotherapies, the availability of data, especially regarding the efficacy of plant-based substances, may often be considered limited due to study designs or endpoints used in clinical trials.
With patient-centred research becoming more important in medical care and research, patient-reported outcomes should not be disregarded because, beyond contagiousness, this is the cause of absenteeism from work or school. Although there is a gradual decline in cases per person per year over the lifespan, the common cold accounts for millions of days lost at school and work.5 Symptomatic relief perceived by the individual patient is the aim of the therapy and is considered beneficial for well-being, stress reduction and sleep quality. Based on the importance of sleep for individual well-being,10 increased quality of sleep is essential to improve immunity.13–19 Data on subjective efficacy endpoints should thus not only be given serious consideration36 but should also become the focus of attention and action in holistic health approaches in the therapy of MURTIs in general and of SARS-CoV-2-related MURTIs in particular.
With various OTC products available, including plant-based medicines, patients can benefit from tailored treatment approaches when experiencing MURTIs. The scheme in Figure 2 summarizes chemical and plant-based actives as classified by World Health Organization Anatomical Therapeutic Chemical/Defined Daily Dose (WHO ATC/DDD) Index Codes108 and by the latest research findings described earlier, and presents available treatment options for the therapy of MURTI symptoms over the illness time course. Treatment options include throat preparations to treat sore throat at the beginning of the illness (day 1); analgesics, non-steroidal anti-inflammatory drugs and antipyretics to treat pains and fever as well as nasal decongestants to treat nasal congestion (days 2–5); and cough suppressants and expectorants to treat cough (days 6–7). Plant-based medicines are often provided as either single substances or as combinations of various essential oils (e.g. inhaled therapeutic vapours), potentially covering all phases of MURTIs with one pharmaceutical product. Therefore, (co)treatment with plant-based combinations is considered suitable to treat multiple symptoms throughout the course of the illness (Figure 2), as shown by in vitro data and/or clinical trials.37,59
Overall, plant-based medicines and honey may provide a safer alternative to synthetic OTC products in a paediatric patient population,87,109,110 with similar or improved efficacy.111,112 Safety and tolerability endpoints for plant-derived substances are reported in the literature66,67,75,113 but are also limited in children,114 with reported adverse events being of a mild irritant nature.115
An estimated one-third of patients in the United States prefer and use ‘natural alternatives’ to chemical drugs in the treatment of the common cold and influenza, including plant-derived essences. Consideration of integrative therapies could thus increase patient compliance and positive patient care experiences.116
Best practice recommendations
The first pillar on which our best practice recommendations to manage MURTIs are based is health literacy. Educated patients can understand and crosslink the interdependency and general importance of a healthy lifestyle, good sleep quality, and their immune system. In the case of MURTIs, healthcare professionals should educate and advise patients about treatment options, facilitating patients to make an educated decision to comply with the selected therapy. Most importantly, patients and caregivers should become aware that antibiotics are inappropriate and ineffective in the treatment of MURTIs,22–27 and vaccines are not available, except for COVID-19, influenza and, most likely soon, respiratory syncytial virus.
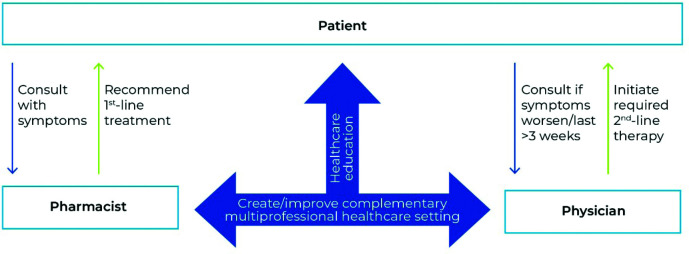
The second pillar consists of healthcare professionals creating and improving a complementary multi-professional healthcare setting. Pharmacists play a fundamental role not only in dealing with MURTI symptoms but also in health promotion and education. They can effectively reach patients and disburden medical doctors.102 Ideally, the patient’s initial consultation is with the pharmacist (Figure 3), who advises the patient to closely monitor a potential worsening of symptoms and may recommend suitable OTC or plant-based drugs.
Figure 3.
Patient-centric management of common cold.
The authors of this position paper suggest a multi-professional, patient-centric approach to managing mild upper respiratory tract infections. Pharmacists and physicians create and improve a complementary healthcare setting, both by assisting with patient healthcare education and by the pharmacist being the primary point of contact for patients within local regulatory boundaries, thus disburdening physicians. The patient is advised to self-monitor recovery or disease progression and seek medical attention from a physician if symptoms worsen or last longer than 3 weeks.
If symptoms worsen, a physician is to be consulted (Figure 3). Home assessment of oxygen saturation could increase patient safety and shorten response times in complicated cases of URTIs. Pulse oximeters have been successfully used for self-monitoring in patients with mild COVID-19;117 they are inexpensive and easy to use118 and could be included in common medicine cabinets like fever thermometers in the future.
Furthermore, home tests for influenza A and B, SARS-CoV-2, and respiratory syncytial virus are available. Thus, acute testing should be advised to monitor for possible post-COVID-19 cases.
The proposed guide for the treatment of patients with MURTIs and/or mild fever includes the following steps:
Help increase self-awareness for symptoms and symptom development: consider signs of infection and illness, for example, by home assessment of fever and monitoring oxygen saturation using a pulse oximeter as appropriate.
Focus on optimization of symptomatic treatment: use adapted continuous (co)treatment(s) targeting multiple symptoms of the natural history of MURTIs, considering the patient’s preferences between plant-derived remedies and chemical drugs, and avoiding initial antibiotic treatment.
If relevant, use appropriate rapid tests (e.g. influenza, SARS-CoV-2): this is important for the identification of viruses during endemics and pandemics where targeted causal therapies are available and to monitor contagiousness.
Advice to seek professional medical attention immediately if symptoms worsen or last longer than 3 weeks.
Conclusion
The COVID-19 pandemic highlighted unmet needs in the management of MURTIs. Antibiotics are still inappropriate for MURTIs, and vaccines are available only for a few viruses, mainly mitigating the symptoms rather than preventing the diseases. Relevant prophylactic measures may include testing and reduced crowd densities in shops and events as well as personal measures such as wearing masks and improved hand hygiene. A strong advancement of patient-centred approaches is required, including the improved appraisal of subjective treatment outcomes within adapted holistic therapies.
Acknowledgements
None.
Footnotes
Contributions: All authors provided scientific input and expert insights. AS wrote the manuscript. All authors reviewed and edited the manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: This work was supported by Procter & Gamble International Operations SA, 47 Route de Saint-Georges, 1213 Petit-Lancy, Geneva, Switzerland. PK received consulting fees from Procter & Gamble, Klosterfrau, Schwabe and Bionorica as well as honoraria from Bionorica. OP reports personal fees from Procter and Gamble during the work presented. Furthermore, he reports grants and/or personal fees from ALK-Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding, B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, ASIT Biotech Tools S.A., Laboratorios LETI/LETI Pharma, GlaxoSmithKline, ROXALL Medizin, Novartis, Sanofi-Aventis and Sanofi-Genzyme, Med Update Europe GmbH, streamedup! GmbH, Pohl-Boskamp, Inmunotek S.L., John Wiley and Sons, AS, Paul-Martini-Stiftung (PMS), Regeneron Pharmaceuticals Inc., RG Aerztefortbildung, Institut für Disease Management, Springer GmbH, AstraZeneca, IQVIA Commercial, Ingress Health, Wort&Bild Verlag, Verlag ME, Altamira Medica AG, Meinhardt Congress GmbH, Deutsche Forschungsgemeinschaft, Thieme, Deutsche AllergieLiga e.V., AeDA, Alfried-Krupp Krankenhaus, Red Maple Trials Inc., Technical University Dresden, ECM Expo & Conference Management, all outside the submitted work; and he is a member of EAACI, Excom and a member of the external board of directors for DGAKI. GER has received grants from GlaxoSmithKline, Chiesi, SEFAC, Arkopharma, Santen Innovation Labs, he has been a speaker at 3rd Congreso SEFAC-SEMERGEN and for Procter & Gamble International Operations, Arkopharma and Santen Innovation Labs as well as attended education events for GlaxoSmithKline and Chiesi and meetings for Procter & Gamble International Operations and SEFAC. FB has been on the scientific Board for AZ, BI, Chiesi, GSK, Menarini group, Sanofi and P&G; received honoraria from AZ, BI, Chiesi, GSK, Menarini group and Sanofi, and is the President of Interasma (Global Asthma Association). A consultancy fee was paid to Laura Sadofsky Procter & Gamble International Operations SA for services related to the European Experts Summit on Respiratory Tract Infections 2022. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2023/07/dic.2023-4-2-COI.pdf
Funding declaration: Preparation of this article was funded by Procter & Gamble International Operations SA, 47 Route de Saint-Georges, 1213 Petit-Lancy, Geneva, Switzerland. Medical writing assistance was provided by Alexandra Kopic, DVM, MSc, Sophinant Medical Writing and Consulting, 2471 Rohrau, Austria.
Correct attribution: Copyright © 2023 Smith A, Kardos P, Pfaar O, Randerath W, Estrada Riolobos G, Braido F, Sadofsky L. https://doi.org/10.7573/dic.2023-4-2. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Submitted; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: 6 Green Lane Business Park, 238 Green Lane, New Eltham, London, SE9 3TL, UK.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights, and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Centers for Disease Control and Prevention. Similarities and differences between Flu and COVID-19. Center for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD); [Accessed February 23, 2023]. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm . [Google Scholar]
- 2.Looi MK, Mahase E. Has covid-19 become milder? BMJ. 2022;379:o2516. doi: 10.1136/bmj.o2516. [DOI] [PubMed] [Google Scholar]
- 3.Kandola A, Weiss K. New coronavirus vs. flu. [Accessed November 3, 2022]. https://www.medicalnewstoday.com/articles/coronavirus-vs-flu .
- 4.News in Health. National Institutes of Health; 2022. Is it Flu, COVID-19, allergies, or a cold? https://newsinhealth.nih.gov/2022/01/it-flu-covid-19-allergies-or-cold . [Google Scholar]
- 5.Heikkinen T, Jarvinen A. The common cold. Lancet. 2003;361(9351):51–59. doi: 10.1016/S0140-6736(03)12162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lessler J, Reich NG, Brookmeyer R, Perl TM, Nelson KE, Cummings DA. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291–300. doi: 10.1016/s1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turner RB. Epidemiology, pathogenesis, and treatment of the common cold. Ann Allergy Asthma Immunol. 1997;78(6):531–539. doi: 10.1016/s1081-1206(10)63213-9. quiz 539–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hendley JO. Epidemiology, pathogenesis, and treatment of the common cold. Semin Pediatr Infect Dis. 1998;9(1):50–55. doi: 10.1016/s1045-1870(98)80051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith AP. Twenty-five years of research on the behavioural malaise associated with influenza and the common cold. Psychoneuroendocrinology. 2013;38(6):744–751. doi: 10.1016/j.psyneuen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirato K, Sato S. Macrophage meets the circadian clock: implication of the circadian clock in the role of macrophages in acute lower respiratory tract infection. Front Cell Infect Microbiol. 2022;12:826738. doi: 10.3389/fcimb.2022.826738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith AP. Sleep and the common cold. J Behav Health. 2012;1(2):114–117. [Google Scholar]
- 12.Meltzer EO. Does rhinitis compromise night-time sleep and daytime productivity? Clin Exp Allergy Rev. 2008;2:67–72. doi: 10.1046/j.1472-9725.2002.00039.x. [DOI] [Google Scholar]
- 13.Prather AA, Leung CW. Association of insufficient sleep with respiratory infection among adults in the United States. JAMA Intern Med. 2016;176(6):850–852. doi: 10.1001/jamainternmed.2016.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besedovsky L, Lange T, Haack M. The sleep-immune crosstalk in health and disease. Physiol Rev. 2019;99(3):1325–1380. doi: 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar D, Erdman D, Keshavjee S, et al. Clinical impact of community-acquired respiratory viruses on bronchiolitis obliterans after lung transplant. Am J Transplant. 2005;5(8):2031–2036. doi: 10.1111/j.1600-6143.2005.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibarra-Coronado EG, Pantaleón-Martínez AM, Velazquéz-Moctezuma J, et al. The bidirectional relationship between sleep and immunity against infections. J Immunol Res. 2015;2015:678164. doi: 10.1155/2015/678164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilder-Smith A, Mustafa FB, Earnest A, Gen L, Macary PA. Impact of partial sleep deprivation on immune markers. Sleep Med. 2013;14(10):1031–1034. doi: 10.1016/j.sleep.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Wentz LM, Ward MD, Potter C, et al. Increased risk of upper respiratory infection in military recruits who report sleeping less than 6 h per night. Mil Med. 2018;183(11–12):e699–e704. doi: 10.1093/milmed/usy090. [DOI] [PubMed] [Google Scholar]
- 19.Dickstein JB, Moldofsky H. Sleep, cytokines and immune function. Sleep Med Rev. 1999;3(3):219–228. doi: 10.1016/s1087-0792(99)90003-5. [DOI] [PubMed] [Google Scholar]
- 20.Larson EL. Warned, but not well armed: preventing viral upper respiratory infections in households. Public Health Nurs. 2007;24(1):48–59. doi: 10.1111/j.1525-1446.2006.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nunes-Silva C, Vilares AT, Schweitzer V, et al. Non-COVID-19 respiratory viral infection. Breathe. 2022;18(1):210151. doi: 10.1183/20734735.0151-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra S, Srivastava P, Sunder S, Mishra AK, Tanti SK. Evaluation and optimisation of antibiotic usage in upper respiratory tract infections in children at a tertiary care outpatient department: a clinical audit. Indian J Pharmacol. 2022;54(1):13–18. doi: 10.4103/ijp.ijp_373_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zawahir S, Le HTT, Nguyen TA, et al. Inappropriate supply of antibiotics for common viral infections by community pharmacies in Vietnam: a standardised patient survey. Lancet Reg Health West Pac. 2022;23:100447. doi: 10.1016/j.lanwpc.2022.100447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sánchez X, Orrico M, Morillo T, Manzano A, Jimbo R, Armijos L. Reducing unnecessary antibiotic prescription through implementation of a clinical guideline on self-limiting respiratory tract infections. PLoS One. 2021;16(4):e0249475. doi: 10.1371/journal.pone.0249475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nowakowska M, van Staa T, Mölter A, et al. Antibiotic choice in UK general practice: rates and drivers of potentially inappropriate antibiotic prescribing. J Antimicrob Chemother. 2019;74(11):3371–3378. doi: 10.1093/jac/dkz345. [DOI] [PubMed] [Google Scholar]
- 26.Piltcher OB, Kosugi EM, Sakano E, et al. How to avoid the inappropriate use of antibiotics in upper respiratory tract infections? A position statement from an expert panel. Braz J Otorhinolaryngol. 2018;84(3):265–279. doi: 10.1016/j.bjorl.2018.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baillie EJ, Merlo G, Magin P, et al. Antibiotic prescribing for upper respiratory tract infections and acute bronchitis: a longitudinal analysis of general practitioner trainees. Fam Pract. 2022;39(6):1063–1069. doi: 10.1093/fampra/cmac052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DRUGDEX System. [Accessed March 8, 2023]. https://www.pharmaceuticalonline.com/doc/drugdex-system-0001 .
- 29.Wisher D. Martindale: the complete drug reference. 37th ed. J Med Libr Assoc. 2012;100:75–76. doi: 10.3163/1536-5050.100.1.018. [DOI] [Google Scholar]
- 30.Loose I, Winkel M. Clinical, double-blind, placebo-controlled study investigating the combination of acetylsalicylic acid and pseudoephedrine for the symptomatic treatment of nasal congestion associated with common cold. Arzneimittelforschung. 2004;54(9):513–521. doi: 10.1055/s-0031-1297006. [DOI] [PubMed] [Google Scholar]
- 31.Eccles R. Efficacy and safety of over-the-counter analgesics in the treatment of common cold and flu. J Clin Pharm Ther. 2006;31(4):309–319. doi: 10.1111/j.1365-2710.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 32.Wat D. The common cold: a review of the literature. Eur J Intern Med. 2004;15(2):79–88. doi: 10.1016/j.ejim.2004.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson GG, Dowling HF, Spiesman IG, Boand AV. Transmission of the common cold to volunteers under controlled conditions. I. The common cold as a clinical entity. AMA Arch Intern Med. 1958;101(2):267–278. doi: 10.1001/archinte.1958.00260140099015. [DOI] [PubMed] [Google Scholar]
- 34.Jaume F, Alobid I, Mullol J, Quintó L. Direct costs of acute rhinosinusitis in Spain: a Prospective and Observational Study (PROSINUS) J Investig Allergol Clin Immunol. 2021;31(6):481–488. doi: 10.18176/jiaci.0525. [DOI] [PubMed] [Google Scholar]
- 35.Kuchar E, Miśkiewicz K, Nitsch-Osuch A, Szenborn L. Pathophysiology of clinical symptoms in acute viral respiratory tract infections. Adv Exp Med Biol. 2015;857:25–38. doi: 10.1007/5584_2015_110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith A, Matthews O. Aromatic ointments for the common cold: what does the science say? Drugs Context. 2022;11:2022-5-6. doi: 10.7573/dic.2022-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witek TJ, Ramsey DL, Carr AN, Riker DK. The natural history of community-acquired common colds symptoms assessed over 4-years. Rhinology. 2015;53(1):81–88. doi: 10.4193/Rhino14.149. [DOI] [PubMed] [Google Scholar]
- 38.Eccles R, Jawad M, Ramsey DL, Hull J. Efficacy of a topical aromatic rub (Vicks VapoRub®)-Speed of action of subjective nasal cooling and relief from nasal congestion. Open J Respir Dis. 2015;5:10–18. doi: 10.4236/ojrd.2015.51002. [DOI] [Google Scholar]
- 39.Proud D, Reynolds CJ, Lacapra S, Kagey-Sobotka A, Lichtenstein LM, Naclerio RM. Nasal provocation with bradykinin induces symptoms of rhinitis and a sore throat. Am Rev Respir Dis. 1988;137(3):613–616. doi: 10.1164/ajrccm/137.3.613. [DOI] [PubMed] [Google Scholar]
- 40.Conti B, Tabarean I, Andrei C, Bartfai T. Cytokines and fever. Front Biosci. 2004;9:1433–1449. doi: 10.2741/1341. [DOI] [PubMed] [Google Scholar]
- 41.Leon LR. Invited review: cytokine regulation of fever: studies using gene knockout mice. J Appl Physiol. 2002;92(6):2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 42.Netea MG, Kullberg BJ, Van der Meer JW. Circulating cytokines as mediators of fever. Clin Infect Dis. 2000;31(Suppl 5):S178–S184. doi: 10.1086/317513. [DOI] [PubMed] [Google Scholar]
- 43.Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983;308(10):553–558. doi: 10.1056/nejm198303103081002. [DOI] [PubMed] [Google Scholar]
- 44.Kotler DP. Cachexia. Ann Intern Med. 2000;133(8):622–634. doi: 10.7326/0003-4819-133-8-200010170-00015. [DOI] [PubMed] [Google Scholar]
- 45.Eccles R. Pathophysiology of nasal symptoms. Am J Rhinol. 2000;14(5):335–338. doi: 10.2500/105065800781329528. [DOI] [PubMed] [Google Scholar]
- 46.Shibayama Y, Skoner D, Suehiro S, Konishi JE, Fireman P, Kaplan AP. Bradykinin levels during experimental nasal infection with rhinovirus and attenuated influenza virus. Immunopharmacology. 1996;33(1–3):311–313. doi: 10.1016/0162-3109(96)00051-3. [DOI] [PubMed] [Google Scholar]
- 47.Eccles R, Lee PC. Cough induced by airway vibration as a model of airway hyperreactivity in patients with acute upper respiratory tract infection. Pulm Pharmacol Ther. 2004;17(6):337–342. doi: 10.1016/j.pupt.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacoby DB. Pathophysiology of airway viral infections. Pulm Pharmacol Ther. 2004;17(6):333–336. doi: 10.1016/j.pupt.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Pfaar O, Klimek L, Jutel M, et al. COVID-19 pandemic: practical considerations on the organization of an allergy clinic-An EAACI/ARIA Position Paper. Allergy. 2021;76(3):648–676. doi: 10.1111/all.14453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dicpinigaitis PV, Eccles R, Blaiss MS, Wingertzahn MA. Impact of cough and common cold on productivity, absenteeism, and daily life in the United States: ACHOO Survey. Curr Med Res Opin. 2015;31(8):1519–1525. doi: 10.1185/03007995.2015.1062355. [DOI] [PubMed] [Google Scholar]
- 51.Kloosterboer SM, McGuire T, Deckx L, Moses G, Verheij T, van Driel ML. Self-medication for cough and the common cold: information needs of consumers. Aust Fam Physician. 2015;44(7):497–501. [PubMed] [Google Scholar]
- 52.Bizot E, Bousquet A, Charpié M, et al. Rhinovirus: a narrative review on its genetic characteristics, pediatric clinical presentations, and pathogenesis. Front Pediatr. 2021;9:643219. doi: 10.3389/fped.2021.643219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillissen A, Wittig T, Ehmen M, Krezdorn HG, de Mey C. A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol® forte in acute bronchitis. Drug Res. 2013;63(1):19–27. doi: 10.1055/s-0032-1331182. [DOI] [PubMed] [Google Scholar]
- 54.Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneimittelforschung. 2007;57(9):607–615. doi: 10.1055/s-0031-1296656. [DOI] [PubMed] [Google Scholar]
- 55.Eccles R. 433 – Cough and common cold. In: Kenakin T, editor. Comprehensive Pharmacology. London: Elsevier; 2022. pp. 745–761. [Google Scholar]
- 56.Johnson DA, Hricik JG. The pharmacology of alpha-adrenergic decongestants. Pharmacotherapy. 1993;13(6 Pt 2):110S–115S. discussion 143S–146S. [PubMed] [Google Scholar]
- 57.Bolser DC. Mechanisms of action of central and peripheral antitussive drugs. Pulm Pharmacol. 1996;9(5–6):357–364. doi: 10.1006/pulp.1996.0047. [DOI] [PubMed] [Google Scholar]
- 58.Muether PS, Gwaltney JM., Jr Variant effect of first- and second-generation antihistamines as clues to their mechanism of action on the sneeze reflex in the common cold. Clin Infect Dis. 2001;33(9):1483–1488. doi: 10.1086/322518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stinson RJ, Morice AH, Sadofsky LR. Modulation of transient receptor potential (TRP) channels by plant derived substances used in over-the-counter cough and cold remedies. Respir Res. 2023;24(1):45. doi: 10.1186/s12931-023-02347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plevkova J, Kollarik M, Poliacek I, et al. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol. 2013;115(2):268–274. doi: 10.1152/japplphysiol.01144.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonvini SJ, Belvisi MG. Cough and airway disease: the role of ion channels. Pulm Pharmacol Ther. 2017;47:21–28. doi: 10.1016/j.pupt.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Packman EW, London SJ. The utility of artificially induced cough as a clinical model for evaluating the antitussive effects of aromatics delivered by inunction. Eur J Respir Dis Suppl. 1980;110:101–109. [PubMed] [Google Scholar]
- 63.Salama SA, Al-Faifi ZE, Masood MF, El-Amier YA. Investigation and biological assessment of rumex vesicarius L. extract: characterisation of the chemical components and antioxidant, antimicrobial, cytotoxic, and anti-dengue vector activity. Molecules. 2022;27(10):3177. doi: 10.3390/molecules27103177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Savickiene N, Jekabsone A, Raudone L, et al. Efficacy of proanthocyanidins from pelargonium sidoides root extract in reducing P. gingivalis viability while preserving oral commensal S. salivarius. Materials. 2018;11(9):1499. doi: 10.3390/ma11091499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jund R, Mondigler M, Steindl H, Stammer H, Stierna P, Bachert C. Clinical efficacy of a herbal drug combination in acute viral rhinosinusitis. MMW Fortschr Med. 2015;157(Suppl 4):6–11. doi: 10.1007/s15006-015-2934-4. [DOI] [PubMed] [Google Scholar]
- 66.Jund R, Mondigler M, Stammer H, Stierna P, Bachert C. Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis. Acta Otolaryngol. 2015;135(1):42–50. doi: 10.3109/00016489.2014.952047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wagner L, Cramer H, Klose P, et al. Herbal medicine for cough: a systematic review and meta-analysis. Forsch Komplementmed. 2015;22(6):359–368. doi: 10.1159/000442111. [DOI] [PubMed] [Google Scholar]
- 68.Kardos P. Phytotherapy in acute bronchitis: what is the evidence? Clin Phytoscience. 2015;1:2. doi: 10.1186/s40816-015-0003-2. [DOI] [Google Scholar]
- 69.Melchior J, Spasov AA, Ostrovskij OV, Bulanov AE, Wikman G. Double-blind, placebo-controlled pilot and phase III study of activity of sstandardised Andrographis paniculata Herba Nees extract fixed combination (Kan jang) in the treatment of uncomplicated upper-respiratory tract infection. Phytomedicine. 2000;7(5):341–350. doi: 10.1016/s0944-7113(00)80053-7. [DOI] [PubMed] [Google Scholar]
- 70.Kurth W. Secure therapeutic effectiveness of the traditional antitussive agent Mintetten in a double-blind study. Med Welt. 1978;29(48):1906–1909. [PubMed] [Google Scholar]
- 71.Kardos P, Bittner CB, Seibel J, Abramov-Sommariva D, Birring SS. Effectiveness and tolerability of the thyme/ivy herbal fluid extract BNO 1200 for the treatment of acute cough: an observational pharmacy-based study. Curr Med Res Opin. 2021;37(10):1837–1844. doi: 10.1080/03007995.2021.1960493. [DOI] [PubMed] [Google Scholar]
- 72.Kardos P, Lehmacher W, Zimmermann A, et al. Effects of Pelargonium sidoides extract EPs 7630 on acute cough and quality of life - a meta-analysis of randomised, placebo-controlled trials. Multidiscip Respir Med. 2022;17:868. doi: 10.4081/mrm.2022.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fürst R, Luong B, Thomsen J, Wittig T. ELOM-080 as add-on treatment for respiratory tract diseases - a review of clinical studies conducted in China. Planta Med. 2019;85(9–10):745–754. doi: 10.1055/a-0942-1993. [DOI] [PubMed] [Google Scholar]
- 74.Esposito C, Garzarella EU, Bocchino B, et al. A standardised polyphenol mixture extracted from poplar-type propolis for remission of symptoms of uncomplicated upper respiratory tract infection (URTI): a monocentric, randomised, double-blind, placebo-controlled clinical trial. Phytomedicine. 2021;80:153368. doi: 10.1016/j.phymed.2020.153368. [DOI] [PubMed] [Google Scholar]
- 75.Ben-Arye E, Dudai N, Eini A, Torem M, Schiff E, Rakover Y. Treatment of upper respiratory tract infections in primary care: a srandomised study using aromatic herbs. Evid Based Complement Alternat Med. 2011;2011:690346. doi: 10.1155/2011/690346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eccles R, Lancashire B, Tolley NS. Experimental studies on nasal sensation of airflow. Acta Otolaryngol. 1987;103(5–6):303–306. doi: 10.3109/00016488709107798. [DOI] [PubMed] [Google Scholar]
- 77.Kamin W, Kieser M. Pinimenthol ointment in patients suffering from upper respiratory tract infections - a post-marketing observational study. Phytomedicine. 2007;14(12):787–791. doi: 10.1016/j.phymed.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 78.Phillipson G, Aspley S, Fietze I. Perceptions of the importance of sleep in common cold – Two online questionnaire-based surveys. SN Compr Clin Med. 2020;2:596–605. doi: 10.1007/s42399-020-00265-5. [DOI] [Google Scholar]
- 79.Hijano DR, Maron G, Hayden RT. Respiratory viral infections in patients with cancer or undergoing hematopoietic cell transplant. Front Microbiol. 2018;9:3097. doi: 10.3389/fmicb.2018.03097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rawlinson WD, Waliuzzaman Z, Carter IW, Belessis YC, Gilbert KM, Morton JR. Asthma exacerbations in children associated with rhinovirus but not human metapneumovirus infection. J Infect Dis. 2003;187(8):1314–1318. doi: 10.1086/368411. [DOI] [PubMed] [Google Scholar]
- 81.Stolz D, Papakonstantinou E, Grize L, et al. Time-course of upper respiratory tract viral infection and COPD exacerbation. Eur Respir J. 2019;54(4):1900407. doi: 10.1183/13993003.00407-2019. [DOI] [PubMed] [Google Scholar]
- 82.Wu D, Bleier BS, Wei Y. Current understanding of the acute exacerbation of chronic Rhinosinusitis. Front Cell Infect Microbiol. 2019;9:415. doi: 10.3389/fcimb.2019.00415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leuschner G, Behr J. Acute exacerbation in interstitial lung disease. Front Med. 2017;4:176. doi: 10.3389/fmed.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Abreu e Silva FA, MacFadyen UM, Williams A, Simpson H. Sleep apnoea during upper respiratory infection and metabolic alkalosis in infancy. Arch Dis Child. 1986;61(11):1056–1062. doi: 10.1136/adc.61.11.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carr BC. Efficacy, abuse, and toxicity of over-the-counter cough and cold medicines in the pediatric population. Curr Opin Pediatr. 2006;18(2):184–188. doi: 10.1097/01.mop.0000193274.54742.a1. [DOI] [PubMed] [Google Scholar]
- 86.Montgomery EJ, Wasserman GS. Toxicity and use of over-the-counter cough and cold medication in the pediatric population. Mo Med. 2008;105(6):514–517. [PubMed] [Google Scholar]
- 87.Paul IM. Therapeutic options for acute cough due to upper respiratory infections in children. Lung. 2012;190(1):41–44. doi: 10.1007/s00408-011-9319-y. [DOI] [PubMed] [Google Scholar]
- 88.Vassilev ZP, Kabadi S, Villa R. Safety and efficacy of over-the-counter cough and cold medicines for use in children. Expert Opin Drug Saf. 2010;9(2):233–242. doi: 10.1517/14740330903496410. [DOI] [PubMed] [Google Scholar]
- 89.Dart RC, Paul IM, Bond GR, et al. Pediatric fatalities associated with over the counter (nonprescription) cough and cold medications. Ann Emerg Med. 2009;53(4):411–417. doi: 10.1016/j.annemergmed.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 90.Baumkötter R, Yilmaz S, Zahn D, et al. Protective behavior and SARS-CoV-2 infection risk in the population - Results from the Gutenberg COVID-19 study. BMC Public Health. 2022;22(1):1993. doi: 10.1186/s12889-022-14310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schoberer D, Osmancevic S, Reiter L, Thonhofer N, Hoedl M. Rapid review and meta-analysis of the effectiveness of personal protective equipment for healthcare workers during the COVID-19 pandemic. Public Health Pract. 2022;4:100280. doi: 10.1016/j.puhip.2022.100280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lichtenwald I. Why hasn’t a more holistic approach to patient care become the norm? 2020. [Accessed March 8, 2023]. https://hitconsultant.net/2020/09/30/holistic-healthcare-cost/
- 93.Smith AP. The psychology of the common cold and Influenza: implications for COVID-19. Int J Clin Virol. 2020;4:27–31. doi: 10.29328/journal.ijcv.1001011. [DOI] [Google Scholar]
- 94.Taylor S. The psychology of pandemics. Annu Rev Clin Psychol. 2022;18:581–609. doi: 10.1146/annurev-clinpsy-072720-020131. [DOI] [PubMed] [Google Scholar]
- 95.Taylor S, Landry CA, Paluszek MM, Fergus TA, McKay D, Asmundson GJG. COVID stress syndrome: concept, structure, and correlates. Depress Anxiety. 2020;37(8):706–714. doi: 10.1002/da.23071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iacobucci G. Covid lockdown: England sees fewer cases of colds, flu, and bronchitis. BMJ. 2020;370:m3182. doi: 10.1136/bmj.m3182. [DOI] [PubMed] [Google Scholar]
- 97.Soo RJJ, Chiew CJ, Ma S, Pung R, Lee V. Decreased Influenza incidence under COVID-19 control measures, Singapore. Emerg Infect Dis. 2020;26(8):1933–1935. doi: 10.3201/eid2608.201229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang H, Zheng Y, de Jonge MI, et al. Lockdown measures during the COVID-19 pandemic strongly impacted the circulation of respiratory pathogens in Southern China. Sci Rep. 2022;12(1):16926. doi: 10.1038/s41598-022-21430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cork T, White S. Exploring community pharmacists’ use of health literacy interventions in their everyday practice. Res Social Adm Pharm. 2022;18(11):3948–3952. doi: 10.1016/j.sapharm.2022.06.007. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira Alfaya FJ, Zarzuelo Romero MJ. Health literacy: a field for pharmaceutical intervention. Res Social Adm Pharm. 2022;18(11):3867–3869. doi: 10.1016/j.sapharm.2022.06.011. [DOI] [PubMed] [Google Scholar]
- 101.Costa S, Romão M, Mendes M, et al. Pharmacy interventions on COVID-19 in Europe: mapping current practices and a scoping review. Res Social Adm Pharm. 2022;18(8):3338–3349. doi: 10.1016/j.sapharm.2021.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ros JM, Recio AO, Gil SP, Arambarri MJ, Eizaguirre JM, Carretero MJ. Primary care consultations: are they all by the doctor? Aten Primaria. 2011;43(10):516–522. doi: 10.1016/j.aprim.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Butler CC, Hood K, Verheij T, et al. Variation in antibiotic prescribing and its impact on recovery in patients with acute cough in primary care: prospective study in 13 countries. BMJ. 2009;338:b2242. doi: 10.1136/bmj.b2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Havers FP, Hicks LA, Chung JR, et al. Outpatient antibiotic prescribing for acute respiratory infections during Influenza seasons. JAMA Netw Open. 2018;1(2):e180243. doi: 10.1001/jamanetworkopen.2018.0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Llor C, Moragas A, Bayona C, et al. Efficacy of anti-inflammatory or antibiotic treatment in patients with non-complicated acute bronchitis and discoloured sputum: randomised placebo controlled trial. BMJ. 2013;347:f5762. doi: 10.1136/bmj.f5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jung N. Klug entscheiden: ... in der Infektiologie. Deutsches Ärzteblatt. 2016;113(13):A-608/B-514/C-510. [Google Scholar]
- 107.Choosing Wisely®. Promoting conversations between patients and clinicians. [Accessed March 8, 2023]. https://www.choosingwisely.org .
- 108.WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. 2023. [Accessed February 23, 2023]. https://www.whocc.no/atc_ddd_index .
- 109.Morice A, Kardos P. Comprehensive evidence-based review on European antitussives. BMJ Open Respir Res. 2016;3(1):e000137. doi: 10.1136/bmjresp-2016-000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Murgia V, Ciprandi G, Votto M, De Filippo M, Tosca MA, Marseglia GL. Natural remedies for acute post-viral cough in children. Allergol Immunopathol. 2021;49(3):173–184. doi: 10.15586/aei.v49i3.71. [DOI] [PubMed] [Google Scholar]
- 111.Cohen HA, Hoshen M, Gur S, Bahir A, Laks Y, Blau H. Efficacy and tolerability of a polysaccharide-resin-honey based cough syrup as compared to carbocysteine syrup for children with colds: a randomised, single-blinded, multicenter study. World J Pediatr. 2017;13(1):27–33. doi: 10.1007/s12519-016-0048-4. [DOI] [PubMed] [Google Scholar]
- 112.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161(12):1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 113.Santhi N, Ramsey DL, Philipson G, Hull D, Revell VL, Dijk D-J. Efficacy of a topical aromatic rub (Vicks VapoRub®) on effects on self-reported and actigraphically assessed aspects of sleep in common cold patients. Open J Respir Dis. 2017;7:83–101. doi: 10.4236/ojrd.2017.72009. [DOI] [Google Scholar]
- 114.Nieber K, Raskopf E, Möller J, et al. Pharmaco-epidemiological research on herbal medicinal products in the paediatric population: data from the PhytoVIS study. Eur J Pediatr. 2020;179(3):507–512. doi: 10.1007/s00431-019-03532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Paul IM, Beiler JS, King TS, Clapp ER, Vallati J, Berlin CM., Jr Vapor rub, petrolatum, and no treatment for children with nocturnal cough and cold symptoms. Pediatrics. 2010;126(6):1092–1099. doi: 10.1542/peds.2010-1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patel VS, Seidman MD. Natural alternatives and the common cold and Influenza. Otolaryngol Clin North Am. 2022;55(5):1035–1044. doi: 10.1016/j.otc.2022.06.009. [DOI] [PubMed] [Google Scholar]
- 117.Clarke J, Flott K, Fernandez Crespo R, et al. Assessing the safety of home oximetry for COVID-19: a multisite retrospective observational study. BMJ Open. 2021;11(9):e049235. doi: 10.1136/bmjopen-2021-049235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.NHS. Using a pulse oximeter to check you are OK. [Accessed November 30, 2022]. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2022/02/pulse-oximeter-easy-read-2022-digital.pdf .
- 119.Liu DX, Liang JQ, Fung TS. Human Coronavirus-229E, -OC43, -NL63, and -HKU1 (Coronaviridae) In: Bamford DH, Zuckerman MA, editors. Encyclopedia of Virology. Cambridge, MA: Academic Press; 2021. pp. 428–440. [Google Scholar]
- 120.Eccles R. Understanding the symptoms of the common cold and influenza. Lancet Infect Dis. 2005;5(11):718–725. doi: 10.1016/s1473-3099(05)70270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]