Abstract
Glycine is a non-essential amino acid with many functions and effects. Glycine can bind to specific receptors and transporters that are expressed in many types of cells throughout an organism to exert its effects. There have been many studies focused on the anti-inflammatory effects of glycine, including its abilities to decrease pro-inflammatory cytokines and the concentration of free fatty acids, to improve the insulin response, and to mediate other changes. However, the mechanism through which glycine acts is not clear. In this review, we emphasize that glycine exerts its anti-inflammatory effects throughout the modulation of the expression of nuclear factor kappa B (NF-κB) in many cells. Although glycine is a non-essential amino acid, we highlight how dietary glycine supplementation is important in avoiding the development of chronic inflammation.
Keywords: glycine, targets, inflammation, immunomodulator
1. Introduction
Glycine, also known as amino acetic acid, is an important component of many proteins and plays a crucial role in the synthesis of many biomolecules, including creatine and purine nucleotides [1]. Glycine was first isolated in 1820 from the acid hydrolysis of gelatine. Its name is derived from the Greek word glykys, meaning sweet, and it is the smallest amino acid (with a molecular weight of 75.067 g/mol). It is located in both the hydrophilic and hydrophobic parts of the polypeptide chain [2,3]. It is abundant in plasma and represents 11.5% of the total amino acids and 20% of the nitrogen in body proteins and accounts for 80% of protein [4,5]. The necessary dietary intake of glycine is ~1.5–3 g/day [6] given that in young men, glycine flux is 34–35 mg/kg/h on average in the fed state; during the post-absorptive state, the glycine flux is decreased by half (around 18 mg/kg/h) [7,8]. Around 35% of glycine in the body comes from endogenous synthesis [9], and the average rate of whole-body de novo glycine synthesis is estimated at 12–15 mg/kg/h, contributing to 81% of the systemic flux [2,7,10]. The physiological glycine plasma concentration ranges from 200 to 300 mol/L [11].
Glycine is synthesized endogenously by the body from serine, choline, threonine, and glyoxylate [12,13]; hence, it has been classified as an unessential amino acid for mammals [4] that has many activities in different systems (Figure 1). Glycine acts as a neurotransmitter and modulates neuronal activity [14]; its main activity is related to the inhibition of different brain regions. For example, in the central nervous system (CNS), glycine binds to chloride-sensitive ion channels to inhibit postsynaptic neurons [15]. It plays an important role in the mechanism of pain transmission: pharmacological treatment or genetic deletion that inhibits glycinergic signaling is sufficient to evoke pain hypersensitivity in living organisms [16]. Glycine has also been associated with the control of motor functions due to its ability to ameliorate motor deficiencies after surgery [17]. Glycine also plays an important role in the regulation of gene expression [18], protein configuration and activity, and several other biological functions [19]. There are other beneficial activities in which glycine is involved: as antacid, modulator of growing throughout the regulation of growth hormone (GH) synthesis; improves muscle tone, collagen synthesis, tissue restore (scar formation) and delaying muscular degeneration [20], in addition, it has been reported that glycine also protects the intestine against the harmful effects of radiotherapy in cancer treatment [21].
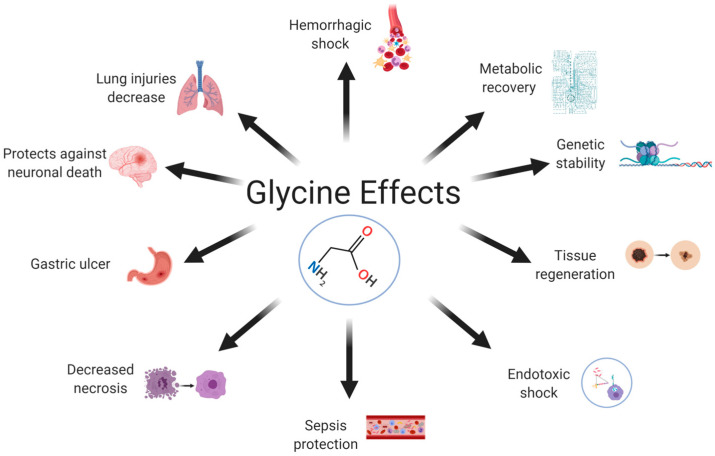
Figure 1.
Glycine effects. Glycine is an aminoacid synthetized endogenous and there has been describe many activities in which it participates that include a variety of systems. Glycine has a protective effect in lung, brain, stomach, and intestine; participates in metabolic process; modulate process of the immune system such as tissue regeneration, decrease necrosis, sepsis protection; and finally, glycine is considerate as a genic expression modulator.
Glycine plays a role in diabetes. It is a secretagogue of glucagon-like peptide-1 (GLP-1) [22], insulin, and glucagon [23] because it has been shown that the effect of ingested glycine on the postprandial glucose concentration facilitates the secretion of insulin by other amino acids [24].
Decreased glycine receptor (GlyR) expression in cells from people with type 2 diabetes mellitus (T2DM) is associated with a disruption of glycine-induced insulin secretion [25]. Clinical studies have shown that higher circulating glycine concentrations help lower the risk of developing T2DM [26].
The objective of this review is to integrate information from basic and clinical studies regarding the role of glycine as a therapeutic agent to regulate the low-grade inflammation associated with disease. We also integrated the possible mechanism throughout glycine could act as a ligand and it has an effect by activation of different pathways related to inflammation process in a variety of cells that belong to different systems.
2. Glycine Targets
According to the International Union of Basic and Clinical Pharmacology (IUPHAR), glycine has the following natural/endogenous targets: GlyRs (with α1, α2, α3, α4, and β subunits), a co-agonist of ionotropic glutamate receptors (GluN1, GluN2A, GluN2B, GluN2C, and GluN2D), G protein-coupled receptor family C group 6 (GPRC6), and transporters, which move this compound across lipid membranes. The transporters include glycine transporter type 1 and 2 (GlyT1 and GlyT2, respectively), proton-coupled amino acid transporter 1, vesicular inhibitory amino acid transporter, proton-coupled amino acid transporter 2, neutral amino acid transport (B0AT1, B0AT2, B0AT3, and NTT4), sodium-coupled neutral amino acid transporter 1, sodium-coupled neutral amino acid transporter 2, sodium-coupled neutral amino acid transporter 4, and sodium-coupled neutral amino acid transporter 5 [27,28,29,30].
3. Receptors
3.1. GlyRs
GlyRs are ligand-activated pentameric ion channels that belong to the Cys-loop family of transmitter-activated ion channels (zinc-activated channels). This family also include γ-aminobutyric acid receptor type A (GABAA); nicotinic acetylcholine receptors; N-methyl-D-aspartate (NMDA) receptors, which are ionotropic glutamate receptors (iGluRs); and serotonin receptor 5-hydroxytryptamine type 3 (5HT3R) [15,31,32]. GlyRs are abundantly expressed throughout the CNS: there are postsynaptic, presynaptic [15], and extrasynaptic GlyRs [33]. There are four known GlyR α subunits (α1–α4) and a single β subunit in vertebrates [34]. Normally, the β subunit is part of a heteromultimeric complex with GlyR α subunits [35].
GlyRs are expressed as homopentamers of five α subunits or as heteropentamers of three α and two β subunits or two α subunits and three β subunits. The receptor is an intrinsic anion channel [34,36,37,38]. As mentioned above, there are four α subunits, namely, α1, α2, α3, and α4, and a single gene coding for the β subunit [39,40]. The four α subunits generally share >90% amino acid sequence homology with each other, but their genes are expressed in specific zones and are developmentally regulated. The α2 subunit is highly expressed in all layers of the cerebral cortex, brain stem, thalamus, spinal cord, hippocampus, diencephalon, and cerebellum during embryonic development [41,42]. There is a change from α2 homomeric GlyRs to α1β heteromeric GlyRs during development [15]. In neonatal period, levels of α1 and α3 expression increase. α1 is prominently expressed in the hypothalamus, colliculi, the spinal cord, and brain stem cerebellar deep nuclei [43,44]. α3 has a relatively lower level of expression than α1 at all developmental stages [45,46]. α4 is an embryonic GlyR subunit isoform [40] but is presumed to be a pseudogene in humans due to a premature stop codon upstream of the final TM4 domain. The β subunit gene is transcribed at all developmental stages and is widely and abundantly distributed in the spinal cord and brain [47,48]. Its distribution is broader than that of α1. The β subunit is indispensable for synaptic clustering [49].
When glycine binds to GlyRs, the channel opens (it is formed by the domine 2 of each subunit of the GlyR) and generates a short-term flux of negative ions into the cell. In this way, the intracellular chlorine concentration increases temporarily, leading to hyperpolarization of the membrane, which prevents the cell from being easily excited (Figure 2). The net effect is inhibitory [50,51]. GlyRs are involved in several processes, including the central regulation of orexigenic signals in obesity [52].
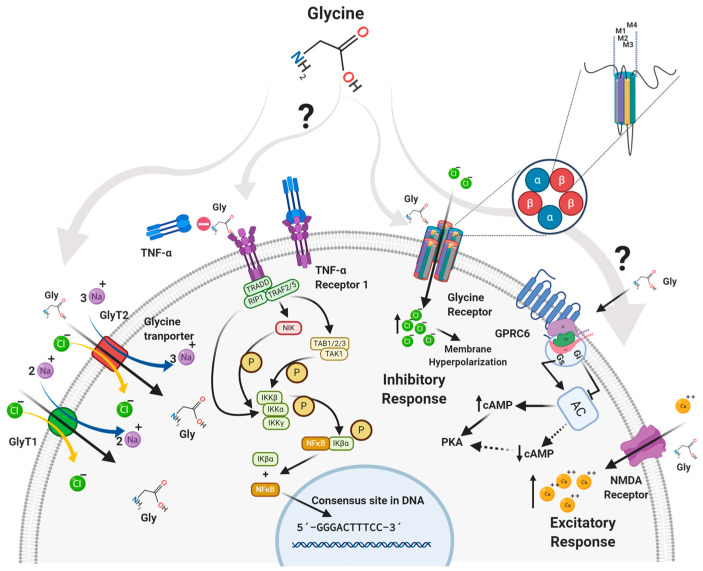
Figure 2.
Glycine targets and pathways. According to the International Union of Basic & Clinical Pharmacology (IUPHAR) glycine has different targets such as Natural/Endogenous Targets: glycine receptor (consisting of glycine receptor α1, α2, α3, α4 and β subunits), ionotropic glutamate receptors co-agonist (GluN1, GluN2A, GluN2B, GluN2C and GluN2D) and GPRC6 Receptor. Transporters moving this compound across a lipid membrane with proton-coupled amino acid transporter 1, vesicular inhibitory amino acid transporter, Proton-coupled Amino acid Transporter 2, GlyT1, GlyT2, B0AT1, B0AT2, B0AT3, NTT4, sodium-coupled neutral amino acid transporter 1, sodium-coupled neutral amino acid transporter 2, sodium-coupled neutral amino acid transporter 4, Sodium-coupled neutral amino acid transporter 5 [27,28,29,30]. These may be the targets involved in the signaling by which glycine exerts its effect on the different cell lines of living organisms.
3.2. NMDA Receptor
The N-methyl-D-aspartate (NMDA) receptors are iGluRs. They share common characteristics with the other two glutamate receptors, namely, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and 2-carboxy-3-carboxymethyl-4-isopropenylpyrrolidine (kainate) receptors. NMDA receptors are heterotetramers composed of two GluN1 subunits and two GluN2 and/or GluN3 subunits [53,54]. Currently, eight splice variants of the GluN1 gene, four GluN2 genes (GluN2A–GluN2AD), and two GluN3 genes (GluN3A and GluN3B) have been identified [54]. NMDA receptors containing the NR2A, NR2B, and NR2D. These subunits are commonly expressed on spinal dorsal horn neurons. In contrast, there are lower levels of expression of NMDA receptors containing the NR2C subunit in this site [55]. These receptors require the concomitant binding of glutamate and glycine or D-serine as a co-agonist [56]. Glycine has an excitatory effect on these receptors [57] (Figure 2).
3.3. GPRC6 Receptor
GPRC6 is a 362 amino acid [58] orphan receptor coupled to a stimulatory Gα subunit (Gαs). It has been associated with the cannabinoid family, class A (similar to rhodopsin) [58,59,60], which phylogenetically belongs to the melanocortin/endothelial differentia-tion/cannabinoid/adenosine (MECA) [61]. It has been shown in oocytes that GPR6 can be activated by glycine, this being a small neutral aliphatic L-α-amino acid that could act as an endogenous agonist in concentrations of 100 µM [62]. GPR6 was previously known as the sphingosine 1-phosphate receptor [63], with sphingosylphosphorylcholine (SPC) considered to be an endogenous ligand [64]. However, it is still considered an orphan receptor [65]. This receptor has a high constitutive activation of adenylyl cyclase—leading to the production of cyclic adenosine monophosphate (cAMP)—and competes with other Gαs-coupled receptors for their corresponding agonists [59,63,66] (Figure 2). In humans, GPRC6 is highly expressed in the hypothalamus [67], leucocytes, skeletal muscles, and testes. In adults, it has medium levels of expression in the heart, kidney, liver, and spleen, and low levels of expression in the pancreas, placenta, ovary, and lung [68]. In previous studies, we found that in adipocytes, glycine has an effect on the expression of GPRC6 [69], and even this receptor has been related to neuroprotective activity [70]; however, there is not much information about the relationship of that receptor with inflammation.
4. Glycine Transporters
There are several known families of glycine transporters, including solute-carrying transporter 36 (SLC36; also known as PATs), SLC38, and SLC6 (also known as GlyTs). Of these families, SLC6 is the only one specific to glycine. The SLC36 family members are expressed primarily in the intestine; the SLC38 family members are widely distributed throughout the body; and the SLC6 family members are expressed primarily in the intestine, kidney, and nervous system [71]. There are two known genes belonging to the SLC6 family, namely, GlyT1 and GlyT2. There are five variants of GlyT1 (a, b, c, d, and e) that differ at their N and C termini due to the use of alternative promoters and splicing, and there are three GlyT2 splice variants (a, b, and c) [37,72,73,74]. Both transporters use the energy stored in the transmembrane Na+ and Cl− concentration gradients to transport glycine against a concentration gradient [75]. The specific stoichiometry is 2 Na+/1 Cl−/1 Gly for GlyT1 (SCL6A9) and 3 Na+/1 Cl−/1 Gly for GlyT2 [76]. These carriers have different mechanisms through which glycine is transported depending on the membrane voltage [77].
4.1. GlyT1
For GlyT1, most Na+ moves when glycine binds to the transporter, while for GlyT2, most Na+ charges move after glycine has dissociated into the cytosol (Figure 2) [77]. GlyT1 is expressed mainly in astrocytes, regulating the glycine concentration in the vicinity of NMDA receptors [78], and oocytes [79]. It is also found in the human intestine and is responsible for 30–50% of glycine uptake in intestinal epithelial cells, maintaining the glycine supply in enterocytes and colonocytes [80]. The kinetics of this transporter are characterized by the transition of the transporter attached to the substrate from the outside to the inside [77].
4.2. GlyT2
GlyT2 is located at the axon terminals of glycinergic neurons, mainly in the spinal cord and brain stem. These transporters facilitate glycine reuptake in presynaptic terminals [81]. The GlyT2-dependent influx of glycine allows for the maintenance of vesicular glycine reserves [82]. GlyT2 kinetics are governed by Na+ binding, which causes a conformational change [77].
5. Effects of Glycine in the Organism
5.1. Neurotransmission
Glycine participates in the rapid excitatory neurotransmission mediated by NMDA receptors. Indeed, the full activation of these receptors requires the binding of both glutamate and glycine [83,84]. This activity is necessary for plasticity processes such as learning, memory, and cognition [85,86]. These receptors are therapeutic targets in counteracting pathologies such as Alzheimer’s disease, Huntington’s disease, amyotrophic lateral sclerosis, schizophrenia, and other psychiatric diseases [87]. Glycine also modulates neurotransmission via GlyT1 and GlyT2 [88,89,90]. Mice lacking GlyT1 show severe respiratory and motor deficiencies due to hyperactive glycinergic signaling [91,92]. On the other hand, the participation of GPR6 due to glycine stimulation could decrease cAMP in striatal tissues and increase dopamine (improving movement) [93], showing a possible potential target for the treatment of Parkinson’s disease [94].
Glycine shows neuroprotective effects on neurons and microglia after ischemic stroke injury. It can inhibit nuclear factor kappa B (NF-κB) p65 and hypoxia-inducible factor 1α (Hif-1α) by activating AKT and downregulating phosphatase and tensin homologue (PTEN). This mechanism suppresses ischemia-induced M1 microglia polarization and inhibits inflammation [95]. Glycine confers protection against neuronal death in in vitro and in vivo experimental conditions [96], and clinical studies have shown that glycine can improve the prognosis of patients after ischemic stroke [97].
5.2. Antioxidant
Glycine effectively protects against alcohol-induced hepatotoxicity by reducing blood alcohol levels and the metabolic products of alcohol, reducing liver damage, and lowering the gastric emptying rate of ethanol [98]. Kupffer cells increase Ca2+ and release prostanoids and inflammatory cytokines in response to LPS stimuli, glycine acts by preventing increases in intracellular levels of Ca2+ in this kind of cell in concentrations comparable to serum levels in glycine-fed animals [99]. Furthermore, this amino acid plays a protective role against the toxic effects of oxidized oil since it has been observed that the co-administration of oxidized oils with glycine cause the recovery of liver structure and function, demonstrating glycine to be a beneficial amino acid that is protective against food toxicity [100].
5.3. Dietary Supplementation
In animal models, dietary supplementation with glycine reduces inflammation, morbidity, and mortality from pathogenic infections [101]. Betaine supplementation is associated with circulating levels of dimethylglycine. Betaine metabolism may be hampered by the activity of dimethylglycine dehydrogenase (DMGDH), which connects betaine to glycine synthesis via the demethylation of dimethylglycine into sarcosine [102]. Glycine supplementation has shown beneficial cardiac effects in a burn model [103] and in a skeletal muscle ischemia model, with decreased reperfusion-mediated necrosis and increased metabolic and functional recovery [104]. The protective effects of glycine have been reported for several sepsis models [105,106] and for hemorrhagic shock [107]. Normal levels of glycine in the body prevent lipopolysaccharide (LPS)-induced endotoxemia by binding to GlyRs on Kupffer cells [108]. Dietary supplementation with glycine decreases adipocyte size, adiposity, and the concentrations of free fatty acids and triglycerides in animal models [109,110]. It also inhibits the nonenzymatic glycation of proteins and hemoglobin in diabetic rats [111] and patients with T2DM [112,113].
5.4. Immunomodulation
Glycine exerts anti-inflammatory and immunomodulatory effects in several cell types [114,115]. However, the underlying mechanisms responsible for these beneficial effects remain unknown. There are some hypotheses about the actions of glycine in living organisms, and accumulating evidence suggests that glycine protects cells from oxidative stress-induced inflammation [116,117]. In a mouse model of cancer cachexia, glycine attenuated oxidative and inflammatory environments [118]. In a model of acute pancreatitis, glycine reduced the severity of pancreatic damage [119]. Moreover, glycine suppressed zymosan-induced joint inflammation [120]. Some studies have shown that supplementation with glycine can reduce acute and systemic allergic responses. Although the exact mechanism of action is still unknown, glycine may exert an effect on key effector cells, such as basophils and mast cells [117].
Glycine inhibits inflammatory responses and modulates the production of cytokines by many innate cells, including monocytes and intestinal and alveolar macrophages [121,122,123]. The best-studied glycine signaling pathway involves GlyRs, but glycine may also utilize GlyR-independent pathways to mediate its cytoprotective function, indicating that the presence of GlyR subunits is not a requirement for the anti-inflammatory effect of glycine [124].
Glycine is a potent therapeutic immunonutrient for various kinds of chronic liver conditions, including alcoholic liver disease [125]. Various insults in the CNS, such as ischemia, can promote neuroinflammation, with the polarization of microglia, the resident macrophages [126,127]. Treatment with glycine can attenuate neuroinflammation, suppressing M1 microglia polarization, the pro-inflammatory state [128], and promoting M2 polarization, the primary anti-inflammatory state [129]. Liu et al., 2019 [95], reported this benefit in an animal model of ischemic stroke: glycine antagonized ischemia-induced M1 polarization and promoted M2 polarization [95].
Glycine mitigates the adverse effects of LPS, peptidoglycans, and peroxisome proliferators. This amino acid modulates the secretion of cytokines and the activation of proteases and increased apoptosis in various animal models [130,131,132].
5.5. Low Glycine Plasma Levels Are Associated with Low-Grade Inflammation
Hyperglycemia or hyperinsulinemia reduce the average rate of glycine synthesis [7]. Based on previous studies, a low plasma glycine concentration is associated with hepatic insulin resistance, obesity, and T2DM [133]. This decrease is due to a reduction in glycine availability via the simultaneous participation of three distinct mechanisms: (a) decreased gut absorption, (b) decreased biosynthesis, and (c) increased catabolism or urine excretion [3].
Obesity and metabolic disorders show elevated plasma glucagon concentrations that reduce circulating glycine and increase its degradation [134,135]. On the other hand, an impaired hepatic branched-chain amino acid metabolism in obesity decreases circulating glycine concentrations [136]. Glycine supplementation (5 g/day or 0.1 g of glycine/kg/day for 14 days in association with N-acetylcysteine) improves the insulin response and glucose tolerance in patients with obesity [23,137].
6. The Role of Glycine in Inflammation
A glycine concentration of 1–3 mM triggers the opening of the GlyR ion channel [138], resulting in rapid hyperpolarization, a decrease in calcium, and a reduction in the synthesis of pro-inflammatory mediators, probably via the tumor necrosis factor α (TNF-α) receptor signaling pathway [51]. The GlyR-dependent pathway can modulate chronic and neuropathic pain [35,139,140] and the anti-inflammatory response (Figure 3) [114].
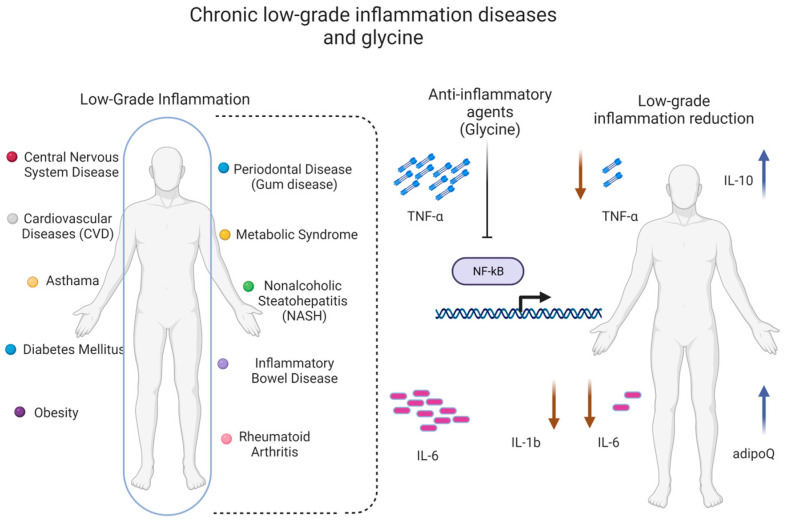
Figure 3.
Chronic low-grade inflammation diseases and glycine. There are diseases that go through a low-grade inflammatory process and maintains decreased glycine concentrations in the body. Glycine, despite not being an essential amino acid, requires its consumption to maintain adequate concentrations in the body and to carry out its activity in the different organs. Glycine decreases the expression of proinflammatory cytokines through the inhibition of NF-kB and favors the expression of anti-inflammatory cytokines, thus reducing the feedback of the chronic inflammatory process that occurs in some diseases.
GlyRs are located on different cell types involved in immune responses, such as macrophages, monocytes, neutrophils, T lymphocytes [114,121,122], hepatic and alveolar macrophages [108], the pancreas, and Kupffer cells [135,141]. In macrophages, T lymphocytes, and neutrophils, GlyR activation suppresses the production of pro-inflammatory cytokines, thus supporting anti-inflammatory properties [121]. Glycine could modulate the low-grade inflammatory process through pathways that involve some of its targets that have already been identified in different cells.
Glycine inhibits the cytokine synthesis stimulated by LPS, reduces serum transaminase levels, and decreases intracellular calcium concentrations by modulating chloride influx inside the cell [125,142]. In addition, there is evidence that glycine administration reduces the infiltration of inflammatory cells and attenuates the elevated concentrations of inflammatory cytokines and chemokines in the hepatic cells of mice via the inhibition of NF-κB [13]. In addition, glycine can upregulate adiponectin expression [143]. Glycine pre-treatment inhibits the inflammation of alveolar cells in LPS-induced lung injury [13,144]. Glycine bioactivity is anti-inflammatory in the lung and other tissues [12]. There is an inverse relationship between glycine plasma levels and systemic inflammation [135,145], and this effect is similar in obesity and diabetes [146].
Many researchers have attempted to describe the mechanism through which glycine exerts its beneficial effects. For decades, glycine has been proposed as an anti-inflammatory agent [114,121] and used as a therapeutic nutrient to treat inflammation related to diseases such as arthritis [132], gastric ulcers [147], melanoma [148], alcoholic liver disease [125], and endotoxic shock [107]. Glycine supplementation inhibits the expression of NF-κB through the inactivation of IκB, an upstream regulator of NF-κB signaling, as well as the production of its downstream pro-inflammatory cytokines and chemokines [13,149]. Glycine blocks the activation of NF-κB; hence, it promotes the downregulation of pro-inflammatory adipokines [107,150].
Glycine inhibits the production of pro-inflammatory cytokines like TNF-α, interleukin- 6 (IL-6), and IL-1β and increases the production of the anti-inflammatory cytokine IL-10 in activated macrophages, leucocytes [131], and T lymphocytes in a pathological state [151]. During endothelial inflammation, glycine can exert anti-inflammatory effects via the inhibition of the activation of NF-κB, degrading IκBα and increasing the expression of E-selectin and the production of IL-6 [152]. Glycine cytoprotection suppresses inflammation by preventing the immunogenic effects of necrosis and directly inhibiting pyroptosis. Moreover, efforts to understand the basis for glycine cytoprotection have led to the discovery of novel upstream immunomodulatory effects of glycine to block the primary activation of multiple types of inflammatory cells. This effect involves receptors that are different from those involved in glycine cytoprotection [124].
Glycine treatment attenuates the production of pro-inflammatory and Th2-skewing cytokines such as IL-13, TNF-α, and IL-4 in the RBL-2H3 basophilic cell line. The glycine effect on the cytokine response following IgE-mediated crosslinking on RBL-cells may be comparable to effects on mast cells [117]. Some studies have shown that the glycine-induced downregulation of NF-κB is mediated, at least partially, by NRF2 signaling [13,95]. The anti-inflammatory properties of glycine have been shown in animal models of septic shock [153]. Moreover, there is evidence that glycine treatment increases adiponectin and IL-10 messenger RNA (mRNA) expression [154].
Dietary supplementation with glycine has been proposed as a potential way to treat conditions with low-grade inflammation, such as obesity [155,156,157]. This approach has been proposed due to its ability to increase the mRNA expression of anti-inflammatory cytokines, such as adiponectin and IL-10 [123,154,158], and because glycine can inhibit the production of pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 [131,158]. In one study, 3 months of glycine treatment attenuated low-grade inflammation by decreasing pro-inflammatory cytokines [143]. Glycine decreases endotoxin-induced TNF-α production by Kupffer cells and alveolar macrophages and also reduces IL-1β and TNF-α expression while simultaneously stimulating IL-10 expression in LPS-activated monocytes [131]. It also interferes with TNF-α-mediated NF-κB activation in human coronary artery endothelial cells and differentiated 3T3-L1 adipocytes [152,156]. In animal models, supplementation with 0.5% glycine has been used to prevent the infiltration of inflammatory cells; to treat synovial hyperplasia in joints, edema, experimental arthritis, and lung inflammation [132,141]; and to inhibit tumor growth [130,148,159].
The pathophysiological mechanisms underlying glycine deficit and its potential clinical repercussions are not yet clear yet. It is important to mention that although glycine is a non-essential amino acid because it can be synthesized endogenously, glycine supplementation helps to maintain homeostasis (Figure 4).
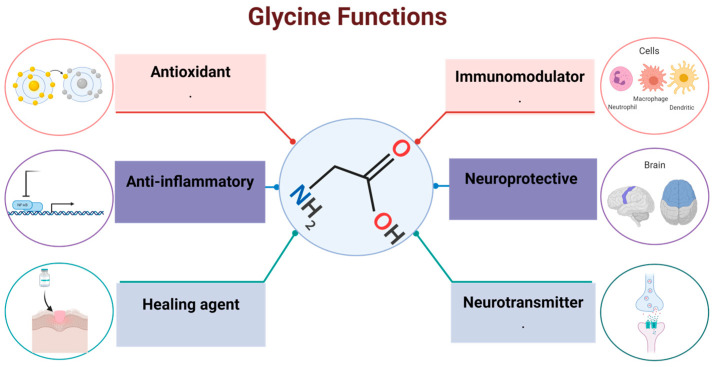
Figure 4.
Glycine functions. Glycine function has been shown in different organs and tissues, from being a protein precursor to adjudicating functions such as antioxidant, immunomodulator, anti-inflammatory, healing agent, as well as, neuroprotective and neurotransmitter in central nervous system.
It this review, we described the importance of glycine as an anti-inflammatory amino acid that not only has beneficial cosmetic effects but is also an alternative medicine for promoting homeostasis in an organism. It has been demonstrated that glycine can modulate the inflammatory response, using different targets in many cells in the entire organism. There are many studies that describe the indirect effects of glycine as a modulator of responses and mechanisms in the organism via dietary supplementation with glycine in animal models and in clinical trials. Another option is including the amino acid as a component of diet using animal models or in clinical trials, finally revealing the indirect role of glycine in reducing the inflammation process and decreasing some symptoms related to low-grade inflammation. However, there are few studies that have evaluated and followed changes due to glycine consumption in the diet over a long period of time, and those that have evaluated how glycine can decrease the risk or development of some diseases related to inflammation process or even the direct effects of glycine on cell activity and the response of cells to specific signals similar to an inflammatory environment.
Acknowledgments
This research was supported by the SIP-IPN-20230866 (Sección de Estudios de Posgrado e Investigación del Instituto Politécnico Nacional).
Author Contributions
Conceptualization, K.A.A.-C. and L.N.A.-V.; methodology, R.A.G.-R. and A.V.F.-Z.; formal analysis, R.R.-N. and F.S.-M.; writing—original draft preparation, K.A.A.-C. and F.H.; writing—review and editing, R.R.-N. and A.G.-M.; funding acquisition, S.V. and R.R.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data Availability Statements are available in section “MDPI Research Data Policies” at https://www.mdpi.com/ethics.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Sección de Estudios de Posgrado e Investigación del Instituto Politécnico Nacional [SIP-IPN-20230866]. CONAHCYT Posdoctoral grant number [2340211] And The APC was funded by the authors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tibbetts A.S., Appling D.R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010;30:57–81. doi: 10.1146/annurev.nutr.012809.104810. [DOI] [PubMed] [Google Scholar]
- 2.Jackson A.A., Badaloo A.V., Forrester T., Hibbert J.M., Persaud C. Urinary excretion of 5-oxoproline (pyroglutamic aciduria) as an index of glycine insufficiency in normal man. Br. J. Nutr. 1987;58:207–214. doi: 10.1079/BJN19870088. [DOI] [PubMed] [Google Scholar]
- 3.Alves A., Bassot A., Bulteau A.L., Pirola L., Morio B. Glycine metabolism and its alterations in obesity and metabolic diseases. Nutrients. 2019;11:1356. doi: 10.3390/nu11061356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu G. Functional amino acids in growth, reproduction, and health. Adv. Nutr. 2010;1:31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Wu Z., Dai Z., Yang Y., Wang J., Wu G. Glycine metabolism in animals and humans: Implications for nutrition and health. Amino Acids. 2013;45:463–477. doi: 10.1007/s00726-013-1493-1. [DOI] [PubMed] [Google Scholar]
- 6.Schmidt J.A., Rinaldi S., Scalbert A., Ferrari P., Achaintre D., Gunter M.J., Appleby P.N., Key T.J., Travis R.C. Plasma concentrations and intakes of amino acids in male meat-eaters, fish-eaters, vegetarians and vegans: A cross-sectional analysis in the EPIC-Oxford cohort. Eur. J. Clin. Nutr. 2016;70:306–312. doi: 10.1038/ejcn.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robert J.J., Bier D.M., Zhao X.H., Matthews D.E., Young V.R. Glucose and insulin effects on de novo amino acid synthesis in young men: Studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982;31:1210–1218. doi: 10.1016/0026-0495(82)90006-3. [DOI] [PubMed] [Google Scholar]
- 8.Lamers Y., Williamson J., Gilbert L.R., Stacpoole P.W., Gregory J.F., III Glycine turnover and decarboxylation rate quantified in healthy men and women using primed, constant infusions of [1, 2-13C2] glycine and [2H3] leucine. J. Nutr. 2007;137:2647–2652. doi: 10.1093/jn/137.12.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Y.M., Yang R.D., Matthews D.E., Wen Z.M., Burke J.F., Bier D.M., Young V.R. Quantitative aspects of glycine and alanine nitrogen metabolism in postabsorptive young men: Effects of level of nitrogen and dispensable amino acid intake. J. Nutr. 1985;115:399–410. doi: 10.1093/jn/115.3.399. [DOI] [PubMed] [Google Scholar]
- 10.Meléndez-Hevia E., de Paz-Lugo P., Cornish-Bowden A., Cárdenas M.L. A weak link in metabolism: The metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis. J. Biosci. 2009;34:853–872. doi: 10.1007/s12038-009-0100-9. [DOI] [PubMed] [Google Scholar]
- 11.Gaggini M., Carli F., Rosso C., Buzzigoli E., Marietti M., Della Latta V., Ciociaro D., Abate M.L., Gambino R., Cassader M., et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology. 2018;67:145–158. doi: 10.1002/hep.29465. [DOI] [PubMed] [Google Scholar]
- 12.Wang W., Wu Z., Lin G., Hu S., Wang B., Dai Z., Wu G. Glycine stimulates protein synthesis and inhibits oxidative stress in pig small intestinal epithelial cells. J. Nutr. 2014;144:1540–1548. doi: 10.3945/jn.114.194001. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Jia H., Jin Y., Liu N., Chen J., Yang Y., Dai Z., Wang C., Wu G., Wu Z. Glycine attenuates LPS-induced apoptosis and inflammatory cell infiltration in mouse liver. J. Nutr. 2020;150:1116–1125. doi: 10.1093/jn/nxaa036. [DOI] [PubMed] [Google Scholar]
- 14.Eulenburg V.S. Glycine transporters: Essential regulators of synaptic transmission. Eur. Neuropsychopharmacol. 2011;21:S230. doi: 10.1016/S0924-977X(11)70352-2. [DOI] [PubMed] [Google Scholar]
- 15.Lynch J.W. Native glycine receptor subtypes and their physiological roles. Neuropharmacology. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- 16.Foster E., Wildner H., Tudeau L., Haueter S., Ralvenius W.T., Jegen M., Johannssen H., Hösli L., Haenraets K., Ghanem A., et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez-Piña R., Nuño-Licona A. Effects of glycine on motor performance in rats after traumatic spinal cord injury. Proc. West. Pharmacol. Soc. 2007;50:131–133. [PubMed] [Google Scholar]
- 18.Luka Z., Cerone R., Phillips J.A., Mudd H.S., Wagner C. Mutations in human glycine N-methyltransferase give insights into its role in methionine metabolism. Hum. Genet. 2002;110:68–74. doi: 10.1007/s00439-001-0648-4. [DOI] [PubMed] [Google Scholar]
- 19.Martínez-Chantar M.L., Vázquez-Chantada M., Ariz U., Martínez N., Varela M., Luka Z., Capdevila A., Rodríguez J., Aransay A.M., Matthiesen R., et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology. 2008;47:1191–1199. doi: 10.1002/hep.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walrand S., Chiotelli E., Noirt F., Mwewa S., Lassel T. Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J. Agric. Food Chem. 2008;56:7790–7795. doi: 10.1021/jf800691f. [DOI] [PubMed] [Google Scholar]
- 21.Picanço E.D.A., Lopes-Paulo F., Marques R.G., Diestel C.F., Caetano C.E.R., de Souza M.V.M., Moscoso G.M., Pazos H.M.F. L-arginine and glycine supplementation in the repair of the irradiated colonic wall of rats. Int. J. Color. Dis. 2011;26:561–568. doi: 10.1007/s00384-011-1154-3. [DOI] [PubMed] [Google Scholar]
- 22.Gameiro A., Reimann F., Habib A.M., O’malley D., Williams L., Simpson A.K., Gribble F.M. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J. Physiol. 2005;569:761–772. doi: 10.1113/jphysiol.2005.098962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gonzalez-Ortiz M., Medina-Santillan R., Martinez-Abundis E., von Drateln C.R. Effect of glycine on insulin secretion and action in healthy first-degree relatives of type 2 diabetes mellitus patients. Horm. Metab. Res. 2001;33:358–360. doi: 10.1055/s-2001-15421. [DOI] [PubMed] [Google Scholar]
- 24.Gannon M.C., Nuttall J.A., Nuttall F.Q. The metabolic response to ingested glycine. Am. J. Clin. Nutr. 2002;76:1302–1307. doi: 10.1093/ajcn/76.6.1302. [DOI] [PubMed] [Google Scholar]
- 25.Yan-Do R., Duong E., Fox J.E.M., Dai X., Suzuki K., Khan S., Bautista A., Ferdaoussi M., Lyon J., Wu X., et al. A glycine-insulin autocrine feedback loop enhances insulin secretion from human β-cells and is impaired in type 2 diabetes. Diabetes. 2016;65:2311–2321. doi: 10.2337/db15-1272. [DOI] [PubMed] [Google Scholar]
- 26.Gao X., Wang Y., Sun G. High dietary choline and betaine intake is associated with low insulin resistance in the Newfoundland population. Nutrition. 2017;33:28–34. doi: 10.1016/j.nut.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Bröer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem. Int. 2006;48:559–567. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Vanslambrouck J.M., Bröer A., Thavyogarajah T., Holst J., Bailey C.G., Bröer S., Rasko J.E. Renal imino acid and glycine transport system ontogeny and involvement in developmental iminoglycinuria. Biochem. J. 2010;428:397–407. doi: 10.1042/BJ20091667. [DOI] [PubMed] [Google Scholar]
- 29.Christopoulos A., Changeux J.-P., Catterall W.A., Fabbro D., Burris T.P., Cidlowski J.A., Olsen R.W., Peters J.A., Neubig R.R., Pin J.-P., et al. International Union of Basic and Clinical Pharmacology. XC. Multisite Pharmacology: Recommendations for the Nomenclature of Receptor Allosterism and Allosteric Ligands. Pharmacol. Rev. 2014;66:918–947. doi: 10.1124/pr.114.008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thwaites D.T., Anderson C.M. The SLC36 family of proton-coupled amino acid transporters and their potential role in drug transport. Br. J. Pharmacol. 2011;164:1802–1816. doi: 10.1111/j.1476-5381.2011.01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen R.W., Sieghart W. International Union of Pharmacology. LXX. Subtypes of γ-aminobutyric acidA receptors: Classification on the basis of subunit composition, pharmacology, and function. Pharmacol. Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velázquez-Flores M.A., Salceda R. Modulación de los receptores ionotrópicos de tipo cys-loop por proteincinasas A y C. Rev. Neurol. 2011;52:81. doi: 10.33588/rn.5203.2010416. [DOI] [PubMed] [Google Scholar]
- 33.Avila A., Nguyen L., Rigo J.M. Glycine receptors and brain development. Front. Cell. Neurosci. 2013;7:184. doi: 10.3389/fncel.2013.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Z., Taran E., Webb T.I., Lynch J.W. Stoichiometry and subunit arrangement of α1β glycine receptors as determined by atomic force microscopy. Biochemistry. 2012;51:5229–5231. doi: 10.1021/bi300063m. [DOI] [PubMed] [Google Scholar]
- 35.Lynch J.W., Callister R.J. Glycine receptors: A new therapeutic target in pain pathways. Curr. Opin. Investig. Drugs. 2006;7:48–53. [PubMed] [Google Scholar]
- 36.Grudzinska J., Schemm R., Haeger S., Nicke A., Schmalzing G., Betz H., Laube B. The β subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Betz H., Laube B. Glycine receptors: Recent insights into their structural organization and functional diversity. J. Neurochem. 2006;97:1600–1610. doi: 10.1111/j.1471-4159.2006.03908.x. [DOI] [PubMed] [Google Scholar]
- 38.Durisic N., Godin A.G., Wever C.M., Heyes C.D., Lakadamyali M., Dent J.A. Stoichiometry of the human glycine receptor revealed by direct subunit counting. J. Neurosci. 2012;32:12915–12920. doi: 10.1523/JNEUROSCI.2050-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matzenbach B., Maulet Y., Sefton L., Courtier B., Avner P., Guenet J.L., Betz H. Structural analysis of mouse glycine receptor alpha subunit genes. Identification and chromosomal localization of a novel variant. J. Biol. Chem. 1994;269:2607–2612. doi: 10.1016/S0021-9258(17)41987-9. [DOI] [PubMed] [Google Scholar]
- 40.Harvey R.J., Schmieden V., Von Holst A., Laube B., Rohrer H., Betz H. Glycine receptors containing the α4 subunit in the embryonic sympathetic nervous system, spinal cord and male genital ridge. Eur. J. Neurosci. 2000;12:994–1001. doi: 10.1046/j.1460-9568.2000.00993.x. [DOI] [PubMed] [Google Scholar]
- 41.Kuhse J., Kuryatov A., Maulet Y., Malosio M., Schmieden V., Betz H. Alternative splicing generates two isoforms of the α2 subunit of the inhibitory glycine receptor. FEBS Lett. 1991;283:73–77. doi: 10.1016/0014-5793(91)80557-J. [DOI] [PubMed] [Google Scholar]
- 42.Liu Q., Wong-Riley M.T. Postnatal development of glycine receptor subunits α1, α2, α3, and β immunoreactivity in multiple brain stem respiratory-related nuclear groups of the rat. Brain Res. 2013;1538:1–16. doi: 10.1016/j.brainres.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malosio M.L., Marquèze-Pouey B., Kuhse J., Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greferath U., Brandstätter J.H., Wässle H., Kirsch J., Kuhse J., Grünert U. Differential expression of glycine receptor subunits in the retina of the rat: A study using immunohistochemistry and in situ hybridization. Vis. Neurosci. 1994;11:721–729. doi: 10.1017/S0952523800003023. [DOI] [PubMed] [Google Scholar]
- 45.Harvey R.J., Depner U.B., Wassle H., Ahmadi S., Heindl C., Reinold H., Smart T.G., Harvey K., Schutz B., Abo-Salem O.M., et al. GlyR α3: An essential target for spinal PGE2-mediated inflammatory pain sensitization. Science. 2004;304:884–887. doi: 10.1126/science.1094925. [DOI] [PubMed] [Google Scholar]
- 46.Manzke T., Niebert M., Koch U.R., Caley A., Vogelgesang S., Hülsmann S., Ponimaskin E., Müller U., Smart T.G., Harvey R.J., et al. Serotonin receptor 1A–modulated phosphorylation of glycine receptor α3 controls breathing in mice. J. Clin. Investig. 2010;120:4118–4128. doi: 10.1172/JCI43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grenningloh G., Pribilla I., Prior P., Multhaup G., Beyreuther K., Taleb O., Betz H. Cloning and expression of the 58 kd β subunit of the inhibitory glycine receptor. Neuron. 1990;4:963–970. doi: 10.1016/0896-6273(90)90149-A. [DOI] [PubMed] [Google Scholar]
- 48.Fujita M., Sato K., Sato M., Inoue T., Kozuka T., Tohyama M. Regional distribution of the cells expressing glycine receptor beta subunit mRNA in the rat brain. Brain Res. 1991;560:23–37. doi: 10.1016/0006-8993(91)91210-R. [DOI] [PubMed] [Google Scholar]
- 49.Meyer G., Kirsch J., Betz H., Langosch D. Identification of a gephyrin binding motif on the glycine receptor β subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 50.Ottersen O.P., Storm-Mathisen J., Laake J.H. Cellular and subcellular localization of glycine studied by quantitative electron microscopic immunocytochemistry. In: Ottersen O.P., Storm-Mathisen J., editors. Glycine Neurotransmission. Wiley; Chichester, UK: 1990. pp. 303–328. [Google Scholar]
- 51.Gundersen Y., Vaagenes P., Dreiem A., Fonnum F. Glysin. Tidsskr. Nor. Legeforening. 2004;124:773–775. [PubMed] [Google Scholar]
- 52.Manousopoulou A., Koutmani Y., Karaliota S., Woelk C.H., Manolakos E.S., Karalis K., Garbis S.D. Hypothalamus proteomics from mouse models with obesity and anorexia reveals therapeutic targets of appetite regulation. Nutr. Diabetes. 2016;6:e204. doi: 10.1038/nutd.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danysz W., Parsons C.G. Glycine and N-methyl-D-aspartate receptors: Physiological significance and possible therapeutic applications. Pharmacol. Rev. 1998;50:597–664. [PubMed] [Google Scholar]
- 54.Paoletti P., Bellone C., Zhou Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 55.Karlsson U., Sjödin J., Angery Möller K., Johansson S., Wiksröm L., Nasström J. Glutamate-induced currents reveal three functionally distinct NMDA receptor populations in rat dorsal horn-effects of peripheral nerve lesion and inflammation. Neuroscience. 2002;112:861–868. doi: 10.1016/S0306-4522(02)00140-9. [DOI] [PubMed] [Google Scholar]
- 56.Le Bail M., Martineau M., Sacchi S., Yatsenko N., Radzishevsky I., Conrod S., Ouares K.A., Wolosker H., Pollegioni L., Billard J.-M., et al. Identity of the NMDA receptor coagonist is synapse specific and developmentally regulated in the hippocampus. Proc. Natl. Acad. Sci. USA. 2015;112:E204–E213. doi: 10.1073/pnas.1416668112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson J.W., Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- 58.Song Z.H., Young W.S., III, Brownstein M.J., Bonner T.I. Molecular cloning of a novel candidate G protein-coupled receptor from rat brain. FEBS Lett. 1994;351:375–379. doi: 10.1016/0014-5793(94)00888-4. [DOI] [PubMed] [Google Scholar]
- 59.Eggerickx D., Denef J.F., Labbé O., Hayashi Y., Refetoff S., Vassart G., Parmentier M., Libert F. Molecular cloning of an orphan G-protein-coupled receptor that constitutively activates adenylate cyclase. Biochem. J. 1995;309:837–843. doi: 10.1042/bj3090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Isawi I.H., Morales P., Sotudeh N., Hurst D.P., Lynch D.L., Reggio P.H. GPR6 structural insights: Homology model construction and docking studies. Molecules. 2020;25:725. doi: 10.3390/molecules25030725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fredriksson R., Lagerström M.C., Lundin L.G., Schiöth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 62.Wellendorph P., Hansen K.B., Balsgaard A., Greenwood J.R., Egebjerg J., Bräuner-Osborne H. Deorphanization of GPRC6A: A promiscuous L-alpha-amino acid receptor with preference for basic amino acids. Mol. Pharmacol. 2005;67:589–597. doi: 10.1124/mol.104.007559. [DOI] [PubMed] [Google Scholar]
- 63.Uhlenbrock K., Gassenhuber H., Kostenis E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 2002;14:941–953. doi: 10.1016/S0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- 64.Ignatov A., Lintzel J., Kreienkamp H.J., Schaller H.C. Sphingosine-1-phosphate is a high-affinity ligand for the G protein-coupled receptor GPR6 from mouse and induces intracellular Ca2+ release by activating the sphingosine-kinase pathway. Biochem. Biophys. Res. Commun. 2003;311:329–336. doi: 10.1016/j.bbrc.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 65.Alexander S.P., Battey J., Benson H.E., Benya R.V., Bonner T.I., Davenport A.P., Eguchi S., Harmar A., Holliday N., Jensen R.T., et al. Class A orphans (version 2019.5) in the IUPHAR/BPS guide to pharmacology database. IUPHAR/BPS Guide Pharmacol. CITE. 2019 doi: 10.2218/gtopdb/F16/2019.5. [DOI] [Google Scholar]
- 66.Martin A.L., Steurer M.A., Aronstam R.S. Constitutive activity among orphan class-A G protein coupled receptors. PLoS ONE. 2015;10:e0138463. doi: 10.1371/journal.pone.0138463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morales P., Isawi I., Reggio P.H. Towards a better understanding of the cannabinoid-related orphan receptors GPR3, GPR6, and GPR12. Drug Metab. Rev. 2018;50:74–93. doi: 10.1080/03602532.2018.1428616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wellendorph P., Bräuner-Osborne H. Molecular cloning, expression, and sequence analysis of GPRC6A, a novel family C G-protein-coupled receptor. Gene. 2004;335:37–46. doi: 10.1016/j.gene.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Gutiérrez-Rojas R.A., Aguayo-Cerón K.A., Vargas-De-León C., Cabrera-Becerra S.E., Almanza-Pérez J.C., Huang F., Villafaña S., Romero-Nava R. Glycine Effect on the Expression Profile of Orphan Receptors GPR21, GPR26, GPR39, GPR82 and GPR6 in a Model of Inflammation in 3T3-L1 Cells. Life. 2022;12:1687. doi: 10.3390/life12111687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laun A.S., Shrader S.H., Brown K.J., Song Z.H. GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin. 2019;40:300–308. doi: 10.1038/s41401-018-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boll M., Daniel H., Gasnier B. The SLC36 family: Proton-coupled transporters for the absorption of selected amino acids from extracellular and intracellular proteolysis. Pflügers Arch. 2004;447:776–779. doi: 10.1007/s00424-003-1073-4. [DOI] [PubMed] [Google Scholar]
- 72.Supplisson S., Roux M.J. Why glycine transporters have different stoichiometries. FEBS Lett. 2002;529:93–101. doi: 10.1016/S0014-5793(02)03251-9. [DOI] [PubMed] [Google Scholar]
- 73.Eulenburg V., Armsen W., Betz H., Gomeza J. Glycine transporters: Essential regulators of neurotransmission. Trends Biochem. Sci. 2005;30:325–333. doi: 10.1016/j.tibs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 74.Gomeza J., Armsen W., Betz H., Eulenburg V. Lessons from the Knocked-out Glycine Transporters. Springer; Berlin/Heidelberg, Germany: 2006. pp. 457–483. [DOI] [PubMed] [Google Scholar]
- 75.Roux M.J., Supplisson S. Neuronal and glial glycine transporters have different stoichiometries. Neuron. 2000;25:373–383. doi: 10.1016/S0896-6273(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 76.López-Corcuera B., Martínez-Maza R., Núñez E., Roux M., Supplisson S., Aragón C. Differential properties of two stably expressed brain-specific glycine transporters. J. Neurochem. 1998;71:2211–2219. doi: 10.1046/j.1471-4159.1998.71052211.x. [DOI] [PubMed] [Google Scholar]
- 77.Erdem F.A., Ilic M., Koppensteiner P., Gołacki J., Lubec G., Freissmuth M., Sandtner W. A comparison of the transport kinetics of glycine transporter 1 and glycine transporter 2. J. Gen. Physiol. 2019;151:1035–1050. doi: 10.1085/jgp.201912318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergeron R., Meyer T.M., Coyle J.T., Greene R.W. Modulation of N-methyl-D-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. USA. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Supplisson S., Bergman C. Control of NMDA receptor activation by a glycine transporter co-expressed in Xenopus oocytes. J. Neurosci. 1997;17:4580–4590. doi: 10.1523/JNEUROSCI.17-12-04580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Howard A., Hirst B.H. The glycine transporter GLYT1 in human intestine: Expression and function. Biol. Pharm. Bull. 2011;34:784–788. doi: 10.1248/bpb.34.784. [DOI] [PubMed] [Google Scholar]
- 81.Liu Q.-R., Corcuera B.L., Mandiyan S., Nelson H., Nelson N. Cloning and expression of a spinal cord-and brain-specific glycine transporter with novel structural features. J. Biol. Chem. 1993;268:22802–22808. doi: 10.1016/S0021-9258(18)41598-0. [DOI] [PubMed] [Google Scholar]
- 82.Carta E., Chung S.-K., James V.M., Robinson A., Gill J.L., Remy N., Vanbellinghen J.-F., Drew C.J.G., Cagdas S., Cameron D., et al. Mutations in the GlyT2 gene (SLC6A5) are a second major cause of startle disease. J. Biol. Chem. 2012;287:28975–28985. doi: 10.1074/jbc.M112.372094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Benveniste M., Mayer M.L. Kinetic analysis of antagonist action at N-methyl-D-aspartic acid receptors. Two binding sites each for glutamate and glycine. Biophys. J. 1991;59:560–573. doi: 10.1016/S0006-3495(91)82272-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clements J.D., Westbrook G.L. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- 85.Mizusawa N., Kimura Y., Ishii A., Yamanari T., Nakazawa S., Teramoto H., Ono T.A. Impact of replacement of D1 C-terminal alanine with glycine on structure and function of photosynthetic oxygen-evolving complex. J. Biol. Chem. 2004;279:29622–29627. doi: 10.1074/jbc.M402397200. [DOI] [PubMed] [Google Scholar]
- 86.Collingridge G.L., Volianskis A., Bannister N., France G., Hanna L., Mercier M., Tidball P., Fang G., Irvine M.W., Costa B.M., et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology. 2013;64:13–26. doi: 10.1016/j.neuropharm.2012.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zafra F., Ibáñez I., Bartolomé-Martín D., Piniella D., Arribas-Blázquez M., Giménez C. Glycine transporters and its coupling with NMDA receptors. Glial Amino Acid Transp. 2017;16:55–83. doi: 10.1007/978-3-319-55769-4_4. [DOI] [PubMed] [Google Scholar]
- 88.Fujita H., Sato K., Wen T.C., Peng Y., Sakanaka M. Differential expressions of glycine transporter 1 and three glutamate transporter mRNA in the hippocampus of gerbils with transient forebrain ischemia. J. Cereb. Blood Flow Metab. 1999;19:604–615. doi: 10.1097/00004647-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 89.Zafra F., Aragón C., Giménez C. Molecular biology of glycinergic neurotransmission. Mol. Neurobiol. 1997;14:117–142. doi: 10.1007/BF02740653. [DOI] [PubMed] [Google Scholar]
- 90.Al-Khrasani M., Mohammadzadeh A., Balogh M., Király K., Barsi S., Hajnal B., Köles L., Zádori Z.S., Harsing L.G., Jr. Glycine transporter inhibitors: A new avenue for managing neuropathic pain. Brain Res. Bull. 2019;152:143–158. doi: 10.1016/j.brainresbull.2019.07.008. [DOI] [PubMed] [Google Scholar]
- 91.Gomeza J., Hülsmann S., Ohno K., Eulenburg V., Szöke K., Richter D., Betz H. Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron. 2003;40:785–796. doi: 10.1016/S0896-6273(03)00672-X. [DOI] [PubMed] [Google Scholar]
- 92.Tsai G., Ralph-Williams R.J., Martina M., Bergeron R., Berger-Sweeney J., Dunham K.S., Jiang Z., Caine S.B., Coyle J.T. Gene knockout of glycine transporter 1: Characterization of the behavioral phenotype. Proc. Natl. Acad. Sci. USA. 2004;101:8485–8490. doi: 10.1073/pnas.0402662101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oeckl P., Hengerer B., Ferger B. G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson’s disease. Exp. Neurol. 2014;257:1–9. doi: 10.1016/j.expneurol.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 94.Oeckl P., Ferger B. Increased susceptibility of G-protein coupled receptor 6 deficient mice to MPTP neurotoxicity. Neuroscience. 2016;337:218–223. doi: 10.1016/j.neuroscience.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 95.Liu R., Liao X.Y., Pan M.X., Tang J.C., Chen S.F., Zhang Y., Lu P.X., Lu L.J., Zou Y.Y., Qin X.P., et al. Glycine exhibits neuroprotective effects in ischemic stroke in rats through the inhibition of M1 microglial polarization via the NF-κB p65/Hif-1α signaling pathway. J. Immunol. 2019;202:1704–1714. doi: 10.4049/jimmunol.1801166. [DOI] [PubMed] [Google Scholar]
- 96.Chen Z., Hu B., Wang F., Du L., Huang B., Li L., Qi J., Wang X. Glycine bidirectionally regulates ischemic tolerance via different mechanisms including NR2A-dependent CREB phosphorylation. J. Neurochem. 2015;133:397–408. doi: 10.1111/jnc.12994. [DOI] [PubMed] [Google Scholar]
- 97.Gusev E.I., Skvortsova V.I., Dambinova S., Raevskiy K.S., Alekseev A.A., Bashkatova V.G., Kovalenko A.V., Kudrin V.S., Yakovleva E.V. Neuroprotective effects of glycine for therapy of acute ischaemic stroke. Cerebrovasc. Dis. 2000;10:49–60. doi: 10.1159/000016025. [DOI] [PubMed] [Google Scholar]
- 98.Ikejima K.E.N.I.C.H.I., Iimuro Y.U.J.I., Forman D.T., Thurman R.G. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol.-Gastrointest. Liver Physiol. 1996;271:G97–G103. doi: 10.1152/ajpgi.1996.271.1.G97. [DOI] [PubMed] [Google Scholar]
- 99.Senthilkumar R., Viswanathan P., Nalini N. Glycine modulates hepatic lipid accumulation in alcohol-induced liver injury. Pol. J. Pharmacol. 2003;55:603–611. [PubMed] [Google Scholar]
- 100.Zeb A., Rahman S.U. Protective effects of dietary glycine and glutamic acid toward the toxic effects of oxidized mustard oil in rabbits. Food Funct. 2017;8:429–436. doi: 10.1039/C6FO01329E. [DOI] [PubMed] [Google Scholar]
- 101.Li P., Yin Y.L., Li D., Kim S.W., Wu G. Amino acids and immune function. Br. J. Nutr. 2007;98:237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- 102.Magnusson M., Wang T.J., Clish C., Engström G., Nilsson P., Gerszten R.E., Melander O. Dimethylglycine deficiency and the development of diabetes mellitus. Diabetes. 2015;64:3010–3016. doi: 10.2337/db14-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Y., Lv S.J., Yan H., Wang L., Liang G.P., Wan Q.X., Peng X. Effects of glycine supplementation on myocardial damage and cardiac function after severe burn. Burns. 2013;39:729–735. doi: 10.1016/j.burns.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 104.Ascher E., Hanson J.N., Cheng W., Hingorani A., Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129:231–235. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- 105.Grotz M.R.W., Pape H.C., van Griensven M., Stalp M., Rohde F., Bock D., Krettek C. Glycine reduces the inflammatory response and organ damage in a two-hit sepsis model in rats. Shock. 2001;16:116–121. doi: 10.1097/00024382-200116020-00006. [DOI] [PubMed] [Google Scholar]
- 106.Yang S., Koo D.J., Chaudry I.H., Wang P. Glycine attenuates hepatocellular depression during early sepsis and reduces sepsis-induced mortality. Crit. Care Med. 2001;29:1201–1206. doi: 10.1097/00003246-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 107.Zhong Z., Enomoto N., Connor H.D., Moss N., Mason R.P., Thurman R.G. Glycine improves survival after hemorrhagic shock in the rat. Shock. 1999;12:54–62. doi: 10.1097/00024382-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 108.Qu W., Ikejima K., Zhong Z., Waalkes M.P., Thurman R.G. Glycine blocks the increase in intracellular free Ca2+ due to vasoactive mediators in hepatic parenchymal cells. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002;283:G1249–G1256. doi: 10.1152/ajpgi.00197.2002. [DOI] [PubMed] [Google Scholar]
- 109.Alvarado-Vásquez N., Zamudio P., Cerón E., Vanda B., Zenteno E., Carvajal-Sandoval G. Effect of glycine in streptozotocin-induced diabetic rats. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2003;134:521–527. doi: 10.1016/S1532-0456(03)00046-2. [DOI] [PubMed] [Google Scholar]
- 110.Hafidi M.E., Pérez I., Zamora J., Soto V., Carvajal-Sandoval G., Banos G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2004;287:R1387–R1393. doi: 10.1152/ajpregu.00159.2004. [DOI] [PubMed] [Google Scholar]
- 111.Ramakrishnan S., Sulochana K.N. Decrease in glycation of lens proteins by lysine and glycine by scavenging of glucose and possible mitigation of cataractogenesis. Exp. Eye Res. 1993;57:623–628. doi: 10.1006/exer.1993.1167. [DOI] [PubMed] [Google Scholar]
- 112.Sandoval G.C., Santillan R.M., Juarez E., Martlnez G.R., Juärez M.E.C. Effect of glycine on hemoglobin glycation in diabetic patients. Proc. West. Pharmacol. Soc. 1999;42:31–32. [PubMed] [Google Scholar]
- 113.Bahmani F., Bathaie S.Z., Aldavood S.J., Ghahghaei A. Glycine therapy inhibits the progression of cataract in streptozotocin-induced diabetic rats. Mol. Vis. 2012;18:439. [PMC free article] [PubMed] [Google Scholar]
- 114.Zhong Z., Wheeler M.D., Li X., Froh M., Schemmer P., Yin M., Bunzendaul H., Bradford B., Lemasters J.J. L-Glycine: A novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care. 2003;6:229–240. doi: 10.1097/00075197-200303000-00013. [DOI] [PubMed] [Google Scholar]
- 115.Razak M.A., Begum P.S., Viswanath B., Rajagopal S. Multifarious Beneficial Effect of Nonessential Amino Acid, Glycine: A Review. Oxidative Med. Cell. Longev. 2017;2017:1716701. doi: 10.1155/2017/1716701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Howard A., Tahir I., Javed S., Waring S.M., Ford D., Hirst B.H. Glycine transporter GLYT1 is essential for glycine-mediated protection of human intestinal epithelial cells against oxidative damage. J. Physiol. 2010;588:995–1009. doi: 10.1113/jphysiol.2009.186262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Bergenhenegouwen J., Braber S., Loonstra R., Buurman N., Rutten L., Knipping K., Savelkoul P.J., Harthoorn L.F., Jahnsen F.L., Garssen J., et al. Oral exposure to the free amino acid glycine inhibits the acute allergic response in a model of cow’s milk allergy in mice. Nutr. Res. 2018;58:95–105. doi: 10.1016/j.nutres.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 118.Ham D.J., Murphy K.T., Chee A., Lynch G.S., Koopman R. Glycine administration attenuates skeletal muscle wasting in a mouse model of cancer cachexia. Clin. Nutr. 2014;33:448–458. doi: 10.1016/j.clnu.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 119.Ceyhan G., Timm A.-K., Bergmann F., Günther A., Aghdassi A., Demir I., Mayerle J., Kern M., Lerch M., Büchler M., et al. Prophylactic glycine administration attenuates pancreatic damage and inflammation in experimental acute pancreatitis. Pancreatology. 2011;11:57–67. doi: 10.1159/000325972. [DOI] [PubMed] [Google Scholar]
- 120.Hartog A., Leenders I., van der Kraan P.M., Garssen J. Anti-inflammatory effects of orally ingested lactoferrin and glycine in different zymosan-induced inflammation models: Evidence for synergistic activity. Int. Immunopharmacol. 2007;7:1784–1792. doi: 10.1016/j.intimp.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 121.Wheeler M.D., Thurman R.G. Production of superoxide and TNF-α from alveolar macrophages is blunted by glycine. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999;277:L952–L959. doi: 10.1152/ajplung.1999.277.5.L952. [DOI] [PubMed] [Google Scholar]
- 122.Froh M., Thurman R.G., Wheeler M.D. Molecular evidence for a glycine-gated chloride channel in macrophages and leukocytes. Am. J. Physiol.-Gastrointest. Liver Physiol. 2002;283:G856–G863. doi: 10.1152/ajpgi.00503.2001. [DOI] [PubMed] [Google Scholar]
- 123.Stoffels B., Türler A., Schmidt J., Nazir A., Tsukamoto T., Moore B.A., Schnurr C., Kalff J.C., Bauer A.J. Anti-inflammatory role of glycine in reducing rodent postoperative inflammatory ileus. Neurogastroenterol. Motil. 2011;23:76-e8. doi: 10.1111/j.1365-2982.2010.01603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weinberg J.M., Bienholz A., Venkatachalam M.A. The role of glycine in regulated cell death. Cell. Mol. Life Sci. 2016;73:2285–2308. doi: 10.1007/s00018-016-2201-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamashina S., Ikejima K., Enomoto N., Takei Y., Sato N. Glycine as a Therapeutic Immuno-Nutrient for Alcoholic Liver Disease. Alcohol. Clin. Exp. Res. 2005;29:162S–165S. doi: 10.1097/01.alc.0000189281.82523.6c. [DOI] [PubMed] [Google Scholar]
- 126.AlShakweer W., Alwelaie Y., Mankung A.M., Graeber M.B. Bone marrow-derived microglia in pilocytic astrocytoma. Front. Biosci. 2011;3:371–379. doi: 10.2741/e252. [DOI] [PubMed] [Google Scholar]
- 127.Xia C.Y., Zhang S., Gao Y., Wang Z.Z., Chen N.H. Selective modulation of microglia polarization to M2 phenotype for stroke treatment. Int. Immunopharmacol. 2015;25:377–382. doi: 10.1016/j.intimp.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 128.Zhu T., Miller A.G., Deliyanti D., Berka D.R., Agrotis A., Campbell D.J., Wilkinson-Berka J.L. Prorenin stimulates a pro-angiogenic and proinflammatory response in retinal endothelial cells and an M1 phenotype in retinal microglia. Clin. Exp. Pharmacol. Physiol. 2015;42:537–548. doi: 10.1111/1440-1681.12376. [DOI] [PubMed] [Google Scholar]
- 129.Xu Y., Xu Y., Wang Y., Wang Y., He L., Jiang Z., Huang Z., Liao H., Li J., Saavedra J.M., et al. Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKb-dependent AMPK activation. Brain Behav. Immun. 2015;50:298–313. doi: 10.1016/j.bbi.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 130.Rose M.L., Cattley R.C., Dunn C., Wong V., Li X., Thurman R.G. Dietary glycine prevents the development of liver tumors caused by the peroxisome proliferator WY-14,643. Carcinogenesis. 1999;20:2075–2081. doi: 10.1093/carcin/20.11.2075. [DOI] [PubMed] [Google Scholar]
- 131.Spittler A., Reissner C.M., Oehler R., Gornikiewicz A., Gruenberger T., Manhart N., Brodowicz T., Mittlboeck M., Boltz-Nitulescu G., Roth E. Immunomodulatory effects of glycine on LPS-treated monocytes: Reduced TNF-alpha production and accelerated IL-10 expression. FASEB J. 1999;13:563–571. doi: 10.1096/fasebj.13.3.563. [DOI] [PubMed] [Google Scholar]
- 132.Li X., Bradford B.U., Wheeler M.D., Stimpson S.A., Pink H.M., Brodie T.A., Schwab J.H., Thurman R.G. Dietary glycine prevents peptidoglycan polysaccharide-induced reactive arthritis in the rat: Role for glycine-gated chloride channel. Infect. Immun. 2001;69:5883–5891. doi: 10.1128/IAI.69.9.5883-5891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Okekunle A.P., Li Y., Liu L., Du S., Wu X., Chen Y., Li Y., Qi J., Sun C., Feng R. Abnormal circulating amino acid profiles in multiple metabolic disorders. Diabetes Res. Clin. Pract. 2017;132:45–58. doi: 10.1016/j.diabres.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 134.Gosmanov N.R., Gosmanov A.R., Gerich J.E. Glucagon Physiology. In: Feingold K.R., Anawalt B., Boyce A., Chrousos G., Dungan K., Grossman A., Hershman J.M., Kaltsas G., Koch C., Kopp P., et al., editors. Endotext [Internet] MDText.com, Inc.; South Dartmouth, MA, USA: 2000. [Google Scholar]
- 135.Yan-Do R., MacDonald P.E. Impaired “glycine”-mia in type 2 diabetes and potential mechanisms contributing to glucose homeostasis. Endocrinology. 2017;158:1064–1073. doi: 10.1210/en.2017-00148. [DOI] [PubMed] [Google Scholar]
- 136.White P.J., Lapworth A.L., An J., Wang L., McGarrah R.W., Stevens R.D., Ilkayeva O., George T., Muehlbauer M.J., Bain J.R., et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Mol. Metab. 2016;5:538–551. doi: 10.1016/j.molmet.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nguyen D., Hsu J.W., Jahoor F., Sekhar R.V. Eect of increasing glutathione with cysteine and glycine supplementation on mitochondrial fuel oxidation, insulin sensitivity, and body composition in older HIV-infected patients. J. Clin. Endocrinol. Metab. 2014;99:169–177. doi: 10.1210/jc.2013-2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beato M. The time course of transmitter at glycinergic synapses onto motoneurons. J. Neurosci. 2008;28:7412–7425. doi: 10.1523/JNEUROSCI.0581-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xiong W., Cui T., Cheng K., Yang F., Chen S.-R., Willenbring D., Guan Y., Pan H.-L., Ren K., Xu Y., et al. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 2012;209:1121–1134. doi: 10.1084/jem.20120242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bregman H., Simard J.R., Andrews K.L., Ayube S., Chen H., Gunaydin H., Guzman-Perez A., Hu J., Huang L., Huang X., et al. discovery and hit-to-lead optimization of tricyclic sulfonamides as potent and efficacious potentiators of glycine receptors. J. Med. Chem. 2017;60:1105–1125. doi: 10.1021/acs.jmedchem.6b01496. [DOI] [PubMed] [Google Scholar]
- 141.Wheeler M., Stachlewitz R.F., Yamashina S., Ikehima K., Morrow A.L., Thurman R.G. Glycine-gated chloride channels in neutrophils attenuate calcium influx and superoxide production. FASEB J. 2000;14:476–484. doi: 10.1096/fasebj.14.3.476. [DOI] [PubMed] [Google Scholar]
- 142.Kawada N., Mizoguchi Y., Kobayashi K., Monna T., Morisawa S., Ueda N., Omoto Y., Takahashi Y., Yamamoto S. Possible induction of fatty acid cyclo-oxygenase in lipopolysaccharide-stimulated rat Kupffer cells. Gastroenterology. 1992;103:1026–1033. doi: 10.1016/0016-5085(92)90039-2. [DOI] [PubMed] [Google Scholar]
- 143.Garcia-Macedo R., Sanchez-Muñoz F., Almanza-Perez J.C., Duran-Reyes G., Alarcon-Aguilar F., Cruz M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur. J. Pharmacol. 2008;587:317–321. doi: 10.1016/j.ejphar.2008.03.051. [DOI] [PubMed] [Google Scholar]
- 144.Ma X., Zhang Y., Jiang D., Yang Y., Wu G., Wu Z. Protective effects of functional amino acids on apoptosis, inflammatory response, and pulmonary fibrosis in lipopolysaccharide-challenged mice. J. Agric. Food Chem. 2019;67:4915–4922. doi: 10.1021/acs.jafc.9b00942. [DOI] [PubMed] [Google Scholar]
- 145.Newgard C.B. Metabolomics and metabolic diseases: Where do we stand? Cell Metab. 2017;25:43–56. doi: 10.1016/j.cmet.2016.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Takashina C., Tsujino I., Watanabe T., Sakaue S., Ikeda D., Yamada A., Sato T., Ohira H., Otsuka Y., Oyama-Manabe N., et al. Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr. Metab. 2016;13:5. doi: 10.1186/s12986-015-0059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tariq M., Al Moutaery A.R. Studies on the antisecretory, gastric anti-ulcer and cytoprotective properties of glycine. Res. Commun. Mol. Pathol. Pharmacol. 1997;97:185–198. [PubMed] [Google Scholar]
- 148.Rose M.L., Madren J., Bunzendahl H., Thurman R.G. Dietary glycine inhibits the growth of B16 melanoma tumors in mice. Carcinogenesis. 1999;20:793–798. doi: 10.1093/carcin/20.5.793. [DOI] [PubMed] [Google Scholar]
- 149.El Kamouni S., El Kebbaj R., Andreoletti P., El Ktaibi A., Rharrassi I., Essamadi A., El Kebbaj M.S., Mandard S., Latruffe N., Vamecq J., et al. Protective Effect of Argan and Olive Oils against LPS-Induced Oxidative Stress and Inflammation in Mice Livers. Int. J. Mol. Sci. 2017;18:2181. doi: 10.3390/ijms18102181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mauriz J.L., Matilla B., Culebras J.M., González P., González-Gallego J. Dietary glycine decreases liver injury after haemorrhagic shock in rats. Br. J. Surg. 2000;87:945–946. doi: 10.1046/j.1365-2168.2000.01544-33.x. [DOI] [Google Scholar]
- 151.Stachlewitz R.F., Li X., Smith S., Bunzendahl H., Graves L.M., Thurman R.G. Glycine Inhibits Growth of T Lymphocytes by an IL-2-Independent Mechanism. J. Immunol. 2000;164:176–182. doi: 10.4049/jimmunol.164.1.176. [DOI] [PubMed] [Google Scholar]
- 152.Hasegawa S., Ichiyama T., Sonaka I., Ohsaki A., Okada S., Wakiguchi H., Kudo K., Kittaka S., Hara M., Furukawa S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2011;167:269–274. doi: 10.1111/j.1365-2249.2011.04519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matilla B., Mauriz J.L., Culebras J.M., Gonzalez-Gallego J., Gonzalez P. La glicina: Un nutriente antioxidante protector celular. Nutr. Hosp. 2002;17:2–9. [PubMed] [Google Scholar]
- 154.Chen J., Ma X., Yang Y., Dai Z., Wu Z., Wu G. Glycine enhances expression of adiponectin and IL-10 in 3T3-L1 adipocytes without affecting adipogenesis and lipolysis. Amino Acids. 2018;50:629–640. doi: 10.1007/s00726-018-2537-3. [DOI] [PubMed] [Google Scholar]
- 155.Alarcon-Aguilar F., Almanza-Perez J., Blancas G., Angeles S., Garcia-Macedo R., Roman R., Cruz M. Glycine regulates the production of pro-inflammatory cytokines in lean and monosodium glutamate-obese mice. Eur. J. Pharmacol. 2008;599:152–158. doi: 10.1016/j.ejphar.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 156.Blancas-Flores G., Alarcón-Aguilar F.J., García-Macedo R., Almanza-Pérez J.C., Flores-Sáenz J.L., Román-Ramos R., Ventura-Gallegos J.L., Kumate J., Zentella-Dehesa A., Cruz M. Glycine suppresses TNF-alpha-induced activation of NF-κB in differentiated 3T3-L1 adipocytes. Eur. J. Pharmacol. 2012;689:270–277. doi: 10.1016/j.ejphar.2012.06.025. [DOI] [PubMed] [Google Scholar]
- 157.Forsythe L.K., Wallace J.M., Livingstone M.B. Obesity and inflammation: The effects of weight loss. Nutr. Res. Rev. 2008;21:117–133. doi: 10.1017/S0954422408138732. [DOI] [PubMed] [Google Scholar]
- 158.Romero-Nava R., Alarcón-Aguilar F.J., Giacoman-Martínez A., Blancas-Flores G., Aguayo-Cerón K.A., Ballinas-Verdugo M.A., Sánchez-Muñoz F., Huang F., Villafaña-Rauda S., Almanza-Pérez J.C. Glycine is a competitive antagonist of the TNF receptor mediating the expression of inflammatory cytokines in 3T3-L1 adipocytes. Inflamm. Res. 2021;70:605–618. doi: 10.1007/s00011-021-01462-1. [DOI] [PubMed] [Google Scholar]
- 159.Amin K., Li J., Chao W.R., Dewhirst M.W., Haroon Z.A. Dietary glycine inhibits angiogenesis during wound healing and tumor growth. Cancer Biol. Ther. 2003;2:173–178. doi: 10.4161/cbt.2.2.280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data Availability Statements are available in section “MDPI Research Data Policies” at https://www.mdpi.com/ethics.